3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(8):2214-2228. doi:10.7150/jca.89788 This issue Cite
Review
Advances in Drug Therapy for Metastatic Pancreatic Ductal Adenocarcinoma
1. Department of Hepatopancreatobiliary Surgery, The Second Affiliated Hospital of Harbin Medical University, Harbin 150086, Heilongjiang, China.
2. Department of General Surgery, Tangdu Hospital, Air Force Medical University, Xi'an 710032, Shanxi, China.
3. Key Laboratory of Basic Pharmacology of Ministry of Education, Zunyi Medical University, Zunyi, 563006, Guizhou, China.
4. Key Laboratory of Functional and Clinical Translational Medicine, Fujian Province University, Xiamen Medical College, Xiamen, 361000, Fujian, China.
5. State Key Laboratory of Chemical Oncogenomics, Key Laboratory of Chemical Genomics, Peking University Shenzhen Graduate School, Shenzhen, 518055, Guangzhou, China.
6. Jiangsu Province Engineering Research Center of Tumor Targeted Nano Diagnostic and Therapeutic Materials, Yancheng Teachers University, Yancheng, 224007, Jiangsu, China.
7. Key Laboratory of Biomarkers and In Vitro Diagnosis Translation of Zhejiang province, Hangzhou,310000, Zhejiang, China.
8. Key Laboratory of Gastrointestinal Cancer, Fujian Medical University, Ministry of Education, School of Basic Medical Sciences, Fujian Medical University, Fuzhou, 350122, Fujian, China.
9. School of Health Administration, Harbin Medical University, Harbin 150086, Heilongjiang, China.
# These authors contributed equally to this work.
Received 2023-9-3; Accepted 2023-12-26; Published 2024-2-25
Abstract
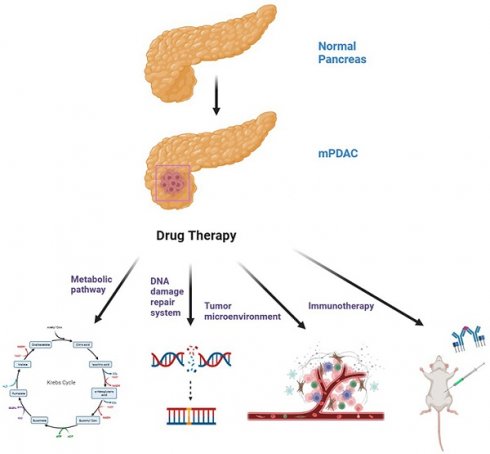
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive disease with a notably poor prognosis. A large number of patients with PDAC develop metastases before they are diagnosed with metastatic pancreatic cancer (mPDAC). For mPDAC, FOLFIRINOX or gemcitabine plus nab-paclitaxel are the current first-line treatments. It is important to note, however, that many patients will fail chemotherapy because of drug resistance. Heterogeneous tumors and complex tumor microenvironments are key factors. As a result, clinical researchers are exploring a variety of alternative treatment modalities. Current understanding of the molecular signature and immune landscape of PDAC has motivated the emergence of different targeted and immune-based therapeutic approaches, some of which have shown promising results. The purpose of this review is to discuss the new targets and new drugs for mPDAC in terms of specific pathogenic factors such as metabolic vulnerability, DNA damage repair system, tumor microenvironment and immune system, in order to identify potential vulnerabilities in mPDAC patients and hopefully improve the prognosis of mPDAC patients.
Keywords: metastatic pancreatic cancer, metabolic pathways, DNA damage systems, tumor microenvironment, immune system
1. Introduction
There is an overall five-year survival rate of only 11% for pancreatic ductal adenocarcinoma (PDAC), which is rather low compared with many other cancer types [1]. Currently, radical surgical resection and systemic palliative chemotherapy are the main treatment strategies for patients with PDAC. Among them, patients with early diagnosis of tumor diameter less than 3 cm have a relatively good prognosis, and the 5-year survival rate after surgery can reach 40% [2]. But the recurrence rate of these patients remains high, with approximately 70% relapsing within two years of treatment [3]. Approximately 50% of patients are diagnosed with progressive pancreatic cancer at an early stage or develop metastases to distant organs that cannot be treated with radical surgery and can only be treated with systemic palliative chemotherapy [4]. The main metastatic sites of PDAC are liver, lung, lymph node, peritoneal and adrenal metastases, of which about 70% of PDAC patients develop liver metastases during their disease [5]. Data show that the median life expectancy for metastatic PDAC (mPDAC) is approximately 1 year, with a 5-year OS rate of less than 2% [6].
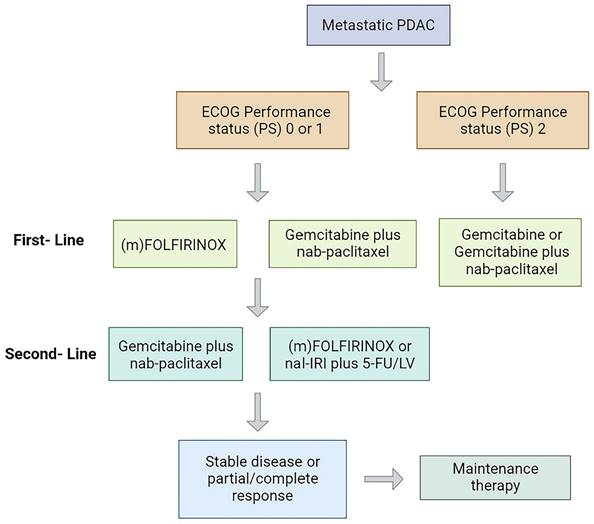
Currently, the standard treatment modality for mPDAC is systemic palliative chemotherapy (Figure 1), and the first-line regimen includes gemcitabine plus nab-paclitaxel (GnP) (ECOG 0-2) or (modified) (m)FOLFIRINOX (ECOG 0-1) with median overall survival (mOS) of 8.5 and 11.1 months, respectively [7-9]. Gemcitabine-based therapy is generally the second-line option for patients treated with FOLFIRINOX in the first line [10]. In October 2015, the Food and Drug Administration (FDA) approved nanoliposomal irinotecan combined with fluorouracil and folinic as a second-line treatment for mPDAC, a regimen that prolonged survival in mPDAC patients previously received gemcitabine-based therapy with an mOS of 6.1 months vs 4.2 months with fluorouracil and folinic acid [11]. Recently, a randomized phase III clinical trial showed that the PARP inhibitor olaparib could be used for maintenance therapy in patients with mPDAC and BRCA1/2 mutations (only 4% to 7% in pancreatic cancer patients) receiving platinum-based chemotherapy and prolonged progression-free survival (PFS) [12]. Although these drugs have produced some efficacy in mPDAC, the long-term survival of patients have not improved significantly [13].
Mutations in genes and altered molecular pathways have become crucial targets for precision therapy of PDAC patients. Numerous researchers have developed strategies to target mutated genes in pancreatic cancer, such as KRAS, NRG1, and NTRK, but most results are unsatisfactory [14]. The genes BRCA and ATM, which are involved in stabilizing the chromosome structure, are defective in some PDAC patients, making them potential therapeutic targets [15]. In addition, therapies such as targeting metabolic pathways, targeting tumor microenvironment (TME) and immune checkpoint inhibition have also shown potential for targeting pancreatic cancer cells [16, 17] (Table 1). In this review, we highlight recent advances in preclinical studies and clinical trials for mPDAC patients.
Treatment strategy in patients with mPDAC. PS, performance status; mFOLFIRINOX, modified FOLFIRINOX.
Ongoing trials evaluating activity and efficacy of novel molecules acting on different targets in PC
| Target | Drug | Population | Additional therapy | Phase | Status | Clinical Trials.gov Identifier | |
|---|---|---|---|---|---|---|---|
| Metabolism | TCA cycle | Devimistat | Metastatic Pancreatic Cancer | mFOLFIRINOX FOLFIRINOX | III | Completed | NCT03504423[30] |
| Asparagine | L-Asparaginase | mPDAC | GemcitabineFOLFIRINOX | II | Completed | NCT02195180[38] | |
| Eryaspase | PDAC | Gemcitabine plus AbraxaneIrinotecan plus 5-FU plus leucovorin | III | Completed | NCT03665441[39] | ||
| Locally Advanced PDACmPDAC | FOLFIRINOX | I | Active, not recruiting | NCT04292743 [40] | |||
| MUC1 | SM-88 | Pancreatic Cancer | Capecitabine Gemcitabine 5-FU | II/III | Completed | NCT03512756[45] | |
| mPDAC | GnP mFOLFIRINOX Pamrevlumab combined with GnP Canakinumab and spartalizumab combined with GnP | III | Recruiting | NCT04229004 | |||
| Autophagy | HCQ | Untreated Resectable PDACBorderline Resectable PDACLocally Advanced PDAC | Paclitaxel protein boundGemcitabineCisplatin | II | Recruiting | NCT04669197 | |
| mPDAC | Binimetinib | I | Recruiting | NCT04132505 [57] | |||
| Metastatic Pancreatic CarcinomaUnresectable Pancreatic Carcinoma | Trametinib | I | Recruiting | NCT03825289[58] | |||
| Advanced PDACmPDAC | Paricalcitol | II | Recruiting | NCT04524702 | |||
| ERK | Temuterkib | Pancreatic CancerAdvanced Cancer | Hydroxychloroquine Sulfate | II | Recruiting | NCT04386057 | |
| DNA repair | PARP (germline or somatic BRCA1/2 or PALB2 mutation) | Olaparib | mPDAC | Pembrolizumab | II | Recruiting | NCT04548752[66] |
| Borderline Resectable PDACLocally Advanced PDACmPDACResectable PDAC | CobimetinibOnvansertibTemuterkib | I | Recruiting | NCT04005690 [67] | |||
| mPDACPDAC | Cediranib Maleate | II | Active, not recruitin | NCT02498613[68] | |||
| Rucaparib | mPDAC | FluorouracilIrinotecan SucrosofateLeucovorin Calcium | I/II | Active, not recruiting | NCT03337087 | ||
| ATM/ATR | Elimusertib | mPDACUnresectable PDAC | Irinotecan Hydrochloride | I | Recruiting | NCT04514497 | |
| Ceralasertib | Locally Advanced Pancreatic CancerMetastatic Pancreatic Cancer | CeralasertibDurvalumab | II | Recruiting | NCT03682289 | ||
| TME | CTGF | Pamrevlumab | Unresectable Pancreatic Carcinoma | GnP FOLFIRINOX | III | Active, not recruiting | NCT03941093 |
| mPDAC | III | Recruiting | NCT04229004 | ||||
| FAK | Defactinib | Resectable PDAC | Pembrolizumab | II | Recruiting | NCT03727880 [96] | |
| Pancreatic Cancer | I/II | Recruiting | NCT02758587 [97] | ||||
| TGF-β | Galunisertib | Metastasis Pancreatic Cancer | Gemcitabine | I/II | Completed | NCT01373164 [101] | |
| Durvalumab | I | Completed | NCT02734160 [102] | ||||
| Vitamin | ATRA | PDAC | GnP | I | Completed | NCT03307148 [107] | |
| II | Not yet recruiting | NCT04241276 | |||||
| CD40 | Mitazalimab | mPDAC | mFOLFIRINOX | I/II | Recruiting | NCT04888312 [138] | |
| CDX-1140 | PDAC | Pembrolizumab Chemotherapy CDX-301 | I | Completed | NCT03329950 | ||
| Immune system | CTLA4 | Tremelimumab | Pancreatic Cancer | Gemcitabine | I | Completed | NCT00556023 [117] |
| Ipilimumab | PDAC | Niraparib Nivolumab | I/II | Active, not recruiting | NCT03404960 [121] | ||
| PD-1 | Nivolumab | Pancreatic Cancer | Carboplatin GnP | I | Completed | NCT02309177 [119, 139] | |
| PD-L1 | Durvalumab | PDAC | Tremelimumab GnP | II | Active, not recruiting | NCT02879318 [120] | |
| mPDAC | Tremelimumab | II | Completed | NCT02558894 [122] | |||
| Vaccine | GVAX | mPDAC | Pembrolizumab Epacadostat Cyclophosphamide CRS-207 | II | Active, not recruiting | NCT03006302 | |
| CAR-T | CAR-T cells directed against CLD18 | PDAC | None | I | Recruiting | NCT03159819 [140] | |
| TOV | Pelareorep | mPDAC | Gemcitabine | II | Completed | NCT00998322 [133] | |
| Carboplatin/Paclitaxel | II | Completed | NCT01280058 [134] | ||||
| PDAC | Pembrolizumab Chemotherapy | I | Completed | NCT02620423 [135] |
2. Targeting metabolic vulnerabilities
Reprogramming of glucose, protein, lipid and nucleic acid metabolism provides tumor cells with energy and responds to redox stress [18]. PDAC cells rely on aerobic glycolysis (Warburg effect) or metabolic changes to adapt to energy and nutrient deprivation and abnormal oxidative stress in the TME [19]. Based on the metabolite profiles of PDAC cells, Daemen et al. [20] classified them into three subtypes: low proliferating, glycolytic, and lipogenic. Glycolysis and glutamine are sensitive to inhibitors of glycolysis. In contrast, lipoproteins are more sensitive to lipid inhibitors, but not to inhibition of the glycolytic pathway. Thus, a deeper understanding of the PDAC metabolic reprogramming process could help to develop better drugs for relevant targets.
2.1 Inhibition of TCA
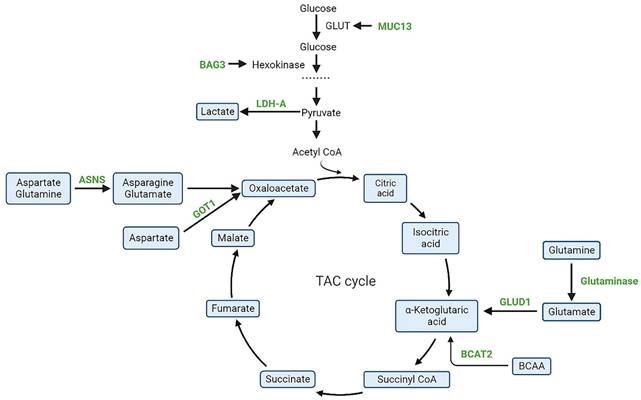
A key pathway in the glycometabolism is glycolysis, which is the primary source of energy for the growth and proliferation of cells. Tricarboxylic acid cycle (TCA) cycle intermediates play an important role in the metabolic abnormalities of PDAC and may be potential therapeutic targets. Currently, molecules related to glycolytic pathways such as ME2/3 [21], PON2 [22], LDH-A [23], BAG3 [24], MUC1/13 [25], long noncoding RNA-PLACT1 [26]and IKKε [27] have been shown to play crucial roles in PDAC (Figure 2). However, more exploration is needed for drug development.Devimistat is a lipid analogue that selectively targets the TCA cycle in tumor cells and thus selectively acts on cancer cells. It inhibits pyruvate dehydrogenase and α-ketoglutarate dehydrogenase complexes to reduce the entry of glucose and glutamine derived carbons into the TCA cycle, ultimately leading to cancer cell death [28]. In a phase I clinical study, 20 mPDAC patients treated with devimistat and mFOLFIRINOX in combination demonstrated better drug tolerance and a 61% objective response rate (ORR) [29]. Subsequently, a global, randomized phase III trial evaluating devimistat in combination with mFOLFIRINOX versus FOLFIRINOX in patients with mPDAC yielded results with no significant improvement in ORR, PFS or OS with the combination (NCT03504423) [30]. In addition, a single arm, open-label, phase I trial evaluated the combination of devimisat with GnP in mPDAC patients and showed that devimisat can be safely administered with GnP and nab-paclitaxel at doses up to 1500 m/g2 [31].
2.2 Inhibition of amino acids metabolism
Studies have shown that glutamine plays an essential role in the growth and proliferation of PDAC cells [32]. Son et al. showed that PDAC cells maintain an intracellular redox state through a non-classical glutamine metabolic pathway in which glutamate-derived aspartate is converted to oxaloacetate, malate, and pyruvate by aspartate transaminase (GOT1). However, in normal cells, glutamate dehydrogenase (GLUD1) can convert glutamine-derived glutamate to α-ketoglutarate in order to enter the TCA cycle [33]. Similarly, Abrego et al. [32] showed that under low PH conditions, PDAC cells deviated from glycolytic metabolism and relied more on oxidative metabolism promoted by increased GOT1 enzyme expression. Zhou et al. [34] suggested that GOT1 plays an important role in coordinating glycolysis and oxidative phosphorylation pathways in KRAS mutated cancer cells. Since GOT1 plays a key role in abnormal glutamine metabolism, it may be a potential anti-cancer target. Wu et al. [35] found that the cardiovascular drug hydrazine hydrochloride can inhibit the catalytic activity of GOT1, but its mechanism needs to be further investigated. The potential of asparagine as a target for PDAC treatment has been discovered. Most cells are capable of transforming asparagine and glutamine into asparagine and glutamate through asparagine synthetase (ASNS). In stark opposition, l-Asparaginase, a chemotherapeutic agent, hydrolyzes asparagine to aspartic acid and ammonia, thus depriving cells of the circulating asparagine and thus influencing the proliferation and growth of cancer cells. Studies have shown that PDAC is a tumor with low ASNS expression, and therefore, patients may benefit from l-Asparaginase treatment [36, 37]. A randomized IIb open-label study revealed that the combination of erythrocyte-encapsulated l-ASNase (eryaspase) and gemcitabine/mFOLFOX-6 as second-line therapy enhanced both overall survival and progression-free survival (NCT02195180) [38], however, independent of ASNS expression. In a randomized, open-label phase III trial (Trybeca-1), the combination of eryaspase with GnP or irinotecan/5-FU failed to meet the primary endpoint of patient OS, however, in the irinotecan/5-FU subgroup, eryaspase group showed good tolerability and survival benefit and warrants additional study (NCT03665441) [39]. Another phase I trial used eryaspase in combination with mFOLFIRINOX for the first-line treatment of locally advanced or mPDAC (NCT04292743), with results to be further observed [40]. In addition, Pathria et al. [41] found that inhibition of asparagine activated receptor tyrosine kinase-MAPK signaling and enhanced translation of transcription factor 4 (ATF4) mRNA in pancreatic cancer cells. Similarly, inhibition of the MAPK pathway reduces ATF4 translation and ASNS expression, increasing the sensitivity of pancreatic tumors to asparagine restriction [42]. Thus, the combination of inhibition of the MAPK pathway and Asparagine may be a new therapeutic measure for PDAC patients with low ASNS expression. Racemetyrosine (SM-88) is a dysfunctional tyrosine derivative that causes disruption of protein synthesis in malignant tumor cells. MUC1 is a key target for this process [43]. Cancer cells display heightened levels of free radicals or reactive oxygen species (ROS). SM-88 controls ROS levels by interfering with tumor cell MUC1 activity, leading to increased toxicity and ultimately triggering apoptotic signaling and cell death [44]. In a prospective, open-label randomized phase II/III clinical trial in metastatic pancreatic cancer, SM-88 in combination with MPS (methoxsalen, phenytoin and sirolimus) was found to be well tolerated, with an overall patient disease control rate (DCR) of 27% (NCT03512756) [45]. DCR is the percentage of patients with advanced tumors who result in a complete response, partial response, or stable disease through a therapeutic intervention. In addition, a multi-center, phase III trial enrolling 825 patients with metastatic pancreatic cancer is currently enrolling (NCT04229004) to evaluate the efficacy of pamrevlumab, a monoclonal antibody to SM-88 or anti-connective tissue growth factor (CTGF), compared to first-line chemotherapy agents GnP or mFOLFIRINOX. Semi-essential amino acid arginine promotes nitric oxide production and protein synthesis in cells [46]. In pancreatic cancer, the rate-limiting enzyme for arginine synthesis, the enzyme argininosuccinate synthetase (ASS1), is commonly absent [47]. Arginine deiminase converts arginine to citrulline and prevents arginine production in cells [48]. Thus, arginine deiminase could be a novel therapeutic approach for PDAC. Preclinical study found that arginine depletion using pegylated arginine deiminase (ADI-PEG20) selectively inhibited the proliferation of ASS1-deficient pancreatic cancer cells and enhanced radiotherapy-induced apoptosis [49]. Moreover, ADI-PEG20 increased the sensitivity of pancreatic cancer to gemcitabine [50]. A single-arm, non-randomized, open-label, phase I/Ib trial showed that the combination of ADI-PEG20, gemcitabine, and nab-paclitaxel was well-tolerated in patients with advanced pancreatic cancer [51]. The potential for ADI-PEG20 and other drugs to augment the activity and anti-tumor capacity of T cells could be a significant factor in the future treatment of advanced pancreatic cancer [52, 53].
Schematic representation of targeted metabolic intermediates and enzymes(green) in PDAC. BAG3, Bcl-2-associated athanogene 3; MUC13, mucin 13; LDH-A, lactate dehydrogenase A; GOT1, aspartate aminotransferase; ASNS, asparagine synthetase; BCAT2, branched-chain amino acid transaminase 2; GLUT, glucose transporter; GLUD1, glutamate dehydrogenase-1.
2.3 Regulating the autophagy
Autophagy is a process that maintains metabolism and cellular homeostasis through the lysosomal breakdown of intracellular organelles and macromolecules. Pancreatic cancer exhibits elevated autophagy under basal conditions, and inhibition of autophagy leads to elevated DNA damage, increased ROS, decreased mitochondrial oxidative phosphorylation, and inhibition of PDAC cell growth [54]. A randomized phase II study showed that preoperative addition of the autophagy inhibitor hydroxychloroquine (HCQ) to GnP chemotherapy improved surgical prognosis in patients with PDAC [55]. The primary endpoint of OS at 1 year was not met in an open-label, phase II randomized clinical trial involving the combination of HCQ and GnP for mPDAC. However, the overall remission rates with HCQ were improved, indicating a potential role for HCQ in the locally advanced PDAC [56]. A phase II study (NCT04669197) is treating locally advanced PDAC with a combination of paclitaxel protein bound + gemcitabine + cisplatin + HCQ and remains to be further evaluated. Preclinical studies have found that KRAS-mutant PDAC cells can activate autophagy to resist the inhibitory effects of MEK. Two studies are currently evaluating the efficacy of HCQ in combination with binimetinib or trametinib in patients with KRAS-mutant advanced or mPDAC (NCT04132505 and NCT03825289) [57, 58]. In addition, the ERK inhibitor temuterkib has shown promise in BRAF- and KRAS-mutant pancreatic cancer models, tested alone or in combination with HCQ (NCT04386057), and paricalcitol and HCQ or in combination with GnP (NCT04524702) in advanced or mPDAC patients.
3. Targeting DNA damage repair system
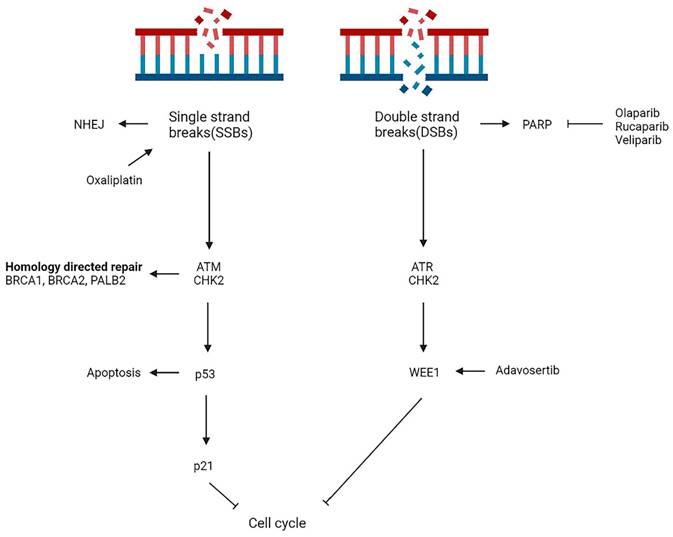
The damage or alteration of DNA molecules is a common phenomenon in cell division and, if not effectively repaired, can result in heritable genetic mutations. DNA damage activates the DNA damage repair (DDR) pathway to restore the structure of damaged DNA molecules, which is essential for maintaining genome stability [59]. Damage to DDR at both endogenous (ROS) and exogenous (ionizing radiation or chemotherapies) factors can result in impaired DNA repair capacity and lead to malignant cellular transformation. Studies have shown that mutations in genes such as BRCA1, BRCA2, PALB2 and ATM cause homologous repair deficiency (HRD), with HRD reaching 14.5-16.5% in patients with PDAC [60, 61].HRD affects DNA double-strand break repair and increases sensitivity to poly ADP-ribose polymerase (PARP) inhibitors. PARP's involvement in DNA single-strand break (SSB) repair is of great significance, and its inhibition results in impaired repair capability and ultimately, the formation of DNA double-strand break (DSB) [62] (Figure 3).
3.1 PARP inhibitors
In a phase II clinical study, oralaparib, a PARP inhibitor, was initially investigated in patients with advanced cancer and BRCA 1/2 mutations. Among the 23 mPDAC patients, the ORR of olaparib was 21.7% and 35% of mPDAC patients had stable disease (SD) lasting ≥ 8 weeks [63]. The POLO trial validated the efficacy of olaparib in mPDAC patients, with a marked enhancement in PFS when compared to placebo, yet the OS remained comparable in both groups [64]. On December 27, 2019, the FDA, having been presented with the evidence from the phase III randomized controlled trial, granted accelerated approval for olaparib to be used as a maintenance treatment for pancreatic cancer patients who have a germline BRCA mutation and have not progressed after 16 weeks of platinum therapy. A phase II clinical trial of olaparib for advanced PDAC patients with DDR deficiency without BRCA mutations (BRCAness) phenotype is underway, with preliminary results indicating an ORR of 20% and mPFS of 24.7 weeks [65]. In addition, a phase II trial (NCT04548752) is evaluating olaparib and pembrolizumab as maintenance therapy for patients with BRCA mutations [66]. Combination therapy with olaparib and the MEK inhibitor cobimetinib is being tested in mPDAC patients (NCT04005690) [67]. The combination of cediranib maleate, an inhibitor of VEGFR tyrosine kinases, and olaparib in patients with solid tumors, including mPDAC (NCT02498613), is currently underway [68].
An investigation of the efficacy and safety of the oral PARP inhibitor olaparib in 19 patients with locally advanced/mPDAC and germline or somatic BRCA1/2 mutations was conducted in a single-arm, phase II RUCAPANC study. Rucaparib demonstrated clinical efficacy with a safety profile. ORR was 15.8% and DCR was 31.6% [69].
In a single-arm, phase II clinical study, the PARP inhibitor veliparib was well-tolerated in phase III/IV PDAC in patients with BRCA1/2 or PALB2 mutations, but without tumor response [70]. The combination of veliparib, oxaliplatin, 5-FU and calcium folinic acid (FOLFOX) is safe for those with mPDAC and has been demonstrated to be effective, particularly in those with BRCA1/2 or PALB2 mutations [71]. In a phase I trial, De Bono et al. assessed the potency of talazoparib, a novel PARP inhibitor, in patients with advanced solid tumors and germline BRCA1/2 mutations. Single agent Talazoparib showed antitumor activity and tolerable safety [72].
Overview of main DNA damage repair pathways in mPDAC. NHEJ, non-homologous end joining; PARP, poly ADP-ribose polymerase.
3.2 ATR or WEE1 inhibitors
ATMs are a key component of the DNA damage response, and they play a central role in activating the response to SSBs and DSBs [73]. Disruption of ATM signals leads cells to rely on downstream ATR and CHK1/2 pathways to repair DNA, thus making them two potential targets for ATM-deficient tumors. In sporadic pancreatic cancer with somatic mutations, the overall mutation frequency of ATM is about 5% [74]. Preclinical studies have found that ATM mutant PDAC cells are particularly sensitive to PARP, ATR and CHK1/2 inhibitors alone or in combination [75, 76]. ATM-deficient tumors are currently being studied in clinical trials (NCT04514497 and NCT03682289). Studies show that WEE1 inhibition increases the effectiveness of DNA damaging agents and enhances the killing effect of combination drugs [77]. A combination of a WEE1 inhibitor with a chemotherapeutic agent or radiation therapy has been evaluated and the results show promising results [78, 79]. The ATM pathway and WEE1 may be crucial research directions for future studies of the DNA damage repair system in mPDAC patients. Routine germline and somatic cell testing, as recommended by the NCCN, should be conducted on all PDAC patients, thereby providing targeted treatment options for those with particular genomic alterations.
4. Targeting TME
PDAC's TME consists of a deeply pro-connective tissue proliferative and fibrotic mesenchyme that includes fibroblasts, stellate cells, endothelial cells, pericytes, neurons, infiltrating immune cells, and extracellular matrix proteins. This dense interstitium causes extreme interstitial fluid pressures, reduced vascularity and hypoxia, creating barriers to drug delivery to tumor cells, in which hyaluronic acid (HA) plays an essential role [80] (Figure 4). PEGPH20, a recombinant pegylated human hyaluronidase enzyme, is one of the most promising drugs. By reducing tumor pressure and vascular compression, PEGPH20 increases antitumor drug penetration and efficacy [80]. However, a recent phase III clinical trial HALO109-301 combining PEGPH20 and GnP in previously untreated patients with hyaluronic acid-high stage IV mPDAC did not achieve the expected endpoints of OS and PFS and has been terminated [81].
The tumor stroma of PDAC. T-cell, T-lymphocyte; NK-cell, natural killer cell; DC cell, dendritic cell.
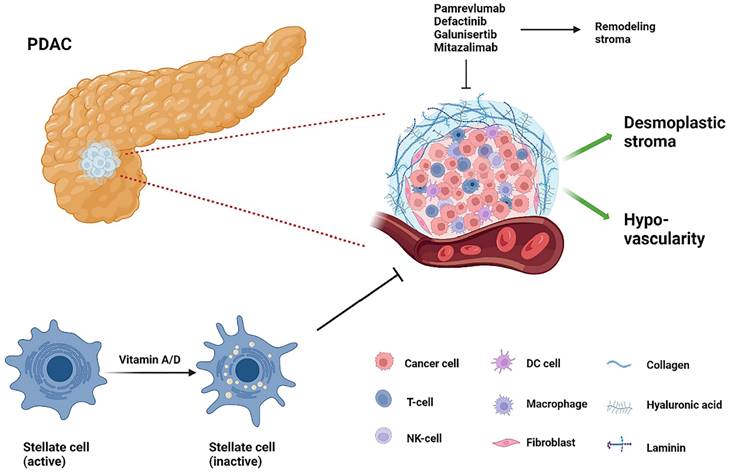
MAESTRO, a phase III trial, revealed that Evofosfamide (TH-302), a selective hypoxia-activated precursor drug, could kill solid tumors with hypoxic microenvironments. Moreover, its combination with gemcitabine improved mPFS and OS in locally advanced or mPDAC, but the difference was not statistically significant [82]. In addition, phase I clinical trials showed that the combination of TH-302 and ipilimumab did not show great efficacy in patients with mPDAC [83]. Ibrutinib, a Bruton tyrosine kinase inhibitor with anti-mesenchymal fibrosis effects, failed to meet the primary endpoint of OS or PFS benefit when combined with GnP in patients with mPDAC [84]. In addition, in patients with PDAC, ibrutinib had limited efficacy in combination with anti-PD-L1 antibodies developed and exported alone or in combination with pembrolizumab [85, 86]. Despite the unsatisfactory results against mesenchymal-targeted drugs, the unique TME profile remains one of the hot spots for mPDAC therapy. Preliminary clinical trials have shown promising results, with final results pending further study [87]. Immune cells are an integral part of TME and we will discuss immune system related treatments in the next subsection. This section will only discuss the rest of the TME mesenchyme in detail.
4.1 CTGF inhibitor
Pamrevlumab (FG-3019), a novel antifibrotic agent, has been found to be effective in suppressing the strong expression of CTGF in pancreatic cancer stroma [88]. Early studies have shown that the combination of pamrevlumab and gemcitabine/erlotinib is well tolerated in locally advanced or mPDAC with no dose-limiting toxicity [89]. In a phase I/II trial, the surgical resection rate for patients with locally advanced PDAC receiving GnP in combination with pamrevlumab was 33%, compared to 8% for GnP-only treatment [90]. A phase III trial is evaluating effect of pamrevlumab in combination with GnP or FOLFIRINOX in patients with advanced PDAC, with results yet to be published (NCT03941093 and NCT04229004). Therefore, pamrevumab has the potential to alter poor outcomes in mPDAC patients.
4.2 FAK inhibitor
A non-receptor tyrosine kinase, focal adhesion kinase (FAK), is a major regulator of adhesion signaling and cell migration, and is commonly expressed in PDAC [91]. It has been found that FAK inhibitors as monotherapy may inhibit tumor adhesion and migration, but do not have a significant effect on the prognosis of patients with advanced cancer, and that combination therapies may buffer the toxicity of other therapies that trigger cancer cell death or immune response [92]. Preclinical data suggested that FAK inhibition synergizes with immune checkpoint inhibitors to increase the infiltration of cytotoxic T cells into tumors [93, 94]. Using defactinib in conjunction with pembrolizumab and gemcitabine, Gillam et al. [95] published preliminary data on a FAK inhibitor combined with pembrolizumab. Among 20 patients with refractory PDAC, DCR was 80%, with one partial remission (PR) and 15 SDs, with mPFS and OS of 3.6 and 7.8 months, respectively. Of the 10 evaluable patients in the maintenance cohort, term response was 70%, with 1 PR and 6 SDs, and mPFS and OS were 5.0 and 8.3 months, respectively. The combination was well tolerated and safe, with good initial efficacy and demonstrated biomarker activity in infiltrating T lymphocytes. Additional clinical trials of defactinib plus pembrolizumab are currently being tested in resectable PC and advanced solid tumors (NCT03727880 [96] and NCT02758587 [97], respectively).
4.3 TGF-β inhibitor
Almost 50% of PDAC patients have mutations in SMAD4, a central component of TGF-β-mediated signalling [98]. TGF-β promotes cell growth, epithelial to mesenchymal cell conversion, extracellular matrix remodelling and immunosuppression. Preclinical studies have shown that inhibition of the TGF-β pathway reduces tumor metastatic progression, fibroblast deposition and induces tumor cell apoptosis [99]. Treatment of pancreatic cancer with galunisertib is being used as monotherapy or in combination with other drugs [100]. Phase II studies of galunisertib in combination with gemcitabine demonstrated acceptable safety profiles (NCT01373164) [101]. Galunisertib and gemcitabine improve OS and PFS in patients with pancreatic cancer, and patients with lower TGF-β levels may benefit more from galunisertib treatment [92]. Melisi et al. [102] published preliminary results from a phase Ib study testing the efficacy of galunisertib and the anti-PD-L1 antibody durvalumab in mPDAC (NCT02734160). The combination of Galunisertib 150 mg twice daily and durvalumab 1500 mg Q4W was well-tolerated, with a DCR of 25%.
4.4 Vitamin analogues
Studies have demonstrated that the regulation of TME by vitamin D is a critical factor in the treatment of pancreatic cancer. Sherman et al. [103] have discovered that the mesenchymal stroma of pancreatic cancer expresses the vitamin D receptor (VDR). VDR, as a key regulator to promote the recovery of pancreatic stellate cells to the resting state, can be combined with gemcitabine to remodel mesenchyme and reduce tumor volume. Paricalcitol, a VDR activator, is currently in clinical trials and awaits publication [104].
All trans retinoic acid (ATRA), a derivative of vitamin A, can break down the dense stroma of pancreatic cancer and allow chemotherapeutic agents to reach the tumor site [105]. Retinoic acid, in a mouse model of pancreatic cancer, induces quiescence of pancreatic stellate cells, reduces the Wnt/β-catenin signalling pathway, and induces apoptosis in the surrounding pancreatic cancer cells [106]. A phase Ib dose escalation and expansion trial in patients with advanced, unresectable PDAC revealed that ATRA, when combined with GnP chemotherapy, was a safe and tolerable stromal-targeting agent (NCT03307148) [107]. This combination will be further examined in a phase II randomised controlled trial in locally advanced PDAC (NCT04241276).
4.5 CD40 agonist
CD40, a member of the tumor necrosis factor receptor superfamily, is primarily expressed in leukocytes and some tumor cells. Studies have shown that CD40 activation prompts macrophages, but not T cells, to rapidly infiltrate tumors, become tumor-killing and promote tumor mesenchymal depletion [108]. A multicentre phase Ib/II study, OTPIMIZE-1 (NCT04888312), was conducted to assess the safety, tolerability, and efficacy of mitazalimab (CD40 agonist) in conjunction with mFOLFIRINOX for the treatment of previously untreated mPDAC.The study is continuing and patients are currently being enrolled at a dose of 900µg/kg in combination with mFOLFIRINOX. In addition, a phase II randomised controlled trial investigated the use of GnP ± nivolumab ± sotigalimab. Antitumor activity was observed in all groups with a 1-year OS > 35%. A phase I trial is currently exploring the efficacy of the agonist CD40 monoclonal antibody CDX-1140 alone or in combination with CDX-301 (FLT3L), pembrolizumab or chemotherapy (NCT03329950).
5. Immunotherapy approaches
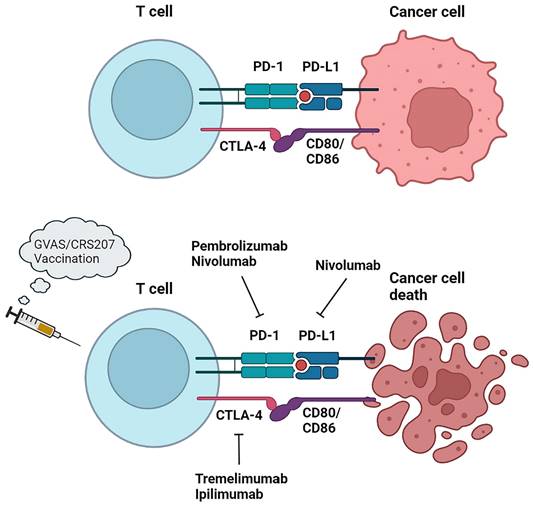
Immunotherapy and related immune checkpoint inhibitors (ICIs) such as programmed cell death protein 1 (PD-1), programmed cell death 1 ligand 1 (PD-L1) or cytotoxic T lymphocyte associated antigen-4 (CTLA-4) have produced positive results in the treatment of various solid tumors. Patients with PDAC respond poorly to immunotherapy due to low immunogenicity and low tumor mutation burden [109]. In addition, the abundant mesenchyme produces a hypoxic microenvironment and activates cancer-associated fibroblasts and secretes TGF-β to drive the recruitment of immunosuppressive cells, thus hampering the role of immunotherapy [110]. In spite of this, studies have found that a small percentage of PDAC patients still suffer from high microsatellite instability (MSI-h) or mismatch repair deficiency (dMMR) [111-113], and these patients, characterised by strong expression of tumor neoantigens and immune checkpoint ligands, have been shown to benefit from ICIs and to improve the rate of survival [114]. The FDA has approved pembrolizumab, a checkpoint inhibitor of anti-PD-1, for MSI/dMMR tumors and is recommended by the NCCN as a second-line treatment [115]. In addition, to overcome existing barriers to immunotherapy, researchers have used ICIs in combination with vaccines or cytotoxic therapies to boost immunogenicity in pancreatic cancer by recruiting and activating effector T cells that kill cancer cells.
5.1 Checkpoint inhibitors
The combination of the CTLA-4 immune checkpoint inhibitor ipilimumab and gemcitabine was safe and well-tolerated in PDAC patients, one of whom had a delayed response [116]. A phase Ib, multisite, open-label, non-randomized trial was conducted to assess the safety and tolerability of tremlimumab in combination with gemcitabine in patients with mPDAC and warrants further study (NCT00556023) [117]. A Ib/II single-center phase study investigating the anti-PD-1 antibody pembrolizumab in combination with GnP demonstrated a 100% DCR in 11 evaluable patients treated with mPDAC chemotherapy [118]. Despite not achieving the primary goal of > 15% complete response, PFS and OS were superior to prior standard dose therapies for GnP. The combination of PD-1 inhibitor nivolumab with GnP has similarly shown great clinical benefit [119]. However, the efficacy of GnP in combination with durvalumab and tremelimumab did not show a greater benefit in the randomised phase II trial (CCTG PA.7) (NCT02879318) [120]. The practical use of ICIs in combination with additional drugs has also shown promise. An evaluation of the combination of ipilimumab and niraparib, a PARP inhibitor, as a maintenance therapy for advanced pancreatic cancer after 16 weeks of chemotherapy with stable outcomes was conducted in a phase I/II trial (NCT03404960) [121]. 59.6% of patients did not progress at 6 months, compared with 44% for the pre-defined endpoint. Ongoing trials of immune checkpoint drug combinations in patients with mPDAC include durvalumab plus tremelimumab (NCT02558894) [122] and an evaluation of efficacy in combination with atezolizumab (NCT03193190) [123].
5.2 Therapeutic vaccines
Cancer vaccines can achieve their killing effect by activating tumor-specific CD8+ T cells. A thick barrier in the TME of mPDAC hinders the infiltration of immune cells. By combining vaccine immunotherapy with therapies targeting TME, clinical regression in patients with mPDAC can be improved, such as by boosting T cell infiltration with hyaluronidase and augmenting immune cells with IL-2, IL-12, and TGF-β [124]. On the other hand, the combination of a cancer vaccine with an adjuvant may also promote the immunogenicity of the vaccine and improve immunosuppressive and specific antigen deficiency in pancreatic cancer. Gene transfected tumor cell vaccine (GVAX), a whole cell vaccine expressing granulocyte-macrophage colony-stimulating factor (GM-CSF), is a gene transfected tumor cell vaccine. The cytokine GM-CSF plays an important role in DC antigen presentation. Combining GVAX with Listeria monocytogenes (Lm)-based vaccines can enhance DC function and enhance cellular immunity [125]. CR-207 is a live attenuator that expresses mesothelin [126]. Based on their study, Le et al. [127] indicated that CRS-207 significantly improved overall survival compared to GVAX alone in patients with mPDAC. A randomized, phase IIb, triple-arm trial was conducted to compare the combination of cyclophosphamide, GVAX and CRS-207, CRS-207 alone, or chemotherapy alone in patients with mPDAC who had been exposed to at least two cytotoxic agents, including gemcitabine, prior to the results. No significant differences in the primary OS endpoint was observed between the three groups (3.4 vs. 5.4 vs. 4.6 months). in the primary OS endpoint was observed between the three groups (3.4 vs. 5.4 vs. 4.6 months) [128]. Combining cancer vaccines with ICIs can also enhance antitumor responses, such as in a phase II trial that combined GVAX with pembrolizumab, epacadostat, and cyclophosphamide, then add CRS-207 in a patient with metastatic pancreas cancer to test efficacy (NCT03006302) (Figure 5). Derived from human telomerase reverse transcriptase (hTERT), an enzyme overexpressed in PDAC, GV1001 is a peptide vaccine. A three-group, open-label, randomised phase III trial (TeloVac) evaluated GV1001 in combination with gemcitabine and capecitabine in a chemotherapy regimen, as well as gemcitabine and capecitabine alone. In the study, which included 1,062 patients with locally advanced or metastatic PDAC, the GV1001 vaccine was not associated with an improved overall survival [129]. Another phase III trial tested GV1001 versus combination gemcitabine and capecitabine in advanced PDAC patients with elevated serum eotaxin, with control patients receiving gemcitabine and capecitabine only. The OS of patients with advanced PDAC was significantly augmented by the trial group, as evidenced by the results [130].
Immunotherapy approaches in PDAC. PD-1, programmed cell death protein 1; PD-L1, programmed cell death 1 ligand 1; CTLA-4, cytotoxic tlymphocyte associated antigen-4.
5.3 CAR T-cells and oncolytic virus therapy
CAR T-cells and oncolytic virus therapy has shown promising results in the treatment of haematological malignancies [131, 132]. A phase I trial (NCT01897415) verified the efficacy and safety of CAR-T therapy for immunotherapy-resistant mPDAC. Claudin-18 is a protein involved in the formation of tight junctions. Claudin 18.2 (CLDN 18.2) isoforms are expressed in 50%-70% of PDAC patients. A single-arm, open-label, first-in-human phase I pilot study (NCT03159819) evaluated the safety and efficacy of CLDN18.2-specific CAR T-cells in CLDN18.2-positive advanced pancreatic or gastric cancer. The results showed an ORR of 33.3%.
Genetically engineered viruses known as tumor oncolytic viruses (TOV) selectively infect and replicate in large numbers, lyse tumor cells without damaging normal cells, and can also provoke a powerful immune response that leads to tumor destruction. Pelareorep is an oncolytic reovirus that induces an anti-tumor immune response by activating the innate and adaptive immune systems. Pelareorep was found to be safe and effective in patients with advanced pancreatic cancer when combined with gemcitabine in a phase II study (NCT00998322) [133]. No distinction in PFS was observed between the two groups when paclitaxel/carboplatin combined with pelareorep was tested in a randomised phase II trial (NCT01280058) compared to paclitaxel/carboplatin alone (4.9 months vs. 5.2 months) [134]. In addition, the effects of pelareorep in combination with immune checkpoint inhibitors are being investigated. A phase Ib/II study (NCT02620423) explored the clinical effects of pelareorep in combination with chemotherapy and pembrolizumab in patients with mPDAC and showed superior safety and anti-tumor activity [135, 136]. In November 2022, Oncolytics reported interim clinical data showing an ORR of 69% in a cohort of first-line advanced or mPDAC patients receiving a combination of pelarep, atezolizumab, and gemcitabine. This ORR is approximately three times the mean ORR reported in historical controlled trials evaluating GnP for pancreatic cancer. The clinical benefit rate was 85%, and as a result, the combination was granted Fast Track status by the FDA.
6. Conclusions and future directions
Treatment of mPDAC is extremely difficult and challenging. Over the past few years, clinical trials for the treatment of mPDAC have proliferated, but with more hotspots and less consensus. In addition to systemic chemotherapy, local treatment (including resection, ablation and embolization) appears to improve survival (hepatic mPDAC 7.8-19 months, pulmonary mPDAC 22.8 -47 months), but more rigorous randomised controlled trials in mPDAC patients are needed to validate this [137]. Researchers are trying to study the new molecular vulnerabilities of mPDAC. Recent advances in mPDAC include targeting metabolic pathways and identifying patients with germline and somatic alterations (e.g. patients with mutations in the BRCA1/2 and PALB2 genes).The POLO trial and FDA approval of olaparib as a maintenance therapy for mPDAC justify this direction. In addition, targeted TME and immune checkpoint inhibitor therapies are also therapeutic priorities for mPDAC and a broad portfolio of clinical immunological agents is yielding favorable results. As a result, future treatments of mPDAC will focus more on these hotspots.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82270599, 81902431); Natural Science Foundation of Heilongjiang Province (LH2022H018); Marshal Initiative Funding of Harbin Medical University (HMUMIF-22008); Thematic Research Support Scheme of State Key Laboratory of Liver Research, The University of Hong Kong (SKLLR/TRSS/2022/08); Beijing Xisike Clinical Oncology Research Foundation (Y-Young2022-0188); Medjaden Academy & Research Foundation for Young Scientists (MJR20220903); Opening Project of State Key Laboratory of Chemical Oncogenomics (NA); Opening Project of Key Laboratory of Basic Pharmacology of Ministry of Education, Zunyi Medicial University (2022-449); Opening Research Fund of Key Laboratory of Gastrointestinal Cancer, Fujian Medical University, Ministry of Education (FMUGIC-202203); Opening Project of Key Laboratory of Environment and Health, Ministry of Education (2022GWKFJJ01); Opening Project of Key Laboratory of Functional and Clinical Translational Medicine, Fujian Province University (XMMC-FCTM202205); Opening Project of Guangxi Laboratory of Enhanced Recovery after Surgery for Gastrointestinal Cancer (GXEKL202204); Opening Project of Key Laboratory of Biomarkers and In Vitro Diagnosis Translation of Zhejiang Province (KFJJ-2022002); Opening Project of Jiangsu Province Engineering Research Center of Tumor Targeted Nano Diagnostic and Therapeutic Materials (JETNM202210); Opening Project of Fujian Provincial Key Laboratory of Innovative Drug Target Research (FJ-YW-2022KF03); Chen Xiao-ping Foundation for the Development of Science and Technology of Hubei Province, China (CXPJJH11900002-058).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022 721
2. Chiorean EG, Coveler AL. Pancreatic cancer: optimizing treatment options, new, and emerging targeted therapies. Drug Des Devel Ther. 2015;9:3529-3545
3. Sinn M, Bahra M, Liersch T, Gellert K, Messmann H, Bechstein W. et al. CONKO-005: Adjuvant Chemotherapy With Gemcitabine Plus Erlotinib Versus Gemcitabine Alone in Patients After R0 Resection of Pancreatic Cancer: A Multicenter Randomized Phase III Trial. J Clin Oncol. 2017;3529:3330-3337
4. Le Large TYS, Bijlsma MF, Kazemier G, van Laarhoven HWM, Giovannetti E, Jimenez CR. Key biological processes driving metastatic spread of pancreatic cancer as identified by multi-omics studies. Semin Cancer Biol. 2017;44:153-169
5. Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;37111:1039-1049
6. Sohal DPS, Mangu PB, Khorana AA, Shah MA, Philip PA, O'Reilly EM. et al. Metastatic Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;3423:2784-2796
7. Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y. et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;36419:1817-1825
8. Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M. et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;36918:1691-1703
9. Sohal DPS, Kennedy EB, Khorana A, Copur MS, Crane CH, Garrido-Laguna I. et al. Metastatic Pancreatic Cancer: ASCO Clinical Practice Guideline Update. Journal of Clinical Oncology. 2018;3624:2545-2556
10. De Dosso S, Siebenhüner AR, Winder T, Meisel A, Fritsch R, Astaras C. et al. Treatment landscape of metastatic pancreatic cancer. Cancer Treat Rev. 2021;96:102180
11. Wang-Gillam A, Li C-P, Bodoky G, Dean A, Shan Y-S, Jameson G. et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016 38710018:545-557
12. Kindler HL, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ. et al. Overall Survival Results From the POLO Trial: A Phase III Study of Active Maintenance Olaparib Versus Placebo for Germline BRCA-Mutated Metastatic Pancreatic Cancer. J Clin Oncol. 2022:JCO2101604.
13. Di Costanzo F, Di Costanzo F, Antonuzzo L, Mazza E, Giommoni E. Optimizing First-Line Chemotherapy in Metastatic Pancreatic Cancer: Efficacy of FOLFIRINOX versus Nab-Paclitaxel Plus Gemcitabine. Cancers (Basel). 2023 152
14. Qian Y, Gong Y, Fan Z, Luo G, Huang Q, Deng S. et al. Molecular alterations and targeted therapy in pancreatic ductal adenocarcinoma. J Hematol Oncol. 2020;131:130
15. Zhu H, Wei M, Xu J, Hua J, Liang C, Meng Q. et al. PARP inhibitors in pancreatic cancer: molecular mechanisms and clinical applications. Mol Cancer. 2020;191:49
16. Chiaravalli M, Reni M, O'Reilly EM. Pancreatic ductal adenocarcinoma: State-of-the-art 2017 and new therapeutic strategies. Cancer Treat Rev. 2017;60:32-43
17. Dorman K, Heinemann V, Kobold S, von Bergwelt-Baildon M, Boeck S. Novel systemic treatment approaches for metastatic pancreatic cancer. Expert Opin Investig Drugs. 2022;313:249-262
18. Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31-46
19. Cao L, Wu J, Qu X, Sheng J, Cui M, Liu S. et al. Glycometabolic rearrangements-aerobic glycolysis in pancreatic cancer: causes, characteristics and clinical applications. J Exp Clin Cancer Res. 2020;39:267
20. Daemen A, Peterson D, Sahu N, McCord R, Du X, Liu B. et al. Metabolite profiling stratifies pancreatic ductal adenocarcinomas into subtypes with distinct sensitivities to metabolic inhibitors. Proc Natl Acad Sci U S A. 2015;11232:E4410-E4417
21. Dey P, Baddour J, Muller F, Wu CC, Wang H, Liao W-T. et al. Genomic deletion of malic enzyme 2 confers collateral lethality in pancreatic cancer. Nature. 2017 5427639:119-123
22. Nagarajan A, Dogra SK, Sun L, Gandotra N, Ho T, Cai G. et al. Paraoxonase 2 Facilitates Pancreatic Cancer Growth and Metastasis by Stimulating GLUT1-Mediated Glucose Transport. Mol Cell. 2017 674
23. Rajeshkumar NV, Dutta P, Yabuuchi S, de Wilde RF, Martinez GV, Le A. et al. Therapeutic Targeting of the Warburg Effect in Pancreatic Cancer Relies on an Absence of p53 Function. Cancer Res. 2015;7516:3355-3364
24. An M-X, Li S, Yao H-B, Li C, Wang J-M, Sun J. et al. BAG3 directly stabilizes Hexokinase 2 mRNA and promotes aerobic glycolysis in pancreatic cancer cells. J Cell Biol. 2017;21612:4091-4105
25. Kumari S, Khan S, Gupta SC, Kashyap VK, Yallapu MM, Chauhan SC. et al. MUC13 contributes to rewiring of glucose metabolism in pancreatic cancer. Oncogenesis. 2018;72:19
26. Ren X, Chen C, Luo Y, Liu M, Li Y, Zheng S. et al. lncRNA-PLACT1 sustains activation of NF-κB pathway through a positive feedback loop with IκBα/E2F1 axis in pancreatic cancer. Mol Cancer. 2020;191:35
27. Zubair H, Azim S, Srivastava SK, Ahmad A, Bhardwaj A, Khan MA. et al. Glucose Metabolism Reprogrammed by Overexpression of IKKε Promotes Pancreatic Tumor Growth. Cancer Res. 2016;7624:7254-7264
28. Lee KC, Maturo C, Perera CN, Luddy J, Rodriguez R, Shorr R. Translational assessment of mitochondrial dysfunction of pancreatic cancer from in vitro gene microarray and animal efficacy studies, to early clinical studies, via the novel tumor-specific anti-mitochondrial agent, CPI-613. Ann Transl Med. 2014;29:91
29. Alistar A, Morris BB, Desnoyer R, Klepin HD, Hosseinzadeh K, Clark C. et al. Safety and tolerability of the first-in-class agent CPI-613 in combination with modified FOLFIRINOX in patients with metastatic pancreatic cancer: a single-centre, open-label, dose-escalation, phase 1 trial. Lancet Oncol. 2017;186:770-778
30. Philip PA, Buyse ME, Alistar AT, Rocha Lima CM, Luther S, Pardee TS. et al. A Phase III open-label trial to evaluate efficacy and safety of CPI-613 plus modified FOLFIRINOX (mFFX) versus FOLFIRINOX (FFX) in patients with metastatic adenocarcinoma of the pancreas. Future Oncol. 2019;1528:3189-3196
31. Alistar AT, Morris B, Harrison L, Bickenbach K, Starker L, Ginder N. et al. A single-arm, open-label, phase I study of CPI-613 (Devimistat) in combination with gemcitabine and nab-paclitaxel for patients with locally advanced or metastatic pancreatic adenocarcinoma. Journal of Clinical Oncology. 2020 3815_suppl:4635-4635
32. Abrego J, Gunda V, Vernucci E, Shukla SK, King RJ, Dasgupta A. et al. GOT1-mediated anaplerotic glutamine metabolism regulates chronic acidosis stress in pancreatic cancer cells. Cancer Lett. 2017;400:37-46
33. Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M. et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013 4967443:101-105
34. Zhou X, Curbo S, Li F, Krishnan S, Karlsson A. Inhibition of glutamate oxaloacetate transaminase 1 in cancer cell lines results in altered metabolism with increased dependency of glucose. BMC Cancer. 2018;18:559
35. Wu Q, Sun Z, Chen Z, Liu J, Ding H, Luo C. et al. The discovery of a non-competitive GOT1 inhibitor, hydralazine hydrochloride, via a coupling reaction-based high-throughput screening assay. Bioorg Med Chem Lett. 2022;73:128883
36. Dufour E, Gay F, Aguera K, Scoazec J-Y, Horand F, Lorenzi PL. et al. Pancreatic tumor sensitivity to plasma L-asparagine starvation. Pancreas. 2012;416:940-948
37. Bachet J-B, Gay F, Maréchal R, Galais M-P, Adenis A, MsC DS. et al. Asparagine Synthetase Expression and Phase I Study With L-Asparaginase Encapsulated in Red Blood Cells in Patients With Pancreatic Adenocarcinoma. Pancreas. 2015;447:1141-1147
38. Hammel P, Fabienne P, Mineur L, Metges J-P, Andre T, De La Fouchardiere C. et al. Erythrocyte-encapsulated asparaginase (eryaspase) combined with chemotherapy in second-line treatment of advanced pancreatic cancer: An open-label, randomized Phase IIb trial. Eur J Cancer. 2020 124
39. Hammel P, El-Hariry I, Macarulla T, Garcia-Carbonero R, Metges J-P, Bouché O. et al. Trybeca-1: A randomized, phase 3 study of eryaspase in combination with chemotherapy versus chemotherapy alone as second-line treatment in patients with advanced pancreatic adenocarcinoma (NCT03665441). Journal of Clinical Oncology. 2022 404_suppl:518-518
40. Yin C, Marshall J, Macke L, Bouker K, He AR, Weinberg BA. et al. A phase I dose-escalation study of eryaspase in combination with modified FOLFIRINOX in locally advanced and metastatic pancreatic ductal adenocarcinoma: Interim update. Journal of Clinical Oncology. 2022 404_suppl:581-581
41. Pathria G, Lee JS, Hasnis E, Tandoc K, Scott DA, Verma S. et al. Translational reprogramming marks adaptation to asparagine restriction in cancer. Nat Cell Biol. 2019;21:1590-1603
42. Pathria G, Lee JS, Hasnis E, Tandoc K, Scott DA, Verma S. et al. Translational reprogramming marks adaptation to asparagine restriction in cancer. Nat Cell Biol. 2019;2112:1590-1603
43. Lemberg KM, Gori SS, Tsukamoto T, Rais R, Slusher BS. Clinical development of metabolic inhibitors for oncology. J Clin Invest. 2022;132:e148550
44. Stega J, Noel MS, Vandell AG, Stega D, Del Priore G, Hoffman S. A first-in-human study of the novel metabolism-based anti-cancer agent SM-88 in subjects with advanced metastatic cancer. Invest New Drugs. 2020;382:392-401
45. Noel MS, Kim S, Hartley ML, Wong S, Picozzi VJ, Staszewski H. et al. A randomized phase II study of SM-88 plus methoxsalen, phenytoin, and sirolimus in patients with metastatic pancreatic cancer treated in the second line and beyond. Cancer Med. 2022;1122:4169-4181
46. Patil MD, Bhaumik J, Babykutty S, Banerjee UC, Fukumura D. Arginine dependence of tumor cells: targeting a chink in cancer's armor. Oncogene. 2016;3538:4957-4972
47. Bowles TL, Kim R, Galante J, Parsons CM, Virudachalam S, Kung H-J. et al. Pancreatic cancer cell lines deficient in argininosuccinate synthetase are sensitive to arginine deprivation by arginine deiminase. Int J Cancer. 2008;1238:1950-1955
48. Endicott M, Jones M, Hull J. Amino acid metabolism as a therapeutic target in cancer: a review. Amino Acids. 2021;538:1169-1179
49. Singh PK, Deorukhkar AA, Venkatesulu BP, Li X, Tailor R, Bomalaski JS. et al. Exploiting Arginine Auxotrophy with Pegylated Arginine Deiminase (ADI-PEG20) to Sensitize Pancreatic Cancer to Radiotherapy via Metabolic Dysregulation. Mol Cancer Ther. 2019;1812:2381-2393
50. Daylami R, Muilenburg DJ, Virudachalam S, Bold RJ. Pegylated arginine deiminase synergistically increases the cytotoxicity of gemcitabine in human pancreatic cancer. J Exp Clin Cancer Res. 2014;331:102
51. Lowery MA, Yu KH, Kelsen DP, Harding JJ, Bomalaski JS, Glassman DC. et al. A phase 1/1B trial of ADI-PEG 20 plus nab-paclitaxel and gemcitabine in patients with advanced pancreatic adenocarcinoma. Cancer. 2017;12323:4556-4565
52. Kim S-H, Roszik J, Grimm EA, Ekmekcioglu S. Impact of l-Arginine Metabolism on Immune Response and Anticancer Immunotherapy. Front Oncol. 2018;8:67
53. Chang KY, Chiang NJ, Wu SY, Yen CJ, Chen SH, Yeh YM. et al. Phase 1b study of pegylated arginine deiminase (ADI-PEG20) plus Pembrolizumab in advanced solid cancers. Oncoimmunology. 2021;101:1943253
54. Yang S, Wang X, Contino G, Liesa M, Sahin E, Ying H. et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;257:717-729
55. Zeh HJ, Bahary N, Boone BA, Singhi AD, Miller-Ocuin JL, Normolle DP. et al. A Randomized Phase II Preoperative Study of Autophagy Inhibition with High-Dose Hydroxychloroquine and Gemcitabine/Nab-Paclitaxel in Pancreatic Cancer Patients. Clin Cancer Res. 2020;2613:3126-3134
56. Karasic TB, O'Hara MH, Loaiza-Bonilla A, Reiss KA, Teitelbaum UR, Borazanci E. et al. Effect of Gemcitabine and nab-Paclitaxel With or Without Hydroxychloroquine on Patients With Advanced Pancreatic Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019;57:993-998
57. Surana R, Lee JJ, Smaglo BG, Zhao D, Lee MS, Wolff RA. et al. Phase I study of hydroxychloroquine plus binimetinib in patients with metastatic pancreatic cancer (the HOPE trial). Journal of Clinical Oncology. 2022 404_suppl:TPS634-TPS634
58. Raufi AG, Wong W, Chen K, Iuga A, Ahmed F, Manji GA. Sustained Partial Response to Inhibition of the Mitogen-Activated Protein Kinase and Autophagy Pathways in Combination With Immune Checkpoint Blockade in KRAS-Mutated Adenocarcinoma of the Small Bowel. JCO Precision Oncology. 2020;4:1122-1127
59. Mateo J, Lord CJ, Serra V, Tutt A, Balmaña J, Castroviejo-Bermejo M. et al. A decade of clinical development of PARP inhibitors in perspective. Ann Oncol. 2019;309:1437-1447
60. Yao W, Maitra A, Ying H. Recent insights into the biology of pancreatic cancer. EBioMedicine. 2020;53:102655
61. Casolino R, Paiella S, Azzolina D, Beer PA, Corbo V, Lorenzoni G. et al. Homologous Recombination Deficiency in Pancreatic Cancer: A Systematic Review and Prevalence Meta-Analysis. J Clin Oncol. 2021;3923:2617-2631
62. Sunada S, Nakanishi A, Miki Y. Crosstalk of DNA double-strand break repair pathways in poly(ADP-ribose) polymerase inhibitor treatment of breast cancer susceptibility gene 1/2-mutated cancer. Cancer Sci. 2018;1094:893-899
63. Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmaña J. et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;333:244-250
64. Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ. et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med. 2019;3814:317-327
65. Golan T, Varadhachary GR, Sela T, Fogelman DR, Halperin N, Shroff RT. et al. Phase II study of olaparib for BRCAness phenotype in pancreatic cancer. Journal of Clinical Oncology. 2018 364_suppl:297-297
66. Lee MS, Pant S. Personalizing Medicine With Germline and Somatic Sequencing in Advanced Pancreatic Cancer: Current Treatments and Novel Opportunities. American Society of Clinical Oncology Educational Book. 2021;41:e153-e165
67. Lopez CD, Kardosh A, Chen EY-s, Pegna GJ, Guimaraes A, Foster B. et al. A window of opportunity trial for metastatic (WOO-M) pancreatic ductal adenocarcinoma (PDAC): A biomarker discovery platform. Journal of Clinical Oncology. 2023 414_suppl:TPS781-TPS781
68. Varadhachary GR, Wolff RA. Current and Evolving Therapies for Metastatic Pancreatic Cancer: Are We Stuck With Cytotoxic Chemotherapy? Journal of Oncology Practice. 2016;129:797-805
69. Shroff RT, Hendifar A, McWilliams RR, Geva R, Epelbaum R, Rolfe L. et al. Rucaparib Monotherapy in Patients With Pancreatic Cancer and a Known Deleterious BRCA Mutation. JCO Precis Oncol. 2018. 2018
70. Lowery MA, Kelsen DP, Capanu M, Smith SC, Lee JW, Stadler ZK. et al. Phase II trial of veliparib in patients with previously treated BRCA-mutated pancreas ductal adenocarcinoma. Eur J Cancer. 2018;89:19-26
71. Pishvaian MJ, Wang H, He AR, Hwang JJ, Smaglo BG, Kim SS. et al. A Phase I/II Study of Veliparib (ABT-888) in Combination with 5-Fluorouracil and Oxaliplatin in Patients with Metastatic Pancreatic Cancer. Clin Cancer Res. 2020;2619:5092-5101
72. de Bono J, Ramanathan RK, Mina L, Chugh R, Glaspy J, Rafii S. et al. Phase I, Dose-Escalation, Two-Part Trial of the PARP Inhibitor Talazoparib in Patients with Advanced Germline BRCA1/2 Mutations and Selected Sporadic Cancers. Cancer Discov. 2017;76:620-629
73. Pritchard CC, Mateo J, Walsh MF, De Sarkar N, Abida W, Beltran H. et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N Engl J Med. 2016;3755:443-453
74. Perkhofer L, Gout J, Roger E, Kude de Almeida F, Baptista Simões C, Wiesmüller L. et al. DNA damage repair as a target in pancreatic cancer: state-of-the-art and future perspectives. Gut. 2021;703:606-617
75. Perkhofer L, Schmitt A, Romero Carrasco MC, Ihle M, Hampp S, Ruess DA. et al. ATM Deficiency Generating Genomic Instability Sensitizes Pancreatic Ductal Adenocarcinoma Cells to Therapy-Induced DNA Damage. Cancer Res. 2017;7720:5576-5590
76. Liang M, Zhao T, Ma L, Guo Y. CHK1 inhibition sensitizes pancreatic cancer cells to gemcitabine via promoting CDK-dependent DNA damage and ribonucleotide reductase downregulation. Oncol Rep. 2018;393:1322-1330
77. O'Connell MJ, Raleigh JM, Verkade HM, Nurse P. Chk1 is a wee1 kinase in the G2 DNA damage checkpoint inhibiting cdc2 by Y15 phosphorylation. EMBO J. 1997;163:545-554
78. Leijen S, van Geel RMJM, Pavlick AC, Tibes R, Rosen L, Razak ARA. et al. Phase I Study Evaluating WEE1 Inhibitor AZD1775 As Monotherapy and in Combination With Gemcitabine, Cisplatin, or Carboplatin in Patients With Advanced Solid Tumors. J Clin Oncol. 2016;3436:4371-4380
79. Cuneo KC, Morgan MA, Sahai V, Schipper MJ, Parsels LA, Parsels JD. et al. Dose Escalation Trial of the Wee1 Inhibitor Adavosertib (AZD1775) in Combination With Gemcitabine and Radiation for Patients With Locally Advanced Pancreatic Cancer. J Clin Oncol. 2019;3729:2643-2650
80. Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;213:418-429
81. Van Cutsem E, Tempero MA, Sigal D, Oh D-Y, Fazio N, Macarulla T. et al. Randomized Phase III Trial of Pegvorhyaluronidase Alfa With Nab-Paclitaxel Plus Gemcitabine for Patients With Hyaluronan-High Metastatic Pancreatic Adenocarcinoma. J Clin Oncol. 2020;3827:3185-3194
82. Van Cutsem E, Lenz H-J, Furuse J, Tabernero J, Heinemann V, Ioka T. et al. Evofosfamide (TH-302) in combination with gemcitabine in previously untreated patients with metastatic or locally advanced unresectable pancreatic ductal adenocarcinoma: Primary analysis of the randomized, double-blind phase III MAESTRO study. Journal of Clinical Oncology. 2016 344_suppl:193-193
83. Hegde A, Jayaprakash P, Couillault CA, Piha-Paul S, Karp D, Rodon J. et al. A Phase I Dose-Escalation Study to Evaluate the Safety and Tolerability of Evofosfamide in Combination with Ipilimumab in Advanced Solid Malignancies. Clin Cancer Res. 2021;2711:3050-3060
84. Tempero M, Oh DY, Tabernero J, Reni M, Van Cutsem E, Hendifar A. et al. Ibrutinib in combination with nab-paclitaxel and gemcitabine for first-line treatment of patients with metastatic pancreatic adenocarcinoma: phase III RESOLVE study. Ann Oncol. 2021;325:600-608
85. Hong D, Rasco D, Veeder M, Luke JJ, Chandler J, Balmanoukian A. et al. A Phase 1b/2 Study of the Bruton Tyrosine Kinase Inhibitor Ibrutinib and the PD-L1 Inhibitor Durvalumab in Patients with Pretreated Solid Tumors. Oncology. 2019;972:102-111
86. Overman M, Javle M, Davis RE, Vats P, Kumar-Sinha C, Xiao L. et al. Randomized phase II study of the Bruton tyrosine kinase inhibitor acalabrutinib, alone or with pembrolizumab in patients with advanced pancreatic cancer. J Immunother Cancer. 2020 81
87. Tran LC, Özdemir BC, Berger MD. The Role of Immune Checkpoint Inhibitors in Metastatic Pancreatic Cancer: Current State and Outlook. Pharmaceuticals (Basel). 2023;16:1411
88. Abbassi R, Schmid RM. Evolving Treatment Paradigms for Pancreatic Cancer. Visc Med. 2019;356:362-372
89. Picozzi VJ, Pipas JM, Koong A, Giaccia A, Bahary N, Krishnamurthi SS, Lopez CD, O'Dwyer PJ, Modelska K, Poolman V. et al. FG-3019, a human monoclonal antibody to CTGF, with gemcitabine/erlotinib in patients with locally advanced or metastatic pancreatic ductal adenocarcinoma. Journal of Clinical Oncology. 2013 314_suppl:213-213
90. Picozzi V, Alseidi A, Winter J, Pishvaian M, Mody K, Glaspy J. et al. Gemcitabine/nab-paclitaxel with pamrevlumab: a novel drug combination and trial design for the treatment of locally advanced pancreatic cancer. ESMO Open. 2020 54
91. Kanteti R, Mirzapoiazova T, Riehm JJ, Dhanasingh I, Mambetsariev B, Wang J. et al. Focal adhesion kinase a potential therapeutic target for pancreatic cancer and malignant pleural mesothelioma. Cancer Biol Ther. 2018;194:316-327
92. Dawson JC, Serrels A, Stupack DG, Schlaepfer DD, Frame MC. Targeting FAK in anticancer combination therapies. Nat Rev Cancer. 2021;215:313-324
93. Jiang H, Liu X, Knolhoff BL, Hegde S, Lee KB, Jiang H. et al. Development of resistance to FAK inhibition in pancreatic cancer is linked to stromal depletion. Gut. 2020;691:122-132
94. Jiang H, Hegde S, Knolhoff BL, Zhu Y, Herndon JM, Meyer MA. et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med. 2016;228:851-860
95. Wang-Gillam A, Lim K-H, McWilliams R, Suresh R, Lockhart AC, Brown A. et al. Defactinib, Pembrolizumab, and Gemcitabine in Patients with Advanced Treatment Refractory Pancreatic Cancer: A Phase I Dose Escalation and Expansion Study. Clin Cancer Res. 2022;2824:5254-5262
96. Davelaar J, Brown Z, Linden S, Rodriguez C, Elmadbouh O, Pachter JA. et al. Trial in progress: A randomized phase II study of pembrolizumab with or without defactinib, a focal adhesion kinase inhibitor, following chemotherapy as a neoadjuvant and adjuvant treatment for resectable pancreatic ductal adenocarcinoma (PDAC). Journal of Clinical Oncology. 2022 4016_suppl:TPS4192-TPS4192
97. Fennell DA, Baas P, Taylor P, Nowak AK, Gilligan D, Nakano T. et al. Maintenance Defactinib Versus Placebo After First-Line Chemotherapy in Patients With Merlin-Stratified Pleural Mesothelioma: COMMAND—A Double-Blind, Randomized, Phase II Study. Journal of Clinical Oncology. 2019;3710:790-798
98. Bailey P, Chang DK, Nones K, Johns AL, Patch A-M, Gingras M-C. et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016 5317592:47-52
99. Ahmed S, Bradshaw A-D, Gera S, Dewan MZ, Xu R. The TGF-β/Smad4 Signaling Pathway in Pancreatic Carcinogenesis and Its Clinical Significance. J Clin Med. 2017 61
100. Ostapoff KT, Cenik BK, Wang M, Ye R, Xu X, Nugent D. et al. Neutralizing murine TGFβR2 promotes a differentiated tumor cell phenotype and inhibits pancreatic cancer metastasis. Cancer Res. 2014;7418:4996-5007
101. Melisi D, Garcia-Carbonero R, Macarulla T, Pezet D, Deplanque G, Fuchs M. et al. TGFβ receptor inhibitor galunisertib is linked to inflammation- and remodeling-related proteins in patients with pancreatic cancer. Cancer Chemother Pharmacol. 2019;835:975-991
102. Melisi D, Oh D-Y, Hollebecque A, Calvo E, Varghese A, Borazanci E. et al. Safety and activity of the TGFβ receptor I kinase inhibitor galunisertib plus the anti-PD-L1 antibody durvalumab in metastatic pancreatic cancer. J Immunother Cancer. 2021 93
103. Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H. et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;1591:80-93
104. Wei D, Wang L, Zuo X, Bresalier RS. Vitamin D: Promises on the Horizon and Challenges Ahead for Fighting Pancreatic Cancer. Cancers (Basel). 2021 1311
105. Brown G. Deregulation of All-Trans Retinoic Acid Signaling and Development in Cancer. Int J Mol Sci. 2023;24:12089
106. Froeling FEM, Feig C, Chelala C, Dobson R, Mein CE, Tuveson DA. et al. Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-β-catenin signaling to slow tumor progression. Gastroenterology. 2011 1414
107. Kocher HM, Basu B, Froeling FEM, Sarker D, Slater S, Carlin D. et al. Phase I clinical trial repurposing all-trans retinoic acid as a stromal targeting agent for pancreatic cancer. Nat Commun. 2020;111:4841
108. Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W. et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011 3316024:1612-1616
109. Tsunedomi R, Shindo Y, Nakajima M, Yoshimura K, Nagano H. The tumor immune microenvironment in pancreatic cancer and its potential in the identification of immunotherapy biomarkers. Expert Rev Mol Diagn. 2023:1-14
110. Hilmi M, Bartholin L, Neuzillet C. Immune therapies in pancreatic ductal adenocarcinoma: Where are we now? World J Gastroenterol. 2018;2420:2137-2151
111. Hu ZI, Shia J, Stadler ZK, Varghese AM, Capanu M, Salo-Mullen E. et al. Evaluating Mismatch Repair Deficiency in Pancreatic Adenocarcinoma: Challenges and Recommendations. Clin Cancer Res. 2018;246:1326-1336
112. Singhi AD, George B, Greenbowe JR, Chung J, Suh J, Maitra A. et al. Real-Time Targeted Genome Profile Analysis of Pancreatic Ductal Adenocarcinomas Identifies Genetic Alterations That Might Be Targeted With Existing Drugs or Used as Biomarkers. Gastroenterology. 2019 1568
113. Latham A, Srinivasan P, Kemel Y, Shia J, Bandlamudi C, Mandelker D. et al. Microsatellite Instability Is Associated With the Presence of Lynch Syndrome Pan-Cancer. J Clin Oncol. 2019;374:286-295
114. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK. et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017 3576349:409-413
115. Sohal DPS, Kennedy EB, Cinar P, Conroy T, Copur MS, Crane CH. et al. Metastatic Pancreatic Cancer: ASCO Guideline Update. J Clin Oncol. 2020:JCO2001364.
116. Kamath SD, Kalyan A, Kircher S, Nimeiri H, Fought AJ, Benson A. et al. Ipilimumab and Gemcitabine for Advanced Pancreatic Cancer: A Phase Ib Study. Oncologist. 2020;255:e808-e815
117. Aglietta M, Barone C, Sawyer MB, Moore MJ, Miller WH, Bagalà C. et al. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann Oncol. 2014;259:1750-1755
118. Weiss GJ, Blaydorn L, Beck J, Bornemann-Kolatzki K, Urnovitz H, Schütz E. et al. Phase Ib/II study of gemcitabine, nab-paclitaxel, and pembrolizumab in metastatic pancreatic adenocarcinoma. Invest New Drugs. 2018 361
119. Wainberg ZA, Hochster HS, Kim EJ, George B, Kaylan A, Chiorean EG. et al. Open-label, Phase I Study of Nivolumab Combined with nab-Paclitaxel Plus Gemcitabine in Advanced Pancreatic Cancer. Clin Cancer Res. 2020;2618:4814-4822
120. Renouf DJ, Loree JM, Knox JJ, Topham JT, Kavan P, Jonker D. et al. The CCTG PA.7 phase II trial of gemcitabine and nab-paclitaxel with or without durvalumab and tremelimumab as initial therapy in metastatic pancreatic ductal adenocarcinoma. Nat Commun. 2022;131:5020
121. Reiss KA, Mick R, Teitelbaum U, O'Hara M, Schneider C, Massa R. et al. Niraparib plus nivolumab or niraparib plus ipilimumab in patients with platinum-sensitive advanced pancreatic cancer: a randomised, phase 1b/2 trial. Lancet Oncol. 2022;238:1009-1020
122. O'Reilly EM, Oh D-Y, Dhani N, Renouf DJ, Lee MA, Sun W. et al. Durvalumab With or Without Tremelimumab for Patients With Metastatic Pancreatic Ductal Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019;510:1431-1438
123. Oh D-Y, Ajani JA, Bang Y-J, Chung HC, Lacy J, Lee J. et al. Phase Ib/II open-label, randomized evaluation of 2L atezolizumab (atezo) + BL-8040 versus control in MORPHEUS-pancreatic ductal adenocarcinoma (M-PDAC) and MORPHEUS-gastric cancer (M-GC). Journal of Clinical Oncology. 2020 384_suppl:712-712
124. Luo W, Yang G, Luo W, Cao Z, Liu Y, Qiu J. et al. Novel therapeutic strategies and perspectives for metastatic pancreatic cancer: vaccine therapy is more than just a theory. Cancer Cell Int. 2020;20:66
125. Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B. et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;145:1455-1463
126. Le DT, Brockstedt DG, Nir-Paz R, Hampl J, Mathur S, Nemunaitis J. et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin Cancer Res. 2012;183:858-868
127. Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, Springett G. et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol. 2015;3312:1325-1333
128. Le DT, Picozzi VJ, Ko AH, Wainberg ZA, Kindler H, Wang-Gillam A. et al. Results from a Phase IIb, Randomized, Multicenter Study of GVAX Pancreas and CRS-207 Compared with Chemotherapy in Adults with Previously Treated Metastatic Pancreatic Adenocarcinoma (ECLIPSE Study). Clin Cancer Res. 2019;2518:5493-5502
129. Middleton G, Silcocks P, Cox T, Valle J, Wadsley J, Propper D. et al. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): an open-label, randomised, phase 3 trial. Lancet Oncol. 2014;158:829-840
130. Jo JH, Kim Y-T, Choi HS, Kim HG, Lee HS, Choi YW. et al. KG 4/2015: A randomized, controlled, multicenter, open-label phase III clinical trial of GV1001 with gemcitabine/capecitabine in previous untreated, eotaxin-high patients with advanced pancreatic ductal adenocarcinoma. Journal of Clinical Oncology. 2021 3915_suppl:4020-4020
131. Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;3658:725-733
132. Beatty GL, O'Hara MH, Nelson AM, McGarvey M, Torigian DA, Lacey SF. et al. Safety and antitumor activity of chimeric antigen receptor modified T cells in patients with chemotherapy refractory metastatic pancreatic cancer. Journal of Clinical Oncology. 2015 3315_suppl:3007-3007
133. Mahalingam D, Goel S, Aparo S, Patel Arora S, Noronha N, Tran H. et al. A Phase II Study of Pelareorep (REOLYSIN®) in Combination with Gemcitabine for Patients with Advanced Pancreatic Adenocarcinoma. Cancers (Basel). 2018 106
134. Noonan AM, Farren MR, Geyer SM, Huang Y, Tahiri S, Ahn D. et al. Randomized Phase 2 Trial of the Oncolytic Virus Pelareorep (Reolysin) in Upfront Treatment of Metastatic Pancreatic Adenocarcinoma. Mol Ther. 2016;246:1150-1158
135. Mahalingam D, Fountzilas C, Moseley JL, Noronha N, Cheetham K, Dzugalo A. et al. A study of REOLYSIN in combination with pembrolizumab and chemotherapy in patients (pts) with relapsed metastatic adenocarcinoma of the pancreas (MAP). Journal of Clinical Oncology. 2017 3515_suppl:e15753-e15753
136. Mahalingam D, Wilkinson GA, Eng KH, Fields P, Raber P, Moseley JL. et al. Pembrolizumab in Combination with the Oncolytic Virus Pelareorep and Chemotherapy in Patients with Advanced Pancreatic Adenocarcinoma: A Phase Ib Study. Clin Cancer Res. 2020;261:71-81
137. Timmer FEF, Geboers B, Nieuwenhuizen S, Schouten EAC, Dijkstra M, de Vries JJJ. et al. Locoregional Treatment of Metastatic Pancreatic Cancer Utilizing Resection, Ablation and Embolization: A Systematic Review. Cancers (Basel). 2021 137
138. Van Laethem J-L, Borbath I, Prenen H, Pico de Coaña Y, Enell Smith K, Nordbladh K. et al. Mitazalimab in combination with mFOLFIRINOX in patients with metastatic pancreatic ductal adenocarcinoma (PDAC): Safety data from part of the OPTIMIZE-1 study. Journal of Clinical Oncology. 2022 4016_suppl:e16237-e16237
139. Goldman JW, Waterhouse DM, George B, O'Dwyer PJ, Bhore R, Banerjee S. et al. Safety and Efficacy Results of a Phase I, Open-Label Study of Concurrent and Delayed Nivolumab in Combination With nab-Paclitaxel and Carboplatin in Advanced Non-small Cell Lung Cancer. Front Oncol. 2019;9:1256
140. Zhan X, Wang B, Li Z, Li J, Wang H, Chen L, et al. Phase I trial of Claudin 18.2-specific chimeric antigen receptor T cells for advanced gastric and pancreatic adenocarcinoma. Journal of Clinical Oncology. 2019;3715_suppl:2509-2509
Author contact
![]() Corresponding authors: Xiangyu Zhong; zhongxiangyuedu.cn. Yi Xu; xuyihrbhku.hk. Yunfu Cui; yfcui7com. Xiaoxue Xu; xuxiaoxue991123com.
Corresponding authors: Xiangyu Zhong; zhongxiangyuedu.cn. Yi Xu; xuyihrbhku.hk. Yunfu Cui; yfcui7com. Xiaoxue Xu; xuxiaoxue991123com.

 Global reach, higher impact
Global reach, higher impact