3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(20):6754-6767. doi:10.7150/jca.101470 This issue Cite
Research Paper
Tumor Heterogeneity of STEAP4 in Malignant Progression of Oral Squamous Cell Carcinoma
1. The Breast Center, Cancer Hospital of Shantou University Medical College, Shantou 515041, China.
2. Department of Physiology, Shantou University Medical College, Shantou 515041, China.
3. Department of General Surgery, the First Affiliated Hospital of Shantou University Medical College, Shantou 515041, China.
Received 2024-7-26; Accepted 2024-9-30; Published 2024-11-4
Abstract
Background: Recent research suggests that STEAP4, a metalloreductase in vivo, plays a crucial role in various types of tumorigeneses, especially in gastrointestinal cancers. However, few oncogenes have been reported in oral squamous cell carcinoma (OSCC). Therefore, this study aimed to explore the potential role of STEAP4 in OSCC.
Methods: The expression level of STEAP4 in OSCC tissues and adjacent normal tissues, was detected using immunohistochemistry. Publicly available online tools were utilized to analyze the expression, prognostic significance, and related enriched pathways of STEAP4 in head and neck squamous cell carcinoma (HNSCC) and OSCC. The relationship between STEAP4 expression and clinicopathological parameters in OSCC patients was validated using the χ2 test and Fisher's exact probability test.
Results: STEAP4 exhibited low expression in both HNSCC and OSCC. Whereas the prognosis for HNSCC patients was favorable, OSCC patients had poor outcomes. Genetic variability analysis revealed no alterations in STEAP4 in OSCC, whereas gene amplification was observed in HNSCC, suggesting tumor heterogeneity in STEAP4 among these cancer types.
Conclusion: STEAP4, as a risk factor associated with poor patient prognosis, shows tumor heterogeneity in OSCC patients, that is potentially related to genetic mutations or differences in histological distribution of oral mucosa. These findings indicate that STEAP4 could serve as an independent predictor for assessing the prognosis of OSCC patients.
Keywords: STEAP4, oral squamous cell carcinoma, biomarker, heterogeneity
Introduction
Six-transmembrane epithelial antigen of prostate 4 (STEAP4), also known as six-transmembrane protein of prostate 2 (STAMP2) and TNFα-induced adipose-associated protein (TNFAIP9), belongs to the prostate six-transmembrane epithelial antigen of prostate (STEAP) family, which has significant roles in regulating glucose metabolism, fatty acid metabolism, and inflammatory responses1-3. During a gene product screen of TNF-α-induced pre-differentiated adipocytes, Korkmaz et al. initially identified and reported STEAP4 in 20121. It is observed that STEAP4 co-localizes with transferrin and transferrin receptor 1 in the Golgi complex and plasma membrane4,5. Soon, numerous studies have indicated a correlation between STEAP4 and obesity, as well as insulin resistance6,7. Orfanou et al. suggested a potential anti-apoptotic role of STEAP4 in breast cancer8. Furthermore, STEAP4 has been implicated in inflammatory arthritis through its regulation of inflammatory cytokines3, and it also modulates the progression of androgen-dependent prostate cancer cells by inhibiting anchorage-dependent cell proliferation9.
On the other hand, oral squamous cell carcinoma (OSCC) is a prevalent malignant tumor, accounting for approximately 90% of all oral malignancies10,11. According to data from the Global Cancer Observatory (GCO), it was projected that by 2020, there would be 377,713 cases of OSCC worldwide, with a majority of cases occurring in Asia12. Moreover, the incidence of OSCC is expected to rise by approximately 40% by 2040, accompanied with an increase in mortality12. Extensive research has identified several risk factors contributing to the development of OSCC, including persistent exposure to smoking13, chronic alcohol abuse14, and chronic betel nut chewing15. Additionally, repeated infection with human papillomavirus (HPV) has been associated with the development of oral potentially malignant diseases (OPMDs)16, such as oral leukoplakia (OL), oral erythema (OE), oral submucous fibrosis (OSMF), and oral lichen planus (OLP). Among these OPMDs, proliferative verrucous mucosal leukoplakia (PVL) is a rare multifocal OL characterized by higher aggressiveness and recurrence rates17. OE, another malignant lesion, exhibits a higher potential for malignant transformation, with approximately 50% of patients progressing to atypical hyperplasia, carcinoma in situ, or invasive tumors18. Although various therapeutic interventions, such as chemotherapy, radiotherapy, immunotherapy, and nanomedicines, have been proposed for OPMDs or OSCC, unraveling the oncogenes and related biomarkers specific to OSCC can significantly contribute to early detection, diagnosis, and treatment of this malignancy, thereby improving the survival and prognosis of OSCC patients.
In this study, we investigated the expression and prognostic value of STEAP4 in OSCC. Additionally, we analyzed the clinicopathological parameters, associated pathways, and prognostic significance of STEAP4 in both HNSCC and OSCC using bioinformatics analytical tools and immunohistochemical staining. Our findings unveiled the tumor heterogeneity of STEAP4 in OSCC, highlighting its potential as an independent predictor of OSCC prognosis.
Materials & Methods
Patient information and ethical statement
A tissue microarray with 44 cases of OSCC tissues and 4 corresponding adjacent normal tissues were obtained for evaluating the expression level of STEAP4 by immunohistochemistry. This study was approved by the Ethics Committee of Shantou University Medical College.
Molecular Structure and Function Prediction of STEAP4
The 3D structure of STEAP4 was predicted using the Swiss-Model website, which utilizes homology modeling (https://swissmodel.expasy.org/). GO database annotation information for the STEAP4 protein (Q687X5) was obtained from the Uniprot database (https://www.uniprot.org/) for functional classification. The STRING database (https://cn.string-db.org/) was used to predict the protein-protein interaction networks (PPIs) of STEAP4, mining the core regulatory networks associated with STEAP4-related genes.
Expression of STEAP4 in different tissues and organs
STEAP4 expression in various tissues and organs was analyzed using the Expression Atlas (https://www.ebi.ac.uk/gxa/about.html), which provides information on gene expression patterns under different biological conditions, including gene knockout and treatment with specific compounds, containing information on microarray and RNA-seq data. Additionally, STEAP4 expression in HNSCC and adjacent normal tissues was analyzed using the GTEx (The Genotype-Tissue Expression) database and the PCAWG (The Pan-Cancer Analysis of Whole Genomes project) database. Bgee (https://www.bgee.org/) retrieved expression and deletion data for STEAP4 across different tissues and organs by integrating multiple data types (RNA-Seq, Affymetrix, in situ hybridization, and EST data). Data were derived from the GTEx dataset and other smaller datasets with consistent data annotation and processing.
Expression of STEAP4 mRNA in HNSCC and prognostic analysis
STEAP4 expression levels in HNSCC were predicted using the ENCORI database (https://rnasysu.com/encori/). The UALCAN portal (https://ualcan.path.uab.edu/) was used to analyze the differential expression of STEAP4 mRNA in HNSCC patients and its relationship to tumor staging from a cohort of TCGA, including 520 primary HNSCC and 44 normal samples.
The PanCanSurvPlot (http://zjyy-oncology.asuscomm.cn:20008/) website was used to predict the overall survival (OS) for STEAP4 in HNSCC patients. Relapse-free survival (RFS) information was evaluated through PrognoScan (http://dna00.bio.kyutech.ac.jp/PrognoScan/). The OShnscc (https://bioinfo.henu.edu.cn/HNSC/HNSC_TCGA.jsp) website, integrating gene expression profiles of 1366 unique HNSCC cases and corresponding clinical information from nine independent cohorts, was used to predict the prognostic value of STEAP4 in TCGA and three GEO datasets (GSE31056, GSE65858, and GSE3292). Univariate Cox regression analysis was performed to show the role of STEAP4 in OS and disease-free survival (DFS) of patients, with hazard ratios (HRs) and their 95% confidence intervals, and log-rank p-values.
Differential expression analysis and enrichment analysis in normal and OSCC tissue
Differentially-expressed genes (DEGs) in normal and OSCC tissues in the GSE31056 dataset were analyzed using the GEO database (https://www.ncbi.nlm.nih.gov/geo/). GO and KEGG enrichment analyses of DEGs was performed using R package.
Expression of STEAP4 mRNA in OSCC and prognostic analysis
STEAP4 expression in DEGs was plotted as a bar graph. The "survival" algorithm of R package was used to calculate the survival of patients in the GSE31056 and GSE27020 datasets, with high and low STEAP4 expression, aiding in evaluating patient prognosis.
Immunohistochemical staining and scoring
Tissue samples underwent deparaffinization in xylene, hydration in graded ethanol, antigenic epitope retrieval in ethylenediaminetetraacetic acid, and blocking of endogenous peroxidase in 3% H2O2. Samples were incubated overnight with an anti-STEAP4 antibody (Proteintech 11944-AP) diluted at 1:400, followed by hematoxylin nuclear re-staining for visualization. Sections were independently observed and evaluated under a bright-field microscope by two individual investigators blinded to patient information. STEAP4 expression was evaluated based on staining intensity (0-3: background color, light yellow, brown, and dark color) and the percentage of positive cells (0-4: 0%, 1%-25%, 26%-50%, 51%-74%, 76%-100%). Patients were categorized into low and high expression groups based on the total score results19. The relationship between STEAP4 level and clinicopathological parameters of OSCC patients was analyzed using the χ2 test and Fisher's exact probability test.
Mutations of STEAP4 in HNSCC and OSCC
Genetic STEAP4 alterations in HNSCC were studied using the HNSCC-TCGA PanCancer Atlas in the cBioPortal platform (https://www.cbioportal.org/)20, which includes 523 samples. Genetic alterations in STEAP4 in OSCC were analyzed using 40 samples from the OSCC dataset (MD Anderson Cancer Discov 2013).
Results
STEAP4 plays dual roles as an oncogene and anti-oncogene in various types of cancers
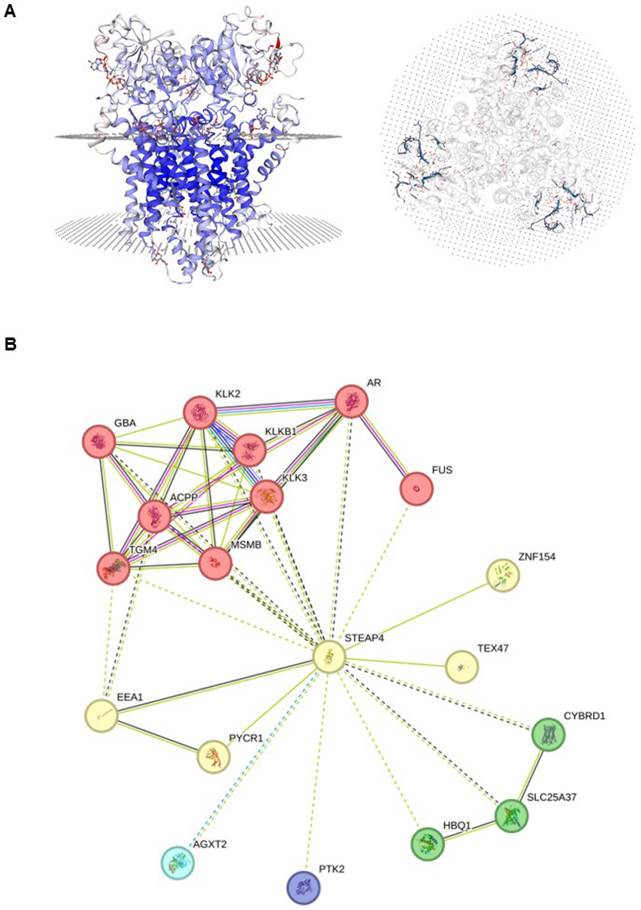
STEAP4 is a protein-coding gene that belongs to the family of STEAP and is situated on chromosome 7q21, comprising five exons and four introns. As shown in Figure 1A, STEAP4 is a transmembrane protein made up of 459 amino acids (52.0 kDa), possessing three conserved motifs in the N-terminal structural domain. These motifs correspond to a dinucleotide-binding structural domain, an NADP oxidoreductase motif, and a pyrrolidine-5-carboxylic acid reductase-like motif, with six transmembrane regions near the COOH-terminal domain1.
As an NADPH-dependent chelating iron-reductase, STEAP4 is found to be located in the Golgi4,5 and can reduce Fe(3+) to Fe(2+) using NAD(+) as an acceptor, which mediates a sequential transmembrane electron transfer from NADPH to FAD, to heme, and finally to Fe(3+) chelates21. Additionally, STEAP4 also reduces Cu(2+) to Cu(1+). GO annotations related to this gene reveal that the cellular components related to STEAP4 are mainly the extracellular exosome, endosome, membrane, and nucleoplasm. The molecular functions of STEAP4 are mainly focused on cupric reductase activity, metal ion binding, and electron transfer activity. STEAP4 also serves to maintain cupric iron homeostasis and assist electron transfer (Table 1).
GO annotations of STEAP4 in UniProt.
| ASPECT | TERM |
|---|---|
| Cellular Component (CC) | early endosome membrane |
| endosome | |
| extracellular exosome | |
| Golgi membrane | |
| membrane | |
| nucleoplasm | |
| plasma membrane | |
| Molecular Function (MF) | cupric reductase activity |
| electron transfer activity | |
| FAD binding | |
| ferric-chelate reductase (NADPH) activity | |
| heme binding | |
| metal ion binding | |
| Biological Process (BP) | copper ion import |
| fat cell differentiation | |
| iron import into cells | |
| protein homotrimerization |
Characteristics of STEAP4 protein. (A) The structure of human STEAP4 from Swiss-Model. (B) Protein-protein interaction (PPI) network of STEAP4. The proteins interacting with STEAP4 were analyzed by k-means clustering that determines the number of proteins based on central points. The red protein cluster indicates mixed protein such as β-trace amino protein and prostate adenocarcinoma. The yellow protein cluster represents protein associated with cupric reductase activity and mitotic spindle midzone. The green protein cluster includes CYBRD1, HBQ1, SLC25A37; The blue protein cluster includes AGXT2; The purple protein cluster includes PTK2, which indicates different protein clusters with unknown function.
Recently, there have been continuous discoveries regarding the function of STEAP4 in various types of cancer. When comparing different tissues and organs, the expression of STEAP4 mRNA and protein was found to be higher in adipose tissues, bone marrow, and prostate, while lower in brain, renal pelvis, and thymus (Supplementary Table 1). Among various diseases, the expression of STEAP4 was found to be elevated in luminal breast tumor and colon mucinous adenocarcinoma, but decreased in chronic lymphocytic leukemia, HNSCC, and breast adenocarcinoma (Supplementary Table 2).
Analysis of PPI networks revealed a close relationship between STEAP4 and the kallikrein-related peptidase (KLK) family, a serine protease family categorized under protein hydrolases (Figure 1B). It has been reported that the KLK family has varying effects on different components of the tumor microenvironment22. KLK3 is widely used as a PSA in the clinic23, and KLK4 and KLK5 are involved in the treatment and prognosis of other cancers, such as ovarian cancers 24, triple-negative breast cancers (TNBC)25 and colorectal cancers (CRC)26. These findings suggest that STEAP4 may exert biological effects through the KLK family, but this conclusion requires further validation.
In summary, STEAP4 exhibits differential expression in various tissues and can function as either a promoter or suppressor of cancer in different cancer types. Therefore, we were interested in exploring the expression of STEAP4 in HNSCC, especially in OSCC, and subsequently conducted further investigations and validations.
STEAP4 serves as a protective factor in HNSCC
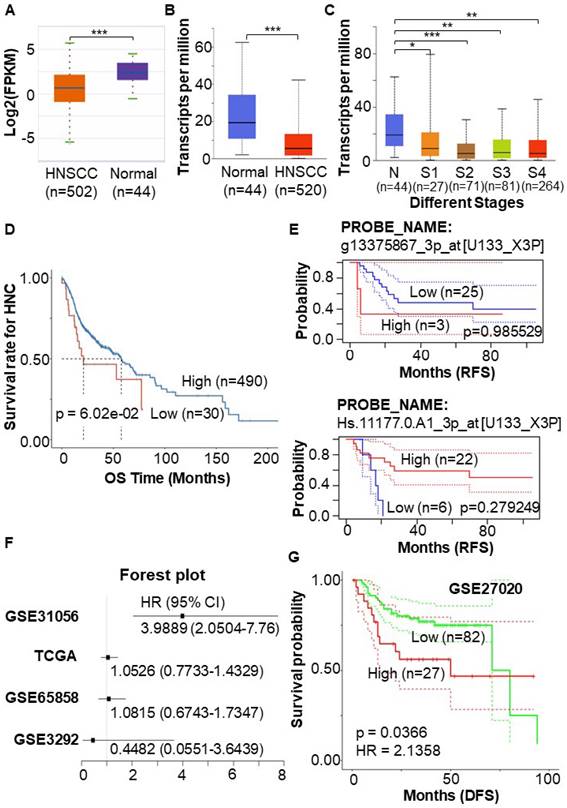
To validate the lower expression of STEAP4 in HNSCC compared to adjacent normal tissues, regardless of HPV positivity27, we utilized different databases to conduct a detailed analysis of STEAP4 expression in HNSCC. The ENCORI database, which statistically analyzed and calibrated data from 44 normal and 502 HNSCC samples, revealed a more pronounced downregulation of STEAP4 in HNSCC (Figure 2A). Another tumor-related database, UALCAN portal, also confirmed this result (Figure 2B). Notably, the expression of STEAP4 in HNSCC tended to be decreased in advanced tumor stages (Figure 2C).
To further investigate the potential role of low STEAP4 expression in HNSCC, we assessed prognostic information, including OS, DFS, and RFS in HNSCC patients. One-way Cox regression analysis indicated that STEAP4 acted as a protective factor in head and neck cancer (HNC). The OS of HNC patients with low expression of STEAP4 showed a downward trend, although the difference was not statistically significant (p = 6.02e-02) (Figure 2D). This may be due to the small sample size applied for survival analysis. To delve deeper, we explored the GSE2837 dataset related to HNSCC in HNC, and statistically analyzed 40 samples from 28 HNSCC patients. Interestingly, different probes yielded varying analytical results, although none of the results were statistically significant (p = 0.279249, and p = 0.985529). In this dataset, STEAP4 was identified to serve as a protective factor (HR = 0.30) or a risk factor (HR = 1.02) for HNSCC, as shown in the RFS (Figure 2E). At this point, the results remain somewhat contentious.
Upon the analysis in OShnscc, it is discovered that in dataset GSE31056, STEAP4 was a risk factor (HR = 3.9889) that predicted poor prognosis in patients (p < 0.0001). TCGA and other datasets GSE65858 also predicted STEAP4 as a risk factor, except for datasets GSE3292 (Figure 2F, Table 2). The result in GSE31056 and TCGA was further confirmed in the GSE27020 dataset (HR = 2.1358, p = 0.0366) (Figure 2G). HNSCC, a specific type of cancer, encompasses OSCC, oropharyngeal squamous cell carcinoma (OPSCC), laryngeal squamous cell carcinoma (LSCC), and nasal and nasopharyngeal squamous cell carcinoma28. So, the contradictory above-mentioned outcomes may be attributed to differences in sample composition. Among these, STEAP4 acted as an independent risk factor in OSCC (GSE31056) and LSCC (GSE27020), and its high expression was positively associated with shorter OS and RFS in these patients (Figure 2F-G). This indicates that OSCC and LSCC exhibit unique tumor heterogeneity within HNSC, potentially reflecting distinct tumor characteristics and gene expression profiles.
Univariate Cox regression analysis of STEAP4 expression with OS in each OShnscc dataset
| Dataset | p-value | HR | 95%CI |
|---|---|---|---|
| GSE31056 | < 0.0001 | 3.9889 | 2.0504 ~ 7.7600 |
| GSE3292 | 0.4529 | 0.4482 | 0.0551 ~ 3.6439 |
| TCGA | 0.7446 | 1.0526 | 0.7733 ~ 1.4329 |
| GSE65858 | 0.7452 | 1.0815 | 0.6743 ~ 1.7347 |
STEAP4 is regarded as a risk factor in OSCC accompanied by worse prognosis
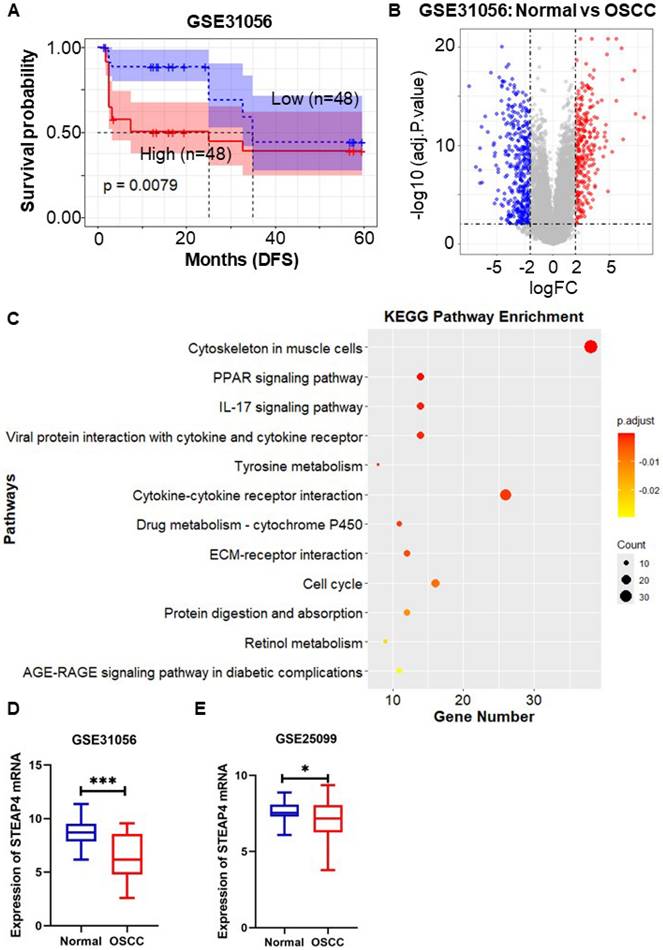
To validate the aforementioned findings, we utilized the OSCC-related dataset GSE31056 to evaluate the survival and prognostic significance of STEAP4, utilizing the Kaplan-Meier method implemented in the R language through the survival package. The dataset comprised 96 tissue samples from 24 patients, encompassing histologically normal tissues, OSCC tissues, and normal adjacent margin tissues. The Kaplan-Meier survival curves depicted the impact of STEAP4 expression on RFS in OSCC patients. Consistent with previous findings, our results indicated that high expression of STEAP4 was associated with decreased RFS (p = 0.0079) (Figure 3A), suggesting that STEAP4 may function as a risk factor in OSCC. The finding indicates that STEAP4 exhibits elevated expression in OSCC, functioning as a potential oncogene.
Expression pattern and prognostic value of STEAP4 in HNSCC. (A & B & C) Expression of STEAP4 in HNSCC in ENCORI (A) and UALCAN (B & C). (D & E) OS and RFS of STEAP4 levels for patients with HNC (D) and HNSCC (E). (F) Correlation of STEAP4 with OS. (G) DFS for STEAP4 levels for patients with HNSCC in GSE27020. Student's t-test was used to estimate the significance of the differences in gene expression levels between groups. * indicates p < 0.05, ** indicates p < 0.01, and *** indicates p < 0.001. HR (Hazard Ratio) was calculated in survival analysis data to estimate the risk ratio of death/remission/recurrence due to the presence of a certain factor.
In order to ascertain the elevated expression of STEAP4 in OSCC, we conducted an analysis of STEAP4 expression in the GEO dataset GSE31056. This analysis identified 682 DEGs from the 96 tissue samples, comprising 277 up-regulated genes and 405 down-regulated genes (Figure 3B). Clustering of DEGs based on functional and signaling pathway analysis, as well as KEGG and GO enrichment analyses, revealed enrichment in external encapsulating structure organization (BP), collagen-containing extracellular matrix (CC), receptor ligand activity (MF), and cytoskeleton in muscle cells, as depicted in Figure 3C and Supplementary Figure 1. Notably, STEAP4 was identified as a down-regulated gene among the DEGs, indicating that its expression was lower in OSCC compared to adjacent normal tissues (Figure 3D), contradicting our initial conjecture. Subsequently, we further investigated the expression of STEAP4 in different OSCC datasets, which revealed that STEAP4 was expressed slightly lower in OSCC than in normal tissues, although the difference was not statistically significant (Figure 3E).
Role of STEAP4 in OSCC. (A) DFS for STEAP4 levels for patients with HNSCC in GSE31056. (B) Volcano plot of DEGs in GSE31056 (p < 0.01, |logFC| = 2). (C) KEGG pathway enrichment analysis of DEGs. (D & E) Expression of STEAP4 in GSE31056 (D) and GSE25099 (E).
Mechanism of tumor heterogeneity of STEAP4 in OSCC
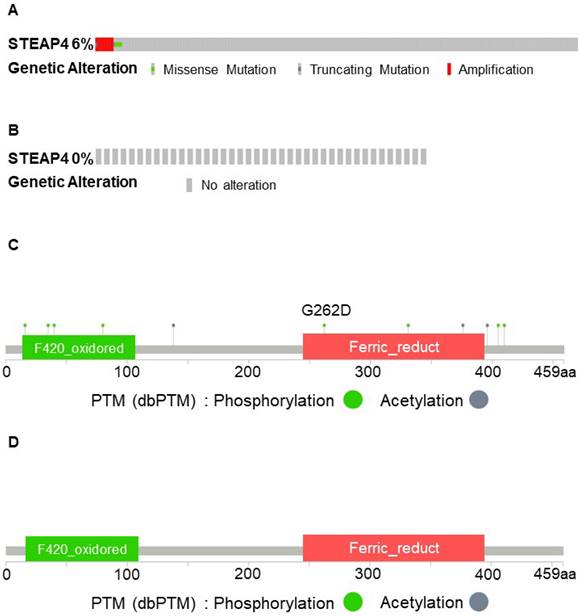
To elucidate the mechanisms underlying the unique expression pattern of STEAP4 in OSCC, we conducted an analysis of genetic alterations in STEAP4 across different datasets within cBioPortal. Varying degrees of gene amplification in HNSCC, accompanied by missense and truncating mutations in most patients across different TCGA cohorts (Figure 4A-B). Interestingly, almost all OSCC patients did not exhibit any mutations in the STEAP4 gene. This led us to speculate that the lack of significant differences in STEAP4 mRNA levels between normal and tumor tissues in OSCC could be attributed to genetic copy number alterations at the transcriptional level (Figure 4C-D). In other words, compared to HNSCC, STEAP4 underwent transcriptional-level mutations, which could potentially explain its transition from a protective factor in HNSCC to a risk factor in OSCC. However, further studies are required to supplement and confirm this conjecture.
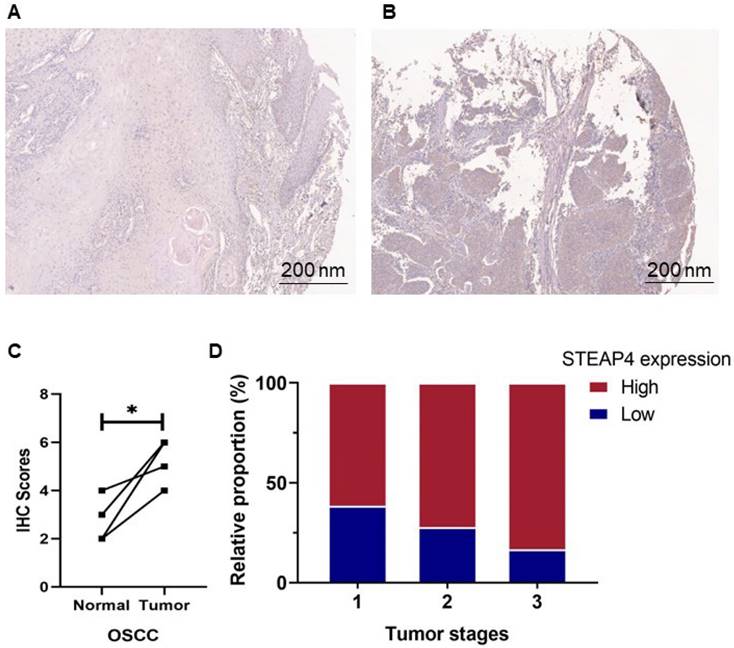
To further investigate the expression and distribution of STEAP4 in the oral cavity, we performed immunohistochemical staining and scoring on OSCC tissues. These samples included 14 with low expression and 30 with high expression of STEAP4, with 4 pairs of corresponding adjacent tissues and cancers. Contrary to mRNA predictions, immunohistochemical staining revealed higher expression levels of STEAP4 in OSCC tissues compared to adjacent normal tissues (Figure 5A-B). Notably, the staining levels of STEAP4 were elevated in cancerous tissues compared to the corresponding adjacent tissues in the four cases (Figure 5C). Clinicopathological analysis showed no statistically significant differences in STEAP4 expression based on gender, age group, tumor site, or various clinical stages. However, the majority of OSCC patients developed tumors in the tongue, with 84.6% of these patients exhibiting high expression of STEAP4 (Table 3). Furthermore, high protein levels of STEAP4 were more common in patients with advanced disease (Figure 5D). These findings suggest that high protein levels of STEAP4 are indicative of a poor prognosis for patients, contradicting the mRNA predictions in the dataset. We hypothesize that this discrepancy may be attributed to systematic biases arising from individual differences in clinical samples or the insufficient sample size of the mRNA dataset, which may not fully reflect the overall picture due to the limited number of OSCC-related studies.
Consistent with these results, the protein expression level scoring of STEAP4 on the Bgee website demonstrated elevated expression on the surface and in the tongue, with scores of 84.98 and 80.74, respectively (Supplementary Figure 2). This aligns with the clinicopathological parameters analyzed earlier. Additionally, recent studies have shown that approximately 40% of OSCC cases occur in the floor of the mouth, lateral margins of the tongue, or the ventral tongue29. Remarkably, in concordance with the immunohistochemical staining results of clinical tissues, we unexpectedly found that normal tongue squamous epithelial cells lacked STEAP4 expression. This phenomenon suggests considerable variation in STEAP4 expression among different oral mucosal tissues, which may contribute to the tumor heterogeneity of STEAP4 in OSCC. These findings not only shed light on the potential molecular mechanisms underlying the presence of STEAP4 in OSCC, but also provide a new direction for the detection and diagnosis of OSCC.
Relationship between STEAP4 protein level and clinicopathological parameters of OSCC patients.
| Clinicopathological parameters | STEAP4 expression | Total | P-value | |
|---|---|---|---|---|
| Low | High | |||
| Gender | 44 | 0.679 | ||
| Male | 7 (29.2%) | 17 (70.8%) | 24 | |
| Female | 7 (35.0%) | 13 (65.0%) | 20 | |
| Age (years) | 0.087 | |||
| ≤ 64 | 5 (20.8%) | 19 (79.2%) | 24 | |
| > 64 | 9 (45.0%) | 11 (55.0%) | 20 | |
| Tumor site | 44 | 0.378 | ||
| Lip | 2 (50.0%) | 2 (50.0%) | 4 | |
| Palate | 3 (37.5%) | 5 (62.5%) | 8 | |
| Buccal | 6 (46.2%) | 7 (53.8%) | 13 | |
| Tongue | 2 (15.4%) | 11 (84.6%) | 13 | |
| Teeth and periodontal tissues | 1 (16.7%) | 5 (83.3%) | 6 | |
| T | 37 | 0.607 | ||
| T1 | 5 (38.5%) | 8 (61.5%) | 13 | |
| T2 | 5 (27.8%) | 13 (72.2%) | 18 | |
| T3 | 1 (16.7%) | 5 (83.3%) | 6 | |
| N | 27 | 0.302 | ||
| N0 | 6 (31.6%) | 13 (68.4%) | 19 | |
| N1-3 | 1 (12.5%) | 7 (87.5%) | 8 | |
| AJCC stage | 38 | 0.479 | ||
| I | 5 (38.5%) | 8 (61.5%) | 13 | |
| II | 4 (30.8%) | 9 (69.2%) | 13 | |
| III | 2 (16.7%) | 10 (83.3%) | 12 | |
| Lymph node invasion | 27 | 0.302 | ||
| Negative | 6 (31.6%) | 13 (68.4%) | 19 | |
| Positive | 1 (12.5%) | 7 (87.5%) | 8 | |
AJCC: American Joint Committee on Cancer; N: Node; T: Tumor.
Discussion
Origin and latest research on STEAP4
The STEAP family of metalloproteinases, involved in iron and copper homeostasis and various cellular processes, contains five known homologs, STEAP1, STEAP1B, STEAP2, STEAP3, and STEAP41,30-32. In mammals, STEAP1-4 also regulate cell proliferation, apoptosis, attenuate oxidative stress, and mediate the transferrin cycle5,33.
STEAP1, a protein of 339 amino acids (39.9 kDa), does not independently exhibit Fe(3+) or Cu(2+) reductase activity due to the absence of an N-terminal NADPH-binding FNO structural domain4,21,34. STEAP1 localizes to cell membranes and is thought to be involved in tight junctions, gap junctions, or cell adhesion, acting as an ion channel or transporter protein, which also facilitates intercellular communication. Its overexpression in cancers may promote cancer cell proliferation and invasion by regulating ion and small molecule concentrations35,36. Blocking STEAP1 with a specific monoclonal antibody in LNCaP cells increased cell death, suggesting that STEAP1 may promote cancer cell proliferation or prevent apoptosis35. As a STEAP1 homologue, STEAP1B also lacks Fe(3+) or Cu(2+) reductase activities, but is distinguished by having two transcripts, STEAP1B1 and STEAP1B230. STEAP1B1 mRNA and STEAP1B2 mRNA are highly expressed in benign prostate cell lines and androgen-dependent PCa cell lines, respectively30.
STEAP2, known as six-transmembrane protein of prostate 1 (STAMP1), possesses an N-terminal NADPH-binding FNO structural domain, giving it iron-copper reductase properties4,5. Highly expressed in the Golgi complex, trans-Golgi network, and plasma membranes31,37, STEAP2 may act as an endogenous or exogenous receptor, such as lipids and proteins, or as a regulator of protein transport and sorting mechanisms31. In vitro and in vivo studies have shown that STEAP2 regulates genes involved in the cell cycle, leading to partial cell cycle arrest in the G0-G1 phase. Knockdown of STEAP2 promotes apoptosis in prostate cancer cells, increasing prostate cancer cell proliferation38.
STEAP3, the unique tumor suppressor in the STEAP family in PCa progression, is involved in transferrin endosomal-mediated iron uptake, which mediates cell death39,40. It participates in apoptosis and cell cycle regulation by interacting with pro-apoptotic factors Nix and Myt1 kinase41. Additionally, STEAP3 mediates the secretion of the translationally controlled tumor protein (TCTP)42, a Ca2þ and microtubule-binding protein implicated in cell cycle progression and malignant transformation43, thereby acquiring antiapoptotic activities 44,45. STEAP3-induced TCTP secretion may also sensitize cancer cells to apoptosis46.
Mutation of STEAP4 in HNSCC and OSCC patients. (A & B) Comparison of mutation percentage and types of STEAP4 in HNSCC (A) and OSCC (B) patients from The Cancer Genome Atlas, PanCancer Atlas. (C & D) Two-dimensional structure of the STEAP4 protein, showing the STEAP4 mutation sites in HNSCC (C) and OSCC (D).
Expression level of STEAP4 at the protein level. (A & B) Representative staining of STEAP4 in normal (A) and OSCC (B) tissues. (C) IHC scores between normal tissues and tumor (p < 0.05). (D) Proportion of Tumor stages with increasing expression level of STEAP4 in OSCC patients.
STEAP4, the latest member of the family, is involved in inflammatory responses, fatty acid metabolism, and glucose metabolism2,47. Overexpression of STEAP4 was initially discovered in white and brown adipose tissues and found to be strongly induced by growth hormone48, TNF-α3, interleukin-6 (IL-6)49 and interleukin-1b (IL-1b)50. Subsequent research has revealed that STEAP4 also plays a crucial role in promoting adipocyte proliferation and apoptosis. Qin et al. conducted a study demonstrating that treatment of preadipocytes with antibodies against STEAP4 resulted in the emergence of apoptotic morphology, followed by mitochondrial damage, leading to cellular swelling and pyrolysis51. Similarly, treatment of adipocytes with antibodies against STEAP4 reduced the rate of cell proliferation and the number of cells entering the S-phase of the cell cycle51. The inflammatory cytokine interleukin 17 (IL17) increases intracellular copper by inducing STEAP4 in CRC, leading to the activation of X-linked inhibitor of apoptosis (XIAP), which inhibits CASP3 activity52. Furthermore, GO annotation analysis indicated that STEAP4 is associated with biological pathways, such as fat cell differentiation (Table 1). These studies have explored the association of STEAP4 with obesity, insulin resistance, inflammation, and prostate cancer progression51,53.
Previous research has shown that STEAP4 is abundantly expressed in prostate cancer cells in the Golgi complex and plasma membrane, with aberrant expression predicting it plays an essential role in the development of prostate cancer 54. STEAP4 may cause the generation of reactive oxygen species (ROS) through its oxidoreductase activity, leading to the expression of transcription factor ATF4, which promotes the growth and progression of prostate cancer, eventually leading to chemoresistance55. ATF4 is a transcription factor involved in tumorigenesis that can be induced by ER, metabolic, oxidative, and other stresses56,57. Furthermore, STEAP4 participates in promoting an LPS-induced inflammatory microenvironment. LPS increases STEAP4 expression through the cGMP-PKG pathway, promoting PCa proliferation58. PPI networking revealed that STEAP4 may exert biological effects through the KLK family. The KLK family was initially discovered in the pancreas by Kraut et al. in 1930, and this family genes are mainly located on chromosome 19q13.3 to 13.4. These genes are involved in the regulation of physiological functions in cardiovascular, renal, and neurological systems, among others59,60. The KLK family consists of 15 members, with KLK1, KLK2, and KLK3 identified as potential biotherapeutic targets for prostate cancer23,61. KLK3, also known as prostate-specific antigen (PSA), has been widely used in clinical settings for many years. Additionally, KLK4 has shown biotherapeutic potential in ovarian cancers and TNBCs 24,25, and KLK5 has been associated with the prognosis of patients with CRCs 26. However, its effect on STEAP4 remains untested.
Recent studies have shown that the STEAP family is involved in processes such as iron and copper uptake and transport, regulation of apoptosis and cell cycle, playing crucial roles in the development, progression and metastasis of different types of cancer27. During the development of PCa, all STEAP members except STEAP3 tend to be overexpressed4. In other types of tumors, the expression of STEAP family members varies27. Research has shown that STEAP4 not only plays a role in the development and progression of breast cancers 62,63, but also participates in the regulation of inflammatory responses in CRC54. Our previous research demonstrated that the expression of STEAP4 is lower in CRC tissues compared to normal tissues, and this expression is positively correlated with immune-related biomarkers27. Conversely, STEAP4 has been shown to act as an oncogene in gastric cancer, and is strongly associated with poor prognosis in patients64. Notably, STEAP4 has rarely been reported in other cancer types, such as BRCA, HNSCC, and OSCC.
Current status and research progress of OSCC
Oral cancer, the most common malignant tumor of the head and neck (excluding non-melanoma skin tumors), has a high global incidence65,66 and a low 5-year survival rate67. Recent studies have shown that the expression levels of specific biomarkers IL-8, IL-6, and TNF-α in saliva are associated with signaling pathways in the development of various oral lesions and oral cancers68. Reports indicate that circadian rhythms significantly affect salivary flow rate, salivary composition, and the expression of cytokines and regulatory factors in oral cancers.
OSCC, the most common type of oral cancers, accounts for about 90% of oral cancer cases69. The 5-year survival rate for OSCC is only 41%, with an annual improvement rate of less than 1%70. Major risk factors for OSCC include smoking, heavy alcohol consumption71, and the presence of OPMDs, such as oral proliferative leukoplakia (PL)72,73. Despite the availability of diagnostic criteria for oral precancerous lesions and the latest clinical grading for oral cancer diagnosis (AJCC 8th edition staging), OSCC diagnosis has high intra- and inter-observer variability74,75, making it challenging to predict the progression of precancerous lesions to OSCC accurately76. Therefore, more accurate biomarkers for predicting early OSCC lesions and cancer progression would enhance OSCC diagnosis and treatment targeting76.
Several studies have reported biomarkers contributing to OSCC diagnosis and prognosis. Li et al. proposed a diagnostic method using mRNAs of certain genes as biomarkers, detecting increased expression of markers, such as DUSP1, H3F3A, IL1B, IL8, OAZ1, S100P, and SAT, with high sensitivity and specificity in OSCC subjects77. Studies have also indicated that miRNAs may play a key role as promising salivary biomarkers with high sensitivity and specificity, and minimally invasive advantages in OSCC diagnosis78. However, the diagnostic performance of miR-136 in OSCC remains inconsistent in reported studies. Liu et al. reported the accuracy of salivary miR-136, miR-27B, and miR-27b for OSCC diagnosis as high as 80.0%, 77.0%, and 78.0%, respectively67. Recent research advances have expanded the understanding of OSCC tumorigenesis and progression by analyzing biomarkers predicting OSCC progression from multiple aspects, including genomics, proteomics, metabolomics, immunomics, and microbiomics, proposing that composite biomarkers discovered through multi-omics integration will enhance the reliability, sensitivity, and specificity of OSCC diagnostic methods and therapeutic regimens79.
Current progress and deficiencies in the research
To date, our team has revealed the potential of STEAP4 as a prognostic biomarker in CRC27 and GC64. In this study, based on TCGA and GEO databases, we used publicly available online analytical tools and bioinformatics software, such as "R language", for data mining. We discovered that STEAP4, as a metalloreductase, exhibits unique tumor heterogeneity in different cancer types and subtypes. PPI network interaction analysis showed that STEAP4 closely interacts with the KLK family, implying that the KLK family may influence STEAP4's role in cancer.
More importantly, we analyzed HNSCC-associated (GSE2837) and OSCC-associated (GSE31056) GEO datasets, finding that STEAP4 mRNA expression was reduced in both HNSCC and OSCC. However, KM survival curve analysis and univariate COX analysis indicated that STEAP4 was a protective factor in HNSCC, but a risk factor in OSCC. We collected clinical tissue samples from OSCC patients for further analysis. Immunohistochemical staining evaluation showed elevated STEAP4 protein levels in OSCC. Clinicopathological parameters indicated that the tongue is the primary site in most OSCC patients, with high STEAP4 levels closely associated with advanced recurrence and metastasis. According to the AJCC 7th edition clinical staging of oral cancers, STEAP4 expression was higher in stage 3 and above. Hereby, we conjectured that, aside from the small sample size of OSCC in datasets and individual differences in clinical organization, genetic mutations and histological differences within the oral cavity between HNSCC and OSCC may account for this inconsistency.
Genetic alterations have been reported to cause aberrant activation of oncogenic pathways in OSCC, such as EGFR80, Wnt/β-catenin81, JAK/STAT82, NOTCH83, PI3K/AKT/mTOR84, MET85, and RAS/RAF/MAPK, as well as blockade of anticancer pathways, such as TP53/RB86and pl6/Rb/cyclin D187. These pathways play crucial roles in OSCC development. However, our current investigation found that STEAP4 was almost not mutated in OSCC, while different degrees of gene copy number variation, such as gene amplification and deletion, occur in HNSCC. Therefore, we conjectured that gene mutations in STEAP4 might be the main reason for STEAP4 heterogeneity in HNSCC and OSCC. In summary, our research verified that STEAP4 exhibits tumor heterogeneity in HNSCC and OSCC and can serve as an independent predictor for assessing the prognosis of OSCC patients.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
We are thankful to Prof. Stanley Lin for his critical and careful editing and proofreading of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China, No. 82273457; Guangdong Basic and Applied Basic Research Foundation No. 2023A1515012762 and 2021A1515010846; Special Grant for Key Area Programs of Guangdong Department of Education, No. 2021ZDZX2040; and Science and Technology Special Project of Guangdong Province, No. 210715216902829.
Author contributions
Jing Liu and Zheng Wu designed the research study; Zheng Wu performed the research; Zheng Wu, Wen-Jia Chen, Yang-Zheng Lan, Ze-Xuan Fang, Yan-Yu Hou, Xin-Ning Yu analyzed the research and wrote the manuscript; Hua-Tao Wu, Jing Liu revised the manuscript critically; and all authors have read and approved the final manuscript.
Institutional review board statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Shantou University Medical College (Approval No. SUMC-2022-075).
Informed consent statement
The informed consent was waived by the Ethics Committee for commercial microarray.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Korkmaz CG, Korkmaz KS, Kurys P. et al. Molecular cloning and characterization of STAMP2, an androgen-regulated six transmembrane protein that is overexpressed in prostate cancer. Oncogene. 2005;24:4934-4945
2. Scarl RT, Lawrence CM, Gordon HM. et al. STEAP4: its emerging role in metabolism and homeostasis of cellular iron and copper. J Endocrinol. 2017;234:R123-R134
3. Moldes M, Lasnier F, Gauthereau X. et al. Tumor Necrosis Factor-α-induced Adipose-related Protein (TIARP), a Cell-surface Protein That Is Highly Induced by Tumor Necrosis Factor-α and Adipose Conversion. J Biol Chem. 2001;276:33938-33946
4. Ohgami RS, Campagna DR, McDonald A. et al. The Steap proteins are metalloreductases. Blood. 2006;108:1388-1394
5. Knutson MD. Steap Proteins: Implications for Iron and Copper Metabolism. Nutr Rev. 2008;65:335-340
6. Zhang CM, Chi X, Wang B. et al. Downregulation of STEAP4, a highly-expressed TNF-alpha-inducible gene in adipose tissue, is associated with obesity in humans. Acta Pharmacol Sin. 2008;29:587-92
7. Arner P, Stenson BM, Dungner E. et al. Expression of six transmembrane protein of prostate 2 in human adipose tissue associates with adiposity and insulin resistance. J Clin Endocrinol Metab. 2008;93:2249-2254
8. Orfanou I-M, Argyros O, Papapetropoulos A. et al. Discovery and Pharmacological Evaluation of STEAP4 as a Novel Target for HER2 Overexpressing Breast Cancer. Front Oncol. 2021;11:608201
9. Tamura T, Chiba J. STEAP4 regulates focal adhesion kinase activation and CpG motifs within STEAP4 promoter region are frequently methylated in DU145, human androgen-independent prostate cancer cells. Int J Mol Med. 2009;24:599-604
10. Ng JH, Iyer NG, Tan M. et al. Changing epidemiology of oral squamous cell carcinoma of the tongue: A global study. Head Neck. 2017;39:297-304
11. Lan G, Yu X, Sun X. et al. Comprehensive analysis of the expression and prognosis for TNFAIPs in head and neck cancer. Sci Rep. 2021;11:15696
12. Romano A, Di Stasio D, Petruzzi M. et al. Noninvasive Imaging Methods to Improve the Diagnosis of Oral Carcinoma and Its Precursors: State of the Art and Proposal of a Three-Step Diagnostic Process. Cancers. 2021;13:2864
13. Ahmadi N, Gao K, Chia N. et al. Association of PD-L1 expression in oral squamous cell carcinoma with smoking, sex, and p53 expression. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;128:631-638
14. Matejcic M, Gunter MJ, Ferrari P. Alcohol metabolism and oesophageal cancer: a systematic review of the evidence. Carcinogenesis. 2017;38:859-872
15. Zhang P, Chua NQE, Dang S. et al. Molecular Mechanisms of Malignant Transformation of Oral Submucous Fibrosis by Different Betel Quid Constituents-Does Fibroblast Senescence Play a Role? Int J Mol Sci. 2022;23:1637
16. Purwanto D, Soedarsono N, Reuwpasssa JO. et al. The prevalence of oral high-risk HPV infection in Indonesian oral squamous cell carcinoma patients. Oral Dis. 2020;26:72-80
17. Cabay RJ, Morton TH, Epstein JB. Proliferative verrucous leukoplakia and its progression to oral carcinoma: a review of the literature. J Oral Pathol Med. 2007;36:255-261
18. Yang SW, Lee YS, Chang LC. et al. Outcome of excision of oral erythroplakia. Brit J Oral Max Surg. 2015;53:142-147
19. Liu J, Wei XL, Huang WH. et al. Cytoplasmic Skp2 expression is associated with p-Akt1 and predicts poor prognosis in human breast carcinomas. PLoS One. 2012;7:e52675
20. Cerami E, Gao J, Dogrusoz U. et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012;2:401-404
21. Oosterheert W, Bezouwen LS, Rodenburg RNP. et al. Cryo-EM structures of human STEAP4 reveal mechanism of iron(III) reduction. Nat Commun. 2018;9:4337
22. Srinivasan S, Kryza T, Batra J. et al. Remodelling of the tumour microenvironment by the kallikrein-related peptidases. Nat Rev Cancer. 2022;22:223-238
23. Zhang Y, Yan L, Zeng J. et al. Pan-cancer analysis of clinical relevance of alternative splicing events in 31 human cancers. Oncogene. 2019;38:6678-6695
24. Gong W, Liu Y, Seidl C. et al. Characterization of kallikrein-related peptidase 4 (KLK4) mRNA expression in tumor tissue of advanced high-grade serous ovarian cancer patients. PLoS One. 2019;14:e0212968
25. Yang F, Aubele M, Walch A. et al. Tissue kallikrein-related peptidase 4 (KLK4), a novel biomarker in triple-negative breast cancer. Biol Chem. 2017;398:1151-1164
26. Lorentz J, Liu SK, Vesprini D. Male Oncology Research and Education program for men at high risk for prostate cancer. Curr Oncol. 2018;25:170-175
27. Fang ZX, Li CL, Chen WJ. et al. Potential of six-transmembrane epithelial antigen of the prostate 4 as a prognostic marker for colorectal cancer. World J Gastrointest Oncol. 2022;14:1675-1688
28. Tang L, Xu M, Zhu H. et al. MiR-299-3p Inhibits Nasopharyngeal Carcinoma Cell Proliferation and Migration by Targeting MMP-2. J Oncol. 2022;2022:2322565
29. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30
30. Gomes IM, Santos CR, Maia CJ. Expression of STEAP1 and STEAP1B in prostate cell lines, and the putative regulation of STEAP1 by post-transcriptional and post-translational mechanisms. Genes Cancer. 2014;5:142-151
31. Korkmaz KS, Elbi C, Korkmaz CG. et al. Molecular Cloning and Characterization of STAMP1, a highly prostate-specific six transmembrane protein that is overexpressed in prostate cancer. J Biol Chem. 2002;277:36689-36696
32. Hubert RS, Vivanco I, Chen E. et al. STEAP: A prostate-specific cell-surface antigen highly expressed in human prostate tumors. Proc Natl Acad Sci. 1999;96:14523-14528
33. Gomes IM, Rocha SM, Gaspar C. et al. Knockdown of STEAP1 inhibits cell growth and induces apoptosis in LNCaP prostate cancer cells counteracting the effect of androgens. Med Oncol. 2018;35:40
34. Oosterheert W, Gros P. Cryo-electron microscopy structure and potential enzymatic function of human six-transmembrane epithelial antigen of the prostate 1 (STEAP1). J Biol Chem. 2020;295:9502-9512
35. Challita-Eid PM, Morrison K, Etessami S. et al. Monoclonal Antibodies to Six-Transmembrane Epithelial Antigen of the Prostate-1 Inhibit Intercellular Communication In vitro and Growth of Human Tumor Xenografts In vivo. Cancer Res. 2007;67:5798-5805
36. Smith P, Rhodes NP, Shortland AP. et al. Sodium channel protein expression enhances the invasiveness of rat and human prostate cancer cells. FEBS letters. 1998;423:19-24
37. Porkka KP, Helenius MA, Visakorpi T. Cloning and Characterization of a Novel Six-Transmembrane Protein STEAP2, Expressed in Normal and Malignant Prostate. Lab Invest. 2002;82:1573-1582
38. Wang L, Jin Y, Arnoldussen YJ. et al. STAMP1 Is Both a Proliferative and an Antiapoptotic Factor in Prostate Cancer. Cancer Res. 2010;70:5818-5828
39. Ohgami RS, Campagna DR, Greer EL. et al. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat Genet. 2005;37:1264-1269
40. Lambe T, Simpson RJ, Dawson S. et al. Identification of a Steap3 endosomal targeting motif essential for normal iron metabolism. Blood. 2009;113:1805-1808
41. Passer BJ, Nancy-Portebois V, Amzallag N. et al. The p53-inducible TSAP6 gene product regulates apoptosis and the cell cycle and interacts with Nix and the Myt1 kinase. Proc Natl Acad Sci. 2003;100:2284-2289
42. Tuynder M, Susini L, Prieur S. et al. Biological models and genes of tumor reversion: cellular reprogramming through tpt1/TCTP and SIAH-1. Proc Natl Acad Sci. 2002;99:14976-14981
43. Gachet Y, Tournier S, Lee M. et al. The growth-related, translationally controlled protein P23 has properties of a tubulin binding protein and associates transiently with microtubules during the cell cycle. J Cell Sci. 1999;112:1257-1271
44. MacDonald SM, Bhisutthibhan J, Shapiro TA. et al. Immune mimicry in malaria: Plasmodium falciparum secretes a functional histamine-releasing factor homolog in vitro and in vivo. Proc Natl Acad Sci. 2001;98:10829-10832
45. Li F, Zhang D, Fujise K. Characterization of Fortilin, a Novel Antiapoptotic Protein. J Biol Chem. 2001;276:47542-47549
46. Lespagnol A, Duflaut D, Beekman C. et al. Exosome secretion, including the DNA damage-induced p53-dependent secretory pathway, is severely compromised in TSAP6/Steap3-null mice. Cell Death Differ. 2008;15:1723-1733
47. Fasshauer M, Kralisch S, Klier M. et al. Insulin resistance-inducing cytokines differentially regulate SOCS mRNA expression via growth factor- and Jak/Stat-signaling pathways in 3T3-L1 adipocytes. J Endocrinol. 2004;181:129-138
48. Fasshauer M, Klein J, Krahlisch S. et al. GH is a positive regulator of tumor necrosis factor alpha-induced adipose related protein in 3T3-L1 adipocytes. J Endocrinol. 2003;178:523-31
49. Fasshauer M, Kralisch S, Klier M. et al. Interleukin-6 is a positive regulator of tumor necrosis factor α-induced adipose-related protein in 3T3-L1 adipocytes. FEbS Lett. 2004;560:153-157
50. Kralisch S, Sommer G, Weise S. et al. Interleukin-1beta is a positive regulator of TIARP/STAMP2 gene and protein expression in adipocytes in vitro. FEBS lett. 2009;583:1196-1200
51. Qin DN, Kou CZ, Ni YH. et al. Monoclonal antibody to the six-transmembrane epithelial antigen of prostate 4 promotes apoptosis and inhibits proliferation and glucose uptake in human adipocytes. Int J Mol Med. 2010;26:803-11
52. Liao Y, Zhao J, Bulek K. et al. Inflammation mobilizes copper metabolism to promote colon tumorigenesis via an IL-17-STEAP4-XIAP axis. Nat Commun. 2020;11:900
53. Chen X, Zhu C, Ji C. et al. STEAP4, a gene associated with insulin sensitivity, is regulated by several adipokines in human adipocytes. Int J Mol Med. 2010;25:361-7
54. Xue X, Bredell BX, Anderson ER. et al. Quantitative proteomics identifies STEAP4 as a critical regulator of mitochondrial dysfunction linking inflammation and colon cancer. Proc Natl Acad Sci. 2017;114:E9608-E9617
55. Jin Y, Wang L, Qu S. et al. STAMP 2 increases oxidative stress and is critical for prostate cancer. Embo Mol Med. 2015;7:315-331
56. Rutkowski DT, Kaufman RJ. All Roads Lead to ATF4. Dev Cell. 2003;4:442-444
57. Lewerenz J, Maher P. Control of Redox State and Redox Signaling by Neural Antioxidant Systems. Antioxid Redox Signal. 2011;14:1449-1465
58. Li W, Yin X, Yan Y. et al. STEAP4 knockdown inhibits the proliferation of prostate cancer cells by activating the cGMP-PKG pathway under lipopolysaccharide-induced inflammatory microenvironment. Int Immunopharmacol. 2021;101:108311
59. Kerr Z, Hayter A, Khan Z. et al. Kallikrein-Related Peptidase mRNA Expression in Adenoid Cystic Carcinoma of Salivary Glands: A Polymerase Chain Reaction Study. Head Neck Pathol. 2020;14:577-87
60. Kishibe M. Physiological and pathological roles of kallikrein-related peptidases in the epidermis. J Dermatol Sci. 2019;95:50-55
61. Emami NC, Kachuri L, Meyers TJ. et al. Association of imputed prostate cancer transcriptome with disease risk reveals novel mechanisms. Nat Commun. 2019;10:3107
62. Wu HT, Chen WJ, Xu Y. et al. The Tumor Suppressive Roles and Prognostic Values of STEAP Family Members in Breast Cancer. BioMed Res Int. 2020;2020:9578484
63. Chen WJ, Wu HT, Li CL. et al. Regulatory Roles of Six-Transmembrane Epithelial Antigen of the Prostate Family Members in the Occurrence and Development of Malignant Tumors. Front Cell Dev Biol. 2021;9:752426
64. Fang ZX, Hou YY, Wu Z. et al. Immune responses of six-transmembrane epithelial antigen of the prostate 4 functions as a novel biomarker in gastric cancer. World J Clin Oncol. 2023;14:297-310
65. Montero PH, Patel SG. Cancer of the oral cavity. Surg Oncol Clin N Am. 2015;24:491-508
66. Zygogianni AG, Kyrgias G, Karakitsos P. et al. Oral squamous cell cancer: early detection and the role of alcohol and smoking. Head Neck Oncol. 2011;3:2
67. Liu CJ, Kao SY, Tu HF. et al. Increase of microRNA miR-31 level in plasma could be a potential marker of oral cancer. Oral Dis. 2010;16:360-364
68. Sahibzada HA, Khurshid Z, Khan RS. et al. Salivary IL-8, IL-6 and TNF-α as Potential Diagnostic Biomarkers for Oral Cancer. Diagnostics. 2017;7:21
69. Bai XX, Zhang J, Wei L. Analysis of primary oral and oropharyngeal squamous cell carcinoma in inhabitants of Beijing, China—a 10-year continuous single-center study. BMC Oral Health. 2020;20:208
70. Tajmirriahi N, Razavi SM, Shirani S. et al. Evaluation of metastasis and 5-year survival in oral squamous cell carcinoma patients in Isfahan (2001-2015). Dent Res J. 2019;16:117-121
71. Radaic A, Ganther S, Kamarajan P. et al. Paradigm shift in the pathogenesis and treatment of oral cancer and other cancers focused on the oralome and antimicrobial-based therapeutics. Periodontol. 2000. 2021;87:76-93
72. Speight PM. Update on Oral Epithelial Dysplasia and Progression to Cancer. Consult Pathol. 2007;1:61-66
73. McCord C, Achita P, Magalhaes M. et al. Progression to malignancy in oral potentially malignant disorders: a retrospective study of 5,036 patients in Ontario, Canada. Oral Surg Oral Med Oral Pathol Oral Radiol. 2023;136:466-477
74. Shubhasini A R, Praveen B N, Hegde U. et al. Inter- and Intra-Observer Variability in Diagnosis of Oral Dysplasia. Asian Pac J Cancer Prev. 2017;18:3251-3254
75. Nevanpää TT, Terävä AE, Laine HK. et al. Malignant transformation of oral epithelial dysplasia in Southwest Finland. Sci Rep. 2022;12:8261
76. Smith J, Rattay T, McConkey C. et al. Biomarkers in dysplasia of the oral cavity: A systematic review. Oral Oncol. 2009;45:647-653
77. Li Y, St John MAR, Zhou X. et al. Salivary transcriptome diagnostics for oral cancer detection. Clin Cancer Res. 2004;10:8442-8450
78. Prasanna R, Balan A, Radhakrishna Pillai M. Diagnostic capability of salivary biomarkers in the assessment of head and neck cancer. Oral Oncol. 2015;51:e86
79. Radaic A, Kamarajan P, Cho A. et al. Biological biomarkers of oral cancer. Periodontol. 2023
80. Ribeiro FAP, Noguti J, Oshima CTF. et al. Effective targeting of the epidermal growth factor receptor (EGFR) for treating oral cancer: a promising approach. Anticancer Res. 2014;34:1547-1552
81. Liu F, Millar SE. Wnt/beta-catenin signaling in oral tissue development and disease. J Dent Res. 2010;89:318-330
82. Huang JS, Yao CJ, Chuang SE. et al. Honokiol inhibits sphere formation and xenograft growth of oral cancer side population cells accompanied with JAK/STAT signaling pathway suppression and apoptosis induction. BMC cancer. 2016;16:245
83. Ali J, Sabiha B, Jan HU. et al. Genetic etiology of oral cancer. Oral Oncol. 2017;70:23-28
84. Simpson DR, Mell LK, Cohen EEW. Targeting the PI3K/AKT/mTOR pathway in squamous cell carcinoma of the head and neck. Oral Oncol. 2015;51:291-298
85. Speight PM, Epstein J, Kujan O. et al. Screening for oral cancer-a perspective from the Global Oral Cancer Forum. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123:680-687
86. Zhang J, Chen T, Yang X. et al. Attenuated TRAF3 Fosters Activation of Alternative NF-κB and Reduced Expression of Antiviral Interferon, TP53, and RB to Promote HPV-Positive Head and Neck Cancers. Cancer Res. 2018;78:4613-4626
87. Vijayalakshmi N, Selvaluxmi G, Majhi U. et al. Alterations found in pl6/Rb/cyclin D1 pathway in the dysplastic and malignant cervical epithelium. Oncol Res. 2007;16:527-533
Author contact
![]() Corresponding authors: Jing Liu, jliu12edu.cn, Hua-Tao Wu, htwuedu.cn.
Corresponding authors: Jing Liu, jliu12edu.cn, Hua-Tao Wu, htwuedu.cn.

 Global reach, higher impact
Global reach, higher impact