Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(17):5492-5505. doi:10.7150/jca.98951 This issue Cite
Research Paper
Comparative analysis of adhesion virulence protein FadA from gut-associated bacteria of colorectal cancer patients (F. nucleatum) and healthy individuals (E. cloacae)
1. Department of Pharmaceutical Sciences, College of Pharmacy, Al Ain University, Al Ain Campus, Al Ain 64141, Abu Dhabi, United Arab Emirates.
2. AAU Health and Biomedical Research Center, Al Ain University, Abu Dhabi Campus, Abu Dhabi P. O. Box 112612, Abu Dhabi, United Arab Emirates.
3. School of Biochemistry and Biotechnology, University of Punjab, Lahore, 52254, Pakistan.
4. Department of Community Health Sciences, College of Applied Medical Sciences, King Saud University, Riyadh, Saudi Arabia.
5. Department of Food Science and Nutrition, College of Food and Agriculture Sciences, King Saud University, Riyadh, Saudi Arabia.
6. University of Bonn, LIMES Institute (AG-Netea), Carl-Troll-Str. 31, 53115 Bonn, Germany.
Received 2024-5-27; Accepted 2024-7-18; Published 2024-8-19
Abstract
Background: Colorectal cancer (CRC) is a gastrointestinal disease linked with GIT microbial dysbiosis. The present study has targeted the comparative analysis of virulent factor FadA from gut-associated bacteria of CRC patients (F. nucleatum) and healthy individuals (E. cloacae).
Methods: For this purpose, FadA protein sequences of fifteen strains of F. nucleatum and four strains of E. cloacae, were retrieved from the UniProt database. These sequences were analysed through VirulentPred, PSLpred, ProtParam, PFP-FunDSeqE, PROTEUS Structure Prediction Server, SWISS-MODEL, SAVES validation server, MEME suite 5.5.0, CAVER Web tool, Webserver VaxinPAD, HPEPDOCK and HDOCK servers.
Results: FadA protein from F. nucleatum was found to exhibit significant differences as compared to E. nucleatum i.e. it exhibited helical configuration, cytoplasmic, periplasmic, outer-membrane and extracellular localisation, 2D structure comprising of 70-96% helix, 0% beta-sheet, 4-30% coils and 17-20 signal peptide residues, hydrophilicity, strongly acidic character and smaller number of antigenic epitopes. In contrast, FadA protein from E. nucleatum was found to have globular 3D configuration, cytoplasmic localisation, 2D structure (30-56% helix, 12-21% beta-sheet, 33-50% coils and 43 signal peptide residues), highly hydrophobic, slightly acidic and more number of antigenic epitopes. Docking analyses of virulent factors revealed their high binding affinities with previously reported inhibitory peptide and FAD-approved drug COX2.
Conclusion: The wide range of differences not only provided us the reason for the role of FadA protein as a virulent factor in F. nucleatum but also might help us in designing virulent FadA protein inhibiting strategies including peptide-based vaccine adjuvants and drugs designing, modification of tunnels and catalytic pockets to reduce substrate binding and FAD approved drugs selection. Inhibition of this virulent factor in CRC patients' gut bacteria might result in oncogenesis regression and reduced death rate.
Keywords: Colorectal cancer, F. nucleatum, E. cloacae, FadA virulence factor
Background
Colorectal cancer (CRC) is the second and third most abundant cancer concerning morbidity (9.2%) and diagnosis (6.1%) as per the research of the Centers for Disease Control and Prevention (CDC). According to a study, by 2030 CRC is expected to increase by 60% with 2.2 million new cases and 1.1 million deaths [1]. It has contributed to 9, 35000 deaths caused by cancer. It is characterized by polyps in the rectum, change in bowel habits, constipation, diarrhoea, rectal bleeding, change in stool colour, shape, abdominal cramps, gas, pain, changed stool consistency, tenesmus, anaemia, loss of appetite and weight, nausea, vomiting, jaundice and fatigue [1]. The risk factors include smoking, physical inactivity, obesity, unhealthy and improper diet routine, family history of diabetes, inflammatory bowel diseases, colon polyps, genetics, race, gender, age and gastrointestinal tract (GIT) microbiome [2, 3].
The composition of the GIT-associated microbial population always varies with oncogenesis. This dysbiosis always involves a shift toward pathogenic microbes including Fusobacterium nucleatum, Escherichia coli, Enterococcus faecalis, Bacteroides fragilis, Salmonella enterica, Streptococcus, Rothia, Faecalibacterium, Porphyromonas, Collinsella, Slackia, Alistipes, Porphyromonas, Prevotella, Methanobrevibacter, Gemella, Mogibacterium, Parvimonas, Peptostreptococcus, Solobacterium, Helicobacter, Klebsiella, Akkermansia and Thermanaerovibrio [4-14]. Following bacteria have been reported to reduce in CRC patients i.e. Ruminococcus, Roseburia, Lactobacillus, Eubacterium, Clostridium, Anaerostipes, Bacteroides, Bifidobacterium, Citrobacter, Treponema, Serratia, Kluyvera and Cronobacter [10-20].
E. cloacae is reported as a harmless GIT commensal microbe normally found in healthy individuals [21]. On the contrary, F. nucleatum is a commensal-turned and obligate pathogen associated with various GIT disorders [22]. It potentiates the development and progression of CRC due to its enrichment in colorectal malignant tumour tissue [23-25]. Being asaccharolytic, it metabolizes the peptides and amino acids into short-chain fatty acids and formyl-methionyl-leucyl-phenylalanine [26]. These metabolites attract myeloid cells in the tumour microenvironment thus contributing to tumour enrichment with myeloid cells. Additionally, metabolic products also promote vascularization and infiltration of immune cells in tumours [9]. Being slightly capable of respiring aerobically, Fusobacterium successfully survives hypoxic tumour conditions [27]. It increases oncogenic micro RNAs [28-30]. It damages DNA and induces single nucleotide polymorphisms (SNPs) by producing reactive oxygen species via its metabolite hydrogen sulfide [31]. It suppresses tumour immune microenvironment via interference with functions of T-cells, macrophages, dendritic cells, neutrophils and natural killer cells (NKCs) [9, 32]. Therefore, it can be considered one of the major non-invasive prognostic, diagnostic and therapy response biomarkers of CRC [9, 33-42].
F. nucleatum being an adhesive pathogen has multiple virulence factors contributing to its colonization of colorectal tissues and CRC incidence. The known virulence factors of F. nucleatum promote adhesion to intestinal epithelial cells via FadA [8]. This factor has a strong connection with CRC due to its association with NF-Kb inflammatory response and cell adhesion and invasion of Fusobacterium [43]. It enables the bacterium to get attached to fibroblasts, and endothelial and epithelial cells [22]. To cause disease, FadA binds the EC5 domain (ASANWTLOYNDP) of the E-cadherin protein leading to its phosphorylation. This activates the Wnt signal in the Wnt/β- cadherin pathway. Wnt binds receptors and disrupts the destruction complex comprising of Axin, APC, GSK-3β, PP2A and Ck1α. This complex under normal circumstances targets β-catenin for ubiquitination and digestion by proteasome. Disruption of this complex inhibits phosphorylation of β-catenin resulting in its stability. The β-catenin gets accumulated in the cytosol and translocated to the nucleus. In the nucleus, β-catenin binds with TCF/LEF transcription factors and activates the transcription of genes involved in inflammation and other cancer-initiating activities [44].
The present study focused on comparative analysis of the FadA protein in F. nucleatum and E. cloacae at the levels of physicochemical properties, sub-cellular localization, conserved domains, antigenic epitopes, functional domains, 2D and 3D structures, the number of catalytic sites and tunnels that might help design strategies for inhibition of tumorigenic roles of F. nucleatum FadA protein.
Methodology
Retrieving the sequences of FadA protein in F. nucleatum and E. cloacae
Sequences of FadA protein in F. nucleatum and E. cloacae were retrieved from the UniProt database (https://www.uniprot.org, accessed on 11 Nov. 2022) [18]. Sequences were retrieved for a total of fifteen strains of F. nucleatum and four strains of E. cloacae (Supplementary data Table 1).
Evaluation of virulence potential
To predict the virulence status of the FadA protein in bacteria documented in the present study VirulentPred tool (http://bioinfo.icgeb.res.in/virulent/, accessed on 13 Nov. 2022) has been consulted [45]. This is a Support Vector Machine (SVM) based method. It was determined based on prediction scores. A positive score indicates virulence while a negative score shows the non-virulent nature of protein.
Phylogenetic tree construction
To analyze the phylogenetic relationship among present study bacteria, the Clustal Omega Multiple Sequence Alignment Tool was used for multiple sequence alignment [46]. Alignment was followed by tree construction using MEGA version 7. Trees were inferred by the neighbour-joining method [47, 48]. The evolutionary distances were computed using the Poisson correction method and are in the units of the number of amino acid substitutions per site [49].
Evaluation of sub-cellular localization
To predict the sub-cellular localization of proteins, the PSLpred tool (webs.iiitd.edu.in/raghava/pslpred/submit.html, accessed on 26 Nov. 2022) was used [50]. The prediction approach used was based on a hybrid approach.
Evaluation of physicochemical properties
To determine the differences between FadA proteins of bacteria documented in the present study, the ProtParam tool (https://web.expasy.org/protparam/, accessed on 12 Dec. 2022) was used [51]. Properties compared include the number of amino acids, molecular weight, theoretical isoelectric point (pI), half-life, instability index, aliphatic index, extinction coefficient and grand average of hydropathy (GRAVY) [51]. The pI value helps in prediction of the acidity or alkalinity of the protein.
Prediction of functional domains
To predict the functional domains in FadA protein sequences of bacteria inhabiting normal and cancerous patients GIT, Functional domain or motif prediction (PFP-FunDSeqE) tool (www.csbio.sjtu.edu.cn/bioinf/PFP-FunDSeqE/#, accessed on 25 Nov. 2022) was used [52].
Prediction of 2D configuration
To predict the 2D configuration of the present study proteins, PROTEUS Structure Prediction Server 2.0 (www.proteus2.ca/proteus2/, accessed on 20 Dec. 2022) was used [53]. This server helped us to determine the helix, beta sheet, coil content, signal peptide and membrane content of proteins [54].
Prediction of 3D configuration
To predict the 3D structure of bacterial proteins, a homology modelling server SWISS-MODEL (https://swissmodel.expasy.org, accessed on 12 Dec. 2022) was used [55].
Validation of 3D structures
To validate the 3D structures of FadA proteins predicted using the SWISS-MODEL, the SAVES validation server (https://saves.mbi.ucla.edu, accessed on 20 Dec. 2022) was used. Two programs of this server i.e. ERRAT and PROCHECK were used.
Prediction of conserved protein motifs
To predict the conserved protein motifs, Multiple Em for Motif Elicitation (MEME) suite 5.5.0 (https://meme.suite.org/meme/tools/meme, accessed on 21 Dec 2022) was used. A total of ten motifs were found using default values of all parameters. For ontology analysis of predicted motifs, Lambda Predict Protein (ƛPP) (https://embed.predictprotein.org/0, accessed on 23 Dec 2022) was used.
Assessment of catalytic pockets and tunnels
To assess the catalytic pockets and tunnels in FadA proteins of bacteria documented in the present study, the CAVER Web tool was used (https://loschmidt.chemi.muni.cz/caverweb, accessed on 20-21 Dec. 2022) [56].
Evaluation of immunomodulatory A-cell epitopes
Webserver VaxinPAD (https://webs.iiitd.edu.in/raghava/vaxinpad/batch.php, accessed on 25 - 27 Dec 2022) was used to explore the immunomodulatory A-cell epitopes in the FadA protein of F. nucleatum and E. cloacae [57].
Assessment of binding affinity of FadA with inhibitor peptide
To determine the binding affinity of FadA protein from virulent F. nucleatum bacteria with a previously reported inhibitor peptide ASANWTIQYND, peptide-protein docking web-server, HPEPDOCK (huanglab.phys.hust.edu.cn/hpepdock/, accessed on 26 & 27 Dec. 2022) was used [8, 58]. To dock FadA protein with cyclooxygenase-2 (COX2), the HDOCK server (http://hdock.phys.hust.edu.cn/, accessed on 31 Dec, 2022) was used [59].
Results
Virulence status prediction
Sequences of the FadA protein retrieved from the UniProt database were analyzed for virulence status. All the sequences from E. cloacae were found to be non-virulent with negative scores while those from F. nucleatum showed virulent status with positive scores (Table 1).
Phylogeny
The optimal tree for the F. nucleatum strains with the sum of branch length 11.14917307 is shown in Supplementary Data Figure 1 (a). According to this tree, F. nucleatum IV and V, F. nucleatum II and III, F. nucleatum I and subsp. polymorphum 3 and F. nucleatum subsp. animalis 7_1 and animalis D11 (I) were closely related as compared to others as they share the same clade with each other.
Virulence status of adhesion virulence factor (FadA) in bacteria documented in present study predicted on the basis of VirulentPred tool
| # | Bacterium | Prediction score | Virulent / Non-virulent |
|---|---|---|---|
| Bacteria from CRC patients | |||
| 1 | F. nucleatum I | 0.5539 | Virulent |
| 2 | F. nucleatum II | 1.0496 | Virulent |
| 3 | F. nucleatum III | 1.0588 | Virulent |
| 4 | F. nucleatum IV | 1.1012 | Virulent |
| 5 | F. nucleatum V | 1.1172 | Virulent |
| 6 | F. nucleatum VI | 1.1403 | Virulent |
| 7 | F. nucleatum 13_3C | 1.0511 | Virulent |
| 8 | F. nucleatum CTI-5 | 1.0936 | Virulent |
| 9 | F. nucleatum subsp. vincentii | 1.1082 | Virulent |
| 10 | F. nucleatum subsp. animalis 7_1 | 1.1398 | Virulent |
| 11 | F. nucleatum subsp. animalis D11 (I) | 1.1275 | Virulent |
| 12 | F. nucleatum subsp. animalis D11 (II) | 1.1280 | Virulent |
| 13 | F.nucleatum subsp. animalis 11_3_2 | 1.1111 | Virulent |
| 14 | F. nucleatum subsp. polymorphum 1 | 0.9287 | Virulent |
| 15 | F. nucleatum subsp. polymorphum 1 | 1.1545 | Virulent |
| Bacteria from healthy human | |||
| 1 | E. cloacae I | -1.018 | Non-virulent |
| 2 | E. cloacae II | -1.009 | Non-virulent |
| 3 | E. cloacae III | -0.962 | Non-virulent |
| 4 | E. cloacae IV | -0.979 | Non-virulent |
The optimal tree for the E. cloacae strains with the sum of branch length 3.79674773 is shown in Supplementary Data Figure 1 (b). According to this tree, E. cloacae I and IV are more closely related to each other as compared to others as they are originating from the same branch point. While E. cloacae III and II are distantly related to each other and from E. cloacae I and IV.
Sub-cellular localization prediction
Assessment of sub-cellular localization of FadA protein using PSLPred analysis tool revealed FadA protein to be localized in the cytoplasm in the case of all four strains of E. cloacae. in the case of colorectal cancer GIT bacteria, in addition to the cytoplasm (F. nucleatum (IV), F. nucleatum (VI), F. nucleatum 13_3C, F. nucleatum CTI-5, F. nucleatum subsp. Vincentii, F. nucleatum subsp. animalis 7_1, F. nucleatum subsp. animalis D11 (I), F. nucleatum subsp. animalis D11 (II) and polymorphum 2) the protein was also found to be localized in periplasmic space (F. nucleatum (II) and F. nucleatum (III)), outer-membrane (F. nucleatum (I) and polymorphum 3) and extracellular membrane (F. nucleatum (V) and F. nucleatum subsp. animalis 11_3_2) (Supplementary data Table 2).
Physicochemical properties prediction
Analysis of the physicochemical properties of FadA proteins revealed a wide range of variations in proteins of F. nucleatum from E. cloacae. In E. cloacae, the pI was observed in the range of 5.97 to 6.90 while in the case of F. nucleatum, the minimum and highest values of pI were observed to be 4.06 and 6.94, respectively. The highest deviation was observed in the case of F. nucleatum I, F. nucleatum II, F. nucleatum III, F. nucleatum IV, F. nucleatum subsp. polymorphum 2 and F. nucleatum subsp. polymorphum 3 (Table 2). F. nucleatum II, F. nucleatum III and F. nucleatum IV showed a very unusual value of half-life i.e. 2 min., as compared to all other bacteria documented in the present study. High deviation of instability index from E. cloacae, was observed in the case of F. nucleatum II (40.2), F. nucleatum V (46.80), F. nucleatum VI (48.33), F. nucleatum 13_3C (52.95), F. nucleatum CTI-5 (49.32), F. nucleatum subsp. vincentii (51.94), F. nucleatum subsp. animalis 7_1 (47.92), F. nucleatum subsp. animalis D11 I (50.36), F. nucleatum subsp. animalis D11 II (50.37), F. nucleatum subsp. animalis 11_3_2 (46.13), F. nucleatum subsp. polymorphum 2 (43.71) and F. nucleatum subsp. polymorphum 3 (69.24). As far as the aliphatic index is concerned, F. nucleatum I (82.79), F. nucleatum II (77.45), F. nucleatum III and F. nucleatum IV (75.79), F. nucleatum V (81.41) and F. nucleatum subsp. polymorphum 3 (75.32) exhibited variation from that of E. cloacae. F. nucleatum III and F. nucleatum IV (2980), F. nucleatum V and F. nucleatum subsp. animalis 11_3_2 (11460), F. nucleatum subsp. animalis D11 II (12950), polymorphum 2 (4470) and polymorphum 3 (1490) was observed to exhibit variation in extinction coefficient values than the GIT inhabiting bacteria from healthy individuals. All the bacteria from CRC patients' gut were found to have very different values of GRAVY i.e. ranging from -0.440 to -1.460 as compared to bacteria from healthy individuals (Table 2).
Functional domains prediction
Functional domain analyses also revealed a large degree of variation of FadA proteins between F. nucleatum and E. cloacae strains. Two fold types were found in E. cloacae protein i.e. NAD(P)-binding Rossmann-fold and (TIM)-barrel. On the other hand, four different types of fold were observed in F. nucleatum strains i.e. 4 helical up and down bundle, DNA-binding 3-helical bundle, EF-hand and 4-helical cytokines (Table 3).
Two-dimensional structures prediction
The 2D structure of F. nucleatum FadA protein was markedly different from that of E. cloacae. i. e. the α-helix content was significantly higher in F. nucleatum (70-96%) as compared to E. cloacae (30-56%). No beta sheets were observed in F. nucleatum while E. cloacae secondary structure exhibited 12-21% beata sheets. Coil content was extremely lower in F. nucleatum (4-16%) than E. cloacae (33-50%). Only E. cloacae III exhibited signal peptide (17%) content while the majority of the F. nucleatum strains were found to have signal peptide content i.e. 14-19% (Table 4, Supplementary data Figure 2).
Three-dimensional structures prediction
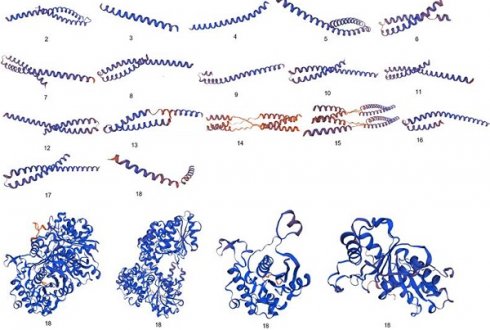
Significant diversity was found between the FadA protein of F. nucleatum and E. cloacae at the level of 3D configuration as is evident from Figure 1. The globular structure was found in E. cloacae proteins while only a helical folding pattern was observed in the case of F. nucleatum. Validation of these structures using Procheck and ERRAT scores is described in detail in Supplementary Data Table 3.
Catalytic pockets and tunnels prediction
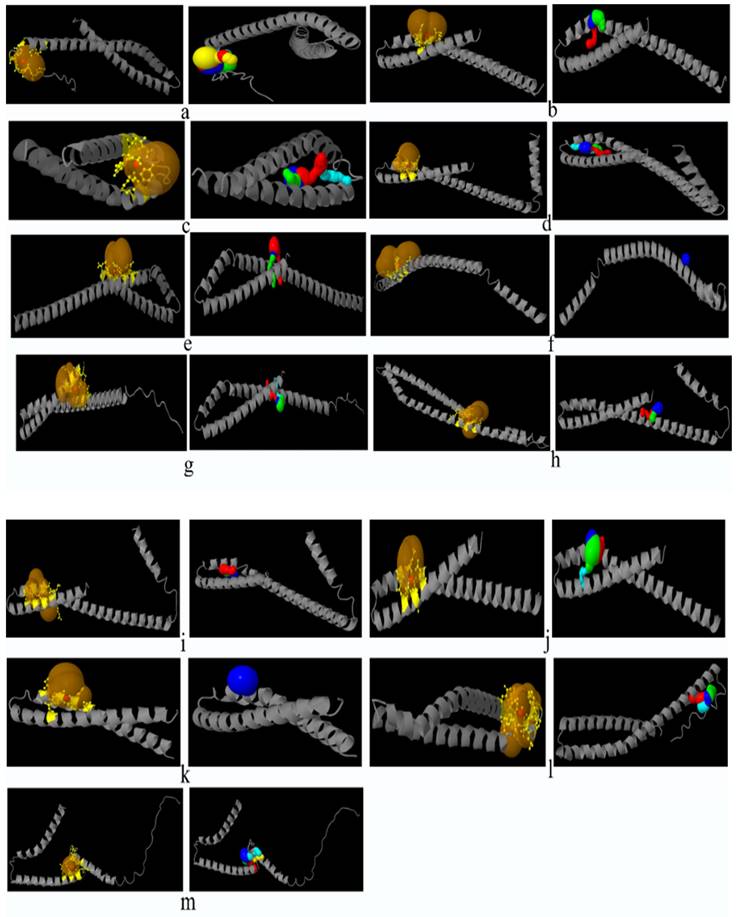
All the bacteria except F. nucleatum II and F. nucleatum III were found to exhibit catalytic pockets and tunnels. The highest number of tunnels was observed in E. cloacae II (Table 5, Figure 2).
Prediction of physicochemical properties of FadA protein from GIT bacteria of colorectal cancer patients and healthy individuals using ProtParam analysis tool
| # | Bacterium | No. of amino acids | Molecular weight | Theoretical pI | Half life (hours) | Instability index | Aliphatic index | Ext. coefficient | GRAVY |
|---|---|---|---|---|---|---|---|---|---|
| Bacteria from CRC patients | |||||||||
| 1 | F. nucleatum I | 129 | 14391.04 | 4.85 | >10 hr | 28.47 | 82.79 | 7450 | -0.577 |
| 2 | F. nucleatum II | 47 | 5111.48 | 4.06 | 2 min | 40.42 | 77.45 | 1490 | -0.636 |
| 3 | F. nucleatum III | 57 | 6343.76 | 4.13 | 2 min | 36.36 | 75.79 | 2980 | -0.740 |
| 4 | F. nucleatum IV | 57 | 6343.76 | 4.13 | 2 min | 36.36 | 75.79 | 2980 | -0.740 |
| 5 | F. nucleatum V | 85 | 10700.26 | 6.94 | >10 hr | 46.80 | 81.41 | 11460 | -1.460 |
| 6 | F. nucleatum VI | 132 | 15735.05 | 5.20 | >10 hr | 48.33 | 88.71 | 14440 | -0.873 |
| 7 | F. nucleatum 13_3C | 131 | 15660.98 | 5.31 | >10 hr | 52.95 | 89.39 | 14440 | -0.878 |
| 8 | F. nucleatum CTI-5 | 102 | 12023.82 | 5.21 | >10 hr | 49.32 | 84.22 | 11460 | -0.825 |
| 9 | F. nucleatum subsp. vincentii | 125 | 14926.11 | 5.16 | >10 hr | 51.94 | 88.24 | 14440 | -0.890 |
| 10 | F. nucleatum subsp. animalis 7_1 | 134 | 15931.30 | 5.20 | >10 hr | 47.92 | 88.88 | 14440 | -0.855 |
| 11 | F. nucleatum subsp. animalis D11 (I) | 128 | 15249.47 | 5.31 | >10 hr | 50.36 | 81.64 | 14440 | -0.963 |
| 12 | F. nucleatum subsp. animalis D11 (II) | 103 | 12663.44 | 5.28 | >10 hr | 50.37 | 82.43 | 12950 | -1.261 |
| 13 | F. nucleatum subsp. animalis 11_3_2 | 83 | 10338.80 | 6.00 | >10 hr | 46.13 | 83.49 | 11460 | -1.431 |
| 14 | F. nucleatum subsp. polymorphum 2 | 122 | 14037.03 | 4.98 | >10 hr | 43.71 | 97.62 | 4470 | -0.440 |
| 15 | F. nucleatum subsp. polymorphum 3 | 126 | 13818.63 | 4.53 | >10 hr | 69.24 | 75.32 | 1490 | -0.498 |
| Bacteria from healthy human | |||||||||
| 1 | E. cloacae I | 387 | 40808.08 | 6.50 | >10 hr | 28.84 | 90.13 | 15470 | 0.106 |
| 2 | E. cloacae II | 729 | 79759.34 | 6.21 | >10 hr | 32.67 | 93.68 | 69330 | -0.080 |
| 3 | E. cloacae III | 252 | 26511.52 | 5.97 | >10 hr | 28.23 | 88.41 | 8480 | 0.099 |
| 4 | E. cloacae IV | 135 | 14481.69 | 6.35 | >10 hr | 33.57 | 91.11 | 6990 | 0.061 |
pI = isoelectric point, GRAVY = grand average of hydropathicity
Functional domains predicted of FadA protein from GIT bacteria of colorectal cancer patients and healthy individuals using PFP-FunDSeqE tool
| # | Bacterium | Predicted fold type |
|---|---|---|
| Bacteria from CRC patients | ||
| 1 | F. nucleatum I | 4 helical up and down bundle |
| 2 | F. nucleatum II | 4 helical up and down bundle |
| 3 | F. nucleatum III | 4 helical up and down bundle |
| 4 | F. nucleatum IV | DNA-binding 3-helical bundle |
| 5 | F. nucleatum V | EF-hand |
| 6 | F. nucleatum VI | 4-helical cytokines |
| 7 | F. nucleatum 13_3C | 4-helical cytokines |
| 8 | F. nucleatum CTI-5 | 4 helical up and down bundle |
| 9 | F. nucleatum subsp. vincentii | 4 helical up and down bundle |
| 10 | F. nucleatum subsp. animalis 7_1 | 4 helical up and down bundle |
| 11 | F. nucleatum subsp. animalis D11 (I) | 4-helical cytokines |
| 12 | F. nucleatum subsp. animalis D11 (II) | DNA-binding 3-helical bundle |
| 13 | F. nucleatum subsp. animalis 11_3_2 | EF-hand |
| 14 | F. nucleatum subsp. polymorphum 2 | DNA-binding 3-helical bundle |
| 15 | F. nucleatum subsp. polymorphum 3 | DNA-binding 3-helical bundle |
| Bacteria from healthy individuals | ||
| 1 | E. cloacae I | NAD(P)-binding Rossmann-fold |
| 2 | E. cloacae II | (TIM)-barrel |
| 3 | E. cloacae III | NAD(P)-binding Rossmann-fold |
| 4 | E. cloacae IV | NAD(P)-binding Rossmann-fold |
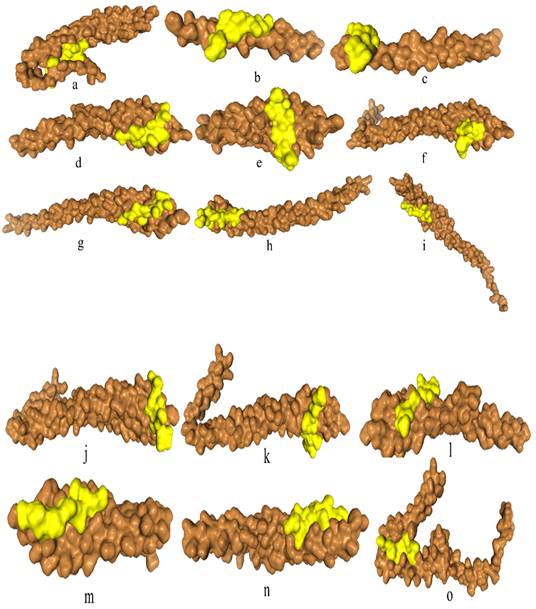
Comparative analysis of 3D configuration of adhesion virulence factor FadA between the F. nucleatum strains belonging to colorectal cancer patients and E. cloacae strains belonging to healthy individuals. a: F. nucleatum (I), b: F. nucleatum (II), c: F. nucleatum (III), d: F. nucleatum (IV), e: F. nucleatum (V), f: F. nucleatum (VI), g: F. nucleatum 13_3C, h: F. nucleatum CTI-5, i: F. nucleatum subsp. Vincentii, j: F. nucleatum subsp. animalis 7_1, k: F. nucleatum subsp. animalis D11 (I), l: F. nucleatum subsp. animalis D11 (II), m: F. nucleatum subsp. animalis 11_3_2, n: F. nucleatum subsp. polymorphum 1, o: F. nucleatum subsp. polymorphum 1, p: Enterobacter cloacae (I), q: Enterobacter cloacae (II), r: Enterobacter cloacae (III), s: Enterobacter cloacae (IV).
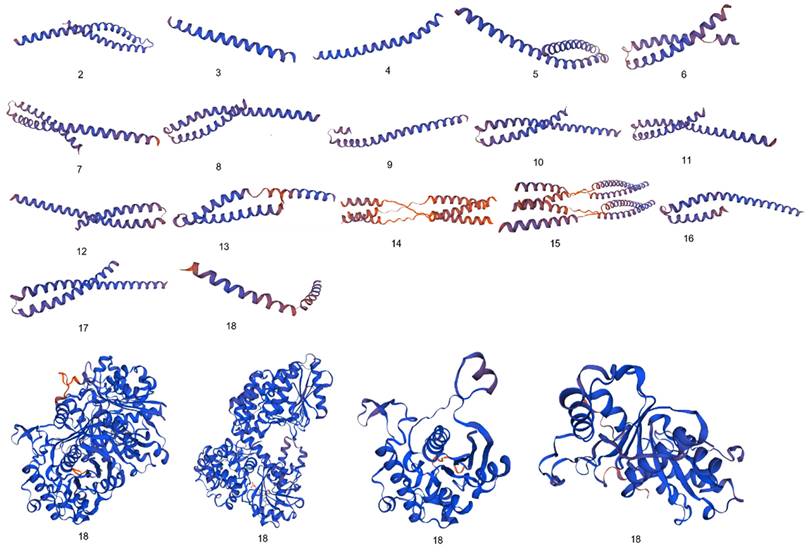
Prediction of secondary structure of FadA protein in present study bacteria
| # | Bacterium | Helix % (residues) | Beta sheet % (residues) | Coil content % (residues) | Signal peptide % (residues) |
|---|---|---|---|---|---|
| Bacteria from CRC patients | |||||
| 1 | F. nucleatum I | 95 (123) | 0 (0) | 5 (6) | 14 (18) |
| 2 | F. nucleatum II | 87 (41) | 0 (0) | 13 (6) | 0 (0) |
| 3 | F. nucleatum III | 89 (51) | 0 (0) | 11 (6) | 0 (0) |
| 4 | F. nucleatum IV | 94 (103) | 0 (0) | 6 (7) | 0 (0) |
| 5 | F. nucleatum V | 92 (78) | 0 (0) | 8 (7) | 0 (0) |
| 6 | F. nucleatum VI | 93 (123) | 0 (0) | 7 (9) | 14 (19) |
| 7 | F. nucleatum 13_3C | 95 (124) | 0 (0) | 5 (7) | 15 (19) |
| 8 | F. nucleatum CTI-5 | 84 (86) | 0 (0) | 16 (16) | 19 (19) |
| 9 | F. nucleatum subsp. vincentii | 93 (116) | 0 (0) | 7 (9) | 0 (0) |
| 10 | F. nucleatum subsp. animalis 7_1 | 92 (123) | 0 (0) | 8 (11) | 14 (19) |
| 11 | F. nucleatum subsp. animalis D11 (I) | 89 (114) | 0 (0) | 11 (14) | 15 (19) |
| 12 | F. nucleatum subsp. animalis D11 (II) | 94 (97) | 0 (0) | 6 (6) | 0 (0) |
| 13 | F. nucleatum subsp. animalis 11_3_2 | 94 (78) | 0 (0) | 6 (5) | 0 (0) |
| 14 | F. nucleatum subsp. polymorphum 2 | 96 (117) | 0 (0) | 4 (5) | 14 (17) |
| 15 | F. nucleatum subsp. polymorphum 3 | 70 (88) | 0 (0) | 30 (38) | 16 (20) |
| Bacteria from healthy individuals | |||||
| 1 | E. cloacae I | 36 (141) | 19 (74) | 44 (172) | 0 |
| 2 | E. cloacae II | 56 (405) | 12 (86) | 33 (238) | 0 |
| 3 | E. cloacae III | 37 (92) | 21 (53) | 42 (107) | 17 (43) |
| 4 | E. cloacae IV | 30 (41) | 19 (26) | 50 (68) | 0 |
Prediction of conserved motifs
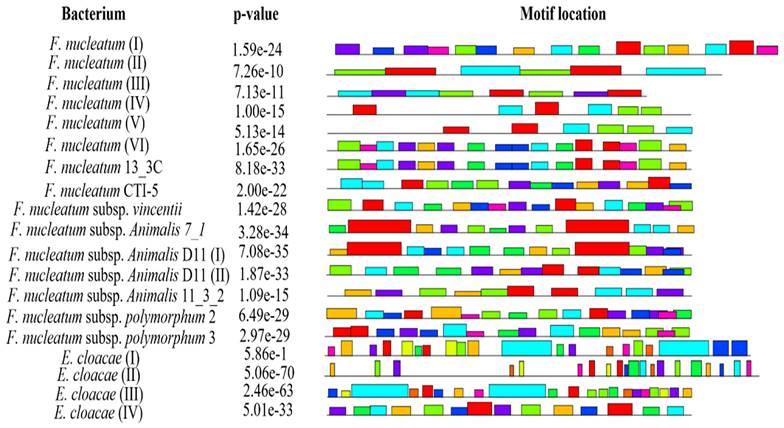
Conserved motif analysis revealed that all the bacteria documented in the present study except F. nucleatum II, F. nucleatum III, F. nucleatum IV and F. nucleatum V exhibited the conserved regions (Figure 3, Supplementary data Table 4).
A-cell epitopes prediction
Epitope assessment revealed that a sufficient number of antigenic peptides were present in FadA protein from healthy individuals' GIT. i.e. E. cloacae I (52), II (40), III (28) and IV (18). However, in most of the bacteria from CRC patients GIT, FadA was found to have very few epitopes like F. nucleatum subsp. polymorphum 2 (1), F. nucleatum subsp. polymorphum 3 (6) and F. nucleatum I (4) or no epitopes i.e. F. nucleatum II and III. Others were found to have epitopes in the range of 12-20 (Supplementary data Table 5).
Pockets and tunnels identified and tunnels parameters of FadA protein of F. nucleatum and E. cloacae
| Tunnels ID | Bacterium | Pocket score | Pocket Volume | Pocket Druggability | Bottle neck radius [Å] | Length [Å] | Curvature | Throughput |
|---|---|---|---|---|---|---|---|---|
| 1 | F. nucleatum I | 100 | 614 | 0.24 | 3.4 | 1.5 | 1.0 | 0.96 |
| 2 | 2.7 | 5.9 | 1.2 | 0.90 | ||||
| 3 | 2.2 | 10.1 | 1.4 | 0.83 | ||||
| 4 | 1.8 | 11.6 | 1.2 | 0.79 | ||||
| 5 | 2.1 | 14.6 | 1.7 | 0.78 | ||||
| 1 | F. nucleatum IV | 100 | 699 | 0.11 | 2.2 | 2.0 | 1.0 | 0.93 |
| 2 | 1.3 | 6.2 | 1.2 | 0.78 | ||||
| 3 | 1.0 | 11.3 | 1.3 | 0.57 | ||||
| 1 | F. nucleatum V | 100 | 470 | 0.03 | 2.4 | 1.5 | 1.0 | 0.95 |
| 2 | 1.1 | 6.4 | 1.3 | 0.67 | ||||
| 3 | 1.2 | 11.2 | 1.3 | 0.64 | ||||
| 4 | 0.9 | 19.1 | 1.3 | 0.37 | ||||
| 1 | F. nucleatum VI | 100 | 913 | 0.44 | 1.9 | 2.0 | 1.0 | 0.93 |
| 2 | 1.1 | 7.8 | 1.1 | 0.65 | ||||
| 3 | 0.9 | 14.7 | 1.7 | 0.41 | ||||
| 4 | 0.9 | 17.1 | 1.6 | 0.38 | ||||
| 1 | F. nucleatum 13_3C | 100 | 769 | 0.06 | 2.1 | 1.4 | 1.0 | 0.95 |
| 2 | 1.0 | 13.2 | 1.3 | 0.43 | ||||
| 3 | 1.0 | 17.3 | 1.5 | 0.43 | ||||
| 1 | F. nucleatum CTI-5 | 100 | 906 | 0.21 | 1.6 | 2.4 | 1.0 | 0.87 |
| 1 | F. nucleatum subsp. vincentii | 100 | 788 | 0.61 | 1.7 | 1.4 | 1.0 | 0.90 |
| 2 | 1.6 | 7.7 | 1.4 | 0.78 | ||||
| 3 | 1.0 | 13.1 | 1.4 | 0.41 | ||||
| 4 | 0.9 | 16.4 | 2.1 | 0.29 | ||||
| 1 | F. nucleatum subsp. animalis 7_1 | 100 | 882 | 0.26 | 2.1 | 2.0 | 1.0 | 0.91 |
| 2 | 1.5 | 3.9 | 1.0 | 0.83 | ||||
| 3 | 1.0 | 12.6 | 1.6 | 0.44 | ||||
| 1 | F. nucleatum subsp. animalis D11 (I) | 100 | 748 | 0.68 | 1.4 | 4.4 | 1.1 | 0.83 |
| 2 | 1.6 | 4.8 | 1.0 | 0.83 | ||||
| 3 | 1.5 | 13.1 | 1.3 | 0.66 | ||||
| 1 | F. nucleatum subsp. animalis D11 (II) | 100 | 598 | 0.38 | 2.2 | 5.2 | 1.6 | 0.91 |
| 2 | 1.4 | 9.2 | 1.6 | 0.82 | ||||
| 3 | 1.2 | 10.6 | 1.5 | 0.74 | ||||
| 4 | 0.9 | 15.5 | 1.5 | 0.40 | ||||
| 1 | F. nucleatum subsp. animalis 11_3_2 | 100 | 664 | 0.10 | 2.3 | 2.5 | 1.0 | 0.94 |
| 1 | F. nucleatum subsp. polymorphum 2 | 100 | 984 | 0.89 | 1.7 | 2.0 | 1.1 | 0.90 |
| 2 | 1.9 | 7.8 | 1.2 | 0.83 | ||||
| 3 | 2.2 | 8.4 | 1.1 | 0.83 | ||||
| 4 | 1.2 | 10.2 | 1.3 | 0.69 | ||||
| 1 | F. nucleatum subsp. polymorphum 3 | 100 | 418 | 0.82 | 2.0 | 2.9 | 1.1 | 0.93 |
| 2 | 1.7 | 8.5 | 1.2 | 0.82 | ||||
| 3 | 1.5 | 11.2 | 1.1 | 0.68 | ||||
| 4 | 1.0 | 14.1 | 1.4 | 0.51 | ||||
| 5 | 0.9 | 12.6 | 1.2 | 0.46 | ||||
| I | E. cloacae I | 100 | 1474 | 0.89 | 2.5 | 1.4 | 1.1 | 0.94 |
| 2 | 1.5 | 7.5 | 1.2 | 0.77 | ||||
| 3 | 0.9 | 8.6 | 1.2 | 0.65 | ||||
| 4 | 0.9 | 13.0 | 1.4 | 0.53 | ||||
| 1 | E. cloacae II | 99 | 1230 | 0.25 | 3.5 | 7.8 | 1.1 | 0.93 |
| 2 | 3.3 | 18.6 | 1.3 | 0.87 | ||||
| 3 | 3.3 | 31.6 | 1.4 | 0.81 | ||||
| 4 | 2.9 | 25.6 | 1.4 | 0.80 | ||||
| 5 | 2.2 | 19.6 | 1.5 | 0.79 | ||||
| 6 | 3.3 | 43.0 | 1.9 | 0.77 | ||||
| 7 | 3.3 | 48.2 | 1.9 | 0.76 | ||||
| 8 | 3.3 | 57.4 | 2.2 | 0.74 | ||||
| 9 | 3.3 | 64.2 | 2.0 | 0.72 | ||||
| 10 | 1.2 | 17.7 | 1.5 | 0.72 | ||||
| 11 | 1.9 | 79.5 | 2.9 | 0.56 | ||||
| 12 | 0.9 | 17.1 | 1.2 | 0.52 | ||||
| 13 | 1.3 | 28.2 | 1.5 | 0.52 | ||||
| 14 | 1.3 | 33.2 | 1.2 | 0.47 | ||||
| 15 | 1.4 | 79.7 | 2.0 | 0.45 | ||||
| 16 | 1.0 | 79.1 | 2.4 | 0.43 | ||||
| 17 | 0.9 | 63.4 | 2.2 | 0.29 | ||||
| 18 | 1.1 | 89.0 | 3.2 | 0.29 | ||||
| 19 | 1.0 | 72.2 | 2.2 | 0.26 | ||||
| 20 | 1.0 | 74.4 | 2.3 | 0.25 | ||||
| 21 | 1.0 | 35.7 | 1.3 | 0.23 | ||||
| 22 | 0.9 | 97.0 | 2.1 | 0.23 | ||||
| 23 | 1.0 | 74.2 | 2.4 | 0.23 | ||||
| 24 | 0.9 | 100.5 | 2.1 | 0.22 | ||||
| 25 | 0.9 | 101.4 | 2.1 | 0.21 | ||||
| 26 | 0.9 | 117.7 | 1.8 | 0.06 | ||||
| 1 | E. cloacae III | 100 | 987 | 0.63 | 2.5 | 1.5 | 1.0 | 0.94 |
| 2 | 2.3 | 8.7 | 1.4 | 0.84 | ||||
| 3 | 1.6 | 8.3 | 1.2 | 0.82 | ||||
| 4 | 1.0 | 6.9 | 1.3 | 0.75 | ||||
| 5 | 1.0 | 5.9 | 1.0 | 0.67 | ||||
| 6 | 1.0 | 19.1 | 1.6 | 0.50 | ||||
| 7 | 0.9 | 13.3 | 1.5 | 0.49 | ||||
| 1 | E. cloacae IV | 100 | 824 | 0.30 | 3.0 | 1.0 | 1.0 | 0.95 |
| 2 | 1.8 | 12.2 | 1.5 | 0.83 | ||||
| 3 | 1.3 | 8.9 | 1.1 | 0.72 | ||||
| 4 | 1.1 | 6.9 | 1.2 | 0.71 |
Energy scores indicating binding affinities of F. nucleatum FadA proteins with reported inhibitor peptide, predicted using HPEPDOCK 2.0 and COX2, predicted using HDOCK server
| # | Bacterium from CRC patients GIT | Energy score (Inhibitory peptide) | Energy score (COX2) |
|---|---|---|---|
| 1 | F. nucleatum I | -168.848 | -263.89 |
| 2 | F. nucleatum II | -136.574 | -204.87 |
| 3 | F. nucleatum III | -147.910 | -224.60 |
| 4 | F. nucleatum IV | -182.402 | -246.10 |
| 5 | F. nucleatum V | -174.946 | -251.38 |
| 6 | F. nucleatum VI | -157.704 | -257.04 |
| 7 | F. nucleatum 13_3C | -209.754 | -242.70 |
| 8 | F. nucleatum CTI-5 | -196.507 | -263.34 |
| 9 | F. nucleatum subsp. vincentii | -188.468 | -224.70 |
| 10 | F. nucleatum subsp. animalis 7_1 | -169.531 | -286.12 |
| 11 | F. nucleatum subsp. animalis D11 (I) | -172.986 | -261.27 |
| 12 | F. nucleatum subsp. animalis D11 (II) | -187.876 | -258.17 |
| 13 | F. nucleatum subsp. animalis 11_3_2 | -168.314 | -263.60 |
| 14 | F. nucleatum subsp. polymorphum 2 | -185.352 | -231.14 |
| 15 | F. nucleatum subsp. polymorphum 3 | -154.926 | -242.45 |
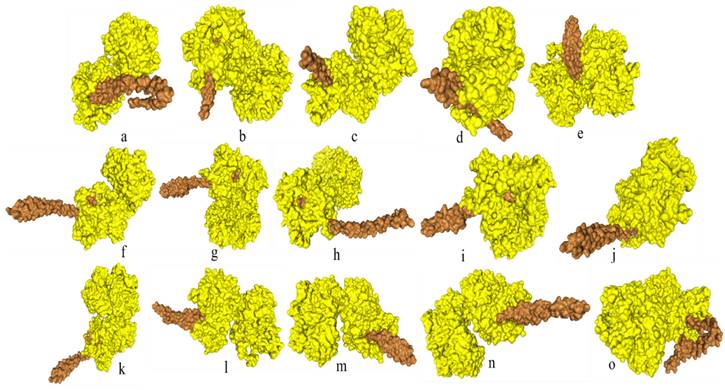
Docking of FadA protein inhibitor peptide
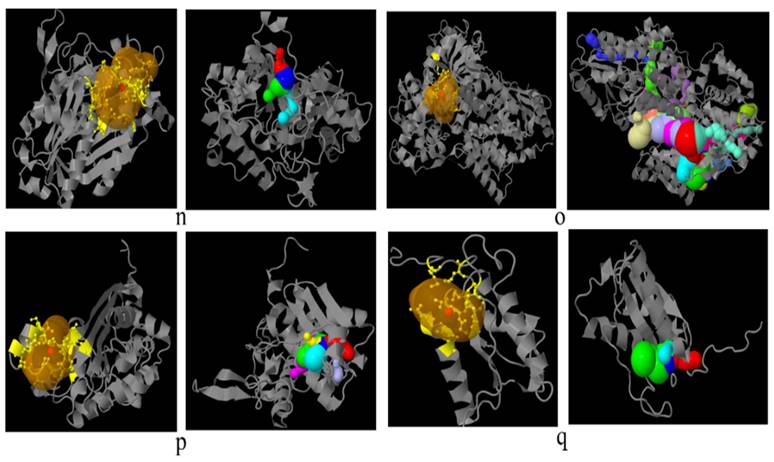
Docking analysis of F. nucleatum FadA proteins with inhibitor peptide showed that all the proteins from virulent bacteria tend to bind with this peptide. Good binding affinity is reflected by the docking energy scores ranging from -136.574 for F. nucleatum II to -209.754 for F. nucleatum 13_3C (Figure 4, Table 6).
Docking of FadA protein with COX2
Docking analysis of F. nucleatum FadA protein with FDA-approved anti-inflammatory drug COX2 revealed a high binding affinity of FadA with this drug with good energy scores ranging from -204.87 to -286.12 (Figure 5).
Discussion
In present study, FadA protein is compared among the two groups of GIT bacteria. In one group (F. nucleatum), this protein causes pathogenicity while in other (E. cloacae) it is non-virulent. F. nucleatum is reported to occur abundantly in gut of CRC patients. Present research analyzed the distinguishing characteristics of FadA which might contribute to its virulent character in F. nucleatum.
FadA has been found virulent in all the strains of F. nucleatum documented in the present study as compared to that of E. cloacae which is consistent to previous findings [21, 22].
According to the present study, E. cloacae-associated FadA protein is localized in the cytoplasm while in six strains of F. nucleatum, the localization is different. Marked deviation in half-life was observed in F. nucleatum II-IV i.e. 2 min. as compared to > 10 hours in other cases. In cases of E. cloacae, an instability index below 40 suggests their poor in-vitro stability as compared to F. nucleatum [60]. An aliphatic index is a measure of protein thermostability [61]. In the present work, FadA in the bacteria from GIT of both the normal and diseased individuals was found to be thermostable. GRAVY values indicated the hydrophilic nature of F. nucleatum FadA as compared to most of the E. cloacae which were hydrophobic with GRAVY score > 0 [62]. In most cases of F. nucleatum, the pI of the FadA virulence factor was observed strongly acidic as compared to slightly acidic pI in cases of E. cloacae-associated FadA.
Prediction of catalytic pockets and tunnels using CAVER Web tool in bacteria documented in present study. a: F. nucleatum (I), b: F. nucleatum (IV), c: F. nucleatum (V), d: F. nucleatum (VI), e: F. nucleatum 13_3C, f: F. nucleatum CTI-5, g: F. nucleatum subsp. Vincentii, h: F. nucleatum subsp. animalis 7_1, i: F. nucleatum subsp. animalis D11 (I), j: F. nucleatum subsp. animalis D11 (II), k: F. nucleatum subsp. animalis 11_3_2, l: F. nucleatum subsp. polymorphum 1, m: F. nucleatum subsp. polymorphum 1, n: Enterobacter cloacae (I), o: Enterobacter cloacae (II), p: Enterobacter cloacae (III), q: Enterobacter cloacae (IV), yellow part: catalytic pocket, multiple colors region = multiple tunnels.
Location of conserved motifs with corresponding combined match p-value for FadA protein of F. nucleatum and E. cloacae, predicted using MEME suite. Each colored block is depicting strength and position of each motif site. Height of block is directly proportional to significance of predicted site. Different motif sites are represented using different colors.
Docking of FadA virulence factor from different strains of F. nucleatum with inhibitory peptide ASANWTIQYND reported in literature. FadA protein and inhibitory peptide are represented in brown and yellow colors, respectively. a: F. nucleatum (I), b: F. nucleatum (II), c: F. nucleatum (III), d: F. nucleatum (IV), e: F. nucleatum (V), f: F. nucleatum (VI), g: F. nucleatum 13_3C, h: F. nucleatum CTI-5, i: F. nucleatum subsp. Vincentii, j: F. nucleatum subsp. animalis 7_1, k: F. nucleatum subsp. animalis D11 (I), l: F. nucleatum subsp. animalis D11 (II), m: F. nucleatum subsp. animalis 11_3_2, n: F. nucleatum subsp. polymorphum 1, o: F. nucleatum subsp. polymorphum 1.
Docking of FadA virulence factor from different strains of F. nucleatum with COX2 anti-inflammatory protein FadA and Cox2 are represented in brown and yellow colors, respectively. a: F. nucleatum (I), b: F. nucleatum (II), c: F. nucleatum (III), d: F. nucleatum (IV), e: F. nucleatum (V), f: F. nucleatum (VI), g: F. nucleatum 13_3C, h: F. nucleatum CTI-5, i: F. nucleatum subsp. Vincentii, j: F. nucleatum subsp. animalis 7_1, k: F. nucleatum subsp. animalis D11 (I), l: F. nucleatum subsp. animalis D11 (II), m: F. nucleatum subsp. animalis 11_3_2, n: F. nucleatum subsp. polymorphum 1, o: F. nucleatum subsp. polymorphum 1.
In the present study, healthy individuals associated with bacterial FadA protein have been found to comprise NAD(P)-binding Rossmann-fold and (TIM)-barrel domains. On the other hand, CRC patients' associated bacteria FadA factors were found to consist of four helical up and down bundles, EF-hand and DNA-binding 3-helical bundle. This finding of the helical domain was consistent with literature where the FadA protein monomer of F. nucleatum has been reported as alpha-helical which gives rise to a hair-pin-like structure [63].
As far as the 2D configuration is concerned, no beta-sheet content, large number of helix residues and low coil content was observed in F. nucleatum FadA protein as compared to that of E. cloacae. Eight strains of F. nucleatum versus only one strain of E. cloacae were found to contain signal peptides. The length of signal peptides ranged between 17-20 residues in F. nucleatum protein. Signal peptide comprising eighteen amino acids i.e. MKKFLLLAVLAVSASAFA has been reported in the FadA protein [64].
Differences between 3D configurations of FadA proteins of two groups of bacteria documented in the present study were understandable. F. nucleatum FadA contained only a helical configuration, while from E. cloacae the globular structure was observed.
The number of tunnels was comparable between FadA proteins of two types of bacteria in the present study, however, only E. cloacae (II) exhibited twenty-six tunnels. Different parameters of catalytic pockets were also analyzed. i. e. druggability, length and bottleneck radius. The binding tendency of a catalytic site of protein for a drug is referred to as druggability. FadA in case of F. nucleatum subsp. polymorphum 2, F. nucleatum subsp. polymorphum 3 and E. cloacae (I), were found to a good therapeutic target drugs with druggability scores closer to 1 [65]. In all other cases, the FadA was not found to be a druggable protein. Tunnel length and curvature reflected the substrate specificity of the protein. Parameters of tunnel and curvature might be used to reduce FadA activity through the inhibition of its substrate binding and catalysis.
Being virulent, FadA from F. nucleatum can be the most appropriate vaccine target against this pathogenic bacterium. Identification of antigenic epitopes could be proven helpful in this regard. In the present study, A-cell epitopes were found to be higher in number in E. cloacae i.e. ranging from 18-52 as compared to F. nucleatum i.e. 0-20. No epitopes were observed in F. nucleatum II and III. Overall, the presence of a variety of epitopes in cases of F. nucleatum VI, F. nucleatum subsp. Vincentii, F. nucleatum subsp. animalis 7_1, F. nucleatum subsp. animalis D11 (I) AND (II) and F. nucleatum subsp. animalis 11_3_2 is consistent with earlier literature reporting antigenic heterogeneity in different strains of F. nucleatum [66]. A protein containing a large number of A-cell epitopes is capable of stimulating the immune system in the host. So, FadA in E. cloacae I, II and III with antigenic peptides 52, 40 and 28, respectively might boost the immune system. The epitopes predicted in virulent FadA from F. nucleatum strains might also be used for designing peptide-based vaccine adjuvants after getting insight into their antigenic potential [57].
According to the literature, the FadA gene is highly conserved not only among different strains of F. nucleatum but also among other oral species of Fusobacterium. Hence, our findings are in agreements with previous reports [64, 67, 69].
Inhibitory peptides might be the promising candidates as immunotherapeutic agents. In the present study, FadA protein from F. nucleatum has been docked with an already reported peptide comprising of eleven amino acid residues i.e. ASANWTIQYND. This peptide has been derived from the EC5 protein and is designated as the inhibitory one [8]. docking analysis revealed the strong binding affinity of FadA proteins with this peptide. Keeping in view, the involvement of FadA protein in inflammation, this protein has been evaluated for binding tendency with an FDA-approved anti-inflammatory drug COX2 [68]. The docking analysis results with energy scores of -224.60 to -286.12 reflected the high binding affinity of FadA virulent factor with COX2. Hence, this drug might be used for successful inhibition of F. nucleatum-associated FadA protein.
This study is limited to in-silico comparison of FadA protein among the healthy and CRC patients gut bacteria. Its findings are significant because they highlighted the pathogenicity associated properties of protein in F. nucleatum. However, validity of these findings should be confirmed in future via experimental studies. For this purpose, fecal samples of healthy and CRC patients will be collected and used for isolation of gut associated bacteria [70]. Bacteria will be targeted for FadA protein extraction and analysis.
Conclusion
Wide range of differences observed in FadA protein not only justifies the virulence associated with F. nucleatum-derived FadA but also suggests multiple strategies to inhibit the oncogenic potential of this protein. Present study revealed that due to the high instability index, F. nucleatum FadA protein tends to remain stable in-vitro, so these proteins can be easily studied and mutated in the laboratory. Tunnels and different attributes of catalytic sites explored might be used to design the drugs targeting FadA virulence factors. Antigenic peptides of virulent FadA proteins might be targeted for peptide-based vaccine designing. Divergence of 2D and 3D structure between virulent and non-virulent FadA protein might help to inactivate the virulency form of this protein through site-directed mutation induction leading to configuration alterations in active regions.
Acknowledgements
The authors extend their appreciation to the Researchers Supporting Project number (RSPD2024R729), King Saud University, Riyadh Saudi Arabia for funding this project.
Funding statement
The authors are grateful to the Researchers Supporting Project number (RSPD2024R729), King Saud University, Riyadh Saudi Arabia for funding this project. The funding body had no role in the study design.
Availability of data and materials
The sequences of FadA protein documented in present study are available at (https://www.uniprot.org) database.
Authors contributions
All authors substantially contributed to the study. NH, FM, HS, SR, TA, and NMA designed the study. NH, FM, HS and SR collected the prime data for the study and FM and MSM analyzed it. FMH, NMA, TA, NH, and HS wrote the first draft of the manuscript. All authors analyzed the results and proofread the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Paschke S, Jafarov S, Staib L, Kreuser ED, Maulbecker-Armstrong C, Roitman M. et al. Are colon and rectal cancer two different tumor entities?. A proposal to abandon the term colorectal cancer. International journal of molecular sciences. 2018;19:2577
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2018;68:394-424
3. Wong MCS, Huang J, Lok V, Wang J, Fung F, Ding H. et al. Differences in incidence and mortality trends of colorectal cancer worldwide based on sex, age, and anatomic location. Clinical Gastroenterology and Hepatology. 2021;19:955-66 e61
4. Sobhani I, Tap J, Roudot-Thoraval F, Roperch JP, Letulle S, Langella P. et al. Microbial dysbiosis in colorectal cancer (CRC) patients. PloS one. 2011;6:e16393
5. Zhang S, Kong C, Yang Y, Cai S, Li X, Cai G. et al. Human oral microbiome dysbiosis as a novel non-invasive biomarker in detection of colorectal cancer. Theranostics. 2020;10:11595
6. Kalasabail S, Engelman J, Zhang LY, El-Omar E, Yim HCH. A Perspective on the Role of Microbiome for Colorectal Cancer Treatment. Cancers. 2021;13:4623
7. Hernández-Luna MA, Lopez-Briones S, Luria-Pérez R. The four horsemen in colon cancer. Journal of Oncology. 2019;2019:5636272
8. Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell host & microbe. 2013;14:195-206
9. Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M. et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell host & microbe. 2013;14:207-15
10. Marchesi JR, Dutilh BE, Hall N, Peters WHM, Roelofs R, Boleij A. et al. Towards the human colorectal cancer microbiome. PloS one. 2011;6:e20447
11. Shah MS, DeSantis TZ, Weinmaier T, McMurdie PJ, Cope JL, Altrichter A. et al. Leveraging sequence-based faecal microbial community survey data to identify a composite biomarker for colorectal cancer. Gut. 2018;67:882-91
12. Richard ML, Liguori G, Lamas B, Brandi G, Da Costa G, Hoffmann TW. et al. Mucosa-associated microbiota dysbiosis in colitis associated cancer. Gut microbes. 2018;9:131-42
13. Wang T, Cai G, Qiu Y, Fei N, Zhang M, Pang X. et al. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. The ISME journal. 2012;6:320-9
14. Yu J, Feng Q, Wong SH, Zhang D, yi Liang Q, Qin Y. et al. Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut. 2017;66:70-8
15. Zhu Q, Jin Z, Wu W, Gao R, Guo B, Gao Z. et al. Analysis of the intestinal lumen microbiota in an animal model of colorectal cancer. PloS one. 2014;9:e90849
16. Chen W, Liu F, Ling Z, Tong X, Xiang C. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PloS one. 2012;7:e39743
17. Meehan CJ, Beiko RG. A phylogenomic view of ecological specialization in the Lachnospiraceae, a family of digestive tract-associated bacteria. Genome biology and evolution. 2014;6:703-13
18. Wu CH, Apweiler R, Bairoch A, Natale DA, Barker WC, Boeckmann B. et al. The Universal Protein Resource (UniProt): an expanding universe of protein information. Nucleic acids research. 2006;34:D187-D91
19. Dai Z, Coker OO, Nakatsu G, Wu WKK, Zhao L, Chen Z. et al. Multi-cohort analysis of colorectal cancer metagenome identified altered bacteria across populations and universal bacterial markers. Microbiome. 2018;6:1-12
20. Candela M, Guidotti M, Fabbri A, Brigidi P, Franceschi C, Fiorentini C. Human intestinal microbiota: cross-talk with the host and its potential role in colorectal cancer. Critical reviews in microbiology. 2011;37:1-14
21. Keller R, Pedroso MZ, Ritchmann R, Silva RM. Occurrence of virulence-associated properties in Enterobacter cloacae. Infection and immunity. 1998;66:645-9
22. Han YW. Fusobacterium nucleatum: a commensal-turned pathogen. Current opinion in microbiology. 2015;23:141-7
23. Sun CH, Li BB, Wang B, Zhao J, Zhang XY, Li TT. et al. The role of Fusobacterium nucleatum in colorectal cancer: from carcinogenesis to clinical management. Chronic diseases and translational medicine. 2019;5:178-87
24. He Z, Tian W, Wei Q, Xu J. Involvement of Fusobacterium nucleatum in malignancies except for colorectal cancer: A literature review. Frontiers in Immunology. 2022;13:968649
25. Rafique A, Koh YS. Association of Fusobacterium nucleatum in the Progression of Colorectal Cancer. Journal of Bacteriology and Virology. 2021;51:39-53
26. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. science. 2009;324:1029-33
27. Kapatral V, Anderson I, Ivanova N, Reznik G, Los T, Lykidis A. et al. Genome sequence and analysis of the oral bacterium Fusobacterium nucleatum strain ATCC 25586. Journal of bacteriology. 2002;184:2005-18
28. Guo S, Chen J, Chen F, Zeng Q, Liu WL, Zhang G. Exosomes derived from Fusobacterium nucleatum-infected colorectal cancer cells facilitate tumour metastasis by selectively carrying miR-1246/92b-3p/27a-3p and CXCL16. Gut. 2021;70:1507-19
29. Feng Y, Zeng D, Tong Y, Lu X, Dun G, Tang B. et al. Alteration of microRNA-4474/4717 expression and CREB-binding protein in human colorectal cancer tissues infected with Fusobacterium nucleatum. PLoS One. 2019;14:e0215088
30. Borowsky J, Haruki K, Lau MC, Dias Costa A, Väyrynen JP, Ugai T. et al. Association of Fusobacterium nucleatum with specific T-cell subsets in the colorectal carcinoma microenvironment. Clinical Cancer Research. 2021;27:2816-26
31. Yoshida Y, Suwabe K, Nagano K, Kezuka Y, Kato H, Yoshimura F. Identification and enzymic analysis of a novel protein associated with production of hydrogen sulfide and L-serine from L-cysteine in Fusobacterium nucleatum subsp. nucleatum ATCC 25586. Microbiology. 2011;157:2164-71
32. Hilmi M, Neuzillet C, Lefèvre JH, Svrcek M, Vacher S, Benhaim L. et al. Prognostic Value of Fusobacterium nucleatum after Abdominoperineal Resection for Anal Squamous Cell Carcinoma. Cancers. 2022;14:1606
33. Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, Strauss J. et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome research. 2012;22:299-306
34. Flanagan L, Schmid J, Ebert M, Soucek P, Kunicka T, Liska V. et al. Fusobacterium nucleatum associates with stages of colorectal neoplasia development, colorectal cancer and disease outcome. European journal of clinical microbiology & infectious diseases. 2014;33:1381-90
35. Ito M, Kanno S, Nosho K, Sukawa Y, Mitsuhashi K, Kurihara H. et al. Association of Fusobacterium nucleatum with clinical and molecular features in colorectal serrated pathway. International journal of cancer. 2015;137:1258-68
36. Fukugaiti MH, Ignacio A, Fernandes MR, Ribeiro Júnior U, Nakano V, Avila-Campos MJ. High occurrence of Fusobacterium nucleatum and Clostridium difficile in the intestinal microbiota of colorectal carcinoma patients. Brazilian Journal of Microbiology. 2015;46:1135-40
37. Li YY, Ge QX, Cao J, Zhou YJ, Du YL, Shen B. et al. Association of Fusobacterium nucleatum infection with colorectal cancer in Chinese patients. World journal of gastroenterology. 2016;22:3227
38. Wong SH, Kwong TNY, Chow TC, Luk AKC, Dai RZW, Nakatsu G. et al. Quantitation of faecal Fusobacterium improves faecal immunochemical test in detecting advanced colorectal neoplasia. Gut. 2017;66:1441-8
39. Suehiro Y, Sakai K, Nishioka M, Hashimoto S, Takami T, Higaki S. et al. Highly sensitive stool DNA testing of Fusobacterium nucleatum as a marker for detection of colorectal tumours in a Japanese population. Annals of clinical biochemistry. 2017;54:86-91
40. Guo S, Li L, Xu B, Li M, Zeng Q, Xiao H. et al. A simple and novel fecal biomarker for colorectal cancer: ratio of Fusobacterium nucleatum to probiotics populations, based on their antagonistic effect. Clinical chemistry. 2018;64:1327-37
41. Proença MA, Biselli JM, Succi M, Severino FE, Berardinelli GN, Caetano A. et al. Relationship between Fusobacterium nucleatum, inflammatory mediators and microRNAs in colorectal carcinogenesis. World journal of gastroenterology. 2018;24:5351
42. Tunsjø HS, Gundersen G, Rangnes F, Noone JC, Endres A, Bemanian V. Detection of Fusobacterium nucleatum in stool and colonic tissues from Norwegian colorectal cancer patients. European Journal of Clinical Microbiology & Infectious Diseases. 2019;38:1367-76
43. Queen J, Domingue JC, White JR, Stevens C, Udayasuryan B, Nguyen TTD. et al. Comparative Analysis of Colon Cancer-Derived Fusobacterium nucleatum Subspecies: Inflammation and Colon Tumorigenesis in Murine Models. Mbio. 2022;13:e02991-21
44. Fardini Y, Wang X, Témoin S, Nithianantham S, Lee D, Shoham M. et al. Fusobacterium nucleatum adhesin FadA binds vascular endothelial cadherin and alters endothelial integrity. Molecular microbiology. 2011;82:1468-80
45. Garg A, Gupta D. VirulentPred: a SVM based prediction method for virulent proteins in bacterial pathogens. BMC bioinformatics. 2008;9:1-12
46. Sievers F, Higgins DG. The clustal omega multiple alignment package. Methods Mol Biol. 2021;2231:3-16
47. Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular biology and evolution. 2016;33:1870-4
48. Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular biology and evolution. 1987;4:406-25
49. Zuckerkandl E, Pauling L. Evolutionary divergence and convergence in proteins. Evolving genes and proteins: Elsevier. 1965 p. 97-166
50. Bhasin M, Garg A, Raghava GPS. PSLpred: prediction of subcellular localization of bacterial proteins. Bioinformatics. 2005;21:2522-4
51. Gasteiger E, Hoogland C, Gattiker A, Wilkins MR, Appel RD, Bairoch A. Protein identification and analysis tools on the ExPASy server. Methods Mol Biol. 1999;112:531-52
52. Shen HB, Chou KC. Predicting protein fold pattern with functional domain and sequential evolution information. Journal of Theoretical Biology. 2009;256:441-6
53. Montgomerie S, Cruz JA, Shrivastava S, Arndt D, Berjanskii M, Wishart DS. PROTEUS2: a web server for comprehensive protein structure prediction and structure-based annotation. Nucleic acids research. 2008;36:W202-W9
54. Montgomerie S, Sundararaj S, Gallin WJ, Wishart DS. Improving the accuracy of protein secondary structure prediction using structural alignment. BMC bioinformatics. 2006;7:1-13
55. Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R. et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic acids research. 2018;46:W296-W303
56. Stourac J, Vavra O, Kokkonen P, Filipovic J, Pinto G, Brezovsky J. et al. Caver Web 1.0: identification of tunnels and channels in proteins and analysis of ligand transport. Nucleic acids research. 2019;47:W414-W22
57. Nagpal G, Chaudhary K, Agrawal P, Raghava GPS. Computer-aided prediction of antigen presenting cell modulators for designing peptide-based vaccine adjuvants. Journal of translational medicine. 2018;16:1-15
58. Zhou P, Jin B, Li H, Huang SY. HPEPDOCK: a web server for blind peptide-protein docking based on a hierarchical algorithm. Nucleic acids research. 2018;46:W443-W50
59. Yan Y, Tao H, He J, Huang SY. The HDOCK server for integrated protein-protein docking. Nature protocols. 2020;15:1829-52
60. Gamage DG, Gunaratne A, Periyannan GR, Russell TG. Applicability of instability index for in vitro protein stability prediction. Protein and Peptide Letters. 2019;26:339-47
61. Ikai A. Thermostability and aliphatic index of globular proteins. The Journal of Biochemistry. 1980;88:1895-8
62. Magdeldin S, Yoshida Y, Li H, Maeda Y, Yokoyama M, Enany S. et al. Murine colon proteome and characterization of the protein pathways. BioData mining. 2012;5:1-14
63. Nithianantham S, Xu M, Yamada M, Ikegami A, Shoham M, Han YW. Crystal structure of FadA adhesin from Fusobacterium nucleatum reveals a novel oligomerization motif, the leucine chain. Journal of Biological Chemistry. 2009;284:3865-72
64. Han YW, Ikegami A, Rajanna C, Kawsar HI, Zhou Y, Li M. et al. Identification and characterization of a novel adhesin unique to oral fusobacteria. Journal of bacteriology. 2005;187:5330-40
65. Alzyoud L, Bryce RA, Al Sorkhy M, Atatreh N, Ghattas MA. Structure-based assessment and druggability classification of protein-protein interaction sites. Scientific reports. 2022;12:1-18
66. Bashir A, Miskeen AY, Bhat A, Fazili KM, Ganai BA. Fusobacterium nucleatum. European Journal of Cancer Prevention. 2015;24:373-85
67. Xu M. et al. FadA from Fusobacterium nucleatum utilizes both secreted and nonsecreted forms for functional oligomerization for attachment and invasion of host cells. Journal of Biological Chemistry. 2007;282(34):25000-25009
68. Huang S. et al. Rapid detection of nusG and fadA in Fusobacterium nucleatum by loop-mediated isothermal amplification. Journal of Medical Microbiology. 2016;65(8):760-769
69. Chen Y. et al. More than just a periodontal pathogen-the research progress on Fusobacterium nucleatum. Frontiers in Cellular and Infection Microbiology. 2022;12:815318
70. Li ZM. et al. Effects of stool sample preservation methods on gut microbiota biodiversity: New original data and systematic review with meta-analysis. Clinical Microbiology. 2023;11(3):e0429722
Author contact
![]() Corresponding author: Dr. Suhail Razak, Department of Community Health Sciences, College of Applied Medical Sciences, King Saud University, Riyadh, Saudi Arabia; Email: Smaraziedu.sa; Phone: +966582759500. Dr. Fatima Muccee, School of Biochemistry and Biotechnology, University of Punjab, Lahore, 52254, Pakistan; E-mail: fatima.sbbedu.pk; Phone: +923314767254.
Corresponding author: Dr. Suhail Razak, Department of Community Health Sciences, College of Applied Medical Sciences, King Saud University, Riyadh, Saudi Arabia; Email: Smaraziedu.sa; Phone: +966582759500. Dr. Fatima Muccee, School of Biochemistry and Biotechnology, University of Punjab, Lahore, 52254, Pakistan; E-mail: fatima.sbbedu.pk; Phone: +923314767254.

 Global reach, higher impact
Global reach, higher impact