3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(8):2340-2353. doi:10.7150/jca.93890 This issue Cite
Research Paper
Unveiling the STAT3-ACC1 axis: a key driver of lipid metabolism and tumor progression in non-small cell lung cancer
1. Department of Respiratory Medicine, Shanghai Tenth People's Hospital, Tongji University School of Medicine, Shanghai200072, China.
2. Department of Integrated Traditional Chinese and Western Medicine, Shanghai Tenth People's Hospital, Tongji University School of Medicine, Shanghai, China.
* First authors.
Abstract
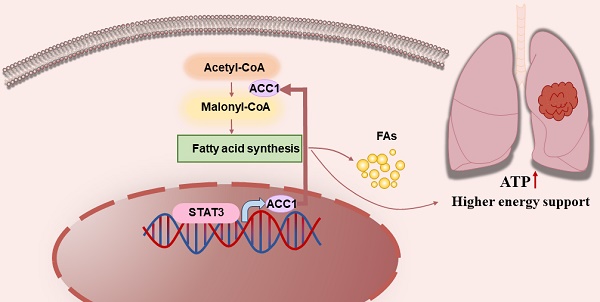
Background and Objective: Lung cancer is a prevalent global malignancy, and investigating the metabolic reprogramming of tumor cells has significant therapeutic implications. This study aims to explore the molecular mechanism driving the progression of non-small cell lung cancer (NSCLC), with a specific emphasis on the STAT3-ACC1-FAS axis involved in fatty acid synthesis.
Methods: The levels of Signal transducer and activator of transcription 3 (STAT3) and acetyl-CoA carboxylase 1 (ACC1) were determined in mouse NSCLC specimens and cell lines using Western blot and qPCR methods. Various assays such as CCK-8, colony formation, EDU, wound-healing, and transwell migration were employed to assess cancer cell proliferation, migration, and invasion. Additionally, a nude mouse xenograft model was utilized for in vivo tumor growth analysis. The interaction between STAT3 and ACC1 was examined through chromatin immunoprecipitation and dual-luciferase assays.
Results: The study observed upregulation of STAT3 and ACC1 in NSCLC tissues. Notably, the suppression of STAT3 and ACC1 inhibited the in vitro progression and lipid synthesis of NSCLC cells. Furthermore, STAT3 enhanced lipid synthesis by upregulating ACC1 expression. Mechanistic assays revealed that this process occurred through direct activation of ACC1 transcription by STAT3. STAT3 played a vital role in regulating lipid metabolism and supporting NSCLC progression.
Conclusion: The findings of this study underscore the significance of the STAT3-ACC1-FAS axis in NSCLC. The activation of ACC1 through STAT3-mediated transcription serves as a crucial mechanism for stimulating the progression of NSCLC tumors and promoting lipid synthesis. Consequently, targeting the STAT3-ACC1 axis may present a promising avenue for the diagnosis and treatment of NSCLC patients.
Keywords: Lung Cancer, Lipid Metabolism, STAT3, ACC1

 Global reach, higher impact
Global reach, higher impact