3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(4):926-938. doi:10.7150/jca.87499 This issue Cite
Review
Vitamin D and Its Receptors in Cervical Cancer
1. Department of Epidemiology and Biostatistics, School of Public Health, Tianjin Medical University, Tianjin, China.
2. Tianjin Key Laboratory of Environment, Nutrition and Public Health, Tianjin, China.
3. Tianjin Center for International Collaborative Research in Environment, Nutrition and Public Health, Tianjin, China.
4. Department of Nutrition and Food Science, School of Public Health, Tianjin Medical University, Tianjin, China.
5. Department of Urology, The Second Hospital of Tianjin Medical University, Tianjin, China.
*These authors have contributed equally to this work and share correspondence and last authorship.
Received 2023-6-26; Accepted 2023-11-22; Published 2024-1-1
Abstract
Several studies have investigated the relationship between vitamin D (VD) and its receptors (VDR) and the risk of cervical cancer. However, the underlying mechanisms that underpin these associations remain incompletely comprehended. In this review, we analyzed the impacts of VD and VDR on cervical cancer and related mechanisms, and discussed the effects of VD, calcium, and other vitamins on cervical cancer. Our literature research found that VD, VDR and their related signaling pathways played indispensable roles in the occurrence and progression of cervical cancer. Epidemiological studies have established associations between VD, VDR, and cervical cancer susceptibility. Current studies have shown that the inhibitory effect of VD and VDR on cervical cancer may be attributed to a variety of molecules and pathways, such as the EAG potassium channel, HCCR-1, estrogen and its receptor, p53, pRb, TNF-α, the PI3K/Akt pathway, and the Wnt/β-catenin pathway. This review also briefly discussed the association between VDR gene polymorphisms and cervical cancer, albeit a comprehensive elucidation of this relationship remains an ongoing research endeavor. Additionally, the potential ramifications of VD, calcium, and other vitamins on cervical cancer has been elucidated, yet further exploration into the precise mechanistic underpinnings of these potential effects is warranted. Therefore, we suggest that further studies should focus on explorations into the intricate interplay among diverse molecular pathways and entities, elucidation of the mechanistic underpinnings of VDR polymorphic loci changes in the context of HPV infection and VD, inquiries into the mechanisms of VD in conjunction with calcium and other vitamins, as well as investigations of the efficacy of VD supplementation or VDR agonists as part of cervical cancer treatment strategies in the clinical trials.
Keywords: vitamin D, vitamin D receptor, cervical cancer
Introduction
Cervical cancer has been identified as the fourth most common cancer and the fourth leading cause of cancer-related deaths among women worldwide, with approximately 600,000 new cases and 340,000 deaths reported annually [1]. The pathogenesis of cervical cancer is a complicated biological process, which involves multi-stage, long-term progression, and multi-factor interactions. Cervical cancer typically originates from healthy cervical tissue and progresses through cervical intraepithelial neoplasia (CIN 1/2/3) to invasive cervical cancer [2, 3]. Although the etiology of cervical cancer remains elusive, high-risk human papillomavirus (HR-HPV) is a significant risk factor, with subtypes 16 and 18 being the most prevalent. Despite the widespread occurrence of human papillomavirus (HPV) infections, more than 90% of women can clear the HPV infection within three years through their immune system after infection. Only 10% of patients may experience persistent HPV infection, and ultimately, less than 1% of those with persistent HPV infection will develop cervical cancer [4]. The reasons for carcinogenesis are possibly related to environmental factors, including dietary patterns and lifestyle choices. Consequently, exploring the factors that lead to persistent HPV infection and ultimately result in the development of cervical cancer has been a prominent research focus in recent years.
The global prevalence of vitamin D deficiency has emerged as a significant health concern, which has raised concerns regarding its potential implications for persistent HPV infection and cervical cancer occurrence. The prevalence of vitamin D (VD) deficiency ranges from 6.9% to 81.8% in European nations and from 2.0% to 87.5% in Asian nations. In more than half the countries, VD deficiency is present in more than 50% of adult individuals [5]. The presence of vitamin D receptors (VDR) and VD-activating enzymes in immune cells, such as monocytes, macrophages, dendritic cells, and lymphocytes, suggests that VD may act as an immunomodulator by binding VDR [6]. Previous research indicated that women with compromised immunity were at elevated risk of harboring persistent HPV infection, which may subsequently progress to CIN and ultimately develop into cervical cancer [2, 3].
It is well known that VD can facilitate bone formation and calcification by stimulating the absorption of calcium and phosphorus by intestinal mucosal cells [7, 8]. Notably, there is a growing body of evidence suggesting that VD and its metabolites may exert a significant role in the prevention or treatment of gynecological cancers, representing a novel and distinct function of VD that diverges from its established function in the regulation of calcium and bone metabolism [9]. However, the precise anticancer mechanism of VD in cervical cancer is still unclear and requires further exploration. This study was proposed to elaborate on previous research regarding VD and VDR and cervical cancer, and to discuss the function and the underlying mechanisms of VD and VDR in cervical cancer.
Metabolic and biological functions of VD
VD is a crucial nutrient required for maintaining various daily activities. It serves as a potent precursor for the steroid hormone that mainly regulates the balance of calcium and phosphorus in the human body and contributes to bone mineralization. In recent years, numerous studies have linked VD deficiency to several extra-skeletal diseases, including cancer, high blood pressure, and autoimmune diseases [10].
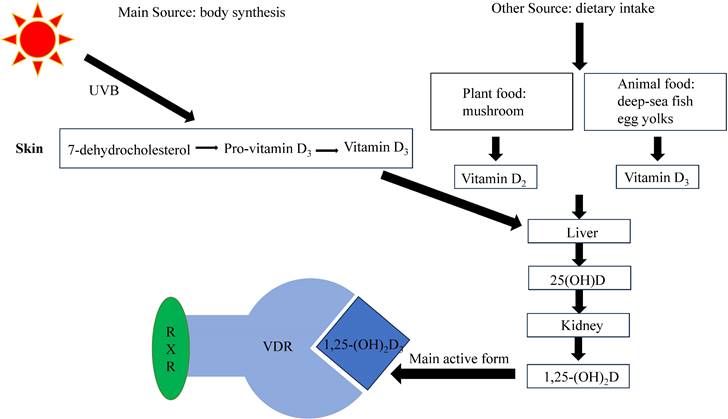
VD can be obtained from two different ways: body synthesis and dietary intake. It is pertinent to note that dietary sources of VD include fatty fish (e.g. salmon: 441IU per 100g), fortified foods such as fortified milk (40IU per 100g) and fortified cheese (301IU per 100g), and to a lesser extent, egg yolks (218IU per 100g) and “sun-dried” mushrooms (154IU per 100g) [11]. However, dietary sources of VD alone are insufficient to meet the body's needs, making the primary source of VD synthesis in the skin via photochemistry in response to ultraviolet B (UVB) radiation.
Currently, several types of VD exist, with VD2 and VD3 being the more important. VD2 (ergocalciferol) is mainly produced in plants, while VD3 (cholecalciferol), synthesized as a prohormone in the skin, is the body's primary source of VD [12]. However, VD itself is not biologically active and needs to undergo two hydroxylation processes to convert into its biologically active form, 1,25-dihydroxyvitamin D [13]. The liver converts VD into 25-hydroxyvitamin D (25(OH)D), which is the main type of VD in the body, and has a long half-life and a high serum concentration. The serum 25(OH)D level serves as an effective indicator of the body's VD levels. The kidneys then convert 25(OH)D into the active metabolite 1,25-dihydroxyvitamin D, of which 1, 25-dihydroxyvitamin D3(1,25-(OH)2D3, calcitriol) is the most active, which exerts its physiological effects by binding to VD receptors (VDRs) (Figure 1).
Both 25(OH)D and 1,25-(OH)2D3 bind to VD binding protein and are transported in the blood. As 1,25-(OH)2D3 has a 1000-fold higher affinity for VDR than 25(OH)D, it is considered the primary effector of VDR binding [14, 15]. The active metabolite 1,25-(OH)2D3 binds to VDR and regulates various physiological processes in the body [16, 17].
The structure and biological function of VDR
VDR, as a transcription factor, reacts to 1,25-(OH)2D3 and mediates its biological effects, such as calcium homeostasis and immune response [18, 19]. When activated by 1,25-(OH)2D3, VDR promotes the expression of genes responsible for enhancing calcium absorption from dietary sources into the bloodstream [18]. Activated VDR can influence the immune system by modulating the production of cytokines, such as interleukin-2 (IL-2), and regulating the differentiation and function of immune cells [19]. VDR can heterodimerize with retinoid X receptor (RXR) to bind to response elements on target genes and thereby modulate gene expression [20] (Figure 1). The VDR gene is located in chromosome 12, with hundreds of single nucleotide polymorphisms. Promoter methylation can cause epigenetic changes, but its effect on VDR expression remains controversial [21]. The occurrence of VDR gene polymorphism may influence VD on various physiological processes [22]. For example, deleterious mutations in the VDR gene may cause inherited 1,25-(OH)2D3 resistant rickets, wherein mutated VDR retains the ability to bind to 1,25-(OH)2D3 but exerts an antagonistic influence on its biological outcomes [23]. VDR gene polymorphism (Fok1) may also be associated with calcium regulation [24]. VDR can also exhibit nongenomic effects that do not involve VD, which may be related to protein-protein interactions [25].
VD and cervical cancer
Several ecological studies have investigated the potential impact of UVB exposure on the risk of cervical cancer through the modulation of VD levels. One such study of more than 70,000 cases of black and white patients by Adams et al using the data from the National Cancer Institute found a negative correlation between UV exposure and cervical cancer incidence in America [26]. Grant et al. also conducted a multifactorial ecological study in Caucasian Americans from 50 states, which demonstrated a negative association between UVB exposure and cervical cancer mortality [27]. Additionally, Chen et al. carried out an ecological study using data from the National Central Cancer Registries (1998-2002), and they reported a 13% decrease in the incidence of cervical cancer for every 10-unit increase in UVB [28], while Grant found a correlation between increased UVB exposure (heat zone and latitude) and reduced mortality of cervical cancer, using data from the National Death Survey in China (1973-1975) [29]. Grant's study in twelve administrative areas in France showed a positive relationship between cervical cancer incidence and latitude [30]. Taken together, the findings of these ecological studies provide evidence supporting the hypothesis that increased VD levels resulting from UVB exposure may play a role in reducing the risk of cervical cancer (Table 1). However, it is important to note that ecological studies inherently lack the capacity to establish a causal relationship, thus underscoring the necessity for additional research in this regard.
Synthesis and metabolism of VD[48]. VD is mostly produced by the skin under the influence of UVB radiation, although it can also be acquired through dietary sources. Upon circulation in the bloodstream, VD undergoes hepatic conversion into 25(OH)D. Subsequently, in the renal tissues, it undergoes further transformation into 1,25-(OH)2D, with 1,25-(OH)2D3 being the most biologically active form. To regulate gene expression, 1,25-(OH)2D3 interacts with VDR and forms complexes with RXR.
Ecological research on the association between UVB exposure and cervical cancer.
| Authors | Year | Sample size | Date source | Results | Reference |
|---|---|---|---|---|---|
| Adams et al | 2016 | 71,209 patients | The National Cancer Institute | The age-adjusted cervical cancer incidence per 100,000 individuals was 4.47, 4.36, and 8.27 for the high (>12363.17 J/m2), medium (10647.77-12363.17 J/m2), and low (<10647.77 J/m2) UV exposure groups, respectively (P<0.001). | [26] |
| Grant et al | 2006 | 50 states | State-averaged data for Caucasians for the periods 1950-1969 and 1970-1994 | A negative association between UVB exposure and age-adjusted cervical cancer mortality (r=-0.46, P=0.005). | [27] |
| Chen et al | 2010 | 30 counties | The Chinese National Central Cancer Registries, 1998-2002 | Association between UVB and cervical cancer incidence (RR=0.87, 95%CI 0.80-0.95). | [28] |
| Grant | 2007 | 39 counties | National Death Survey, 1973-1975 in China | A negative association between heat zone and cervical cancer mortality (β=-0.61, P<0.001), a positive association between latitude and mortality (β=0.69, P<0.001). | [29] |
| Grant | 2010 | 21 continental regions of France | The 12 cancer registries and a publication of the Fédération Nationale des Observatoires Régionaux de la Santé, etc | A positive association between latitude and cervical cancer incidence (r=0.60, P=0.004). | [30] |
Abbreviations: RR, risk ratio; CI, confidence interval.
Epidemiological research on VD deficiency and HPV infection.
| Authors | Year | Sample size | Design | Results | Reference |
|---|---|---|---|---|---|
| Özgü et al | 2016 | 85 patients | Case-control study | Women with abnormal cervical smear results and positive HPV DNA had lower levels of VD compared to women with negative HPV DNA. | [32] |
| Gupta et al | 2021 | 4343 females | Cross-sectional study | VD deficiency was associated with higher HPV infection (low-risk HPV infection: OR=1.41, 95%CI 1.23-1.61; HR-HPV: OR=1.25, 95%CI 1.04-1.49). | [33] |
| Chu et al | 2021 | 7699 females | Cohort study | HPV positive women had lower serum VD levels (P<0.01). | [34] |
| Shim et al | 2016 | 2353 females | Cross-sectional study | For every 10 ng/mL reduction in serum 25(OH)D levels, the risk of high-risk HPV infection increased (OR=1.14, 95%CI 1.02-1.27). | [35] |
| Troja et al | 2020 | 404 females | Cohort study | Serum VD levels were not associated with HR-HPV infection. | [36] |
Abbreviations: OR, odds ratio; CI, confidence interval.
The inhibitory effect of VD on cervical cancer may be related to its potential association with HPV infection. VD has been shown to activate genes and pathways involved in both innate and adaptive immunity, indicating its role in the immune process [18]. Previous studies have demonstrated that VD may be utilized as a prophylactic and adjuvant therapy for diseases caused by impaired immune homeostasis [31]. Özgü et al. have suggested that VD deficiency may be a risk factor of HPV DNA persistence and related CIN [32]. In a cross-sectional survey of 4,343 women, Gupta et al. discovered that serum VD deficiency was associated with a higher risk of HR-HPV infection [33]. In a cohort study involving 7,699 female adults Chu et al. found that women with HR-HPV infection had lower serum VD levels [34]. Shim et al. observed an increased in HR-HPV infection rates for every 10ng/ml fall in serum 25(OH)D levels in a cross-sectional study of 2353 women in the United States (OR=1.14, 95%CI: 1.02-1.27) [35], while Troja et al. found no correlation between HR-HPV infection rates and the increase of serum 25(OH)D level by 10ng/ml in a narrower age range of 30-50 years old [36]. It may be that age range has some influence on the association between VD and HPV infection [35, 36]. Nevertheless, these findings have suggested that persistent HPV infection is linked to decreased immune function, and VD may reduce HPV infection rates by improving the body's immunity, thus reducing cervical cancer morbidity and mortality (Table 2).
Numerous epidemiological studies have investigated the relationship between VD and cervical cancer. In a case-control study of 405 cervical cancer patients and 2,025 age-matched controls, Hosono et al. found that Japanese women who consumed higher doses of VD had a lower incidence of invasive cervical cancer, but not CIN3[37]. A randomized, double-blind, placebo-controlled parallel clinical trial involving 58 patients with CIN1 in Iran by Vahedpoor et al. revealed that long-term use of VD supplements for six months was associated with a higher proportion of CIN1 regression than the placebo group [38]. Meanwhile, Vahedpoor et al. [39] conducted the similar randomized controlled trial on 58 patients with CIN2/3 and found that the same VD supplement for six months had a positive impact on reducing the recurrence rate in CIN1/2/3. However, when CIN1 was excluded, the difference of recurrence rate between the intervention and placebo groups was no longer statistically significant. The same dose and duration of VD supplementation did not produce the same effect in CIN2/3 patients as in CIN1 patients, possibly due to the greater severity of cervical lesions and more severe symptoms in CIN2/3, which may require a higher dose and longer period of VD supplementation. While these studies have provided a foundation for further investigation, larger and more diverse sample sizes are generally preferred to draw more robust conclusions (Table 3).
Epidemiological research on VD and cervical cancer.
| Authors | Year | Sample size | Design | Results | Reference |
|---|---|---|---|---|---|
| Hosono et al | 2010 | 405 cervical neoplasias and 2025 age-matched non-cancer controls in Japan | Case-control study | Compared with Q1, VD intake had an inverse association in invasive cervical cancer (Q2: OR=1.03, 95%CI 0.74-1.44; Q3: OR=0.80, 96%CI 0.56-1.15; Q4: OR=0.64, 95%CI 0.43-0.94, Ptrend=0.013). However, there were no association between VD intake and CIN3 (Ptrend=0.109). | [37] |
| Vahedpoor et al | 2017 | 58 CIN1 patients | Randomized Controlled Trial | CIN1 regression rate of VD group vs. placebo group: 84.6% vs. 53.8% (P=0.01). | [38] |
| Vahedpoor et al | 2018 | 58 CIN2/3 patients | Randomized Controlled Trial | Comparing the VD group's CIN1/2/3 recurrence rate to the placebo group: 18.5% vs. 48.1% (P=0.02). However, the changes were not statistically significant (P=0.15) when CIN1 was excluded from the analysis. | [39] |
Abbreviations: Q, quartile; OR, odds ratio; CI, confidence interval.
Research on VDR and risk of cancer.
| Authors | Year | Sample size | Design | Results | Reference |
|---|---|---|---|---|---|
| Deuster et al | 2017 | - | Systematic review | Compared with normal tissues, VDR was upregulated in cancers such as endometrial cancer, ovarian cancer, cervical cancer and vulvar cancer. | [9] |
| Huss et al | 2019 | 718 patients | Cohort study | VDR positive expression was associated with favorable tumor characteristics such as smaller tumor size and lower tumor grade. High VDR expression was associated with a reduced risk of breast cancer death. | [41] |
| Hendrickson et al | 2011 | 841 patients | Cohort study | Men with the highest VDR expression had a significantly reduced risk of fatal prostate cancer compared to the lowest quartile (HR=0.17; 95% CI, 0.07- 0.41). | [42] |
| Ditsch et al | 2012 | 82 patients | Cohort study | Patients with higher VDR expression levels had higher overall survival. | [43] |
| Friedrich et al | 2002 | 50 cervical cancer tissues and 15 Benign cervical tissues | Case-control study | There were no significant correlations between the expression of VDR and tumor stage, histological type, proliferation marker KI-67 and tumor suppressor gene p53 expression. | [44] |
| Friedrich et al | 2003 | - | Case-control study | The expression level of VDR was higher in cervical cancer tissues compared with normal tissues. However, upregulation of VDR was not associated with cell proliferation. There was no statistical association between VDR expression and histological type or grade. | [45] |
| Krishnan et al | 2012 | - | Systematic review | Calcitriol reduced the expression of aromatase, and the combination of calcitriol and aromatase inhibitors may have a better anti-cancer effect. | [46] |
Abbreviations: HR, hazard ratio; CI, confidence interval.
According to animal experimentation, the administration of calcitriol alone demonstrated a suppressive effect on cervical tumors in mice. However, there was no evidence indicating that calcitriol enhanced the therapeutic efficacy of radiation treatment [40]. There are few animal studies on the effects of VD on cervical cancer, but the current study found that VD may possess anti-carcinogenic properties in the context of cervical cancer, which warrants further investigation to elucidate the underlying mechanisms [40].
VDR and cervical cancer
The literature on the relationship between cervical cancer and the VDR is limited. A review has reported the role of VDR upregulation in gynecological cancers, with elevated expression levels of VDR in comparison to normal tissues observed in endometrial cancer, ovarian cancer, cervical cancer, and vulvar cancer [9]. Contrasting the differences in VDR expression levels in invasive breast cancer tissues, a cohort study has revealed a negative correlation between VDR expression levels and the degree of tumor malignancy. That is to say, as the degree of malignancy worsens, VDR expression levels decrease [41]. High VDR expression was inversely associated with the malignancy of prostate cancer and the risk of cancer-related mortality [42]. Another cohort study indicated that increased VDR expression was associated with improved overall survival rates, suggesting that VDR levels could serve as a prognostic marker [43]. However, Friedrich et al. did not find a significant association between VDR expression levels and cervical cancer pathological staging, differentiation, and lymph node metastasis [44, 45]. This disparity might arise from the limited small size of 50 cervical cancer tissues and 15 benign cervical tissues in the study [44]. In a review study, combining VDR agonists with standard treatment modalities like aromatase inhibitors has been proposed to enhance the treatment response in breast cancer [46]. Therefore, further in-depth investigation is required to elucidate the role of VDR upregulation in cervical cancer progression, prognosis and response to treatment (Table 4).
Mechanisms underlying the VD-VDR signaling in cervical cancer
While epidemiological studies have demonstrated associations between VD, VDR and a reduced risk of cervical cancer, and certain mechanisms have been investigated, the precise mechanisms by which VD and VDR influencing cervical cancer remain incompletely understood and warrant further exploration.
Regulation of gene expression by the VD-VDR signaling
Several studies have reported that the VD-VDR signaling in cervical cancer cells can directly impact the expression of key oncogenes and tumor suppressor genes [47, 48]. For instance, VDR activation has been shown to downregulate oncogenes like human cervical cancer oncogene-1 (HCCR-1) and upregulate tumor suppressors like p21 and p53, leading to cell cycle arrest and inhibition of cell proliferation [47, 49]. Wang et al. conducted research revealing that calcitriol induced cell cycle arrest in the G1 phase, thereby inhibiting the proliferation of HeLaS3 cells, through the down regulation of HCCR-1 expression, concomitant with an increase in the expression and promoter activity of p21[47]. Notably, p21 is known to interact with p53, leading to the inhibition of cell proliferation [49]. These findings have suggested that VD may play a crucial role in inhibiting the HCCR-1 oncogene of cervical cancer and potentially act as an anti-cervical cancer agent.
Furthermore, the VD-VDR signaling also influences the expression of genes related to immune responses. It can upregulate genes involved in antigen presentation, such as major histocompatibility complex (MHC) class II molecules, enhancing the immune recognition of cancer cells [50, 51]. Additionally, VDR can downregulate genes associated with inflammation, thus reducing the pro-inflammatory environment within the tumor microenvironment [52]. Previous research also indicated that VDR regulated genes involved in DNA repair pathways. Activation of VDR has been shown to reduce nitrosylation of DNA repair enzymes, thereby maintaining genomic stability [53].
Regulation of cellular processes by the VD-VDR signaling
In cervical cancer, the VD-VDR signaling has been shown to exhibit inhibitory effects by regulating various cellular processes [47, 54-58], such as cell proliferation, differentiation, and apoptosis. Several studies have investigated associations between VD, VDR and cervical cancer in vitro studies [47, 54-58]. Calcitriol was found to induce HeLa cells to stop in G0/G1 phase and inhibited proliferation, by using the cell counting kit-8 assay and flow cytometry [47]. Conversely, when employing the ki67 nuclear antigen method and flow cytometry, it was found that cholecalciferol had no effect on SiHa and Caski cells proliferation. Instead, it led to an increased in the sub-G1 phase, while no effect was observed on the G0/G1 phase [54, 55]. Furthermore, 25-hydroxycholecalciferol had no effect on SiHa cell proliferation by the ki67 nuclear antigen method. However, it did induce an augmentation in the sub-G1 phase, albeit without instigating cell cycle arrest as elucidated through flow cytometry analysis [57]. These findings lead to a plausible hypothesis that the two activation pathways of cholecalciferol in vivo, mediated through the liver and kidney, may have specific impacts on cellular behavior. This conjecture is supported by the observed differential effects of various forms of vitamin D on cell proliferation and the cell cycle.
Moreover, the VD-VDR signaling plays an important role in activating both intrinsic and extrinsic apoptosis pathways, which highlights its potential as a therapeutic target for inducing programmed cell death in cervical cancer cells. The VD-VDR signaling has been found to activate the intrinsic apoptosis pathway in cervical cancer cells. This involves the release of pro-apoptotic factors from the mitochondria, such as cytochrome c, which triggers the formation of the apoptosome and subsequently leads to caspase activation and apoptosis [59, 60]. In addition to the intrinsic pathway, the VD-VDR signaling can also influence the extrinsic apoptosis pathway. Studies have shown that VDR activation can upregulate death receptors like Fas and Fas ligand (FasL), leading to the activation of caspase and initiation of extrinsic apoptosis [61, 62]. While these findings listed above are often based on specific cancer cell lines, it's critical to acknowledge that the response to VDR activation may differ among various cervical cancer subtypes, and extrapolating these results to clinical setting requires caution.
The VD-VDR signaling can also modulate the expression of Bcl-2 family proteins, which are critical regulators of apoptosis. VDR activation has been reported to decrease the expression of anti-apoptotic proteins like Bcl-2 and increase the expression of pro-apoptotic proteins like Bcl-2-associated X protein (Bax), tilting the balance in favor of apoptosis induction [63, 64]. However, it's important to note that the interplay between these pathways can be complex and context-dependent, involving multiple factors and feedback loops.
Epigenetic changes and the VD-VDR signaling
Epigenetic alterations, including DNA methylation, can affect VDR expression and activity. Studies have shown that promoter hypermethylation of the VDR gene can lead to reduced VDR expression in cervical cancer cells. This epigenetic silencing of VDR can impair its tumor-suppressive functions, including the regulation of gene expression and apoptosis [65]. Additionally, epigenetic changes can also affect the expression of genes involved in cervical cancer pathogenesis and progression. For example, DNA methylation of tumor suppressor genes can lead to their silencing, promoting uncontrolled cell growth. VDR signaling may play a role in regulating the epigenetic status of some of these genes [63]. However, it's essential to recognize that epigenetic modifications are highly context-dependent and can vary among individuals. Moreover, while altered VDR expression due to methylation changes is observed, the downstream effects on gene expression and apoptosis may differ among cervical cancer subtypes.
Epigenetic modifications of histones, such as histone acetylation and methylation, can also influence VDR-mediated effects. Altered histone marks at VDR target gene promoters can impact VDR binding and subsequent gene regulation [66, 67]. This highlights the intricate interplay between epigenetic modifications and the VD-VDR signaling in cervical cancer. Nevertheless, the exact mechanisms by which histone modifications interact with VDR signaling in cervical cancer cells remain an active area of research. The specificity and dynamics of these interactions need further elucidation.
Interactions with other signaling pathways
VD and Ether à go-go potassium channels
The Ether à go-go (EAG) family of potassium channels has been implicated in promoting cancer cell proliferation and is expressed in a variety of cancer types [68, 69]. EAG1, a member of this family [70], is expressed at low levels in normal tissues but is significantly overexpressed in various cancer types. Inhibition of EAG1 expression has been shown to reduce cell proliferation [71]. Notably, EAG1 expression has been detected in cervical cancer tissues and cell lines, with increased expression as the severity of CIN increases [68, 72]. Previous research has demonstrated that calcitriol can downregulate the expression of EAG1 mRNA and protein in SiHa cells, leading to reduced proliferation. Moreover, this effect was more pronounced in cells transfected with VDR expression vectors, indicating that VDR played a crucial role in mediating the inhibition of EAG1 gene expression [73]. Further studies on SiHa and C33A cells have revealed that calcitriol inhibited EAG1 gene expression at the transcriptional level, with the involvement of VDR [74].
Various studies have reported that estrogen could play a role as a co-factor in the increased risk of cervical cancer in women with HPV DNA+[75]. Specially, Díaz et al. discovered that the expression of estrogen receptor-α (ERα) led to a strong upregulation of EAG1 expression in HeLa cells [76], in response to both estradiol and anti-estrogen. Conversely, another study found that estradiol, through G protein-coupled receptor 30 (GPR30), rather than ERα, contributes to the destabilization of genome structure in HPV-infected cells, potentially promoting carcinogenesis [77]. This discrepancy may be due to different concentrations of estradiol, resulting in distinct mechanisms of action, which may be explained by the dual effects of estrogen concentrations. Moreover, Dupuis et al. confirmed that estrogen had the capacity to enhance the effect of VD by facilitating the expression of VDR. VD, on the other hand, can down-regulate aromatase expression, reducing the level of estrogen [78]. Consequently, as subsequent studies unfold, the determination of the optimal estrogen concentration may have substantial significance for influencing VD in cervical cancer. In previous studies, the HPV oncoproteins E6 and E7 caused the loss of cell protein p53 and retinoblastoma protein pRb[79]. It is noteworthy that EAG1 may be down-regulated by p53 and pRb pathways [76], indicating a potential synergistic effect of estrogen and HPV on EAG1 expression.
The HERG potassium channel, a member of the EAG potassium channel family, has been identified in various cancer types, and HERG mRNA has been detected in HeLa cells [70]. Suzuki et al. have observed the expression of the HERG gene in C33A cells, and HERG channel inhibitors have been shown to reduce the G2/M phase cell ratio [80]. Furthermore, VD has been reported to up-regulate tumor necrosis factor α (TNF-α) in cancer cells [81], leading to an increase in intracellular reactive oxygen species [82]. Nevertheless, it is noteworthy that reactive oxygen species may increase the outward current of HERG potassium channels, while its scavengers can reduce the outward current at rest [83]. Therefore, it is hypothesized that VD may have a modulatory effect on the HERG potassium channel.
Wnt/β-catenin pathway
The abnormal activation of the Wnt/β-catenin signaling pathway is implicated in irregular cell proliferation and differentiation, which can contribute to tumorigenesis. Studies have revealed an elevated rate of abnormal β-catenin protein expression along with the progression of CIN and the development of cervical cancer [84]. Notably, HPV E6 and E7 oncoproteins have been shown to up-regulate β-catenin expression, activate the regulated Wnt pathway, and thereby promote cervical cancer progression [85, 86]. In related research, VD has exhibited inhibitory effects on the progression of various cancer, such as melanoma [87], Kaposi's sarcoma [88], oral squamous cell carcinoma [89], and ovarian cancer [90], by modulating the Wnt/β-catenin signaling pathway through its interaction with VDR. Therefore, it is reasonable to speculate that VD may inhibit the progression of cervical cancer by binding with VDR, potentially implicating the Wnt/β-catenin signaling pathway as a target for intervention.
PI3K-AKT pathway and PI3K-AKT- mTOR pathway
The phosphatidylinositol 3-kinase/Akt (PI3K/AKT) pathway has the capacity to modulate the expression of key oncogenes (e.g. HCCR-1) and tumor suppressor genes (e.g. p53) [91] and elicit the transition between epithelial and stromal tissues [92], thereby serving as a facilitator in the onset and advancement of cervical tumors. While studies have demonstrated VD's ability to impede Non-Small-Cell Lung Cancer progression through the PI3K/AKT pathway [93]. This pathway's involvement in cervical cancer remains unexplored. However, the association between this pathway and HCCR-1[94], which is associated with cervical cancer progression, strengthens the hypothesis that the PI3K/AKT pathway may be instrumental in mediating VD's effects on cervical cancer.
The PI3K-AKT-mTOR pathway is an extension of the PI3K/AKT pathway and includes the mammalian Target of Rapamycin (mTOR) as a key downstream component. The extended pathway is known to regulate essential cellular functions including proliferation, differentiation, and apoptosis [95]. In various cancer contexts, VD has been shown to engage with VDR and inhibit tumor progression through the PI3K-AKT-mTOR pathway, as observed in Kaposi's sarcoma cells [96] and non-small cell lung cancer [97]. Aberrant activation of the PI3K-AKT-mTOR pathway has been documented in cervical cancer [98].
Notably, VD has demonstrated the ability to impede the growth of HeLa cells by suppressing autophagy and altering mitochondrial homeostasis through modulation of the PI3K-AKT-mTOR pathway [58]. However, it is crucial to highlight that studies on the PI3K-AKT-mTOR pathway have primarily focused on HeLa cells and has not encompassed other cervical cancer cell lines, such as Caski, SiHa and C33A cells. Therefore, it remains inconclusive whether the observed effects of VD are attributed to specific characteristics of HeLa cells, such as their HPV typing.
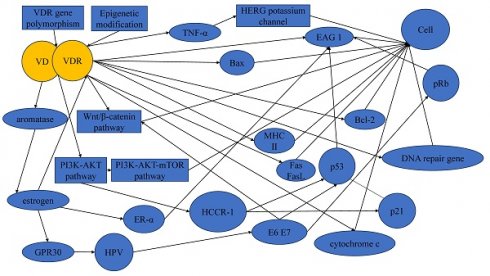
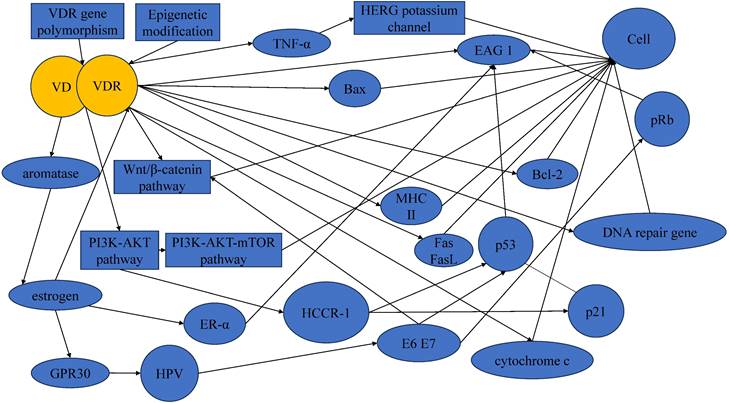
It is crucial to acknowledge that the development and progression of cervical cancer involve a complex interplay of multiple factors, and the VD-VDR signaling represents one aspect of this complexity (Figure 2). Thus, the translation of these findings into clinical applications may face challenges related to the specificity, safety, and potential side effects of VDR-targeted therapies.
Mechanisms underlying the protective actions of VD in cervical cancer.
VDR gene polymorphism and cervical cancer
The antitumor effect of VDR is influenced by gene polymorphism, which can affect the activity of the VD-VDR complex [99, 100]. Certain gene polymorphisms may reduce VDR activity and responsiveness to calcitriol, thereby leading to the progression of cervical cancer. Genetic polymorphic loci such as APa1, Bsm1, Taq1, Fok1, Cdx2 have been extensively studies in the context of tumors [101]. Investigations have shown that VDR gene polymorphism is associated with ovarian cancer (Fok1, Apa1), breast cancer (Bsm1, Fok1) and other tumors [102]. Phuthong et al. have detected VDR polymorphism (Fok1, Apa1, and Taq1) in 204 patients with cervical squamous cell carcinoma and 204 healthy controls matched by age, and found that Taq1 was associated with cervical cancer in northeastern Thailand, and Taq1 and Fok1 may interact to affect the development of cervical cancer but no association in APa1[103]. Meanwhile, Li et al. found that Fok1 and Taq1 polymorphisms were associated with an increased risk of CIN2+ (CIN2, CIN3 and cervical cancer) within the Shanxi population [104]. It is imperative to acknowledge certain limitations in these studies, such as their exclusive focus on cervical squamous cell carcinoma [103], or their restriction to HPV16+ CIN2+ patients, without distinguishing between CIN and cervical cancer patients [104]. Notably, these studies did not consider gene-environment interactions, for example, skin color may affect the process of skin synthesis of VD under light, and circulating VD levels may interact with the VDR gene variants to have an impact on cervical cancer risk. Future research should expand the scope of investigation to include other subtypes of cervical cancer, and consider the intricate interplay between genetic and environmental factors. Such endeavors will undoubtedly advance our understanding of the association between VDR gene polymorphism and cervical cancer.
Persistent HPV infection is linked to the impairment of immune function. VDR is expressed in multiple cell types, including immune cells [31], indicating a potential association between VDR and HPV infection. Previous studies have suggested that VDR gene polymorphism is related to viral infection [105], and the polymorphism of the VDR gene may influence the effect of VD by affecting its activity and expression. Nevertheless, to date, empirical examinations that examine the relationship between VDR gene polymorphism and HPV infection remain conspicuously absent from the extant literature, thereby warranting consideration as a prospective avenue of research in forthcoming investigations.
Interaction of VD with calcium and other vitamins
Although vitamins and minerals are micronutrients, their impact on human physiological processes is profound. Minerals and vitamins, such as calcium (Ca), vitamin A (VA), vitamin B (VB), vitamin C (VC), VD, vitamin E (VE) and vitamin K (VK), have been reported as potential preventive measures against the progression of cervical cancer [9, 106-110]. Studies have highlighted the potential significance of VD in impeding cervical cancer progression, warranting further exploration of the interactions of VD with Ca and other vitamins.
VD has been recognized for its traditional role in the regulation of calcium and bone metabolism. VD may collaborate with calcium by regulating calcium metabolism in the body. Exploring the synergistic effects of Ca and VD in cervical cancer holds therapeutic promise, particularly considering the significant role of Ca signaling channels in cervical cancer [111].
VA plays an anti-cancer role through its antioxidant capacity [112]. Retinoic acid, a metabolite of VA in the body, exerts its function through the retinoic acid receptor (RAR)-RXR heterodimer, formed by RXR and the nuclear receptor of the RAR family. This complex interacts with retinoic acid response elements (RAREs) within the promoters of retinoic acid-responsive genes [113]. Given that both VA and VD interact with RXR, the possibility of antagonistic effects arises. Epidemiological studies have shown that VA levels in both dietary intake (OR=0.59 95%CI 0.49-0.72) and blood (OR=0.60 95%CI 0.41-0.89) are inversely associated with cervical cancer risk [114]. Moreover, VA has been shown to inhibit the transcription level of HPV oncogenes [115]. Therefore, it is reasonable to speculate that VA can affect EAG1 by affecting HPV oncogenes, but the competitive relationship of RXR also needs to be considered.
Folate, a member of the B vitamin group, mainly uses three folate transporters for cellular uptake: the reduced folate carrier (RFC), the proton-coupled folate transporter (PCFT) and folate receptors (FOLRs). Studies have suggested that VD3 and its receptors can increase the expression of PCFT, thus increasing folate intake [116]. However, conflicting findings exist, with some studies reporting no effect of VD on folate levels in the body [117]. The effect of VD on folic acid metabolism is still controversial, potentially involving undiscovered mechanisms warranting further investigation. Notably, folate receptor ɑ (FRɑ), one of the FOLRs, displays increased expression with the progression of cervical lesions and is highly expressed in cervical cancer [118, 119]. FRɑ exerts its influence on p53 and p21 through the ERK1/2 signaling pathway [118]. Given that VD can also influence p53 and p21 through various mechanisms, it is reasonable to postulate potential interactions between folate and VD in the context of cervical cancer, although over-supplementation of folate may have a positive effect on precancerous changes [120].
VC exerts its anti-cancer effects by acting on redox imbalances, epigenetic reprogramming an oxygen-sensing regulation of cancer cells [121]. Patients with cervical cancer have been reported to exhibit lower levels of VC in both blood levels and dietary intake compared to normal controls [122-124]. VC can potentially impact cervical cancer through pathways involving TNF-α and p21[125], which may interact with the anti-cancer pathway of VD.
VE hinders cancer progression by promoting apoptosis and inhibiting cell proliferation and invasion [126]. Epidemiological studies have shown that VE levels in both dietary intake (OR=0.68 95%CI:0.49-0.94) and blood (OR=0.52 95%CI:0.40-0.69) are inversely associated with cervical cancer risk [127]. VE consists mainly of tocopherols and tocotrienols, with tocopherol notably inhibiting the AKT signaling pathway to exert its anticancer effects [126]. Tocotrienol, another component of VE, can inhibit cell proliferation through several pathways, including the PI3K/AKT pathway, the Wnt/β-catenin pathway and TNF-ɑ [128]. The potential relationship between VE and the action pathway of VD suggests the possibility of a synergistic role against cervical cancer.
VK can promote apoptosis, induce cell cycle arrest and overcome drug resistance by inhibiting P-glycoprotein, offering potential value when combined with VD in the treatment of cervical cancer [129]. A cross-sectional survey of more than 10,000 women in the United States suggests an association between VK intake and HPV infection, although the relationship appears non-linear [130]. VK can produce reactive oxygen species, which leads to apoptosis [131, 132]. Given that VD also influences reactive oxygen species, the potential synergy in inhibiting cervical cancer progression warrants consideration.
Conclusions
In summary, VD and VDR emerge as potential pivotal factors in the occurrence and progression of cervical cancer, potentially reducing disease risk. Although epidemiological studies have established associations between VD, VDR and cervical cancer susceptibility, the empirical support for their preventive efficacy remains notably reliant on a paucity of clinical trial studies. Existing studies underscores that the inhibitory effect of VD on cervical cancer may be mediated through various pathways and factors, including but not limited to the EAG potassium channel, HCCR-1, estrogen and its receptor, p53, pRb, TNF-α, the PI3K/Akt pathway, and the Wnt/β-catenin pathway. However, the extant literature in the realm of cervical cancer remains limited, with a conspicuous dearth in investigations exploring the intricate interplay among diverse molecular pathways and entities.
In addition, while the association between VDR gene polymorphisms and cervical cancer has been elucidated to some extent, there remains an empirical void concerning the mechanistic underpinnings of these polymorphic loci changes in the context of HPV infection and VD. Moreover, an avenue of research conspicuously absent pertains to the relationship between genetic polymorphisms, dietary intake of VD and calcium, and their collective influence on cervical cancer.
VD, as a vitamin, has a certain inhibitory effect on cervical cancer, thereby eliciting interest in the potential contributions of other vitamins in the context of this malignancy. This review briefly alludes to the possible effects of other vitamins on cervical cancer, and explores potential synergistic mechanisms between VD and its vitamin counterparts. While VD's traditional role lies in the regulation of calcium metabolism, the intricate interplay between VD, Ca, and other vitamins has received limited attention in the current literature. Many of the proposed mechanistic processes still require confirmation through future studies.
In conclusion, VD presents a promising avenue for novel therapeutic approaches to cervical cancer. Further research endeavors should seek to elucidate the potential synergistic benefits of combining VD with other anticancer medications, thereby advancing our understanding of effective treatment strategies in this context.
Abbreviations
CIN: cervical intraepithelial neoplasia; HR-HPV: high-risk human papillomavirus; HPV: human papillomavirus; VD: vitamin D; VDR: vitamin D receptor; UVB: ultraviolet B; RXR: retinoid X receptor; RR: risk ratio; CI: confidence interval; OR: odds ratio; Q: quartile; HR: hazard ratio; HCCR-1: human cervical cancer oncogene-1; MHC: major histocompatibility complex; FasL: Fas ligand; Bax: Bcl-2-associated X protein; EAG: Ether à go-go; Erα: estrogen receptor-α; GPR30: G protein-coupled receptor 30; TNF-α: tumor necrosis factor α; PI3K/AKT: phosphatidylinositol 3-kinase /Akt; mTOR: mammalian target of the rapamycin; Ca: calcium; VA: vitamin A; VB: vitamin B; VC: vitamin C; VE : vitamin E; VK: vitamin K; RAR: retinoic acid receptor; RAREs: retinoic acid response elements; RFC: reduced folate carrier; PCFT: proton-coupled folate transporter; FOLRs: folate receptors; FRɑ: Folate receptor α.
Acknowledgements
Funding
This study was supported by the National Natural Science Foundation of China (grant number:82003440, 82173519, 72174144). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Author contributions
Xu-mei Zhang, Juan Xie and Zhuo-yu Sun designed this conceptualization. Han-yu Dong and Shi-yue Chen wrote, reviewed and revised of the manuscript. Qi-liang Cai and Xiao-shan Liang provided materials and technical support. All authors contributed to critical revision of the manuscript for important intellectual content and gave final approval.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-49
2. Ghittoni R, Accardi R, Chiocca S, Tommasino M. Role of human papillomaviruses in carcinogenesis. Ecancermedicalscience. 2015;9:526-34
3. Ono A, Koshiyama M, Nakagawa M, Watanabe Y, Ikuta E, Seki K. et al. The Preventive Effect of Dietary Antioxidants on Cervical Cancer Development. Medicina (Kaunas). 2020;56:604-15
4. Hu Z, Ma D. The precision prevention and therapy of HPV-related cervical cancer: new concepts and clinical implications. Cancer Med. 2018;7:5217-36
5. Sooriyaarachchi P, Jeyakumar DT, King N, Jayawardena R. Impact of vitamin D deficiency on COVID-19. Clin Nutr ESPEN. 2021;44:372-8
6. Ao T, Kikuta J, Ishii M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules. 2021;11:1624-32
7. Ziemińska M, Sieklucka B, Pawlak K. Vitamin K and D Supplementation and Bone Health in Chronic Kidney Disease-Apart or Together? Nutrients. 2021;13:809-42
8. Botelho J, Machado V, Proença L, Delgado AS, Mendes JJ. Vitamin D Deficiency and Oral Health: A Comprehensive Review. Nutrients. 2020;12:1471-86
9. Deuster E, Jeschke U, Ye Y, Mahner S, Czogalla B. Vitamin D and VDR in Gynecological Cancers-A Systematic Review. Int J Mol Sci. 2017;18:2328-39
10. Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080s-6s
11. Holden JM, Lemar LE. Assessing vitamin D contents in foods and supplements: challenges and needs. Am J Clin Nutr. 2008;88:551s-3s
12. Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135:317-22
13. Deluca HF. History of the discovery of vitamin D and its active metabolites. Bonekey Rep. 2014;3:479-86
14. Tsoukas CD, Provvedini DM, Manolagas SC. 1,25-dihydroxyvitamin D3: a novel immunoregulatory hormone. Science. 1984;224:1438-40
15. Kroner Jde C, Sommer A, Fabri M. Vitamin D every day to keep the infection away? Nutrients. 2015;7:4170-88
16. Carlberg C. The physiology of vitamin D-far more than calcium and bone. Front Physiol. 2014;5:335-6
17. Christakos S, Dhawan P, Liu Y, Peng X, Porta A. New insights into the mechanisms of vitamin D action. J Cell Biochem. 2003;88:695-705
18. Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59:881-6
19. van Etten E, Stoffels K, Gysemans C, Mathieu C, Overbergh L. Regulation of vitamin D homeostasis: implications for the immune system. Nutr Rev. 2008;66:S125-34
20. Haussler MR, Whitfield GK, Kaneko I, Haussler CA, Hsieh D, Hsieh JC. et al. Molecular mechanisms of vitamin D action. Calcif Tissue Int. 2013;92:77-98
21. Fetahu IS, Höbaus J, Kállay E. Vitamin D and the epigenome. Front Physiol. 2014;5:164-75
22. Valdivielso JM, Fernandez E. Vitamin D receptor polymorphisms and diseases. Clin Chim Acta. 2006;371:1-12
23. Fang Y, van Meurs JB, d'Alesio A, Jhamai M, Zhao H, Rivadeneira F. et al. Promoter and 3'-untranslated-region haplotypes in the vitamin d receptor gene predispose to osteoporotic fracture: the rotterdam study. Am J Hum Genet. 2005;77:807-23
24. Abrams SA, Griffin IJ, Hawthorne KM, Chen Z, Gunn SK, Wilde M. et al. Vitamin D receptor Fok1 polymorphisms affect calcium absorption, kinetics, and bone mineralization rates during puberty. J Bone Miner Res. 2005;20:945-53
25. Hii CS, Ferrante A. The Non-Genomic Actions of Vitamin D. Nutrients. 2016;8:135-48
26. Adams S, Lin J, Brown D, Shriver CD, Zhu K. Ultraviolet Radiation Exposure and the Incidence of Oral, Pharyngeal and Cervical Cancer and Melanoma: An Analysis of the SEER Data. Anticancer Res. 2016;36:233-7
27. Grant WB, Garland CF. The association of solar ultraviolet B (UVB) with reducing risk of cancer: multifactorial ecologic analysis of geographic variation in age-adjusted cancer mortality rates. Anticancer Res. 2006;26:2687-99
28. Chen W, Clements M, Rahman B, Zhang S, Qiao Y, Armstrong BK. Relationship between cancer mortality/incidence and ambient ultraviolet B irradiance in China. Cancer Causes Control. 2010;21:1701-9
29. Grant WB. Does solar ultraviolet irradiation affect cancer mortality rates in China? Asian Pac J Cancer Prev. 2007;8:236-42
30. Grant WB. An ecological study of cancer incidence and mortality rates in France with respect to latitude, an index for vitamin D production. Dermatoendocrinol. 2010;2:62-7
31. Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and immune function. Nutrients. 2013;5:2502-21
32. Özgü E, Yılmaz N, Başer E, Güngör T, Erkaya S, Yakut H. Could 25-OH vitamin D deficiency be a reason for HPV infection persistence in cervical premalignant lesions? J Exp Ther Oncol. 2016;11:177-80
33. Gupta A, Villa A, Feldman S, Citow B, Sroussi H. Site and sex-specific differences in the effect of vitamin D on human papillomavirus infections: analyses of NHANES 2009-2014. Sex Transm Infect. 2021;97:75-6
34. Chu TW, Jhao JY, Lin TJ, Lin TW, Wang CL, Chang HS. et al. Vitamin D in gynecological diseases. J Chin Med Assoc. 2021;84:1054-9
35. Shim J, Pérez A, Symanski E, Nyitray AG. Association Between Serum 25-Hydroxyvitamin D Level and Human Papillomavirus Cervicovaginal Infection in Women in the United States. J Infect Dis. 2016;213:1886-92
36. Troja C, Hoofnagle AN, Szpiro A, Stern JE, Lin J, Winer RL. Serum Concentrations of Emerging Vitamin D Biomarkers and Detection of Prevalent High-Risk HPV Infection in Mid-adult Women. Cancer Epidemiol Biomarkers Prev. 2020;29:1468-74
37. Hosono S, Matsuo K, Kajiyama H, Hirose K, Suzuki T, Kawase T. et al. Association between dietary calcium and vitamin D intake and cervical carcinogenesis among Japanese women. Eur J Clin Nutr. 2010;64:400-9
38. Vahedpoor Z, Jamilian M, Bahmani F, Aghadavod E, Karamali M, Kashanian M. et al. Effects of Long-Term Vitamin D Supplementation on Regression and Metabolic Status of Cervical Intraepithelial Neoplasia: a Randomized, Double-Blind, Placebo-Controlled Trial. Horm Cancer. 2017;8:58-67
39. Vahedpoor Z, Mahmoodi S, Samimi M, Gilasi HR, Bahmani F, Soltani A. et al. Long-Term Vitamin D Supplementation and the Effects on Recurrence and Metabolic Status of Cervical Intraepithelial Neoplasia Grade 2 or 3: A Randomized, Double-Blind, Placebo-Controlled Trial. Ann Nutr Metab. 2018;72:151-60
40. Zhang F, Yu Y, Song S, Wang M, Ma Y, Xing L. Calcitriol does not significantly enhance the efficacy of radiation of human cervical tumors in mice. Eur J Gynaecol Oncol. 2015;36:452-6
41. Huss L, Butt ST, Borgquist S, Elebro K, Sandsveden M, Rosendahl A. et al. Vitamin D receptor expression in invasive breast tumors and breast cancer survival. Breast Cancer Res. 2019;21:84-96
42. Hendrickson WK, Flavin R, Kasperzyk JL, Fiorentino M, Fang F, Lis R. et al. Vitamin D receptor protein expression in tumor tissue and prostate cancer progression. J Clin Oncol. 2011;29:2378-85
43. Ditsch N, Toth B, Mayr D, Lenhard M, Gallwas J, Weissenbacher T. et al. The association between vitamin D receptor expression and prolonged overall survival in breast cancer. J Histochem Cytochem. 2012;60:121-9
44. Friedrich M, Meyberg R, Axt-Fliedner R, Villena-Heinsen C, Tilgen W, Schmidt W. et al. Vitamin D receptor (VDR) expression is not a prognostic factor in cervical cancer. Anticancer Res. 2002;22:299-304
45. Friedrich M, Rafi L, Mitschele T, Tilgen W, Schmidt W, Reichrath J. Analysis of the vitamin D system in cervical carcinomas, breast cancer and ovarian cancer. Recent Results Cancer Res. 2003;164:239-46
46. Krishnan AV, Swami S, Feldman D. The potential therapeutic benefits of vitamin D in the treatment of estrogen receptor positive breast cancer. Steroids. 2012;77:1107-12
47. Wang G, Lei L, Zhao X, Zhang J, Zhou M, Nan K. Calcitriol Inhibits Cervical Cancer Cell Proliferation Through Downregulation of HCCR1 Expression. Oncol Res. 2014;22:301-9
48. Avila E, Noriega-Mejía BJ, González-Macías J, Cortes-Hernández U, García-Quiroz J, García-Becerra R. et al. The Preventive Role of the Vitamin D Endocrine System in Cervical Cancer. Int J Mol Sci. 2023;24:8665-79
49. Shamloo B, Usluer S. p21 in Cancer Research. Cancers (Basel). 2019;11:1178-96
50. Wang SY, Chen LY, Wang SJ, Lin CK, Ho CK. Growth inhibition and differentiation in HL-60 leukemia cells induced by 1,25-dihydroxyvitamin D3 and tumor necrosis factor alpha. Exp Hematol. 1991;19:1025-30
51. Lathers DM, Clark JI, Achille NJ, Young MR. Phase 1B study to improve immune responses in head and neck cancer patients using escalating doses of 25-hydroxyvitamin D3. Cancer Immunol Immunother. 2004;53:422-30
52. Díaz L, Díaz-Muñoz M, García-Gaytán AC, Méndez I. Mechanistic Effects of Calcitriol in Cancer Biology. Nutrients. 2015;7:5020-50
53. Pawlowska E, Wysokinski D, Blasiak J. Nucleotide Excision Repair and Vitamin D-Relevance for Skin Cancer Therapy. Int J Mol Sci. 2016;17:372-92
54. Bhoora S, Pillay TS, Punchoo R. Cholecalciferol induces apoptosis via autocrine metabolism in epidermoid cervical cancer cells. Biochem Cell Biol. 2022;100:387-402
55. Bhoora S, Pather Y, Marais S, Punchoo R. Cholecalciferol Inhibits Cell Growth and Induces Apoptosis in the CaSki Cell Line. Med Sci (Basel). 2020;8:12-27
56. González-Duarte RJ, Cázares-Ordoñez V, Díaz L, Ortíz V, Larrea F, Avila E. The expression of RNA helicase DDX5 is transcriptionally upregulated by calcitriol through a vitamin D response element in the proximal promoter in SiHa cervical cells. Mol Cell Biochem. 2015;410:65-73
57. Punchoo R, Dreyer G, Pillay TS. 25-Hydroxycholecalciferol Inhibits Cell Growth and Induces Apoptosis in SiHa Cervical Cells via Autocrine Vitamin D Metabolism. Biomedicines. 2023;11:871-90
58. Setiawan I, Lesmana R, Goenawan H, Suardi D, Gatera VA, Abdulah R. et al. Calcitriol potentially alters HeLa cell viability via inhibition of autophagy. J Cancer Res Ther. 2022;18:1144-51
59. Simboli-Campbell M, Narvaez CJ, Tenniswood M, Welsh J. 1,25-Dihydroxyvitamin D3 induces morphological and biochemical markers of apoptosis in MCF-7 breast cancer cells. J Steroid Biochem Mol Biol. 1996;58:367-76
60. Narvaez CJ, Welsh J. Role of mitochondria and caspases in vitamin D-mediated apoptosis of MCF-7 breast cancer cells. J Biol Chem. 2001;276:9101-7
61. Swami S, Krishnan AV, Wang JY, Jensen K, Horst R, Albertelli MA. et al. Dietary vitamin D₃ and 1,25-dihydroxyvitamin D₃ (calcitriol) exhibit equivalent anticancer activity in mouse xenograft models of breast and prostate cancer. Endocrinology. 2012;153:2576-87
62. Osborne JE, Hutchinson PE. Vitamin D and systemic cancer: is this relevant to malignant melanoma? Br J Dermatol. 2002;147:197-213
63. So JY, Suh N. Targeting cancer stem cells in solid tumors by vitamin D. J Steroid Biochem Mol Biol. 2015;148:79-85
64. Johnson CS, Hershberger PA, Trump DL. Vitamin D-related therapies in prostate cancer. Cancer Metastasis Rev. 2002;21:147-58
65. Zhong W, Gu B, Gu Y, Groome LJ, Sun J, Wang Y. Activation of vitamin D receptor promotes VEGF and CuZn-SOD expression in endothelial cells. J Steroid Biochem Mol Biol. 2014;140:56-62
66. Bao B, Wang Z, Ali S, Kong D, Banerjee S, Ahmad A. et al. Over-expression of FoxM1 leads to epithelial-mesenchymal transition and cancer stem cell phenotype in pancreatic cancer cells. J Cell Biochem. 2011;112:2296-306
67. Li Z, Jia Z, Gao Y, Xie D, Wei D, Cui J. et al. Activation of vitamin D receptor signaling downregulates the expression of nuclear FOXM1 protein and suppresses pancreatic cancer cell stemness. Clin Cancer Res. 2015;21:844-53
68. Farias LM, Ocaña DB, Díaz L, Larrea F, Avila-Chávez E, Cadena A. et al. Ether a go-go potassium channels as human cervical cancer markers. Cancer Res. 2004;64:6996-7001
69. Urrego D, Tomczak AP, Zahed F, Stühmer W, Pardo LA. Potassium channels in cell cycle and cell proliferation. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130094-102
70. Asher V, Sowter H, Shaw R, Bali A, Khan R. Eag and HERG potassium channels as novel therapeutic targets in cancer. World J Surg Oncol. 2010;8:113-21
71. Hemmerlein B, Weseloh RM, Mello de Queiroz F, Knötgen H, Sánchez A, Rubio ME. et al. Overexpression of Eag1 potassium channels in clinical tumours. Mol Cancer. 2006;5:41-53
72. Ortiz CS, Montante-Montes D, Saqui-Salces M, Hinojosa LM, Gamboa-Dominguez A, Hernández-Gallegos E. et al. Eag1 potassium channels as markers of cervical dysplasia. Oncol Rep. 2011;26:1377-83
73. Avila E, García-Becerra R, Rodríguez-Rasgado JA, Díaz L, Ordaz-Rosado D, Zügel U. et al. Calcitriol down-regulates human ether a go-go 1 potassium channel expression in cervical cancer cells. Anticancer Res. 2010;30:2667-72
74. Cázares-Ordoñez V, González-Duarte RJ, Díaz L, Ishizawa M, Uno S, Ortíz V. et al. A cis-acting element in the promoter of human ether à go-go 1 potassium channel gene mediates repression by calcitriol in human cervical cancer cells. Biochem Cell Biol. 2015;93:94-101
75. Moreno V, Bosch FX, Muñoz N, Meijer CJ, Shah KV, Walboomers JM. et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet. 2002;359:1085-92
76. Díaz L, Ceja-Ochoa I, Restrepo-Angulo I, Larrea F, Avila-Chávez E, García-Becerra R. et al. Estrogens and human papilloma virus oncogenes regulate human ether-à-go-go-1 potassium channel expression. Cancer Res. 2009;69:3300-7
77. Ogawa M, Hashimoto K, Kitano S, Yamashita S, Toda A, Nakamura K. et al. Estrogen induces genomic instability in high-risk HPV-infected cervix and promotes the carcinogenesis of cervical adenocarcinoma. Biochem Biophys Res Commun. 2023;659:80-90
78. Dupuis ML, Pagano MT, Pierdominici M, Ortona E. The role of vitamin D in autoimmune diseases: could sex make the difference? Biol Sex Differ. 2021;12:12-23
79. Shillitoe EJ. Papillomaviruses as targets for cancer gene therapy. Cancer Gene Ther. 2006;13:445-50
80. Suzuki T, Takimoto K. Selective expression of HERG and Kv2 channels influences proliferation of uterine cancer cells. Int J Oncol. 2004;25:153-9
81. Golovko O, Nazarova N, Tuohimaa P. Vitamin D-induced up-regulation of tumour necrosis factor alpha (TNF-alpha) in prostate cancer cells. Life Sci. 2005;77:562-77
82. Wang J, Wang H, Zhang Y, Gao H, Nattel S, Wang Z. Impairment of HERG K(+) channel function by tumor necrosis factor-alpha: role of reactive oxygen species as a mediator. J Biol Chem. 2004;279:13289-92
83. Taglialatela M, Castaldo P, Iossa S, Pannaccione A, Fresi A, Ficker E. et al. Regulation of the human ether-a-gogo related gene (HERG) K+ channels by reactive oxygen species. Proc Natl Acad Sci U S A. 1997;94:11698-703
84. Yang JZ, Zhang XH, Wu WX, Yan X, Liu YL, Wang JL. et al. Expression of EP-CAM, beta-catenin in the carcinogenesis of squamous cell carcinoma of uterine cervix. Zhonghua Zhong Liu Za Zhi. 2003;25:372-5
85. Yang M, Wang M, Li X, Xie Y, Xia X, Tian J. et al. Wnt signaling in cervical cancer? J Cancer. 2018;9:1277-86
86. Bello JO, Nieva LO, Paredes AC, Gonzalez AM, Zavaleta LR, Lizano M. Regulation of the Wnt/β-Catenin Signaling Pathway by Human Papillomavirus E6 and E7 Oncoproteins. Viruses. 2015;7:4734-55
87. Muralidhar S, Filia A, Nsengimana J, Poźniak J, O'Shea SJ, Diaz JM. et al. Vitamin D-VDR Signaling Inhibits Wnt/β-Catenin-Mediated Melanoma Progression and Promotes Antitumor Immunity. Cancer Res. 2019;79:5986-98
88. Tapia C, Suares A, De Genaro P, González-Pardo V. In vitro studies revealed a downregulation of Wnt/β-catenin cascade by active vitamin D and TX 527 analog in a Kaposi's sarcoma cellular model. Toxicol In vitro. 2020;63:104748-57
89. Oak ASW, Bocheva G, Kim TK, Brożyna AA, Janjetovic Z, Athar M. et al. Noncalcemic Vitamin D Hydroxyderivatives Inhibit Human Oral Squamous Cell Carcinoma and Down-regulate Hedgehog and WNT/β-Catenin Pathways. Anticancer Res. 2020;40:2467-74
90. Fu Y, Katsaros D, Biglia N, Wang Z, Pagano I, Tius M. et al. Vitamin D receptor upregulates lncRNA TOPORS-AS1 which inhibits the Wnt/β-catenin pathway and associates with favorable prognosis of ovarian cancer. Sci Rep. 2021;11:7484-97
91. Cho GW, Shin SM, Namkoong H, Kim HK, Ha SA, Hur SY. et al. The phosphatidylinositol 3-kinase/Akt pathway regulates the HCCR-1 oncogene expression. Gene. 2006;384:18-26
92. Ha SA, Kim HK, Yoo J, Kim S, Shin SM, Lee YS. et al. Transdifferentiation-inducing HCCR-1 oncogene. BMC Cell Biol. 2010;11:49-57
93. Yiyan S, Yang S, Li D, Li W. Vitamin D Affects the Warburg Effect and Stemness Maintenance of Non- Small-Cell Lung Cancer Cells by Regulating the PI3K/AKT/mTOR Signaling Pathway. Curr Cancer Drug Targets. 2022;22:86-95
94. Cho GW, Shin SM, Kim HK, Ha SA, Kim S, Yoon JH. et al. HCCR-1, a novel oncogene, encodes a mitochondrial outer membrane protein and suppresses the UVC-induced apoptosis. BMC Cell Biol. 2007;8:50-61
95. Polivka J Jr, Janku F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol Ther. 2014;142:164-75
96. Suares A, Tapia C, González-Pardo V. VDR agonists down regulate PI3K/Akt/mTOR axis and trigger autophagy in Kaposi's sarcoma cells. Heliyon. 2019;5:e02367-e79
97. Songyang Y, Song T, Shi Z, Li W, Yang S, Li D. Effect of vitamin D on malignant behavior of non-small cell lung cancer cells. Gene. 2021;768:145309-18
98. Bahrami A, Hasanzadeh M, Hassanian SM, ShahidSales S, Ghayour-Mobarhan M, Ferns GA. et al. The Potential Value of the PI3K/Akt/mTOR Signaling Pathway for Assessing Prognosis in Cervical Cancer and as a Target for Therapy. J Cell Biochem. 2017;118:4163-9
99. Anderson LN, Cotterchio M, Cole DE, Knight JA. Vitamin D-related genetic variants, interactions with vitamin D exposure, and breast cancer risk among Caucasian women in Ontario. Cancer Epidemiol Biomarkers Prev. 2011;20:1708-17
100. Vaughan-Shaw PG, O'Sullivan F, Farrington SM, Theodoratou E, Campbell H, Dunlop MG. et al. The impact of vitamin D pathway genetic variation and circulating 25-hydroxyvitamin D on cancer outcome: systematic review and meta-analysis. Br J Cancer. 2017;116:1092-110
101. Gnagnarella P, Raimondi S, Aristarco V, Johansson HA, Bellerba F, Corso F. et al. Vitamin D Receptor Polymorphisms and Cancer. Adv Exp Med Biol. 2020;1268:53-114
102. Köstner K, Denzer N, Müller CS, Klein R, Tilgen W, Reichrath J. The relevance of vitamin D receptor (VDR) gene polymorphisms for cancer: a review of the literature. Anticancer Res. 2009;29:3511-36
103. Phuthong S, Settheetham-Ishida W, Natphopsuk S, Ishida T. Genetic Polymorphisms of Vitamin D Receptor Gene are Associated with Cervical Cancer Risk in Northeastern Thailand. Asian Pac J Cancer Prev. 2020;21:2935-9
104. Li D, Liu Y, Kong D, Papukashvili D, Rcheulishvili N, Zhao H. et al. Vitamin D Receptor Gene Polymorphisms and the Risk of CIN2+ in Shanxi Population. Biomed Res Int. 2022;2022:6875996-6004
105. Laplana M, Royo JL, Fibla J. Vitamin D Receptor polymorphisms and risk of enveloped virus infection: A meta-analysis. Gene. 2018;678:384-94
106. Chih HJ, Lee AH, Colville L, Binns CW, Xu D. A review of dietary prevention of human papillomavirus-related infection of the cervix and cervical intraepithelial neoplasia. Nutr Cancer. 2013;65:317-28
107. Sedjo RL, Roe DJ, Abrahamsen M, Harris RB, Craft N, Baldwin S. et al. Vitamin A, carotenoids, and risk of persistent oncogenic human papillomavirus infection. Cancer Epidemiol Biomarkers Prev. 2002;11:876-84
108. Wang Z, Yang A, Yang J, Zhao W, Wang Z, Wang W. et al. Dietary nutrient intake related to higher grade cervical intraepithelial neoplasia risk: a Chinese population-based study. Nutr Metab (Lond). 2020;17:100-13
109. Giuliano AR, Siegel EM, Roe DJ, Ferreira S, Baggio ML, Galan L. et al. Dietary intake and risk of persistent human papillomavirus (HPV) infection: the Ludwig-McGill HPV Natural History Study. J Infect Dis. 2003;188:1508-16
110. Gholamalizadeh M, Ardekanizadeh NH, Aghakhaninejad Z, Mohammadi S, Majidi N, Masoumvand M. et al. The effects of dietary supplements in patients with cervical cancer: a comprehensive systematic review. Eur J Obstet Gynecol Reprod Biol X. 2023;19:100217-22
111. Lei J, Deng F, Ding H, Fu M, Xu T, Ji B. et al. Recent Developments on the Roles of Calcium Signals and Potential Therapy Targets in Cervical Cancer. Cells. 2022;11:3003-15
112. Kim JA, Jang JH, Lee SY. An Updated Comprehensive Review on Vitamin A and Carotenoids in Breast Cancer: Mechanisms, Genetics, Assessment, Current Evidence, and Future Clinical Implications. Nutrients. 2021;13:3162-93
113. Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8:685-98
114. Zhang X, Dai B, Zhang B, Wang Z. Vitamin A and risk of cervical cancer: a meta-analysis. Gynecol Oncol. 2012;124:366-73
115. Agarwal C, Rorke EA, Irwin JC, Eckert RL. Immortalization by human papillomavirus type 16 alters retinoid regulation of human ectocervical epithelial cell differentiation. Cancer Res. 1991;51:3982-9
116. Eloranta JJ, Zaïr ZM, Hiller C, Häusler S, Stieger B, Kullak-Ublick GA. Vitamin D3 and its nuclear receptor increase the expression and activity of the human proton-coupled folate transporter. Mol Pharmacol. 2009;76:1062-71
117. Brandsch C, Zibolka J, Frommhagen M, Lehmann U, Dierkes J, Kühne H. et al. Vitamin D is not linked to folate status and mRNA expression of intestinal proton-coupled folate transporter. Eur J Nutr. 2014;53:1115-22
118. Nawaz FZ, Kipreos ET. Emerging roles for folate receptor FOLR1 in signaling and cancer. Trends Endocrinol Metab. 2022;33:159-74
119. Liu C, Ding L, Bai L, Chen X, Kang H, Hou L. et al. Folate receptor alpha is associated with cervical carcinogenesis and regulates cervical cancer cells growth by activating ERK1/2/c-Fos/c-Jun. Biochem Biophys Res Commun. 2017;491:1083-91
120. Shulpekova Y, Nechaev V, Kardasheva S, Sedova A, Kurbatova A, Bueverova E. et al. The Concept of Folic Acid in Health and Disease. Molecules. 2021;26:3731-59
121. Ngo B, Van Riper JM, Cantley LC, Yun J. Targeting cancer vulnerabilities with high-dose vitamin C. Nat Rev Cancer. 2019;19:271-82
122. Naidu MS, Suryakar AN, Swami SC, Katkam RV, Kumbar KM. Oxidative stress and antioxidant status in cervical cancer patients. Indian J Clin Biochem. 2007;22:140-4
123. Gonçalves TL, Erthal F, Corte CL, Müller LG, Piovezan CM, Nogueira CW. et al. Involvement of oxidative stress in the pre-malignant and malignant states of cervical cancer in women. Clin Biochem. 2005;38:1071-5
124. Kim J, Kim MK, Lee JK, Kim JH, Son SK, Song ES. et al. Intakes of vitamin A, C, and E, and beta-carotene are associated with risk of cervical cancer: a case-control study in Korea. Nutr Cancer. 2010;62:181-9
125. Markowska A, Antoszczak M, Markowska J, Huczyński A. Role of Vitamin C in Selected Malignant Neoplasms in Women. Nutrients. 2022;14:882-98
126. Yang CS, Luo P, Zeng Z, Wang H, Malafa M, Suh N. Vitamin E and cancer prevention: Studies with different forms of tocopherols and tocotrienols. Mol Carcinog. 2020;59:365-89
127. Hu X, Li S, Zhou L, Zhao M, Zhu X. Effect of vitamin E supplementation on uterine cervical neoplasm: A meta-analysis of case-control studies. PLoS One. 2017;12:e0183395-e409
128. Sailo BL, Banik K, Padmavathi G, Javadi M, Bordoloi D, Kunnumakkara AB. Tocotrienols: The promising analogues of vitamin E for cancer therapeutics. Pharmacol Res. 2018;130:259-72
129. Gul S, Maqbool MF, Maryam A, Khan M, Shakir HA, Irfan M. et al. Vitamin K: A novel cancer chemosensitizer. Biotechnol Appl Biochem. 2022;69:2641-57
130. Jiang Y, Xu S, Lan J, Zhang J, Chen T. Dietary Vitamin K Intake and HPV-Infection Status Among American Women: A Secondary Analysis from National Health and Nutrition Examination Survey Data From 2003 to 2016. Int J Public Health. 2022;67:1604616-23
131. Xin Y, Guo W, Yang C, Huang Q, Zhang P, Zhang L. et al. Photodynamic Effects of Vitamin K3 on Cervical Carcinoma Cells Activating Mitochondrial Apoptosis Pathways. Anticancer Agents Med Chem. 2021;21:91-9
132. de Carvalho Scharf Santana N, Lima NA, Desoti VC, Bidóia DL, de Souza Bonfim Mendonça P, Ratti BA. et al. Vitamin K3 induces antiproliferative effect in cervical epithelial cells transformed by HPV 16 (SiHa cells) through the increase in reactive oxygen species production. Arch Gynecol Obstet. 2016;294:797-804
Author contact
![]() Corresponding authors: Xumei Zhang, Department of Nutrition and Food Science, School of Public Health, Tianjin Medical University, No 22. Qixiangtai Road, Heping District, Tianjin 300070, Tianjin, China; E-mail: zhangxumeiedu.cn; Juan Xie, Department of Epidemiology and Biostatistics, School of Public Health, Tianjin Medical University, No 22. Qixiangtai Road, Heping District, Tianjin 300070, Tianjin, China; E-mail: xiejuanedu.cn; Zhuoyu Sun, Department of Epidemiology and Biostatistics, School of Public Health, Tianjin Medical University, No 22. Qixiangtai Road, Heping District, Tianjin 300070, Tianjin, China; E-mail: sunzhuoyuedu.cn.
Corresponding authors: Xumei Zhang, Department of Nutrition and Food Science, School of Public Health, Tianjin Medical University, No 22. Qixiangtai Road, Heping District, Tianjin 300070, Tianjin, China; E-mail: zhangxumeiedu.cn; Juan Xie, Department of Epidemiology and Biostatistics, School of Public Health, Tianjin Medical University, No 22. Qixiangtai Road, Heping District, Tianjin 300070, Tianjin, China; E-mail: xiejuanedu.cn; Zhuoyu Sun, Department of Epidemiology and Biostatistics, School of Public Health, Tianjin Medical University, No 22. Qixiangtai Road, Heping District, Tianjin 300070, Tianjin, China; E-mail: sunzhuoyuedu.cn.

 Global reach, higher impact
Global reach, higher impact