Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(14):4643-4655. doi:10.7150/jca.96859 This issue Cite
Review
Nanotechnology as a new strategy for the diagnosis and treatment of gliomas
1. Department of Neurosurgery, The First People's Hospital of Shuangliu District (West China Airport Hospital of Sichuan University), Chengdu 610200, China.
2. Department of Neurosurgery, West China Hospital of Sichuan University, Chengdu 610041, China.
3. Southwest Medical University, Luzhou 646000, China.
Received 2024-4-1; Accepted 2024-6-19; Published 2024-7-2
Abstract
Glioma is the most common malignant tumor of the central nervous system (CNS), and is characterized by high aggressiveness and a high recurrence rate. Currently, the main treatments for gliomas include surgical resection, temozolomide chemotherapy and radiotherapy. However, the prognosis of glioma patients after active standardized treatment is still poor, especially for glioblastoma (GBM); the median survival is still only 14.6 months, and the 5-year survival rate is only 4-5%. The current challenges in glioma treatment include difficulty in complete surgical resection, poor blood‒brain barrier (BBB) drug permeability, therapeutic resistance, and difficulty in tumor-specific targeting. In recent years, the rapid development of nanotechnology has provided new directions for diagnosing and treating gliomas. Nanoparticles (NPs) are characterized by excellent surface tunability, precise targeting, excellent biocompatibility, and high safety. In addition, NPs can be used for gene therapy, photodynamic therapy, and antiangiogenic therapy and can be combined with biomaterials for thermotherapy. In recent decades, breakthroughs in diagnosing and treating gliomas have been made with various functional NPs, and NPs are expected to become a new strategy for glioma diagnosis and treatment. In this paper, we review the main obstacles in the treatment of glioma and discuss the potential and challenges of the latest nanotechnology in the diagnosis and treatment of glioma.
Keywords: glioma, glioblastoma, blood‒brain barrier, nanotechnology, nanoparticles
Introduction
The annual prevalence of gliomas is 3-6.4 / 100,000 people, accounting for 23.3% of all central nervous system (CNS) tumors and 78.3% of malignant tumors. The World Health Organization (WHO) classifies gliomas into grades I-IV according to the degree of malignancy [1]. Of these, glioblastoma (GBM) has the worst prognosis, with a median overall survival (OS) of approximately 15 months [2,3]. The standard treatment options for glioma include surgical resection and temozolomide (TMZ) radiotherapy. However, even with this combination, patients with glioma still have a poor prognosis and a high recurrence rate [4]. The invasive growth of gliomas makes complete surgical resection a challenge, which is the main reason for the postoperative recurrence of gliomas and the necessity of adjuvant therapy [4,5]. On the other hand, the blood-brain barrier (BBB), therapeutic resistance, and genetic heterogeneity are the main challenges currently facing glioma treatment [6-9]. Therefore, the development of new approaches for the treatment and diagnosis of gliomas is urgent and necessary.
Nanotechnology refers to the study of the properties and interactions of substances at the atomic and molecular levels at the nanoscale (1-100 nanometers), and the use of these properties to intersect with multiple disciplines [10]. With the continuous development of nanotechnology in recent decades, nanotechnology has shown great promise in diagnosing and treating glioma. Nanoparticles (NPs) have shown potential advantages in a variety of cancer therapies due to various benefits such as their small size, modifiable surface, and few toxic side effects [11,12]. NPs with the appropriate modifications can successfully deliver drugs to the brain, which is the most important advantage of NPs for CNS disease treatment. For example, lipid-based NPs (LBNPs) with low immunogenicity, high biocompatibility, and enhanced BBB crossing ability have become the main nanocarriers for intracranial disease drug delivery [13]. In addition, nanocarrier systems for delivering glioma-specific receptor inhibitors can efficiently inhibit the signaling axis abnormally activated by gliomas, thereby reducing glioma therapeutic resistance and preventing its progression [14]. Some magnetic NPs (MNPs) have uniquely high permeability and distinctive and prominent magnetism when subjected to magnetic fields have been used for detection of biomarkers, and may become an important means for the diagnosis of a variety of diseases, including glioma [15-18]. In short, NPs have a wide range of applications and are expected to become a new modality for glioma diagnosis and treatment.
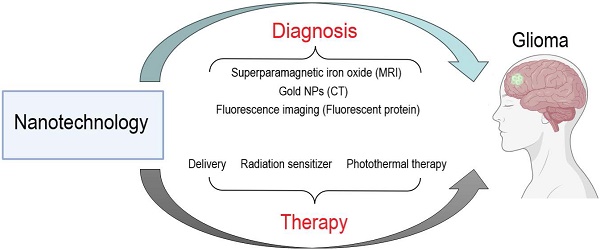
This review systematically discusses the ongoing challenges in glioma treatment and summarizes the potential of nanotechnology-based glioma diagnosis and treatment (Figure 1). This review concludes with an outlook on the future direction of nanotechnology use in glioma therapy to provide a theoretical basis and new insights for glioma research.
Potential of nanotechnology in the diagnosis and treatment of glioma.
Current Status of Glioma Treatment
Currently, the traditional treatments for gliomas include surgical excision, radiotherapy, total body treatments, localized treatment, and supportive therapy (Table 1). The standardized treatment regimens used in recent decades for glioma treatment include maximum safe range surgical resection, radiation therapy (2 Gray/day, five days/week for six weeks) concurrently with daily temozolomide (TMZ) at 75 mg/m2, and six subsequent cycles of TMZ (150-200 mg/m2) [19]. However, the prognosis for patients is still not ideal, as the median overall survival of GBM patients is only 14.6 months, and the 5-year survival rate is only 4-5% [20]. There are several reasons for this. First, the maximum safe margin of excision affects the efficacy of subsequent radiation and chemotherapy treatments. Additionally, tumor location affects the extent of resection, and subjective judgment by the attending physician during the procedure also affects the extent of resection [21,22]. Therefore, preoperative imaging is essential for determining the extent of surgical resection. Second, although TMZ is a first-line therapeutic agent for glioma, patients are prone to drug resistance, and the drugs have limited effectiveness with long-term use [23]. Glioma patients respond differently to TMZ depending on the methylation of the O6-methylguanine-DNA methyltransferase (MGMT) promoter [24]. Long-term alkylating agent therapy increases the risk of myelodysplasia and increases the cost of care. Moreover, DNA damage induced by radiotherapy under hypoxic conditions can be repaired by intracellular DNA repair enzymes, thereby increasing cellular tolerance to radiotherapy [25].
In summary, standardized treatments have inherent limitations. The addition of bevacizumab, which targets vascular growth factors, to radiotherapy/TMZ has been shown to improve progression-free survival (PFS), improve quality of life and reduce steroid requirements. However, it does not significantly improve overall patient survival [26,27]. Treatment with electric fields (TTFs) in combination with TMZ as adjuvant therapy has been approved, but its cost, treatment compliance, and skin toxicity limit its use [28,29]. The slow development of targeted therapies and immunotherapies for glioma is related to its high heterogeneity and mechanisms governing immunosuppression and acquired resistance. In contrast, with the rapid advancement of nanotechnology, nanomaterials with unique physical, chemical, and biological properties are gaining prominence in biomedicine, providing new and exciting therapeutic strategies for glioma treatment.
Challenges in Glioma Treatment
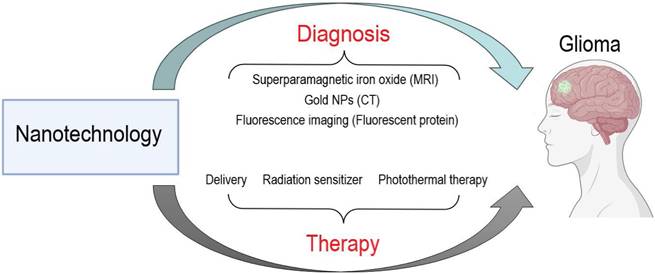
To date, effective therapies that improve the long-term survival of glioma patients are lacking. This paragraph summarizes the major challenges currently facing glioma treatment, including the blood‒brain barrier, therapeutic resistance, and genetic heterogeneity (Figure 2).
BBB
The BBB is the barrier between plasma and brain cells formed by capillary walls and glia of the central nervous system. It is essential for maintaining the stability of the brain's internal environment [30]. First, the BBB allows small molecules necessary to sustain neural function, such as glucose, amino acids and organic ions, to enter the brain [31]. On the other hand, the BBB effectively prevents toxic substances in the peripheral blood from entering the brain, protecting brain tissue from damage [32]. However, the BBB likewise precludes the transit of most drugs into the brain, making it difficult for chemotherapeutic agents to reach therapeutically effective concentrations in brain tissue. Increasing the dose can cause severe toxic side effects, which severely limits the treatment of CNS disorders [33].
The integrity of the BBB may be affected by gliomas, resulting in enhanced permeability and substance transport capacity [34]. However, such changes exist only in localized regions affected by gliomas and result in a relatively low degree of increase in BBB permeability, which is inadequate for many therapeutic agents to enter the brain. Although research targeting BBB permeability has undergone rapid development, including modes of drug delivery that increase permeability and bypass the BBB, progress in conducting clinical trials has been very limited. To date, nanoparticle delivery systems remain the most promising option for overcoming the challenges posed by the BBB. For example, nanoparticles modified by hydrophilic moieties or BBB endothelial cell surface-specific receptors exhibit strong trans-BBB capabilities and are considered to be the most desirable drug delivery system for glioma therapy [35].
Summary of traditional glioma treatments
| Treatment Strategies | Specific measures | Limitations | Improvements | |
|---|---|---|---|---|
| Surgical Excision | Gross Total Resection (GTR). GTR improves survival outcomes and increases survival rates. | Due to the infiltrative growth of GBM in the brain, the tumor boundaries that could not be accurately identified by conventional imaging techniques, the surgery failed to completely resect the infiltrated area of the tumor (47). | Tumor fluorescence with 5-aminolevulinic acid (5-ALA) allows for more complete tumor resection, thereby improving progression-free survival in patients with GBM. | |
| Radiotherapy | Routine postoperative RT was combined with TMZ to deliver 60 Gy of radiotherapy in 2-Gy fractions over a 6-week period (9). Standard first-line chemotherapy consists of TMZ (75 mg/m2 per day) during radiotherapy followed by 6 cycles of temozolomide (150-200 mg/m2 every 28 days for 1-5 days) (17). | At present, other radiation dose options have been explored, but there are no clear benefits. (67) | NA | |
| Total Bodies Treatments | TMZ | Drug toxicities of TMZ include nausea and myelosuppression. | Longer or dose-intensive regimens of temozolomide have not been studied to prove beneficial. | |
| Procarbazine, Lomustine, Vincristine | Salvage chemotherapy used in combination may have some activity but is limited by greater toxicity. | NA | NA | |
| Bevacizumab | Bevacizumab improved PFS but did not prolong OS. | Bevacizumab in combination with lomustine improved PFS, but again there was no OS benefit. | ||
| Localized Treatment | Tumor-Treating Fields (TTF) | The addition of TTF to temozolomide maintenance chemotherapy improves in terms of progression-free survival and overall survival there (26). | Non-blinding and delays in randomization are the major concerns. Cost, treatment compliance and skin toxicity are other barriers that limit the adoption of this treatment modality. | NA |
| Supportive Therapy | Antiepileptic Treatment | Most patients with GBM require long-term antiepileptic therapy, and the principle of treatment should be seizure control at low doses to avoid side effects and minimize drug-drug interactions. | Levetiracetam has been most extensively studied in GBM patients and is more an'quan relative to other drugs (20,21). | NA |
| Corticosteroids | Corticosteroids are commonly used to reduce peritumoral vasogenic edema; side effects limit long-term corticosteroid use | NA |
Major challenges in the treatment of glioma.
Therapeutic Resistance
Numerous anticancer drugs have been used to treat gliomas but the prognosis of glioma patients has remained poor, which may be attributed to unique resistance mechanisms. Acquired chemoresistance in tumor cells is caused by drug-induced genetic and epigenetic changes [36]. Studies have shown that tumor cells with stem cell characteristics are directly involved in brain tumor recurrence and drug resistance [37]. During glioma treatment, TMZ kills drug-sensitive tumor cells. At the same time, drug-resistant tumor stem cells may proliferate in large numbers and become the dominant cell population. MGMT directly repairs TMZ-induced damage to tumor cell DNA, a significant cause of glioma drug resistance [38]. Clinical studies have shown that patients with methylated MGMT gene promoters are more sensitive to TMZ treatment [39]. Methylation of the MGMT gene promoter has become a predictive marker for the effectiveness of treatment with alkylating agents such as TMZ. DNA base excision repair is primarily involved in non-large-scale DNA damage repair and is an alternative pathway for glioma drug resistance [40]. When DNA is damaged by TMZ, poly (ADP-ribose) polymerase-1 (PARP-1) binds to gaps in DNA single- or double-strand breaks and catalyzes the cleavage of β-nicotinamide adenine dinucleotide to generate nicotinamide and ADP-ribose [40].
Nanotechnology-based approaches have made new advances in overcoming these resistance mechanisms in the last decade. For example, nanotechnology can improve drug delivery and cellular uptake and enhance glioma sensitivity to chemotherapeutic agents [41]. In addition, nanotechnology enables delivery of siRNAs and peptides, which can significantly inhibit glioma resistance to TMZ or other chemotherapy drugs [41]. A recent report claimed that combining multimodal neuro- and nanotechnology-enabled precision immunotherapies with existing systemic immunotherapies could overcome therapeutic resistance may ultimately provide a major breakthrough against GBM [42]. From this perspective, the development of nanotechnology in combination with existing therapies might become an emerging treatment modality for gliomas.
Genetic Heterogeneity
Glioma exhibits substantial genetic heterogeneity, which is thought to be a key factor driving therapeutic resistance and tumor recurrence. Gliomas may have different mutations or expression levels of genes, including those related to oncogenic signaling axes, metabolism, and immune response [43-45]. The generation of phenotypic heterogeneity in gliomas differs from the hierarchical differentiation process of normal stem cells. Neural stem cells irreversibly give rise to stereotypical progenitors and differentiated cells during unidirectional stratification. However, there is little difference between glioma stem cells and stem cells that give rise to diverse cell populations; these cells are highly plastic [46]. Glioma cells have multiple states, including neural progenitor cell (NPC)-like, oligodendrocyte progenitor cell (OPC)-like, astrocyte (Astro)-like, and mesenchymal (Mes)-like differentiation-like states [47,48]. The genetic heterogeneity of gliomas allows gliomas to acquire different abilities in hypoxic environments, immune escape mechanisms, and chemotherapeutic drug sensitivities [49-51]. Therefore, recognizing the distinct genetic makeup of each patient's tumor is critical for developing strategies for personalized medicine. With the development of molecular diagnostics, the classification of various glioma subtypes has been improved, which has potential implications for the development of personalized nanotechnology-based therapies. For example, designing NPs that specifically bind to surface receptors expressed on glioma cells could be effective in promoting cellular uptake and improving therapeutic efficacy [52]. In addition, cellular and molecular studies focused on understanding the GBM microenvironment, especially in the aggressive peripheral regions, provide a wealth of information for the design of specific nanotherapeutics. Several potentially effective nanotherapeutics are currently in clinical trials. For example, a Phase II clinical trial is currently studying the effects of a combination of TMZ and intravenous targeted p53 gene therapy (a cationic liposome, SGT-53) (NCT02340156). Although it is hoped that these trials will lead to promising new treatments for glioma, more research is needed to determine ultimate efficacy, feasibility and safety.
Nanotechnology for Glioblastoma Diagnosis
Accurate diagnosis is the most crucial step in glioma treatment. Since gliomas grow invasively in the brain, it is challenging to accurately locate the exact tumor boundary using clinical imaging techniques. The failure of surgery to completely remove the tumor is an essential reason for the high recurrence rate and high mortality rate of GBM [53]. Therefore, there is an urgent need for more advanced GBM diagnosis and treatment methods, and nanotechnology has shown great potential in glioma prediction, diagnosis, imaging and therapy.
To achieve a more accurate diagnosis of gliomas, the small particle size, photosensitivity, and magnetic properties of nanomaterials are considerable advantages. In addition, nanomaterials can carry many radioisotopes, improving the specificity and sensitivity of imaging, and glioma visualization contributes significantly to the accuracy of glioma diagnosis. Magnetic resonance imaging (MRI), computed tomography (CT), and optical imaging are the most commonly used methods for diagnostic glioma imaging. In recent years, many studies have investigated the combined use of nanotechnology and imaging techniques for diagnosing gliomas.
Nanotechnology for MRI
MRI is a relatively new medical imaging technique widely used to provide physiological and anatomical information with high spatial and soft-tissue resolution by recognizing differences in the relaxation of hydrogen atoms abundant in flowing water in living organisms. Currently, MRI is the predominant test for the diagnosis of gliomas. However, as clinical needs increase, the background noise, sensitivity and overall resolution of gadolinium-based contrast agents are no longer sufficient for glioma diagnosis. Highly efficient nanoscale contrast agents with high magnetic relaxation and specificity have received much attention and may improve the sensitivity of MRI and better differentiate between diseased and healthy tissues.
Some essential properties of nanomaterials, such as BBB permeability, biocompatibility, specific targeting and low toxicity, make them ideal candidates for MRI of intracranial lesions. Metal-based NPs, such as gadolinium, manganese, and iron, have advanced the study of MRI contrast agents due to their paramagnetic and superparamagnetic properties. Tan et al. developed a superparamagnetic iron oxide (SPIO) nanoprobe for enhanced t2-weighted MRI, and covalent modification with interleukin-6 receptor-targeting peptide (I6P7) allowed the particles to pass through the blood‒brain barrier and to better identify low-grade gliomas [54]. There is a need to thoroughly investigate the potential of magnetic nanomaterials for MRI-guided drug delivery in the diagnosis and treatment of malignant brain tumors in the future. In addition, various fluorinated contrast agents are considered promising imaging techniques for cancer diagnosis due to their excellent soft tissue resolution, deep tissue penetration, and virtually no endogenous interference. In recent years, researchers have designed a variety of fluorine MRI (19F MRI) contrast agents and demonstrated satisfactory relaxation efficiencies by further integrating various nanomaterials [55]. To avoid the limitations of a single imaging method, the development of multimodal imaging methods in which nanoparticles are combined with multiple imaging methods has recently become favored. For example, MnO NPs with excitation-dependent fluorescence have been synthesized by the thermal decomposition of manganese-based compounds, and this method of combining MRI and fluorescence imaging has shown equally impressive advantages [56]. Although there is still a long way to go before these nanomaterials can be used as alternatives to gadolinium-based contrast agents or other commercial materials in clinical applications, these studies support the use of nanotechnology for the clinical diagnosis of gliomas.
Computerized Tomography Nanoprobe
Electron computed tomography (ECT), single electron emission computed tomography (SPECT) and positron emission tomography (PET) are all commonly used imaging methods for the clinical diagnosis of gliomas. X-rays often require the addition of contrast material to better visualize the differences between glioma and normal brain tissue. The most commonly used iodine agent has a short imaging time, low spatial resolution and poor specificity. Compared with iodine agents, gold nanoparticles have been shown to provide sharper and more stable images [57]. Gold nanoparticles are also relatively easy to modify, allowing for better penetration of the BBB and specific targeting of gliomas. In addition, nanomaterials can bind to radioisotopes and target molecules, increasing the amount of contrast agent available to the tumor lesion. Injection of radioisotope-labeled nanomaterials into gliomas has been reported in both imaging and treatment of tumors [58]. In conclusion, nanotechnology-based radiation modalities may be a practical breakthrough for providing more detailed and precise anatomical information.
Fluorescent Nanoprobe
In a study combining chronic brain opening and two-photon microscopy, Zhang et al. established a mouse glioma model in situ in a pure syncytiotrophoblast. They investigated the dynamics of nanoparticles during the long-term growth of gliomas. The results showed that silicon nanoparticles (SNPs-PEG-RGD-FITC) bound by intravenous injection of integrin αvβ3 had high penetration and retention in solid gliomas, revealing the dynamic real-time observation of nanoparticles in a mouse glioma model and laying a technical foundation for exploring the targeting and infiltration of nanomaterials in gliomas [59]. A recent study reported a glioma targeting and redox activatable theranostic nanoprobe (Co-NP-RGD1/1) for magnetic resonance (MR) and fluorescence (FL) bimodal imaging-guided on-demand synergistic chemotherapy/photodynamic therapy (Chemo-PDT) of orthotopic gliomas [60]. In an in vitro model, Co-NP-RGD administered intravenously could be delivered to in situ glioma cells across the BBB in the presence of cRGD-targeting groups on the surface, thus generating an enhanced MRI contrast signal for the localization of in situ gliomas in the brain [60]. Sheng et al. developed a new novel multimodal nanoprobe of NIR-II fluorescent molecules with aggregation-induced emission (AIE) properties based on the principle of NIR-II fluorescence imaging [61]. They achieved high-resolution, high signal-to-noise ratio dual-modal molecular imaging of gliomas via NIR two-region fluorescence and NIR one-region photoacoustic imaging in a mouse glioma model. In addition, other domestic and international studies have made considerable research progress in multimodal nanoprobe guidance for determining the intraoperative boundaries of gliomas, which provides a new guidance technique for the resection of gliomas [62].
Nanotechnology for Molecular Diagnostics
The rapid development of whole genome sequencing (WGS) has accelerated the development of precision medicine by providing unprecedented information on genotypes and phenotypes of diseases. The search for disease-related biomarkers has become a hot topic in recent years, especially in tumor research. Molecular biomarkers (e.g., genes, RNAs, and proteins) have been widely used for disease prediction, diagnosis, and prognostic assessment [63]. In recent years, molecular pathology of gliomas has made significant progress. In 2016, WHO has included molecular pathology into the pathological diagnostic system of gliomas, which provides a differential basis for tumors that are difficult to be clearly diagnosed by histology, which is conducive to a better judgment of the clinical prognosis of patients [64]. For example, IDH mutations, combined chromosome 1p/19q deletions and MGMT promoter methylation are newly identified molecular variants that have the potential to become new targets for future glioma therapy [64]. Surface plasmon resonance (SPR) sensing is a novel analytical technique developed based on optical principles and has been shown to have great potential for studying molecular interactions. Recent studies have found that SPR measurement of patient miRNA-182 levels in combination with nanotechnology is expected to aid in the diagnosis of gliomas [65]. In addition, CRISPR/Cas13a RNA-editing system is a novel molecular diagnostic tool with the advantages of rapidity, high sensitivity, and programmability. Wu et al. reported the potential of an in vivo imaging method based on CRISPR/Cas13a RNA-editing system in the diagnosis of glioma [66].
In summary, the successful application of nanotechnology in glioma imaging can improve the accuracy of glioma diagnosis and provide a new direction and idea for the accurate diagnosis of glioma in the future. However, the application of nanotechnology to the diagnosis of glioma still has limitations and challenges to be solved. The potential limitations of current nanotechnology mainly include: (i) lack of target specificity, (ii) adhesive interactions with non-target structures, and (iii) biosafety. For example, non-specific adsorption of metal nanoparticles limits their further application in SPR [67]. Therefore, in response to the limitations faced by nanotechnology, we expect the next generation of nanomedicine to continue to develop new technologies to overcome these challenges.
Nanomaterials for Glioblastoma Treatment
Nanodrug Delivery Across the BBB
Chemotherapy-based nanocarrier technology has become an important research direction to improve the transport of chemotherapeutic drugs across the BBB and increase the efficacy of chemotherapy in GBM treatment (Table 2). Lahann et al. constructed novel synthetic protein nanoparticles (SPNPs) using polymerized human serum albumin (HSA) and the cell-penetrating peptide iRGD [68]. The results of mouse experiments showed that synthetic protein nanoparticles (SPNPs) carrying signal transducer and activation of transcription 3 factor (STAT3i) successfully crossed the blood‒brain barrier to reach tumors and enhanced the effect of standardized treatment of GBM after intravenous injection [68]. This is the first study to demonstrate that systemic or intravenous delivery of therapeutic agents can cross the blood-brain barrier to reach brain tumors, providing new hope for treating GBM.
Liposomes are considered to be self-assembling colloidal nanocarriers capable of targeted drug delivery to glioma tissues and can be used as carriers for glioma chemotherapeutic agents. For example, human ferritin (HFn) can efficiently cross the BBB by transcytosis through binding to the transferrin receptor (TfR1) on the BBB [69]. Different glioma tumor-targeting peptides (including RGE, Pep-1 and CGKRK) were genetically engineered at the N-terminal end of the protein subunit of the heavy chain HFn to confer tumor-targeting and tissue-permeable properties to HFn nanocarriers, while the peptide-modified protein subunits could still self-assemble to form nanocarriers. Modification with the permeable peptide RGE significantly improved the ability of HFn nanocarriers to penetrate deep into tumors and target tumor cells without affecting the ability of HFn nanoparticles to efficiently cross the BBB, proving that RGE-HFn nanocarriers can be used as tumor-targeting and tissue-permeable nanocarriers. HFn nanocarriers can be used as an ideal drug delivery vehicle for targeting gliomas in situ [70]. Finally, RGE-HFn nanoparticles encapsulating SR717 also showed excellent antiglioma efficacy in animal models, significantly prolonging the survival of the animals. However, its efficacy in humans needs to be further confirmed. Mesenchymal-epithelial transition is associated with poor prognosis, aberrant aggressiveness, and tumor resistance in patients with gliomas and is a possible therapeutic target for gliomas [71].
Summary of nanotechnology-based drug delivery across the BBB
| Nanomedicine | Components | Mechanism of action | Outcomes |
|---|---|---|---|
| STAT3i SPNPs | Synthetic protein nanoparticle (SPNP) particles of polymerized human serum albumin (HSA) equipped with the cell-penetrating peptide iRGD, which contains siRNAs targeting signal transducer and activator of transcription 3 factor (STAT3i) (57). | STAT3 is a critical factor associated with GBM progression, and free siRNA provided a slight benefit with prolonged median survival when combined with radiotherapy (57). | STAT3i SPNPs combined with radiotherapy induces dendritic cell (DC) activation by enhancing the expression of MHC II, which is involved in antigen presentation, and induces an immune response to kill the tumor (57). |
| SR717@RGE-HFnNP | Heavy chain ferritin (HFn) binds tumor-targeting peptides including RGE, Pep-1 and CGKRK to form glioma-targeting RGE-HFn nanocarriers. Then SR717@RGE-HFnNP was formed after the STING agonist SR717 was encapsulated within the RGE-HFn nanocarriers (58). | STING is involved in a variety of diseases. Activation of STING induces an effective immune response against pathogenic infections and cancers, while aberrant activation of STING triggers autoimmune and inflammatory diseases (58). | The biomimetic nanocarrier successfully crossed the BBB and delivered the STING agonist to the glioma lesions in the brain, which effectively activated the STING pathway and triggered the immunomodulatory effect, thus effectively inhibiting the growth of glioma and improving the survival rate of glioma-loaded mice (58). |
| Den-cMBP5 and Den-cMBP10(29) | cMBP is a MET receptor-specific targeting peptide. cMBP peptides were coupled to G4 dendrimers and Den-cMBP5 and Den-cMBP10 were prepared (61). | cMBP is a MET receptor-specific targeting peptide that competes with HGF for binding to the MET site, which prevents the dimerization of the MET receptor and inhibits the activation of its downstream signaling pathway. However, the application of the peptide in targeted therapy is limited by its short cycle time and easy degradation. | The inhibitor exhibited extremely high binding affinity for MET and effectively inhibited glioma growth by blocking MET downstream signaling. |
| ATMO-21@SpAcDex NPs(30) | Antisense miRNA-21 oligonucleotide (ATMO-21) achieves high loading in bradykinin ligand agonist-modified spermine-modified acetylated dextran nanoparticles (SpAcDex NPs). | SpAcDex NPs inhibit tumor growth by activating G protein-coupled receptors, which temporarily open the BBB and deliver ATMO-21. | An in vivo in situ glioma model confirmed that ATMO-21 released from SpAcDex NPs inhibits tumor growth and is an effective therapeutic strategy for GBM. |
| RGD-RBC-NCs (33) | Targeted peptides are modified on the surface of red blood cell (RBC) membranes. As RBC membrane-encapsulated drug nanocrystalline NCs (RBC-NCs), RGD-RBC-NCs were formed after modification by tumor-targeting peptide c (RGDyK). | NA | Active target recognition capability to increase drug accumulation at the tumor site improves the efficacy of chemotherapeutic agents against subcutaneous graft tumors and gliomas in situ. |
| AMD3100-SPNPs(35) | SPNPs encapsulate the extracellular transcription peptide iRGD (AMD3100-SPNPs) (67). | CXCR4 antagonist (AMD3100) has made an attractive target for anti-GBM therapy. | Blocking CXCL12/CXCR4 signaling. This inhibits GBM proliferation and reduces the infiltration of CXCR4+ M-MDSCs into the tumor microenvironment. |
| Adenosine 2A receptor (A2AR) nano-agonists(36) | A2AR targets neovascularization in glioma-infiltrated areas, prompting vascular endothelial cell cytoskeleton contraction through specific activation of the A2AR signaling pathway, opening tight junctions between endothelial cells, and transiently increasing blood-brain barrier permeability. | Imaging monitors blood-brain barrier permeability and injects the chemotherapeutic drug paclitaxel to increase the drug's efficacy while decreasing its toxicity to normal tissues. |
Polymeric nanoparticles are considered promising materials for drug delivery. Wu et al. coupled epithelial transition factor (MET)-targeted cMBP peptides to G4 dendrimers to form new nanoinhibitors [72]. These subsequent experiments showed that this nanoinhibitor effectively reduced glioma cell proliferation and invasion in an in vitro model by blocking MET signaling at significantly reduced levels of phosphorylated MET (p-MET) and its downstream signaling proteins, such as p-AKT and p-ERK1/2. Sun et al. developed a novel targeted antisense miRNA-21 oligonucleotide (ATMO-21) delivery system for GBM therapy: bradykinin ligand agonist-modified spermine-modified acetylated dextran nanoparticles (B1L@SpAcDex-ATMO-21 NPs). It can improve the therapeutic effect of drugs on GBM by activating G protein-coupled receptors expressed in tumor blood vessels and tumor cells, resulting in increased BBB permeability and drug transport and accumulation at the tumor site [73].
X-ray-induced photodynamic therapy (XPDT) is highly advantageous for treating deep-seated tumors [74]. Based on this idea, Li et al. developed a new CaF2 nanocrystal oscillator with a dendritic polymer as a framework to build a nanoplatform with a dual-core satellite structure that is capable of programmatically and spatially separating the dual loading of therapeutic agents, permitting the combination of XPDT and antiangiogenic therapies, thus generating a restorative system capable of synergistically combating tumors [75]. Chai et al. prepared a bionic nanodelivery system composed of high-drug-loading-capacity erythrocyte membrane-coated drug nanocrystals, namely, membrane-coated drug NCs (RBC-NCs), by an antibiotic-biotin interaction insertion method [76]. The nanodelivery system can increase target recognition, increase drug accumulation at the tumor site and improve the efficacy of chemotherapeutic drugs against subcutaneous graft tumors and gliomas in situ. C-X-C motif chemokine ligand-12/C-X-C motif chemokine receptor-4 (CXCR4-CXCL12) is associated with progression in GBM [77]. Although AMD3100 can be used as a GBM therapeutic target and CXCR4 antagonist, achieving successful transport through the BBB and high drug concentrations at the tumor site is difficult. Therefore, Alghamri et al. prepared synthetic protein nanoparticles (SPNPs) coated with transcystic peptides (iRGDs) (AMD3100-SPNPs) and experimentally demonstrated that AMD3100-SPNPs could inhibit GBM cell proliferation and infiltration by blocking CXCL12/CXCR4 signaling [78]. The existence of the blood‒brain barrier prevents most drugs from entering the brain and exerting their therapeutic effects, which is a common challenge in treating almost all central nervous system diseases. In contrast, nanomedicines improve therapeutic efficacy by enabling effective drug concentrations in the tumor region of the brain through different means, including BBB permeability reduction or tumor-targeted trans-BBB transport nanodelivery systems. Therefore, the use of a nanodrug delivery platform is a promising direction for GBM therapy.
Nanomaterials Improve GBM Sensitivity to Treatment
While the first-line chemotherapeutic agent for glioma, TMZ, improves survival in patients with glioma by methylating DNA and inducing cytotoxicity, the BBB and acquisition of drug resistance impact treatment outcomes [79-81]. Chemotherapy or postoperative adjuvant chemotherapy can improve patient survival to a certain extent. Scholtyssek et al. conducted a meta-analysis and reported that the 1-year survival of patients treated with a combination of radiotherapy and chemotherapy was greater than that of patients treated with radiotherapy alone and that chemotherapy can indeed prolong patient survival [82]. Therefore, improving the sensitivity of gliomas to TMZ is a viable future research direction.
Several recent studies on nanomedicine delivery platforms have shown possible avenues for improving resistance to TMZ. Zhang's team developed a new nanoformulation called RPDGs targeting GBM cells, which utilizes the process of reducing Pt (IV) to Pt (II) to deplete glutathione (GSH) to further increase the level of reactive oxygen species (ROS) in tumor cells, promoting apoptosis and iron death. The anti-GBM effect of RPDGs was confirmed in both in vivo and in vitro models [83]. Platinum compounds are the mainstays of combination drugs used for precision therapy and immunotherapy and have potent anticancer effects. The mechanism of action is mainly the formation of intra- and interstrand cross-links with DNA, which are difficult to repair by MGMT enzymes and are not cross-resistant to DNA-alkylating agents [84]. Recently, in Wang et al.'s study, platinum was synthesized by designing and synthesizing the reduction-responsive polymer poly(1,2,4,5-cyclohexane tetracarboxylic acid dianhydride-co-hydroxyethyl disulfide)-polyethylene glycol (poly(CHTA-co-HD)-PEG), which encapsulates a tetravalent platinum prodrug of oxaliplatin (OxaPt(IV)) as well as the DNA-embedding agent 56MESS to form platinum drugs, creating a variety of platinum-containing NPs [85]. Platinum-containing nanopharmaceuticals can reverse the drug resistance to TMZ at the cellular level, and in an animal in situ tumor model, combined with convection-enhanced drug delivery technology, they can increase the survival period of animals and reverse the resistance of GBM to TMZ [85].
Sonodynamic therapy (SDT) is an emerging treatment method in which ultrasound waves can be precisely applied to the deep tumor area, thus effectively stimulating the acoustic sensitizers enriched in the tumor tissue to generate reactive oxygen species (ROS), resulting in deep and noninvasive treatment while causing minor damage to normal tissues; this method is now used to treat a wide range of diseases [86]. Previous studies have demonstrated that it is possible to abrogate the downregulation of chemoresistance-related markers (e.g., HSV-1, p53, and P-gp), reduce drug resistance and improve drug sensitivity by drug and chemo-photothermal combination therapy, which can significantly enhance anticancer efficacy [87]. Chen et al. combined polyglutamic acid (PGA) with a chemotherapeutic drug-cum-acoustic sensitizer (adriamycin, DOX), wrapped them in a brain glioma cell membrane, and prepared bionic nanoacoustic sensitizers (MDNPs) with the functions of homologous targeting of brain gliomas and ultrasound-triggered acoustic chemotherapy [88]. The MDNPs had suitable homologous targeting ability and effectively penetrated the BBB when combined with ultrasound. Moreover, they reached the intracranial tumor site, induced tumor cell apoptosis, downregulated drug resistance-related factors, disrupted chemotherapy resistance, increased sensitivity to chemotherapy, and significantly prolonged the survival time of mice [88].
In the treatment of glioma, in addition to killing tumor cells, radiotherapy (RT) can have toxic effects on surrounding normal brain cells, thus affecting the efficacy of RT [89]. Tumor hypoxia is one of the most important reasons for resistance to radiotherapy [90,91]. Therapeutic radionuclides have the potential to increase the therapeutic efficacy of glioma radiation. The liposome-encapsulated radionuclide rhenium has the potential to promote the retention of radioisotopes in tissues. Nanoliposomes help radionuclide rhenium deliver radiation to tumor cells through convection-enhanced delivery [92]. The FDA approved the Rhenium-186-NanoLiposome (RNL) for the treatment of patients with recurrent glioblastoma. RNL administers high doses of radiation directly to brain tumors at concentrations up to 25 times that of external beam radiation while being safe, effective and convenient. The results of the phase 1 ReSPECT glioma trial showed that RNL at doses exceeding 100 Gy improved overall survival in patients with recurrent gliomas [93]. In conclusion, existing nanotechnology can enhance the sensitivity of glioma to radiotherapy in various ways and open up new avenues for treating glioma.
Novel Nanomedicine Applications in GBM Therapy
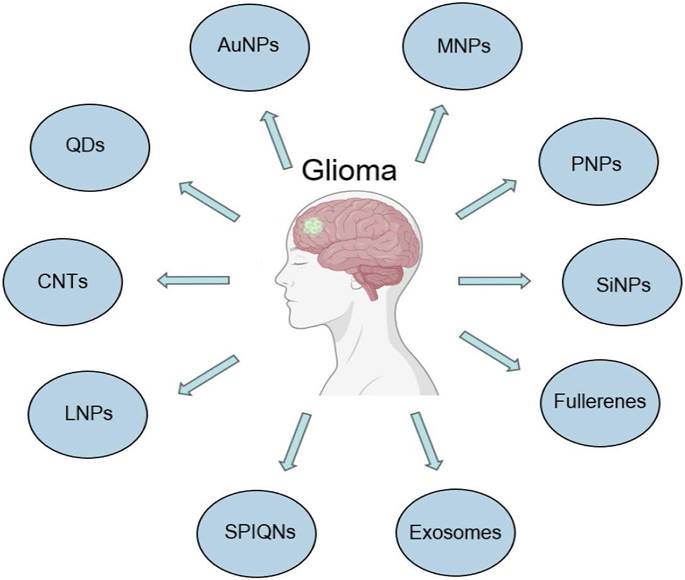
As research on nanotechnology continues to progress and intersect with that in the medical field, many new nanomaterials continue to be synthesized and investigated for applications in glioma therapy (Figure 3). Exosomes are cell-derived small extracellular vesicles (sEVs) approximately 100 nanometers in size that transport specific substances to target cells [94]. Exosomes, which are enriched in proteins, lipids, DNA, RNA and glucose, regulate the extracellular matrix and signal to other cells, affecting all areas of cell biology [95]. Exosomes are able to cross the BBB, which makes them possible therapeutic agents and diagnostic tools for gliomas [96,97]. Serum exosomal EGFRvIII mRNA has been studied in patients with gliomas and may provide sufficient diagnostic information [98]. Conventional nanoparticles (e.g., liposomes or metal particles) have relatively poor bioactivity, compatibility and tumor selectivity with limited benefits [99].
In contrast, exosomes, as a novel nanomedicine delivery technology with low toxicity, improved biocompatibility, and suitable stability, can be potential carriers and effectively stimulate antiglioma immune responses [99]. Zhu et al. designed sEVs with dual-targeting functionalization of angiopep-2 and TAT and applied them to the therapeutic study of glioma [100]. Angiopep-2 peptide can specifically target low-density lipoprotein receptor (LRP-1), which is highly expressed on the surface of glioma cells and cerebrovascular endothelial cells [101]. TAT enhances sEV BBB permeability and tumor tissue penetration. Furthermore, in a mouse glioma model in situ, dual-targeted functionalized sEVs were shown to effectively penetrate the blood‒brain barrier and target glioma lesion areas [100]. In conclusion, dual-targeted functionalized sEVs can improve the efficiency of drug treatment for in situ gliomas, significantly inhibit glioma growth, and effectively prolong the survival of tumor-bearing mice, which provides encouraging prospects for the treatment of gliomas.
Moreover, bionic nanorobots will also play an essential role in medicine in the future. Deng et al. generated natural killer (NK) cell membranes wrapped with aggregation-induced luminescence (AIE) organic semiconductor skeletal materials with near-infrared two-region fluorescence properties to fabricate NK cell-mimicking AIE nanorobots (NK@AIE dots) and evaluated their efficacy in the diagnosis and treatment of glioma [102]. NK@AIEdots cross the BBB by regulating the tight junctions (TJs) between BBB epidermal cells through specific interactions with the cells on the BBB, increasing BBB permeability, and enriching glioma cells by the particular recognition of NK cell membranes and glioma cell membrane surface receptors to achieve high signal-to-noise ratio near-infrared fluorescence imaging of brain tumors. The thermal effect generated by NK@AIEdots under near-infrared light irradiation significantly inhibited the growth of glioma tumors. In addition, a recently reported technique of mechanical nanosurgery using magnetic carbon nanotubes (mCNTs) and precise magnetic field control was developed based on the principle that mCNTs are enriched in GBM, and mechanical stimulation using magnetic field control can destroy the cellular structure and lead to tumor cell death [103].
Recently developed nanomaterials in the diagnosis and treatment of glioblastoma.
Recent studies have demonstrated that nanoimmunotherapy, a type of nanoparticle-based tumor immunotherapy, has great potential as a personalized and synergistic treatment regimen due to its unique biological properties that facilitate precise targeting, local drug delivery, and enhanced therapeutic efficacy. Exosome-based nanoimmunotherapy targeting tumor-associated macrophages (TAMs) is a promising immunotherapeutic strategy that uses nanosized exosomes as a delivery system for specific therapeutic agents targeting TAMs to modulate their behavior and inhibit the recruitment or deplete or reprogram them from a tumor-promoting phenotype to a tumor-suppressing phenotype [104].
Novel Nanomedicine Applications in GBM Therapy advantages and disadvantages of the Nanotechnology on the diagnosis and treatment of gliomas
More and more advances have been made in novel nanotechnology-based diagnostic and therapeutic systems for gliomas. Next there is an urgent need to validate the efficacy of these nanotechnologies in clinical practice. AGuIX NPs in combination with radiotherapy have been found to be clinically beneficial for glioma patients in recent phase Ib/II clinical trials (NCT04881032) [105]. However, the only NP available for clinical application to date, ferumoxytol, has caused rare but severe allergic reactions [106]. Therefore, the application of nanotechnology to the clinical diagnosis and treatment of gliomas still faces many challenges.
Nanotechnology offers several advantages in the diagnosis and treatment of gliomas, though it also presents some challenges. This technology can integrate multiple functions, including imaging diagnostics, drug delivery, and photothermal therapy, to achieve comprehensive diagnostic and therapeutic solutions. One of its key benefits is improved penetration of the blood-brain barrier (BBB), allowing for targeted drug delivery directly to tumor cells, thereby minimizing the impact on healthy cells. Additionally, encapsulating drugs within nanoparticles can extend their half-life and enhance their solubility and stability. However, there are several significant disadvantages associated with the use of nanotechnology in glioma diagnosis and treatment: 1. Biocompatibility and Toxicity: The long-term biocompatibility and potential toxicity of nanomaterials within the body remain critical concerns. 2. Cost and Complexity: The production of nanotechnology products typically requires sophisticated, high-cost equipment and complex processes, limiting widespread application. 3. Clinical Translation: The process of translating nanotechnology from research to clinical practice is lengthy and intricate. 4. Regulatory Challenges: The approval and oversight of nano-medical products necessitate new evaluation standards and regulatory frameworks. 5. Environmental Impact: The disposal of nanomaterials poses environmental challenges. Despite these challenges, nanotechnology remains a highly promising solution for the diagnosis and treatment of gliomas. As technological advancements continue and further research is conducted, it is anticipated that many of the current limitations will be addressed, thereby enhancing the application of nanotechnology in the medical field.
Conclusion and Future Expectations
New treatment modalities are urgently needed as the 5-year survival rate of GBM patients is still very low, and improvements in existing treatments are difficult to achieve [107]. In recent decades, substantial progress has been made in nanotechnology [108]. The use of nanotechnology to develop novel therapeutic strategies for cancer treatment is promising [109]. With respect to precision medicine, nanotechnology has excellent potential for GBM diagnosis and treatment. It offers the possibility of individually tailored treatment regimens for each patient, and it provides new options for the treatment of deep-seated tumors as well as for the elimination of residual microtumours or individual tumor cells, which are the leading cause of cancer recurrence [110].
In the future, nanomaterials are expected to be more effective than current cancer treatments, including the surgical removal of tumors, chemotherapy and radiation. Nanocarrier drugs offer many advantages over conventional medicines. They better regulate the pharmacokinetics and drug distribution profiles and can improve drug uptake and intracellular penetration in target cells/tissues [111]. However, medical nanocarriers must have suitable biocompatibility and biodegradability and an extended drug circulation half-life [112]. They must target specific cells or tissues. In addition, accumulation in off-target organs of the body should be avoided, and clearance strategies should be developed to prevent damage at the time of use. Although there are various methods for generating nanoparticles, ultimately, they must be easy to assemble, cost-effective, homogeneous, strictly conform to clinical trials, highly tissue specific, and safe.
Author contributions
Jun Lei- Acquisition study concept and design- manuscript writing; Yiyang Huang, Yichuan Zhao, Zhi Zhou, and Lei Mao- Study concept and design; Yanhui Liu- revision of the manuscript for important intellectual content and study supervision.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Śledzińska P, Bebyn M, Szczerba E. et al. Glioma 2021 WHO Classification: The Superiority of NGS Over IHC in Routine Diagnostics. Mol Diagn Ther. 2022;26(6):699-713
2. Sung H, Ferlay J, Siegel RL. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249
3. van den Bent MJ, Geurts M, French PJ. et al. Primary brain tumours in adults. Lancet. 2023;402:1564-1579
4. Tseng CL, Zeng KL, Mellon EA. et al. Evolving concepts in margin strategies and adaptive radiotherapy for glioblastoma: A new future is on the horizon. Neuro Oncol. 2024;26:S3-S16
5. Mohamed AA, Alshaibi R, Faragalla S. et al. Updates on management of gliomas in the molecular age. World J Clin Oncol. 2024;15:178-194
6. Lombardo SM, Schneider M, Türeli AE. et al. Key for crossing the BBB with nanoparticles: the rational design. Beilstein J Nanotechnol. 2020;11:866-883
7. Zhao C, Zhu X, Tan J. et al. Lipid-based nanoparticles to address the limitations of GBM therapy by overcoming the blood-brain barrier, targeting glioblastoma stem cells, and counteracting the immunosuppressive tumor microenvironment. Biomed Pharmacother. 2024;171:116113
8. Markouli M, Strepkos D, Papavassiliou KA. et al. Bivalent Genes Targeting of Glioma Heterogeneity and Plasticity. Int J Mol Sci. 2021;22:540
9. Wang J, Wang Z, Zhang G. et al. Blood-brain barrier-crossing dendrimers for glioma theranostics. Biomater Sci. 2024;12:1346-1356
10. Daraee H, Eatemadi A, Abbasi E. et al. Application of gold nanoparticles in biomedical and drug delivery. Artif Cells Nanomed Biotechnol. 2016;44(1):410-22
11. Zhang Y, Gu X, Huang L. et al. Enhancing precision medicine: Bispecific antibody-mediated targeted delivery of lipid nanoparticles for potential cancer therapy. Int J Pharm. 2024;654:123990
12. Choi Y, Son W, Han Y. et al. Glycan targeting nanoparticle for photodynamic immunotherapy of melanoma. Acta Pharm Sin B. 2023;13:1903-1918
13. Maher R, Moreno-Borrallo A, Jindal D. et al. Intranasal Polymeric and Lipid-Based Nanocarriers for CNS Drug Delivery. Pharmaceutics. 2023;15:746
14. Jia W, Tian H, Jiang J. et al. Brain-Targeted HFn-Cu-REGO Nanoplatform for Site-Specific Delivery and Manipulation of Autophagy and Cuproptosis in Glioblastoma. Small. 2023;19:e2205354
15. Chen Z, Wu C, Yuan Y. et al. CRISPR-Cas13a-powered electrochemical biosensor for the detection of the L452R mutation in clinical samples of SARS-CoV-2 variants. J Nanobiotechnology. 2023;21(1):141
16. Xue T, Liang W, Li Y. et al. Ultrasensitive detection of miRNA with an antimonene-based surface plasmon resonance sensor. Nat Commun. 2019;10(1):28
17. Zheng F, Chen Z, Li J. et al. A Highly Sensitive CRISPR-Empowered Surface Plasmon Resonance Sensor for Diagnosis of Inherited Diseases with Femtomolar-Level Real-Time Quantification. Adv Sci (Weinh). 2022;9(14):e2105231
18. Chen Z, Li J, Li T. et al. A CRISPR/Cas12a-empowered surface plasmon resonance platform for rapid and specific diagnosis of the Omicron variant of SARS-CoV-2. Natl Sci Rev. 2022;9(8):nwac104
19. Ungan G, Pons-Escoda A, Ulinic D. et al. Early pseudoprogression and progression lesions in glioblastoma patients are both metabolically heterogeneous. NMR Biomed. 2024;37:e5095
20. Ostrom QT, Gittleman H, Truitt G. et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011-2015. Neuro Oncol. 2018;20:iv1-iv86
21. Kamali A, Flanders AE, Brody J. et al. Tracing superior longitudinal fasciculus connectivity in the human brain using high resolution diffusion tensor tractography. Brain Struct Funct. 2014;219:269-81
22. Orringer D, Lau D, Khatri S. et al. Extent of resection in patients with glioblastoma: limiting factors, perception of resectability, and effect on survival. J Neurosurg. 2012;117:851-9
23. Meng J, Qian W, Yang Z. et al. p53/E2F7 axis promotes temozolomide chemoresistance in glioblastoma multiforme. BMC Cancer. 2024;24:317
24. Tomar MS, Kumar A, Srivastava C. et al. Elucidating the mechanisms of Temozolomide resistance in gliomas and the strategies to overcome the resistance. Biochim Biophys Acta Rev Cancer. 2021;1876:188616
25. Wu Y, Song Y, Wang R. et al. Molecular mechanisms of tumor resistance to radiotherapy. Mol Cancer. 2023;22:96
26. Ameratunga M, Pavlakis N, Wheeler H. et al. Anti-angiogenic therapy for high-grade glioma. Cochrane Database Syst Rev. 2018;11:CD008218
27. Vredenburgh JJ, Desjardins A, Herndon JE 2nd. et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253-9
28. Stupp R, Taillibert S, Kanner AA. et al. Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA. 2015;314:2535-43
29. Bernard-Arnoux F, Lamure M, Ducray F. et al. The cost-effectiveness of tumor-treating fields therapy in patients with newly diagnosed glioblastoma. Neuro Oncol. 2016;18:1129-36
30. Tian M, Zhan Y, Cao J. et al. Targeting blood-brain barrier for sepsis-associated encephalopathy: Regulation of immune cells and ncRNAs. Brain Res Bull. 2024;209:110922
31. Peng X, Luo Z, He S. et al. Blood-Brain Barrier Disruption by Lipopolysaccharide and Sepsis-Associated Encephalopathy. Front Cell Infect Microbiol. 2021;11:768108
32. Sun P, Hamblin MH, Yin KJ. Non-coding RNAs in the regulation of blood-brain barrier functions in central nervous system disorders. Fluids Barriers CNS. 2022;19:27
33. Angom RS, Nakka NMR, Bhattacharya S. Advances in Glioblastoma Therapy: An Update on Current Approaches. Brain Sci. 2023;13:1536
34. Kofman AV, Abounader R. When tumor cells make blood vessels: implications for glioblastoma therapy. Future Oncol. 2011;7:841-3
35. Meng X, Qiu D. Surface morphology regulation of colloidal Nanoparticles: A convenient Kinetically-Controlled seeded growth strategy. J Colloid Interface Sci. 2023;633:284-290
36. Ye L, Gu L, Wang Y. et al. Identification of TMZ resistance-associated histone post-translational modifications in glioblastoma using multi-omics data. CNS Neurosci Ther. 2024;30:e14649
37. Adhikaree J, Moreno-Vicente J, Kaur AP. et al. Resistance Mechanisms and Barriers to Successful Immunotherapy for Treating Glioblastoma. Cells. 2020;9:263
38. Tancredi A, Gusyatiner O, Bady P. et al. BET protein inhibition sensitizes glioblastoma cells to temozolomide treatment by attenuating MGMT expression. Cell Death Dis. 2022;13:1037
39. Zappe K, Pühringer K, Pflug S. et al. Association between MGMT Enhancer Methylation and MGMT Promoter Methylation, MGMT Protein Expression, and Overall Survival in Glioblastoma. Cells. 2023;12:1639
40. Bahrami K, Kärkkäinen J, Bibi S. et al. Specific transport of temozolomide does not override DNA repair-mediated chemoresistance. Eur J Pharm Sci. 2024;195:106661
41. Adylova A, Kapanova G, Datkhayeva Z. et al. Nanotechnology-based cancer chemoprevention in glioblastoma. Folia Neuropathol. 2023;61(3):235-241
42. Kudruk S, Forsyth CM, Dion MZ. et al. Multimodal neuro-nanotechnology: Challenging the existing paradigm in glioblastoma therapy. Proc Natl Acad Sci U S A. 2024;121(8):e2306973121
43. Larionova TD, Bastola S, Aksinina TE. et al. Alternative RNA splicing modulates ribosomal composition and determines the spatial phenotype of glioblastoma cells. Nat Cell Biol. 2022;24:1541-1557
44. Ren Y, Huang Z, Zhou L. et al. Spatial transcriptomics reveals niche-specific enrichment and vulnerabilities of radial glial stem-like cells in malignant gliomas. Nat Commun. 2023;14:1028
45. Mathur R, Wang Q, Schupp PG. et al. Glioblastoma evolution and heterogeneity from a 3D whole-tumor perspective. Cell. 2024;187:446-463.e16
46. Nicholson JG, Fine HA. Diffuse Glioma Heterogeneity and Its Therapeutic Implications. Cancer Discov. 2021;11:575-590
47. Zamler DB, Hu J. Primitive Oligodendrocyte Precursor Cells Are Highly Susceptible to Gliomagenic Transformation. Cancer Res. 2023;83:807-808
48. Niklasson M, Bergström T, Jarvius M. et al. Mesenchymal transition and increased therapy resistance of glioblastoma cells is related to astrocyte reactivity. J Pathol. 2019;249:295-307
49. von Manstein V, Groner B. Tumor cell resistance against targeted therapeutics: the density of cultured glioma tumor cells enhances Stat3 activity and offers protection against the tyrosine kinase inhibitor canertinib. Medchemcomm. 2016;8:96-102
50. Gangoso E, Southgate B, Bradley L. et al. Glioblastomas acquire myeloid-affiliated transcriptional programs via epigenetic immunoediting to elicit immune evasion. Cell. 2021;184:2454-2470.e26
51. Jackson CM, Choi J, Lim M. Mechanisms of immunotherapy resistance: lessons from glioblastoma. Nat Immunol. 2019;20:1100-1109
52. Wadajkar AS, Dancy JG, Hersh DS. et al. Tumor-targeted nanotherapeutics: overcoming treatment barriers for glioblastoma. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(4):10.1002 /wnan.1439
53. Sanai N, Polley MY, McDermott MW. et al. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115:3-8
54. Tan J, Sun W, Lu L. et al. I6P7 peptide modified superparamagnetic iron oxide nanoparticles for magnetic resonance imaging detection of low-grade brain gliomas. J Mater Chem B. 2019;7:6139-6147
55. Li Y, Cui J, Li C. et al. 19 F MRI Nanotheranostics for Cancer Management: Progress and Prospects. ChemMedChem. 2022;17:e202100701
56. Fu C, Duan X, Cao M. et al. Targeted Magnetic Resonance Imaging and Modulation of Hypoxia with Multifunctional Hyaluronic Acid-MnO2 Nanoparticles in Glioma. Adv Healthc Mater. 2019;8:e1900047
57. Jackson PA, Rahman WN, Wong CJ. et al. Potential dependent superiority of gold nanoparticles in comparison to iodinated contrast agents. Eur J Radiol. 2010;75:104-9
58. Silindir M, Erdoğan S, Özer AY. et al. Nanosized multifunctional liposomes for tumor diagnosis and molecular imaging by SPECT/CT. J Liposome Res. 2013;23:20-7
59. Zhang W, Chen X, Ding D. et al. Real-time in vivo imaging reveals specific nanoparticle target binding in a syngeneic glioma mouse model. Nanoscale. 2022;14:5678-5688
60. An R, Liu L, Wei S. et al. Controlling Disassembly of Paramagnetic Prodrug and Photosensitizer Nanoassemblies for On-Demand Orthotopic Glioma Theranostics. ACS Nano. 2022;16:20607-20621
61. Sheng Z, Guo B, Hu D. et al. Bright Aggregation-Induced-Emission Dots for Targeted Synergetic NIR-II Fluorescence and NIR-I Photoacoustic Imaging of Orthotopic Brain Tumors. Adv Mater. 2018;28:e1800766
62. Tang T, Chang B, Zhang M. et al. Nanoprobe-mediated precise imaging and therapy of glioma. Nanoscale Horiz. 2021;6:634-650
63. O'Connor JP, Aboagye EO, Adams JE. et al. Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol. 2017;14(3):169-186
64. Wagner MW, Jabehdar Maralani P, Bennett J. et al. Brain Tumor Imaging in Adolescents and Young Adults: 2021 WHO Updates for Molecular-based Tumor Types. Radiology. 2024;310(2):e230777
65. Chen S, He Y, Liu L. et al. DNA walking system integrated with enzymatic cleavage reaction for sensitive surface plasmon resonance detection of miRNA. Sci Rep. 2022;12(1):16093
66. Wu Y, Wang Y, Zhou J. et al. Universal theranostic CRISPR/Cas13a RNA-editing system for glioma. Theranostics. 2023;13(15):5305-5321
67. Jebelli A, Oroojalian F, Fathi F. et al. Recent advances in surface plasmon resonance biosensors for microRNAs detection. Biosens Bioelectron. 2020;169:112599
68. Gregory JV, Kadiyala P, Doherty R. et al. Systemic brain tumor delivery of synthetic protein nanoparticles for glioblastoma therapy. Nat Commun. 2020;11:5687
69. Wang B, Tang M, Yuan Z. et al. Targeted delivery of a STING agonist to brain tumors using bioengineered protein nanoparticles for enhanced immunotherapy. Bioact Mater. 2022;16:232-248
70. Huang CW, Chuang CP, Chen YJ. et al. Integrin α2β1-targeting ferritin nanocarrier traverses the blood-brain barrier for effective glioma chemotherapy. J Nanobiotechnology. 2021;19:180
71. Majc B, Sever T, Zarić M. et al. Epithelial-to-mesenchymal transition as the driver of changing carcinoma and glioblastoma microenvironment. Biochim Biophys Acta Mol Cell Res. 2020;1867:118782
72. Wu Y, Fan Q, Zeng F. et al. Peptide-Functionalized Nanoinhibitor Restrains Brain Tumor Growth by Abrogating Mesenchymal-Epithelial Transition Factor (MET) Signaling. Nano Lett. 2018;18:5488-5498
73. Zheng T, Wang W, Mohammadniaei M. et al. Anti-MicroRNA-21 Oligonucleotide Loaded Spermine-Modified Acetalated Dextran Nanoparticles for B1 Receptor-Targeted Gene Therapy and Antiangiogenesis Therapy. Adv Sci (Weinh). 2022;9:e2103812
74. He L, Yu X, Li W. Recent Progress and Trends in X-ray-Induced Photodynamic Therapy with Low Radiation Doses. ACS Nano. 2022;16:19691-19721
75. Jiang Z, He L, Yu X. et al. Antiangiogenesis Combined with Inhibition of the Hypoxia Pathway Facilitates Low-Dose, X-ray-Induced Photodynamic Therapy. ACS Nano. 2021;15:11112-11125
76. Chai Z, Ran D, Lu L. et al. Ligand-Modified Cell Membrane Enables the Targeted Delivery of Drug Nanocrystals to Glioma. ACS Nano. 2019;13:5591-5601
77. Wei R, Li J, Lin W. et al. Nanoparticle-mediated blockade of CXCL12/CXCR4 signaling enhances glioblastoma immunotherapy: Monitoring early responses with MRI radiomics. Acta Biomater. 2024;14:S1742-7061 (24)00067-9
78. Alghamri MS, Banerjee K, Mujeeb AA. et al. Systemic Delivery of an Adjuvant CXCR4-CXCL12 Signaling Inhibitor Encapsulated in Synthetic Protein Nanoparticles for Glioma Immunotherapy. ACS Nano. 2022;16:8729-8750
79. Gao X, Yue Q, Liu Y. et al. Image-guided chemotherapy with specifically tuned blood brain barrier permeability in glioma margins. Theranostics. 2018;8:3126-3137
80. Lu C, Wei Y, Wang X. et al. DNA-methylation-mediated activating of lncRNA SNHG12 promotes temozolomide resistance in glioblastoma. Mol Cancer. 2020;19:28
81. Oldrini B, Vaquero-Siguero N, Mu Q. et al. MGMT genomic rearrangements contribute to chemotherapy resistance in gliomas. Nat Commun. 2020;11:3883
82. Scholtyssek F, Zwiener I, Schlamann A. et al. Reirradiation in progressive high-grade gliomas: outcome, role of concurrent chemotherapy, prognostic factors and validation of a new prognostic score with an independent patient cohort. Radiat Oncol. 2013;8:161
83. Zhang Y, Xi K, Fu X. et al. Versatile metal-phenolic network nanoparticles for multitargeted combination therapy and magnetic resonance tracing in glioblastoma. Biomaterials. 2021;278:121163
84. Rottenberg S, Disler C, Perego P. The rediscovery of platinum-based cancer therapy. Nat Rev Cancer. 2021;21:37-50
85. Wang Y, Jiang Y, Wei D. et al. Nanoparticle-mediated convection-enhanced delivery of a DNA intercalator to gliomas circumvents temozolomide resistance. Nat Biomed Eng. 2021;5:1048-1058
86. Bunevicius A, Pikis S, Padilla F. et al. Sonodynamic therapy for gliomas. J Neurooncol. 2022;156:1-10
87. He H, Liu L, Zhang S. et al. Smart gold nanocages for mild heat-triggered drug release and breaking chemoresistance. J Control Release. 2020;323:387-397
88. Chen H, Zhang S, Fang Q. et al. Biomimetic Nanosonosensitizers Combined with Noninvasive Ultrasound Actuation to Reverse Drug Resistance and Sonodynamic-Enhanced Chemotherapy against Orthotopic Glioblastoma. ACS Nano. 2023;17:421-436
89. Lawrie TA, Gillespie D, Dowswell T. et al. Long-term neurocognitive and other side effects of radiotherapy, with or without chemotherapy, for glioma. Cochrane Database Syst Rev. 2019;8:CD013047
90. Horsman MR, Mortensen LS, Petersen JB. et al. Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol. 2012;9:674-87
91. Moeller BJ, Richardson RA, Dewhirst MW. Hypoxia and radiotherapy: opportunities for improved outcomes in cancer treatment. Cancer Metastasis Rev. 2007;26:241-8
92. Woodall RT, Hormuth Ii DA, Wu C. et al. Patient specific, imaging-informed modeling of rhenium-186 nanoliposome delivery via convection-enhanced delivery in glioblastoma multiforme. Biomed Phys Eng Express. 2021;7:10.1088 /2057-1976/ac02a6
93. Phillips WT, Goins B, Bao A. et al. Rhenium-186 liposomes as convection-enhanced nanoparticle brachytherapy for treatment of glioblastoma. Neuro Oncol. 2012;14:416-25
94. Zhang L, Yu D. Exosomes in cancer development, metastasis, and immunity. Biochim Biophys Acta Rev Cancer. 2019;187:455-468
95. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977
96. Morad G, Carman CV, Hagedorn EJ. et al. Tumor-Derived Extracellular Vesicles Breach the Intact Blood-Brain Barrier via Transcytosis. ACS Nano. 2019;13:13853-13865
97. Wu X, Wang X, Wang J. et al. The Roles of Exosomes as Future Therapeutic Agents and Diagnostic Tools for Glioma. Front Oncol. 2021;11:733529
98. Skog J, Würdinger T, van Rijn S. et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2018;10:1470-6
99. Xu B, Cui Y, Wang W. et al. Immunomodulation-Enhanced Nanozyme-Based Tumor Catalytic Therapy. Adv Mater. 2020;32:e2003563
100. Zhu Z, Zhai Y, Hao Y. et al. Specific anti-glioma targeted-delivery strategy of engineered small extracellular vesicles dual-functionalised by Angiopep-2 and TAT peptides. J Extracell Vesicles. 2022;11:e12255
101. Thirumurugan S, Dash P, Liu X. et al. Angiopep-2-decorated titanium-alloy core-shell magnetic nanoparticles for nanotheranostics and medical imaging. Nanoscale. 2022;14:14789-14800
102. Deng G, Peng X, Sun Z. et al. Natural-Killer-Cell-Inspired Nanorobots with Aggregation-Induced Emission Characteristics for Near-Infrared-II Fluorescence-Guided Glioma Theranostics. ACS Nano. 2020;14:11452-11462
103. Wang X, Gong Z, Wang T. et al. Mechanical nanosurgery of chemoresistant glioblastoma using magnetically controlled carbon nanotubes. Sci Adv. 2023;9:eade5321
104. Luo H, Zhang H, Mao J. et al. Exosome-based nanoimmunotherapy targeting TAMs, a promising strategy for glioma. Cell Death Dis. 2023;14:235
105. Thivat E, Casile M, Moreau J. et al. Phase I/II study testing the combination of AGuIX nanoparticles with radiochemotherapy and concomitant temozolomide in patients with newly diagnosed glioblastoma (NANO-GBM trial protocol). BMC Cancer. 2023;23(1):344
106. Wu WE, Chang E, Jin L. et al. Multimodal In vivo Tracking of Chimeric Antigen Receptor T Cells in Preclinical Glioblastoma Models. Invest Radiol. 2023;58(6):388-395
107. Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet. 2018;392:432-446
108. Bayda S, Adeel M, Tuccinardi T. et al. The History of Nanoscience and Nanotechnology: From Chemical-Physical Applications to Nanomedicine. Molecules. 2019;25:112
109. Chen T, Ren L, Liu X. et al. DNA Nanotechnology for Cancer Diagnosis and Therapy. Int J Mol Sci. 2018;19:1671
110. Haddad AF, Aghi MK, Butowski N. Novel intraoperative strategies for enhancing tumor control: Future directions. Neuro Oncol. 2022;24:S25-S32
111. Fang RH, Gao W, Zhang L. Targeting drugs to tumours using cell membrane-coated nanoparticles. Nat Rev Clin Oncol. 2023;20:33-48
112. Chen G, Roy I, Yang C. et al. Nanochemistry and Nanomedicine for Nanoparticle-based Diagnostics and Therapy. Chem Rev. 2016;116:2826-85
Author contact
![]() Corresponding author: Yanhui Liu, E-mail: liuyhedu.cn.
Corresponding author: Yanhui Liu, E-mail: liuyhedu.cn.

 Global reach, higher impact
Global reach, higher impact