Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(20):6741-6753. doi:10.7150/jca.95775 This issue Cite
Research Paper
Genistein Induces Ferroptosis in Colorectal Cancer Cells via FoxO3/SLC7A11/GPX4 Signaling Pathway
1. Department of Colorectal & Anal Surgery, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, China.
2. The Affiliated Nanhua Hospital, Department of Gastrointestinal Surgery, Hengyang Medical College, University of South China, Hengyang, Hunan 421001, China.
3. Guangxi Key Laboratory of Diabetic Systems Medicine, Department of Histology and Embryology, Guilin Medical University, Guilin 541199, Guangxi, China.
Received 2024-2-28; Accepted 2024-10-4; Published 2024-11-4
Abstract
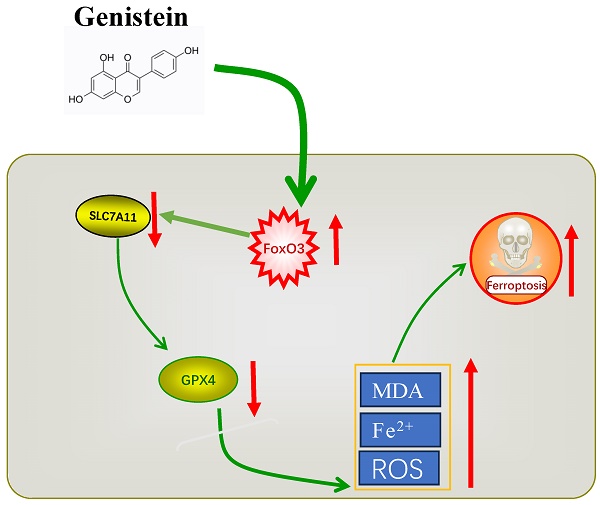
Colorectal cancer is among the most frequently diagnosed cancers with high mortality rates and poses a serious threat to human health. Genistein (Gen) has been found to have anti-colorectal cancer effects, however, the molecular mechanisms by which genistein elicits its effects on colorectal cancer (CRC) cells have not been fully elucidated. In this study, we investigated the oxidative state of colorectal cancer cells during the antitumor action of Genistein and whether it can exert its antitumor effects through ferroptosis. Current research on the oxidative state of Genistein indicates that it exhibits both antioxidant and pro-oxidant properties. Different drug concentrations were applied to colorectal cancer cells, after which cell viability and key markers of ferroptosis, including reactive oxygen species (ROS), malondialdehyde (MDA), and Fe2+, were measured. We found that genistein significantly reduced the viability of colorectal cancer cells, and the expression of ferroptosis markers increased in a concentration-dependent manner. Subsequently, we treated cells with the ferroptosis inhibitor fer-1 in combination with genistein and observed a partial reversal of ferroptosis markers. These findings suggest that genistein exerts its antitumor effect by promoting iron-dependent oxidative damage-induced ferroptosis. To further elucidate the mechanism underlying ferroptosis modulation, we examined the protein and mRNA expression levels of the classical key ferroptosis molecules SLC7A11 and GPX4. We found that the expression levels of these molecules decreased, with GPX4 exhibiting a greater decrease. Overexpression of GPX4 reversed the pro-ferroptotic effect of genistein, indicating that genistein promotes ferroptosis occurrence by downregulating GPX4 expression. When the drug was applied to colorectal cancer cells, the expression of the transcription factor FoxO3 increased. Treatment of cells with the FoxO3 inhibitor JY-2 in combination with other drugs resulted in antagonism of ferroptosis markers. These findings suggest that genistein induces ferroptosis in colorectal cancer cells through the FoxO3/SLC7A11/GPX4 signaling pathway, thereby inhibiting tumor growth.
Keywords: Genistein, Colorectal cancer, Ferroptosis, FoxO3.
1. Introduction
Colorectal cancer (CRC) is one of the most common and deadly cancers worldwide, and its incidence is increasing annually. With the popularity of screening methods, an increasing number of young people are being diagnosed with CRC [1]. In the United States, the incidence of CRC in adults aged >20-49 years has increased by nearly 45% [2]. The reasons for this increasing incidence are associated with unhealthy lifestyles, dietary habits, and underlying diseases, including smoking, alcohol consumption, sedentary behavior, a Western diet, and diabetes [3-6]. Shockingly, individuals under 50 years old with diabetes have a 27% greater risk of developing tumors than healthy individuals [7]. Moreover, due to late-stage diagnosis in the majority of CRC patients [8], the optimal surgical window is missed, and the efficacy of existing chemotherapy regimens is limited for metastatic CRC [9]. Therefore, there is a need to explore new chemotherapeutic methods to achieve better outcomes and reduce the incidence and mortality rate of CRC. Dietary supplements have attracted attention because they can enhance physical fitness and reduce the probability of developing disease [10]. Dietary supplements are substances that contain nutritional or physiologically active ingredients, including plant or herbal extracts, vitamins, minerals, amino acids, etc. These supplements also have antioxidant, anti-inflammatory, anticancer, and immune regulatory functions and play important roles in cancer prevention and prognosis. Studies have shown that dietary supplements can prevent carcinogenesis and inhibit the growth of CRC cells [11], thus potentially providing new strategies for CRC treatment.
Genistein (Gen) is a natural isoflavone that can be extracted from various plants [12]. It not only has anticancer activity but also has multiple biological effects, such as antidiabetic, anti-inflammatory, and antioxidant effects. Among these, its anticancer activity has been extensively studied in breast cancer, CRC, gastric cancer, and liver cancer, among others [13]. Rasheed, S's study indicated that consuming Gen in the diet can reduce the risk of CRC by 23% [14]. Therefore, Gen holds great research value in the prevention and treatment of CRC. Research has suggested that Gen achieves its effects on CRC prevention and treatment by inhibiting cell proliferation, invasion, and migration, blocking the progression of the cell cycle at the G2/M phase [15], inhibiting angiogenesis [16], exerting antioxidant effects [17], suppressing inflammation [18], promoting apoptosis [19], inhibiting topoisomerase II [20], and activating estrogen receptors [21]. Several studies suggest that Gen has antioxidant effects, stabilizes cellular antioxidant status, and scavenges oxygen free radicals, thereby inhibiting carcinogenesis by preventing oxidative damage in the tissue microenvironment [17, 22]. However, research has also shown that Gen can weaken tumor proliferation by promoting oxidative damage in prostate cancer [23] and acute myeloid leukemia in humans [24]. The latest research on Gen in CRC indicates that treatment with Gen leads to a significant increase in endogenous reactive oxygen species (ROS) in SW620 cells, and the accumulation of ROS leads to cell death, which is caused by decreased expression of antioxidant enzymes and weakened capacity to scavenge oxygen free radicals [25]. Therefore, our study aimed to further explore the dynamic process of oxidative stress during the anticancer effects of Gen in CRC and whether this process tends toward oxidative accumulation or an antioxidative state.
Ferroptosis is a programmatic cell death process driven by iron-dependent lipid peroxidation accumulation [26]. It plays a crucial role in the treatment of various cancers, not only by directly inducing tumor cell death but also by reversing the drug resistance of tumors to chemotherapy [27-29], thus enhancing the therapeutic efficacy of chemotherapy drugs. Therefore, ferroptosis is considered an effective target for treating different types of cancer. However, the mechanism of action of genistein, a compound that has anticancer properties, is complex, and it is unclear whether it triggers ferroptosis. Interestingly, ferroptosis is characterized by lipid peroxidation accumulation and an impact on antioxidant enzymes, which aligns with the changes observed in cells upon the introduction of genistein. Therefore, it is reasonable to speculate that genistein could exert its anticancer effects through ferroptosis. This study sought to investigate whether genistein can exert anticancer effects through ferroptosis and how it influences ferroptosis in CRC cells.
2. Material and Methods
2.1 Cell culture and treatment
Human colorectal cancer cell lines HCT116 and HT-29 were obtained from the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in DMEM (#PYG0073, Boster, China) and McCoy's 5A (#PYG0025, Bode Biology, China) media supplemented with 10% fetal bovine serum (#11011-8611, Zhejiang Tianhang, China), 100 IU/mL penicillin, and 100 IU/mL streptomycin in a humidified incubator at 37°C with 5% CO2. Genistein (#G0272; Tokyo Chemical Industry Co., Ltd.), the ferroptosis inhibitor ferrostatin-1 (#HY-100579; MedChemExpress), and JY-2 (#HY-153347; MedChemExpress) were dissolved in dimethyl sulfoxide (DMSO). At specified concentrations and time points, the cells that reached 70% confluency were treated with these compounds, and the DMSO concentration was maintained below one thousandth throughout the treatment process.
2.2 Cell viability
Cell viability was assessed using a Cell Counting Kit-8 (#AR1160, Boster, China). Cancer cells were grown in 96-well plates at a density of 5000 cells/well. After treatment, 10 μL of CCK-8 solution was added, and the mixture was incubated at 37 °C for 1 h. The absorbance was measured at 450 nm using a microplate reader, and each experiment was repeated three times.
2.3 Levels of Fe2+ and MDA
The MDA and Fe2+ contents of the treated cells were analyzed using a Lipid Peroxidation MDA Assay Kit (#S0131S; Shanghai Biyun Tian, China) and a Ferrozine Colorimetric Assay Kit for Iron Detection (#ADS-W-QT027; Jiangsu Feiya, China), respectively, according to the manufacturer's instructions. All the results were normalized to the corresponding total cell numbers.
2.4 ROS levels
Intracellular ROS levels in HCT116 and HT-29 cells treated with the indicated compounds were determined using the oxidation-sensitive fluorescent probe dichlorodihydrofluorescein diacetate (DCFH-DA) (#E004-1-1; Nanjing built, China). A fluorescence microscope (Bio-Rad, Singapore) was used for photographic processing.
2.5 Western blotting
The logarithmic growth phase cells were seeded in a six-well plate at a density of 500,000 cells per well. After treatment, the cells were collected and lysed in RIPA (#R0020, Solarbio China) buffer. The total protein concentration of each sample was determined using a BCA protein concentration assay kit (#AR0146, Boster, China). Equal amounts of protein from each sample were separated via 10% sodium dodecyl sulfate‒polyacrylamide gel electrophoresis and transferred to a PVDF membrane. Next, the membrane was blocked with 5% nonfat milk at room temperature for 1 h and then incubated with primary antibodies against GPX4 (#BM5231), SLC7A11 (#BM5318), FoxO3 (#PB9196), and β-actin (#BM0627) overnight at 4 °C. All antibodies were purchased from Boster (Wuhan, China) and diluted 1:1000. The membranes were then incubated with HRP-conjugated anti-mouse IgG (#BA1050, 1/10000) and HRP-conjugated anti-rabbit IgG (#BA1054, 1/10000) secondary antibodies at room temperature for 1 h, followed by band visualization using a Universal Hood Ⅱ (Bio-Rad, USA) and analysis of the results with ImageJ software.
2.6 RT‒qPCR
Total RNA was extracted using TRNzol (#DP424, TIANGEN, Beijing, China), and cDNA was synthesized by reverse transcription using the FastKing One Step RT‒qPCR Kit (#KR118, TIANGEN, Beijing, China). Real-time quantitative PCR (RT‒qPCR) was performed using RealUniversal Color Fluorescent Quantitative SYBR Green Premix (Cat#FP201; TIANGEN, Beijing, China) on a CFX Connect Real-Time System (Bio-Rad, Singapore) data analysis system. The PCR procedure was as follows: 1 cycle of 95 °C for 15 min, followed by 40 cycles of 95 °C for 10 s and 60 °C for 30 s. GAPDH was used as an internal reference gene. The relative expression levels of the transcripts were calculated using the 2-ΔΔCT method. The sequences of primers used were as follows: GPX4 (forward: 5′-AGAGATCAAAGAGTTCGCCGC-3′, reverse: 5′-TCTTCATCCACTTCCACAGCG-3′), SLC7A11 (forward: 5′-GCTGTGATATCCCTGGCATT-3′, reverse: 5′-GGCGTCTTTAAAGTTCTGCG-3′), FoxO3 (forward: 5'- ATGGCAGAGGCACCAGCCTCC-3', reverse: 5'- TCAGCCTGGTACCCAGCTTTG-3′), and GAPDH (forward: 5′-CCAGGTGGTCTCCTCTGA-3′, reverse: 5′-GCTGTAGCCAAATCGTTGT-3′). All the reagents were purchased from TIANGEN (Beijing, China).
2.7 Plasmid transfection
The cloning vector PcDNA3.1(+) with the cloning sites KPNI and EcoRI, designated Y5844, was obtained from the host bacterium Top10. Both the plasmid and the control vector were purchased from GenePharma (Suzhou, China). For cell transfection, Lip3000 reagent (#BL632B; Biosharp, China) was used. After transfection, the transfection medium was replaced with regular culture medium containing Gen after 6h. Total protein and total RNA were extracted 72h later.
2.8 Hematoxylin-eosin staining and immunohistochemistry
Hematoxylin and eosin (HE) staining and immunohistochemistry (IHC) were performed using standard methods. The primary antibody used was against GPX4 (1:100 dilution; #BM5231; Abcam), which was incubated overnight at 4 °C. Staining was performed using a Universal Two-Step Test Kit (Mouse/Rabbit) (#PV-9000, Zhongshan Golden Bridge, China) and a DAB Staining Kit (#ZLI-9018, Zhongshan Golden Bridge, China). Images were randomly taken from 3 samples in each group and captured using an inverted microscope for analysis.
2.9 Statistical analysis
The Western blots were processed for grayscale analysis using ImageJ2x. The statistical significance of differences was analyzed using one-way ANOVA. Statistical analysis was performed using GraphPad Prism (version 8.0.2, GraphPad Software). All the data are presented as the means ± standard deviations from three independent experiments. p < 0.05 was considered to indicate statistical significance.
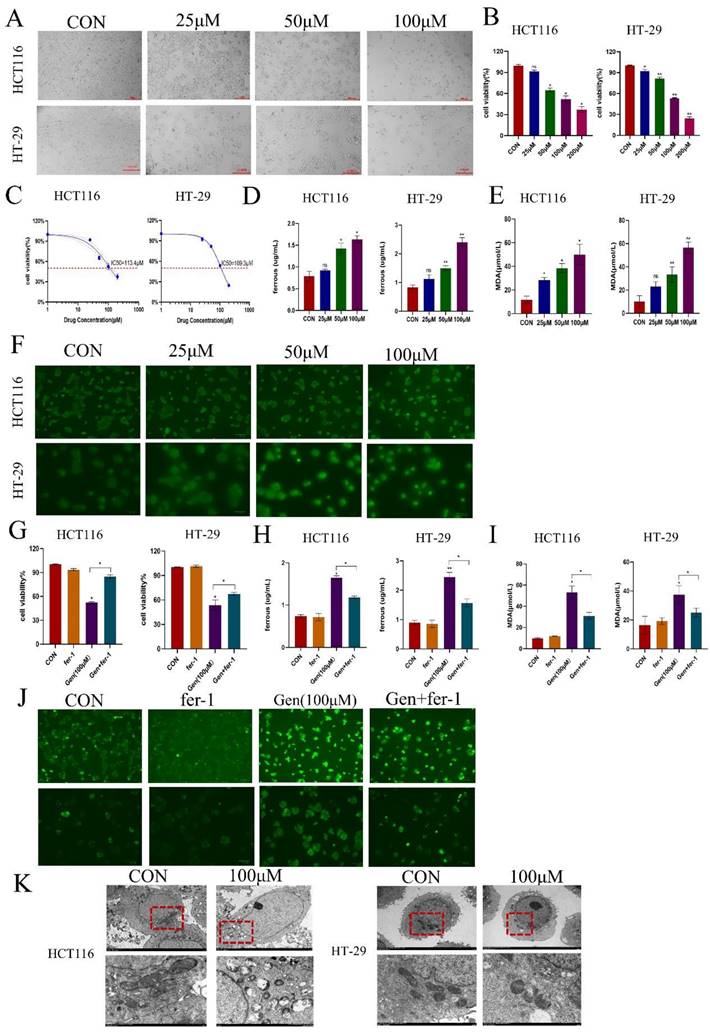
Gen exerts antitumor effects by promoting ferroptosis in CRC cells. (A) Results of inverted microscope shots taken 72 h after treatment of cells with Gen. (B) A CCK-8 assay was used to determine the viability of HCT116 and HT-29 cells after 72 h of Gen treatment. (C) The IC50 of Gen on HCT116 and HT-29 cells was determined via the CCK-8 assay. (D-F) Relative levels of Fe2+(D), ROS (E), and MDA (F) in HCT116 and HT-29 cells after 72 h of treatment with different Gen concentrations. (G) Cell viability was determined by a CCK-8 assay in which cancer cells were pretreated with the ferroptosis inhibitor fer-1 (10 μM) for 12 h and subsequently treated with Gen (100 μM) for 72 h. (H-J) Relative levels of intracellular Fe2+ (H), MDA (I), and ROS (J) in cancer cells pretreated with the ferroptosis inhibitor fer-1 (10 μM) for 12 h and subsequently treated with Gen (100 μM) for 72 h. (K) Morphological changes in mitochondria in the control and 100 μM drug groups were observed via transmission electron microscopy. The data are presented as the means ± SDs (n=3); *p<0.05; **p<0.01.
3. Results
3.1 Gen exerts its antitumor effect by inducing ferroptosis in CRC cells
To investigate the effect of Gen on cell proliferation, we treated HCT116 and HT-29 cells with different concentrations of Gen for 72 h. After treatment with Gen at concentrations ranging from 25 to 200 μM, the viability of the HCT116 and HT-29 cells significantly decreased in a concentration-dependent manner (Figure 1A-B). The IC50 values for HCT116 and HT-29 cells were 113.4 μM and 108.1 μM, respectively (Figure 1C). Experiments conducted with 25, 50, and 100 μM Gen demonstrated that this natural compound upregulated the levels of Fe2+, MDA, and ROS in the cells (Figure 1D-F). Additionally, the addition of the ferroptosis inhibitor fer-1 in the presence of the drug significantly restored cell viability (Figure 1G) and led to a decrease in Fe2+, MDA, and ROS levels to varying degrees (Figure 1H-J). Furthermore, via transmission electron microscopy, it was evident that cells treated with 100 μM Gen exhibited noticeable mitochondrial damage, including mitochondrial ridge shrinkage, swelling, and even rupture (Figure 1K). Therefore, Gen appears to inhibit the growth of colorectal cancer cells by inducing ferroptosis.
3.2 Gen promotes ferroptosis in CRC cells by affecting SLC7A11/GPX4
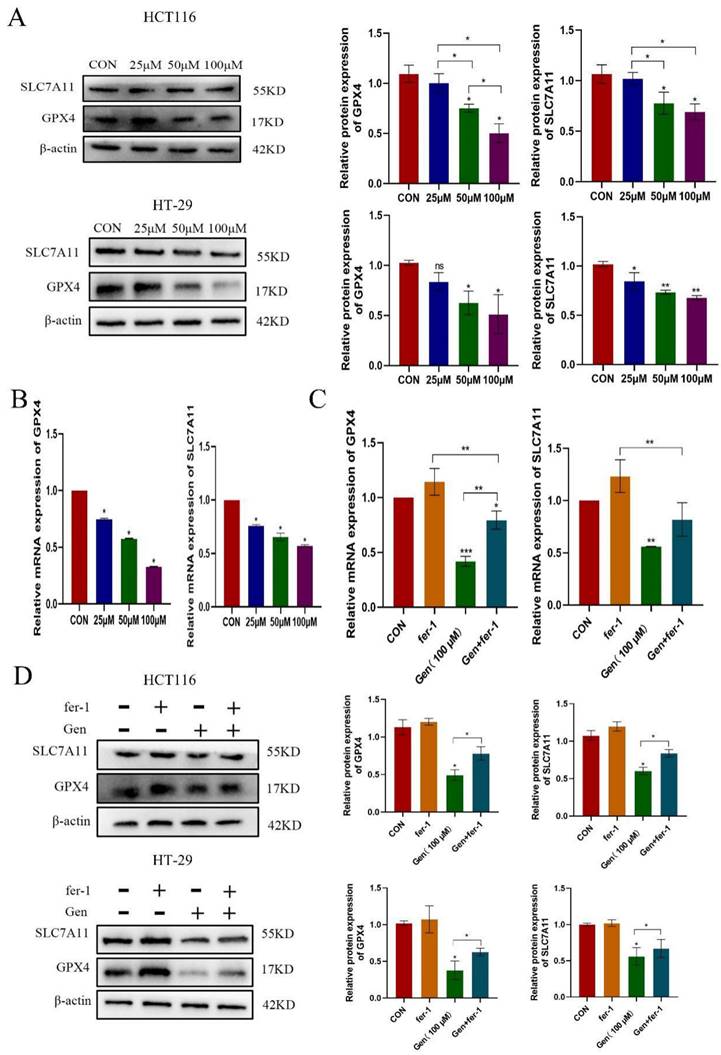
To confirm the mechanism by which Gen affects ferroptosis in cells, we treated HCT116 and HT-29 cells with different concentrations of Gen and collected cell lysates and total RNA to examine the changes in the ferroptosis-related proteins SLC7A11 and GPX4. We found that the protein (Figure 2A) and mRNA (Figure 2B) levels of SLC7A11 and GPX4 decreased as the drug concentration increased. Next, we pretreated HCT116 and HT-29 cells with 10 μM fer-1 for 12 h and then treated them with 100 μM Gen for 72 h. Fer-1 significantly counteracted the effects of Gen on the protein levels of SLC7A11 and GPX4 (Figure 2D) as well as the mRNA levels (Figure 2C) in HCT116 and HT-29 cells. These results demonstrated that Gen promotes ferroptosis in CRC cells by affecting SLC7A11/GPX4.
3.3 Gen promotes ferroptosis in CRC cells by downregulating GPX4
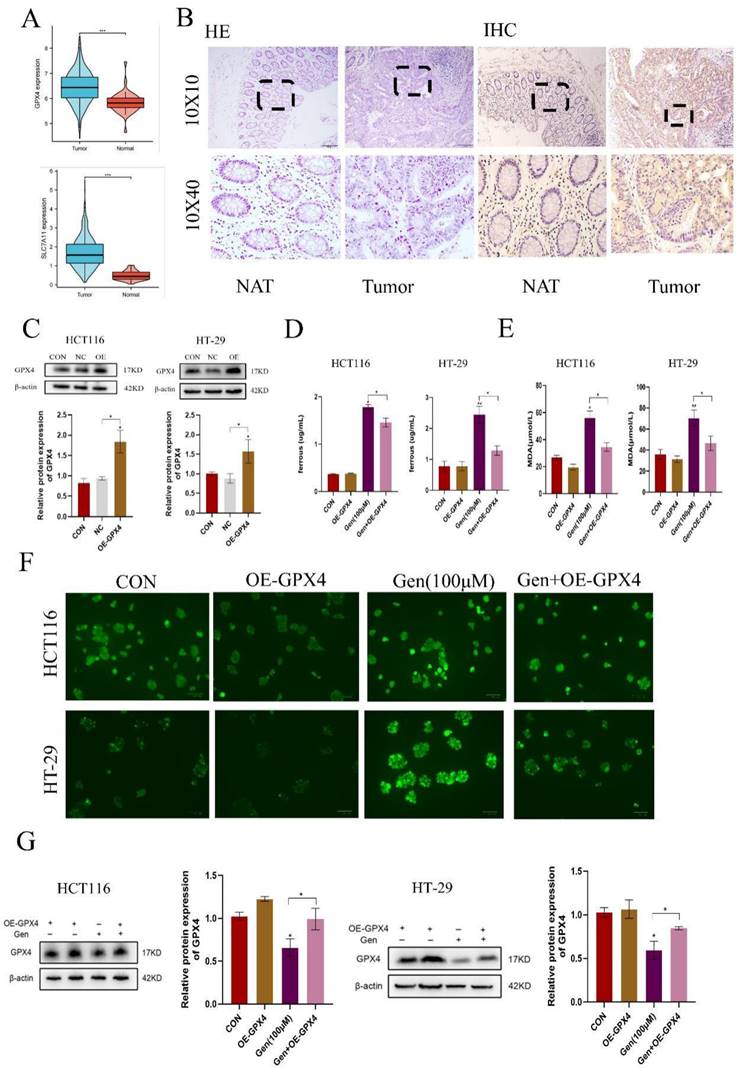
Following drug treatment, we observed that the changes in the expression of GPX4, a classic protein associated with ferroptosis, were more pronounced than those in the SLC7A11 cells at both the mRNA and protein levels. Therefore, we first analyzed the differential expression of GPX4 and SLC7A11 using the TAGG database and found that both were highly expressed in cancer patients (Figure 3A). We also confirmed these findings by analyzing GPX4 in human CRC specimens (Figure 3B). After validating the overexpression of GPX4 (Figure 3C) and cotreating cells with Gen, we observed that OE-GPX4 significantly antagonized the effects of Gen on Fe2+, MDA, and ROS levels (Figure 3D-F) as well as on the levels of GPX4 (Figure 3G). Therefore, Gen induces ferroptosis in cancer cells by downregulating GPX4.
3.4 The binding specificity coefficient between Gen and FoxO3 is high, and there is a negative correlation between FoxO3 and GPX4
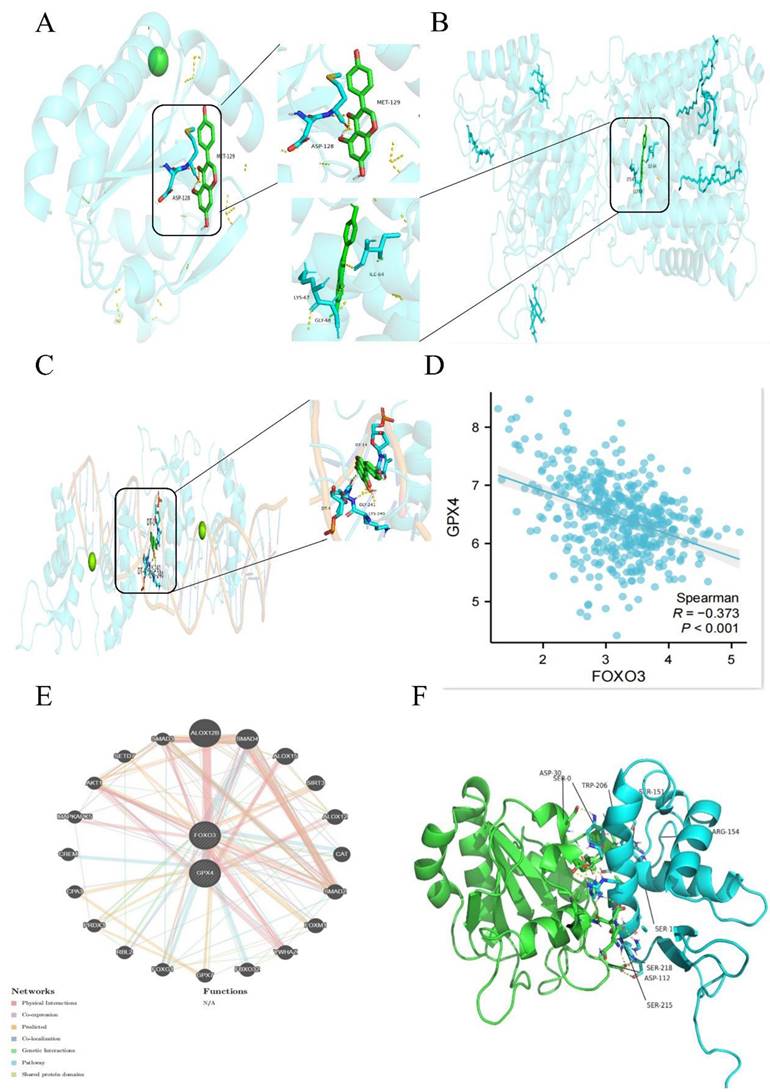
To investigate whether Gen directly affects GPX4 to influence ferroptosis, we obtained the structures of the small molecule ligands and target protein receptors from the PubChem and PDB databases. Preprocessing was conducted using PyMOL and AutoDock software, and the active pockets were identified. Molecular docking was employed to predict the binding modes of the small molecule ligands and target macromolecular receptors. The docking results were evaluated based on binding energy, focusing on key target proteins. The specificity coefficient of the drug binding to GPX4 and SLC7A11 was not high (Figure 4A-B), while the specificity coefficient with the transcription factor FoxO3 was as high as 9.5 (Figure 4C). Detailed tabular data can be found in the Supplementary Materials (S1-S3). The COAD dataset retrieved from the TCGA database was analyzed, and the expression data of "GPX4" and "FoxO3" in tumor patients were extracted. Spearman correlation analysis was used to explore the coexpression relationships of the genes. These parameters were negatively correlated (Figure 4D). Additionally, the GeneMANIA database was used to identify genes that may interact with GPX4 and FoxO3, and the coexpression network was further evaluated, suggesting the possible existence of other intermediate mediators (Figure 4E and 4F).
3.5 Gen promotes ferroptosis by activating FoxO3
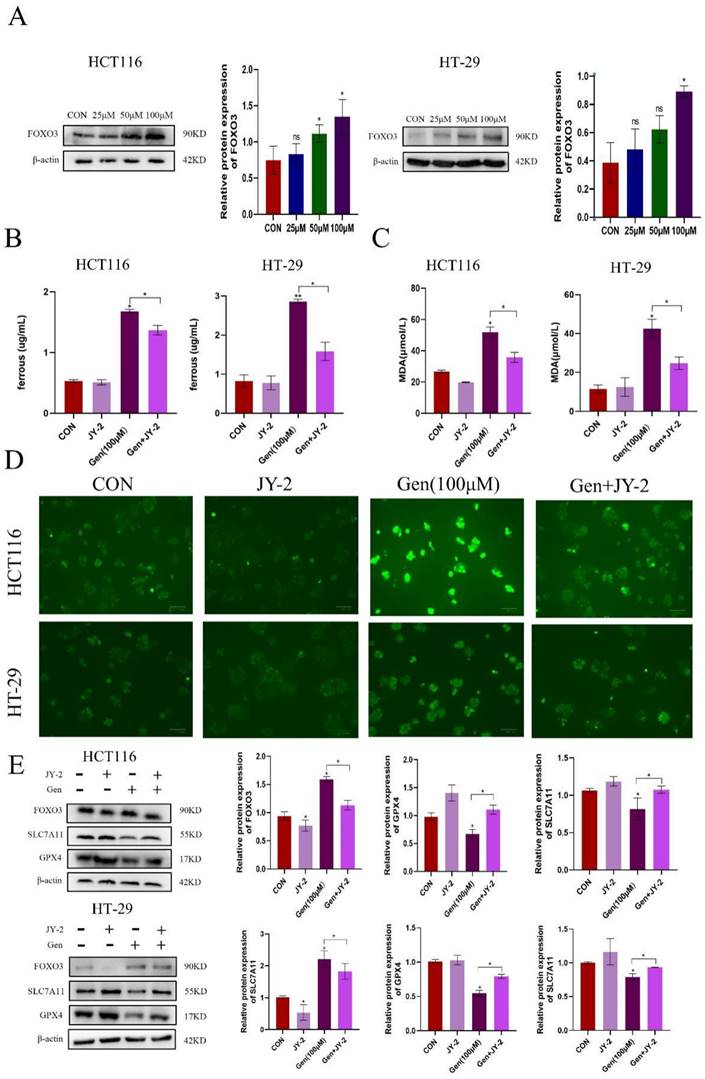
After treating HCT116 and HT-29 cells with different concentrations of Gen, the expression level of the transcription factor FoxO3 gradually increased (Figure 5A). Subsequently, we cotreated HCT116 and HT-29 cells with the FoxO3 inhibitors JY-2 and Gen and found that JY-2 antagonized the impact of Gen on ferroptosis (Figure 5B-D), as did the mRNA and protein levels of SLC7A11 and GPX4 in HCT116 and HT-29 cells (Figure 5E). In summary, Gen promotes ferroptosis in CRC cells through the FoxO3/SLC7A11/GPX4 signaling pathway, achieving an antitumor effect.
Gen promotes ferroptosis in CRC cells by affecting SLC7A11/GPX4. (A) Changes in the protein levels of SLC7A11 and GPX4 after treatment of HCT116 and HT-29 cells with different concentrations of drugs. (B-C) Changes in mRNA levels of SLC7A11 and GPX4 in HCT116 cells in drug concentration gradient and fer-1 co-treatment groups. (D) Changes in the protein levels of intracellular SLC7A11 and GPX4 after pretreatment of HCT116 and HT-29with fer-1 (10 μM), a ferroptosis inhibitor, for 12 h and subsequent addition of Gen (100 μM) for 72 h. Protein levels were determined by Western blotting and normalized to β-actin levels. Relative mRNA levels, determined by RT‒qPCR, were normalized to GAPDH levels. Experiments involving mRNA refer to the results obtained from HCT116 cell experiments. The data are presented as the means ± SDs (n = 3). * p < 0.05; **p<0.01.
Gen promotes ferroptosis in CRC cells by downregulating GPX4. (A) Differential analysis of GPX4 and SLC7A11 in the TCGA database revealed that both are highly expressed in cancer patients. (B) GPX4 was also found to be highly expressed in CRC patient samples by immunohistochemistry. (C) Overexpression of GPX4 was validated at the protein levels. (D-F) Relative levels of intracellular Fe2+ (D), MDA (E), and ROS (F) after cotreatment with OE-GPX4 and Gen. (G) Changes in the relative protein levels of GPX4. Protein levels were determined by Western blotting and normalized to β-actin levels. The data are presented as the means ± SDs (n = 3). * p < 0.05; **p<0.01.
High Gen and FoxO3 binding specificity coefficients and negative correlation between FoxO3 and GPX4. (A-C) The specific binding coefficients of GPX4 (A), SLC7A11 (B), and FoxO3 (C) were measured using the PubChem database. (D) Spearman correlation analysis was performed to explore the coexpression relationships. (E-F) The GeneMANIA database was used to identify genes that may interact with GPX4 and FoxO3, and the coexpression network was further evaluated.
Gen promotes ferroptosis by activating FoxO3. (A) Changes in the expression of FoxO3 after treatment of HCT116 and HT-29 cells with different concentrations of Gen. (B-D) Relative levels of intracellular Fe2+ (B), MDA (C), and ROS (D) after cotreatment of HCT116 and HT-29 cells with the FoxO3 inhibitor JY-2 and Gen. (E) Relative levels of protein of FoxO3, GPX4, and SLC7A11 in cells after cotreatment of HCT116 and HT-29 cells with the FoxO3 inhibitor JY-2 and Gen. The protein concentrations were determined by Western blotting and normalized to the β-actin level. The data are presented as the means ± SDs (n = 3). * p < 0.05; **p<0.01.
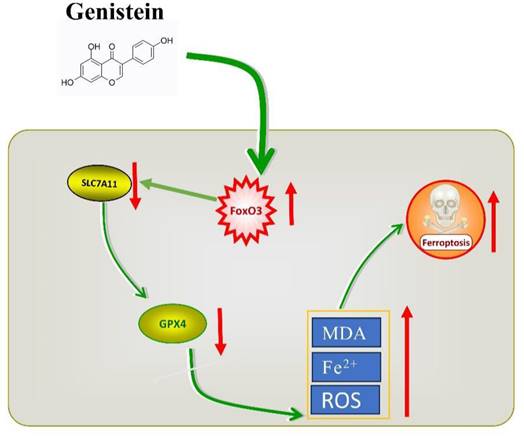
Graphical summary of the current research.
4. Discussion
Iron-dependent cell death, known as ferroptosis, is influenced by iron metabolism, lipid metabolism, and amino acid metabolism. The XC-/GPX4 pathway is the most extensively studied regulatory system [30]. The XC- system primarily consists of solute carrier family 3 member 2 (SLC3A2) and solute carrier family 7 member 11 (SLC7A11, also known as xCT). SLC7A11 plays a key role in transporting cystine into cells, which is subsequently utilized for the synthesis of glutathione. Glutathione is a crucial antioxidant that counteracts the accumulation of reactive oxygen species during ferroptosis. GPX4, utilizes glutathione as a reducing cofactor to detoxify lethal reactive oxygen species and protects cells from oxidative damage [31]. Due to the promising application of ferroptosis in cancer therapy and overcoming drug resistance, ferroptosis has become a potential target for various types of therapeutic agents. Gen exhibits antitumor activity in CRC cells and shares common characteristics with ferroptosis, such as lipid peroxidation accumulation and an impact on antioxidant enzymes upon its introduction. Therefore, in this study, we investigated whether ferroptosis plays a role in the antitumor activity of Gen and how it exerts its effects. Our research results demonstrated that Gen induces ferroptosis by inhibiting the FoxO3/SLC7A11/GPX4 pathway, thus inhibiting the proliferation of CRC cells.
Gen can play a therapeutic role in the prevention and treatment of a variety of diseases. Some studies have shown that the risk of CRC in Asian populations is significantly lower than that in Western countries, possibly due to the characteristics of the Asian diet. Asians consume a wide range of soybean products, and the main ingredient in soybeans is Gen (phytoestrogens), which not only plays a preventive role in CRC but also against diabetes, cardiovascular function, adenomatous polyposis, and other diseases [32]. A large body of literature has also proven the therapeutic effects of Gen, and our findings are consistent with those of our predecessors in terms of its antiproliferative effects on CRC tumors. In addition, our study revealed that the levels of MDA, Fe2+, and ROS, which are related indicators of ferroptosis, increased with increasing concentration, and the levels of GPX4 and SLC7A11, which are related proteins of the XC-/GPX4 pathway, decreased after GEN was applied to CRC cells. Moreover, when Gen was used in combination with fer-1, a ferroptosis inhibitor, the viability of HCT116 and HT-29 cells increased, and the levels of MDA, Fe2+, and ROS decreased. Therefore, Gen was able to exert anti-CRC effects by inducing ferroptosis.
Therefore, based on our findings, when Gen acts on HCT116 and HT-29 cells, it leads to ROS accumulation, and the oxidative state becomes more apparent at higher concentrations. However, in a study by Vasudevan Sekar et al. [17], Gen activated the Nrf-2/HO-1 signaling pathway to reduce cellular oxidative stress, thereby inhibiting carcinogenesis. This difference may be caused by the fact that the mice were treated with a drug that was administered by gavage. The gastrointestinal barrier can affect the absorption of drug concentration. Due to the low bioavailability of Gen, increasing its concentration in the serum can improve its anticancer effects. There have been reports of the development of an immune conjugate (Gen-17. A) composed of Gen and monoclonal antibody 17. A, which can maintain the Gen concentration to a certain extent and enhance its antitumor activity [33]. Gen-PEG-SiHNM (Gen-loaded polyethylene glycol-modified silica hybrid nanomaterial) enhances the antioxidant and antiproliferative effects of Gen on HT29 human colon cancer cells by regulating endogenous antioxidant enzymes and H2O2 production. Furthermore, the synthesized Gen-PEG-SiHNM also reduces the incidence of side effects [34]. Therefore, the antioxidative or pro-oxidative effects of Gen may be related to the concentration of the drug administered. In this study, the drug was directly applied to the cancer cells, achieving an effective concentration that directly acts on the cancer cells.
Figure 1G-H shows that the decrease in GPX4 expression was significantly greater than that in SLC7A11 expression at both the mRNA and protein levels. Therefore, we believe that Gen not only affects the antioxidant function of GPX4 by affecting solely cysteine transport through SLC7A11 but also affects the expression of GPX4 through complex mechanisms. However, Gen-induced ferroptosis in CRC cells is clearly related to GPX4 and SLC7A11. Therefore, we validated their relationship through the overexpression of GPX4 (OE-GPX4) in the opposite direction. After OE-GPX4, a trend similar to that of fer-1 was observed, indicating that Gen can induce ferroptosis by downregulating GPX4.
Ferroptosis is accompanied by the production of a large amount of ROS, which is closely related to mitochondrial dysfunction [35]. When mitochondrial function is impaired, ATP production decreases, which activates the AMPK/FoxO3 pathway to participate in the regulation of mitochondrial function and energy homeostasis. Forkhead box O3 (FoxO3) is a transcription factor involved in the regulation of various physiological processes, such as cell metabolism, mitochondrial function, and oxidative stress [36]. To explore whether Gen directly affects GPX4 to induce ferroptosis, we used molecular docking tools and found that the drug had a low specific binding coefficient with GPX4 and SLC7A11, while the coefficient for the transcription factor FoxO3 was as high as 9.5. It was also found that FoxO3 is negatively correlated with GPX4 and that there are other intermediate mediators affecting them. In addition, a study on energy stress showed that FoxO3 can directly bind to the promoter of SLC7A11 to inhibit its expression and regulate glutamate metabolism [37]. However, whether FoxO3 in CRC affects changes in SLC7A11 and GPX4 to regulate ferroptosis is still unclear. Therefore, we detected changes in the content of FoxO3 in CRC cells treated with different concentrations of Gen and found that the expression of FoxO3 increased with increasing concentrations of the drug. Furthermore, after treatment with JY-2, a FoxO3 inhibitor, the expression of GPX4 and SLC7A11, which decreased in response to Gen treatment, increased, and the expression of ROS, MDA, and Fe2+ also decreased, indicating that Gen promotes ferroptosis by inhibiting the expression of SLC7A11 and GPX4 through activation of FoxO3.
Therefore, the antitumor effect of Gen is significant. However, this drug is currently rarely used in clinical treatment and remains in the development stage. In addition to using it alone, it may also be considered in combination with first-line drugs for colorectal cancer treatment, such as oxaliplatin and 5-FU, to enhance therapeutic efficacy. As Gen is a natural compound with high drug safety, combining it with other drugs might increase its efficacy and reduce the side effects of chemotherapy drugs, which will be a key focus of future research. Additionally, during further in vivo and clinical trials, appropriate modifications to the administration route should be made to avoid affecting the experimental results due to bioavailability issues.
5. Conclusion
In conclusion, our research findings indicate that Gen promotes ferroptosis in CRC cells by activating FoxO3 and further inhibiting the XC-/GPX4 axis. Gen, as a naturally occurring compound, not only has disease prevention properties but also shows potential as a CRC therapeutic drug. It offers several advantages, including easy accessibility, economic benefits, broad functionality, and high safety (Gen is classified by the FDA as a substance generally recognized as safe [32]), making it a valuable candidate for further development.
Abbreviations
Gen: Genistein; ROS: Reactive oxygen species; MDA: Malondialdehyde; Fe2+: Ferrous ion; fer-1: ferrostatin-1; RT‒qPCR: Real-time RT-PCR; IHC: Immunohistochemistry; HE: Hematoxylin-Eosin Staining; RIPA: Radio Immunoprecipitation Assay; PVDF: Poly (vinylidene fluoride).
Supplementary Material
Supplementary tables.
Acknowledgements
Thanks for the technical support offered by Central Laboratory and The First Affiliated Hospital of Guangxi Medical University; Department of Gastrointestinal Surgery, Hengyang Medical College, University of South China.
Funding
This work was supported by Provincial Natural Science Foundation of Hunan (2020JJ4552).
Author contributions
Sen Zhang: Conception and Design of the work; Longferi Liu: Conception and Design of the work, Article revision; Yuan Qiu: Data curation, Data Analysis, Writing-original draft; Zehao Peng: Supplementary Experiments; Zhongchao Yu: Case collection; Hengzhe Lu: Article revision; Rongjun Xie and Zhongcheng Mo: Immunohistochemistry and Hematoxylin-Eosin Staining; All authors reviewed the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Fedewa SA, Anderson WF, Miller KD, Ma J, Rosenberg PS. et al. Colorectal Cancer Incidence Patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017;109(8):djw322
2. Stoffel EM, Murphy CC. Epidemiology and Mechanisms of the Increasing Incidence of Colon and Rectal Cancers in Young Adults. Gastroenterology. 2020;158:341-53
3. Nimptsch K, Wu K. Is Timing Important? The Role of Diet and Lifestyle during Early Life on Colorectal Neoplasia. Curr Colorectal Cancer Rep. 2018;14:1-11
4. Rosato V, Bosetti C, Levi F, Polesel J, Zucchetto A, Negri E. et al. Risk factors for young-onset colorectal cancer. Cancer Causes Control. 2013;24:335-41
5. Kim JY, Jung YS, Park JH, Kim HJ, Cho YK, Sohn CI. et al. Different risk factors for advanced colorectal neoplasm in young adults. World J Gastroenterol. 2016;22:3611-20
6. Nguyen LH, Liu PH, Zheng X, Keum N, Zong X, Li X. et al. Sedentary Behaviors, TV Viewing Time, and Risk of Young-Onset Colorectal Cancer. JNCI Cancer Spectr. 2018;2:pky073
7. Kim NH, Jung YS, Yang HJ, Park SK, Park JH, Park DI. et al. Prevalence of and Risk Factors for Colorectal Neoplasia in Asymptomatic Young Adults (20-39 Years Old). Clin Gastroenterol Hepatol. 2019;17:115-22
8. Zheng R, Zhang K, Tan S, Gao F, Zhang Y, Xu W. et al. Exosomal circLPAR1 functions in colorectal cancer diagnosis and tumorigenesis through suppressing BRD4 via METTL3-eIF3h interaction. Mol Cancer. 2022;21(1):49
9. Hu J, Li J, Yue X, Wang JC, Wang JF, Liu JZ. et al. Targeting BCRP/ABCG2 by RNA interference enhances the chemotherapy sensitivity of human colon cancer side population cells. J Huazhong U Sci-Med. 2017;37:231-6
10. Tan S, Meng Q, Jiang Y, Zhuang Q, Xi Q, Xu J. et al. Impact of oral nutritional supplements in post-discharge patients at nutritional risk following colorectal cancer surgery: A randomised clinical trial. Clin Nutr. 2021;40:47-53
11. Johal J, Han CY, Joseph R, Munn Z, Agbejule OA, Crawford-Williams F. et al. Dietary Supplements in People with Metastatic Cancer Who Are Experiencing Malnutrition, Cachexia, Sarcopenia, and Frailty: A Scoping Review. Nutrients. 2022;14(13):2642
12. Wei TT, Chandy M, Nishiga M, Zhang A, Kumar KK, Thomas D. et al. Cannabinoid receptor 1 antagonist genistein attenuates marijuana-induced vascular inflammation. Cell. 2022;185:2387-9
13. Sharifi-Rad J, Quispe C, Imran M, Rauf A, Nadeem M, Gondal TA. et al. Genistein: An Integrative Overview of Its Mode of Action, Pharmacological Properties, and Health Benefits. Oxid Med Cell Longev. 2021;2021:3268136
14. Rasheed S, Rehman K, Shahid M, Suhail S, Akash MSH. Therapeutic potentials of genistein: New insights and perspectives. J Food Biochem. 2022;46:e14228
15. Zhang Z, Wang CZ, Du GJ, Qi LW, Calway T, He TC. et al. Genistein induces G2/M cell cycle arrest and apoptosis via ATM/p53-dependent pathway in human colon cancer cells. Int J Oncol. 2013;43:289-96
16. Zhang L, Zhang J, Gong Y, Lv L. Systematic and experimental investigations of the anti-colorectal cancer mediated by genistein. Biofactors. 2020;46:974-82
17. Sekar V, Anandasadagopan SK, Ganapasam S. Genistein regulates tumor microenvironment and exhibits anticancer effect in dimethyl hydrazine-induced experimental colon carcinogenesis. Biofactors. 2016;42:623-37
18. Dariya B, Muppala S, Srivani G, Momin S, Alam A, Saddala MS. Targeting STAT proteins via computational analysis in colorectal cancer. Mol Cell Biochem. 2021;476:165-74
19. Qin J, Teng J, Zhu Z, Chen J, Huang WJ. Genistein induces activation of the mitochondrial apoptosis pathway by inhibiting phosphorylation of Akt in colorectal cancer cells. Pharm Biol. 2016;54:74-9
20. Salti GI, Grewal S, Mehta RR, Das Gupta TK, Boddie AW Jr, Constantinou AI. Genistein induces apoptosis and topoisomerase II-mediated DNA breakage in colon cancer cells. Eur J Cancer. 2000;36:796-802
21. Bielecki A, Roberts J, Mehta R, Raju J. Estrogen receptor-beta mediates the inhibition of DLD-1 human colon adenocarcinoma cells by soy isoflavones. Nutr Cancer. 2011;63:139-50
22. Ganai AA, Farooqi H. Bioactivity of genistein: A review of in vitro and in vivo studies. Biomed Pharmacother. 2015;76:30-8
23. Wang G, Zhang D, Yang S, Wang Y, Tang Z, Fu X. Co-administration of genistein with doxorubicin-loaded polypeptide nanoparticles weakens the metastasis of malignant prostate cancer by amplifying oxidative damage. Biomater Sci. 2018;6:827-35
24. Sanchez Y, Amran D, de Blas E, Aller P. Regulation of genistein-induced differentiation in human acute myeloid leukaemia cells (HL60, NB4) Protein kinase modulation and reactive oxygen species generation. Biochem Pharmacol. 2009;77:384-96
25. Alorda-Clara M, Torrens-Mas M, Morla-Barcelo PM, Roca P, Sastre-Serra J, Pons DG. et al. High Concentrations of Genistein Decrease Cell Viability Depending on Oxidative Stress and Inflammation in Colon Cancer Cell Lines. Int J Mol Sci. 2022;23(14):7526
26. Liang D, Feng Y, Zandkarimi F, Wang H, Zhang Z, Kim J. et al. Ferroptosis surveillance independent of GPX4 and differentially regulated by sex hormones. Cell. 2023;186:2748-64 e22
27. Wang Y, Zheng L, Shang W, Yang Z, Li T, Liu F. et al. Wnt/beta-catenin signaling confers ferroptosis resistance by targeting GPX4 in gastric cancer. Cell Death Differ. 2022;29:2190-202
28. Wang Y, Zhao G, Condello S, Huang H, Cardenas H, Tanner EJ. et al. Frizzled-7 Identifies Platinum-Tolerant Ovarian Cancer Cells Susceptible to Ferroptosis. Cancer Res. 2021;81:384-99
29. Wang Y, Zhang L, Yao C, Ma Y, Liu Y. Epithelial Membrane Protein 1 Promotes Sensitivity to RSL3-Induced Ferroptosis and Intensifies Gefitinib Resistance in Head and Neck Cancer. Oxid Med Cell Longev. 2022;2022:4750671
30. Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347-64
31. Shen LD, Qi WH, Bai JJ, Zuo CY, Bai DL, Gao WD. et al. Resibufogenin inhibited colorectal cancer cell growth and tumorigenesis through triggering ferroptosis and ROS production mediated by GPX4 inactivation. Anat Rec (Hoboken). 2021;304:313-22
32. Pintova S, Dharmupari S, Moshier E, Zubizarreta N, Ang C, Holcombe RF. Genistein combined with FOLFOX or FOLFOX-Bevacizumab for the treatment of metastatic colorectal cancer: phase I/II pilot study. Cancer Chemother Pharmacol. 2019;84:591-8
33. Gentile MS, Vasu C, Green A, Murillo G, Das Gupta TK, Constantinou AI. et al. Targeting colon cancer cells with genistein-17.1A immunoconjugate. Int J Oncol. 2003;22:955-9
34. Pool H, Campos-Vega R, Herrera-Hernandez MG, Garcia-Solis P, Garcia-Gasca T, Sanchez IC. et al. Development of genistein-PEGylated silica hybrid nanomaterials with enhanced antioxidant and antiproliferative properties on HT29 human colon cancer cells. Am J Transl Res. 2018;10:2306-23
35. Gao M, Yi J, Zhu J, Minikes AM, Monian P, Thompson CB. et al. Role of Mitochondria in Ferroptosis. Mol Cell. 2019;73:354-63 e3
36. Wang M, Jiang R, Liu J, Xu X, Sun G, Zhao D. et al. 20(s)-ginseonside-Rg3 modulation of AMPK/FoxO3 signaling to attenuate mitochondrial dysfunction in a dexamethasone-injured C2C12 myotube-based model of skeletal atrophy in vitro. Mol Med Rep. 2021;23(5):306
37. Zhong S, Chen W, Wang B, Gao C, Liu X, Song Y. et al. Energy stress modulation of AMPK/FoxO3 signaling inhibits mitochondria-associated ferroptosis. Redox Biol. 2023;63:102760
Author contact
![]() Corresponding author: Sen Zhang, Department of Colorectal & Anal Surgery, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, China. E-mail: Liulongfeiedu.cn.
Corresponding author: Sen Zhang, Department of Colorectal & Anal Surgery, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, China. E-mail: Liulongfeiedu.cn.

 Global reach, higher impact
Global reach, higher impact