Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(19):6196-6203. doi:10.7150/jca.101165 This issue Cite
Research Paper
The lower incidence of cervical cancer in type 2 diabetes mellitus with sodium-glucose cotransporter 2 inhibitors utilization
1. Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan.
2. Division of Cardiology, Department of Internal Medicine, Camillian Saint Mary's Hospital Luodong, Luodong, Yilan, Taiwan.
3. Center for General Education, Chung Shan Medical University, Taichung, Taiwan.
4. School of Medicine, Chung Shan Medical University, Taichung, Taiwan.
5. Division of Cardiology, Department of Internal Medicine, Chung Shan Medical University Hospital, Taichung, Taiwan.
6. Department of Medical Research, Chung Shan Medical University Hospital, Taichung, Taiwan.
7. Department of Ophthalmology, Nobel Eye Institute, Taipei, Taiwan.
8. Whole-Genome Research Core Laboratory of Human Diseases, Chang Gung Memorial Hospital, Keelung, Taiwan.
9. Department of Medical Biotechnology and Laboratory Science, College of Medicine, Chang Gung University, Taoyuan, Taiwan.
Received 2024-7-19; Accepted 2024-10-5; Published 2024-10-14
Abstract
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are medications with anti-inflammatory effects used to treat type 2 diabetes mellitus (T2DM). Cervical cancer is the most common gynecological cancer and is characterized by elevated inflammatory status. Accordingly, this study aimed to investigate the potential association between SGLT2 inhibitor use and cervical cancer development. In this retrospective cohort study, female patients with T2DM were divided into 2 groups: SGLT2 inhibitor users and a control group of non-SGLT2 inhibitor users. After propensity score matching, the SGLT2 inhibitor group and control group each had 136 212 patients. Cox proportional hazards regression was conducted to obtain the adjusted hazard ratio (aHR) and 95% confidence interval (CI) for cervical cancer between the 2 groups. Overall, 148 and 191 cases of cervical cancer were identified in the SGLT2 inhibitor and control groups, respectively. The incidence of cervical cancer was significantly lower in the SGLT2 inhibitor group than in the control group (aHR, 0.77; 95% CI, 0.62-0.96, P = 0.0179). In a subgroup analysis stratified by type of oral medication, the effect of SGLT2 inhibitors on cervical cancer development exhibited a significant difference compared with a biguanide group (aHR, 0.77; 95% CI, 0.63-0.95) and a sulfonylurea group (aHR, 0.69; 95% CI, 0.50-0.94) groups. In conclusion, the use of SGLT2 inhibitors in patients with T2DM is associated with reduced risk of cervical cancer development.
Keywords: SGLT2 inhibitors, epidemiology, cervical cancer, age, type 2 diabetes mellitus
Introduction
Type 2 diabetes mellitus (T2DM) is a common metabolic disease characterized by increased blood glucose levels [1]. The primary pathophysiology for persistent hyperglycemia and subsequent T2DM involves the endogenous resistance of body cells to insulin stimulation [2]. Treating T2DM includes the use of oral medications such as alpha-glucosidase inhibitors and biguanides, with insulin injections indicated for severe T2DM cases [3]. In recent years, sodium-glucose cotransporter 2 (SGLT2) inhibitors have been widely used for T2DM management, reducing hyperglycemia by 0.5% to 1.0% [4, 5].
In addition to their anti-hyperglycemic function, SGLT2 inhibitors also protect other organs, as reported in previous publications [4, 6]. Administration of SGLT2 inhibitors has been demonstrated to have neuroprotective effects and may be applied to treat cognitive impairment [7]. The rate of myocardial infarction is considerably lower in T2DM individuals using SGLT2 inhibitors than in nonusers [8]. Additionally, SGLT2 inhibitors have demonstrated protective effects on the kidneys, improving glomerular filtration rates [9]. Regarding eye health, SGLT2 inhibitor use can reduce the risks of dry eye disease and diabetic retinopathy development [10-12].
Cervical cancer, a major cause of cancer-related mortality, primarily originates at the junction of the uterus and vagina [13-15]. Known risk factors for cervical cancer include early age at first sexual intercourse, smoking, and previous human papillomavirus infection [16-18]. However, few studies have evaluated the relationship between cervical cancer and SGLT2 inhibitor use in the T2DM population. Given that SGLT2 inhibitors can suppress inflammation, a pathophysiology of cervical cancer [19, 20], they may have a protective effect against cervical cancer formation.
This study investigated a potential correlation between SGLT2 inhibitor use and the incidence of cervical cancer. Data from Taiwan's National Health Insurance Research Database (NHIRD) were used for our analysis.
Materials and Methods
Data source
Our study adhered to the Declaration of Helsinki of 1964 and its subsequent amendments. In addition, this study was approved by the National Health Insurance Administration of Taiwan and the Institutional Review Board of Chung Shan Medical University Hospital (project code: CS1-20113). The requirement for written informed consent was waived by both institutions. Taiwan's NHIRD contains the claimed medical records of more than 23 million Taiwanese individuals for the period from January 1, 2000, to December 31, 2020. The data preserved in Taiwan's NHIRD are stratified by International Classification of Diseases-Ninth Revision (ICD-9) and International Classification of Diseases-Tenth Revision (ICD-10) diagnostic codes, age, sex, medical department codes, urbanization level, educational level, image examination codes, laboratory examination codes, procedure codes, and international ATC codes for medicine.
Patient selection
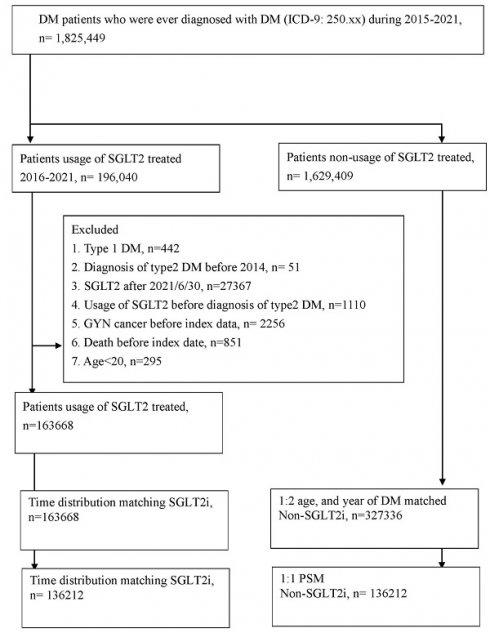
This study was a retrospective cohort study. Patients with T2DM using SGLT2 inhibitors were defined on the basis of the following criteria: (1) the presence of ICD-9/ICD-10 codes indicating a T2DM diagnosis made between 2015 and 2021, (2) visits to an internal medicine doctor for more than 3 months, (3) the arrangement of glycated hemoglobin exams before T2DM diagnosis, and (4) the use of SGLT2 inhibitors such as dapagliflozin, canagliflozin, empagliflozin, or ertugliflozin as determined by ATC codes. The index date of our study was 6 months after the start of SGLT2 inhibitor use. The following exclusion criteria were applied to standardize our study population: (1) missing demographic data, (2) anti-hyperglycemic medicine use before T2DM diagnosis, (3) being aged below 20 years or above 100 years, and (4) the presence of gynecological cancers before the index date. Each patient with T2DM using SGLT2 inhibitors was matched to 2 T2DM participants who did not use SGLT2 inhibitors, who served as the control group. Propensity score matching (PSM) was applied to match the SGLT2 inhibitor group and the control group on the basis of demographics, comorbidities, and T2DM-related medication use. Consequently, 136 212 individuals were enrolled in the SGLT2 inhibitor group, and another 136 212 were enrolled in the control group. A flowchart illustrating the patient selection process is depicted in Figure 1.
Primary outcome
The primary outcome of our study was the occurrence of cervical cancer as defined by the following criteria: (1) cervical cancer diagnosis based on ICD-9/ICD-10 diagnostic codes, (2) presence of pelvic exam procedure codes before or on the same day as the cervical cancer diagnosis, (3) occurrence of procedure codes indicating computed tomography or pelvic ultrasound exam before or on the same day as the cervical cancer diagnosis, and (4) diagnosis of cervical cancer by gynecologists.
Covariate enrollment
Several covariates were considered in the present statistical analysis to adjust for their potential effects on the development of cervical cancer. These covariates were age, urbanization, hypertension, coronary heart disease, ischemic stroke, hyperlipidemia, hemorrhagic stroke, peripheral vascular disease, human papillomavirus infection, chronic kidney disease, diabetic retinopathy, and diabetic neuropathy, as diagnosed on the basis of ICD-9 or ICD-10 diagnostic codes. The number of comorbidities was integrated into the adjusted Diabetes Complications Severity Index (aDCSI) to assess T2DM severity. The use of various T2DM medications (as determined on the basis of ATC codes)—including alpha-glucosidase inhibitors, biguanides, sulfonylureas, thiazolidinediones, dipeptidyl peptidase-4 (DPP4) inhibitors, statins, and insulin—were included in the analysis. To ensure that the comorbidity and medication durations in the T2DM population were sufficiently long to influence cervical cancer risk, only comorbidities and medications present for more than 2 years before the index date in the NHIRD were considered in our analysis.
The flowchart of participant selection. NHIRD: National Health Insurance Research Database, N: number, PSM: propensity score-matching, SGLT2: sodium-glucose cotransporter 2.
Statistical analysis
Statistical analyses were conducted using SAS Statistics version 9.4 (SAS Institute, Cary, NC, USA). Descriptive analyses were employed to obtain the demographics, comorbidities, and T2DM medication distributions of the SGLT2 inhibitor and control groups. Absolute standardized differences (ASDs) were calculated to compare the parameters of the 2 groups, with an ASD value greater than 0.1 indicating a significant difference in our study. Cox proportional hazards regression was then performed to obtain adjusted hazard ratios (aHRs) with 95% confidence intervals (CIs) for cervical cancer incidence in the SGLT2 inhibitor group and the control group. This analysis was adjusted for the influence of demographics, comorbidities, and T2DM medication use. Statistical significance was set at P < 0.05.
Results
Table 1 presents a comparison of basic features between the SGLT2 inhibitor group and the control group. The age distributions of the 2 groups were statistically identical (ASD < 0.1). Regarding comorbidities, the distributions of aDCSI scores were not significantly different between the 2 groups, likely because of the PSM procedure. Additionally, the rates of T2DM-related medication use—including use of sulfonylureas, alpha-glucosidase inhibitors, thiazolidinediones, DPP4 inhibitors, and insulin—were similar between the SGLT2 inhibitor group and the control group (all ASDs < 0.1; Table 1).
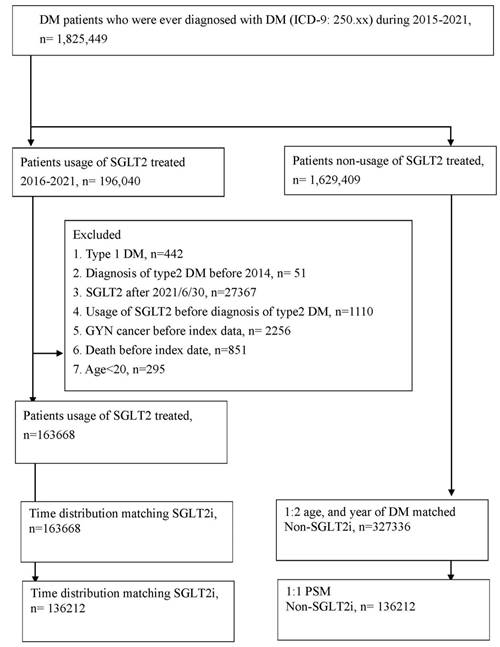
After the follow-up period, 172 and 443 cervical cancer events were reported in the SGLT2 inhibitor group and control group, respectively, after 2:1 sex and age matching (aHR, 0.77; 95% CI, 0.63-0.93, P = 0.0081; Table 2). Through PSM, 148 and 191 cervical cancer episodes were revealed to have occurred in the SGLT2 inhibitor group and control group, respectively (Table 2). In the multivariable analysis, which adjusted for all covariates, the incidence of cervical cancer was significantly lower in the SGLT2 inhibitor group than in the control group (aHR, 0.77; 95% CI, 0.62-0.96, P = 0.0179; Tables 2 and 3). The cumulative probability of cervical cancer was lower in the SGLT2 inhibitor group than in the control group after both 2:1 sex and age matching (P = 0.0020) and PSM (P = 0.0109) (Figure 2). In the subgroup analysis stratified by oral medication, 159 and 264 events of cervical cancer were identified in the SGLT2 inhibitor group and the biguanide group, respectively (Table 4). The incidence of cervical cancer was significantly lower in the SGLT2 inhibitor group than in the biguanide group (aHR, 0.77; 95% CI, 0.63-0.95; Table 4). Furthermore, the effect of SGLT2 inhibitors on cervical cancer development exhibited a significant difference when compared with the sulfonylurea group (aHR, 0.69; 95% CI, 0.50-0.94; Table 4).
Baseline characteristics of the features in Non-SGLT2 inhibitors user and SGLT-2 inhibitor user.
| 2:1 sex age matching | After PSM | |||||
|---|---|---|---|---|---|---|
| Non- SGLT2 | SGLT2 | ASD | Non- SGLT2 | SGLT2 | ASD | |
| N | 327336 | 163668 | 136212 | 136212 | ||
| Age | 0.0000 | 0.0679 | ||||
| 20-39 | 17670 (5.40%) | 8835 (5.40%) | 4249 (3.12%) | 6028 (4.43%) | ||
| 40-49 | 36722 (11.22%) | 18361 (11.22%) | 13266 (9.74%) | 14401 (10.57%) | ||
| 50-59 | 80116 (24.48%) | 40058 (24.48%) | 33503 (24.60%) | 33603 (24.67%) | ||
| 60-69 | 111622 (34.10%) | 55811 (34.10%) | 49297 (36.19%) | 47772 (35.07%) | ||
| 70-79 | 60504 (18.48%) | 30252 (18.48%) | 27020 (19.84%) | 25898 (19.01%) | ||
| >=80 | 20702 (6.32%) | 10351 (6.32%) | 8877 (6.52%) | 8510 (6.25%) | ||
| Urbanization | 0.0453 | 0.0370 | ||||
| Urban | 191091 (58.38%) | 98397 (60.12%) | 79727 (58.53%) | 80828 (59.34%) | ||
| Sub-urban | 103456 (31.61%) | 50052 (30.58%) | 43112 (31.65%) | 42377 (31.11%) | ||
| Rural | 32789 (10.02%) | 15219 (9.30%) | 13373 (9.82%) | 13007 (9.55%) | ||
| aDCSI score | 0.2851 | 0.0000 | ||||
| 0 | 200570 (61.27%) | 77452 (47.32%) | 67685 (49.69%) | 67559 (49.60%) | ||
| 1-2 | 101960 (31.15%) | 69994 (42.77%) | 56413 (41.42%) | 56284 (41.32%) | ||
| >=3 | 24806 (7.58%) | 16222 (9.91%) | 12114 (8.89%) | 12369 (9.08%) | ||
| Comorbidities | ||||||
| Hypertension | 169611 (51.82%) | 101028 (61.73%) | 0.2011 | 84407 (61.97%) | 83114 (61.02%) | 0.0195 |
| CAD | 30924 (9.45%) | 23051 (14.08%) | 0.1443 | 16787 (12.32%) | 17363 (12.75%) | 0.0128 |
| Hyperlipidemia | 167061 (51.04%) | 108528 (66.31%) | 0.3140 | 89765 (65.90%) | 88625 (65.06%) | 0.0176 |
| Ischemic stroke | 13958 (4.26%) | 7799 (4.77%) | 0.0241 | 6535 (4.80%) | 6391 (4.69%) | 0.0050 |
| Hemorrhage stroke | 2289 (0.70%) | 1164 (0.71%) | 0.0014 | 927 (0.68%) | 904 (0.66%) | 0.0021 |
| Kidney disease | 32156 (9.82%) | 16497 (10.08%) | 0.0086 | 13266 (9.74%) | 13354 (9.80%) | 0.0022 |
| Rheumatoid arthritis | 3557 (1.09%) | 1449 (0.89%) | 0.0204 | 1286 (0.94%) | 1270 (0.93%) | 0.0012 |
| Systemic lupus erythematosus | 736 (0.22%) | 252 (0.15%) | 0.0163 | 207 (0.15%) | 211 (0.15%) | 0.0008 |
| Sicca/Sjogren syndrome | 3856 (1.18%) | 1471 (0.90%) | 0.0276 | 1343 (0.99%) | 1282 (0.94%) | 0.0046 |
| Ankylosing spondylitis | 2489 (0.76%) | 1185 (0.72%) | 0.0042 | 1005 (0.74%) | 984 (0.72%) | 0.0018 |
| COPD | 8539 (2.61%) | 4241 (2.59%) | 0.0011 | 3460 (2.54%) | 3461 (2.54%) | 0.0001 |
| Medication | ||||||
| NSAIDs | 194966 (59.56%) | 99533 (60.81%) | 0.0256 | 82456 (60.54%) | 82479 (60.55%) | 0.0004 |
| Corticosteroids | 65684 (20.07%) | 34487 (21.07%) | 0.0249 | 28065 (20.60%) | 28109 (20.64%) | 0.0008 |
| PPI | 28343 (8.66%) | 15088 (9.22%) | 0.0196 | 11822 (8.68%) | 12035 (8.84%) | 0.0055 |
| Aspirin | 55706 (17.02%) | 38670 (23.63%) | 0.1648 | 30556 (22.43%) | 30661 (22.51%) | 0.0019 |
| Statin | 161792 (49.43%) | 119710 (73.14%) | 0.5020 | 96479 (70.83%) | 96160 (70.60%) | 0.0052 |
| Alpha-blockers | 5612 (1.71%) | 2996 (1.83%) | 0.0088 | 2420 (1.78%) | 2408 (1.77%) | 0.0007 |
| Beta- blockers | 86740 (26.50%) | 54864 (33.52%) | 0.1537 | 43319 (31.80%) | 43556 (31.98%) | 0.0037 |
| CCBs | 87183 (26.63%) | 44789 (27.37%) | 0.0165 | 38064 (27.94%) | 37294 (27.38%) | 0.0126 |
| ACEI | 13903 (4.25%) | 9126 (5.58%) | 0.0615 | 7152 (5.25%) | 7221 (5.30%) | 0.0023 |
| ARBs | 132465 (40.47%) | 91676 (56.01%) | 0.3149 | 74360 (54.59%) | 73926 (54.27%) | 0.0064 |
| Biguanides | 179428 (54.81%) | 149456 (91.32%) | 0.9028 | 124185 (91.17%) | 122073 (89.62%) | 0.0526 |
| Sulfonylureas | 73873 (22.57%) | 66089 (40.38%) | 0.3908 | 51793 (38.02%) | 51245 (37.62%) | 0.0083 |
| Alpha glucosidase inhibitors | 22845 (6.98%) | 27730 (16.94%) | 0.3107 | 17947 (13.18%) | 19291 (14.16%) | 0.0287 |
| Thiazolidinediones | 23704 (7.24%) | 29103 (17.78%) | 0.3227 | 19725 (14.48%) | 20718 (15.21%) | 0.0205 |
| DPP4 | 54086 (16.52%) | 62791 (38.36%) | 0.5048 | 41485 (30.46%) | 44649 (32.78%) | 0.0500 |
| Insullin | 38605 (11.79%) | 39236 (23.97%) | 0.3219 | 25876 (19.00%) | 27684 (20.32%) | 0.0334 |
| GLP-1 | 3215 (0.98%) | 3566 (2.18%) | 0.0961 | 2587 (1.90%) | 2666 (1.96%) | 0.0042 |
COPD: chronic obstructive pulmonary disease, CAD: Coronary Artery Disease, GLP-1: Glucagon-like peptide-1
ASD: absolute standardized difference, PSM: propensity score matching.
Incidence rate of cervical cancer in Non-SGLT-2 inhibitors user and control groups
| 2:1 sex age matching | After PSM | |||||
|---|---|---|---|---|---|---|
| Non- SGLT2 | SGLT2 | P value | Non- SGLT2 | SGLT2 | P value | |
| N | 327336 | 163668 | 136212 | 136212 | ||
| Follow up person months | 11192382 | 5706217 | 4668288 | 4777101 | ||
| New case | 443 | 172 | 191 | 148 | ||
| Incidence rate* (95% C.I.) | 0.40 (0.36-0.43) | 0.30 (0.26-0.35) | 0.41 (0.36-0.47) | 0.31 (0.26-0.36) | ||
| Crude Relative risk (95% C.I.) | reference | 0.76 (0.64-0.91) | 0.0027 | reference | 0.76 (0.61-0.94) | 0.0119 |
| Adjusted HR* (95% C.I.)† | reference | 0.77 (0.63-0.93) | 0.0081 | reference | 0.77 (0.62-0.96) | 0.0179 |
*Incidence rate, per 10,000 person-months
† adjusted hazard ratio, the covariates including year of index, sex, age, Urbanization, Insurance property, aDCSI score, co-morbidities, and medication at baseline.
(A) 2:1 sex age matching. (B) After PSM.
Multiple Cox regression to estimate the hazard ratio in this study.
| aHR(95% CI ) | ||
|---|---|---|
| 2:1 sex age matching | After PSM | |
| Study | ||
| Non-SGLT2 | reference | reference |
| SGLT2 | 0.77 (0.63-0.93) | 0.77 (0.62-0.96) |
| Age | ||
| 20-39 | reference | reference |
| 40-49 | 3.65 (1.74-7.63) | 5.40 (1.29-22.66) |
| 50-59 | 3.93 (1.92-8.04) | 6.48 (1.59-26.45) |
| 60-69 | 4.25 (2.08-8.68) | 6.85 (1.68-27.92) |
| 70-79 | 4.44 (2.14-9.22) | 7.69 (1.86-31.75) |
| >=80 | 5.98 (2.78-12.85) | 7.19 (1.65-31.29) |
| Comorbidity (ref: non) | ||
| Hypertension | 1.12 (0.91-1.38) | 1.04 (0.79-1.37) |
| CAD | 0.94 (0.70-1.26) | 0.86 (0.59-1.25) |
| Hyperlipidemia | 1.00 (0.83-1.20) | 1.06 (0.83-1.35) |
| Ischemic stroke | 1.09 (0.74-1.61) | 1.19 (0.72-1.97) |
| Hemorrhage stroke | 1.67 (0.82-3.42) | 1.73 (0.62-4.77) |
| Kidney disease | 1.26 (0.94-1.68) | 1.56 (1.09-2.24) |
| Rheumatoid arthritis | 1.31 (0.65-2.67) | 2.26 (1.00-5.12) |
| Sicca/Sjogren syndrome | 1.61 (0.85-3.03) | 0.61 (0.15-2.47) |
| Ankylosing spondylitis | 0.69 (0.22-2.15) | 0.43 (0.06-3.07) |
| COPD | 0.93 (0.56-1.54) | 1.05 (0.54-2.06) |
Incidence rate of cervical cancer by oral medications.
| 2:1 sex age matching | ||
|---|---|---|
| Biguanides | SGLT2 | |
| N | 179428 | 149456 |
| Follow up person months | 6351688 | 5262900 |
| New case | 264 | 159 |
| Incidence rate*(95% C.I.) | 0.42 (0.37-0.47) | 0.30 (0.26-0.35) |
| Crude Relative risk (95% C.I.) | reference | 0.73 (0.60-0.88) |
| Adjusted HR* (95% C.I.)† | reference | 0.77 (0.63-0.95) |
| Sulfonylureas | SGLT2 | |
| N | 73873 | 66089 |
| Follow up person months | 2701750 | 2347102 |
| New case | 122 | 67 |
| Incidence rate*(95% C.I.) | 0.45 (0.38-0.54) | 0.29 (0.22-0.36) |
| Crude Relative risk (95% C.I.) | reference | 0.63 (0.47-0.85) |
| Adjusted HR* (95% C.I.)† | reference | 0.69 (0.50-0.94) |
Discussion
Our study demonstrated an association between the use of SGLT2 inhibitors and the risk of cervical cancer development. The use of SGLT2 inhibitors can reduce the likelihood of several morbidities [8, 9, 12]. The most crucial mechanism of SGLT2 inhibitors is their anti-hyperglycemic function, which can reduce glycated hemoglobin levels by up to 1.0% [4]. When used together with other anti-diabetic medications, the concurrent use of SGLT2 inhibitors and DPP4 inhibitors reduced the glycated hemoglobin level by 0.71% compared with DPP4 inhibitor monotherapy [21]. Additionally, the use of SGLT2 inhibitors was demonstrated to reduce the inflammatory response in a model of autoimmune myocarditis [22]. Specifically, SGLT2 inhibitors perform their anti-inflammatory function by downregulating the expression of several pro-inflammatory cytokines [6]. In addition to inflammation suppression, SGLT2 inhibitors have antioxidant properties, which can reduce oxidative stress and related myocardium fibrosis [23]. Furthermore, SGLT2 inhibitors can hinder the formation of reactive oxygen species in experimental diabetic kidney disease [19], and the antioxidant characteristic of SGLT2 inhibitors could alter the development of several diseases including liver diseases, neural defects, and neoplasm [24]. If we focus on the correlation between SGLT2 inhibitors and cancers, both the SGLT2 inhibitors and malignancy precipitate euglycemic diabetic ketoacidosis while the interaction between SGLT2 inhibitors and cancer on euglycemic diabetic ketoacidosis risk remains unclear [25]. Besides, previous studies demonstrated an inconclusive correlation between the risk of breast and bladder cancers and the usage of SGLT2 inhibitors [26, 27]. On the other side, some preclinical researches exhibited that the SGLT2 inhibitors have anti-proliferative effects on several malignancies [28], and the possibilities of lung cancer and non-melanoma skin cancer may be reduced by SGLT2 inhibition [27]. Moreover, the SGLT2 inhibitors were proven to trigger the apoptosis of cervical cancer call via modulating the sonic hedgehog signaling molecule expression in an experimental study [29]. Regarding cervical cancer, inflammatory cytokines contribute to cervical cancer formation [20], and tumor-associated macrophages, altered under inflammatory conditions, facilitate cervical cancer metastasis [30]. Oxidative stress is involved in the pathogenesis of cervical cancer [31], and antioxidants have potential for use in cervical cancer management [32]. Additionally, hyperglycemic status can lead to cervical cancer development and poor prognosis for cervical cancer [33, 34]. Because SGLT2 inhibitors can reduce pathways related to cervical cancer development [4, 19], their use in the T2DM population may correlate with a lower rate of cervical cancer. Our study's results support this opinion.
In our study, the use of SGLT2 inhibitors in the T2DM population was associated with a reduced likelihood of cervical cancer occurrence. Previous studies have indicated that SGLT2 inhibitors can reduce the proliferation of certain malignancies, including breast and liver cancer cells [35, 36]. Regarding the correlation between cervical cancer and SGLT2 inhibitors, the previous experimental research and bioinformatic analysis demonstrated that the application of SGLT2 inhibitors can reduce the cervical cancer cell growth and migration [27-29, 37, 38]. Nevertheless, the association between SGLT2 inhibitors utilization and the incidence of subsequent cervical cancer in real world had not been reported. To our knowledge, our results could be a preliminary experience that revealed the negative correlation between SGLT2 inhibitors utilization and consecutive cervical cancer occurrence in patients with T2DM clinically. Comparing to previous experimental studies [27-29, 37, 38], we expanded their results and exhibited that the SGLT2 inhibitor may indeed related to the lower risk of cervical cancer in real world, which is the novelty of our study. In our study, patients with T2DM diagnosed with cervical cancer within 6 months of SGLT2 inhibitor use were excluded to ensure a clear temporal relationship between SGLT2 inhibitor use and the occurrence of cervical cancer. Furthermore, established risk factors such as human papillomavirus infection and T2DM severity were adjusted for in our multivariable regression analysis to account for these confounders [15, 16, 34]. Correspondingly, SGLT2 inhibitor use may be an independent protective factor against cervical cancer development. The mechanism by which SGLT2 inhibitors suppress liver cancer cells involves the regulation of inflammatory cytokines such as interleukin-8 and tissue inhibitors of metalloproteinase-1 [36]. Therefore, the relationship between SGLT2 inhibitor use and reduced incidence of cervical cancer may be primarily due to its anti-inflammatory effects. Further studies are required to clarify this concept and to explore the underlying mechanisms.
T2DM is a common metabolic disease affecting more than 10% of the global population [3]. Additionally, the prevalence of T2DM is increasing, with projections suggesting that approximately 700 million individuals will be diagnosed with T2DM by 2040 [2]. SGLT2 inhibitors have demonstrated their efficacy in reducing serum glucose levels, and they are frequently used for T2DM management [3, 39]. In the United States, more than 10% of patients with T2DM use SGLT2 inhibitors to reduce their serum glucose concentration [40]. Cervical cancer is the third-to-fourth leading malignancy among women following breast cancer and colorectal cancer [41, 42]. In 2018, approximately 570 000 cases of cervical cancer were diagnosed, resulting in 311 000 deaths [13]. Given the considerable prevalence of both SGLT2 inhibitor use and cervical cancer [39, 43], investigating the potential association between them is crucial.
Our study had several limitations. First, the NHIRD is a claims database that does not contain actual medical records. Consequently, crucial information—such as the serum glucose levels and glycated hemoglobin concentrations of patients with T2DM, the severity of T2DM-related complications, treatment responses after SGLT2 inhibitor use, details of other comorbidities such as human papillomavirus infection and sexual intercourse, actual sites of cervical cancer, imaging results related to cervical cancer, pathological reports of cervical cancer, responses to treatment for cervical cancer, and recurrence of cervical cancer (if any)—was not considered in our study. Second, the retrospective design of our study might have contributed to low homogeneity between the groups despite the application of PSM. Additionally, although smoking is a well-known risk factor for cervical cancer development [41], we could not include this covariate in our analysis because the related code is rarely entered by physicians in clinical practice. Similarly, the use of oral contraceptives, another risk factor for cervical cancer formation [15], was not included in our research because such contraceptives are self-paid in Taiwan.
In conclusion, the use of SGLT2 inhibitors in patients with T2DM correlated with a significantly reduced incidence of subsequent cervical cancer after adjustment for multiple covariates. Accordingly, SGLT2 inhibitors can be recommended for patients with both T2DM and preexisting risk factors for cervical cancer. In the field of cancer intervention, the usage of SGLT2 inhibitors might be applied as a new preventive or adjuvant intervention for the T2DM population with precancerous cervix conditions or cervical cancer. Further large-scale prospective clinical trial to evaluate the exact role of SGLT2 inhibitors on cervical cancer in real world and the association between SGLT2 inhibitors utilization and the treatment outcome of cervical cancer is mandatory.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH. et al. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40-50
2. Lovic D, Piperidou A, Zografou I, Grassos H, Pittaras A, Manolis A. The Growing Epidemic of Diabetes Mellitus. Curr Vasc Pharmacol. 2020;18:104-9
3. Xu B, Li S, Kang B, Zhou J. The current role of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes mellitus management. Cardiovasc Diabetol. 2022;21:83
4. Tentolouris A, Vlachakis P, Tzeravini E, Eleftheriadou I, Tentolouris N. SGLT2 Inhibitors: A Review of Their Antidiabetic and Cardioprotective Effects. Int J Environ Res Public Health. 2019;16:2965
5. Brown E, Heerspink HJL, Cuthbertson DJ, Wilding JPH. SGLT2 inhibitors and GLP-1 receptor agonists: established and emerging indications. Lancet. 2021;398:262-76
6. Cowie MR, Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol. 2020;17:761-72
7. Pawlos A, Broncel M, Woźniak E, Gorzelak-Pabiś P. Neuroprotective Effect of SGLT2 Inhibitors. Molecules. 2021;26:7213
8. Jiang K, Xu Y, Wang D, Chen F, Tu Z, Qian J. et al. Cardioprotective mechanism of SGLT2 inhibitor against myocardial infarction is through reduction of autosis. Protein Cell. 2022;13:336-59
9. Vallon V, Verma S. Effects of SGLT2 Inhibitors on Kidney and Cardiovascular Function. Annu Rev Physiol. 2021;83:503-28
10. Yao YP, Yang PJ, Lee CY, Huang JY, Yang SF, Lin HY. Utilization of sodium-glucose cotransporter 2 inhibitors on dry eye disease severity in patients with type 2 diabetes mellitus. Int J Med Sci. 2023;20:1705-10
11. Su YC, Hung JH, Chang KC, Sun CC, Huang YH, Lee CN. et al. Comparison of Sodium-Glucose Cotransporter 2 Inhibitors vs Glucagonlike Peptide-1 Receptor Agonists and Incidence of Dry Eye Disease in Patients With Type 2 Diabetes in Taiwan. JAMA Netw Open. 2022;5:e2232584
12. Sha W, Wen S, Chen L, Xu B, Lei T, Zhou L. The Role of SGLT2 Inhibitor on the Treatment of Diabetic Retinopathy. J Diabetes Res. 2020;2020:8867875
13. Buskwofie A, David-West G, Clare CA. A Review of Cervical Cancer: Incidence and Disparities. J Natl Med Assoc. 2020;112:229-32
14. Shrestha AD, Neupane D, Vedsted P, Kallestrup P. Cervical Cancer Prevalence, Incidence and Mortality in Low and Middle Income Countries: A Systematic Review. Asian Pac J Cancer Prev. 2018;19:319-24
15. Aballéa S, Beck E, Cheng X, Demarteau N, Li X, Ma F. et al. Risk factors for cervical cancer in women in China: A meta-model. Womens Health (Lond). 2020;16:1745506520940875
16. Olusola P, Banerjee HN, Philley JV, Dasgupta S. Human Papilloma Virus-Associated Cervical Cancer and Health Disparities. Cells. 2019;8:622
17. Moore DH. Cervical cancer. Obstet Gynecol. 2006;107:1152-61
18. Stumbar SE, Stevens M, Feld Z. Cervical Cancer and Its Precursors: A Preventative Approach to Screening, Diagnosis, and Management. Prim Care. 2019;46:117-34
19. Winiarska A, Knysak M, Nabrdalik K, Gumprecht J, Stompór T. Inflammation and Oxidative Stress in Diabetic Kidney Disease: The Targets for SGLT2 Inhibitors and GLP-1 Receptor Agonists. Int J Mol Sci. 2021;22:10822
20. Deivendran S, Marzook KH, Radhakrishna Pillai M. The role of inflammation in cervical cancer. Adv Exp Med Biol. 2014;816:377-99
21. Li D, Shi W, Wang T, Tang H. SGLT2 inhibitor plus DPP-4 inhibitor as combination therapy for type 2 diabetes: A systematic review and meta-analysis. Diabetes Obes Metab. 2018;20:1972-6
22. Long Q, Li L, Yang H, Lu Y, Yang H, Zhu Y. et al. SGLT2 inhibitor, canagliflozin, ameliorates cardiac inflammation in experimental autoimmune myocarditis. Int Immunopharmacol. 2022;110:109024
23. Li C, Zhang J, Xue M, Li X, Han F, Liu X. et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol. 2019;18:15
24. Tsai KF, Chen YL, Chiou TT, Chu TH, Li LC, Ng HY. et al. Emergence of SGLT2 Inhibitors as Powerful Antioxidants in Human Diseases. Antioxidants (Basel). 2021;10:1166
25. Somagutta MR, Agadi K, Hange N, Jain MS, Batti E, Emuze BO. et al. Euglycemic Diabetic Ketoacidosis and Sodium-Glucose Cotransporter-2 Inhibitors: A Focused Review of Pathophysiology, Risk Factors, and Triggers. Cureus. 2021;13:e13665
26. Lin HW, Tseng CH. A Review on the Relationship between SGLT2 Inhibitors and Cancer. Int J Endocrinol. 2014;2014:719578
27. Zhang L, Xue B, Yu F, Yin Y, Jin S. Deciphering the Causal Relationship between Sodium-glucose Cotransporter 2 Inhibition and Cancer Risks: A Comprehensive Mendelian Randomization Study. J Cancer. 2024;15:3903-12
28. Dutka M, Bobiński R, Francuz T, Garczorz W, Zimmer K, Ilczak T. et al. SGLT-2 Inhibitors in Cancer Treatment-Mechanisms of Action and Emerging New Perspectives. Cancers (Basel). 2022;14:5811
29. Xie Z, Wang F, Lin L, Duan S, Liu X, Li X. et al. An SGLT2 inhibitor modulates SHH expression by activating AMPK to inhibit the migration and induce the apoptosis of cervical carcinoma cells. Cancer Lett. 2020;495:200-10
30. Liu Y, Li L, Li Y, Zhao X. Research Progress on Tumor-Associated Macrophages and Inflammation in Cervical Cancer. Biomed Res Int. 2020;2020:6842963
31. Zahra K, Patel S, Dey T, Pandey U, Mishra SP. A study of oxidative stress in cervical cancer- an institutional study. Biochem Biophys Rep. 2021;25:100881
32. Silva GÁ F, Nunes RAL, Morale MG, Boccardo E, Aguayo F, Termini L. Oxidative stress: therapeutic approaches for cervical cancer treatment. Clinics (Sao Paulo). 2018;73:e548s
33. Chen YH, Wang PH, Chen PN, Yang SF, Hsiao YH. Molecular and Cellular Mechanisms of Metformin in Cervical Cancer. Cancers (Basel). 2021;13:2545
34. Chen S, Tao M, Zhao L, Zhang X. The association between diabetes/hyperglycemia and the prognosis of cervical cancer patients: A systematic review and meta-analysis. Medicine (Baltimore). 2017;96:e7981
35. Komatsu S, Nomiyama T, Numata T, Kawanami T, Hamaguchi Y, Iwaya C. et al. SGLT2 inhibitor ipragliflozin attenuates breast cancer cell proliferation. Endocr J. 2020;67:99-106
36. Kaji K, Nishimura N, Seki K, Sato S, Saikawa S, Nakanishi K. et al. Sodium glucose cotransporter 2 inhibitor canagliflozin attenuates liver cancer cell growth and angiogenic activity by inhibiting glucose uptake. Int J Cancer. 2018;142:1712-22
37. Kogot-Levin A, Riahi Y, Abramovich I, Mosenzon O, Agranovich B, Kadosh L. et al. Mapping the metabolic reprogramming induced by sodium-glucose cotransporter 2 inhibition. JCI Insight. 2023;8:e164296
38. Wicik Z, Nowak A, Jarosz-Popek J, Wolska M, Eyileten C, Siller-Matula JM. et al. Characterization of the SGLT2 Interaction Network and Its Regulation by SGLT2 Inhibitors: A Bioinformatic Analysis. Front Pharmacol. 2022;13:901340
39. Nelinson DS, Sosa JM, Chilton RJ. SGLT2 inhibitors: a narrative review of efficacy and safety. J Osteopath Med. 2021;121:229-39
40. Mahtta D, Ramsey DJ, Lee MT, Chen L, Al Rifai M, Akeroyd JM. et al. Utilization Rates of SGLT2 Inhibitors and GLP-1 Receptor Agonists and Their Facility-Level Variation Among Patients With Atherosclerotic Cardiovascular Disease and Type 2 Diabetes: Insights From the Department of Veterans Affairs. Diabetes Care. 2022;45:372-80
41. Johnson CA, James D, Marzan A, Armaos M. Cervical Cancer: An Overview of Pathophysiology and Management. Semin Oncol Nurs. 2019;35:166-74
42. Mayadev JS, Ke G, Mahantshetty U, Pereira MD, Tarnawski R, Toita T. Global challenges of radiotherapy for the treatment of locally advanced cervical cancer. Int J Gynecol Cancer. 2022;32:436-45
43. Kojalo U, Tisler A, Parna K, Kivite-Urtane A, Zodzika J, Stankunas M. et al. An overview of cervical cancer epidemiology and prevention in the Baltic States. BMC Public Health. 2023;23:660
Author contact
![]() Corresponding authors: Shun-Fa Yang, Ph.D. or Shih-Chi Su, Ph.D., Institute of Medicine, Chung Shan Medical University, Taichung 402, Taiwan; Tel: +886-4-24739595 ext. 34253; Fax: +886-4-24723229; E-mail: ysfedu.tw (Shun-Fa Yang); E-mail: ssu1org.tw (Shih-Chi Su).
Corresponding authors: Shun-Fa Yang, Ph.D. or Shih-Chi Su, Ph.D., Institute of Medicine, Chung Shan Medical University, Taichung 402, Taiwan; Tel: +886-4-24739595 ext. 34253; Fax: +886-4-24723229; E-mail: ysfedu.tw (Shun-Fa Yang); E-mail: ssu1org.tw (Shih-Chi Su).

 Global reach, higher impact
Global reach, higher impact