Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(19):6383-6415. doi:10.7150/jca.98426 This issue Cite
Review
Unlocking the Secrets of Extracellular Vesicles: Orchestrating Tumor Microenvironment Dynamics in Metastasis, Drug Resistance, and Immune Evasion
1. Department of Medical Laboratory Technology, Prince Fahad Bin Sultan Chair for Biomedical Research, Faculty of Applied Medical Sciences, University of Tabuk, Tabuk, Saudi Arabia.
2. Watson Crick Center for Molecular Medicine, Islamic University of Science and Technology, J & K, India.
3. Department of Biochemistry, Faculty of Science, University of Tabuk, Tabuk, Saudi Arabia.
4. Department of Surgical Oncology, Faculty of Medicine, University of Tabuk, Tabuk, Saudi Arabia.
5. Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, University of Bisha, Bisha, Saudi Arabia.
6. Department of Biochemistry, College of Medicine, University of Bisha, Bisha, Saudi Arabia.
7. Department of Biology, Faculty of Science, University of Tabuk, Tabuk, Saudi Arabia.
8. King Khalid hospital, MOH, Tabuk 71491, Saudi Arabia.
*Equal contribution as 1st author.
Received 2024-5-14; Accepted 2024-9-27; Published 2024-10-14
Abstract
Extracellular vehicles (EVs) are gaining increasing recognition as central contributors to the intricate landscape of the tumor microenvironment (TME). This manuscript provides an extensive examination of the multifaceted roles played by EVs in shaping the TME, with a particular emphasis on their involvement in metastasis, drug resistance, and immune evasion. Metastasis, the process by which cancer cells disseminate to distant sites, remains a formidable challenge in cancer management. EVs, encompassing exosomes and microvesicles, have emerged as critical participants in this cascade of events. They facilitate the epithelial-to-mesenchymal transition (EMT), foster pre-metastatic niche establishment, and enhance the invasive potential of cancer cells. This manuscript delves into the intricate molecular mechanisms underpinning these processes, underscoring the therapeutic potential of targeting EVs to impede metastasis. Drug resistance represents a persistent impediment to successful cancer treatment. EVs are instrumental in intrinsic and acquired drug resistance, acting as mediators of intercellular communication. They ferry molecules like miRNAs and proteins, which confer resistance to conventional chemotherapy and targeted therapies. This manuscript scrutinizes the diverse strategies employed by EVs in propagating drug resistance while also considering innovative approaches involving EV-based drug delivery systems to counteract this phenomenon. Immune evasion is a hallmark of cancer, and EVs are central in sculpting the immunosuppressive milieu of the TME. Tumor-derived EVs thwart immune responses through various mechanisms, including T cell dysfunction induction, the expansion of regulatory T cells (Tregs), and polarization of macrophages towards an immunosuppressive phenotype. In addition, the manuscript explores the diagnostic potential of EVs as biomarkers and their role as therapeutic agents in immune checkpoint blockade therapies. This manuscript provides a comprehensive overview of EV's pivotal role in mediating intricate interactions within the TME, ultimately influencing cancer progression and therapeutic outcomes. A profound understanding of EV-mediated processes in metastasis, drug resistance, and immune evasion opens up promising avenues for developing innovative therapeutic strategies and identifying valuable biomarkers in the ongoing battle against cancer.
Keywords: Exosomal miRNAs, Extracellular vesicles, tumor microenvironment, epithelial-to-mesenchymal transition, Intercellular Communication
1. Introduction
Cancer poses a significant threat to human health and is a leading cause of death worldwide [1]. Despite extensive research, effective treatments for many types of tumors remain elusive. Clinical tumor therapy faces challenges of limited effectiveness and specificity. Thus, there is an urgent need to develop innovative approaches for precise tumor treatment. Throughout the evolution of molecular medicine, scientists and healthcare professionals have delved into the intricate molecular pathways and mechanisms governing cancer initiation, growth, and advancement [2].
Extracellular Vesicles (EVs) are tiny spherical structures with double-layered membranes, comprised of a wide range of biomolecules such as proteins, nucleic acids, lipids, and metabolites [3, 4], and their composition varies depending on the cell or tissue type of origin [5]. Initially believed to be primarily involved in disposing of cellular waste [6], EVs have become vital mediators of intercellular communication, actively engaging in a wide range of normal physiological and pathological processes [7]. Their growing recognition stems from their capacity to convey bioactive cargo from one cell to another, facilitating intricate intercellular signaling in healthy or diseased states [4].
A tumor microenvironment (TME) is a complex mixture of cancer cells and an array of non-cancerous cells called cancer-associated stromal cells (CASCs), including immune cells (such as monocytes/macrophages, dendritic cells (DC), neutrophils, natural killer (NK) cells, T cells, and B cells), cancer-associated fibroblasts (CAFs), endothelial cells (EndC), neurons, vasculature, secreted molecules, and the extracellular matrix (ECM) [8-11]. Extensive interactions occur between these cells within the TME throughout the stages of cancer development [12]. While growth factors, signaling molecules, cytokines, chemokines, and their receptors on both tumor and CASCs facilitate intercellular communication within the TME [13], recently, it has become evident that EVs play a crucial role in intercellular communication in cancers supplying essential nutrients [14], and promoting or inhibiting tumor growth, depending on their cellular origin [1]. Both tumor-derived extracellular vesicles (T-EVs) and extracellular vesicles from CASCs (CASCs-EV) reshape their surrounding microenvironment into a supportive ecosystem to promote tumor development, including proliferation invasion, metastasis, angiogenesis, resistance to treatments such as chemo-radiotherapy, targeted therapy, immunotherapy, and anti-angiogenesis therapy across different types of tumors [15-17], immune evasion, pre-metastatic niche formation, and distant metastasis [18-24]. Furthermore, although there has been a significant increase in the availability of cancer treatment drugs in recent decades, drug resistance remains a significant hurdle. Cancer patients often respond well to drug therapy initially, but resistance eventually develops and significantly contributes to cancer-related deaths [25]. The role of EVs in cancer drug resistance has gained recognition over time. EVs are implicated in drug resistance across various treatment modalities, including radiotherapy, chemotherapy, immunotherapy, targeted therapy, and anti-angiogenesis therapy. Several pathways have been identified as contributors to EV-mediated drug resistance. One mechanism involves drug efflux pumps within EVs, which sequester anticancer drugs, reducing their concentration in cancer cells [26, 27]. Specific drug efflux pumps, found abundantly in EVs, can be transferred between cells during chemotherapy, indicating a shared mechanism for conveying drug resistance. Moreover, extracellular vesicles released by drug-resistant cancer cells transport nucleic acids and proteins to susceptible cells, fostering phenomena such as epithelial-mesenchymal transition (EMT), autophagy, metabolic changes, and the emergence of cancer stem cell properties, all associated with acquiring drug resistance [28]. EVs can also act as bait, capturing monoclonal antibodies targeting cancer-associated ligands or receptors [28]. Moreover, within the TME, cancer cells actively communicate with CASCs through EVs, leading to therapeutic resistance and cancer progression [29]. Hence, gaining a more profound insight into the mechanisms of EV-mediated drug resistance has become a pivotal research focus. Cargo within EVs from cancer patients' bodily fluids has been employed as biomarkers for evaluating treatment efficacy. Furthermore, researchers are investigating EVs as a potential target for reversing cancer drug resistance and exploiting their distinctive biological traits as a promising means of delivering therapies to combat resistance.
In this review, we present current insights into the formation, release, and uptake of EVs, focusing on the release of EVs by various cellular components within the TME. We summarize the characteristics of both tumor-derived EVs (T-EVs) and EVs from CASCs and their impact on tumor progression and metastasis within the TME. Specifically, we delve into how EVs facilitate cell-to-cell communication and trigger downstream signaling cascades within the TME, including promoting cancer development. We also incorporate the deregulation of exosomal miRNAs in many cancer types, such as gastric, lung, colorectal, liver, breast, and cervical malignancies. In addition, we investigate the role that EVs play in the development of drug resistance in cancer, as well as the molecular mechanisms that underlie this phenomenon, including the participation of EV-mediated CASCs, miRNAs, lncRNAs, proteins, mRNAs, DNA, and involvement of drug efflux pump in EVs. We also emphasize recent breakthroughs in utilizing EVs to monitor responses to cancer therapy, such as using exosomal miRNAs, lncRNAs, and cirRNAs as biomarkers. Additionally, we discussed EVs potential to tackle drug resistance in cancer treatment via targeting key proteins involved in the biogenesis, secretion, and uptake of EVs and EVs as drug delivery vehicles to overcome drug resistance.
2. Role of extracellular vesicles and intercellular communication
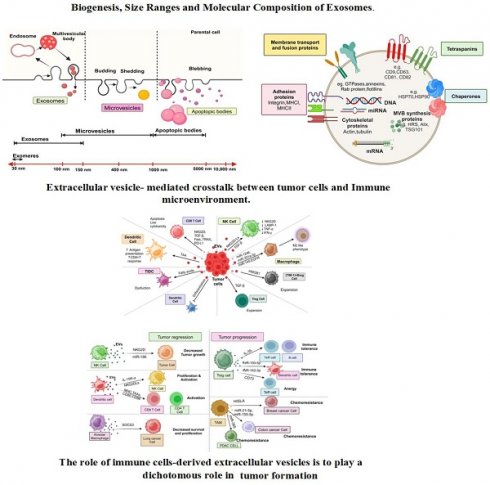
EVs are diverse vesicular structures secreted by various types of cells, either in a continuous or regulated manner, into the extracellular environment [4]. These EVs can be found in human biofluids, including blood-derived serum/plasma, urine, saliva, and milk, both in normal physiological conditions and during pathological states [30, 31] and serve as a novel means of intercellular communication between different cell types, facilitating the exchange of biological information and influencing cellular processes. These EVs are classified into three primary subtypes-exosomes, microvesicles (MVs) or microparticles (MPs), and apoptotic bodies-according to their origin and size [3, 32] (Figure 1A). While the microvesicles (MVs) arise through cell membrane outward budding and fission [33], exosomes form via fusion between multivesicular bodies (MVBs) containing intraluminal vesicles (ILVs) and the cell's plasma membrane [34] and apoptotic bodies are released during cell apoptosis [29]. Exosomes typically measure between 30 to 150 nm, apoptotic bodies range from 1000 to 5000 nm, and microvesicles fall within the size range of 100-1000 nm, sharing characteristics with both exosomes and apoptotic bodies [35].
Recently, even smaller particles known as exomeres, measuring less than 35 nm, have been described, although little is known about their physiological roles, biogenesis, and composition [36]. EVs can be classified based on their origin into tumor-derived EVs or mesenchymal stem cell (MSC)-derived EVs (MSC-EVs); their functions into pro-apoptotic EVs or immune-suppressing/stimulating EVs, and based on the presence of specific surface biomarkers, such as CD63+, CD9+, CD81+, or EpCAM+ EVs [37].
Biogenesis, Size Ranges and Molecular Composition of Exosomes. (A) This schematic illustrates the distinct biogenesis pathways of exosomes, microvesicles, and apoptotic bodies. Exosomes originate from the endosomal pathway, where intraluminal vesicles (ILVs) are formed within multivesicular bodies (MVBs) and are eventually released as exosomes upon MVB fusion with the cell membrane. Microvesicles, conversely, are formed through direct outward budding of the plasma membrane, leading to the release of larger vesicles. Apoptotic bodies are generated during programmed cell death (apoptosis) and contain cellular components, including organelles, membrane fragments, and cytoplasmic contents. (B) This section provides information on the characteristic size ranges reported for different vesicle types. Exosomes typically range in size from approximately 30 to 150 nanometers. Additionally, it outlines the molecular composition of exosomes, which includes a diverse cargo of proteins, lipids, nucleic acids (such as miRNA and mRNA), and surface markers, all of which play crucial roles in intercellular communication and modulating various biological processes.
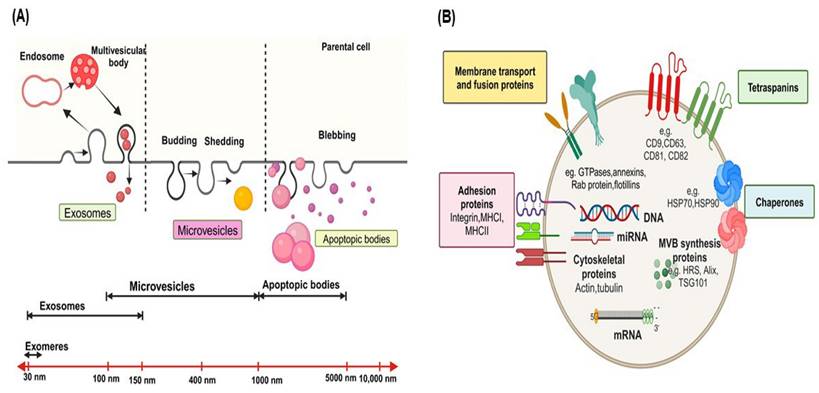
EVs contain various categories of proteins, such as membrane transport and fusion proteins (Figure 1B), including GTPases, annexins, flotillins, and Rab proteins like Rab2, Rab7, Rab11; Tetraspanins like CD9, CD63, CD81, CD82; Chaperones, such as heat-shock proteins (HSP) like HSP70 and HSP90; adhesion proteins like integrins; major histocompatibility complex (MHC I and II) molecules; cytoskeletal proteins like actin, tubulin, and moesin; proteins involved in multivesicular body synthesis, such as HRS, Alix, and tumor susceptibility gene 101 (TSG101); lipid-related proteins [5, 18, 38-42]. In addition, proteins like TSG101, Alix, CD9, CD63, CD81, and HSP70 are highly enriched in EVs and commonly used as markers for detecting and purifying EVs [4]. EVs contain lipids, including phosphatidylserine, sphingolipids, and cholesterol [43-45]. Significantly, phosphatidylserine is uniquely located on the outer surface of the EV membrane, in contrast to its typical position on the inner side of the plasma membrane [44, 46].
The lipid membrane of EVs protects the enclosed nucleic acids, including DNA, mRNA, miRNAs, circular-RNA (circRNAs), and other non-coding RNAs like long non-coding RNAs (lncRNAs) [47, 48], shielding them from degradation by extracellular nucleases [3]. These nucleic acids mediate the formation of either a pro-tumoral or anti-tumoral TME, ultimately influencing tumor progression [49]. These nucleic acids can serve as identifiers of the donor cells from which the EVs originated and are frequently investigated as disease diagnostic and monitoring biomarkers [50]. EVs also contain intact metabolites, such as amino acids, lipids, and intermediates of the tricarboxylic acid (TCA) cycle [51]. These metabolites are capable of reprogramming the metabolism of recipient cells. For example, EVs derived from CAFs carry substantial amounts of amino acids and TCA-cycle intermediates. The internalization of these metabolites by nutrient-deprived cancer cells can promote central carbon metabolism, contributing to tumor growth and cancer progression [51]. As a result, researchers are exploring the potential of EV metabolites as biomarkers for various diseases [52]. In addition, EVs are characterized by unique properties, including highly biocompatible due to their natural origin, the ability to cross biological barriers, low toxicity, and minimal immunogenicity [53]. Due to these characteristics, EVs are promising clinical biomarkers and nanocarriers for non-invasive early disease diagnosis, monitoring, and treatment [9, 53, 54].
Following their release from donor cells into the extracellular space, EVs interact with recipient cells and undergo various processes, carrying out their role in intercellular communication upon being taken up by recipient cells [55]. It's noteworthy that different types of recipient cells exhibit varying abilities to internalize the same kind of EVs, and the efficiency of EV uptake by the same cell types is also influenced by the source or origin of the EVs. This highlights that the selectivity of EV uptake depends on the specific recipient cell type and the identity of the EVs [56, 57].
Upon contact with recipient cell plasma membranes, EVs are internalized via various endocytic pathways, including micropinocytosis, phagocytosis, lipid raft- or clathrin-caveolae mediated endocytosis [58-61]. Surface proteins like integrins, such as integrin-associated protein (IPA) or CD47, are commonly found on EVs and mediate their uptake [62]. Other factors involved in EV internalization include β3 integrin [63], heparan sulfate proteoglycans [64], and survivin [65]. Once internalized, EVs travel to multivesicular bodies (MVBs) through endocytic routes, where they can either fuse with lysosomes for degradation, supporting recipient cell metabolism, or release/recycle their cargo through endosomes [3]. EVs can serve as messengers without being internalized or delivering their contents to recipient cells. For instance, EVs enriched with MHC-II from B lymphocytes can present antigens, initiating antigen-specific T cell responses based on the T cell-activating function of MHC-peptide complexes on EV surfaces rather than their enclosed contents [4].
3. Interactions of EV with tumor microenvironment cells
Cancer progression and the development of tumors are influenced by complex and intricate crosstalk between malignant cells and various CASCs within the TME [8, 66]. Historically, it was thought that tumor cells directed and instructed CASCs in the TME to adapt and function in ways that support and nourish cancer cells [67]. However, recent research has revealed that CASCs can reprogram tumor cells [68, 69], highlighting the bidirectional nature of communication within the TME. These interactions can involve direct cell-to-cell contact and the secretion of signaling molecules that either inhibit or promote tumor progression, depending on the specific signals involved. In the TME, direct cellular junctions, such as cell-cell junctions, are often involved in these interactions. For instance, synaptic connections between neurons and tumor cells in brain tumors enable intercellular signaling, accelerating tumor colonization and progression [70-72]. Gap junction proteins, connexins, found between DC cells and tumor cells facilitate the transfer of antigenic peptides from tumor cells to enhance DC-mediated T cell responses, thereby suppressing tumor growth [73]. Furthermore, non-cellular components of the TME, including the ECM, cytokines, chemokines, and growth factors, actively participate in intercellular interactions [74, 75]. For example, chemokines like CCL2, CCL3, and CCL5 can promote different aspects of tumor development by modulating immune cell activity [76], while others like CXCL8 and CXCL14 mediate more significant anti-tumoral effects [77, 78]. The discovery of diverse elements in the TME has dramatically enhanced our comprehension of cancer biology's molecular mechanisms. Furthermore, the growing recognition of EVs in the TME highlights their role as messengers between tumor cells and the microenvironment, significantly influencing local environmental changes [79].
3.1 EV-mediated interaction of tumor cells with endothelial cells
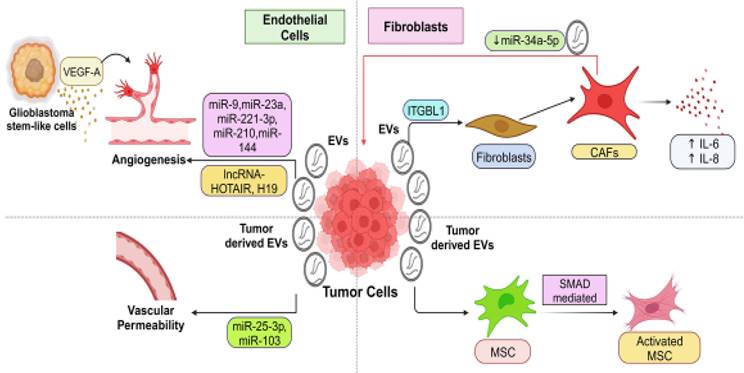
T- EVs regulated pro-tumoral functions of endothelial cells (EndCs) are observed in various cancer types, including hepatocellular carcinoma (HCC) [80], nasopharyngeal carcinoma (NPC) [81, 82], glioblastoma (GBM) [83, 84], colorectal cancer (CRC) [85-87], and lung cancer (LC) [88] (Figure 2). In EndCs, EVs can be internalized through a process similar to endocytosis and may deliver regulatory biomolecules. Consequently, by secreting EVs, tumor cells can influence EndC functions such as sprouting, branching, and tubular-like structure formation, proliferation, and migration [89, 90]. As tumors grow, recruiting new blood vessels is crucial to provide cancer cells with the necessary nutrients and oxygen, a process known as tumor angiogenesis [91, 92]. Several growth factors, including fibroblast growth factors (FGF) [93], vascular endothelial growth factor (VEGF) [94], and platelet-derived growth factors (PDGF) [95], play critical roles in regulating tumor angiogenesis. For instance, EVs derived from GBM stem-like cells carry high levels of VEGF-A, inducing angiogenic potential in human brain EndCs [96]. EVs containing miRNAs and lncRNAs participate in regulating the pro-tumoral functions of EndCs (Table 1). Through the release of ncRNAs, T-EVs can influence angiogenesis. For example, melanoma (MeL)-derived EVs containing miR-9 stimulate EndC migration and tumor angiogenesis by reducing the suppressor of cytokine signaling 5 (SOCS5) levels and activating the JAK/STAT pathway in EndCs [89]. Another miRNA, miR-23a, promotes angiogenesis by targeting SIRT1 in recipient EndCs [97]. Vesicular miR-221-3P from cervical cancer (CC) inhibits thrombospondin-2 expression in human umbilical vein endothelial cells (HUVECs), enhancing angiogenesis and tumor growth [49]. Furthermore, vesicular miR-21-5p from CRC promotes angiogenesis by activating the β-catenin signaling pathway and increasing VEGFA expression [87]. MiR-210, abundant in EVs from malignant tumors, stimulates tubular-like structure formation in EndCs, enhancing pro-angiogenic effects and hastening tumor growth. In cases of HCC, the plentiful miR-210 can transfer to HUVECs [80], triggering angiogenesis by reducing SMAD4 and STAT6 expression [80, 98]. Additionally, miR-144 plays a pivotal role in angiogenesis in NPC, with vesicular miR-144 suppressing FBXW7 and elevating HIF-1α and VEGFA in recipient cells [82]. LncRNAs like HOTAIR and H19 are also involved in angiogenesis. T-EVs carrying lncRNA-HOTAIR induce angiogenesis by upregulating VEGF-A expression in EndCs. LncRNA-H19, closely associated with HCC [99] and liver metastases [100], enhances angiogenesis by upregulating VEGF production [83, 101] in EndCs after being transmitted via T-EVs. Additionally, tumor-derived EVs can increase vascular permeability in endothelial barriers, facilitating cancer cell extravasation and metastasis. For instance, EVs from CRC cells contain miRNA-25-3p, targeting Transcription factor Kruppel-like factors 2 and 4 (KLF2) and KLF4 in EndCs to upregulate vascular permeability [86]. Similarly, miRNA-103 transported via EVs enhances vascular permeability and promotes metastasis in HCC by targeting transcripts encoding junction proteins [102].
Role of Tumor derived EVs in Cancer Angiogenesis
| EVs Cargo | Cancer type | Molecular Mechanisms of action | Ref. | |
|---|---|---|---|---|
| miR-9 | MeL | Activation of JAK/ STAT pathway | Downregulation of suppressor of cytokine signalling 5 (SOCS5) levels | [89] |
| miR-221-3P | CC | Inhibition of Thrombospondin-2 expression | ---- | [49] |
| miR-21-5p | CRC | Activation of β-catenin signaling pathway, upregulation of VEGFA | Downregulation of Krev interaction trapped protein 1 | [87] |
| miR-210 | HCC | upregulation of STAT6 | Downregulation of SMAD4 | [80, 98] |
| miR-144 | NPC | upregulation of VEGFA and HIF-1α | Downregulation of FBXW7 | [82] |
| LncRNA-HOTAIR | GBM | upregulation of VEGF-A | ---- | [83] |
| LncRNA-H19 | LiC | upregulation of VEGF | ---- | [402] |
| miRNA-25- 3p | CRC | ---- | Down regulation of KLF2, KLF4 | [86] |
| miRNA-103 | HCC | ---- | Down regulation of Cadherin, p120-catenin, zonula occludens-1 | [102] |
Abbreviations: MeLC, Melanoma cancer; CC, Cervical cancer; CRC, Colorectal cancer; HCC, Hepatocellular carcinoma; NPC, Nasopharyngeal carcinoma; GBM, Glioblastoma; LiC, Liver cancer; CRC, Colorectal cancer; SOCS5, suppressor of cytokine signalling 5; VEGFA, Vascular endothelial growth factor A; STAT, Signal transducer and activator of transcription.
3.2 EV-mediated interaction of tumor cells with fibroblasts
T-EVs play a crucial role in inducing the transformation of resident fibroblasts into CAFs (Figure 2). CAFs originate from resident fibroblasts, MSCs, and cells undergoing EMT after exposure to tumor-derived EVs [103]. CAFs play a central role in shaping the tumor-promoting environment involved in malignant tumor initiation, ECM remodeling, tumor advancement, and metastasis [104, 105]. EVs originating from Hodgkin lymphoma can transform healthy fibroblasts into disease-associated CAFs by initiating the NF-κB signaling pathway, producing neo-angiogenic factors [106]. EVs containing integrin beta-like 1 (ITGBL1) from CRC cells trigger the transformation of local fibroblasts into CAFs through the TNFAIP3-mediated NF-κB signaling pathway. These activated CAFs release various pro-inflammatory cytokines, such as IL-6 and IL-8, which facilitate the creation of pre-metastatic sites and support the metastatic process [107]. Several studies have highlighted the importance of functional biomolecule delivery in regulating the transformation of CAFs [108-112]. For example, EVs originating from chronic lymphocytic leukemia (CLL) cells prompt the conversion of fibroblasts into CAFs by transferring regulatory proteins and miRNAs from the parent cells, ultimately accelerating tumor growth. [108]. It's important to note that MSCs and resident fibroblasts EV can also differentiate into an activated, myofibroblast-like phenotype, contributing to tumor progression within the TME [113]. EVs derived from BC, ovarian cancer (OVC), and gastric cancer (GC) cells have demonstrated the ability to convert adipose tissue-derived MSCs into an activated, myofibroblast-like state with pro-tumor functions, frequently involving the SMAD-mediated signaling pathway [114-116]. However, the specific EV cargoes and underlying molecular mechanisms involved in these processes require further investigation. Furthermore, fibroblasts, including CAFs, can signal cancer cells via EVs once activated. They transport lipids and proteins from CAFs to nearby cells, including cancer cells, increasing tumor growth [117]. Enhanced migration and invasion of cancer cells following education by CAF-derived EVs are attributed to their tumor-promoting actions [118]. One study revealed that CAF-derived EVs contain lower levels of miRNA-34a-5p compared to EVs from normal fibroblasts. MiRNA-34a-5p can directly target AXL, limiting cancer cell proliferation and progression. Deleting miRNA-34a-5p in CAF-derived EVs increased AXL expression in recipient cancer cells, activating the AKT/GSK-3/β-catenin signaling cascade and promoting EMT and metastasis [119]. Most research indicates a reinforcing loop where cancer cells and fibroblasts mutually enhance their support through EV-mediated communication, ultimately driving tumor advancement. Disrupting these EV-mediated interactions between cancer cells and fibroblasts could potentially serve as a therapeutic approach.
Extracellular vesicle- mediated crosstalk between tumor cells with endothelial cells and fibroblasts in the TME. This figure illustrates the intricate cell-cell communication network mediated by EVs within the TME. Tumor and stromal cells, comprising endothelial cells fibroblasts, engage in EV-based signaling that profoundly influences tumor and stromal cell behavior, ultimately creating a favorable TME conducive to tumor growth and progression. EVs originating from tumor cells are shown to be actively involved in communicating with endothelial cells and fibroblasts within the TME. Tumor-derived EVs carry specific cargo, including various bioactive molecules and genetic material, which are transferred to endothelial cells and fibroblasts. This EV-mediated signaling enhances angiogenesis, which promotes the formation of new blood vessels (angiogenesis) that supply nutrients and oxygen to the tumor. Transferring specific molecules can also stimulate fibroblasts to create a supportive matrix that facilitates tumor invasion and metastasis.
3.3 EV-mediated interaction between tumor cells and immune microenvironment
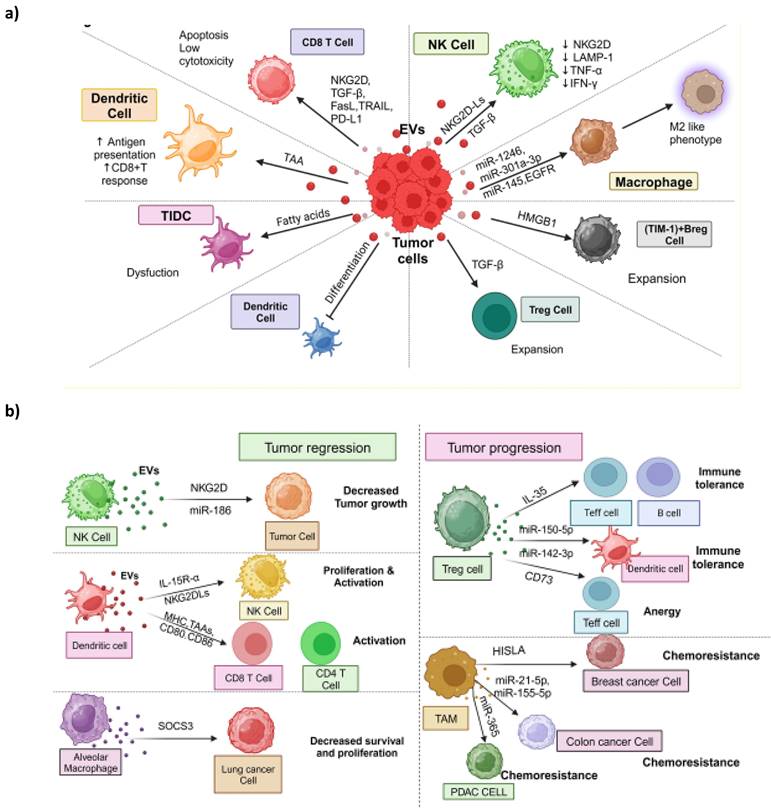
Within the TME, immune cells play a crucial role in detecting and responding to cancer cells [120]. However, cancer cells can manipulate signaling pathways within these immune cells, rendering them immunosuppressive and promoting tumor cell survival and proliferation [121]. Emerging evidence suggests that T-EVs are essential signaling molecules contributing to abnormal immune responses in the TME. While specific subsets of T-EVs have immune-stimulatory effects targeting immune cells in the tumor immune microenvironment (TIME) [18, 122-124], they are generally involved in reshaping the TIME (Figure 3a). In addition, we discussed the dual role of immune cell-derived EVs in the tumor microenvironment; some are involved in tumor regression, and others in tumor progression (Figure 3b).
3.3.1 Involving NK cells
NK cells are among the first responders in detecting and responding to cancer cells. NK cells express the receptor NKG2D, which allows them to recognize stress-associated molecules on the surface of cancer cells, aiding in cancer cell detection [125]. Studies have shown that EVs derived from PCa cells can inhibit NK cell activity. These EVs carry NKG2D ligands on their surface, leading to the downregulation of NKG2D on NK cells and a reduction in NK cell cytotoxicity [126]. Additionally, T-EVs can downregulate the levels of lysosome-associated membrane protein 1(LAMP1), tumor necrosis alpha (TNF-α), and INF-γ, collectively attenuating NK cell cytotoxicity and inhibiting the expression of CD71 and CD98, thereby limiting glucose uptake in NK cells [151] (Figure 3a). Moreover, EVs derived from pancreatic cancer (PC) cells are rich in TGF-β1, which can hinder the expression of CD107a and IFN-γ in NK cells. Mechanistically, TGF-β1-carrying EVs activate the TGF β-Smad2/3 pathway in NK cells, further impairing NK cell-mediated cytotoxicity [127]. Conversely, NK cells release EVs that contain functional molecules like NKG2D/CD94, perforin, and granzymes, contributing to NK cell cytotoxicity and presenting a potential therapeutic approach to enhance NK cell anti-cancer actions [128] (Figure 3b). For instance, EVs derived from NK cells carry functional miR-186, which can target genes such as v-Myc avian myelocytomatosis viral oncogene neuroblastoma derived homolog (MYCN), Aurora-A kinase (AURKA), transforming growth factor-beta (TGF-β) receptor type 1 (TGFBR1), and transforming growth factor-beta (TGF-β) receptor type 2 (TGFBR2) in neuroblastoma or NK cells. This inhibition suppresses neuroblastoma's tumorigenic potential and prevents TGF-β-dependent inhibition of NK cells [129].
3.3.2 Involving dendritic cells
DCs are crucial antigen-presenting cells coordinating immune responses (Figure 3a). T-EVs carry and transfer tumor antigens to DCs, leading to robust CD8+ T cell-dependent anti-tumor immune responses [130]. Additionally, EVs derived from macrophages transfer antigens to DCs, promoting CD4+ T-cell responses [131]. In these scenarios, EVs typically enhance anti-tumor activity by facilitating antigen presentation. However, within the TME, DC function can be compromised due to various immunosuppressive factors [132]. For example, T-EVs containing fatty acids can induce metabolic changes in tumor-infiltrating dendritic cells (TIDCs), causing immune dysfunction by promoting fatty acid oxidation and inhibiting glucose uptake by TIDCs [133]. Furthermore, several studies have demonstrated that tumor-derived EVs can inhibit the differentiation of DCs from myeloid precursors in the bone marrow [134] and from monocytes [135] while promoting the expansion of myeloid-derived suppressor cells (MDSCs) [135, 136]. Nonetheless, it's important to note that not all EV uptake by DCs leads to enhanced immunity. Some research has found that EVs derived from T cells can down-regulate peptide/MHC antigen I and induce apoptosis in DCs, thus suppressing the CD8+ cytotoxic T cell response [137].
On the other hand, EVs derived from DCs have been shown to stimulate immune responses [138] (Figure 3b). These DC-derived EVs may carry NKG2DLs (NKG2D ligands) and functional IL-15 receptor alpha (IL-15 Rα), promoting NK cell proliferation and activation, ultimately resulting in anti-metastatic effects mediated by NK1.1+ cells [139]. Moreover, DC-derived EVs containing MHC I and II, T cell costimulatory molecules, and tumor-associated antigens can prime cytotoxic T lymphocytes (CTLs) in vivo and suppress the growth of established murine tumors [140]. Similarly, exosomes derived from DCs expressing antigens from hepatocellular carcinoma (HCC) induce an effective antigen-specific immune response and reshape the tumor microenvironment from immunoinhibitory to immuno-stimulatory, leading to tumor shrinkage [141]. Additionally, a study demonstrated that EVs derived from activated DCs via TLR 4 signaling stimulated macrophages and DCs, resulting in stronger anti-tumor immunity in vivo [142]. These findings suggest that DC-derived EVs hold potential utility for cancer immunotherapy.
Extracellular vesicle- mediated crosstalk between tumor cells and Immune microenvironment. This diagram shows the complex web of cell-to-cell communication inside the TME that EVs facilitate. Both tumor and immune cells participate in EV-based signaling, which has a significant impact on the behavior of both types of cells, thereby contributing to TME (A) Tumor-Derived EVs' Role in the Tumor Immune Microenvironment: the pivotal role of tumor-derived EVs in shaping the Tumor Immune Microenvironment (TIME) is depicted. Tumor cells release EVs that contain various signaling molecules, such as cytokines, chemokines, and antigens. These EVs interact with immune cells, including T, B, and myeloid cells, within the TME. Tumor-derived EVs can have immunosuppressive effects, dampening the immune response and promoting immune evasion by inhibiting the activation of cytotoxic T cells and promoting the expansion of regulatory T cells (Tregs). Conversely, some EVs can have pro-inflammatory effects, further fuelling the immune response and contributing to chronic inflammation within the TME. (B) The role of immune cells-derived extracellular vesicles is to play a dichotomous role in tumor formation. This figure illustrates the dual roles of immune cell-derived EVs in the tumor microenvironment. On the left side, NK cell-derived EVs containing miR-186 suppress neuroblastoma tumorigenic growth. Dendritic cell (DC)-derived EVs possess IL-15Rα and NKG2D ligands, promoting NK cell proliferation and activation. DC-derived EVs carrying MHC molecules, tumor-associated antigens, and costimulatory molecules activate CD8+ and CD4+ T cells. Alveolar macrophage-derived EVs transfer suppressors of cytokine signaling 3 (SOCS3), repressing the survival and proliferation of lung cancer cells. On the right side, immune-suppressive cell-derived EVs exhibit pro-tumoral effects. Treg cell-derived exosomes loaded with IL-35 propagate immune tolerance through effects on effector T cells and B cells. miR-150-5p and miR-142-3p from Treg cells via EVs induce a tolerogenic phenotype in DCs. Treg cell-derived exosomes carrying CD73 induce effector T cell anergy. EVs derived from tumor-associated macrophages (TAM) containing miRNAs or lncRNAs promote tumor cell migration, invasion, and chemoresistance. For example, TAM-derived EVs containing lncRNA HISLA induce chemoresistance in breast cancer cells. This figure highlights immune cell-derived EVs' complex and dualistic nature in promoting antitumor immune responses and driving tumor progression.
3.3.3 Involving macrophages
Macrophages are a notable immune cell population in the TME, playing a crucial role in immune-oncological dynamics, often engaging in significant interactions through EVs. In the TME, tumor-associated macrophages (TAMs) primarily exhibit a tumor-promoting M2-like phenotype characterized by immunosuppression and the facilitation of tumor growth and advancement [143]. Macrophage polarization, i.e., conversion of antitumor M1 subtype to M2 subtype, is influenced by various factors, and tumor-derived EVs containing functional components like miRNA, lncRNAs, and proteins have emerged as critical regulators of macrophage polarization (Figure 3a). For example, EVs from epithelial OVC can transfer various miRNAs to macrophages, inducing an M2 phenotype, especially in the hypoxic TME [144]. Hypoxic EVs from PC and GBM induce M2-like macrophages [145, 146]. In GBM-derived EVs, an increased content of miR-1246 plays a role in inducing M2 macrophage polarization by activating the STAT3 signaling pathway, suppressing the NF-κB signaling pathway [146], and elevating TGF-β activity [147]. Hypoxic EVs from PC cells are enriched with miR-301a-3p, which down-regulates PTEN expression and activates the PI3Kγ pathway, increasing CD163 expression, a marker of M2 macrophages [145]. The M2 macrophage phenotype creates a TIME and promotes tumor cell migration and invasion. EVs from CRC cells have transferred miR-145, resulting in the down-regulation of histone deacetylase 11 in macrophages and subsequent M2 polarization [148]. Macrophage-derived EVs, on the other hand, have received less attention regarding their effects on other cells but are potentially significant (Figure 3b). For example, EVs from TAMs can enhance glycolysis and chemoresistance in BC cells by delivering HIF-1α-stabilizing lncRNA (HISLA) [149]. The transfer of exosomal miR-365 from TAMs can influence pyrimidine metabolism and cytidine deaminase expression in pancreatic cancer (PC) cells, ultimately leading to chemotherapy resistance [150].
Additionally, EVs originating from M2 macrophages have demonstrated the ability to enhance the invasion and migration of CRC cells by transmitting miR-155-5p and miR-21-5p [151]. Interestingly, in certain cases, macrophage-derived EVs may exert anti-tumor effects. For instance, EVs from alveolar macrophages (AMs) hinder the proliferation and survival of LC cells by delivering suppressors of cytokine signaling 3 (SOCS3) [152]. Further investigation is required to fully comprehend the multifaceted role of macrophage-derived EVs in the TME.
3.3.4 Involving T-cells
T cells have pivotal and diverse roles in cancer immunology. Cytotoxic T cells and helper T cells are at the forefront of tumor immunity, while T regulatory cells (Tregs) contribute to the evasion of the immune response. Within the TME, T-EVs act as immune suppressors, fostering a pro-tumor environment by promoting the recruitment and activation of Tregs [49] (Figure 3a). Notably, T-EVs containing transforming growth factor-beta (TGF-β) play a role in expanding Tregs from peripheral blood precursors, and these developed Tregs can, in turn, produce TGF-β to exert immunosuppressive effects [153, 154]. In certain instances, such as in NPC, T-EVs recruit Tregs to the TME and transform T cells into Tregs, intensifying immunosuppression [155]. The up-regulation of CCL20 transcription by NPC cells attracts Tregs [155]. Cytotoxic T cells are pivotal for targeting cancer cells by detecting tumor-associated antigens on cancer cells and inducing apoptosis in them through the secretion of granzymes and perforins [156, 157]. However, T-EVs can hinder the function of CD8+ cytotoxic T cells by down-regulating the expression of NKG2D, thereby facilitating immune evasion [126]. Moreover, exosomes released by colorectal cancer (CRC) cells, carrying Fas ligand (FasL) and TNF-related apoptosis-inducing ligand (TRAIL), can trigger apoptosis in activated CD8+ T cells, further promoting immune escape [158]. Tumor cells often evade immune surveillance by engaging inhibitory immune checkpoint signaling through the interaction of programmed death-ligand 1 (PD-L1) on their surface with programmed death-1 (PD-1) on effector T cells. T-EVs carry PD-L1 on their surface, which suppresses the function of CD8+ T cells, aiding immune evasion [159, 160]. Immunosuppressive cells release EVs with immunosuppressive functions (Figure 3b). For instance, Treg cell-derived EVs bear IL-35 on their surface, inducing an immunosuppressive phenotype in recipient T and B cells, resulting in secondary immune suppression [161]. Additionally, Treg cell-derived exosomes containing CD73 can convert extracellular adenosine-5-monophosphate (AMP) into adenosine, inhibiting the production of IL-2 and interferon-gamma (IFN-γ), thereby suppressing the function of effector T cells [162]. Several miRNAs also contribute to Treg cell-derived EV-mediated immunosuppression, such as miR-150-5p and miR-142-3p, which induce a tolerogenic phenotype in DCs [163]. Although less extensively studied in this context, B cells [164] and neutrophils [164] also partake in EV-mediated interactions within the TIME. Tumor-derived EVs can modulate regulatory B (Breg) cells, expanding TIM-1+ Breg cells that secrete IL-10 and impair CD8+ T cell functions [165]. Regarding neutrophils, reports have demonstrated that T-EVs can influence neutrophils to support tumor progression [166-168].
4. Exosomal-miRNAs' and their functions in various carcinomas
There has been recent speculation that tumoral cells produce excessive exosomes. Exosomes are an important group of extracellular vesicles that help with cell-to-cell contact and the transfer of various biomolecules. Recipient cells may be exposed to miRNAs via exosomes, which then form a RISC complex that can either degrade the target mRNAs or prevent the translation of the associated proteins. As a result, miRNAs produced from exosomes play a significant role in recipient cell gene regulation. It may impede tumor development, invasion, metastasis, angiogenesis, and treatment resistance by interfering with the microenvironment and tumor immunity. Consequently, exosomal miRNAs play a crucial role in controlling the advancement of cancer. Exosome composition, in particular the quantity of microRNAs (miRNAs) within these vesicles, exhibits a pattern unique to each disease that reflects pathogenic processes and can be used as a predictive and diagnostic indicator [169]. It is documented that exosome-mediated delivery of miRNAs are found to be linked to the etiology of lung, gastrointestinal, breast and cervical carcinoma (Table 2).
Exosomal miRNAs in various cancers
| Cancer type | Exosomal miRNA | Expression pattern | Molecular Pathways & targets | Observation | Ref. |
|---|---|---|---|---|---|
| NSCLC | miR-3180-3p | Down | FOXP4, Flotillin-1 | Inhibits the proliferation and metastasis | [170] |
| miR-770 | (-) | MAP3K1, Arginase-1, iNOS, IL-10, TGF-β, E/N-cadherin, Vimentin | Decreases invasion | [171] | |
| miR-338-3p | Down | MAPK, ERK5, CHL1, MEK4, JNK, p38 | Suppresses metastasis | [172]. | |
| miR-3157-3p | Up | KLF2, TSG101, TIMP, VEGF, Occludin, CD63, ZO-1, Claudin-5, MMP-2/9 | Enhances angiogenesis vascular permeability and metastasis | [173] | |
| miR-1260b | Up | PARP, Caspase-3, HIPK2 | Inhibiting HIPK2 could promote tumor metastasis. | [174] | |
| miR-196a-5p, miR-155 | (-) | TNF-α, IRF4, IRF5, Arg-1, TSG101, E-cadherin, Vimentin, RASSF4 | Enhance the metastasis | [175] | |
| GC | miR-590-5p | Down | CD63, CD9 | Considered as a diagnostic marker for GC. | [176] |
| miR-122-5p | Down | HSP70, GIT1, Twist1, TSG101, E-cadherin | Inhibits the tumorigenicity | [177] | |
| miR-10b-5p | Up | AKT, PTEN, TGFβR1, S6, KLF11 | Mediate communication between GC cells and fibroblasts. | [178] | |
| CRC | miR-21-5p | Up | GM130, KRIT1, TSG101 | Induces angiogenesis and vascular permeability. | [87] |
| miR-22-3p | Down | PI3K, CD63, AKT, RAP2B, CD9, HSP70 | Suppresses CRC proliferation and invasion | [403] | |
| miR-10a | Down | IL-6/8, IL-1β | Exosomal-miR-10a derived from CRC cells could decrease the migration of lung fibroblasts, and levels of IL-6, IL-8, and IL-1β. | [404] | |
| HCC | miR-638 | (-) | ZO-1, Snail, E/N-cadherin, VE-cadherin | Considered as a prognostic marker of HCC. | [181] |
| miR-15a | (-) | SALL4, TSG101, HLA-DR, PCNA, MMP-2/9, Caspase-3 | ImpedeS HCC progression. | [182] | |
| CC | miR-1468-5p | Up | TSG101, PD-1, PD-L1, HSP70, IFN-γ, HMBOX1, STAT3, SOCS1/2/3, JAK2 | Immunosuppressive reprogramming of lymphatic vessels could accelerate tumor immune escape | [184] |
| miR-663b | Up | Vinculin, TSG101, CD81, CD63 | Increase angiogenesis in vascular endothelial cells | [185] | |
| BC | miR-138-5p | (-) | TNF-α, IL-6, IL-1β, KDM6B, | Regulates the polarization of tumor-associated macrophages. | [405] |
| miR-500a-5p | (-) | α-SMA, USP28, FSP1, FAP, E/N-cadherin, Vimentin, FN1, ZEB1, Snail, Slug | Increases BC cell proliferation and metastasis | [406] | |
| miR-21, miR-106b, miR-1246, miR-373, miR-96, miR-17-5p, and miR-10b | PTEN, PI3K, mapsin, PDCD4, HODX10, TBX5, DYRK1A, Syndecan-1, CCNG2, CD44, HBP1, TCF, LEF, ErbB2, FUT6, and Wnt/β-Catenin | Promotes invasion and migration | [407-417] | ||
| miR-10a, miR-564, miR-217, miR-34c, miR-1226-3p, miR-21, miR-148b-3p, miR-19a-3p, miR-19b, miR-1486-3p, miR-100, miR-503, miR-17/20, and miR-148a | Akt, GNA12, GYS1, SRF, PIK/MAPK, mTOR, GIT1, KLF5, FZD8, Wnt-β-Catenin, AQP5, FOSL1, mucin1, TRIM29, CCND2/CCND3, E2F1, IL-8, and CCND1 | Suppresses invasion and migration | [418-425] | ||
| miR-155 and miR-132 | Down | VHL/HIF, RAS, and VEGF | Promotes angiogenesis | [426, 427] | |
| miR-16, miR-503, and miR-100 | (-) | VEGF, FGF2, VEGFA, mtor, and HIF-1α | Suppresses angiogenesis | [428-430] | |
| miR-7641 | (-) | CD9, CD63 | Enhances BC progression and metastasis | [431] | |
| miR-18b | (-) | α-SMA, β-Catenin, MMP-3/9, E/N-cadherin, Snail, Vimentin, Zeb1/2, Slug, ICAM-1 | Enhances BC invasion and metastasis. | [432] |
Abbreviations: NSCLC, Non-Small Cell Lung Carcinoma; GC, Gastric Cancer; CRC, Colorectal cancer; HCC, Hepatocellular carcinoma; CC, Cervical cancer; BC, Breast cancer.
Exosomal microRNAs, depending on whether they are upregulated or downregulated, play critical roles in either the development or prevention of NSCLC through a variety of signaling pathways. For instance, Exosomal miR-3180- 3p can stop lung cancer cells from proliferating and from being able to spread by inhibiting the expression of FOXP4 [170]. Similar to this, miR-770 transported via exosomes limits M2 macrophage polarization by affecting MAP3K1 expression. This would lessen the lung cancer cells' capacity for invasion [171]. In addition, exosome-transferred miR-338-3p is found to inhibit CHL1 via the MAPK signaling pathway, which in turn suppresses lung cancer metastasis [172]. Conversely, lung cancer-originated exosomal miR-3157-3p has also been shown to upregulate vascular permeability and to increase angiogenic and metastatic potential [173]. Moreover, exosomal miR-1260b generated from lung cancer has been demonstrated to upregulate these cells' capacity for metastasis by inhibiting HIPK2 expression [174]. Furthermore, this kind of cancer may spread more easily if tumor-associated macrophages secrete miR-155 and miR-196a-5p in their exosomes [175].
Exosome-mediated delivery of miRNAs is also implicated in the etiology of gastro-intestinal carcinoma. For instance, it has been shown that in patients with gastric cancer, there is a corelation between exosomal miR-590-5p down-regulation and a low overall survival rate. Studies conducted in vitro have demonstrated that over-expression of miR-590-5p inhibits the migration and invasion of cells in gastric cancer cells [176]. Another downregulated miRNA found in serum-derived exosomes from individuals with this kind of malignancy is miR-122-5p. By suppressing GIT1 expression, exosome-mediated transport of miR-122-5p may limit the ability of gastric cancer cells to proliferate and spread [177]. In contrast to early-stage samples, tissues and serum exosomes from advanced stages of gastric cancer have been found to have an overexpression of miR-10b-5p. miR-10b-5p could target KLF11 in fibroblasts and PTEN in stomach cancer cells. The suppression of miR-10b-5p increases PTEN levels and represses PI3K/AKT/mTORC1 signaling in gastric cancer cells, which lowers the cells' capacity to form colonies and their viability [178]. Numerous miRNAs that have been found to influence the growth of colorectal cancer have been found in exosomes produced from cancer. For example, miR-21-5p transferred from colorectal cancer cells to endothelial cells via exosomes suppressed KRIT1 expression in recipient HUVEC cells. This, in turn, induces the activity of β-catenin signals and VEGF-A and Ccnd1 expression. In their entirety, these modifications in expression promote angiogenesis and increase vascular permeability. Additionally, it has been demonstrated that patients with colorectal cancer have higher quantities of this miRNA in circulating exosomes than do healthy donors [87]. An analysis of serum exosomal miR-92a-3p and miR-17-5p for detecting primary and metastatic colorectal cancer (CRC) revealed a direct correlation between the stages of CRC17 and the up-regulation of these miRNAs [179]. Another study found that in colon cancer patients at stages II and III, low expression of circulating exosomal miR-4772-3p, isolated from serum, had been thought to be a predictive biomarker for cancer recurrence [180]. In hepatocellular carcinoma, reduced expression of VE-cadherin and ZO-1 in endothelial cells has demonstrated the predictive usefulness of exosomal levels miR-638 in serum samples [181]. Conversely, mesenchymal stem cells have been demonstrated to secrete exosomal miR-15a, which can suppress SALL4 levels and obstruct the advancement of this particular cancer [182].
Furthermore, exosome-derived miRNAs also have the potential to impact the etiology of breast and cervical cancers. One of the leading causes of breast cancer patients' poor overall survival rates is metastasis. Exosomal miRNAs are essential for nearly all stages of numerous biological processes related to breast cancer [183]. In various investigations, exosomal miRNAs have been shown to have a dual function in breast cancer metastasis and associated processes. Within the framework of cervical cancer, cancer cells produce exosomes containing miR-1468-5p, which causes immunological escape by converting lymphatic capillaries to immunosuppressive [184]. Furthermore, by preventing the production of vinculin in vascular endothelial cells, the delivery of miR-663b by these exosomes increases the angiogenic potential of cervical cancer cells [185].
5. Extracellular vesicles from TME cells induce therapeutic resistance in cancers
Drug resistance continues to be a significant obstacle to successful cancer treatment, contributing to around 90% of cancer-related fatalities. EVs induce resistance to therapy by sequestering or removing drugs from cancer cells and regulating signaling pathways associated with processes like EMT, autophagy, metabolism, and the maintenance of cancer stemness [29]. This section focuses on the contribution of EV-mediated miRNAs and lncRNAs to drug resistance Table 3.
List of T-EVs cargos and their cancer drug resistance mechanisms
| Cargo type | EV cargos | Cancer type | Targets | Drug resistant to | Outcome | Ref. |
|---|---|---|---|---|---|---|
| miRNAs | miR-221 & miR-222 | BC | p27 and ERα | Tamoxifen | Induced drug resistance in sensitive cells | [188] |
| miR-210 | PC | mTOR signaling pathway | Gemcitabine | Activate mTOR signaling pathway | [189] | |
| miR-31-5P | RCC | MutL homolog 1 (MLH1) | Sorafenib | Downregulate MLH1 expression | [191] | |
| miR-500a-3p | GC | FBXW7 | Cisplatin | Enhance stemness properties and resistance | [190] | |
| miR-222-3P | NSCLC | SOCS3 | Gemcitabine | Enhance the proliferation, gemcitabine resistance, migration, invasion, and anti-anoikis of sensitive cells | [192] | |
| miR-761 | SS | TRIP6, LMNA, SIRT3 | Pazopanib | Confer increased resistance | [193] | |
| miR-46146 | CRC | PDCD10 | Oxaliplatin | Contribute to the chemoresistance transfer | [433] | |
| miR-21 | CRCs | PDCD4 | 5-FU | Downregulate TPM1 and PTEN; promote proliferation and invasion | [195] | |
| OSCC | PTEN and PDCD4 | Cisplatin | Decrease the DNA damage repair signaling | [194] | ||
| miR-19b & miR-20a | ALL | --- | Daunorubicin | Transfer resistance from chemoresistant cells to sensitive cells | [196] | |
| miR-208a | LC | p21 | Radiotherapy | Promote cell proliferation and induce RR | [200] | |
| miR-603 | GBM | IGF1 and IGF1R | Radiotherapy | Promote the CSC state and upregulate DNA repair to promote acquired resistance | [201]. | |
| miR-195-5p, miR-203a-3p, miR-9-5p, | BC | ONECUT2 | Docetaxel & Doxorubicin | Promote BC stemness | [197] | |
| miR-21-5p, miR-1246, miR-1229-5p & miR-96-5p | CRC | --- | Oxaliplatin, 5FU | Confer increased resistance | [199] | |
| lncRNAs | SNHG14 | BC | Bcl-2/Bax | Trastuzumab | Transfer resistance from chemoresistant cells to sensitive cells | [203] |
| RP11-838N2.4 | NSCLC | --- | Erlotinib | Contribute to the chemoresistance transfer | [204] | |
| Linc-VLDLR | EC | ABCG2 | Sorafenib, DOX & Camptothecin | Promote cell viability and cell cycle progression | [207] | |
| Linc-ROR | HCC | --- | Sorafenib, DOX, & Camptothecin | Reduce chemotherapy-induced cell death | [205] | |
| H19 | NSCLC | --- | Gefitinib | Reduce gefitinib-induced cell cytotoxicity | [208] | |
| NSCLC | miR-615-3p/ATG7 axis | Erlotinib | Facilitate erlotinib resistance | [209] | ||
| BC | --- | DOX | Promote cell viability, colony-forming ability, and reduce apoptosis | [210] | ||
| Lnc-SOX2 | NSCLC | miR-627-3p/Smads signaling pathway | EGFR-TKI | Enhance EGFR-TKI resistance | [211] | |
| Proteins | p-STAT3 | CRC | ---- | 5-FU | Contribute to acquired 5-FU resistance | [212] |
| TrpC5 | BC | P-glycoprotein | DOX | Stimulate P-gp production | [213] | |
| survivin | PC | ---- | PTX, ERK inhibitor & Cloroquine | Promotes cancer cell survival and therapy resistance | [220] | |
| PD-L1 | --- | ---- | Anti-PD1 Ab | Suppress function of CD8 T cells | [29] | |
| Annexin A6 | BC | EGFR | Gemcitabin | Inhibit ubiquitination and degradation of EGFR and induce GeM resistance | [319] | |
| mRNAs | VEGF & VEGFR | Leukaemia | VEGFR/ glycolysis pathway | Ara-C | Induce glycolysis in HUVECs and lead to vascular remodelling | [224] |
| ZEB1 | NSCLC | ---- | Cisplatin and gemcitabine | Induce a mesenchymal phenotype in recipient cells | [225] | |
| GK5 | LC | SREBP1/SCD1 signaling pathway | Gefitinib-resistant | Inhibit mitochondrial damage, caspase activation, cell cycle arrest, and apoptosis | [226] | |
| DNA | mtDNA | BC | Estrogen receptor -independent (OXPHOS) | Hormone | Promote an exit from dormancy of therapy-induced cancer stem-like cells and lead to endocrine therapy resistance | [227] |
| Drug efflux pumps | ABCB1 (P-gp) | BC | ---- | Docetaxel and Doxorubicin | Confers drug resistance transfer | [231, 232] |
| OVC & PCa | ---- | taxane | Confers drug resistance in the drug sensitive cancer cells | [233] | ||
| PCa | ---- | Docetaxel | Promotes drug resistance transfer | [234] | ||
| ABCA3 | AML | Promotes Drug Export Genes Expression and ROS Inhibition | Idarubicin | Induces expression of drug efflux pump and mediate acquired chemoresistance | [235] | |
| ABCG2 | laryngeal cancer | ---- | Cisplatin | Induces drug resistance | [236] |
Abbreviations: BC, Breast cancer; PC, Pancreatic cancer; RCC, Renal cell carcinoma; GC, Gastric cancer; NSCLC, Non-small cell lung cancer; CRC, Colorectal cancer; OSCC, Oral squamous cell carcinoma; LC, Lung cancer; GBM, Glioblastoma; CRC, Colorectal cancer; EC, Esophageal cancer; HCC, Hepatocellular carcinoma; LC, Lung cancer; OVC, Ovarian cancer; PCa, Prostate cancer; AML, Acute myeloid leukaemia; MLH1,MutL homolog 1; 5-FU, 5-fluorouracil; PDCD4, programmed cell death factor 4; DOX, doxorubicin; ABCG2, ATP-binding cassette transporter G2; PTX, Paclitaxel; GeM, gemcitabine; HUVECs, Human umbilical vein endothelial cells.
5.1 Resistance due to EV-mediated miRNAs
miRNAs are frequently dysregulated in cancer and are implicated in drug resistance [186, 187]. When EVs transfer miRNAs from drug-resistant cancer cells to drug-sensitive ones, they alter gene expression in the recipient cells, making them more resistant to drugs. Examples include miR-221 and miR-222 from tamoxifen-resistant BC cells [188], miR-210 from gemcitabine (GeM)-resistant PC stem cells [189], miR-500a-3p from cisplatin-resistant GC cells [190], miR-31-5P from sorafenib resistant renal cell carcinoma (RCC) [191], miR-222-3P from GeM resistant non-small cell lung cancer (NSCLC) cells [192], miR-761 from pazopanib resistant synovial sarcoma (SS) [193], miR-46146 from oxaliplatin resistant CRCs [193], miR-21 from 5-fluorouracil (5-FU) resistant CRCs [194], and miR-21 from cisplatin-resistant OSCC [195]. These miRNAs can target genes associated with drug response, leading to drug resistance.
Moreover, miRNAs carried by EVs play a significant role in fostering multidrug resistance (MDR) in acute myeloid leukemia (AML) cells. For example, miR-19b and miR-20a display distinct expression patterns between AML cells that responded to chemotherapy and those that developed chemoresistance [196]. Additionally, BC cells release a variety of EV miRNAs following chemotherapy, including miR-203a-3p, miR-195-5p, and miR-9-5p. These specific miRNAs target the transcription factor ONECUT2 [197], leading to the emergence of cancer stem cell-like characteristics and chemotherapy resistance. EVs obtained from docetaxel-resistant prostate cancer (PCa) cell lines contain an abundance of miR-598, miR-34-a, miR-148a, miR-146a, and miR-34. Among these, miR-34 plays a regulatory role in BCL-2, potentially impacting apoptosis in response to drug treatment [198]. Similarly, higher miR-21-5p, miR-1246, miR-1229-5p, and miR-96-5p levels were detected in drug-resistant CRC cells and the EVs of chemoresistant patients compared to their sensitive counterparts. The transmission of these miRNAs via EVs contributes to the development of drug resistance across various cancer types, presenting both challenges and opportunities for comprehending and surmounting drug resistance in cancer therapy [199]. In addition to chemotherapeutic resistance, miRNA in EVs plays diverse roles in radiotherapy resistance (RR). For instance, miR-208a is upregulated in the serum of LC patients after radiotherapy [200]. Exosomes transport it and induce RR by targeting p21 and activating LC cells' Akt/mTOR pathway. Another study identified miR-603 as the most altered miRNA in GBM samples before and after radiation treatment. Ionizing radiation triggers the release of miR-603 via EVs, reducing IGF1 and IGF1R expression, promoting a cancer stem-cell state, enhancing DNA repair, and leading to RR in GBM [201].
5.2 Resistance due to EV-mediated lncRNAs
EVs deliver lncRNAs to recipient cells, modulating their response to drugs. Examples include lncRNA HOTAIR in EVs from GBM patients conferring resistance to TMZ lncRNA NEAT1 from BC, promoting resistance to cisplatin, PTX, and 5FU. Drug-resistant ALK translocated LC cells secrete lncRNAs MEG3 and XIST in EVs, inducing drug resistance in other cell subpopulations and maintaining intratumoral heterogeneity [202]. The transmission of EVs carrying lncRNAs like SNHG14 in BC contributes to drug resistance by activating the Bcl-2/Bax signaling pathway while inhibiting apoptosis [203]. Similarly, EV-mediated transfer of lncRNA RP11-838N2.4 may promote erlotinib resistance in NSCLC cells [204]. In HCC, EVs contain lncRNAs such as VLDR and ROR, contributing to drug resistance [205, 206]. Additionally, EVs transmit linc-VLDLR in esophageal cancer (EC) cells, promoting drug resistance by upregulating ABCG2 expression [207]. EVs from HCC cells also enrich lncRNA ROR, reducing chemotherapy-induced cell death [190]. Moreover, lncRNA H19 delivered via exosomes promotes resistance to gefitinib in NSCLC cells [208], erlotinib resistance in NSCLC [209], and doxorubicin resistance in BC [210]. Exosomes released by NSCLC cells transport lncRNA SOX2 overlapping transcript (SOX2-OT) to macrophages, promoting M2 polarization and enhancing EGFR-TKI resistance in NSCLC cells [211].
5.3 Resistance due to EV-mediated proteins
EVs also carry functional proteins that play a role in developing drug resistance in cancer cells (Table 3). One study suggests that CRC cells acquire resistance to 5-FU by transferring phosphorylated STAT3 (signal transducer and activator of transcription 3) via exosomes [212]. Another study demonstrates that intercellular transfer of TrpC5 (transient receptor potential channel 5) through EVs enables recipient cells to acquire Ca2+-permeable channels, leading to increased production of P-glycoprotein (P-gp/ABCB1) and conferring chemoresistance to initially sensitive cells [213]. Furthermore, EVs can transfer oncoproteins from drug-resistant to drug-sensitive cells. For example, in NPC, EVs rich in EGFR promote EGFR overexpression and the downregulation of intracellular ROS levels via the PI3K/AKT pathway, promoting the metastatic potential of poorly metastatic NPC cells [214]. In addition, EVs produced by the K562 CML cell line contain the BCR-ABL oncoprotein, which can support the survival of BaF3 cells under IL-3 deprivation, protecting them from cell cycle arrest and apoptosis [215].
Resistance to drugs is transmitted by exchanging anti-apoptotic proteins via EVs, such as survivin. Survivin functions by inhibiting caspases, thus preventing cell death, and it is often highly expressed in various types of cancers [216]. Notably, survivin is identified in EVs derived from PCa, CC, and BCs [217-219], and its presence is associated with unfavorable clinical outcomes [216]. Exosomal survivin, for instance, has been demonstrated to enhance the survival of PCa cells under conditions of serum starvation and impairs the effectiveness of paclitaxel (PTX) treatment [220].
In cancer therapy, antibodies targeting PD-1/PD-L1 have demonstrated effectiveness [221]. However, anti-PD-1 therapy encounters challenges due to its low response rates and PD-L1-mediated immune evasion mechanisms. Research has indicated that metastatic MeL cells release EVs that express PD-L1, and this expression can be amplified by IFN-γ, leading to the inhibition of CD8+ T cell function and the promotion of cancer cell proliferation. In patients with metastatic MeL, the levels of circulating exosomal PD-L1 are positively associated with IFN-γ levels and exhibit changes during anti-PD-1 therapy, suggesting that exosomal PD-L1 may serve as a predictive marker for the outcomes of anti-PD-L1 treatment [29]. Furthermore, the expression of PD-L1 on immune cells like monocytes and NK cells contributes to the deactivation of cytotoxic T-cell responses [4, 222].
Additionally, studies indicate that exosomes derived from drug-resistant cancer cells have enriched in annexin A6 protein, which inhibits the ubiquitination and degradation of EGFR. This induction of resistance to GeM is observed in triple-negative BC. In CLL, ncRNA hY4 from CLL-derived EVs can induce the expression of PD-L1 on monocytes through a TLR7-dependent mechanism [223]. Inhibiting TLR signaling can counteract the upregulation of PD-L1 induced by CLL-derived EVs and may offer a potential therapeutic strategy against resistance [4]. These studies emphasize the role of proteins within EVs from cancer cells in propagating resistance to anticancer drugs.
5.4 Resistance due to EV-mediated mRNAs and DNA
EVs containing mRNAs and DNA have emerged as active regulators of cancer progression (Table 3). For instance, exosomes derived from AML cells carry both VEGF and VEGF receptor (VEGFR) mRNA, promoting proangiogenic activity in HUVECs by enhancing glycolysis. This results in vascular remodeling and the development of chemoresistance [224]. In another study, exosomes containing ZEB1 mRNA, a key transcription factor in the EMT, are transferred from chemoresistant NSCLC cells to chemosensitive cells, offering a new mechanism for inducing chemoresistance [225]. Furthermore, research has shown that gefitinib-resistant (GR) cells exhibit higher glycerol kinase 5 (GK5) mRNA levels than GR-sensitive cells. Exosomal GK5 mRNA plays a role in mediating gefitinib resistance, and silencing GK5 can restore drug sensitivity [226]. These findings suggest that mRNAs carried by EVs can be transferred to recipient cells, where they are translated into proteins, contributing to the acquisition of chemoresistance. Additionally, EVs containing mitochondrial DNA (mtDNA) have been associated with therapy-induced transition of cancer stem-like cells from dormant to activated, resulting in resistance to hormonal therapy through an oxidative phosphorylation-dependent mechanism [227].
5.5 Resistance due to drug efflux pumps in EVs
Tumor cells can exploit EVs to transmit chemoresistance through the horizontal transfer of drug efflux pumps (Table 3). Many instances of MDR in cancer therapy are associated with increased drug efflux pump expression [227, 228]. Members of the ATP-binding protein family typically regulate drug efflux across the plasma membrane. These proteins use ATP to pump drugs out of the cytoplasm, preventing their accumulation in cancer cells [229, 230]. Emerging evidence suggests that EVs harbor a variety of drug efflux pumps, including transporters belonging to the ATP-binding cassette (ABC) superfamily. Notable examples include ABC transporter G2 (ABCG2/BCRP, BC-resistant protein), ABC transporter C1 (ABCC1/MRP1, MDR-associated protein 1), ABC transporter B1 (P-gp/ABCB1/MDR1, MDR protein 1), and ABCA3. These pumps within EVs directly contribute to the active removal of drugs [215]. Furthermore, it is proposed that these drug efflux pumps can be transferred from drug-resistant cancer cells to drug-sensitive ones through EVs, ultimately acquiring drug resistance [26, 231]. ABCB1 (P-gp) is the most extensively studied and associated with resistance to multiple chemotherapeutic drugs among the ABC transporter family. Mechanistically, P-gp is enclosed within the vesicular membrane, which is then transferred to recipient cells and exposed on their surface [26]. For instance, when P-gp-carrying EVs from resistant BC cells are exposed to sensitive cells, they transfer resistance to cytotoxic drugs like docetaxel and doxorubicin in vitro [231, 232]. Notably, EVs containing P-gp from OVC and PCa cells confer taxane resistance to drug-sensitive cancer cells [233]. Similarly, the acquisition of docetaxel resistance in recipient cells by transferring P-gp via EVs from docetaxel-resistant PCa cells has also been documented [234]. In addition to P-gp, other members of the ABC transporter family, including ABCC1, ABCA3, and ABCG2, have been implicated in horizontal transfer mediated by EVs, contributing to drug resistance in AML [196, 235, 236] and laryngeal cells [236], respectively [196, 235, 236]. Remarkably, the persistence of this intercellular transfer of drug resistance extends beyond the half-life of the drug efflux pumps themselves. Consequently, while the transfer of ABC transporters via EVs can provide partial insights into the development of drug resistance in previously sensitive cells, additional mechanisms facilitating EV-mediated intercellular transfer of drug resistance probably exist. These mechanisms might involve the regulation of these pumps at the protein level within recipient cells, offering a more comprehensive understanding of the phenomenon [237]. In addition to pumping drugs, EVs also play a role in sequestering drugs, limiting their intracellular concentration and potentially leading to sublethal drug levels within the cells [238-244]. For instance, EVs derived from MCF7 BC cells and Balm3arege-B-cell-lymphoma cells accumulate DOX and release it into the surrounding media, reducing its intracellular concentration [245].
Similarly, BC cells have demonstrated the ability to expel the DOX into the extracellular environment via vesicle formation [246]. Notably, ABC transporters have been identified on the membranes of EV-like structures, aiding in drug sequestration [242]. This phenomenon has been observed in regions of cell-to-cell contact between adjacent cells in mitoxantrone-resistant human BC cell lines that express ABCG2, a transporter capable of sequestering mitoxantrone [242]. Additionally, EVs have been found to accumulate other ABCG2 substrates, such as imidazoacridinones and methotrexate, further underscoring the role of ABCG2-overexpressing EVs in MDR [240]. Interestingly, EVs can also facilitate the removal of drugs from the extracellular space. For instance, they can reduce the extracellular levels of anti-cancer therapeutic antibodies by presenting specific antigens on their surfaces. B-cell lymphoma-derived EVs, for example, carry the CD-20 receptor, enabling them to bind to the anti-CD20 chimeric antibody rituximab and shield target cells from its effects [27]. A similar phenomenon is observed in epithelial cell adhesion molecule (EpCam)-positive BC cells treated with the EpCam-specific antibody C215, suggesting a potential connection between EV release and tumor progression [247]. Although some studies have explored drug sequestration by EVs using cancer cell models, the evidence from in vivo models and patients remains limited. Thus, further research is necessary to unveil the mechanisms underpinning drug sequestration by EVs released by cancer cells and to ascertain the clinical significance of this process in cancer patients.
5.6 Resistance due to CASC-released EVs
Cancer associated stromal cells (CASCs) residing within the TME also releases EVs, facilitating the transfer of drug resistance capabilities (Table 4).
5.6.1 Involvement of CAFs released EVs
EVs derived from CAFs contain many molecules, including long lncRNAs, miRNAs, proteins, and circRNAs, and they play a significant role in conferring drug resistance to recipient cancer cells. CAFs containing lncRNA H19 induce resistance in CRC cells to oxaliplatin by activating the β-catenin pathway [248]. Similarly, CAFs harboring lncRNA CCAL promote resistance of CRC cells to oxaliplatin and 5-FU by activating the Wnt/β-catenin signaling pathway [249]. miRNAs are also transferred from CAFs to cancer cells via EVs, contributing to chemotherapy resistance. For instance, miR-21 present in EVs released by CAFs induces paclitaxel chemoresistance in OVC cells by downregulating the expression of apoptotic peptidase activating factor (APAF1) mRNA [250]. MiR-21 is also involved in CAF EV-induced GeM resistance in PC [251]. CAFs secrete EVs containing miR-92a-3p, which, upon transfer to CRC cells, activates the Wnt/β-catenin pathway and inhibits mitochondrial apoptosis, thereby promoting resistance to combined oxaliplatin and 5-FU treatment [252]. CAFs also transfer miR-522 to GC cells, inhibiting ferroptosis by regulating arachidonate lipoxygenase 15 (ALOX15) expression and lipid-ROS levels, ultimately suppressing cell death and promoting resistance to cisplatin and paclitaxel [253]. Additionally, CAFs-derived EVs induce resistance in NSCLC cells to cisplatin through the intercellular transfer of miRNA-130a, which is packaged into EVs with the assistance of pumilio homolog 2 (PUM2), an RNA-binding protein [254].
Furthermore, miR-20a is enriched in CAF-derived exosomes and targets the PTEN/PI3K/Akt pathway to promote cisplatin resistance in NSCLC [255]. CAFs containing miR-196a within the cargo of EVs confer cisplatin resistance in head and neck squamous cell carcinoma (HNSCC) by targeting p27 and ING5, key regulators of cell cycle and apoptosis [256]. CAF-derived EVs also contain miR-106b, critical in gemcitabine resistance by directly targeting TP53INP1 in PC [257]. Moreover, EVs containing miR-93-5p released by CAFs confer resistance by downregulating FOXA1 and upregulating TGFβ3 [258]. In addition to nucleic acids, CAF-derived EVs mediate drug resistance by transferring proteins and circRNAs.
List of EVs cargos from stromal cells in the TME with recipient cancer cells and resistance mechanisms identified.
| Donor TME cell type | EVs cargo | Recipient cancer cell type | Drugs resistance to | Resistance mechanism identified | Ref. |
|---|---|---|---|---|---|
| CAFs | lncRNA H19 | CRC | Oxaliplatin | Activation of the β-catenin pathway | [248] |
| lncRNA CCAL | CRC | Oxaliplatin | Activation of the Wnt/β-catenin signaling pathway | [249] | |
| miR-21 | OVC | Paclitaxel | Downregulation of APAF1 | [250] | |
| PC | Gemcitabine | --- | [251] | ||
| miR-92a-3p | CRC | Oxaliplatin & 5FU | Activatation of Wnt/β-catenin pathway and inhibits apoptosis | [252] | |
| miR-522 | GC | Cisplatin and Paclitaxel | Targeting ALOX15 and lipid-ROS | [253] | |
| miRNA-130a | NSCLC | Cisplatin | ---- | [254] | |
| miR-20a | NSCLC | Cisplatin | Targeting PTEN/PI3K/Akt pathway | [255] | |
| miR-196a | HNSCC | Cisplatin | Targeting CDKN1B and ING5 | [256] | |
| miR-106b | PC | Gemcitabine | Targeting TP53INP1 | [257] | |
| miR-93-5p | CRC | Radioresistance | Downregulation of FOXA1 and upregulation of TGFB3 | [258] | |
| Annexin A6 | GC | Cisplatin | Activation of β1 integrin-FAK-YAP signaling | [259] | |
| Wnt | CRC | Oxaliplatin | Activation of Wnt/β-catenin pathway | [260] | |
| cricN4BP2L2 | CRC | Oxaliplatin | Activation of PI3K/Akt/mTOR pathway | [261] | |
| MSCs | miR-222/223 | BC | Carboplatin | Promotion of quiescence | [263] |
| lncPSMA3-AS1 | MM | Bortezomib | Increasing PSMA3 expression | [264] | |
| miR23b | BC | Docetaxel | Decreased MARCKS expression | [265] | |
| miR-155 | MM | --- | Upregulation of MRP1, ABCG2, and P-gp | [267] | |
| TAMs | miR-21 | GC | Cisplatin | PI3K/Akt signaling enhancement | [269] |
| miR-223 | OVC | Cisplatin | PTEN-PI3K/Akt signaling activation | [270] | |
| Fibronectin; chitinase 3-like-1 | PC | Gemcitabine | Activation of the ERK signaling | [271] | |
| miR-365 | PC | Gemcitabine | Upregulation of triphospho-nucleotide and inducing the expression of enzyme cytidine deaminase | [150] | |
| GATA3 | OVC | Cisplatin | Induces polarization of macrophages | [272] | |
| LncRNA CRNDE | GC | Cisplatin | Inhibition of PTEN expression | [273] | |
| CAAs | miR-23a/b | HCC | 5-FU | Confers resistance by targeting the VHL/HIF axis | [274] |
| miR-21 | OVC | --- | Regulating APAF1 | [250] | |
| MTTP | CRC | Oxaliplatin | Suppresses Ferroptosis and activation of MTTP/PRAP1/ZEB1 axis | [276] | |
| MDSCs & Treg | miR-126a | BC | DOX | --- | [278] |
| miR-208b | CRC | Oxaliplatin | Targeting PDCD4 to promote Treg expansion | [280] |
Abbreviations: CRC, Colorectal cancer; GC, Gastric cancer; NSCLC, Non-small cell lung cancer; HNSCC, Head and neck squamous cell carcinoma; PC, Pancreatic cancer; BC, Breast cancer; MM, Multiple myeloma; OVC, Ovarian cancer; HCC, Hepatocellular carcinoma;PDCD4, programmed cell death factor 4;P-gp, P-glycoprotein; PDCD4, programmed cell death factor 4; DOX, Doxorubicin; ABCG2, ATP-binding cassette transporter G2; CAF, cancer-associated fibroblasts; MSC, Mesenchymal stem cell; TAM, Tumor associated macrophages; CAA, cancer associated adipocytes; MDSCs Myeloid derived suppressor cells; Treg, T-regulatory cells; ; 5-FU, 5-fluorouracil.
For instance, CAF-derived EVs contain Annexin A6, enhancing drug resistance in GC cells to cisplatin by activating the β1 integrin-adhesion kinase (FAK)-YAP signaling pathway [259]. These EVs can also carry Wnts, glycoproteins that activate the Wnt/β-catenin pathway, leading to resistance in CRC cells to oxaliplatin in vitro and in vivo [260]. Furthermore, CAF-derived circN4BP2L2 binds to EIF4A3, activating the PI3K/Akt/mTOR pathway and inducing oxaliplatin resistance in CRC cells [261]. These findings underscore the active role of CAFs within the TME in regulating therapy resistance through the transmission of various molecules via EVs, representing a complex and multifaceted resistance mechanism in cancer therapy.
5.6.2 Involvement of MSC-released EVs
MSC-EVs also play a role in promoting tumor progression by enhancing vascularization and influencing the TME. These MSC-EVs, primarily containing miRNAs and lncRNAs, mediate therapy resistance through intercellular communication [262]. MSC-EVs containing miR-222/223 can alter the cell cycle of BC cells, inducing quiescence and dormancy, compromising the response to chemotherapy [263]. The presence of the lncRNA lncPSMA3-AS1 in MSC-EVs has conferred resistance to the proteasome inhibitor bortezomib in multiple myeloma (MM) cells by increasing PSMA3 expression [264]. Another example involves miR-23b, found in EVs originating from bone marrow MSCs (BMSCs) and inducing resistance in BC cells to DOX [265]. Furthermore, EVs derived from BMSCs mediate drug resistance in MM cells [266]. These EVs carry miR-155, which promotes stemness in MM cells and upregulates drug resistance genes such as MRP1, ABCG2, and P-gp, ultimately contributing to increased drug resistance [267].
5.6.3 Involvement of TAMs and CAAs released EVs
TAMs play a significant role in regulating cancer drug resistance [268]. EVs derived from TAMs (TAM-EVs) containing miRNAs are implicated in drug resistance. For instance, miR-21 and miR-223 from TAM exosomes induce cisplatin resistance in GC [269] and OVC [270]. Proteins from TAM-EV, such as fibronectin and chitinase 3-like-1, impact PC cells sensitivity to GeM, possibly by activation of the ERK pathway [271]. miR-365 originating from TAM-EV also plays a role in GeM resistance in PC by impacting nucleotide pools and cytidine deaminase expression [150]. Reports indicate that TAMs secrete the transcription factor GATA3 via EVs, which regulates macrophage polarization and contributes to cisplatin resistance in OVC cells [272]. Furthermore, exosomes derived from M2 macrophages enriched with the lncRNA CRNDE reduce cisplatin sensitivity in GC cells by inhibiting PTEN expression [273]. Similarly, EVs released by adipocytes are known to transfer miR-23a/b to hepatocellular carcinoma (HCC) cells, leading to resistance against 5-fluorouracil (5-FU) by targeting the VHL/HIF axis [274]. Exosomes enriched with miR-21 from cancer-associated adipocytes (CAAs) are transferred to OVC cells, promoting chemotherapy resistance through regulating APAF1 [250]. Additionally, CAAs from MM cells contain LOC606724 and SNHG1, which protect against apoptotic damage induced by chemotherapeutic drugs, ultimately contributing to therapy resistance [275]. Moreover, adipocyte-derived exosomal MTTP inhibits ferroptosis and promotes oxaliplatin resistance in CRC through the MTTP/PRAP1/ZEB1 axis [276].
5.6.4 Involvement of MDSCs and Treg-released EVs
MDSC-derived exosomes (MDSCs-Exos) are involved in various aspects of cancer, including immunosuppression, tumor angiogenesis, metastasis, resistance to immunotherapy, and drug resistance [277, 278]. MeL-derived EVs induce the conversion of monocytes into monocytic MDSCs through miRNA-mediated transcriptional regulation. This process involves miR-146a, miR-100, miR-125b, and miR-155, which are enriched in the plasma of multiple myeloma (MM) patients and are closely associated with the clinical efficacy of immune checkpoint inhibitors (ICIs) [279]. Moreover, exosomal miR-126a released from MDSCs induced by DOX treatment can confer resistance to BC cells against DOX therapy and promote lung metastasis [278]. While the relationship between Treg-EVs and chemotherapy resistance though not fully characterized, recent research has provided insights into this aspect. CRC cells secrete exosomal miR-208b, which is transferred to recipient T cells. In these T cells, miR-208b targets programmed cell death factor 4 (PDCD4), leading to Treg expansion associated with oxaliplatin resistance and tumor growth promotion [280].
Similarly, EndCs within the TME can also play a role in contributing to drug resistance [281]. Antiangiogenesis therapies (AATs) have demonstrated effectiveness against various malignancies, but the subsequent emergence of cancer vasculogenesis and disease progression often limits their efficacy. For instance, Vandetanib, a vasculogenesis inhibitor, has been shown to induce the release of exosomes enriched with VEGF. These VEGF-rich exosomes promote the formation of endothelial vessels and angiogenic mimicry in HCC. This suggests that the development of vasculogenesis and disease progression following AATs may involve communication between EndCs and cancer cells mediated by VEGF-rich exosomes [282]. Furthermore, EVs carry various membrane-associated proteins with angiogenic properties. For example, EPHB2, a protein found on small EVs secreted by HNSCC cells, can stimulate cancer angiogenesis by interacting with ephrin-B2 on the surface of EndCs, activating the STAT3 signaling pathway and exacerbating resistance to anti-angiogenesis therapy [283]. On the flip side, human microvascular EndCs have been reported to promote chemotherapy resistance in NPC cells through the secretion of EVs [284]. These studies underscore the diverse mechanisms through which EVs derived from various non-cancer cell populations, including MSCs, TAMs, and CAAs, can contribute to therapy resistance in different cancer types by transferring molecules to cancer cells within the TME.
6. Role of EVs in monitoring cancer therapy response
Due to their ease of detection in various bodily fluids and unique bioactive contents, EVs have paved the way for developing novel cancer biomarkers for purposes like diagnosis, prognosis, and predicting treatment efficacy [285-288] (Table 5).
6.1 Exosomal microRNAs as a biomarker
miR-425-3p has emerged as one of the most differentially expressed miRNAs in serum exosomes of platinum-resistant patients compared to platinum-sensitive individuals. It has been linked to poor responsiveness and reduced progression-free survival (PFS) in NSCLC patients, underscoring its potential as a biomarker for predicting the response to platinum-based chemotherapy [285]. Similarly, elevated levels of miR-21 in circulating exosomes are a potential biomarker in various malignancies, spanning LivC, GC, BC, CRC, OVC, and ECs. Increased exosomal miR-21 in urine is associated with urothelial carcinoma of the bladder (UCB), and PCa [289-294]. Additionally, an elevated level of exosomal miR-146a-5p has demonstrated its potential as a robust predictor for cisplatin response, with lower levels indicating a higher recurrence rate in NSCLC patients [295]. Furthermore, several miRNAs have been identified in serum-derived exosomes from HCC patients, with some showing positive associations with HCC progression and poor survival rates [296, 297]. In contrast, others like miR-638 exhibit downregulation and negatively correlate with more aggressive tumor behavior [286, 298-301]. These findings highlight the promising role of EV-derived miRNAs as cancer biomarkers, offering valuable insights into diagnosis and prognosis.
EV-associated miRNAs have shown promise as predictive indicators for assessing therapeutic responses and patient outcomes in various cancer contexts. For example, in PCa, reduced levels of miR-34a in EVs have been linked to poor survival, potentially related to how EV-miR-34a responds to DOX treatment [198]. However, most EV-miRNAs in PCa appear to play a protective role. Studies have identified specific miRNAs, including hsa-let-7a-5p, hsa-miR-21-5p [302], miR-493-5p, miR-323a-3p, miR-411-5p, miR-494-3p, miR-379-5p, miR-654-3p, miR-409-3p, miR-543, and miR-200c-3p, which, when present at higher levels in serum EVs, are associated with a more favorable response to carbon ion radiotherapy (CIRT). Increased expression of these miRNAs predicts better therapy outcomes for patients undergoing CIRT [303]. Moreover, the abundance of exosomal miR-146a-5p has been identified as a robust predictor for cisplatin response, while elevated levels of exosomal miR-425-3p indicate cisplatin resistance in LC [295, 304]. In OVC, the analysis of EV-related miRNAs in plasma from patients resistant to platinum therapy has revealed several differentially expressed miRNAs in plasma EVs, including miR-181a, miR-21, miR-1908, miR-486, and miR-223. These EV-derived miRNAs hold promise as predictors of platinum resistance [305]. In OVC, miR-21-3p, miR-21-5p, and miR-891-5p are enriched in EVs and contribute to resistance to carboplatin-based chemotherapy. Detecting these miRNAs in EVs from biofluids may offer a means of assessing patient responses to chemotherapy, thereby providing valuable information for treatment decisions [306]. Exosomal miR-155 and miR-1246 is used to distinguish trastuzumab-resistant and sensitive patients. Their upregulation is closely associated with shorter DFS in early-stage patients and PFS in metastatic patients [307]. Detecting exosomal miRNA levels holds great promise for personalized treatment and post-treatment disease monitoring. miRNAs like let-7 and miR-497, considered tumor suppressors, have been linked to better outcomes in response to chemotherapy or radiotherapy [308]. A study by Svedman et al. revealed that levels of let-7g-5p and miR-497-5p increased in plasma EVs following targeted therapy in MM patients. These increases correlated with improved disease control, aligning with their roles in inhibiting tumor progression [308]. Furthermore, androgen receptor splice variant 7 (AR-V7), a variant of the androgen receptor [291], has been discovered in plasma EVs and is associated with shorter PFS and overall survival (OS) in metastatic castration-resistant PCa. This finding suggests that AR-V7 in EVs may be a predictive marker for resistance to hormonal therapy in these patients [309].
EV Associated Biomarkers for predicting prognosis and therapeutic response
| Cargo type | Potential EV biomarkers | Cancer Type | Therapy involved | Change | Potential application | Ref. |
|---|---|---|---|---|---|---|
| miRNAs | miR-425-3p | NSCLC | Chemotherapy | Upregulation | Predicting Low responsiveness | [285] |
| miR-146a-5p | NSCLC | Chemotherapy | Downregulation | Predicting recurrence | [295] | |
| miR-638 | HCC | Downregulation | Predicting poor prognosis | [286] | ||
| miR-34a | PCa | Chemotherapy | Downregulation | Indicating therapy failure | [198] | |
| hsa-let-7a-5p; hsa-miR-21-5p | PCa | Radiotherapy | Upregulation | Elevated radiation response | [302] | |
| 9 miRNAs | PCa | Radiotherapy | Upregulation | Predicting better therapy efficacy | [434] | |
| miR-181a, miR-21, miR-1908, miR-486, & miR-223 | OVC | Chemotherapy | Upregulation | Predicting primary platinum resistance | [305] | |
| miR-21-3p, miR-21-5p, miR-891-5p | OVC | Chemotherapy | Upregulation | Correlate with the risk of OVC relapse | [306] | |
| let-7g-5p; miR-497-5p | MeL | Targeted therapy | Upregulation | Correlated with better disease control | [308] | |
| mRNA | AR-V7 mRNA | PCa | Hormonal therapy | NA | Predicting therapy resistance | [309] |
| lncRNA | UCA1 | CRC | Chemotherapy | Upregulation | Predicting drug resistance | [310] |
| HILAR | RCC | --- | Upregulation | Correlated with metastasis | [311] | |
| CRNDE-h | CRC | --- | Upregulation | Correlated with metastasis | [287] | |
| SOX2OT | NSCLC | --- | Upregulation | Predicting prognosis | [312] | |
| ZFAS1 | GC | --- | Upregulation | Correlate with higher metastatic risk | [313] | |
| circRNAs | circ_0008928 | NSCLC | Chemotherapy | Upregulation | Predicting drug resistance | [314] |
| circPRMT5 | UCB | --- | Upregulation | Correlated with metastasis | [315] | |
| Proteins | Glypican 1 | PC | --- | Upregulation | Diagnosis and monitoring | [317] |
| Contactin 1 | MeL | --- | Upregulation | Diagnosis | [288] | |
| TRPC5 | BC | chemotherapy | Upregulation | Predicting chemoresistance | [318] | |
| Annexin A6 | BC | chemotherapy | Upregulation | Predicting poor response | [319] | |
| PD-L1 | MeL | Immunotherapy | Upregulation | Predicting treatment responses | [159] | |
| PD-1 and CD28 | MeL | Immunotherapy | Upregulation | Predicting good treatment responses | [323] | |
| PSA, PSMA, 5T4 | PCa | Hormonal therapy | Downregulation | Monitoring treatment-related responses | [324] | |
| MCSP, MCAM, LNGFR, ErbB3 | MeL | Targeted therapy | NA | Monitoring treatment-related responses | [325] |
Abbreviations: NSCLC, Non-small cell lung cancer; HCC, Hepatocellular carcinoma; PCa, Prostate cancer; OVC, Ovarian cancer; MeLC, Melanoma cancer; CRC, Colorectal cancer; RCC, Renal cell carcinoma; GC, Gastric cancer; UCB, Urothelial Carcinoma of the Bladder; PC, Pancreatic cancer; BC, Breast cancer.
6.2 EV-associated lncRNAs and circRNAs as biomarkers
Several deregulated lncRNAs and circRNAs found in EVs play crucial roles in cancer drug resistance and have the potential to serve as novel biomarkers. For example, a study conducted by Yang et al. demonstrated that circulating exosomal lncRNA UCA1 could predict the efficacy of cetuximab treatment in CRC patients. Patients with a poor response to treatment exhibited significantly higher levels of circulating exosomal UCA1 than those with a favorable response [310]. In renal cancer carcinoma (RCC) patients, the expression of vesicular lncRNA HILAR was notably higher in those with metastasis than those without metastasis [311]. Another study revealed a significant correlation between increased expression of EV-associated lncRNA CRNDE-h and regional lymph node metastasis and distant metastasis in CRC patients [287]. Furthermore, elevated levels of vesicular lncRNA-SOX2OT were associated with shorter overall survival rates in NSCLC patients [312]. Zinc finger antisense 1 (ZFAS1), which belongs to competing endogenous lncRNAs, was enriched in GC patients' serum EVs. High levels of vesicular ZFAS1 may be linked to a higher risk of lymphatic metastasis in GC patients [313]. Another study demonstrated that circ_0008928 contributes to cisplatin resistance in NSCLC by regulating the miR-488/HK2 axis. The levels of circ_0008928 were upregulated in serum exosomes of cisplatin-resistant patients [314]. Similarly, the upregulated expression of circPRMT5, a circRNA specific to UCB, was more likely to occur in patients with lymph node metastasis than in those without metastasis in EVs derived from serum and urine [315]. Despite their potential value, detecting exosomal RNAs in early-stage cancers is challenging due to their low expression levels. A nanoparticle-based biochips method has been developed to capture circulating EVs without isolation. It enables the visualization and amplification of encapsulated RNAs in situ in a single step, making it advantageous for early cancer detection [316].
6.3 EV-associated proteins as biomarkers
EV proteins hold great promise as biomarkers for assessing cancer therapy effectiveness. For example, Glypican-1 (GPC1) is overexpressed explicitly in EVs derived from tumors. Detecting serum-derived EVs containing GPC1 can differentiate between healthy individuals, patients with benign PC, and those with early- and late-stage PC, offering high specificity and sensitivity [317]. Similarly, contactin-1 is found at elevated levels in plasma EVs from MeL patients compared to those from healthy individuals, suggesting that detecting these differently expressed proteins in MeL cancer-derived EVs could play a crucial role in tumor diagnosis and monitoring [288]. Studies on EV proteins in BC have also revealed interesting insights. For instance, the transfer of transient receptor potential channel 5 (TRPC5) via EVs stimulates the production of the p-gp, contributing to chemoresistance in non-resistant cells. Increased TRPC5 expression in plasma EVs predicts low chemotherapy response and a strong association with the development of acquired chemoresistance before tumor progression [213, 318]. Moreover, overexpression of annexin A6 is linked to a poor response to GeM-based chemotherapy [319]. Emerging evidence suggests that exosomal PD-L1 plays a role in immunosuppression and may be a potential predictor for anti-PD-1 therapy in MeL and NSCLC. High levels of EV-PD-L1 may indicate T cell exhaustion before treatment, and increased PD-L1 in EVs may predict improved anti-tumor immunity following immunotherapy in responders [159, 320-322]. This phenomenon was not observed in non-responder MeL patients [159]. Additionally, higher expression of PD-1 and CD28 in immune cell-secreted EVs may predict a positive response to immunotherapy in metastatic MeL, offering a new source of EVs for potential biomarker discovery [323]. Furthermore, researchers have demonstrated that PCa markers, such as PSA, PSMA (prostate-specific membrane antigen), and 5T4 (oncofetal glycoprotein), are differentially positive in urinary EVs from cancer patients compared to healthy volunteers. Furtehrmore, PSA, PSMA, and 5T4 were found to decrease after therapy, suggesting that PCa markers in EVs have the potential as biomarkers for monitoring patients post-therapy. However, further validation in larger cohorts is necessary [324]. Studies have also explored specific proteins associated with MeL therapy and progression in EVs, including MeL chondroitin sulfate proteoglycan (MCSP), MeL cell adhesion molecule (MCAM), low-affinity nerve growth factor receptor (LNGFR), and receptor tyrosine-protein kinase (ErbB3). These proteins exhibited changes in different MeL patients during and after targeted therapy, but their precise functions in patients undergoing treatments require further investigation [325]. CD31, a marker for EndC-derived EVs that regulate T-cell responses, has also been studied. Low levels of CD31+ endothelial-derived EVs in responders to ICIs corresponded to an activated immune response.
In contrast, high levels may indicate endothelial-induced tumor immune escape in non-responders with NSCLC [326]. In summary, EV-associated biomolecules have the potential to serve as valuable biomarkers for assessing therapy response (Table 5). Although the accessibility of EVs makes them appealing for biomarker discovery, clinical validation is still needed for their routine use.
7. Impeding EV biogenesis, trafficking, release, and recipient cell uptake; novel approach to cancer treatment
Recently, EVs have been utilized as a focal point for combating drug resistance. Potential strategies encompass reshaping the composition of EVs, disrupting EV-mediated interactions within the TME, impeding the processes of EV biogenesis and release, enhancing the degradation of EVs, capturing circulating EVs originating from tumors, and hindering the uptake of EVs by recipient cells [327]. Additionally, investigations have delved into using exosomes derived from specific cell types and the potential engineering of EVs to serve as a delivery tool for overcoming drug resistance. Disrupting the proteins involved in EV biogenesis and secretion represents a promising avenue for cancer treatment [124, 328-330] (Figure 4). This includes directing attention to endosomal sorting complexes required for transport proteins (ESCRT-dependent), neutral sphingomyelinases (ESCRT-independent), tetraspanin family proteins, as well as proteins governing the trafficking and secretion of small EVs (sEVs), such as Rab family proteins, soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs), Rac-1, and actin cytoskeletal proteins [329, 331, 332]. Large EVs (LEVs) can potentially be inhibited by targeting small GTPase RhoA [333], membrane cholesterol [334], and the calcium-dependent enzymatic machinery responsible for the externalization of phosphatidylserine [335].
This figure illustrates strategies for overcoming drug resistance by targeting key proteins involved in the biogenesis, secretion, and uptake of EVs. Red lines represent therapeutic agents that interfere with EV biogenesis, secretion, or internalization processes, aiming to disrupt the pro-tumoral functions of EVs. Additionally, a green dotted line signifies an activator of ISGylation, a process that promotes the fusion of multivesicular bodies (MVBs) with lysosomes for degradation, thus mitigating the effects of EVs.
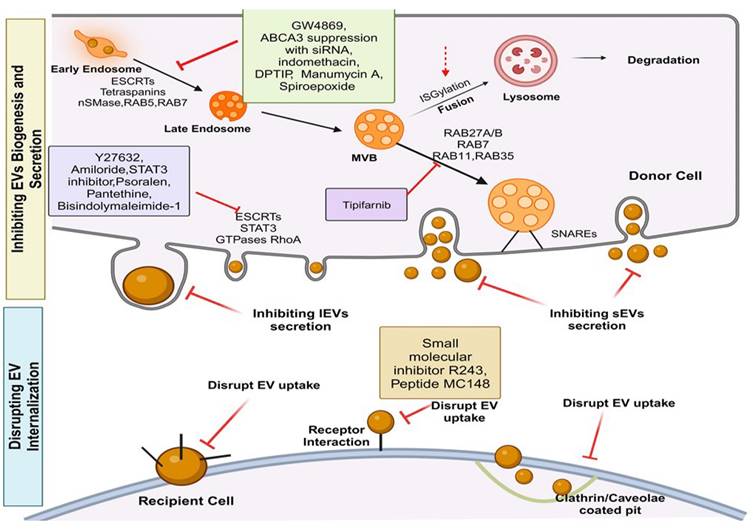
Pharmacological agents have been explored for their potential to inhibit EVs originating from tumors. For example, targeting oncoproteins like STAT3, which play a role in EV biogenesis and secretion, can be accomplished using compounds like amiloride or STAT3 inhibitors. Combining these inhibitors with chemotherapy has shown promising results, leading to increased apoptosis and reduced proliferation in chemoresistant OVC cells [336]. Psoralen, a compound derived from Fructus Psoraleae, can reduce the production and release of exosomes by modulating PPAR and p53 signaling pathways, thereby overcoming resistance to DOX in BC [337]. Senescent stromal cells, contributors to cancer resistance through the excessive production of EVs, can be targeted by activating SIRT1 using SRT2104, thus curbing EV production [338]. Moreover, various drugs and compounds have been examined for their potential to inhibit EV biogenesis and release. These include the ROCK inhibitor Y27632, which reorganizes the cellular cytoskeleton and acts as an inhibitor of EV formation [333, 339, 340]. The membrane-neutral sphingomyelinase (nSMase) inhibitor GW4869 has demonstrated the ability to block ceramide-mediated EV biogenesis [341-343], resulting in the reversal of drug resistance in OVC cells [344], and decreased tumor progression in MeL [345]. D-pantethine, an inhibitor of cholesterol synthesis, has been found to reduce EV release [346], potentially due to reduced cellular cholesterol levels or the inhibition of phosphatidylserine (PS) translocation [347]. Moreover, drugs manumycin A, spiroepoxide, and 2,6-Dimethoxy-4-(5-Phenyl-4-Thiophen-2-yl-1H-Imidazol-2 yl)-Phenol (DPTIP), have been shown to selectively block the release of EVs from various cancer cells by targeting neutral sphingomyelinases or ESCRT machinery [347]. Additionally, the drug Bisindolylmaleimide-1 (BIM-1) has been shown to significantly inhibit EV release from BC and PCa cells [335], potentially by reducing the externalization of PS [335, 348]. Tipifarnib is another compound with the potential to modulate EV biogenesis and secretion in PCa cells by inhibiting essential proteins (ALIX, nSMase2, and Rab27a) involved in EV production [349]. Several other compounds, including U0126, clopidogrel, and calpeptin, have been reported to reduce EV formation and release [46].
An additional strategy to combat cancer drug resistance involves inhibiting the internalization of exosomes by target cells. For instance, MM cells internalize exosomes from BMSC, leading to reduced sensitivity to bortezomib and a chemotherapy drug. MM and BMSCs communicate through macropinocytosis and Clathrin- and caveolin-dependent endocytosis. Inhibitors of these endocytic processes in MM cells suppress the internalization of BMSC-derived exosomes, enhancing the effectiveness of bortezomib by attenuating exosome-mediated chemoresistance [350]. A recent study uncovered a new way that cells take up EVs involving the interaction between a cell receptor called CCR8, the sugars on EVs' surfaces, and a molecule called CCL18. Blocking CCR8 using specific inhibitors can prevent EV uptake by GBM cells and make them more responsive to temozolomide (TMZ), a chemotherapy drug used to treat GBM [351].
Functional transfer of EVs containing MDR transporters is associated with the desensitization of cancer cells to chemotherapeutics. Therefore, reducing the proportion of these vesicular pumps could be a valuable therapeutic approach. For example, ABCA3, which promotes the secretion of EVs and the development of drug resistance, can be suppressed using siRNA or indomethacin. Inhibition of ABCA3 leads to decreased exosome production, increased intracellular drug retention, altered subcellular drug accumulation, prolonged nuclear drug retention, and enhanced sensitivity of cancer cells to drugs like DOX and pixantrone [352]. Moreover, administering a proton pump inhibitor to metastatic MeL cells has improved the effectiveness of cisplatin treatment. This improvement is attributed to a reduction in the production of EVs and an increased retention of chemotherapy drugs within the tumor cells [237, 353].
Exosomal PD-L1 molecules inhibit T-cell activation in lymph nodes, contributing to cancer immune evasion. Strategies to target exosomal PD-L1 are being explored to overcome cancer resistance to immunotherapy. In one study, removing PD-L1 from cancer cell-derived exosomes inhibited cancer growth, even in models resistant to anti-PD-L1 antibodies [247]. Combining anti-PD-L1 antibodies with the blockade of exosomal PD-L1 has demonstrated synergistic effects in suppressing cancer growth [247]. In another study, the use of macitentan effectively inhibited the production of EV PD-L1 in BC cells. This inhibition enhanced antitumor immune responses and improved therapeutic outcomes when macitentan was used with anti-PD-L1 antibody treatment [248]. Furthermore, a phototheranostic approach involving metal-phenolic networks was employed to deliver a ferroptosis inducer (Fe3+) and an exosome inhibitor (GW4869). This strategy combined photothermal therapy with exosome-based immunotherapy, reactivating T cells by counteracting exosomal PD-L1-mediated suppression and ultimately inducing robust antitumor immunity in metastatic MeL [249].
8. EVs as potential drug delivery vehicles
Efficient cancer treatment necessitates the precise and targeted delivery of therapeutic agents to cancer cells [354]. EVs have gained attention as potential drug delivery vehicles due to their high drug-loading capacity and specific targeting capabilities, promising to overcome drug resistance in cancer treatment [29]. Exosomal miRNAs have emerged as valuable tools in cancer diagnosis and therapy [355, 356]. Research has demonstrated that loading EVs with miRNAs designed to target cancer-promoting genes can effectively inhibit cancer growth and reverse drug resistance. For instance, in the case of GBM, resistance to TMZ is associated with the loss of miR-151a. Introducing miR-151a into TMZ-resistant cells through exosomal delivery inhibits XRCC4-mediated DNA repair, thereby increasing sensitivity to therapy [357]. Similarly, exosomal delivery of miR-122, miR-146a, and miR-567 enhances the sensitivity of HCC cells to sorafenib [358], OVC cells to docetaxel [359], and BC cells to trastuzumab [359], respectively. In addition to delivering miRNAs, EVs can carry miRNA inhibitors to achieve anti-tumor effects. For example, elevated levels of miR-374a-5p in oxaliplatin-resistant GC cells are associated with poor prognosis. A miR-374a-5p inhibitor via exosome delivery promotes cell apoptosis and overcomes drug resistance [360]. Similarly, in cisplatin-resistant GC cells with high levels of miR-214, exosome-mediated delivery of a miR-214 inhibitor reverses chemoresistance and inhibits cancer growth [361]. Furthermore, miR-21 induces 5-FU resistance in CRC by downregulating hMSH2 (human DNA MutS homolog 2) [362]. Exosomal delivery of a miR-21 inhibitor combined with 5-FU treatment enhances the sensitivity of HER2+ cancer cells to 5-FU [363]. Additionally, exosome-mediated delivery of a miR-21 inhibitor reduces the resistance of BC cells to DOX by blocking the function of oncogenic miR-21 [364].
EVs can load and deliver siRNAs to tumor cells, including those within the brain, bypassing the blood-brain barrier (BBB) [365-367]. For instance, exosomal delivery of siRNA efficiently silences circRNA-SORE in HCC cells. This circRNA promotes PRP19-mediated degradation of YBX1, enhancing sorafenib-induced apoptosis [368]. In sorafenib-resistant cancer cells, exosomal siRNA targeting GRP78 increases the sensitivity of drug-resistant HCC cells to sorafenib [369]. Notably, engineered exosomes loaded with siRNAs have been used to target PC cells and showed significant tumor inhibition in preclinical models, with clinical trials underway [370, 371]. Furthermore, exosomes loaded with siRNA targeting metastasis-associated protein 1 (MTA1) enhance the therapeutic effect of GeM by inhibiting HIF-α and autophagy in luminal-b BC [372]. Similarly, exosome-mediated delivery of siRNA against carnitine palmitoyltransferase 1A (CPT1A) enhances the response to oxaliplatin in CRC by inhibiting CPT1A and downstream fatty acid oxidation (FAO) [373]. The delivery of si-cMet via exosomes has significantly reversed cisplatin resistance in GC [374]. These findings illustrate that EV-mediated delivery of siRNAs can effectively target drug-resistant cancer cells, restoring their sensitivity to treatment. Furthermore, in the case of oxaliplatin-resistant CRC, exosomal delivery of circ-FBXW7, which binds to miR-128-3p, has demonstrated the potential to render CRC cells sensitive to oxaliplatin and inhibit drug efflux, presenting a promising therapeutic strategy [375]. Additionally, the lncRNA PGM5 antisense RNA1 (PGM5AS1) has been found to inhibit acquired oxaliplatin tolerance in CRCs. The co-encapsulation of oxaliplatin and PGM5AS1 in exosomes represents an efficient approach to reversing oxaliplatin resistance [376]. Furthermore, a membrane fusion technique has been developed to load macromolecular proteins into EVs for targeted delivery. In one example, researchers employed exosomal delivery of Survivin-T34A in PC, leading to increased sensitivity to GeM. Survivin-T34A, a dominant-negative mutant of survivin, triggers caspase activation and cell apoptosis by dissociating the caspase9/survivin protein complex [377].
The recombinant form of TRAIL (rTRAIL) has been extensively studied in clinical trials [378]. However, the limited bioavailability of rTRAIL has posed challenges for its clinical application [379]. EV-mediated delivery of TRAIL (EV-T) in a membrane-bound form has shown enhanced ability to induce cell apoptosis. Importantly, T-EV has demonstrated significant apoptotic effects in TRAIL-resistant cancer cells, suggesting that EV-TRAIL could serve as a viable alternative to rTRAIL for cancer treatment, yielding superior therapeutic outcomes [380]. Furthermore, combining T-EV with other drugs, such as SCH727965 (dinaciclib), an effective CDK inhibitor, has been investigated to sensitize the cellular response pathways in cancer cells. Dinaciclib has substantially augmented the cytotoxic effects of T-EV in cancer cells expressing death receptor 5. Combining a low dose of T-EV with dinaciclib has led to a complete cancer regression in mouse models. These findings underscore the potential of combining T-EV with dinaciclib as a promising approach to enhance the effectiveness of cancer therapy [381].
9. EV-mediated drug delivery to overcome cancer drug resistance
Recent research has proved that EVs can serve as delivery vehicles to augment drug accumulation within tumor tissues, extend drug circulation in the bloodstream, enhance therapeutic effectiveness, and mitigate systemic toxicity [382-388]. One promising avenue involves using exosomes as a platform for delivering the chemotherapeutic drug paclitaxel (PTX) to combat MDR cancer. Exosomes derived from macrophages are loaded with PTX through sonication, resulting in a formulation known as exoPTX. ExoPTX enhances PTX solubility and circumvents drug resistance mediated by P-gp. This exosome-mediated delivery approach exhibits high PTX loading and sustained release within resistant cancer cells, thereby increasing cytotoxicity compared to free PTX [419]. Furthermore, EVs loaded with PTX have demonstrated effective targeting and antitumor effects in LC, BC, and PCs [383, 386, 389, 390]. Notably, PTX's limited ability to traverse the BBB has been addressed through the use of PTX-loaded exosomes, improving the treatment of GBM [391]. Autologous PCa cell-derived EVs have also been explored as efficient carriers for PTX, enhancing its cytotoxic effects [382]. Exosomes derived from BMSCs loaded with PTX and GeM were employed to overcome chemotherapy resistance in PC. This approach demonstrated low systemic toxicity, excellent tissue penetration, and improved anticancer efficacy, offering a prospective strategy for targeted PC therapy [392]. DOX is known for its effectiveness against various cancers but is limited by poor biocompatibility and cardiotoxicity. EVs have been shown to mitigate DOX's cardiotoxicity by reducing its accumulation in the heart, making it a potential means of enhancing its clinical application [393, 394]. DOX-loaded EVs derived from various sources, including macrophages, DCs, HEK293 cells, and red blood cells, have exhibited superior anticancer effects compared to free DOX [385, 389, 395, 396]. Furthermore, to enhance drug delivery strategies, researchers have explored the development of co-delivery systems involving EVs and inorganic nanocarriers, aiming to leverage the advantages of both approaches. The combination of EVs with hydrogels and liposomes has gained significant attention in recent studies. This synergistic approach improves the targeting capabilities of EVs and enhances drug encapsulation efficiency, surpassing the performance of standalone inorganic nanocarriers like gold nanoparticles and therapeutic nano-bioconjugates [433-434]. For example, Li et al. have developed hybrid nanoparticles inspired by biology. These nanoparticles merge tumor exosomes expressing CD47 with cRGD-modified liposomes to co-deliver the chemotherapeutic drug triptolide (TP) and miR-497. This innovative combination exhibits potent anticancer activity through a synergistic mechanism involving inhibiting the PI3K/Akt/mTOR signaling pathway, induction of excessive ROS production, and repolarization of macrophages from an M2 to an M1 phenotype. This approach effectively overcomes chemoresistance in OVC [397]. In summary, EVs serve as an effective nano delivery platform for various anticancer drugs, potentially mitigating their original limitations in clinical use.
Apart from acting as delivery tools, EVs have been engineered with functional nanomaterials to enhance their efficiency, specificity, and safety in cancer therapy [398]. For instance, in the case of PTX delivery, researchers have loaded PTX into exosomes (exoPTX) and modified them with aminoethylanisamide-polyethylene glycol (AA-PEG). This modification enhances loading capacity and targeting capabilities, significantly improving the therapeutic effectiveness of PTX [383]. In treating metastatic peritoneal cancer, thermo-peritoneal chemotherapy has proven effective but is hindered by drug delivery challenges and the development of resistance. Innovative approaches in drug delivery involve the genetic engineering of fibroblasts to produce exosomes expressing CD47, which are then combined with thermosensitive liposomes (referred to as gETLNPs) for the delivery of docetaxel. This novel combination therapy enhances the targeted delivery of cancer drugs and successfully overcomes drug resistance [399]. Additionally, researchers have engineered EVs derived from HEK293T cells by attaching a hyaluronic acid derivative with octadecyl tails (referred to as lipHA) to the EV membrane, resulting in engineered EVs known as lipHA-engineered hEVs. These modified EVs efficiently deliver chemotherapeutic drugs to drug-resistant cancer cells through CD44-mediated targeting. Simultaneously, they inhibit drug efflux by suppressing P-gp expression, effectively reversing cancer drug resistance [400]. Researchers have genetically engineered fibroblasts to produce exosomes expressing CD47, fused with thermosensitive liposomes (gETLNPs) to deliver docetaxel. This innovative combination therapy enhances cancer-targeted drug delivery and successfully overcomes drug resistance [399]. Furthermore, EVs derived from HEK293T cells have been engineered by attaching a hyaluronic acid derivative with octadecyl tails (lipHA) to the EV membrane, resulting in lipHA-engineered hEVs. These engineered EVs deliver chemotherapeutic drugs to drug-resistant cancer cells through CD44-mediated targeting. Simultaneously, they inhibit drug efflux by suppressing P-gp expression, thus effectively reversing cancer drug resistance [400]. In addition to drug delivery, functionalized EVs have shown promise in radiotherapy sensitization. For instance, engineered exosomes from M1 macrophages (M1Exos) is used as sensitizers. These M1Exos were modified with catalases to alleviate tumor hypoxia and loaded with an anti-PD-L1 nanobody and a DNA damage repair inhibitor. This combination leads to effective remodeling of the immunosuppressive TME, ultimately enhancing radiotherapy efficacy [401].
10. Concluding remarks and future perspectives
The rapid expansion of research on EVs has shed considerable light on their versatile and pivotal roles within the TME. This comprehensive review revisits the intricate biology of EVs, encompassing their composition and uptake, which are fundamental for deciphering EV-mediated intercellular communication within the complex TME between cancer cells and CASCs, delineating their involvement in metabolic reprogramming, horizontal transfer of malignant traits, and their substantial contribution to resistance against anticancer therapeutics within the intricate TME.
Central to the discourse is the pivotal role of EV constituents, spanning proteins, nucleic acids, lipids, and metabolites, in orchestrating cell-cell communication dynamics within the TME. While much emphasis has been placed on the delivery of proteins and nucleic acids following EV internalization, there is an imperative need for further investigation into the intricate mechanisms governing the sorting and export of these bioactive cargo molecules. Understanding how these EV cargoes evade degradation post-internalization and subsequently exert their functional impact is paramount. Notably, it is worth acknowledging that not all facets of EV-mediated communication rely solely on internalization. The significance of EV-cell surface signaling cannot be understated. Technological advancements have allowed for a more confident characterization of EVs, even with reduced input material. However, adherence to rigorous guidelines that substantiate the 'extracellular' and 'vesicular' nature of isolated particles remains essential.
The TME, a complex milieu encompassing cancer cells, CASCs, and non-cellular components, hinges upon EV-mediated interactions, leading to mutual education of cancer cells and CASCs. These interactions profoundly affect the existing functions of these cellular constituents and the acquisition of novel traits. For instance, EVs have demonstrated the capacity to activate fibroblasts into CAFs and fine-tune cancer cells to promote proliferation and metastasis. Conversely, certain immune cell-derived EVs have exhibited the ability to enhance anticancer activities. Furthermore, EV-mediated signaling within the TME significantly influences angiogenesis and vascular permeability, creating a hospitable environment conducive to tumor development. Intriguingly, neurons have emerged as participants in the EV-mediated crosstalk, as cancer-derived EVs can reprogram neurons into a pro-tumorigenic phenotype. EVs also carry proteinases that contribute to extracellular matrix degradation, exacerbated by the hypoxic and acidic conditions often found within the TME. These findings offer promising avenues for targeted cancer treatment, with standard EV-mediated positive feedback loops between cancer cells and TME constituents representing attractive therapeutic targets. Beyond the modulation of specific TME components, EVs play a pivotal role in reshaping the cancer-associated metabolic landscape, conferring malignant traits, and fostering resistance to various therapeutic modalities. These multifaceted processes involve EV-mediated communication across diverse cell types, underscoring the importance of comprehensive, combination therapeutic strategies. Despite the notable progress made in EV research, many challenges remain. These encompass the standardization of EV isolation protocols, the assessment of clinical efficacy, and the need to classify various EV subtypes accurately. Furthermore, there is a critical need to develop accurate and highly sensitive tools for detecting single tumor-derived EVs, along with a deeper understanding of the trafficking and uptake of tumor EVs.
In summary, the expanding body of evidence from preclinical and clinical studies underscores the immense potential of EVs as diagnostic cancer biomarkers and as carriers for targeted drug delivery. However, several scientific challenges persist, including refining EV-based drug delivery strategies and large-scale purification techniques. To fully harness the potential of EVs in cancer therapeutics, concerted efforts from researchers, clinicians, and regulatory authorities are imperative. The ongoing progress in EV research undoubtedly holds the promise of translating laboratory findings into clinically viable tools for cancer management within the intricate TME.
Abbreviations
AML: Acute myeloid leukemia
BMSCs: Bone marrow MSCs
CAAs: Cancer-associated adipocytes
CAS: Cancer-associated stromal cells
CLL: Chronic lymphocytic leukemia
CC: Cervical cancer
CRC: Colorectal cancer
EndCs: Endothelial cells
EV: Extracellular vesicles
EC: Esophageal cancer
EMT: Epithelial-mesenchymal transition
ECM: Extracellular matrix
FGF: Fibroblast growth factors
GBM: Glioblastoma
GC: Gastric cancer
HCC: Hepatocellular carcinoma
HSP: Heat-shock proteins
ICIs: Immune checkpoint inhibitors
HNSCC: Head and neck squamous cell carcinoma
LC: Lung cancer
MeL: Melanoma
MDSCs: Myeloid-derived suppressor cells
MSCs: Mesenchymal stem cells
MHC: Major histocompatibility complex
NSCLC: Non-small cell lung cancer
NPC: Nasopharyngeal carcinoma
OVC: Ovarian cancer
PDGF: Platelet-derived growth factors
PC: Pancreatic cancer
PCa: Prostate cancer
RCC: Renal cell carcinoma
SS: Synovial Sarcoma
TME: Tumor microenvironment
TIME: Tumor immune microenvironment
TAMs: Tumor-associated macrophages
TGF-β: Transforming growth factor-beta
UCB: Urothelial carcinoma of the bladder
VEGF: Vascular endothelial growth factor
Acknowledgements
Figures were created using BioRender.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Wu M, Wang M, Jia H, Wu P. Extracellular vesicles: emerging anti-cancer drugs and advanced functionalization platforms for cancer therapy. Drug delivery. 2022;29:2513-38
2. Maman S, Witz IP. A history of exploring cancer in context. Nature reviews Cancer. 2018;18:359-76
3. van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nature reviews Molecular cell biology. 2018;19:213-28
4. Zhang DX, Vu LT, Ismail NN, Le MTN, Grimson A. Landscape of extracellular vesicles in the tumour microenvironment: Interactions with stromal cells and with non-cell components, and impacts on metabolic reprogramming, horizontal transfer of neoplastic traits, and the emergence of therapeutic resistance. Seminars in cancer biology. 2021;74:24-44
5. Mathivanan S, Lim JW, Tauro BJ, Ji H, Moritz RL, Simpson RJ. Proteomics analysis of A33 immunoaffinity-purified exosomes released from the human colon tumor cell line LIM1215 reveals a tissue-specific protein signature. Molecular & cellular proteomics: MCP. 2010;9:197-208
6. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). The Journal of biological chemistry. 1987;262:9412-20
7. Yuana Y, Sturk A, Nieuwland R. Extracellular vesicles in physiological and pathological conditions. Blood reviews. 2013;27:31-9
8. Sullivan R, Maresh G, Zhang X, Salomon C, Hooper J, Margolin D. et al. The Emerging Roles of Extracellular Vesicles As Communication Vehicles within the Tumor Microenvironment and Beyond. Frontiers in endocrinology. 2017;8:194
9. Wiklander OPB, Brennan M, Lötvall J, Breakefield XO, El Andaloussi S. Advances in therapeutic applications of extracellular vesicles. Science translational medicine. 2019;11:eaav8521
10. Bhat AA, Nisar S, Maacha S, Carneiro-Lobo TC, Akhtar S, Siveen KS. et al. Cytokine-chemokine network driven metastasis in esophageal cancer; promising avenue for targeted therapy. Molecular cancer. 2021;20:2
11. Bhat AA, Yousuf P, Wani NA, Rizwan A, Chauhan SS, Siddiqi MA. et al. Tumor microenvironment: an evil nexus promoting aggressive head and neck squamous cell carcinoma and avenue for targeted therapy. Signal transduction and targeted therapy. 2021;6:12
12. da Cunha BR, Domingos C, Stefanini ACB, Henrique T, Polachini GM, Castelo-Branco P. et al. Cellular Interactions in the Tumor Microenvironment: The Role of Secretome. Journal of Cancer. 2019;10:4574-87
13. Anderson NM, Simon MC. The tumor microenvironment. Current biology: CB. 2020;30:R921-r5
14. Shah R, Patel T, Freedman JE. Circulating Extracellular Vesicles in Human Disease. The New England journal of medicine. 2018;379:2180-1
15. Bandari SK, Tripathi K, Rangarajan S, Sanderson RD. Therapy-induced chemoexosomes: Sinister small extracellular vesicles that support tumor survival and progression. Cancer letters. 2020;493:113-9
16. Xavier CPR, Caires HR, Barbosa MAG, Bergantim R, Guimarães JE, Vasconcelos MH. The Role of Extracellular Vesicles in the Hallmarks of Cancer and Drug Resistance. Cells. 2020;9:1141
17. Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ. Extracellular vesicles in cancer - implications for future improvements in cancer care. Nature reviews Clinical oncology. 2018;15:617-38
18. Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nature reviews Immunology. 2009;9:581-93
19. Xie F, Zhou X, Fang M, Li H, Su P, Tu Y. et al. Extracellular Vesicles in Cancer Immune Microenvironment and Cancer Immunotherapy. Advanced science (Weinheim, Baden-Wurttemberg, Germany). 2019;6:1901779
20. Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487-95
21. Ciardiello C, Cavallini L, Spinelli C, Yang J, Reis-Sobreiro M, de Candia P. et al. Focus on Extracellular Vesicles: New Frontiers of Cell-to-Cell Communication in Cancer. International journal of molecular sciences. 2016;17:175
22. Bebelman MP, Smit MJ, Pegtel DM, Baglio SR. Biogenesis and function of extracellular vesicles in cancer. Pharmacology & therapeutics. 2018;188:1-11
23. Benito-Martin A, Di Giannatale A, Ceder S, Peinado H. The new deal: a potential role for secreted vesicles in innate immunity and tumor progression. Frontiers in immunology. 2015;6:66
24. van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacological reviews. 2012;64:676-705
25. Assaraf YG, Brozovic A, Gonçalves AC, Jurkovicova D, Linē A, Machuqueiro M. et al. The multi-factorial nature of clinical multidrug resistance in cancer. Drug resistance updates: reviews and commentaries in antimicrobial and anticancer chemotherapy. 2019;46:100645
26. Bebawy M, Combes V, Lee E, Jaiswal R, Gong J, Bonhoure A. et al. Membrane microparticles mediate transfer of P-glycoprotein to drug sensitive cancer cells. Leukemia. 2009;23:1643-9
27. Aung T, Chapuy B, Vogel D, Wenzel D, Oppermann M, Lahmann M. et al. Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15336-41
28. Anastasiadou E, Slack FJ. Cancer. Malicious exosomes. Science (New York, NY). 2014;346:1459-60
29. Yang Q, Xu J, Gu J, Shi H, Zhang J, Zhang J. et al. Extracellular Vesicles in Cancer Drug Resistance: Roles, Mechanisms, and Implications. Advanced science (Weinheim, Baden-Wurttemberg, Germany). 2022;9:e2201609
30. Merchant ML, Rood IM, Deegens JKJ, Klein JB. Isolation and characterization of urinary extracellular vesicles: implications for biomarker discovery. Nature reviews Nephrology. 2017;13:731-49
31. Iwai K, Minamisawa T, Suga K, Yajima Y, Shiba K. Isolation of human salivary extracellular vesicles by iodixanol density gradient ultracentrifugation and their characterizations. Journal of extracellular vesicles. 2016;5:30829
32. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. The Journal of cell biology. 2013;200:373-83
33. Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends in cell biology. 2015;25:364-72
34. Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annual review of cell and developmental biology. 2014;30:255-89
35. Špilak A, Brachner A, Kegler U, Neuhaus W, Noehammer C. Implications and pitfalls for cancer diagnostics exploiting extracellular vesicles. Advanced drug delivery reviews. 2021;175:113819
36. Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H. et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nature cell biology. 2018;20:332-43
37. Willms E, Cabañas C, Mäger I, Wood MJA, Vader P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Frontiers in immunology. 2018;9:738
38. Simons M, Raposo G. Exosomes-vesicular carriers for intercellular communication. Current opinion in cell biology. 2009;21:575-81
39. Poliakov A, Spilman M, Dokland T, Amling CL, Mobley JA. Structural heterogeneity and protein composition of exosome-like vesicles (prostasomes) in human semen. The Prostate. 2009;69:159-67
40. Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochimica et biophysica acta. 2012;1820:940-8
41. Conde-Vancells J, Rodriguez-Suarez E, Embade N, Gil D, Matthiesen R, Valle M. et al. Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. Journal of proteome research. 2008;7:5157-66
42. Subra C, Grand D, Laulagnier K, Stella A, Lambeau G, Paillasse M. et al. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. Journal of lipid research. 2010;51:2105-20
43. Verderio C, Gabrielli M, Giussani P. Role of sphingolipids in the biogenesis and biological activity of extracellular vesicles. Journal of lipid research. 2018;59:1325-40
44. Haraszti RA, Didiot MC, Sapp E, Leszyk J, Shaffer SA, Rockwell HE. et al. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. Journal of extracellular vesicles. 2016;5:32570
45. Pfrieger FW, Vitale N. Cholesterol and the journey of extracellular vesicles. Journal of lipid research. 2018;59:2255-61
46. Catalano M, O'Driscoll L. Inhibiting extracellular vesicles formation and release: a review of EV inhibitors. Journal of extracellular vesicles. 2020;9:1703244
47. Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature cell biology. 2007;9:654-9
48. Guescini M, Genedani S, Stocchi V, Agnati LF. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. Journal of neural transmission (Vienna, Austria: 1996). 2010;117:1-4
49. Bao Q, Huang Q, Chen Y, Wang Q, Sang R, Wang L. et al. Tumor-Derived Extracellular Vesicles Regulate Cancer Progression in the Tumor Microenvironment. Frontiers in molecular biosciences. 2021;8:796385
50. Momen-Heravi F, Getting SJ, Moschos SA. Extracellular vesicles and their nucleic acids for biomarker discovery. Pharmacology & therapeutics. 2018;192:170-87
51. Zhao H, Yang L, Baddour J, Achreja A, Bernard V, Moss T. et al. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. eLife. 2016;5:e10250
52. Clos-Garcia M, Loizaga-Iriarte A, Zuñiga-Garcia P, Sánchez-Mosquera P, Rosa Cortazar A, González E. et al. Metabolic alterations in urine extracellular vesicles are associated to prostate cancer pathogenesis and progression. Journal of extracellular vesicles. 2018;7:1470442
53. S ELA, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nature reviews Drug discovery. 2013;12:347-57
54. Zhou B, Xu K, Zheng X, Chen T, Wang J, Song Y. et al. Application of exosomes as liquid biopsy in clinical diagnosis. Signal transduction and targeted therapy. 2020;5:144
55. Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nature cell biology. 2019;21:9-17
56. Horibe S, Tanahashi T, Kawauchi S, Murakami Y, Rikitake Y. Mechanism of recipient cell-dependent differences in exosome uptake. BMC cancer. 2018;18:47
57. Chivet M, Javalet C, Laulagnier K, Blot B, Hemming FJ, Sadoul R. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. Journal of extracellular vesicles. 2014;3:24722
58. Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF. et al. Cellular internalization of exosomes occurs through phagocytosis. Traffic (Copenhagen, Denmark). 2010;11:675-87
59. Costa Verdera H, Gitz-Francois JJ, Schiffelers RM, Vader P. Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. Journal of controlled release: official journal of the Controlled Release Society. 2017;266:100-8
60. Svensson KJ, Christianson HC, Wittrup A, Bourseau-Guilmain E, Lindqvist E, Svensson LM. et al. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. The Journal of biological chemistry. 2013;288:17713-24
61. Tian T, Zhu YL, Zhou YY, Liang GF, Wang YY, Hu FH. et al. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. The Journal of biological chemistry. 2014;289:22258-67
62. Kaur S, Singh SP, Elkahloun AG, Wu W, Abu-Asab MS, Roberts DD. CD47-dependent immunomodulatory and angiogenic activities of extracellular vesicles produced by T cells. Matrix biology: journal of the International Society for Matrix Biology. 2014;37:49-59
63. Fuentes P, Sesé M, Guijarro PJ, Emperador M, Sánchez-Redondo S, Peinado H. et al. ITGB3-mediated uptake of small extracellular vesicles facilitates intercellular communication in breast cancer cells. Nature communications. 2020;11:4261
64. Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:17380-5
65. Gonda A, Kabagwira J, Senthil GN, Ferguson Bennit HR, Neidigh JW, Khan S. et al. Exosomal survivin facilitates vesicle internalization. Oncotarget. 2018;9:34919-34
66. Zanconato F, Cordenonsi M, Piccolo S. YAP and TAZ: a signalling hub of the tumour microenvironment. Nature reviews Cancer. 2019;19:454-64
67. Burgos-Panadero R, Lucantoni F, Gamero-Sandemetrio E, Cruz-Merino L, Álvaro T, Noguera R. The tumour microenvironment as an integrated framework to understand cancer biology. Cancer letters. 2019;461:112-22
68. Yan L, Wang P, Fang W, Liang C. Cancer-associated fibroblasts-derived exosomes-mediated transfer of LINC00355 regulates bladder cancer cell proliferation and invasion. Cell biochemistry and function. 2020;38:257-65
69. Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC. et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527:100-4
70. Venkataramani V, Tanev DI, Strahle C, Studier-Fischer A, Fankhauser L, Kessler T. et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature. 2019;573:532-8
71. Venkatesh HS, Morishita W, Geraghty AC, Silverbush D, Gillespie SM, Arzt M. et al. Electrical and synaptic integration of glioma into neural circuits. Nature. 2019;573:539-45
72. Zeng Q, Michael IP, Zhang P, Saghafinia S, Knott G, Jiao W. et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature. 2019;573:526-31
73. Mendoza-Naranjo A, Saéz PJ, Johansson CC, Ramírez M, Mandakovic D, Pereda C. et al. Functional gap junctions facilitate melanoma antigen transfer and cross-presentation between human dendritic cells. Journal of immunology (Baltimore, Md: 1950). 2007;178:6949-57
74. Haralambiev L, Wien L, Gelbrich N, Kramer A, Mustea A, Burchardt M. et al. Effects of Cold Atmospheric Plasma on the Expression of Chemokines, Growth Factors, TNF Superfamily Members, Interleukins, and Cytokines in Human Osteosarcoma Cells. Anticancer research. 2019;39:151-7
75. Henke E, Nandigama R, Ergün S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Frontiers in molecular biosciences. 2019;6:160
76. Robinson SC, Scott KA, Balkwill FR. Chemokine stimulation of monocyte matrix metalloproteinase-9 requires endogenous TNF-alpha. European journal of immunology. 2002;32:404-12
77. Sukkurwala AQ, Martins I, Wang Y, Schlemmer F, Ruckenstuhl C, Durchschlag M. et al. Immunogenic calreticulin exposure occurs through a phylogenetically conserved stress pathway involving the chemokine CXCL8. Cell death and differentiation. 2014;21:59-68
78. Tessema M, Klinge DM, Yingling CM, Do K, Van Neste L, Belinsky SA. Re-expression of CXCL14, a common target for epigenetic silencing in lung cancer, induces tumor necrosis. Oncogene. 2010;29:5159-70
79. Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM. Exosome mediated communication within the tumor microenvironment. Journal of controlled release: official journal of the Controlled Release Society. 2015;219:278-94
80. Lin XJ, Fang JH, Yang XJ, Zhang C, Yuan Y, Zheng L. et al. Hepatocellular Carcinoma Cell-Secreted Exosomal MicroRNA-210 Promotes Angiogenesis in vitro and in vivo. Molecular therapy Nucleic acids. 2018;11:243-52
81. Zhang K, Liu D, Zhao J, Shi S, He X, Da P. et al. Nuclear exosome HMGB3 secreted by nasopharyngeal carcinoma cells promotes tumour metastasis by inducing angiogenesis. Cell death & disease. 2021;12:554
82. Tian X, Liu Y, Wang Z, Wu S. miR-144 delivered by nasopharyngeal carcinoma-derived EVs stimulates angiogenesis through the FBXW7/HIF-1α/VEGF-A axis. Molecular therapy Nucleic acids. 2021;24:1000-11
83. Ma X, Li Z, Li T, Zhu L, Li Z, Tian N. Long non-coding RNA HOTAIR enhances angiogenesis by induction of VEGFA expression in glioma cells and transmission to endothelial cells via glioma cell derived-extracellular vesicles. American journal of translational research. 2017;9:5012-21
84. Wang ZF, Liao F, Wu H, Dai J. Glioma stem cells-derived exosomal miR-26a promotes angiogenesis of microvessel endothelial cells in glioma. Journal of experimental & clinical cancer research: CR. 2019;38:201
85. Huang Z, Feng Y. Exosomes Derived From Hypoxic Colorectal Cancer Cells Promote Angiogenesis Through Wnt4-Induced β-Catenin Signaling in Endothelial Cells. Oncology research. 2017;25:651-61
86. Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J. et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nature communications. 2018;9:5395
87. He Q, Ye A, Ye W, Liao X, Qin G, Xu Y. et al. Cancer-secreted exosomal miR-21-5p induces angiogenesis and vascular permeability by targeting KRIT1. Cell Death Dis. 2021;12:576
88. Hsu YL, Hung JY, Chang WA, Lin YS, Pan YC, Tsai PH. et al. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene. 2017;36:4929-42
89. Zhuang G, Wu X, Jiang Z, Kasman I, Yao J, Guan Y. et al. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. The EMBO journal. 2012;31:3513-23
90. French KC, Antonyak MA, Cerione RA. Extracellular vesicle docking at the cellular port: Extracellular vesicle binding and uptake. Seminars in cell & developmental biology. 2017;67:48-55
91. Garcia-Bermudez J, Williams RT, Guarecuco R, Birsoy K. Targeting extracellular nutrient dependencies of cancer cells. Molecular metabolism. 2020;33:67-82
92. De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nature reviews Cancer. 2017;17:457-74
93. Korc M, Friesel RE. The role of fibroblast growth factors in tumor growth. Current cancer drug targets. 2009;9:639-51
94. Goel HL, Mercurio AM. VEGF targets the tumour cell. Nature reviews Cancer. 2013;13:871-82
95. Xue Y, Lim S, Yang Y, Wang Z, Jensen LD, Hedlund EM. et al. PDGF-BB modulates hematopoiesis and tumor angiogenesis by inducing erythropoietin production in stromal cells. Nature medicine. 2011;18:100-10
96. Treps L, Perret R, Edmond S, Ricard D, Gavard J. Glioblastoma stem-like cells secrete the pro-angiogenic VEGF-A factor in extracellular vesicles. Journal of extracellular vesicles. 2017;6:1359479
97. Sruthi TV, Edatt L, Raji GR, Kunhiraman H, Shankar SS, Shankar V. et al. Horizontal transfer of miR-23a from hypoxic tumor cell colonies can induce angiogenesis. Journal of cellular physiology. 2018;233:3498-514
98. Lin XJ, Chong Y, Guo ZW, Xie C, Yang XJ, Zhang Q. et al. A serum microRNA classifier for early detection of hepatocellular carcinoma: a multicentre, retrospective, longitudinal biomarker identification study with a nested case-control study. The Lancet Oncology. 2015;16:804-15
99. Matouk IJ, DeGroot N, Mezan S, Ayesh S, Abu-lail R, Hochberg A. et al. The H19 non-coding RNA is essential for human tumor growth. PloS one. 2007;2:e845
100. Yu FJ, Zheng JJ, Dong PH, Fan XM. Long non-coding RNAs and hepatocellular carcinoma. Molecular and clinical oncology. 2015;3:13-7
101. Nakamura K, Martin KC, Jackson JK, Beppu K, Woo CW, Thiele CJ. Brain-derived neurotrophic factor activation of TrkB induces vascular endothelial growth factor expression via hypoxia-inducible factor-1alpha in neuroblastoma cells. Cancer research. 2006;66:4249-55
102. Fang JH, Zhang ZJ, Shang LR, Luo YW, Lin YF, Yuan Y. et al. Hepatoma cell-secreted exosomal microRNA-103 increases vascular permeability and promotes metastasis by targeting junction proteins. Hepatology (Baltimore, Md). 2018;68:1459-75
103. Darby IA, Laverdet B, Bonté F, Desmoulière A. Fibroblasts and myofibroblasts in wound healing. Clinical, cosmetic and investigational dermatology. 2014;7:301-11
104. Ishii G, Ochiai A, Neri S. Phenotypic and functional heterogeneity of cancer-associated fibroblast within the tumor microenvironment. Advanced drug delivery reviews. 2016;99:186-96
105. Guo W, Gao Y, Li N, Shao F, Wang C, Wang P. et al. Exosomes: New players in cancer (Review). Oncology reports. 2017;38:665-75
106. Dörsam B, Bösl T, Reiners KS, Barnert S, Schubert R, Shatnyeva O. et al. Hodgkin Lymphoma-Derived Extracellular Vesicles Change the Secretome of Fibroblasts Toward a CAF Phenotype. Frontiers in immunology. 2018;9:1358
107. Ji Q, Zhou L, Sui H, Yang L, Wu X, Song Q. et al. Primary tumors release ITGBL1-rich extracellular vesicles to promote distal metastatic tumor growth through fibroblast-niche formation. Nature communications. 2020;11:1211
108. Paggetti J, Haderk F, Seiffert M, Janji B, Distler U, Ammerlaan W. et al. Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer-associated fibroblasts. Blood. 2015;126:1106-17
109. Fang T, Lv H, Lv G, Li T, Wang C, Han Q. et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nature communications. 2018;9:191
110. Giusti I, Di Francesco M, D'Ascenzo S, Palmerini MG, Macchiarelli G, Carta G. et al. Ovarian cancer-derived extracellular vesicles affect normal human fibroblast behavior. Cancer biology & therapy. 2018;19:722-34
111. Yang Z, Jin P, Xu S, Zhang T, Yang X, Li X. et al. Dicer reprograms stromal fibroblasts to a pro-inflammatory and tumor-promoting phenotype in ovarian cancer. Cancer letters. 2018;415:20-9
112. Zhou X, Yan T, Huang C, Xu Z, Wang L, Jiang E. et al. Melanoma cell-secreted exosomal miR-155-5p induce proangiogenic switch of cancer-associated fibroblasts via SOCS1/JAK2/STAT3 signaling pathway. Journal of experimental & clinical cancer research: CR. 2018;37:242
113. Kim W, Barron DA, San Martin R, Chan KS, Tran LL, Yang F. et al. RUNX1 is essential for mesenchymal stem cell proliferation and myofibroblast differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:16389-94
114. Cho JA, Park H, Lim EH, Lee KW. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. International journal of oncology. 2012;40:130-8
115. Cho JA, Park H, Lim EH, Kim KH, Choi JS, Lee JH. et al. Exosomes from ovarian cancer cells induce adipose tissue-derived mesenchymal stem cells to acquire the physical and functional characteristics of tumor-supporting myofibroblasts. Gynecologic oncology. 2011;123:379-86
116. Gu J, Qian H, Shen L, Zhang X, Zhu W, Huang L. et al. Gastric cancer exosomes trigger differentiation of umbilical cord derived mesenchymal stem cells to carcinoma-associated fibroblasts through TGF-β/Smad pathway. PloS one. 2012;7:e52465
117. Santi A, Caselli A, Ranaldi F, Paoli P, Mugnaioni C, Michelucci E. et al. Cancer associated fibroblasts transfer lipids and proteins to cancer cells through cargo vesicles supporting tumor growth. Biochimica et biophysica acta. 2015;1853:3211-23
118. Dourado MR, Korvala J, Åström P, De Oliveira CE, Cervigne NK, Mofatto LS. et al. Extracellular vesicles derived from cancer-associated fibroblasts induce the migration and invasion of oral squamous cell carcinoma. Journal of extracellular vesicles. 2019;8:1578525
119. Li YY, Tao YW, Gao S, Li P, Zheng JM, Zhang SE. et al. Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine miR-34a-5p. EBioMedicine. 2018;36:209-20
120. Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes & development. 2018;32:1267-84
121. Tauriello DVF, Batlle E. Targeting the Microenvironment in Advanced Colorectal Cancer. Trends in cancer. 2016;2:495-504
122. Whiteside TL. Exosomes and tumor-mediated immune suppression. The Journal of clinical investigation. 2016;126:1216-23
123. Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nature reviews Immunology. 2014;14:195-208
124. Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G. et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nature medicine. 2012;18:883-91
125. Schmiedel D, Mandelboim O. NKG2D Ligands-Critical Targets for Cancer Immune Escape and Therapy. Frontiers in immunology. 2018;9:2040
126. Lundholm M, Schröder M, Nagaeva O, Baranov V, Widmark A, Mincheva-Nilsson L. et al. Prostate tumor-derived exosomes down-regulate NKG2D expression on natural killer cells and CD8+ T cells: mechanism of immune evasion. PloS one. 2014;9:e108925
127. Zhao J, Schlößer HA, Wang Z, Qin J, Li J, Popp F. et al. Tumor-Derived Extracellular Vesicles Inhibit Natural Killer Cell Function in Pancreatic Cancer. Cancers. 2019;11:874
128. Federici C, Shahaj E, Cecchetti S, Camerini S, Casella M, Iessi E. et al. Natural-Killer-Derived Extracellular Vesicles: Immune Sensors and Interactors. Frontiers in immunology. 2020;11:262
129. Neviani P, Wise PM, Murtadha M, Liu CW, Wu CH, Jong AY. et al. Natural Killer-Derived Exosomal miR-186 Inhibits Neuroblastoma Growth and Immune Escape Mechanisms. Cancer research. 2019;79:1151-64
130. Wolfers J, Lozier A, Raposo G, Regnault A, Théry C, Masurier C. et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nature medicine. 2001;7:297-303
131. Xu Y, Liu Y, Yang C, Kang L, Wang M, Hu J. et al. Macrophages transfer antigens to dendritic cells by releasing exosomes containing dead-cell-associated antigens partially through a ceramide-dependent pathway to enhance CD4(+) T-cell responses. Immunology. 2016;149:157-71
132. Tran Janco JM, Lamichhane P, Karyampudi L, Knutson KL. Tumor-infiltrating dendritic cells in cancer pathogenesis. Journal of immunology (Baltimore, Md: 1950). 2015;194:2985-91
133. Yin X, Zeng W, Wu B, Wang L, Wang Z, Tian H. et al. PPARα Inhibition Overcomes Tumor-Derived Exosomal Lipid-Induced Dendritic Cell Dysfunction. Cell reports. 2020;33:108278
134. Yu S, Liu C, Su K, Wang J, Liu Y, Zhang L. et al. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. Journal of immunology (Baltimore, Md: 1950). 2007;178:6867-75
135. Valenti R, Huber V, Filipazzi P, Pilla L, Sovena G, Villa A. et al. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer research. 2006;66:9290-8
136. Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP. et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. The Journal of clinical investigation. 2010;120:457-71
137. Xie Y, Zhang H, Li W, Deng Y, Munegowda MA, Chibbar R. et al. Dendritic cells recruit T cell exosomes via exosomal LFA-1 leading to inhibition of CD8+ CTL responses through downregulation of peptide/MHC class I and Fas ligand-mediated cytotoxicity. Journal of immunology (Baltimore, Md: 1950). 2010;185:5268-78
138. Liu Q, Rojas-Canales DM, Divito SJ, Shufesky WJ, Stolz DB, Erdos G. et al. Donor dendritic cell-derived exosomes promote allograft-targeting immune response. The Journal of clinical investigation. 2016;126:2805-20
139. Viaud S, Terme M, Flament C, Taieb J, André F, Novault S. et al. Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha. PloS one. 2009;4:e4942
140. Gutiérrez-Vázquez C, Villarroya-Beltri C, Mittelbrunn M, Sánchez-Madrid F. Transfer of extracellular vesicles during immune cell-cell interactions. Immunological reviews. 2013;251:125-42
141. Lu Z, Zuo B, Jing R, Gao X, Rao Q, Liu Z. et al. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. Journal of hepatology. 2017;67:739-48
142. Matsumoto A, Asuka M, Takahashi Y, Takakura Y. Antitumor immunity by small extracellular vesicles collected from activated dendritic cells through effective induction of cellular and humoral immune responses. Biomaterials. 2020;252:120112
143. Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. Journal of hematology & oncology. 2019;12:76
144. Chen X, Zhou J, Li X, Wang X, Lin Y, Wang X. Exosomes derived from hypoxic epithelial ovarian cancer cells deliver microRNAs to macrophages and elicit a tumor-promoted phenotype. Cancer letters. 2018;435:80-91
145. Wang X, Luo G, Zhang K, Cao J, Huang C, Jiang T. et al. Hypoxic Tumor-Derived Exosomal miR-301a Mediates M2 Macrophage Polarization via PTEN/PI3Kγ to Promote Pancreatic Cancer Metastasis. Cancer research. 2018;78:4586-98
146. Qian M, Wang S, Guo X, Wang J, Zhang Z, Qiu W. et al. Hypoxic glioma-derived exosomes deliver microRNA-1246 to induce M2 macrophage polarization by targeting TERF2IP via the STAT3 and NF-κB pathways. Oncogene. 2020;39:428-42
147. Cooks T, Pateras IS, Jenkins LM, Patel KM, Robles AI, Morris J. et al. Mutant p53 cancers reprogram macrophages to tumor supporting macrophages via exosomal miR-1246. Nature communications. 2018;9:771
148. Shinohara H, Kuranaga Y, Kumazaki M, Sugito N, Yoshikawa Y, Takai T. et al. Regulated Polarization of Tumor-Associated Macrophages by miR-145 via Colorectal Cancer-Derived Extracellular Vesicles. Journal of immunology (Baltimore, Md: 1950). 2017;199:1505-15
149. Chen F, Chen J, Yang L, Liu J, Zhang X, Zhang Y. et al. Extracellular vesicle-packaged HIF-1α-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells. Nature cell biology. 2019;21:498-510
150. Binenbaum Y, Fridman E, Yaari Z, Milman N, Schroeder A, Ben David G. et al. Transfer of miRNA in Macrophage-Derived Exosomes Induces Drug Resistance in Pancreatic Adenocarcinoma. Cancer research. 2018;78:5287-99
151. Lan J, Sun L, Xu F, Liu L, Hu F, Song D. et al. M2 Macrophage-Derived Exosomes Promote Cell Migration and Invasion in Colon Cancer. Cancer research. 2019;79:146-58
152. Speth JM, Penke LR, Bazzill JD, Park KS, de Rubio RG, Schneider DJ. et al. Alveolar macrophage secretion of vesicular SOCS3 represents a platform for lung cancer therapeutics. JCI insight. 2019;4:e131340
153. Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ, Whiteside TL. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. Journal of immunology (Baltimore, Md: 1950). 2009;183:3720-30
154. Yen EY, Miaw SC, Yu JS, Lai IR. Exosomal TGF-β1 is correlated with lymphatic metastasis of gastric cancers. American journal of cancer research. 2017;7:2199-208
155. Mrizak D, Martin N, Barjon C, Jimenez-Pailhes AS, Mustapha R, Niki T. et al. Effect of nasopharyngeal carcinoma-derived exosomes on human regulatory T cells. Journal of the National Cancer Institute. 2015;107:363
156. Martínez-Lostao L, Anel A, Pardo J. How Do Cytotoxic Lymphocytes Kill Cancer Cells? Clinical cancer research: an official journal of the American Association for Cancer Research. 2015;21:5047-56
157. Prajapati K, Perez C, Rojas LBP, Burke B, Guevara-Patino JA. Functions of NKG2D in CD8(+) T cells: an opportunity for immunotherapy. Cellular & molecular immunology. 2018;15:470-9
158. Huber V, Fais S, Iero M, Lugini L, Canese P, Squarcina P. et al. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape. Gastroenterology. 2005;128:1796-804
159. Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W. et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382-6
160. Poggio M, Hu T, Pai CC, Chu B, Belair CD, Chang A. et al. Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory. Cell. 2019;177:414-27.e13
161. Sullivan JA, Tomita Y, Jankowska-Gan E, Lema DA, Arvedson MP, Nair A. et al. Treg-cell-derived IL-35-coated extracellular vesicles promote infectious tolerance. Cell Rep. 2020;30:1039-51 e5
162. Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A. et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257-65
163. Tung SL, Boardman DA, Sen M, Letizia M, Peng Q, Cianci N. et al. Regulatory T cell-derived extracellular vesicles modify dendritic cell function. Sci Rep. 2018;8:6065
164. Singel KL, Segal BH. Neutrophils in the tumor microenvironment: trying to heal the wound that cannot heal. Immunological reviews. 2016;273:329-43
165. Ye L, Zhang Q, Cheng Y, Chen X, Wang G, Shi M. et al. Tumor-derived exosomal HMGB1 fosters hepatocellular carcinoma immune evasion by promoting TIM-1(+) regulatory B cell expansion. Journal for immunotherapy of cancer. 2018;6:145
166. Zhang X, Shi H, Yuan X, Jiang P, Qian H, Xu W. Tumor-derived exosomes induce N2 polarization of neutrophils to promote gastric cancer cell migration. Molecular cancer. 2018;17:146
167. Liu Y, Gu Y, Han Y, Zhang Q, Jiang Z, Zhang X. et al. Tumor Exosomal RNAs Promote Lung Pre-metastatic Niche Formation by Activating Alveolar Epithelial TLR3 to Recruit Neutrophils. Cancer cell. 2016;30:243-56
168. Hwang WL, Lan HY, Cheng WC, Huang SC, Yang MH. Tumor stem-like cell-derived exosomal RNAs prime neutrophils for facilitating tumorigenesis of colon cancer. Journal of hematology & oncology. 2019;12:10
169. Ghafouri-Fard S, Shoorei H, Dong P, Poornajaf Y, Hussen BM, Taheri M. et al. Emerging functions and clinical applications of exosomal microRNAs in diseases. Noncoding RNA Res. 2023;8:350-62
170. Chen T, Liu Y, Chen J, Zheng H, Chen Q, Zhao J. Exosomal miR-3180-3p inhibits proliferation and metastasis of non-small cell lung cancer by downregulating FOXP4. Thorac Cancer. 2021;12:372-81
171. Liu J, Luo R, Wang J, Luan X, Wu D, Chen H. et al. Tumor Cell-Derived Exosomal miR-770 Inhibits M2 Macrophage Polarization via Targeting MAP3K1 to Inhibit the Invasion of Non-small Cell Lung Cancer Cells. Front Cell Dev Biol. 2021;9:679658
172. Tian W, Yang X, Yang H, Lv M, Sun X, Zhou B. Exosomal miR-338-3p suppresses non-small-cell lung cancer cells metastasis by inhibiting CHL1 through the MAPK signaling pathway. Cell Death Dis. 2021;12:1030
173. Ma Z, Wei K, Yang F, Guo Z, Pan C, He Y. et al. Tumor-derived exosomal miR-3157-3p promotes angiogenesis, vascular permeability and metastasis by targeting TIMP/KLF2 in non-small cell lung cancer. Cell Death Dis. 2021;12:840
174. Kim DH, Park H, Choi YJ, Kang MH, Kim TK, Pack CG. et al. Exosomal miR-1260b derived from non-small cell lung cancer promotes tumor metastasis through the inhibition of HIPK2. Cell Death Dis. 2021;12:747
175. Li X, Chen Z, Ni Y, Bian C, Huang J, Chen L. et al. Tumor-associated macrophages secret exosomal miR-155 and miR-196a-5p to promote metastasis of non-small-cell lung cancer. Transl Lung Cancer Res. 2021;10:1338-54
176. Zheng GD, Xu ZY, Hu C, Lv H, Xie HX, Huang T. et al. Exosomal miR-590-5p in Serum as a Biomarker for the Diagnosis and Prognosis of Gastric Cancer. Front Mol Biosci. 2021;8:636566
177. Jiao Y, Zhang L, Li J, He Y, Zhang X, Li J. Exosomal miR-122-5p inhibits tumorigenicity of gastric cancer by downregulating GIT1. Int J Biol Markers. 2021;36:36-46
178. Yan T, Wang X, Wei G, Li H, Hao L, Liu Y. et al. Exosomal miR-10b-5p mediates cell communication of gastric cancer cells and fibroblasts and facilitates cell proliferation. J Cancer. 2021;12:2140-50
179. Fu F, Jiang W, Zhou L, Chen Z. Circulating Exosomal miR-17-5p and miR-92a-3p Predict Pathologic Stage and Grade of Colorectal Cancer. Transl Oncol. 2018;11:221-32
180. Liu C, Eng C, Shen J, Lu Y, Takata Y, Mehdizadeh A. et al. Serum exosomal miR-4772-3p is a predictor of tumor recurrence in stage II and III colon cancer. Oncotarget. 2016;7:76250-60
181. Yokota Y, Noda T, Okumura Y, Kobayashi S, Iwagami Y, Yamada D. et al. Serum exosomal miR-638 is a prognostic marker of HCC via downregulation of VE-cadherin and ZO-1 of endothelial cells. Cancer Sci. 2021;112:1275-88
182. Ma YS, Liu JB, Lin L, Zhang H, Wu JJ, Shi Y. et al. Exosomal microRNA-15a from mesenchymal stem cells impedes hepatocellular carcinoma progression via downregulation of SALL4. Cell Death Discov. 2021;7:224
183. Liu Q, Peng F, Chen J. The Role of Exosomal MicroRNAs in the Tumor Microenvironment of Breast Cancer. Int J Mol Sci. 2019;20:3884
184. Zhou C, Wei W, Ma J, Yang Y, Liang L, Zhang Y. et al. Cancer-secreted exosomal miR-1468-5p promotes tumor immune escape via the immunosuppressive reprogramming of lymphatic vessels. Mol Ther. 2021;29:1512-28
185. You X, Sun W, Wang Y, Liu X, Wang A, Liu L. et al. Cervical cancer-derived exosomal miR-663b promotes angiogenesis by inhibiting vinculin expression in vascular endothelial cells. Cancer Cell Int. 2021;21:684
186. Giovannetti E, Erozenci A, Smit J, Danesi R, Peters GJ. Molecular mechanisms underlying the role of microRNAs (miRNAs) in anticancer drug resistance and implications for clinical practice. Critical reviews in oncology/hematology. 2012;81:103-22
187. Iorio MV, Croce CM. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO molecular medicine. 2012;4:143-59
188. Wei Y, Lai X, Yu S, Chen S, Ma Y, Zhang Y. et al. Exosomal miR-221/222 enhances tamoxifen resistance in recipient ER-positive breast cancer cells. Breast cancer research and treatment. 2014;147:423-31
189. Yang Z, Zhao N, Cui J, Wu H, Xiong J, Peng T. Exosomes derived from cancer stem cells of gemcitabine-resistant pancreatic cancer cells enhance drug resistance by delivering miR-210. Cellular oncology (Dordrecht). 2020;43:123-36
190. Lin H, Zhang L, Zhang C, Liu P. Exosomal MiR-500a-3p promotes cisplatin resistance and stemness via negatively regulating FBXW7 in gastric cancer. Journal of cellular and molecular medicine. 2020;24:8930-41
191. He J, He J, Min L, He Y, Guan H, Wang J. et al. Extracellular vesicles transmitted miR-31-5p promotes sorafenib resistance by targeting MLH1 in renal cell carcinoma. International journal of cancer. 2020;146:1052-63
192. Wei F, Ma C, Zhou T, Dong X, Luo Q, Geng L. et al. Exosomes derived from gemcitabine-resistant cells transfer malignant phenotypic traits via delivery of miRNA-222-3p. Molecular cancer. 2017;16:132
193. Shiozawa K, Shuting J, Yoshioka Y, Ochiya T, Kondo T. Extracellular vesicle-encapsulated microRNA-761 enhances pazopanib resistance in synovial sarcoma. Biochemical and biophysical research communications. 2018;495:1322-7
194. Liu T, Chen G, Sun D, Lei M, Li Y, Zhou C. et al. Exosomes containing miR-21 transfer the characteristic of cisplatin resistance by targeting PTEN and PDCD4 in oral squamous cell carcinoma. Acta biochimica et biophysica Sinica. 2017;49:808-16
195. Sun LH, Tian D, Yang ZC, Li JL. Exosomal miR-21 promotes proliferation, invasion and therapy resistance of colon adenocarcinoma cells through its target PDCD4. Scientific reports. 2020;10:8271
196. Bouvy C, Wannez A, Laloy J, Chatelain C, Dogné JM. Transfer of multidrug resistance among acute myeloid leukemia cells via extracellular vesicles and their microRNA cargo. Leukemia research. 2017;62:70-6
197. Shen M, Dong C, Ruan X, Yan W, Cao M, Pizzo D. et al. Chemotherapy-Induced Extracellular Vesicle miRNAs Promote Breast Cancer Stemness by Targeting ONECUT2. Cancer research. 2019;79:3608-21
198. Corcoran C, Rani S, O'Driscoll L. miR-34a is an intracellular and exosomal predictive biomarker for response to docetaxel with clinical relevance to prostate cancer progression. The Prostate. 2014;74:1320-34
199. Jin G, Liu Y, Zhang J, Bian Z, Yao S, Fei B. et al. A panel of serum exosomal microRNAs as predictive markers for chemoresistance in advanced colorectal cancer. Cancer chemotherapy and pharmacology. 2019;84:315-25
200. Tang Y, Cui Y, Li Z, Jiao Z, Zhang Y, He Y. et al. Radiation-induced miR-208a increases the proliferation and radioresistance by targeting p21 in human lung cancer cells. Journal of experimental & clinical cancer research: CR. 2016;35:7
201. Ramakrishnan V, Xu B, Akers J, Nguyen T, Ma J, Dhawan S. et al. Radiation-induced extracellular vesicle (EV) release of miR-603 promotes IGF1-mediated stem cell state in glioblastomas. EBioMedicine. 2020;55:102736
202. Kwok HH, Ning Z, Chong PW, Wan TS, Ng MH, Ho GYF. et al. Transfer of Extracellular Vesicle-Associated-RNAs Induces Drug Resistance in ALK-Translocated Lung Adenocarcinoma. Cancers. 2019;11:104
203. Dong X, Bai X, Ni J, Zhang H, Duan W, Graham P. et al. Exosomes and breast cancer drug resistance. Cell Death Dis. 2020;11:987
204. Zhang W, Cai X, Yu J, Lu X, Qian Q, Qian W. Exosome-mediated transfer of lncRNA RP11-838N2.4 promotes erlotinib resistance in non-small cell lung cancer. International journal of oncology. 2018;53:527-38
205. Takahashi K, Yan IK, Kogure T, Haga H, Patel T. Extracellular vesicle-mediated transfer of long non-coding RNA ROR modulates chemosensitivity in human hepatocellular cancer. FEBS open bio. 2014;4:458-67
206. Takahashi K, Yan IK, Wood J, Haga H, Patel T. Involvement of extracellular vesicle long noncoding RNA (linc-VLDLR) in tumor cell responses to chemotherapy. Molecular cancer research: MCR. 2014;12:1377-87
207. Chen Y, Liu L, Li J, Du Y, Wang J, Liu J. Effects of long noncoding RNA (linc-VLDLR) existing in extracellular vesicles on the occurrence and multidrug resistance of esophageal cancer cells. Pathology, research and practice. 2019;215:470-7
208. Lei Y, Guo W, Chen B, Chen L, Gong J, Li W. Tumor-released lncRNA H19 promotes gefitinib resistance via packaging into exosomes in non-small cell lung cancer. Oncology reports. 2018;40:3438-46
209. Pan R, Zhou H. Exosomal Transfer of lncRNA H19 Promotes Erlotinib Resistance in Non-Small Cell Lung Cancer via miR-615-3p/ATG7 Axis. Cancer management and research. 2020;12:4283-97
210. Wang X, Pei X, Guo G, Qian X, Dou D, Zhang Z. et al. Exosome-mediated transfer of long noncoding RNA H19 induces doxorubicin resistance in breast cancer. Journal of cellular physiology. 2020;235:6896-904
211. Zhou D, Xia Z, Xie M, Gao Y, Yu Q, He B. Exosomal long non-coding RNA SOX2 overlapping transcript enhances the resistance to EGFR-TKIs in non-small cell lung cancer cell line H1975. Human cell. 2021;34:1478-89
212. Zhang Q, Liu RX, Chan KW, Hu J, Zhang J, Wei L. et al. Exosomal transfer of p-STAT3 promotes acquired 5-FU resistance in colorectal cancer cells. Journal of experimental & clinical cancer research: CR. 2019;38:320
213. Ma X, Chen Z, Hua D, He D, Wang L, Zhang P. et al. Essential role for TrpC5-containing extracellular vesicles in breast cancer with chemotherapeutic resistance. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:6389-94
214. Li F, Zhao X, Sun R, Ou J, Huang J, Yang N. et al. EGFR-rich extracellular vesicles derived from highly metastatic nasopharyngeal carcinoma cells accelerate tumour metastasis through PI3K/AKT pathway-suppressed ROS. Journal of extracellular vesicles. 2020;10:e12003
215. Xavier CPR, Belisario DC, Rebelo R, Assaraf YG, Giovannetti E, Kopecka J. et al. The role of extracellular vesicles in the transfer of drug resistance competences to cancer cells. Drug resistance updates: reviews and commentaries in antimicrobial and anticancer chemotherapy. 2022;62:100833
216. Garg H, Suri P, Gupta JC, Talwar GP, Dubey S. Survivin: a unique target for tumor therapy. Cancer cell international. 2016;16:49
217. Khan S, Aspe JR, Asumen MG, Almaguel F, Odumosu O, Acevedo-Martinez S. et al. Extracellular, cell-permeable survivin inhibits apoptosis while promoting proliferative and metastatic potential. British journal of cancer. 2009;100:1073-86
218. Khan S, Jutzy JM, Aspe JR, McGregor DW, Neidigh JW, Wall NR. Survivin is released from cancer cells via exosomes. Apoptosis: an international journal on programmed cell death. 2011;16:1-12
219. Khan S, Jutzy JM, Valenzuela MM, Turay D, Aspe JR, Ashok A. et al. Plasma-derived exosomal survivin, a plausible biomarker for early detection of prostate cancer. PloS one. 2012;7:e46737
220. Chang WH, Nguyen TT, Hsu CH, Bryant KL, Kim HJ, Ying H. et al. KRAS-dependent cancer cells promote survival by producing exosomes enriched in Survivin. Cancer letters. 2021;517:66-77
221. Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S. et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell. 2017;168:542
222. Vari F, Arpon D, Keane C, Hertzberg MS, Talaulikar D, Jain S. et al. Immune evasion via PD-1/PD-L1 on NK cells and monocyte/macrophages is more prominent in Hodgkin lymphoma than DLBCL. Blood. 2018;131:1809-19
223. Haderk F, Schulz R, Iskar M, Cid LL, Worst T, Willmund KV. et al. Tumor-derived exosomes modulate PD-L1 expression in monocytes. Science immunology. 2017;2:eaah5509
224. Wang B, Wang X, Hou D, Huang Q, Zhan W, Chen C. et al. Exosomes derived from acute myeloid leukemia cells promote chemoresistance by enhancing glycolysis-mediated vascular remodeling. Journal of cellular physiology. 2019;234:10602-14
225. Lobb RJ, van Amerongen R, Wiegmans A, Ham S, Larsen JE, Möller A. Exosomes derived from mesenchymal non-small cell lung cancer cells promote chemoresistance. International journal of cancer. 2017;141:614-20
226. Zhou J, Qu G, Zhang G, Wu Z, Liu J, Yang D. et al. Glycerol kinase 5 confers gefitinib resistance through SREBP1/SCD1 signaling pathway. Journal of experimental & clinical cancer research: CR. 2019;38:96
227. Sansone P, Savini C, Kurelac I, Chang Q, Amato LB, Strillacci A. et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:E9066-e75
228. Ferreira RJ, dos Santos DJ, Ferreira MJ. P-glycoprotein and membrane roles in multidrug resistance. Future medicinal chemistry. 2015;7:929-46
229. Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nature reviews Cancer. 2010;10:147-56
230. Ughachukwu P, Unekwe P. Efflux pump-mediated resistance in chemotherapy. Annals of medical and health sciences research. 2012;2:191-8
231. Dong Y, Pan Q, Jiang L, Chen Z, Zhang F, Liu Y. et al. Tumor endothelial expression of P-glycoprotein upon microvesicular transfer of TrpC5 derived from adriamycin-resistant breast cancer cells. Biochemical and biophysical research communications. 2014;446:85-90
232. Lv MM, Zhu XY, Chen WX, Zhong SL, Hu Q, Ma TF. et al. Exosomes mediate drug resistance transfer in MCF-7 breast cancer cells and a probable mechanism is delivery of P-glycoprotein. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35:10773-9
233. Mir B, Goettsch C. Extracellular Vesicles as Delivery Vehicles of Specific Cellular Cargo. Cells. 2020;9:1601
234. Corcoran C, Rani S, O'Brien K, O'Neill A, Prencipe M, Sheikh R. et al. Docetaxel-resistance in prostate cancer: evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PloS one. 2012;7:e50999
235. Barzegar M, Allahbakhshian Farsan M, Amiri V, Mohammadi S, Shahsavan S, Mirzaeian A. et al. AML-derived Extracellular Vesicles Confer De Novo Chemoresistance to Leukemic Myeloblast Cells by Promoting Drug Export Genes Expression and ROS Inhibition. Iranian journal of pharmaceutical research: IJPR. 2021;20:384-97
236. Zhao Y, Chen Y, Wang J, Liu L. Effects of ATP-binding cassette transporter G2 in extracellular vesicles on drug resistance of laryngeal cancer cells in in vivo and in vitro. Oncology letters. 2021;21:364
237. Fontana F, Carollo E, Melling GE, Carter DRF. Extracellular Vesicles: Emerging Modulators of Cancer Drug Resistance. Cancers. 2021;13:749
238. Chen VY, Posada MM, Blazer LL, Zhao T, Rosania GR. The role of the VPS4A-exosome pathway in the intrinsic egress route of a DNA-binding anticancer drug. Pharmaceutical research. 2006;23:1687-95
239. Cvjetkovic A, Jang SC, Konečná B, Höög JL, Sihlbom C, Lässer C. et al. Detailed Analysis of Protein Topology of Extracellular Vesicles-Evidence of Unconventional Membrane Protein Orientation. Scientific reports. 2016;6:36338
240. Goler-Baron V, Assaraf YG. Structure and function of ABCG2-rich extracellular vesicles mediating multidrug resistance. PloS one. 2011;6:e16007
241. Gong J, Luk F, Jaiswal R, George AM, Grau GE, Bebawy M. Microparticle drug sequestration provides a parallel pathway in the acquisition of cancer drug resistance. European journal of pharmacology. 2013;721:116-25
242. Ifergan I, Scheffer GL, Assaraf YG. Novel extracellular vesicles mediate an ABCG2-dependent anticancer drug sequestration and resistance. Cancer research. 2005;65:10952-8
243. Muralidharan-Chari V, Kohan HG, Asimakopoulos AG, Sudha T, Sell S, Kannan K. et al. Microvesicle removal of anticancer drugs contributes to drug resistance in human pancreatic cancer cells. Oncotarget. 2016;7:50365-79
244. Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A. et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. The Journal of biological chemistry. 2009;284:34211-22
245. Shedden K, Xie XT, Chandaroy P, Chang YT, Rosania GR. Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer research. 2003;63:4331-7
246. Kumar MA, Baba SK, Sadida HQ, Marzooqi SA, Jerobin J, Altemani FH, Algehainy N, Alanazi MA, Abou-Samra AB, Kumar R, Al-Shabeeb Akil AS, Macha MA, Mir R, Bhat AA. Extracellular vesi-cles as tools and targets in therapy for diseases. Signal Transduct Target Ther. 2024;9(1):27
247. Battke C, Ruiss R, Welsch U, Wimberger P, Lang S, Jochum S. et al. Tumour exosomes inhibit binding of tumour-reactive antibodies to tumour cells and reduce ADCC. Cancer immunology, immunotherapy: CII. 2011;60:639-48
248. Ren J, Ding L, Zhang D, Shi G, Xu Q, Shen S. et al. Carcinoma-associated fibroblasts promote the stemness and chemoresistance of colorectal cancer by transferring exosomal lncRNA H19. Theranostics. 2018;8:3932-48
249. Deng X, Ruan H, Zhang X, Xu X, Zhu Y, Peng H. et al. Long noncoding RNA CCAL transferred from fibroblasts by exosomes promotes chemoresistance of colorectal cancer cells. International journal of cancer. 2020;146:1700-16
250. Au Yeung CL, Co NN, Tsuruga T, Yeung TL, Kwan SY, Leung CS. et al. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nature communications. 2016;7:11150
251. Zhang L, Yao J, Li W, Zhang C. Micro-RNA-21 Regulates Cancer-Associated Fibroblast-Mediated Drug Resistance in Pancreatic Cancer. Oncology research. 2018;26:827-35
252. Hu JL, Wang W, Lan XL, Zeng ZC, Liang YS, Yan YR. et al. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Molecular cancer. 2019;18:91
253. Zhang H, Deng T, Liu R, Ning T, Yang H, Liu D. et al. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Molecular cancer. 2020;19:43
254. Zhang T, Zhang P, Li HX. CAFs-Derived Exosomal miRNA-130a Confers Cisplatin Resistance of NSCLC Cells Through PUM2-Dependent Packaging. International journal of nanomedicine. 2021;16:561-77
255. Shi L, Zhu W, Huang Y, Zhuo L, Wang S, Chen S. et al. Cancer-associated fibroblast-derived exosomal microRNA-20a suppresses the PTEN/PI3K-AKT pathway to promote the progression and chemoresistance of non-small cell lung cancer. Clinical and translational medicine. 2022;12:e989
256. Qin X, Guo H, Wang X, Zhu X, Yan M, Wang X. et al. Exosomal miR-196a derived from cancer-associated fibroblasts confers cisplatin resistance in head and neck cancer through targeting CDKN1B and ING5. Genome biology. 2019;20:12
257. Fang Y, Zhou W, Rong Y, Kuang T, Xu X, Wu W. et al. Exosomal miRNA-106b from cancer-associated fibroblast promotes gemcitabine resistance in pancreatic cancer. Experimental cell research. 2019;383:111543
258. Chen X, Liu J, Zhang Q, Liu B, Cheng Y, Zhang Y. et al. Exosome-mediated transfer of miR-93-5p from cancer-associated fibroblasts confer radioresistance in colorectal cancer cells by downregulating FOXA1 and upregulating TGFB3. Journal of experimental & clinical cancer research: CR. 2020;39:65
259. Uchihara T, Miyake K, Yonemura A, Komohara Y, Itoyama R, Koiwa M. et al. Extracellular Vesicles from Cancer-Associated Fibroblasts Containing Annexin A6 Induces FAK-YAP Activation by Stabilizing β1 Integrin, Enhancing Drug Resistance. Cancer research. 2020;80:3222-35
260. Hu YB, Yan C, Mu L, Mi YL, Zhao H, Hu H. et al. Exosomal Wnt-induced dedifferentiation of colorectal cancer cells contributes to chemotherapy resistance. Oncogene. 2019;38:1951-65
261. Qu Z, Yang KD, Luo BH, Zhang F. CAFs-secreted exosomal cricN4BP2L2 promoted colorectal cancer stemness and chemoresistance by interacting with EIF4A3. Experimental cell research. 2022;418:113266
262. Adamo A, Dal Collo G, Bazzoni R, Krampera M. Role of mesenchymal stromal cell-derived extracellular vesicles in tumour microenvironment. Biochimica et biophysica acta Reviews on cancer. 2019;1871:192-8
263. Bliss SA, Sinha G, Sandiford OA, Williams LM, Engelberth DJ, Guiro K. et al. Mesenchymal Stem Cell-Derived Exosomes Stimulate Cycling Quiescence and Early Breast Cancer Dormancy in Bone Marrow. Cancer research. 2016;76:5832-44
264. Xu H, Han H, Song S, Yi N, Qian C, Qiu Y. et al. Exosome-Transmitted PSMA3 and PSMA3-AS1 Promote Proteasome Inhibitor Resistance in Multiple Myeloma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2019;25:1923-35
265. Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi RU. et al. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Science signaling. 2014;7:ra63
266. Wang J, Hendrix A, Hernot S, Lemaire M, De Bruyne E, Van Valckenborgh E. et al. Bone marrow stromal cell-derived exosomes as communicators in drug resistance in multiple myeloma cells. Blood. 2014;124:555-66
267. Gao X, Zhou J, Wang J, Dong X, Chang Y, Jin Y. Mechanism of exosomal miR-155 derived from bone marrow mesenchymal stem cells on stemness maintenance and drug resistance in myeloma cells. Journal of orthopaedic surgery and research. 2021;16:637
268. Gong J, Jaiswal R, Dalla P, Luk F, Bebawy M. Microparticles in cancer: A review of recent developments and the potential for clinical application. Seminars in cell & developmental biology. 2015;40:35-40
269. Zheng P, Chen L, Yuan X, Luo Q, Liu Y, Xie G. et al. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. Journal of experimental & clinical cancer research: CR. 2017;36:53
270. Zhu X, Shen H, Yin X, Yang M, Wei H, Chen Q. et al. Macrophages derived exosomes deliver miR-223 to epithelial ovarian cancer cells to elicit a chemoresistant phenotype. Journal of experimental & clinical cancer research: CR. 2019;38:81
271. Xavier CPR, Castro I, Caires HR, Ferreira D, Cavadas B, Pereira L. et al. Chitinase 3-like-1 and fibronectin in the cargo of extracellular vesicles shed by human macrophages influence pancreatic cancer cellular response to gemcitabine. Cancer letters. 2021;501:210-23
272. El-Arabey AA, Denizli M, Kanlikilicer P, Bayraktar R, Ivan C, Rashed M. et al. GATA3 as a master regulator for interactions of tumor-associated macrophages with high-grade serous ovarian carcinoma. Cellular signalling. 2020;68:109539
273. Xin L, Zhou LQ, Liu C, Zeng F, Yuan YW, Zhou Q. et al. Transfer of LncRNA CRNDE in TAM-derived exosomes is linked with cisplatin resistance in gastric cancer. EMBO reports. 2021;22:e52124
274. Liu Y, Tan J, Ou S, Chen J, Chen L. Adipose-derived exosomes deliver miR-23a/b to regulate tumor growth in hepatocellular cancer by targeting the VHL/HIF axis. Journal of physiology and biochemistry. 2019;75:391-401
275. Wang Z, He J, Bach DH, Huang YH, Li Z, Liu H. et al. Induction of m(6)A methylation in adipocyte exosomal LncRNAs mediates myeloma drug resistance. Journal of experimental & clinical cancer research: CR. 2022;41:4
276. Zhang Q, Deng T, Zhang H, Zuo D, Zhu Q, Bai M. et al. Adipocyte-Derived Exosomal MTTP Suppresses Ferroptosis and Promotes Chemoresistance in Colorectal Cancer. Advanced science (Weinheim, Baden-Wurttemberg, Germany). 2022;9:e2203357
277. Geis-Asteggiante L, Belew AT, Clements VK, Edwards NJ, Ostrand-Rosenberg S, El-Sayed NM. et al. Differential Content of Proteins, mRNAs, and miRNAs Suggests that MDSC and Their Exosomes May Mediate Distinct Immune Suppressive Functions. Journal of proteome research. 2018;17:486-98
278. Deng Z, Rong Y, Teng Y, Zhuang X, Samykutty A, Mu J. et al. Exosomes miR-126a released from MDSC induced by DOX treatment promotes lung metastasis. Oncogene. 2017;36:639-51
279. Huber V, Vallacchi V, Fleming V, Hu X, Cova A, Dugo M. et al. Tumor-derived microRNAs induce myeloid suppressor cells and predict immunotherapy resistance in melanoma. The Journal of clinical investigation. 2018;128:5505-16
280. Ning T, Li J, He Y, Zhang H, Wang X, Deng T. et al. Exosomal miR-208b related with oxaliplatin resistance promotes Treg expansion in colorectal cancer. Molecular therapy: the journal of the American Society of Gene Therapy. 2021;29:2723-36
281. Hida K, Maishi N, Sakurai Y, Hida Y, Harashima H. Heterogeneity of tumor endothelial cells and drug delivery. Advanced drug delivery reviews. 2016;99:140-7
282. Zeng Y, Yao X, Liu X, He X, Li L, Liu X. et al. Anti-angiogenesis triggers exosomes release from endothelial cells to promote tumor vasculogenesis. Journal of extracellular vesicles. 2019;8:1629865
283. Sato S, Vasaikar S, Eskaros A, Kim Y, Lewis JS, Zhang B. et al. EPHB2 carried on small extracellular vesicles induces tumor angiogenesis via activation of ephrin reverse signaling. JCI insight. 2019;4:e132447
284. Huang L, Hu C, Chao H, Zhang Y, Li Y, Hou J. et al. Drug-resistant endothelial cells facilitate progression, EMT and chemoresistance in nasopharyngeal carcinoma via exosomes. Cellular signalling. 2019;63:109385
285. Yuwen D, Ma Y, Wang D, Gao J, Li X, Xue W. et al. Prognostic Role of Circulating Exosomal miR-425-3p for the Response of NSCLC to Platinum-Based Chemotherapy. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2019;28:163-73
286. Shi M, Jiang Y, Yang L, Yan S, Wang YG, Lu XJ. Decreased levels of serum exosomal miR-638 predict poor prognosis in hepatocellular carcinoma. Journal of cellular biochemistry. 2018;119:4711-6
287. Liu T, Zhang X, Gao S, Jing F, Yang Y, Du L. et al. Exosomal long noncoding RNA CRNDE-h as a novel serum-based biomarker for diagnosis and prognosis of colorectal cancer. Oncotarget. 2016;7:85551-63
288. Pietrowska M, Zebrowska A, Gawin M, Marczak L, Sharma P, Mondal S. et al. Proteomic profile of melanoma cell-derived small extracellular vesicles in patients' plasma: a potential correlate of melanoma progression. Journal of extracellular vesicles. 2021;10:e12063
289. Tomimaru Y, Eguchi H, Nagano H, Wada H, Kobayashi S, Marubashi S. et al. Circulating microRNA-21 as a novel biomarker for hepatocellular carcinoma. Journal of hepatology. 2012;56:167-75
290. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science (New York, NY). 2020;367:eaau6977
291. Tsujiura M, Ichikawa D, Komatsu S, Shiozaki A, Takeshita H, Kosuga T. et al. Circulating microRNAs in plasma of patients with gastric cancers. British journal of cancer. 2010;102:1174-9
292. Afonso MB, Rodrigues PM, Simão AL, Castro RE. Circulating microRNAs as Potential Biomarkers in Non-Alcoholic Fatty Liver Disease and Hepatocellular Carcinoma. Journal of clinical medicine. 2016;5:30
293. Liu W, Chen S, Liu B. Diagnostic and prognostic values of serum exosomal microRNA-21 in children with hepatoblastoma: a Chinese population-based study. Pediatric surgery international. 2016;32:1059-65
294. Thind A, Wilson C. Exosomal miRNAs as cancer biomarkers and therapeutic targets. Journal of extracellular vesicles. 2016;5:31292
295. Yuwen DL, Sheng BB, Liu J, Wenyu W, Shu YQ. MiR-146a-5p level in serum exosomes predicts therapeutic effect of cisplatin in non-small cell lung cancer. European review for medical and pharmacological sciences. 2017;21:2650-8
296. Sohn W, Kim J, Kang SH, Yang SR, Cho JY, Cho HC. et al. Serum exosomal microRNAs as novel biomarkers for hepatocellular carcinoma. Experimental & molecular medicine. 2015;47:e184
297. Fornari F, Ferracin M, Trerè D, Milazzo M, Marinelli S, Galassi M. et al. Circulating microRNAs, miR-939, miR-595, miR-519d and miR-494, Identify Cirrhotic Patients with HCC. PloS one. 2015;10:e0141448
298. Xue X, Zhao Y, Wang X, Qin L, Hu R. Development and validation of serum exosomal microRNAs as diagnostic and prognostic biomarkers for hepatocellular carcinoma. Journal of cellular biochemistry. 2019;120:135-42
299. Liu W, Hu J, Zhou K, Chen F, Wang Z, Liao B. et al. Serum exosomal miR-125b is a novel prognostic marker for hepatocellular carcinoma. OncoTargets and therapy. 2017;10:3843-51
300. Sugimachi K, Matsumura T, Hirata H, Uchi R, Ueda M, Ueo H. et al. Identification of a bona fide microRNA biomarker in serum exosomes that predicts hepatocellular carcinoma recurrence after liver transplantation. British journal of cancer. 2015;112:532-8
301. Qu Z, Wu J, Wu J, Ji A, Qiang G, Jiang Y. et al. Exosomal miR-665 as a novel minimally invasive biomarker for hepatocellular carcinoma diagnosis and prognosis. Oncotarget. 2017;8:80666-78
302. Malla B, Aebersold DM, Dal Pra A. Protocol for serum exosomal miRNAs analysis in prostate cancer patients treated with radiotherapy. Journal of translational medicine. 2018;16:223
303. Wu H, Zeng C, Ye Y, Liu J, Mu Z, Xie Y. et al. Exosomes from Irradiated Nonsmall Cell Lung Cancer Cells Reduced Sensitivity of Recipient Cells to Anaplastic Lymphoma Kinase Inhibitors. Molecular pharmaceutics. 2018;15:1892-900
304. Wu H, Zhou J, Mei S, Wu D, Mu Z, Chen B. et al. Circulating exosomal microRNA-96 promotes cell proliferation, migration and drug resistance by targeting LMO7. Journal of cellular and molecular medicine. 2017;21:1228-36
305. Kuhlmann JD, Chebouti I, Kimmig R, Buderath P, Reuter M, Puppel SH. et al. Extracellular vesicle-associated miRNAs in ovarian cancer - design of an integrated NGS-based workflow for the identification of blood-based biomarkers for platinum-resistance. Clinical chemistry and laboratory medicine. 2019;57:1053-62
306. Alharbi M, Sharma S, Guanzon D, Lai A, Zuñiga F, Shiddiky MJA. et al. miRNa signature in small extracellular vesicles and their association with platinum resistance and cancer recurrence in ovarian cancer. Nanomedicine: nanotechnology, biology, and medicine. 2020;28:102207
307. Zhang Z, Zhang L, Yu G, Sun Z, Wang T, Tian X. et al. Exosomal miR-1246 and miR-155 as predictive and prognostic biomarkers for trastuzumab-based therapy resistance in HER2-positive breast cancer. Cancer chemotherapy and pharmacology. 2020;86:761-72
308. Svedman FC, Lohcharoenkal W, Bottai M, Brage SE, Sonkoly E, Hansson J. et al. Extracellular microvesicle microRNAs as predictive biomarkers for targeted therapy in metastastic cutaneous malignant melanoma. PloS one. 2018;13:e0206942
309. Del Re M, Biasco E, Crucitta S, Derosa L, Rofi E, Orlandini C. et al. The Detection of Androgen Receptor Splice Variant 7 in Plasma-derived Exosomal RNA Strongly Predicts Resistance to Hormonal Therapy in Metastatic Prostate Cancer Patients. European urology. 2017;71:680-7
310. Yang YN, Zhang R, Du JW, Yuan HH, Li YJ, Wei XL. et al. Predictive role of UCA1-containing exosomes in cetuximab-resistant colorectal cancer. Cancer cell international. 2018;18:164
311. Hu G, Ma J, Zhang J, Chen Y, Liu H, Huang Y. et al. Hypoxia-induced lncHILAR promotes renal cancer metastasis via ceRNA for the miR-613/206/ 1-1-3p/Jagged-1/Notch/CXCR4 signaling pathway. Molecular therapy: the journal of the American Society of Gene Therapy. 2021;29:2979-94
312. Ni J, Zhang X, Li J, Zheng Z, Zhang J, Zhao W. et al. Tumour-derived exosomal lncRNA-SOX2OT promotes bone metastasis of non-small cell lung cancer by targeting the miRNA-194-5p/RAC1 signalling axis in osteoclasts. Cell death & disease. 2021;12:662
313. Pan L, Liang W, Fu M, Huang ZH, Li X, Zhang W. et al. Exosomes-mediated transfer of long noncoding RNA ZFAS1 promotes gastric cancer progression. Journal of cancer research and clinical oncology. 2017;143:991-1004
314. Shi Q, Ji T, Ma Z, Tan Q, Liang J. Serum Exosomes-Based Biomarker circ_0008928 Regulates Cisplatin Sensitivity, Tumor Progression, and Glycolysis Metabolism by miR-488/HK2 Axis in Cisplatin-Resistant Nonsmall Cell Lung Carcinoma. Cancer Biother Radiopharm. 2023;38:558-71
315. Chen X, Chen RX, Wei WS, Li YH, Feng ZH, Tan L. et al. PRMT5 Circular RNA Promotes Metastasis of Urothelial Carcinoma of the Bladder through Sponging miR-30c to Induce Epithelial-Mesenchymal Transition. Clinical cancer research: an official journal of the American Association for Cancer Research. 2018;24:6319-30
316. Hu J, Sheng Y, Kwak KJ, Shi J, Yu B, Lee LJ. A signal-amplifiable biochip quantifies extracellular vesicle-associated RNAs for early cancer detection. Nature communications. 2017;8:1683
317. Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J. et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177-82
318. Wang T, Ning K, Lu TX, Sun X, Jin L, Qi X. et al. Increasing circulating exosomes-carrying TRPC5 predicts chemoresistance in metastatic breast cancer patients. Cancer science. 2017;108:448-54
319. Li T, Tao Z, Zhu Y, Liu X, Wang L, Du Y. et al. Exosomal annexin A6 induces gemcitabine resistance by inhibiting ubiquitination and degradation of EGFR in triple-negative breast cancer. Cell death & disease. 2021;12:684
320. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568-71
321. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A. et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. The New England journal of medicine. 2016;375:1823-33
322. Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Molecular cancer therapeutics. 2015;14:847-56
323. Tucci M, Passarelli A, Mannavola F, Stucci LS, Ascierto PA, Capone M. et al. Serum exosomes as predictors of clinical response to ipilimumab in metastatic melanoma. Oncoimmunology. 2018;7:e1387706
324. Mitchell PJ, Welton J, Staffurth J, Court J, Mason MD, Tabi Z. et al. Can urinary exosomes act as treatment response markers in prostate cancer? Journal of translational medicine. 2009;7:4
325. Wang J, Wuethrich A, Sina AA, Lane RE, Lin LL, Wang Y. et al. Tracking extracellular vesicle phenotypic changes enables treatment monitoring in melanoma. Science advances. 2020;6:eaax3223
326. Brocco D, Lanuti P, Pieragostino D, Cufaro MC, Simeone P, Bologna G. et al. Phenotypic and Proteomic Analysis Identifies Hallmarks of Blood Circulating Extracellular Vesicles in NSCLC Responders to Immune Checkpoint Inhibitors. Cancers. 2021;13:585
327. Milman N, Ginini L, Gil Z. Exosomes and their role in tumorigenesis and anticancer drug resistance. Drug resistance updates: reviews and commentaries in antimicrobial and anticancer chemotherapy. 2019;45:1-12
328. Cox TR, Rumney RMH, Schoof EM, Perryman L, Høye AM, Agrawal A. et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature. 2015;522:106-10
329. Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A. et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nature cell biology. 2010;12:19-30 sup pp 1-13
330. Wang T, Gilkes DM, Takano N, Xiang L, Luo W, Bishop CJ. et al. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E3234-42
331. Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G. et al. Pre-metastatic niches: organ-specific homes for metastases. Nature reviews Cancer. 2017;17:302-17
332. Fader CM, Sánchez DG, Mestre MB, Colombo MI. TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochimica et biophysica acta. 2009;1793:1901-16
333. Li B, Antonyak MA, Zhang J, Cerione RA. RhoA triggers a specific signaling pathway that generates transforming microvesicles in cancer cells. Oncogene. 2012;31:4740-9
334. Del Conde I, Shrimpton CN, Thiagarajan P, López JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604-11
335. Kosgodage US, Trindade RP, Thompson PR, Inal JM, Lange S. Chloramidine/Bisindolylmaleimide-I-Mediated Inhibition of Exosome and Microvesicle Release and Enhanced Efficacy of Cancer Chemotherapy. International journal of molecular sciences. 2017;18:1007
336. Dorayappan KDP, Wanner R, Wallbillich JJ, Saini U, Zingarelli R, Suarez AA. et al. Hypoxia-induced exosomes contribute to a more aggressive and chemoresistant ovarian cancer phenotype: a novel mechanism linking STAT3/Rab proteins. Oncogene. 2018;37:3806-21
337. Wang X, Xu C, Hua Y, Sun L, Cheng K, Jia Z. et al. Exosomes play an important role in the process of psoralen reverse multidrug resistance of breast cancer. Journal of experimental & clinical cancer research: CR. 2016;35:186
338. Han L, Long Q, Li S, Xu Q, Zhang B, Dou X. et al. Senescent Stromal Cells Promote Cancer Resistance through SIRT1 Loss-Potentiated Overproduction of Small Extracellular Vesicles. Cancer research. 2020;80:3383-98
339. Sapet C, Simoncini S, Loriod B, Puthier D, Sampol J, Nguyen C. et al. Thrombin-induced endothelial microparticle generation: identification of a novel pathway involving ROCK-II activation by caspase-2. Blood. 2006;108:1868-76
340. Abid Hussein MN, Böing AN, Sturk A, Hau CM, Nieuwland R. Inhibition of microparticle release triggers endothelial cell apoptosis and detachment. Thrombosis and haemostasis. 2007;98:1096-107
341. Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F. et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science (New York, NY). 2008;319:1244-7
342. Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. The Journal of biological chemistry. 2010;285:17442-52
343. Fonseka P, Chitti SV, Sanwlani R, Mathivanan S. Sulfisoxazole does not inhibit the secretion of small extracellular vesicles. Nature communications. 2021;12:977
344. Cao YL, Zhuang T, Xing BH, Li N, Li Q. Exosomal DNMT1 mediates cisplatin resistance in ovarian cancer. Cell biochemistry and function. 2017;35:296-303
345. Matsumoto A, Takahashi Y, Nishikawa M, Sano K, Morishita M, Charoenviriyakul C. et al. Accelerated growth of B16BL6 tumor in mice through efficient uptake of their own exosomes by B16BL6 cells. Cancer science. 2017;108:1803-10
346. Roseblade A, Luk F, Ung A, Bebawy M. Targeting microparticle biogenesis: a novel approach to the circumvention of cancer multidrug resistance. Current cancer drug targets. 2015;15:205-14
347. Chitti SV, Nedeva C, Manickam R, Fonseka P, Mathivanan S. Extracellular Vesicles as Drug Targets and Delivery Vehicles for Cancer Therapy. Pharmaceutics. 2022;14:2822
348. Stratton D, Moore C, Zheng L, Lange S, Inal J. Prostate cancer cells stimulated by calcium-mediated activation of protein kinase C undergo a refractory period before re-releasing calcium-bearing microvesicles. Biochemical and biophysical research communications. 2015;460:511-7
349. Datta A, Kim H, McGee L, Johnson AE, Talwar S, Marugan J. et al. High-throughput screening identified selective inhibitors of exosome biogenesis and secretion: A drug repurposing strategy for advanced cancer. Scientific reports. 2018;8:8161
350. Tu C, Du Z, Zhang H, Feng Y, Qi Y, Zheng Y. et al. Endocytic pathway inhibition attenuates extracellular vesicle-induced reduction of chemosensitivity to bortezomib in multiple myeloma cells. Theranostics. 2021;11:2364-80
351. Berenguer J, Lagerweij T, Zhao XW, Dusoswa S, van der Stoop P, Westerman B. et al. Glycosylated extracellular vesicles released by glioblastoma cells are decorated by CCL18 allowing for cellular uptake via chemokine receptor CCR8. Journal of extracellular vesicles. 2018;7:1446660
352. Koch R, Aung T, Vogel D, Chapuy B, Wenzel D, Becker S. et al. Nuclear Trapping through Inhibition of Exosomal Export by Indomethacin Increases Cytostatic Efficacy of Doxorubicin and Pixantrone. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016;22:395-404
353. Marleau AM, Chen CS, Joyce JA, Tullis RH. Exosome removal as a therapeutic adjuvant in cancer. Journal of translational medicine. 2012;10:134
354. Li L, Zhang L, Knez M. Comparison of two endogenous delivery agents in cancer therapy: Exosomes and ferritin. Pharmacological research. 2016;110:1-9
355. Shtam TA, Kovalev RA, Varfolomeeva EY, Makarov EM, Kil YV, Filatov MV. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell communication and signaling: CCS. 2013;11:88
356. Wang Y, Liu J, Ma J, Sun T, Zhou Q, Wang W. et al. Exosomal circRNAs: biogenesis, effect and application in human diseases. Molecular cancer. 2019;18:116
357. Zeng A, Wei Z, Yan W, Yin J, Huang X, Zhou X. et al. Exosomal transfer of miR-151a enhances chemosensitivity to temozolomide in drug-resistant glioblastoma. Cancer letters. 2018;436:10-21
358. Qiu L, Wang J, Chen M, Chen F, Tu W. Exosomal microRNA-146a derived from mesenchymal stem cells increases the sensitivity of ovarian cancer cells to docetaxel and taxane via a LAMC2-mediated PI3K/Akt axis. International journal of molecular medicine. 2020;46:609-20
359. Han M, Hu J, Lu P, Cao H, Yu C, Li X. et al. Exosome-transmitted miR-567 reverses trastuzumab resistance by inhibiting ATG5 in breast cancer. Cell death & disease. 2020;11:43
360. Ji R, Zhang X, Gu H, Ma J, Wen X, Zhou J. et al. miR-374a-5p: A New Target for Diagnosis and Drug Resistance Therapy in Gastric Cancer. Molecular therapy Nucleic acids. 2019;18:320-31
361. Wang X, Zhang H, Bai M, Ning T, Ge S, Deng T. et al. Exosomes Serve as Nanoparticles to Deliver Anti-miR-214 to Reverse Chemoresistance to Cisplatin in Gastric Cancer. Molecular therapy: the journal of the American Society of Gene Therapy. 2018;26:774-83
362. Valeri N, Gasparini P, Braconi C, Paone A, Lovat F, Fabbri M. et al. MicroRNA-21 induces resistance to 5-fluorouracil by down-regulating human DNA MutS homolog 2 (hMSH2). Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21098-103
363. Liang G, Zhu Y, Ali DJ, Tian T, Xu H, Si K. et al. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. Journal of nanobiotechnology. 2020;18:10
364. Bose RJC, Uday Kumar S, Zeng Y, Afjei R, Robinson E, Lau K. et al. Tumor Cell-Derived Extracellular Vesicle-Coated Nanocarriers: An Efficient Theranostic Platform for the Cancer-Specific Delivery of Anti-miR-21 and Imaging Agents. ACS nano. 2018;12:10817-32
365. Limoni SK, Moghadam MF, Moazzeni SM, Gomari H, Salimi F. Engineered Exosomes for Targeted Transfer of siRNA to HER2 Positive Breast Cancer Cells. Applied biochemistry and biotechnology. 2019;187:352-64
366. Aqil F, Munagala R, Jeyabalan J, Agrawal AK, Kyakulaga AH, Wilcher SA. et al. Milk exosomes - Natural nanoparticles for siRNA delivery. Cancer letters. 2019;449:186-95
367. Yang T, Fogarty B, LaForge B, Aziz S, Pham T, Lai L. et al. Delivery of Small Interfering RNA to Inhibit Vascular Endothelial Growth Factor in Zebrafish Using Natural Brain Endothelia Cell-Secreted Exosome Nanovesicles for the Treatment of Brain Cancer. The AAPS journal. 2017;19:475-86
368. Xu J, Ji L, Liang Y, Wan Z, Zheng W, Song X. et al. CircRNA-SORE mediates sorafenib resistance in hepatocellular carcinoma by stabilizing YBX1. Signal transduction and targeted therapy. 2020;5:298
369. Li H, Yang C, Shi Y, Zhao L. Exosomes derived from siRNA against GRP78 modified bone-marrow-derived mesenchymal stem cells suppress Sorafenib resistance in hepatocellular carcinoma. Journal of nanobiotechnology. 2018;16:103
370. Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA. et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498-503
371. Mendt M, Kamerkar S, Sugimoto H, McAndrews KM, Wu CC, Gagea M. et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI insight. 2018;3:e99263
372. Li P, Cao G, Huang Y, Wu W, Chen B, Wang Z. et al. siMTA1-Loaded Exosomes Enhanced Chemotherapeutic Effect of Gemcitabine in Luminal-b Type Breast Cancer by Inhibition of EMT/HIF-α and Autophagy Pathways. Frontiers in oncology. 2020;10:541262
373. Lin D, Zhang H, Liu R, Deng T, Ning T, Bai M. et al. iRGD-modified exosomes effectively deliver CPT1A siRNA to colon cancer cells, reversing oxaliplatin resistance by regulating fatty acid oxidation. Molecular oncology. 2021;15:3430-46
374. Zhang Q, Zhang H, Ning T, Liu D, Deng T, Liu R. et al. Exosome-Delivered c-Met siRNA Could Reverse Chemoresistance to Cisplatin in Gastric Cancer. International journal of nanomedicine. 2020;15:2323-35
375. Xu Y, Qiu A, Peng F, Tan X, Wang J, Gong X. Exosomal transfer of circular RNA FBXW7 ameliorates the chemoresistance to oxaliplatin in colorectal cancer by sponging miR-18b-5p. Neoplasma. 2021;68:108-18
376. Hui B, Lu C, Wang J, Xu Y, Yang Y, Ji H. et al. Engineered exosomes for co-delivery of PGM5-AS1 and oxaliplatin to reverse drug resistance in colon cancer. Journal of cellular physiology. 2022;237:911-33
377. Aspe JR, Diaz Osterman CJ, Jutzy JM, Deshields S, Whang S, Wall NR. Enhancement of Gemcitabine sensitivity in pancreatic adenocarcinoma by novel exosome-mediated delivery of the Survivin-T34A mutant. Journal of extracellular vesicles. 2014 3
378. Herbst RS, Eckhardt SG, Kurzrock R, Ebbinghaus S, O'Dwyer PJ, Gordon MS. et al. Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:2839-46
379. Micheau O, Shirley S, Dufour F. Death receptors as targets in cancer. British journal of pharmacology. 2013;169:1723-44
380. Yuan Z, Kolluri KK, Gowers KH, Janes SM. TRAIL delivery by MSC-derived extracellular vesicles is an effective anticancer therapy. Journal of extracellular vesicles. 2017;6:1265291
381. Ke C, Hou H, Li J, Su K, Huang C, Lin Y. et al. Extracellular Vesicle Delivery of TRAIL Eradicates Resistant Tumor Growth in Combination with CDK Inhibition by Dinaciclib. Cancers. 2020;12:1157
382. Saari H, Lázaro-Ibáñez E, Viitala T, Vuorimaa-Laukkanen E, Siljander P, Yliperttula M. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. Journal of controlled release: official journal of the Controlled Release Society. 2015;220:727-37
383. Kim MS, Haney MJ, Zhao Y, Yuan D, Deygen I, Klyachko NL. et al. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomedicine: nanotechnology, biology, and medicine. 2018;14:195-204
384. Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL. et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine: nanotechnology, biology, and medicine. 2016;12:655-64
385. Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ. et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383-90
386. Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E. et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. Journal of controlled release: official journal of the Controlled Release Society. 2014;192:262-70
387. Zhang M, Xiao B, Wang H, Han MK, Zhang Z, Viennois E. et al. Edible Ginger-derived Nano-lipids Loaded with Doxorubicin as a Novel Drug-delivery Approach for Colon Cancer Therapy. Molecular therapy: the journal of the American Society of Gene Therapy. 2016;24:1783-96
388. Kuerban K, Gao X, Zhang H, Liu J, Dong M, Wu L. et al. Doxorubicin-loaded bacterial outer-membrane vesicles exert enhanced anti-tumor efficacy in non-small-cell lung cancer. Acta pharmaceutica Sinica B. 2020;10:1534-48
389. Haney MJ, Zhao Y, Jin YS, Li SM, Bago JR, Klyachko NL. et al. Macrophage-Derived Extracellular Vesicles as Drug Delivery Systems for Triple Negative Breast Cancer (TNBC) Therapy. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2020;15:487-500
390. Wang P, Wang H, Huang Q, Peng C, Yao L, Chen H. et al. Exosomes from M1-Polarized Macrophages Enhance Paclitaxel Antitumor Activity by Activating Macrophages-Mediated Inflammation. Theranostics. 2019;9:1714-27
391. Salarpour S, Forootanfar H, Pournamdari M, Ahmadi-Zeidabadi M, Esmaeeli M, Pardakhty A. Paclitaxel incorporated exosomes derived from glioblastoma cells: comparative study of two loading techniques. Daru: journal of Faculty of Pharmacy, Tehran University of Medical Sciences. 2019;27:533-9
392. Zhou Y, Zhou W, Chen X, Wang Q, Li C, Chen Q. et al. Bone marrow mesenchymal stem cells-derived exosomes for penetrating and targeted chemotherapy of pancreatic cancer. Acta pharmaceutica Sinica B. 2020;10:1563-75
393. Toffoli G, Hadla M, Corona G, Caligiuri I, Palazzolo S, Semeraro S. et al. Exosomal doxorubicin reduces the cardiac toxicity of doxorubicin. Nanomedicine (London, England). 2015;10:2963-71
394. Hadla M, Palazzolo S, Corona G, Caligiuri I, Canzonieri V, Toffoli G. et al. Exosomes increase the therapeutic index of doxorubicin in breast and ovarian cancer mouse models. Nanomedicine (London, England). 2016;11:2431-41
395. Schindler C, Collinson A, Matthews C, Pointon A, Jenkinson L, Minter RR. et al. Exosomal delivery of doxorubicin enables rapid cell entry and enhanced in vitro potency. PloS one. 2019;14:e0214545
396. Zhang G, Huang X, Xiu H, Sun Y, Chen J, Cheng G. et al. Extracellular vesicles: Natural liver-accumulating drug delivery vehicles for the treatment of liver diseases. Journal of extracellular vesicles. 2020;10:e12030
397. Li L, He D, Guo Q, Zhang Z, Ru D, Wang L. et al. Exosome-liposome hybrid nanoparticle codelivery of TP and miR497 conspicuously overcomes chemoresistant ovarian cancer. Journal of nanobiotechnology. 2022;20:50
398. Zhang X, Zhang H, Gu J, Zhang J, Shi H, Qian H. et al. Engineered Extracellular Vesicles for Cancer Therapy. Advanced materials (Deerfield Beach, Fla). 2021;33:e2005709
399. Lv Q, Cheng L, Lu Y, Zhang X, Wang Y, Deng J. et al. Thermosensitive Exosome-Liposome Hybrid Nanoparticle-Mediated Chemoimmunotherapy for Improved Treatment of Metastatic Peritoneal Cancer. Advanced science (Weinheim, Baden-Wurttemberg, Germany). 2020;7:2000515
400. Liu J, Ye Z, Xiang M, Chang B, Cui J, Ji T. et al. Functional extracellular vesicles engineered with lipid-grafted hyaluronic acid effectively reverse cancer drug resistance. Biomaterials. 2019;223:119475
401. Ma X, Yao M, Gao Y, Yue Y, Li Y, Zhang T. et al. Functional Immune Cell-Derived Exosomes Engineered for the Trilogy of Radiotherapy Sensitization. Advanced science (Weinheim, Baden-Wurttemberg, Germany). 2022;9:e2106031
402. Conigliaro A, Costa V, Lo Dico A, Saieva L, Buccheri S, Dieli F. et al. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Molecular cancer. 2015;14:155
403. Wang Y, Lin C. Exosomes miR-22-3p Derived from Mesenchymal Stem Cells Suppress Colorectal Cancer Cell Proliferation and Invasion by Regulating RAP2B and PI3K/AKT Pathway. J Oncol. 2021;2021:3874478
404. Wang J, Liu Y, Li Y, Zheng X, Gan J, Wan Z. et al. Exosomal-miR-10a derived from colorectal cancer cells suppresses migration of human lung fibroblasts, and expression of IL-6, IL-8 and IL-1β. Mol Med Rep. 2021;23:84
405. Xun J, Du L, Gao R, Shen L, Wang D, Kang L. et al. Cancer-derived exosomal miR-138-5p modulates polarization of tumor-associated macrophages through inhibition of KDM6B. Theranostics. 2021;11:6847-59
406. Chen B, Sang Y, Song X, Zhang D, Wang L, Zhao W. et al. Exosomal miR-500a-5p derived from cancer-associated fibroblasts promotes breast cancer cell proliferation and metastasis through targeting USP28. Theranostics. 2021;11:3932-47
407. Huang Q, Gumireddy K, Schrier M, le Sage C, Nagel R, Nair S. et al. The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat Cell Biol. 2008;10:202-10
408. Zhu S, Wu H, Wu F, Nie D, Sheng S, Mo YY. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 2008;18:350-9
409. Ibrahim SA, Yip GW, Stock C, Pan JW, Neubauer C, Poeter M. et al. Targeting of syndecan-1 by microRNA miR-10b promotes breast cancer cell motility and invasiveness via a Rho-GTPase- and E-cadherin-dependent mechanism. Int J Cancer. 2012;131:E884-96
410. Chen W, Cai F, Zhang B, Barekati Z, Zhong XY. The level of circulating miRNA-10b and miRNA-373 in detecting lymph node metastasis of breast cancer: potential biomarkers. Tumour Biol. 2013;34:455-62
411. Eichelser C, Stückrath I, Müller V, Milde-Langosch K, Wikman H, Pantel K. et al. Increased serum levels of circulating exosomal microRNA-373 in receptor-negative breast cancer patients. Oncotarget. 2014;5:9650-63
412. Singh R, Pochampally R, Watabe K, Lu Z, Mo YY. Exosome-mediated transfer of miR-10b promotes cell invasion in breast cancer. Mol Cancer. 2014;13:256
413. Feng YH, Tsao CJ. Emerging role of microRNA-21 in cancer. Biomed Rep. 2016;5:395-402
414. Hong Y, Liang H, Uzair Ur R, Wang Y, Zhang W, Zhou Y. et al. miR-96 promotes cell proliferation, migration and invasion by targeting PTPN9 in breast cancer. Sci Rep. 2016;6:37421
415. Kim J, Siverly AN, Chen D, Wang M, Yuan Y, Wang Y. et al. Ablation of miR-10b Suppresses Oncogene-Induced Mammary Tumorigenesis and Metastasis and Reactivates Tumor-Suppressive Pathways. Cancer Res. 2016;76:6424-35
416. Shi J. Considering Exosomal miR-21 as a Biomarker for Cancer. J Clin Med. 2016;5:42
417. Xu YF, Hannafon BN, Khatri U, Gin A, Ding WQ. The origin of exosomal miR-1246 in human cancer cells. RNA Biol. 2019;16:770-84
418. Yu Z, Willmarth NE, Zhou J, Katiyar S, Wang M, Liu Y. et al. microRNA 17/20 inhibits cellular invasion and tumor metastasis in breast cancer by heterotypic signaling. Proc Natl Acad Sci U S A. 2010;107:8231-6
419. Bovy N, Blomme B, Frères P, Dederen S, Nivelles O, Lion M. et al. Endothelial exosomes contribute to the antitumor response during breast cancer neoadjuvant chemotherapy via microRNA transfer. Oncotarget. 2015;6:10253-66
420. Jiang Q, He M, Guan S, Ma M, Wu H, Yu Z. et al. MicroRNA-100 suppresses the migration and invasion of breast cancer cells by targeting FZD-8 and inhibiting Wnt/β-catenin signaling pathway. Tumour Biol. 2016;37:5001-11
421. Mutlu M, Saatci Ö, Ansari SA, Yurdusev E, Shehwana H, Konu Ö. et al. miR-564 acts as a dual inhibitor of PI3K and MAPK signaling networks and inhibits proliferation and invasion in breast cancer. Sci Rep. 2016;6:32541
422. Tao WY, Wang CY, Sun YH, Su YH, Pang D, Zhang GQ. MicroRNA-34c Suppresses Breast Cancer Migration and Invasion by Targeting GIT1. J Cancer. 2016;7:1653-62
423. Ke K, Lou T. MicroRNA-10a suppresses breast cancer progression via PI3K/Akt/mTOR pathway. Oncol Lett. 2017;14:5994-6000
424. Zhou W, Song F, Wu Q, Liu R, Wang L, Liu C. et al. miR-217 inhibits triple-negative breast cancer cell growth, migration, and invasion through targeting KLF5. PLoS One. 2017;12:e0176395
425. Park EJ, Jung HJ, Choi HJ, Jang HJ, Park HJ, Nejsum LN. et al. Exosomes co-expressing AQP5-targeting miRNAs and IL-4 receptor-binding peptide inhibit the migration of human breast cancer cells. Faseb j. 2020;34:3379-98
426. Kong W, He L, Richards EJ, Challa S, Xu CX, Permuth-Wey J. et al. Upregulation of miRNA-155 promotes tumour angiogenesis by targeting VHL and is associated with poor prognosis and triple-negative breast cancer. Oncogene. 2014;33:679-89
427. Kontomanolis E, Mitrakas A, Giatromanolaki A, Kareli D, Panteliadou M, Pouliliou S. et al. A pilot study on plasma levels of micro-RNAs involved in angiogenesis and vascular maturation in patients with breast cancer. Med Oncol. 2017;34:20
428. Lee JK, Park SR, Jung BK, Jeon YK, Lee YS, Kim MK. et al. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One. 2013;8:e84256
429. Zhou B, Ma R, Si W, Li S, Xu Y, Tu X. et al. MicroRNA-503 targets FGF2 and VEGFA and inhibits tumor angiogenesis and growth. Cancer Lett. 2013;333:159-69
430. Pakravan K, Babashah S, Sadeghizadeh M, Mowla SJ, Mossahebi-Mohammadi M, Ataei F. et al. MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. Cell Oncol (Dordr). 2017;40:457-70
431. Shen S, Song Y, Zhao B, Xu Y, Ren X, Zhou Y. et al. Cancer-derived exosomal miR-7641 promotes breast cancer progression and metastasis. Cell Commun Signal. 2021;19:20
432. Yan Z, Sheng Z, Zheng Y, Feng R, Xiao Q, Shi L. et al. Cancer-associated fibroblast-derived exosomal miR-18b promotes breast cancer invasion and metastasis by regulating TCEAL7. Cell Death Dis. 2021;12:1120
433. Xu Y, Zhu M. Novel exosomal miR-46146 transfer oxaliplatin chemoresistance in colorectal cancer. Clinical & translational oncology: official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2020;22:1105-16
434. Yu Q, Li P, Weng M, Wu S, Zhang Y, Chen X. et al. Nano-Vesicles are a Potential Tool to Monitor Therapeutic Efficacy of Carbon Ion Radiotherapy in Prostate Cancer. Journal of biomedical nanotechnology. 2018;14:168-78
Author contact
![]() Corresponding author: DR RASHID MIR, Associate Professor, Department of Medical Lab Technology, Faculty of Applied Medical Sciences, Supervisor - Division of Cancer Molecular Biology, Prince Fahd Bin Sultan Research Chair, University of Tabuk, Kingdom of Saudi Arabia, Tabuk 71491; Mobile: +966580225811; Email: rashidmirtabukcom.
Corresponding author: DR RASHID MIR, Associate Professor, Department of Medical Lab Technology, Faculty of Applied Medical Sciences, Supervisor - Division of Cancer Molecular Biology, Prince Fahd Bin Sultan Research Chair, University of Tabuk, Kingdom of Saudi Arabia, Tabuk 71491; Mobile: +966580225811; Email: rashidmirtabukcom.

 Global reach, higher impact
Global reach, higher impact