Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(19):6315-6325. doi:10.7150/jca.100791 This issue Cite
Research Paper
Serum CHI3L1 Levels Predict Overall Survival of Hepatocellular Carcinoma Patients after Hepatectomy
1. Department of Epidemiology and Health Statistics, School of Public Health, Guangxi Medical University, Nanning, Guangxi, 530021, China.
2. Department of Scientific Research, Guangxi Medical University Cancer Hospital, Nanning, Guangxi, 530021, China.
3. Hepatobiliary Surgery Department, Guangxi Medical University Cancer Hospital, Nanning, Guangxi, 530021, China.
4. Department of Experimental Research, Guangxi Medical University Cancer Hospital, Nanning, Guangxi, 530021, China.
5. Department of Cancer Prevention and Control, Guangxi Medical University Cancer Hospital, Nanning, Guangxi, 530021, China.
6. Department of Disease Process Management, Guangxi Medical University Cancer Hospital, Nanning, Guangxi, 530021, China.
7. Department of Clinical Research, Guangxi Medical University Cancer Hospital, Nanning, Guangxi, 530021, China.
8. Department of Respiratory Oncology, Guangxi Medical University Cancer Hospital, Nanning, Guangxi, 530021, China
9. Zhejiang University, Zhejiang-California International Nanosystems Institute (ZCNI) Proprium Research Center, Hangzhou, Zhejiang, 310058, China
10. University of Washington School of Medicine, Seattle, WA, 98195, USA.
11. Key Laboratory of Early Prevention and Treatment for Regional High-Frequency Tumor (Guangxi Medical University), Ministry of Education, Nanning, Guangxi, 530021, China.
12. Key Cultivated Laboratory of Cancer Molecular Medicine, Health Commission of Guangxi Zhuang Autonomous Region, Nanning, Guangxi, 530021, China.
#Yanji Jiang and Wenfeng Gong contributed equally to this work and share the first authorship.
Received 2024-7-11; Accepted 2024-9-21; Published 2024-10-14
Abstract
Objective: The Chitinase 3-like protein 1 (CHI3L1) is currently used as a biomarker for the diagnosis of liver fibrosis. However, its prognostic value for hepatocellular carcinoma (HCC) patients remains controversial. In this study, we aimed to investigate the prognostic value of the CHI3L1 in HCC patients after hepatectomy.
Methods: In total, 753 HCC patients who underwent curative hepatectomy between January 2017 to August 2021 were retrospectively recruited. The probability of overall survival (OS) was evaluated by the Kaplan-Meier method and compared between groups using the log-rank test. Cox proportional hazard regression analysis was used to determine the independent prognostic factors. A prognostic nomogram was constructed for further examine the clinical utility of CHI3L1 in HCC.
Results: Kaplan-Meier analysis revealed that elevated serum CHI3L1 levels were associated with worse overall survival of HCC patients. Multivariate Cox regression analysis showed that the high-CHI3L1 group (≥198.94 ng/ml) was associated with a shorter survival time compared with that in the low-CHI3L1 group (< 198.94 ng/ml) after adjustment for potential confounding factors (HR =1.43, 95% CI = 1.05-1.94, P = 0.024). Additionally, the nomogram had sufficient calibration and discriminatory power in the training cohort, with C-indexes of 0.723 (95% CI: 0.673-0.772). The validation cohort showed similar results. Finally, we demonstrated that the AUC of the nomogram was 0.752 (95% CI: 0.683-0.821), which had better predictive ability than AFP (AUC: 0.644, 95% CI: 0.577-0.711).
Conclusion: Our results confirmed that the CHI3L1 could serve as an independent predictor for OS in HCC patients after hepatectomy. The nomogram showed a good performance in prognosis prediction of HCC.
Keywords: Hepatocellular carcinoma, CHI3L1, Nomogram, Overall survival
Introduction
Primary liver cancer (PLC) is a common malignancy that ranks sixth among all types of cancer. PLC is the third leading cause of cancer death worldwide [1]. By 2025, liver cancer is estimated to affect more than 1 million people worldwide each year [2]. The most common histologic subtype of liver cancer, hepatocellular carcinoma (HCC), accounts for 75%∼85% of the cases [1]. Hepatectomy is an established curative treatment for patients with HCC, particularly for small HCC. However, the 5-year overall survival (OS) rate of patients with HCC is very unsatisfactory, remaining less than 50% after radical resection [3]. Thus, it is imperative that novel biomarkers are identified to accurately predict the survival and to improve the management of HCC patients.
CHI3L1 (Chitinase 3-like protein 1, or YKL-40) is a 40-kDa secreted glycoprotein with a highly conserved heparin and chitin-binding lectin, belonging to the mammalian chitinase family [4]. It is now well-established that CHI3L1 is endogenously expressed in a wide variety of cells, including neutrophils, macrophages, differentiated smooth muscle cell, endothelial cells, and tumor cells [5]. Up to now, CHI3L1 is known to be stimulated by mediators such as interleukin (IL) -13, IL-6, IL-1β, and IFN-γ [6]. It is also a fibroblast growth factor that is highly enriched in liver and significantly correlated with the degree of liver fibrosis [7]. Intriguingly, previous studies have revealed that CHI3L1 could regulate various biological and cellular processes, including angiogenesis, inflammation, tissue remodeling, and is involved in the development of cancers [8-11]. In fact, CHI3L1 is elevated at both the mRNA and the protein levels in a variety of cancers and in many animal tumor models, and its levels were correlated with the stages and the outcomes of multiple types of primary and secondary carcinomas [12-16]. Consequently, CHI3L1 has been established as a promising biomarker for diagnosing different diseases and for prognosis. Studies have found that the HCC patients with higher CHI3L1 had significantly lower rates of OS and disease-free survival (DFS) than those with lower levels of CHI3L1, suggesting that CHI3L1 is an independent predictor for survival in HCC patients [17,18]. However, these associations remain controversial since some other studies have found no relationship between CHI3L1 overexpression and survival in patients with liver cancer [19]. In addition, it is worthwhile to study whether the combined use of CHI3L1 and other clinical indicators can improve the accuracy of prognosis prediction of HCC patients.
Here, we conducted this retrospective study to evaluate the prognostic value of the CHI3L1, alone or with other clinical indicators, in predicting OS of HCC patients undergoing hepatectomy. In the end, we developed and validated a prognostic nomogram for predicting OS of HCC patients by integrating serum CHI3L1 level and other independent prognostic factors.
Materials and Methods
Patient selection
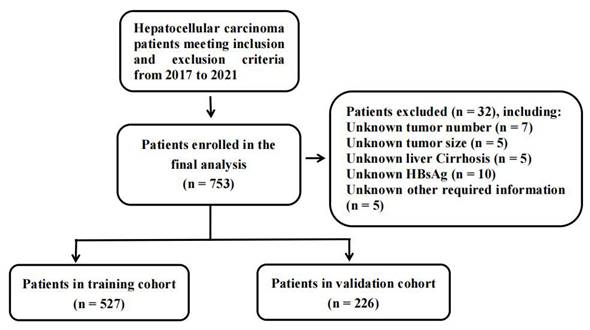
Between January 2017 to August 2021, 753 patients diagnosed with HCC were recruited from the Guangxi Medical University Cancer Hospital. The selection criteria were as follows: (1) pathological diagnosis of HCC; (2) age from 18 to 85 years; (3) underwent complete surgical resection. Patients meeting the following criteria were excluded: (1) HCC with distant metastasis; (2) preoperative treatment performed with chemotherapy, radiotherapy, or transcatheter arterial chemoembolization; (3) no follow-up or incomplete clinical data. This study was approved by the Institutional Review Board of the Guangxi Medical University Cancer Hospital (reference no. LW2024028). All patients provided written informed consent.
Sample collection and assay of serum CHI3L1 level
Peripheral blood samples were collected before surgery without any treatment and centrifuged at 3000r/min for 10 min. The serum was separated from plasma and then stored at -80°C. Serum CHI3L1 levels in HCC patients were measured by enzyme linked immunosorbent assay (ELISA). The test kits were provided by Hangzhou Proprium Biotech Company Limited (Hangzhou, China). The analyses were carried out following the manufacturer's instructions.
Data collection and follow-up
Based on hospital electronic medical records, we collected the information of HCC patients underwent hepatectomy retrospectively. The characteristics of the patients included their age, sex, height, weight, body mass index (BMI), smoking status, alcohol consumption, history of hypertension and diabetes mellitus (DM), and the family history of liver cancer. The clinicopathological parameters included tumor number, tumor size, Child-Pugh grade, cirrhosis, and Barcelona Clinic Liver Cancer (BCLC) stage. According to the Chinese Guideline for surgical treatment for HCC patients [20], some patients with BCLC stage B or C stage were ultimately included in the current study. The laboratory examinations included hepatitis B surface antigen (HBsAg), hepatitis B e antigen (HBeAg), alpha-foetoprotein (AFP), des-gamma-carboxy prothrombin (DCP), neutrophil-to-lymphocyte ratio (NLR), and international normalized ratio (INR).
A flowchart for inclusion and exclusion of HCC patients in the study.
The patients were followed up every three months following surgery for the first two years, and then every 6 months thereafter via phone calls until the death of the patient or till August 2023. Patients' overall survival (OS) was the primary endpoint of the study. Follow-up time was defined as the time between the date of hepatectomy and the date of death or the last effective follow-up.
Statistical analysis
Categorical variables were expressed as N (%), and chi-square test was used to test differences in the distribution of covariates between groups. Time-dependent receive operating characteristic (ROC) curve analysis was used to determine the optimal cutoff value of continuous variable CHI3L1, NLR, and INR. The survival curves of CHI3L1 were calculated using the Kaplan-Meier method and assessed using the log-rank test. We used univariate Cox regression analysis to identify potential risk factors associated with OS. Variables with P < 0.05 in univariable analysis were entered into multivariable Cox regression model, and then backward stepwise selection was performed to select independent risk factors. Results were reported using the P values, and the hazard ratio (HR) with their respective 95% CI.
To study whether CHI3L1 in combination with other clinical parameters performs better than CHI3L1 alone in predicting OS in HCC patients undergoing hepatectomy, we constructed a nomogram. We randomly divided all HCC patients into the training (n = 527) and the validation (n = 226) cohorts with a ratio of 7 to 3. A nomogram for predicting 1-, 3-, and 5-year survival rates in HCC patients was constructed combining CHI3L1 with other independent risk factors. The performance of the nomogram in predicting OS was evaluated by Harrell's concordance index (C-index) and the area under the curve (AUC) of the ROC curves. The value of the C-index and AUC range from 0.5 (no discrimination at all) to 1.0 (perfect discrimination). Additionally, we used calibration curves to compare the nomogram-predicted survival rates with the actual survival rates in the 1-, 3-, and 5-year time periods. Finally, according to the median of risk score, all patients were divided into the high-risk and low-risk groups, and the prognostic differences between the high-risk and low-risk groups were analyzed by log-rank test. SPSS 25.0 (IBM, Chicago, IL, USA) and R software (version 4.3.2) were used for statistical analysis of the data. A two tailed P value < 0.05 was considered statistically significant.
Results
CHI3L1 was associated significantly with age, sex, tumor size, the HCC biomarker DCP, and NLR
A total of 753 HCC patients were included for this study and a flowchart showing the inclusion and exclusion criteria was shown in Figure 1. The clinical and pathologic characteristics grouped by CHI3L1 were summarized in Table 1. The majority of patients (87.6%) were males, while the minority (12.4%) were females. The median age of the patients was 52 years (range, 24 - 83 years). We found that CHI3L1 was significantly associated with age (P < 0.001), sex (P = 0.002), tumor size (P < 0.001), the HCC biomarker DCP (P < 0.001), and NLR (P = 0.003) (Table 1). Patients were divided into the high- and the low-CHI3L1 expression groups by the optimal cutoff value of 198.94 for CHI3L1 based on the time-dependent ROC curve analysis. 468 patients (62.2%) and 285 patients (37.8%) fell into the low-CHI3L1 group (<198.94 ng/ml) and high-CHI3L1 group (≥198.94 ng/ml), respectively.
Baseline characteristics of the study patients according to different CHI3L1 group
| Characteristics | Overall | Low-CHI3L1 group | High-CHI3L1 group | P value |
|---|---|---|---|---|
| (N=753) | (N =468) | (N =285) | ||
| Age, n (%) | <0.001 | |||
| <52 | 376 (49.9) | 267 (57.1) | 109 (38.2) | |
| ≥52 | 377 (50.1) | 201 (42.9) | 176 (61.8) | |
| Sex, n (%) | 0.002 | |||
| female | 93 (12.4) | 44 (9.4) | 49 (17.2) | |
| male | 660 (87.6) | 424 (90.6) | 236 (82.8) | |
| Smoking, n (%) | 0.296 | |||
| No | 423 (56.2) | 256 (54.7) | 167 (58.6) | |
| Yes | 330 (43.8) | 212 (45.3) | 118 (41.4) | |
| Alcohol consumption, n (%) | 0.142 | |||
| No | 461 (61.2) | 277 (59.2) | 184 (64.6) | |
| Yes | 292 (38.8) | 191 (40.8) | 101 (35.4) | |
| BMI [kg/m2, n (%)] | 0.812 | |||
| 18.5 ~ 25 | 247 (32.8) | 155 (33.1) | 92 (32.3) | |
| Others | 506 (67.2) | 313 (66.9) | 193 (67.7) | |
| Hypertension, n (%) | 0.485 | |||
| No | 653 (86.7) | 409 (87.4) | 244 (85.6) | |
| Yes | 100 (13.3) | 59 (12.6) | 41 (14.4) | |
| Diabetes | 0.814 | |||
| No | 698 (92.7) | 433 (92.5) | 265 (93.0) | |
| Yes | 55 (7.3) | 35 (7.5) | 20 (7.0) | |
| Family history of liver cancer, n (%) | 0.607 | |||
| No | 649 (86.2) | 401 (85.7) | 248 (87.0) | |
| Yes | 104 (13.8) | 67 (14.3) | 37 (13.0) | |
| Liver Cirrhosis, n (%) | 0.439 | |||
| No | 254 (33.7) | 153 (32.7) | 101 (35.4) | |
| Yes | 499 (66.3) | 315 (67.3) | 184 (64.6) | |
| BCLC, n (%) | 0.070 | |||
| 0-A | 485 (64.4) | 313 (66.9) | 172 (60.4) | |
| B-C | 268 (35.6) | 155 (33.1) | 113 (39.6) | |
| Child-Pugh grade, n (%) | 0.370 | |||
| A | 726 (96.4) | 449 (95.9) | 277 (97.2) | |
| B | 27 (3.6) | 19 (4.1) | 8 (2.8) | |
| Tumor number, n (%) | 0.696 | |||
| Solitary | 600 (79.7) | 375 (80.1) | 225 (78.9) | |
| Multiple | 153 (20.3) | 93 (19.9) | 60 (21.1) | |
| Tumor size [cm, n (%)] | <0.001 | |||
| <5 | 278 (36.9) | 199 (42.5) | 79 (27.7) | |
| ≥5 | 475 (63.1) | 269 (57.5) | 206 (72.3) | |
| HBeAg | 0.963 | |||
| Negative | 40 (5.3) | 25 (5.3) | 15 (5.3) | |
| Positive | 713 (94.7) | 443 (94.7) | 270 (94.7) | |
| HBsAg | 0.968 | |||
| Negative | 130 (17.3) | 81 (17.3) | 49 (17.2) | |
| Positive | 623 (82.7) | 387 (82.7) | 236 (82.8) | |
| AFP [ng/ml, n (%)] | 0.239 | |||
| <400 | 464 (61.6) | 296 (63.2) | 168 (58.9) | |
| ≥400 | 289 (38.4) | 172 (36.8) | 117 (41.1) | |
| DCP [ng/ml, n (%)] | <0.001 | |||
| <40 | 203 (27.0) | 149 (31.8) | 54 (18.9) | |
| ≥40 | 550 (73.0) | 319 (68.2) | 231 (81.1) | |
| NLR | 0.003 | |||
| <3.34 | 633 (84.1) | 408 (87.2) | 225 (78.9) | |
| ≥3.34 | 120 (15.9) | 60 (12.8) | 60 (21.1) | |
| INR | 0.135 | |||
| <1.05 | 503 (66.8) | 322 (68.8) | 181 (63.5) | |
| ≥1.05 | 250 (33.2) | 146 (31.2) | 104 (36.5) |
BCLC, Barcelona Clinic Liver Cancer; AFP, alpha-fetoprotein; DCP, des-gamma-carboxy prothrombin; NLR, neutrophil-to-lymphocyte ratio; INR, international normalized ratio.
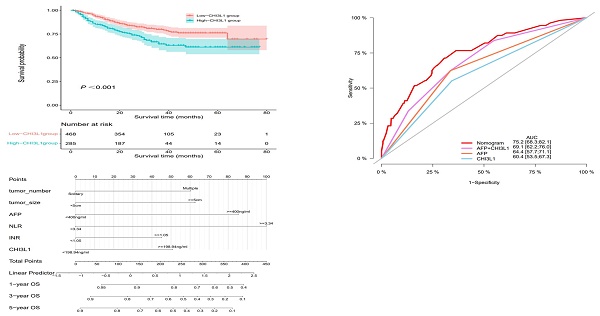
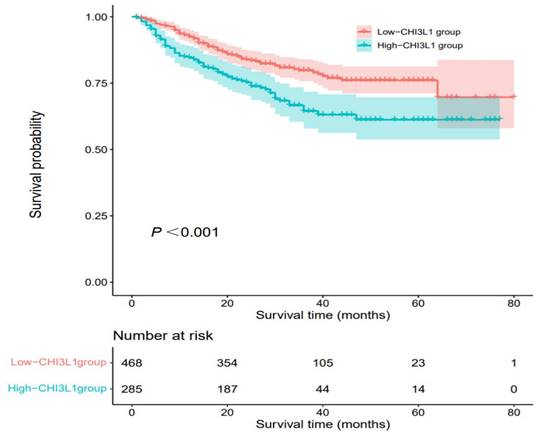
High-CHI3L1 level predicted shorter OS of HCC patients
The median OS was 27 months (range, 1 month to 80 months) and death occurred in 170 (22.6%) patients. According to Kaplan-Meier analysis, we found that the OS of HCC patients in the high-CHI3L1 group was shorter than that in the low-CHI3L1 group (P < 0.001, Figure 2). Other variables associated with OS in study cohorts analyzed by univariate and multivariate Cox regression analyses were summarized in Table 2. The high-CHI3L1 group (≥198.94 ng/ml) had a shorter survival time compared with the low-CHI3L1 group (< 198.94 ng/ml) after adjustment for potential confounding factors (HR =1.43, 95% CI = 1.05-1.94, P = 0.024). We also found that the tumor size (HR =1.61, 95% CI = 1.10-2.35, P = 0.013), the biomarker AFP (HR =1.70, 95% CI = 1.25-2.31, P < 0.001) and DCP (HR =1.67, 95% CI = 1.06-2.62, P = 0.026), and NLR (HR =2.07, 95% CI = 1.47-2.91, P < 0.001), INR (HR =1.58, 95% CI = 1.16-2.14, P = 0.003) were all independent prognostic factors for HCC survival.
Construction of a nomogram for improved performance in OS prediction for HCC patients and its validation
We divided 753 HCC patients randomly divided into the training (n = 527) and the validation (n = 226) cohorts. The clinical and pathologic characteristics of the HCC patients in the study cohort are described in Table S1. Clearly, there were no differences in the baseline characteristics between the training cohort and the validation cohort, except for the tumor number (P = 0.017). Univariate and multivariate Cox regression analyses were conducted in the training cohort and the result of OS-related variables were summarized in Table 3. All significant prognostic factors for OS (P < 0.05) in the univariable analysis were enrolled in the multivariable analysis. In addition, considering that the tumor number (P = 0.050) is closely related to the survival of HCC, it is also included in the multivariate model. Finally, multivariate regression analysis revealed that the tumor number (HR =1.66, 95% CI = 1.08-2.57, P = 0.022), the tumor size (HR =1.61, 95% CI = 1.08-2.57, P = 0.013), the traditional HCC biomarker AFP (HR =1.97, 95% CI = 1.35-2.87, P < 0.001), NLR (HR =2.34, 95% CI = 1.55-3.52, P < 0.001), INR (HR =1.45, 95% CI = 1.00-2.49, P = 0.041) and CHI3L1 (HR =1.46, 95% CI = 1.00-2.13, P = 0.049) were all independent prognostic factors.
Kaplan-Meier analyses for OS of HCC patients based on preoperative CHI3L1.
Univariable and multivariable Cox regression analysis of variables associated with OS in study patients (N=753)
| Variables | Univariate | Multivariable | |||
|---|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | ||
| Age (years) | |||||
| <52 vs. ≥52 | 0.84 (0.62-1.14) | 0.264 | |||
| Sex | |||||
| Male vs. female | 0.69 (0.46-1.04) | 0.076 | |||
| Smoking | |||||
| No vs. Yes | 1.18 (0.87-1.59) | 0.291 | |||
| Alcohol consumption | |||||
| No vs. Yes | 1.08 (0.791-1.46) | 0.641 | |||
| BMI (kg/m2) | |||||
| Others vs. 18.5-25 | 1.23 (0.88-1.72) | 0.220 | |||
| Hypertension | |||||
| No vs. Yes | 0.69 (0.41-1.15) | 0.154 | |||
| Diabetes | |||||
| No vs. Yes | 0.96 (0.53-1.72) | 0.886 | |||
| Family history of liver cancer | |||||
| No vs. Yes | 1.07 (0.702-1.64) | 0.745 | |||
| Liver cirrhosis | |||||
| No vs. Yes | 1.14 (0.82-1.58) | 0.429 | |||
| BCLC stage | |||||
| 0-A vs. B-C | 1.26 (0.93-1.72) | 0.135 | |||
| Child-Pugh grade | |||||
| A vs. B | 0.72 (0.30-1.76) | 0.474 | |||
| Tumor number | |||||
| solitary vs. multiple | 1.46 (1.03-2.05) | 0.032 | 1.39 (0.98-1.95) | 0.063 | |
| Tumor size (cm) | |||||
| <5 vs.≥5 | 2.22 (1.55-3.18) | <0.001 | 1.61 (1.10-2.35) | 0.013 | |
| HBeAg | |||||
| Negative vs.Positive | 0.78 (0.45-1.37) | 0.391 | |||
| HBsAg | |||||
| Negative vs.Positive | 1.17 (0.77-1.77) | 0.466 | |||
| AFP (ng/ml) | |||||
| <400 vs. ≥400 | 2.08 (1.54-2.81) | <0.001 | 1.70 (1.25-2.31) | <0.001 | |
| DCP (ng/ml) | |||||
| <40 vs.≥40 | 2.39 (1.56-3.69) | <0.001 | 1.67 (1.06-2.62) | 0.026 | |
| NLR | |||||
| <3.34 vs. ≥3.34 | 2.68 (1.93-3.74) | <0.001 | 2.07 (1.47-2.91) | <0.001 | |
| INR | |||||
| <1.05 vs. ≥1.05 | 1.72 (1.27-2.33) | <0.001 | 1.58 (1.16-2.14) | 0.003 | |
| CHI3L1 (ng/ml) | |||||
| <198.94 vs. ≥198.94 | 1.77 (1.31-2.39) | <0.001 | 1.43 (1.05-1.94) | 0.024 | |
OS, overall survival; HR, hazard ratio; BCLC, Barcelona Clinic Liver Cancer; AFP, alpha-fetoprotein; DCP, des-gamma-carboxy prothrombin; NLR, neutrophil-to-lymphocyte ratio; INR, international normalized ratio.
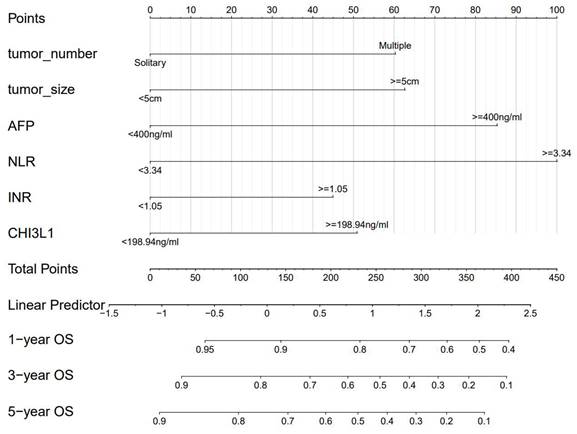
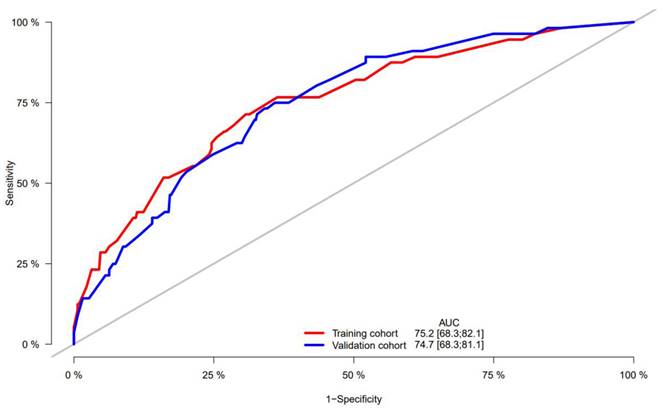
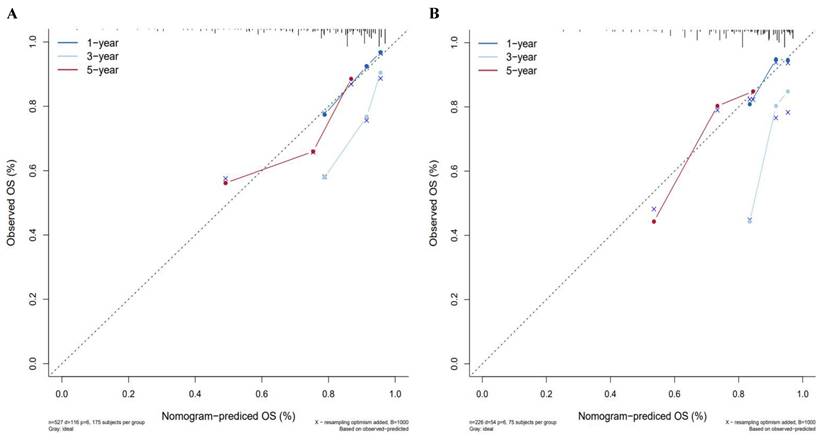
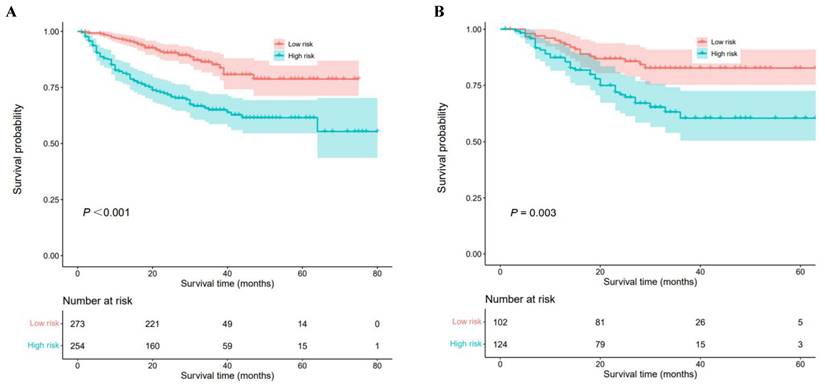
A nomogram was constructed to predict 1-, 3-, and 5-year OS, on the basis of the 6 variables described above (Figure 3). The Harrell's C-index for OS prediction was 0.723 (95% CI: 0.673-0.772) in the training cohort and 0.668 (95% CI: 0.588-0.748) in the validation cohort. The AUC value of the ROC analysis was 0.752 (95% CI: 0.683-0.821) in the training cohort and 0.747 (95% CI: 0.683-0.811) in the validation cohort (Figure 4). Overall, the calibration curves for 1-, 3-, and 5-year OS fitted well between the results from the prediction by the nomogram and the actual OS time in the training and the validation cohorts (Figure 5). Based on the median value of the total risk scores calculated by the nomogram, all patients could be stratified into the low-risk group (<2.80) and the high-risk group (≥2.80). In both the training and validation cohorts, the OS of HCC patients in the high-risk group was shorter than that in the low-risk group (Figure 6).
Univariable and multivariable Cox regression analysis of variables associated with OS in train cohort (N=527)
| Variables | Univariate | Multivariable | |||
|---|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | ||
| Age (years) | |||||
| <52 vs. ≥52 | 0.98 (0.68-1.41) | 0.926 | |||
| Sex | |||||
| Male vs. female | 0.66 (0.41-1.06) | 0.086 | |||
| Smoking | |||||
| No vs. Yes | 1.02 (0.71-1.47) | 0.912 | |||
| Alcohol consumption | |||||
| No vs. Yes | 1.01 (0.69-1.46) | 0.968 | |||
| BMI (kg/m2) | |||||
| Others vs. 18.5-25 | 1.26 (0.84-1.87) | 0.264 | |||
| Hypertension | |||||
| No vs. Yes | 0.77 (0.42-1.4) | 0.394 | |||
| Diabetes | |||||
| No vs. Yes | 0.94 (0.46-1.92) | 0.861 | |||
| Family history of liver cancer | |||||
| No vs. Yes | 1.01 (0.61-1.70) | 0.957 | |||
| Liver cirrhosis | |||||
| No vs. Yes | 1.11 (0.75-1.64) | 0.594 | |||
| BCLC stage | |||||
| 0-A vs. B-C | 1.11 (0.76-1.62) | 0.592 | |||
| Child-Pugh grade | |||||
| A vs. B | 0.64 (0.21-2.03) | 0.453 | |||
| Tumor number | |||||
| solitary vs. multiple | 1.54 (1.00-2.37) | 0.050 | 1.66 (1.08-2.57) | 0.022 | |
| Tumor size (cm) | |||||
| <5 vs. ≥5 | 2.18 (1.43-3.34) | <0.001 | 1.61 (1.10-2.35) | 0.013 | |
| HBeAg | |||||
| Negative vs. Positive | 1.09 (0.52-2.29) | 0.813 | |||
| HBsAg | |||||
| Negative vs. Positive | 1.18 (0.71-1.98) | 0.527 | |||
| AFP (ng/ml) | |||||
| <400 vs. ≥400 | 2.22 (1.54-3.20) | <0.001 | 1.97 (1.35-2.87) | <0.001 | |
| DCP (ng/ml) | |||||
| <40 vs. ≥40 | 2.36 (1.42-3.89) | <0.001 | 1.54 (0.91-2.62) | 0.108 | |
| NLR | |||||
| <3.34 vs. ≥3.34 | 2.90 (1.95-4.31) | <0.001 | 2.34 (1.55-3.52) | <0.001 | |
| INR | |||||
| <1.05 vs. ≥1.05 | 1.56 (1.08-2.25) | 0.018 | 1.45 (1.00-2.49) | 0.041 | |
| CHI3L1 (ng/ml) | |||||
| <198.94 vs. ≥198.94 | 1.91 (1.32-2.75) | <0.001 | 1.46 (1.00-2.13) | 0.049 | |
OS, overall survival; HR, hazard ratio; BCLC, Barcelona Clinic Liver Cancer; AFP, alpha-fetoprotein; DCP, des-gamma-carboxy prothrombin; NLR, neutrophil-to-lymphocyte ratio; INR, international normalized ratio.
The nomogram performed better in predicting OS in HCC patients than the AFP and CHI3L1
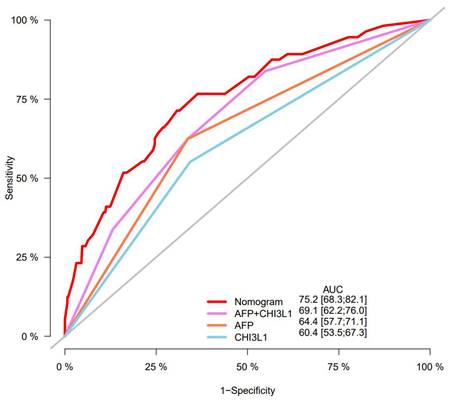
Furthermore, predictive ability of the nomogram was compared with AFP and CHI3L1 by ROC curves (Figure 7). We found that the nomogram (AUC:0.752) performed better in predicting OS in patients with HCC than the AFP (AUC:0.644), and CHI3L1 (AUC:0.604).
Nomogram for OS in HCC patients after hepatectomy. OS, overall survival; AFP, alpha-fetoprotein; DCP, des-gamma-carboxy prothrombin; NLR, neutrophil-to-lymphocyte ratio; INR, international normalized ratio.
ROC curve of the nomogram in the training and validation cohorts.
Calibration curves for predicting HCC patient survival in the training and validation cohorts. (A) 1-, 3-, and 5-year survival rate in the training cohort, (B) 1-, 3-, and 5-year survival rate in the validation cohort.
Kaplan-Meier analyses for overall survival of HCC patients with different risk groups. (A) Survival curves of HCC patients in the training cohort, (B) Survival curves of HCC patients in the validation cohort.
Predictive ability of the nomogram was compared with AFP and CHI3L1 by ROC curves.
Discussion
Hepatocellular carcinoma is highly heterogeneous and has a high recurrence rate, resulting in poor survival rate after surgery. It is important to identify prognostic markers or simple prognostic models for better HCC patient management. Elevated serum CHI3L1 levels were found in patients with different pathological processes characterized by inflammation, fibrosis, and tissue remodeling [21]. Studies have shown that deficiency in CHI3LI could improve liver fibrosis by promoting apoptosis and inhibiting the aggregation of liver macrophages [22]. Qiu et al. found that the overexpression of CHI3L1 can intensify the proliferation, migration and invasion of HCC cells, and proposed a hypothesis that CHI3L1 may active TGF-β signaling pathway by binding to interleukin-13 receptor subunit α2 (IL-13Rα2) [23]. In addition, it has been reported that elevated CHI3L1 accelerates HCC tumor progression by promoting ROS synthesis and inducing lipid peroxide (LPO) accumulation [24]. In this study, we found that serum CHI3L1 level was associated with overall survival in HCC patients. The survival time of the HCC patients with low serum CHI3L1 levels was better than those with high serum CHI3L1 levels (P < 0.001). Univariate and multivariate Cox regression analyses indicated that CHI3LI was an independent predictor for OS in HCC. In addition, serum CHI3L1 expression was significantly associated with age, sex, tumor size, DCP, and NLR. These findings are consistent with those previously reported [17-18,25-27]. Thus, we demonstrated that the serum CHI3L1 is a promising and valuable prognostic biomarker for patients with HCC after hepatic resection.
In the present study, we also confirmed previously reported risk factors for the prognosis in HCC patients: tumor size, AFP, DCP, INR, and NLR. Tumor size >5 cm was a significant prognostic factor in HCC, especially in HBV-associated patients [28]. AFP is extensively studied as a biomarker for diagnosis and prognosis in patients with HCC. However, only about 10% of early HCC patients are known to have elevated AFP, suggesting that AFP alone is a limited prognostic factor [29]. Even worse, there were about 38.1-39.4 % of HCC are AFP-negative, rendering AFP useless for these patients [30,31]. The combination of serum CHI3L1 and AFP was able to predict outcomes of HCC patients undergoing TACE than either alone [26]. DCP, also known as PIVKA-II, is an immature form of prothrombin that does not have any clotting function and is caused by an acquired defect in post-translational carboxylation of prothrombin precursors [32]. Studies demonstrated that elevated DCP levels were associated with large tumor sizes and recurrences, poor differentiation, and intrahepatic metastasis in HCC patients [33,34]. In addition, BALAD and BALAD2 scores derived from DCP, AFP, AFP-L3, albumin and bilirubin have been used as a prognostic system for HCC and have good application value [35,36]. Previous studies have demonstrated a sex difference in the subgroup with lower INR and showed that the lower level of INR for male patients with HCC showed a favorable overall survival [37]. Moreover, preoperative INR could predict the recurrence of early HCC after liver resection [38]. However, the optimal cutoff value for INR in predicting the prognosis of HCC remains unclear. Our results suggest that INR≥1.05 may serve as the cutoff value for possible adverse postoperative outcomes in HCC patients. Consistent with our findings, a multicenter, multinational study found that high NLR values were associated with poor survival in HCC patients, and its combination with AFP is a useful prognostic marker for HCC [39].
Numerous nomograms have been developed and validated for predicting prognosis in various malignancies [40-42]. Thus, we constructed a prognostic nomogram for further examine the clinical utility of CHI3L1 in HCC. The nomogram showed adequate discrimination and good consistency in the training cohort (C-index, 0.723; AUC: 0.752) and in the validation cohort (C-index, 0.668; AUC: 0.747). Based on the risk score, all patients could be divided into the low-risk group (<2.80) and the high-risk group (≥2.80). Kaplan-Meier curves revealed that the high-risk group of HCC patients had a poor OS (P<0.05). Interestingly, the prediction accuracy of the nomogram established in our study was superior to that of the single AFP and CHI3L1, which also proved that the combined model is more accurate and effective than single index model.
There are a few limitations to this study. First, the data was only collected from one institution, which could lead to selection bias; thus, the results need to be further verified by a prospective, multicenter study with a large sample size. Second, the follow-up samples after hepatectomy were not collected, which make it impossible to assess the effect of postoperative serum CHI3L1 on overall survival. Third, the findings may be subject to potential confounding due to lack of clinical characteristics, such as treatment methods, TNM stage, and vascular invasion.
Conclusions
In summary, we identified that CHI3L1 may serve as a good predictor of a poor prognosis in HCC patients after hepatectomy. Moreover, the nomogram we constructed is a powerful tool for predicting HCC prognosis, superior to single AFP. The findings of our study should be further externally validation in other large, independent populations.
Supplementary Material
Supplementary table.
Acknowledgements
Funding
The current study was supported by the Key Project of Guangxi Natural Science Foundation (2024GXNSFDA010040) ; Guangxi Medical and health appropriate Technology opening and popularization and application project (S2021022); Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor, Ministry of Education (GKE-ZZ202137); Guangxi University Young and middle-aged Teachers' Basic Research Ability Improvement Project (2021KY0112); Key R&D Program of Scientific Research and Technical Development Project of Qingxiu District (2022009); National Natural Science Foundation of China (81960613).
Data acquisition
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Author contributions
YJ and WG conceived and designed the study. YL, ZZ collected clinical data. XL carried out follow-up of the cases. YJ wrote original drafts of the paper. QL and MQ handled the statistical analysis and interpretation. BL, XQ and HY made revisions to the article. All authors reviewed the manuscript. All the authors read and approved the final manuscript.
Ethics approval and consent to participate
The current study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of the Guangxi Medical University Cancer Hospital (Approval number: LW2024028). All participants signed informed consent documentation in the study.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sung H, Ferlay J, Siegel RL. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249
2. Llovet JM, Kelley RK, Villanueva A. et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6
3. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301-1314
4. Hakala BE, White C, Recklies AD. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J Biol Chem. 1993;268:25803-10
5. Lee CG, Elias JA. Role of breast regression protein-39/YKL-40 in asthma and allergic responses. Allergy Asthma Immunol Res. 2010;2(1):20-27
6. Ma B, Akosman B, Kamle S. et al. CHI3L1 regulates PD-L1 and anti-CHI3L1-PD-1 antibody elicits synergistic antitumor responses. J Clin Invest. 2021;131(21):e137750
7. Li Y, Li C, Zhang L. et al. Serum CHI3L1 as a diagnostic marker and risk factor for liver fibrosis in HBeAg-negative chronic hepatitis B. Am J Transl Res. 2022;14(6):4090-4096
8. Wang S, Hu M, Qian Y. et al. CHI3L1 in the pathophysiology and diagnosis of liver diseases. Biomed Pharmacother. 2020;131:110680
9. Kornblit B, Hellemann D, Munthe-Fog L. et al. Plasma YKL-40 and CHI3L1 in systemic inflammation and sepsis-experience from two prospective cohorts. Immunobiology. 2013;218(10):1227-1234
10. Li F, Liu A, Zhao M, Luo L. Astrocytic Chitinase-3-like protein 1 in neurological diseases: Potential roles and future perspectives. J Neurochem. 2023;165(6):772-790
11. Francescone RA, Scully S, Faibish M. et al. Role of YKL-40 in the angiogenesis, radioresistance, and progression of glioblastoma. J Biol Chem. 2011;286(17):15332-15343
12. Bergmann OJ, Johansen JS, Klausen TW. et al. High serum concentration of YKL-40 is associated with short survival in patients with acute myeloid leukemia. Clin Cancer Res. 2005;11(24 Pt 1):8644-8652
13. Cintin C, Johansen JS, Christensen IJ, Price PA, Sørensen S, Nielsen HJ. High serum YKL-40 level after surgery for colorectal carcinoma is related to short survival. Cancer. 2002;95(2):267-274
14. Johansen JS, Drivsholm L, Price PA, Christensen IJ. High serum YKL-40 level in patients with small cell lung cancer is related to early death. Lung Cancer. 2004;46(3):333-340
15. Høgdall EV, Ringsholt M, Høgdall CK. et al. YKL-40 tissue expression and plasma levels in patients with ovarian cancer. BMC Cancer. 2009;9:8
16. Brasso K, Christensen IJ, Johansen JS. et al. Prognostic value of PINP, bone alkaline phosphatase, CTX-I, and YKL-40 in patients with metastatic prostate carcinoma. Prostate. 2006;66(5):503-513
17. Jiang Z, Wang S, Jin J. et al. The clinical significance of serum chitinase 3-like 1 in hepatitis B-related chronic liver diseases. J Clin Lab Anal. 2020;34(5):e23200
18. Zhao T, Su Z, Li Y, Zhang X, You Q. Chitinase-3 like-protein-1 function and its role in diseases. Signal Transduct Target Ther. 2020;5(1):201
19. Wang S, Chen S, Jin M. et al. Diagnostic and prognostic value of serum Chitinase 3-like protein 1 in hepatocellular carcinoma. J Clin Lab Anal. 2022;36(2):e24234
20. General Office of National Health Commission. General Office of National Health Commission. Standard for diagnosis and treatment of primary liver cancer (2022 edition). J Clin Hepatol. 2022;38(2):288-303
21. Johansen JS. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan Med Bull. 2006;53(2):172-209
22. Higashiyama M, Tomita K, Sugihara N. et al. Chitinase 3-like 1 deficiency ameliorates liver fibrosis by promoting hepatic macrophage apoptosis. Hepatol Res. 2019;49(11):1316-1328
23. Qiu QC, Wang L, Jin SS. et al. CHI3L1 promotes tumor progression by activating TGF-β signaling pathway in hepatocellular carcinoma. Sci Rep. 2018;8(1):15029
24. Lu D, Lin Z, Wang R. et al. Multi-omics profiling reveals Chitinase-3-like protein 1 as a key mediator in the crosstalk between sarcopenia and liver cancer. Redox Biol. 2022;58:102538
25. Zhu CB, Chen LL, Tian JJ. et al. Elevated serum YKL-40 level predicts poor prognosis in hepatocellular carcinoma after surgery. Ann Surg Oncol. 2012;19(3):817-825
26. Zhu CB, Wang C, Chen LL. et al. Serum YKL-40 independently predicts outcome after transcatheter arterial chemoembolization of hepatocellular carcinoma. PLoS One. 2012;7(9):e44648
27. Pan JJ, Ge YS, Xu GL. et al. The expression of chitinase 3-like 1: a novel prognostic predictor for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2013;139(6):1043-1054
28. Hwang S, Lee YJ, Kim KH. et al. The Impact of Tumor Size on Long-Term Survival Outcomes After Resection of Solitary Hepatocellular Carcinoma: Single-Institution Experience with 2558 Patients. J Gastrointest Surg. 2015;19(7):1281-1290
29. Singal AG, Hoshida Y, Pinato DJ. et al. International Liver Cancer Association (ILCA) White Paper on Biomarker Development for Hepatocellular Carcinoma. Gastroenterology. 2021;160(7):2572-2584
30. Zhang B, Zhang B, Zhang Z. et al. 42,573 cases of hepatectomy in China: a multicenter retrospective investigation. Sci China Life Sci. 2018;61(6):660-670
31. Chan MY, She WH, Dai WC. et al. Prognostic value of preoperative alpha-fetoprotein (AFP) level in patients receiving curative hepatectomy- an analysis of 1,182 patients in Hong Kong. Transl Gastroenterol Hepatol. 2019;4:52
32. Durazo FA, Blatt LM, Corey WG. et al. Des-gamma-carboxyprothrombin, alpha-fetoprotein and AFP-L3 in patients with chronic hepatitis, cirrhosis and hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23(10):1541-1548
33. Norman JS, Li PJ, Kotwani P, Shui AM, Yao F, Mehta N. AFP-L3 and DCP strongly predict early hepatocellular carcinoma recurrence after liver transplantation. J Hepatol. 2023;79(6):1469-1477
34. Tang W, Miki K, Kokudo N. et al. Des-gamma-carboxy prothrombin in cancer and non-cancer liver tissue of patients with hepatocellular carcinoma. Int J Oncol. 2003;22(5):969-975
35. Toyoda H, Kumada T, Osaki Y. et al. Staging hepatocellular carcinoma by a novel scoring system (BALAD score) based on serum markers. Clin Gastroenterol Hepatol. 2006;4(12):1528-1536
36. Wongjarupong N, Negron-Ocasio GM, Mara KC. et al. BALAD and BALAD-2 predict survival of hepatocellular carcinoma patients: a North American cohort study. HPB (Oxford). 2021;23(5):762-769
37. Lai MW, Chu YD, Lin CL. et al. Is there a sex difference in postoperative prognosis of hepatocellular carcinoma? BMC Cancer. 2019;19(1):250
38. Tortajada P, Doamba R, Cano L. et al. Resectable and transplantable hepatocellular carcinoma: Integration of liver stiffness assessment in the decision-making algorithm. Surgery. 2022;172(6):1704-1711
39. Dirican A, Uncu D, Sekacheva M. et al. A multicentre, multinational study of clinical characteristics and prognosis of hepatocellular carcinoma. East Mediterr Health J. 2023;29(6):462-473
40. Hong K, Cen K, Chen Q, Dai Y, Mai Y, Guo Y. Identification and validation of a novel senescence-related biomarker for thyroid cancer to predict the prognosis and immunotherapy. Front Immunol. 2023;14:1128390
41. Liu H, Li Z, Zhang Q. et al. Multi-institutional development and validation of a nomogram to predict prognosis of early-onset gastric cancer patients. Front Immunol. 2022;13:1007176
42. Lu H, Wu J, Liang L, Wang X, Cai H. Identifying a Novel Defined Pyroptosis-Associated Long Noncoding RNA Signature Contributes to Predicting Prognosis and Tumor Microenvironment of Bladder Cancer. Front Immunol. 2022;13:803355
Author contact
![]() Corresponding authors: Hongping Yu, Guangxi Medical University Cancer Hospital, 71, Hedi Road, Nanning, Guangxi 530021, China. Email: yuhongpinggxmu.edu.cn. Xiaoqiang Qiu, Department of Epidemiology and Health Statistics, School of Public Health, Guangxi Medical University, No.22 Shuangyong Road, Nanning 530021, Guangxi, China. Email: xqqiu9999com. Biaoyang Lin, Zhejiang University, 866 Yuhangtang Road, Hangzhou Zhejiang 310058, China. Email: bylinwashington.edu.
Corresponding authors: Hongping Yu, Guangxi Medical University Cancer Hospital, 71, Hedi Road, Nanning, Guangxi 530021, China. Email: yuhongpinggxmu.edu.cn. Xiaoqiang Qiu, Department of Epidemiology and Health Statistics, School of Public Health, Guangxi Medical University, No.22 Shuangyong Road, Nanning 530021, Guangxi, China. Email: xqqiu9999com. Biaoyang Lin, Zhejiang University, 866 Yuhangtang Road, Hangzhou Zhejiang 310058, China. Email: bylinwashington.edu.

 Global reach, higher impact
Global reach, higher impact