3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(5):1213-1224. doi:10.7150/jca.90970 This issue Cite
Research Paper
Gene Expression Profile Analysis of the Molecular Mechanism of HOXD10 Regulation of Epithelial Ovarian Cancer Cells
1. Department of Obstetrics and Gynecology, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung City 807, Taiwan.
2. Graduate Institute of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung City 807, Taiwan.
3. Department of Obstetrics and Gynecology, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung 833, Taiwan.
4. Graduate Institute of Clinical Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung City 807, Taiwan.
5. Department of Obstetrics and Gynecology, Chia-Yi Chang Gung Memorial Hospital, Chiayi 613, Taiwan.
6. Department of Internal Education, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung 833, Taiwan.
†These authors contributed equally to this study.
Abstract
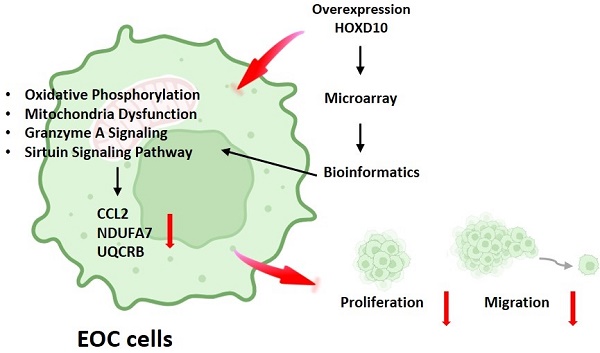
Epithelial ovarian cancer (EOC) is the most common type of ovarian cancer. Although studies have reported that downregulation of HOXD10 expression may contribute to the migration and invasion abilities in EOC, much about its regulation remains to be fully elucidated. The present study aimed to identify different gene expression profiles associated with HOXD10 overexpression in EOC cells. The present study confirmed that HOXD10 overexpression effectively inhibited the proliferation and motility of the TOV21G and TOV112D cells. Further, we overexpress HOXD10 in TOV112D cells, the different gene expression (DEGs) profiles induce by HOXD10 was analyze by the Human OneArray microarray. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG), ingenuity pathway analysis (IPA) was used to perform the pathway enrichment analysis for the DEGs. Integrated bioinformatics analysis showed that the DEGs were enriched for terms related to oxidative phosphorylation and mitochondrial function pathways. Dysfunction oxidative phosphorylation metabolic pathway occurs frequently in many tumors. We validated the expression of NDUFA7, UQCRB and CCL2 using qPCR, involving in metabolism-related pathway, were significantly changed by HOXD10 overexpression in EOC. The detailed regulatory mechanism that links HOXD10 and the oxidative phosphorylation genes is not yet fully understood, our findings provide novel insight into HOXD10-mediated pathways and their effects on cancer metabolism, carcinogenesis, and the progression of EOC. Thus, the data suggest that strategies to interfere with metabolism-related pathways associated with cancer drug resistance could be considered for the treatment of ovarian tumors.
Keywords: epithelial ovarian cancer, HOXD10, microarray, oxidative phosphorylation

 Global reach, higher impact
Global reach, higher impact