3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(20):6784-6797. doi:10.7150/jca.100179 This issue Cite
Research Paper
YAP Promotes Chemoresistance to 5-FU in Colorectal Cancer Through mTOR/GLUT3 Axis
1. Department of General Surgery, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China.
2. Department of General Surgery, First Medical Center, Chinese PLA General Hospital, Beijing, China.
3. Medical School of Chinese PLA, Beijing, China.
4. Department of Gastrointestinal Surgery, Affiliated Hospital of Jiangsu University, Zhenjiang, Jiangsu,China.
5. School of Medicine, Nankai University, Tianjin, China.
6. Department of General Surgery, Linfen Central Hospital, Linfen, Shanxi, China.
* These authors contributed equally to this work.
Received 2024-6-27; Accepted 2024-9-28; Published 2024-11-4
Abstract
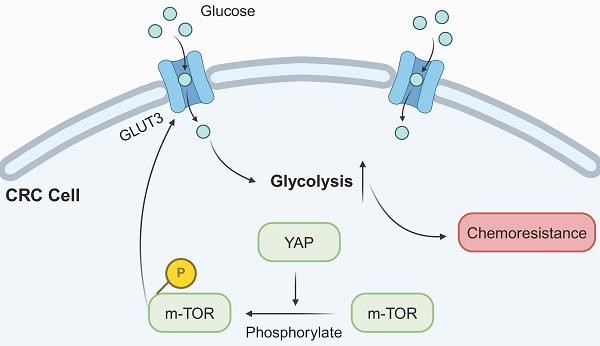
Background: Although chemoresistance constitutes a significant barrier to the effectiveness of chemotherapy in colorectal cancer (CRC), its precise mechanisms remain unclear. YAP functions as an oncogene in various malignancies. However, the relationship between YAP and chemoresistance in CRC needs clarification.
Methods: The expression level of YAP in CRC tissues was assessed through immunohistochemistry (IHC), and the impact of YAP on CRC cell chemoresistance was evaluated using the Cell Counting Kit-8, EdU, and flow cytometry assays. Meanwhile, tumor proliferation was assessed in vivo by analyzing the expression of PCNA and Ki-67 in subcutaneous tumors via IHC. In addition, the TUNEL assay was employed to evaluate tumor apoptosis levels and western blot was utilized to detect the mTOR/GLUT3 pathway-related protein expression to provide insights into the underlying mechanism.
Results: YAP was highly expressed in CRC tissues and correlated with patient prognosis and clinicopathological features. Bioinformatic analysis based on the TCGA database revealed that YAP was associated with DNA replication, glycolysis, and the mTOR pathway. Meanwhile, YAP could enhance chemoresistance and glycolysis in CRC cells both in vitro and in vivo. Additional mechanistic experiments unveiled that YAP promoted CRC cell chemoresistance via the mTOR/GLUT3 axis.
Conclusion: This study validated the role of YAP as an oncogene in CRC, as it promoted chemoresistance through the mTOR/GLUT3 axis. These results suggested YAP as a potential target for promoting the efficacy of chemotherapy in patients with CRC.
Keywords: YAP, chemoresistance, colorectal cancer, glycolysis, mTOR pathway, GLUT3.
Introduction
Colorectal cancer (CRC) is one of the most prevalent malignant tumors of the digestive system. Recent global data underscore its prominence: it ranks third in incidence and second in mortality among all malignant tumors, next to only lung cancer [1]. Although surgery remains the primary treatment modality for CRC, the lack of early diagnostic markers often leads to diagnoses at an advanced stage, depriving many patients of surgical intervention [2]. The efficacy of 5-fluorouracil (5-FU), an important component of adjuvant therapy in patients with inoperable CRC, is significantly limited because of the development of chemoresistance [3]. Thus, it is of utmost significance to delve into the molecular mechanisms of chemoresistance and devise strategies to enhance CRC cell sensitivity to 5-FU treatment and thereby improve overall survival.
The Hippo pathway, which is intricately involved in tumor initiation, progression, metastasis, and chemoresistance, has emerged as a critical player in these complex processes [4]. YAP, a downstream effector in the Hippo pathway, is centrally involved in these processes, as the nuclear entry of unphosphorylated YAP regulates downstream gene expression, thereby serving as a critical node for the biological functions of the Hippo pathway [5, 6]. YAP, in an oncogenic role, participates in various malignancies and affects cellular processes and tissue-level dynamics. For example, MEKK3 in human pancreatic cancer cells could stimulate epithelial-mesenchymal transition and extend survival in a pancreatic cancer mouse model via YAP regulation [7]. At the tissue level, YAP expression was increased in the tumor tissues of patients with esophageal squamous cell carcinoma and led to poor prognosis, particularly concerning the N stage of these patients [8]. Additionally, studies have highlighted the contribution of YAP to chemoresistance in small cell lung cancer, indicating that it is a potential target for treating chemoresistant small cell lung cancer [9]. Moreover, YAP expression was closely related to doxorubicin efficacy in breast cancer [10]. While previous research demonstrated that YAP plays a vital role in the promotion of CRC cell proliferation and metastasis in vitro [11], the relationship between YAP and chemoresistance in CRC remains unclear and needs further investigation.
Glucose transporters (GLUTs), specialized transport proteins that facilitate glucose entry into cells, maintain the energy required for tumor cell metabolism. Of note, the expression of GLUT3, closely linked to the EGFR signaling pathway in lung adenocarcinoma, is increased in various malignancies, including CRC [12-14]. Although previous research suggested that the expression level of GLUT3 in CRC tissues is associated with the prognosis of patients [15], the specific mechanism of YAP and GLUT3 in regulating the biological behavior of CRC cells was not explored. Thus, this aspect needs to be explored.
The mammalian target of rapamycin (mTOR), which is known to form two distinct complexes (mTORC1 and mTORC2), is aberrantly activated in multiple cancers. The involvement of the PI3K/AKT/mTOR pathway in malignant tumor behaviors such as proliferation, migration, and stemness has been well documented [16-18]. Indeed, targeting the mTOR pathway has shown promise in cancer treatment in both research and clinical settings [19-21]. Recent research has established the close relationship among the mTOR pathway, glycolysis, and GLUT3 expression in tumor cells [22]. However, the intricate interplay among YAP, the mTOR pathway, and glycolysis requires further investigation.
The present study findings indicated heightened YAP expression in CRC tissues, which correlated with patient prognosis and clinicopathological features. In addition, in vitro and in vivo experiments confirmed the inhibitory effect of YAP on the therapeutic efficacy of 5-FU in chemoresistant CRC cells. Mechanistically, YAP enhanced 5-FU-resistant CRC cells by activating the mTOR pathway to promote GLUT3 expression. Thus, inhibiting the YAP/mTOR/GLUT3 axis in conjunction with 5-FU may be a promising treatment strategy for patients with CRC.
Materials and methods
Clinical tissue specimens
One hundred and ten patients who underwent radical CRC resection at the Department of Gastrointestinal Surgery of the First Affiliated Hospital of Soochow University from 2014 to 2016 were randomly included retrospectively. Inclusion criteria are as follows: (1) The primary site of the tumor is the colon or rectum, with postoperative pathological diagnosis confirming colorectal adenocarcinoma; (2) Patients have undergone radical resection of colorectal cancer, encompassing complete excision of the tumor mass and a thorough dissection of surrounding lymph nodes; (3) Patients have not received neoadjuvant chemoradiotherapy or targeted therapy as adjuvant treatment prior to surgery. Exclusion criteria include: (1) Postoperative pathological diagnosis reveals signet-ring cell carcinoma, mucinous adenocarcinoma, or any other pathological subtypes; (2) Patients who have undergone neoadjuvant therapy before surgery. The primary tumor tissues and adjacent normal tissues, located >2 cm from the tumor edge, were resected. Colorectal adenocarcinoma was diagnosed by two independent pathologists. Written informed consent was obtained from all enrolled patients, and the study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (2020076).
Cell culture
The human CRC cell lines SW480, LOVO, HCT116, SW620, and HT29 were purchased from the Cell Bank of the Chinese Academy of Sciences. Complete medium was prepared with 10% fetal bovine serum and 1% penicillin-streptomycin. The RPMI-1640 complete medium was used for cell culture. Meanwhile, 5-FU-resistant HCT116 (HCT116R) and SW480 (SW480R) cells were obtained by increasing the 5-FU concentration from 0.01 to 80 μM for 6 months. All cells were cultured at 37℃ in a constant temperature incubator with 5% CO2.
Relationship between YAP and clinic-pathological factors in CRC patients.
| YAP | |||
|---|---|---|---|
| Negative | Positive | P | |
| Age | 0.235 | ||
| ≤60 | 28 | 17 | |
| >60 | 33 | 32 | |
| Gender | 0.079 | ||
| Male | 40 | 24 | |
| Female | 21 | 25 | |
| Tumor size (cm) | 0.162 | ||
| <5 | 38 | 24 | |
| ≥5 | 23 | 25 | |
| Depth of tumor invasion | 0.366 | ||
| T1-2 | 17 | 10 | |
| T3-4 | 44 | 39 | |
| Lymph node metastasis | <0.001* | ||
| No | 50 | 23 | |
| Yes | 11 | 26 | |
| Degree of differentiation | 0.122 | ||
| Well | 54 | 38 | |
| Poor | 7 | 11 | |
| Venous invasion | 0.024* | ||
| Negative | 60 | 43 | |
| Positive | 1 | 6 | |
| Neural invasion | 0.309 | ||
| Negative | 56 | 42 | |
| Positive | 5 | 7 | |
| TNM staging | <0.001* | ||
| I-II | 48 | 22 | |
| III-IV | 13 | 27 | |
*p<0.05
Cell transfection
Overexpression plasmids, shRNA, and negative control plasmids designed and manufactured by GenePharma (Suzhou, China) were obtained. First, 5-FU-resistant CRC cells were seeded into six-well plates and transfected using LipofectamineTM 3000 (Invitrogen, USA) on the second day at 80% confluence according to the manufacturer's instructions. Then, to establish cell lines exhibiting stable interference with YAP expression, a lentiviral packaging kit was utilized to cotransfect HEK-293T cells with both the packaging vectors and specialized interference plasmids. The shRNA and negative control sequences are presented in Supplementary Table 1.
Quantitative real-time PCR (qRT-PCR)
Total RNA from cells was extracted using the TRIzol reagent (Thermo Fisher Scientific, USA) according to the manufacturer's instructions. After NanoDrop2000 quantification, samples with purity between 1.8 and 2.0 were used for subsequent experiments. RNA was reverse-transcribed into cDNA using the HiScriptIIQ Select RT Super Mix with random primers (Vazyme, China) for qPCR. The TB Green Premix Ex Taq II (Takara, Japan) kit was used for the subsequent PCR reaction. The 20-µL reaction system contained 10 µL TB Green Premix Ex Taq II, 0.8 µL forward primer (10 µM), 0.8 µL reverse primer (10 µM), 0.4 µL ROX reference dye, and template cDNA, which were mixed for amplification on ABI7500 (Applied Biosystems, USA). GAPDH served as the internal control, and the 2-ΔΔct method was used to assess the expression of target genes. The relevant primer sequences are provided in Supplementary Table 2.
Results of univariate and multivariate analyses of postoperative patients' survival by Cox's proportional hazard model.
| Characteristics | n | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age (≤60/>60) | 45/65 | 0.614 | 0.354-1.063 | 0.081 | |||
| Gender (Male/Female) | 64/46 | 0.930 | 0.551-1.568 | 0.784 | |||
| Tumor size (<5/≥5) | 62/48 | 0.556 | 0.331-0.932 | 0.026* | 0.658 | 0.369-1.173 | 0.156 |
| Depth of tumor invasion (T1-2/T3-4) | 27/83 | 0.344 | 0.163-0.727 | 0.005* | 0.668 | 0.282-1.585 | 0.361 |
| Lymph node metastasis (Negative/Positive) | 73/37 | 0.179 | 0.105-0.305 | <0.001* | 0.271 | 0.145-0.508 | <0.001* |
| Degree of differentiation (Well/Poor) | 92/18 | 0.436 | 0.235-0.810 | 0.009* | 0.737 | 0.367-1.481 | 0.392 |
| Venous invasion (Negative/Positive) | 103/7 | 0.382 | 0.163-0.899 | 0.028* | 3.341 | 1.028-10.853 | 0.045* |
| Neural invasion (Negative/Positive) | 98/12 | 0.324 | 0.167-0.628 | 0.001* | 0.413 | 0.168-1.018 | 0.055 |
| YAP expression | 70/40 | 0.317 | 0.186-0.540 | <0.001* | 0.383 | 0.215-0.682 | 0.001* |
*p<0.05
Western blot
Cell and tissue proteins were extracted using RIPA containing 1% PMSF. After quantification using the BCA method (Beyotime, China), the proteins were separated using SDS-polyacrylamide gel electrophoresis and subsequently transferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% skimmed milk for 2 h at room temperature to prevent nonspecific binding. Subsequently, the membranes were incubated with a primary antibody overnight at 4℃ and then with an HRP-conjugated antibody for 1 h at room temperature. Protein bands were visualized using an ECL western blotting substrate (Beyotime, China). The antibodies used for western blot are listed in Supplementary Table 3.
Cell Counting Kit-8 (CCK-8) assay
The cell proliferation capacity was assessed using the CCK-8 assay. In brief, 3000 CRC cells were seeded in 96-well plates. At 24, 48, 72, and 96 h, 10 µL CCK-8 reagent (Vazyme, China) was added to each well and incubated for 1 h at 37℃ in the dark. Finally, absorbance was measured at 450 nm using a microplate reader.
EdU assay
First, a 100 μL suspension containing 2 × 104 CRC cells was seeded into a 96-well plate. Next, proliferating cells were stained using the EdU Image Kit (Abbkine, China) and 4',6-diamino-2-phenylindole according to the kit instructions. The stained cells were observed and enumerated under an inverted fluorescence microscope.
Colony formation assay
Chemoresistant CRC cells were resuspended and seeded in six-well plates at 1000 cells per well. After 10 days of continuous culture, the cells were fixed with 4% paraformaldehyde and stained with crystal violet. Photographs were captured and the number of colonies were counted.
Flow cytometry
Flow cytometry was employed to detect apoptosis in CRC cells. The Muse Annexin V & Dead Cell Kit (Luminex, USA) was utilized to stain the markers of the apoptotic process as per the manufacturer's instructions.
Immunohistochemistry (IHC)
The human and mouse tissues were embedded in paraffin and fixed and sectioned. The antibodies used for IHC are listed in Supplementary Table 3. IHC staining was performed using the Metal Enhanced DAB Substrate Kit (Solarbio, China) according to the manufacturer's protocol. Staining intensity scores ranging from 0 to 3 represented negative, weak, moderate, and strong staining intensities, respectively, whereas staining extent scores ranging from 0 to 4 represented 0%-5%, 6%-25%, 26%-50%, 51%-75%, and >75% positive cells, respectively. The final score was calculated by multiplying the staining intensity score with the staining extent score. A final score of <4 was considered negative expression and that of >4 was deemed positive expression.
TUNEL assay
The TUNEL assay was used to evaluate apoptosis in subcutaneous tumor tissue using the One Step TUNEL Apoptosis Assay Kit (Beyotime, China) according to the manufacturer's instructions. A fluorescence microscope was used to observe dUTP labeled with the green fluorescent probe fluorescein (FITC).
Glycolysis
The lactate, pyruvate, and ATP production as well as the glucose uptake rate were used to reflect cellular glycolysis levels. The Glucose Uptake Colorimetric Assay Kit, ATP Colorimetric Assay Kit, Lactate Assay Kit II, and Pyruvate Colorimetric Assay Kit (Biovision, CA, USA) were used to detect the above indicators as per the respective kit instructions. A microplate reader was used to measure absorbance according to the respective optimal wavelength.
Xenograft tumors in mice
Xenograft tumors in nude mice were developed as described previously [23]. Four-week-old male BALB/C nude mice were purchased from Shanghai SLRC Laboratory Animal Co., Ltd. (Shanghai, China). The mice were subjected to experiments after 1 week of adaptive growth in a specific pathogen-free environment. During adaptive growth, the animals had unlimited access to sterile water and food. The environment was maintained at a stable temperature of 18°C-22°C, humidity of 40%-70%, and light/dark cycle of 12/12 h. The mice were randomly divided into three groups: HCT116 (wild type [WT]), HCT116R, and HCT116R-KD. Next, 5 × 106 treated HCT116 cells were injected subcutaneously into the right flanks of the animals. Approximately 2 weeks after the injection, mice in both groups received 50 mg/kg 5-FU via an intraperitoneal injection every 3 days. The subcutaneous tumor volume was measured every 3 days, and the tumors were extracted for weight measurement and subsequent experiments after 28 days.
Bioinformatic analysis
To investigate YAP-related genes in CRC based on the TCGA database, the online platform LinkedOmics (https://www.linkedomics.org/admin.php) was employed. The following parameters were set: cancer type, colorectal adenocarcinoma (COADREAD); sample cohort, TCGA_COADREAD; data type, RNAseq; platform, Hiseq RNA; attribute, YAP1; and statistical method: Spearman correlation test. Subsequently, Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Set Enrichment Analysis (GSEA) were performed.
Statistical analysis
All experiments were repeated three times. The SPSS 23.0, GraphPad Prism 8, and R software were employed for data analysis. Data are presented as means ± standard deviation, and Student's t-test (unpaired, two-tailed) was used to compare means between two groups. The IHC scores of YAP in CRC tumors and the adjacent normal tissues were analyzed using the Chi-squared test or Fisher's exact test. Finally, survival curves were generated using the Hiplot online website (https://hiplot-academic.com/). P < 0.05 indicated statistical significance.
Results
YAP is highly expressed in CRC tissues
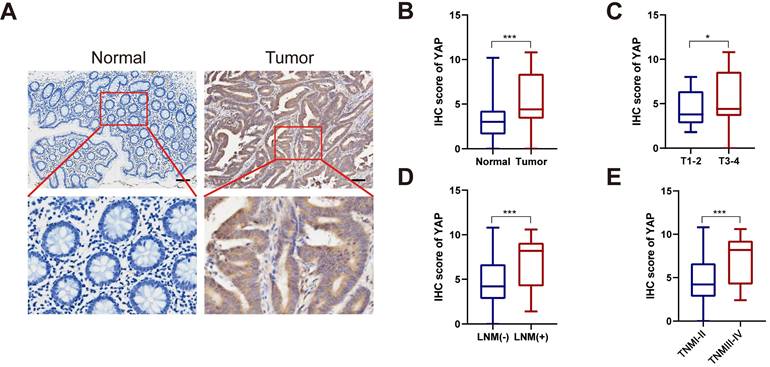
To investigate YAP expression in CRC, tumor tissues and the adjacent normal tissues from 110 patients with CRC were retrospectively analyzed. YAP protein expression in CRC tissues was assessed via IHC. As illustrated in Figure 1A-B, YAP was abnormally upregulated in tumor tissues compared with the adjacent normal tissues. In addition, subgroup analysis revealed elevated YAP levels in the tumor tissues of patients with T stage 3-4, lymph node metastasis, and TNM stage III-IV compared with patients with T stage 1-2, no lymph node metastasis, and TNM stage I-II (Figure 1C-E). This finding suggested a potential correlation between YAP expression and CRC stage. Meanwhile, a chi-squared test used to analyze the relationship between the YAP expression and clinicopathological characteristics of patients with CRC indicated that YAP expression correlated with lymph node metastasis, venous invasion, and TNM staging but not with age, gender, tumor size, depth of tumor invasion, degree of differentiation, and neural invasion (Table 1).
YAP expression is associated with prognosis
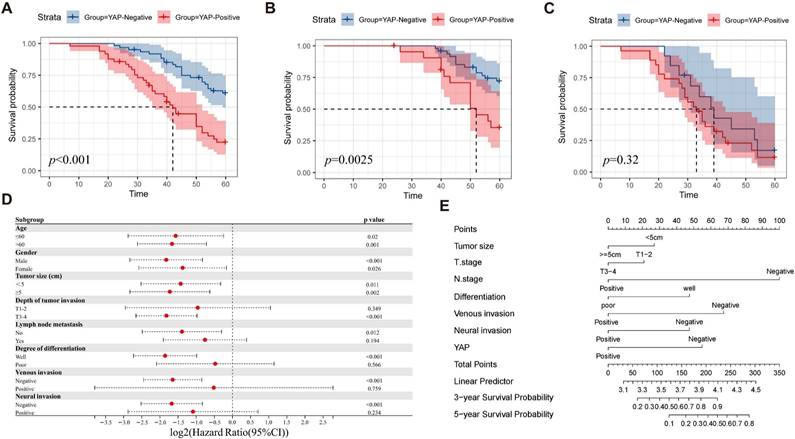
To evaluate the impact of YAP expression on patient prognosis, the patients were divided into YAP positive and negative groups based on their IHC scores. The Kaplan-Meier survival analysis demonstrated significantly reduced survival time in the positive group compared with the negative group (Figure 2A). In addition, subgroup analysis indicated that YAP expression was associated with the prognosis of patients with TNM stage I-II but not with TNM stage III-IV (Figure 2B-C). This result suggested that YAP can be used as a prognostic marker for patients with CRC, particularly for those with early stage disease. Meanwhile, univariate analysis revealed that the tumor size, depth of tumor invasion, lymph node metastasis, degree of differentiation, venous invasion, neural invasion, and YAP expression were the independent risk factors of prognosis. Multivariate analysis confirmed that lymph node metastasis, venous invasion, and YAP expression were strongly associated with prognosis (Table 2). To better identify the effect of YAP expression level on the prognosis of patients with CRC, the patients were categorized according to the clinicopathological characteristics and a Cox regression model was used to predict the relationship between YAP and prognosis. The results showed that YAP positivity could predict the survival of patients with CRC, regardless of age, gender or tumor size (Figure 2D). In addition, YAP was associated with poor survival in patients with CRC with T3-4, negative lymph node metastasis, well differentiation, negative venous invasion, and negative neural invasion (Figure 2D). For a comprehensive evaluation of the effect of YAP expression on prognosis, a nomogram model was constructed, which considered various clinicopathological characteristics. The model revealed that YAP plays a crucial role in predicting three-year and five-year survival rates in CRC patients (Figure 2E).
YAP is related to DNA replication and mTOR pathways
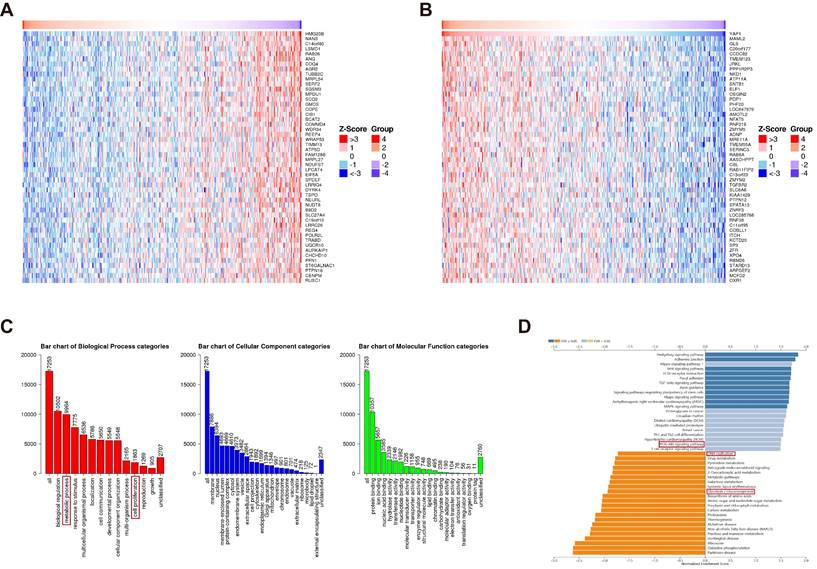
Although YAP has emerged as a potential diagnostic and prognostic marker of CRC, the underlying mechanisms remain unclear. Utilizing the TCGA database, genes positively and negatively correlated with YAP in CRC were screened using LinkedOmics (Figure 3A-B). The subsequent GO enrichment analysis of these genes revealed associations with cell proliferation and metabolic processes, suggesting that YAP may be considered an oncogene that regulates cell metabolism (Figure 3C). The KEGG analysis demonstrated that the YAP-related genes were enriched in the DNA replication and PI3K/AKT/mTOR pathways (Figure 3D). mTOR pathway activation has been shown to induce improper DNA replication in various cancer cells, which contributes to drug resistance [23]. Thus, it was hypothesized that YAP, acting as an oncogene, is involved in CRC cell drug resistance.
YAP promotes 5-FU resistance in CRC cells
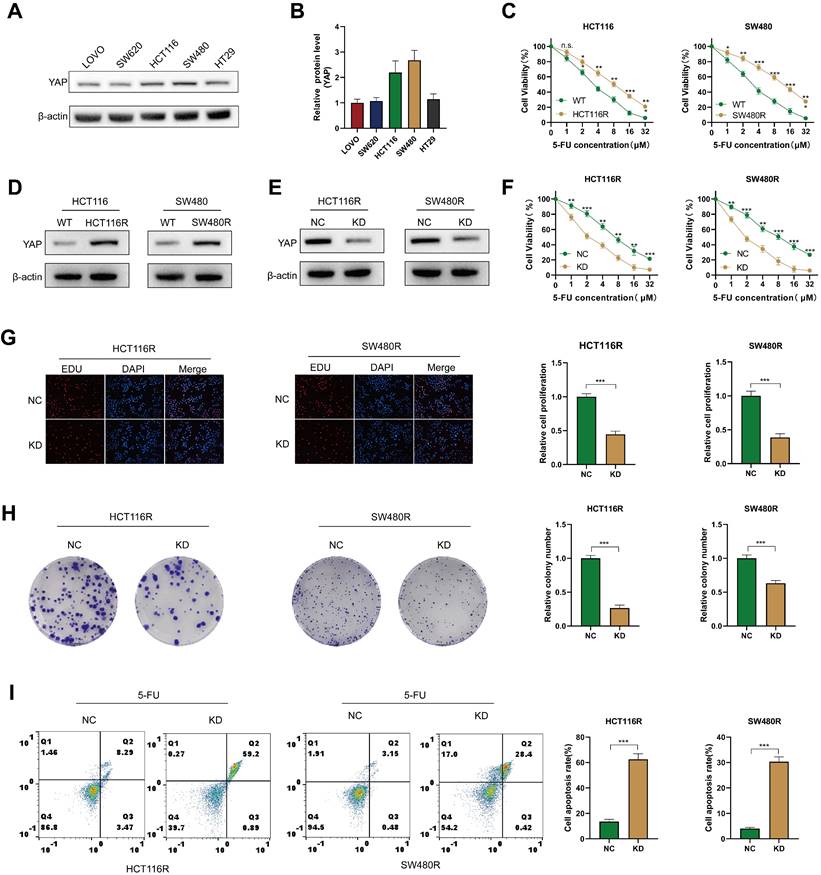
To explore the role of YAP in CRC chemoresistance in vitro, YAP expression was assessed in different CRC cell lines via western blot. The results revealed that HCT116 and SW480 exhibited the highest YAP expression. Therefore, these cell lines were selected for subsequent experiments (Figure 4A-B). First, 5-FU-resistant CRC cells (including HCT116R and SW480R) were produced, and increased resistance to 5-FU was observed in these cells groups compared with cells in the WT groups (Figure 4C). In addition, western blot analysis revealed elevated YAP expression in chemoresistant HCT116R and SW480R cells (Figure 4D). To investigate the impact of YAP on chemoresistance, YAP expression was stably knocked down in chemoresistant cells using siRNA, which was verified via western blot (Figure 4E). The CCK-8 assay demonstrated that YAP knockdown significantly enhanced the effect of 5-FU on chemoresistant cells (Figure 4F). Moreover, the EdU and colony formation assays revealed an increased proliferation of YAP-knockdown chemoresistant cells (Figure 4G-H) and flow cytometry revealed that YAP inhibited apoptosis in chemoresistant cells (Figure 4I). These results affirmed that YAP promoted chemoresistance in CRC cells in vitro.
YAP expression in CRC tissues. (A) Representative IHC staining of YAP in CRC tissues and adjacent normal tissues. (B) IHC scores of YAP in CRC tissues and adjacent normal tissues. (C) IHC scores of YAP in T1-2 and T3-4 CRC tissues. (D) IHC scores of YAP in LNM (-) and LNM (+) CRC tissues. (E) IHC scores of YAP in TNM I-II and TNM III-IV CRC tissues.
Correlation between YAP and prognosis of patients with CRC. Overall survival analysis (A) shows significantly lower survival in patients with CRC with positive YAP expression, particularly in TNM stage I-II (B) compared with TNM stage III-IV (C). Subgroup analysis (D) identifies factors that influence survival. Nomogram (E) predicts the survival of patients with CRC.
Downregulation of YAP reprograms glycolysis in chemoresistant CRC cells
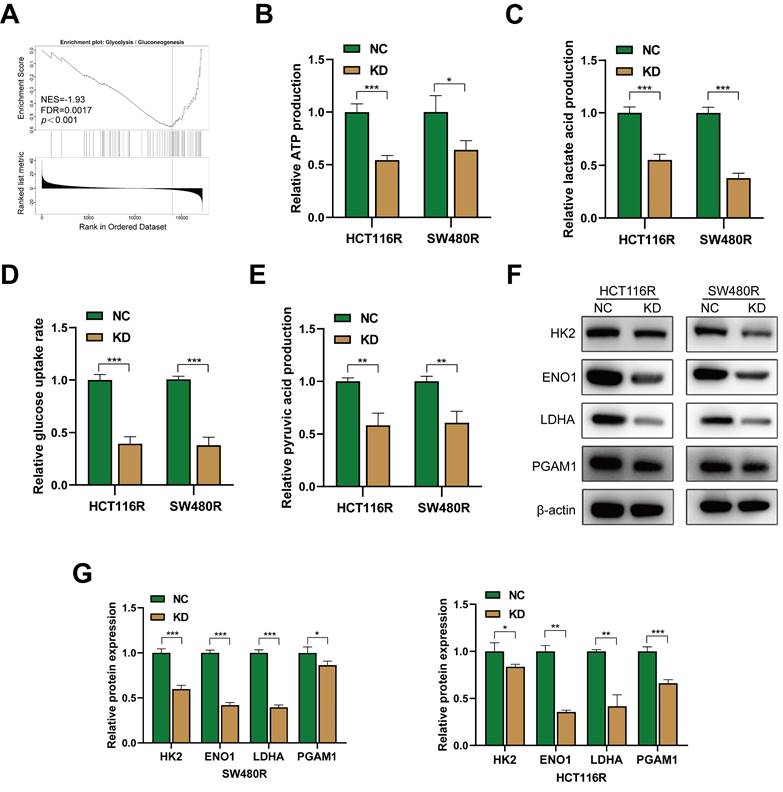
The KEGG enrichment results indicated that YAP-related genes in CRC were primarily enriched in the glycolysis pathway. GSEA results revealed that YAP was associated significantly with the glycolysis pathway (Figure 5A). Thus, it was hypothesized that YAP influences chemoresistance in CRC cells by modulating glucose metabolism. To evaluate this, a series of glycolytic assays were performed to examine the effect of YAP on the glycolysis levels of chemoresistant CRC cells. YAP inhibition significantly reduced the lactate, pyruvate, and ATP production and decreased glucose uptake rate in chemoresistant CRC cells (Figure 5B-E). In addition, western blot showed that YAP knockdown significantly inhibited the expression of glycolysis-related genes in chemoresistant CRC cells (Figure 5F-G). Taken together, YAP participated in the glycolytic reprogramming of chemoresistant CRC cells.
YAP promotes 5-FU resistance in vivo
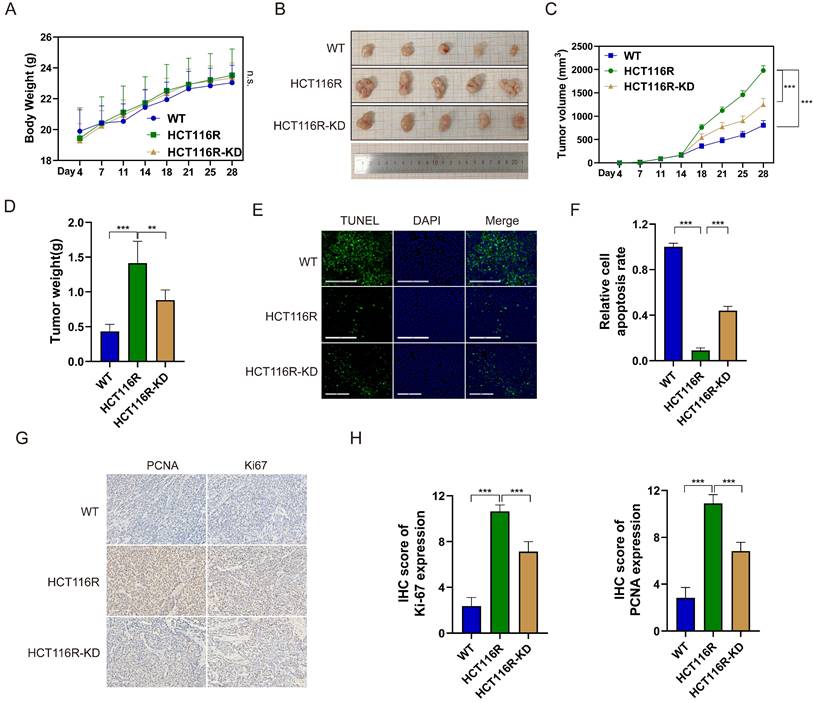
To assess the impact of YAP on 5-FU efficacy in CRC cells in vivo, subcutaneous tumor xenograft models were established. The mice were randomly divided into the three groups of HCT116 (WT), HCT116R, and HCT116R-KD and injected with HCT116, HCT116R, and HCT116R cells transfected with sh-YAP, respectively. All mouse groups received intraperitoneal 5-FU from day 7 after injection. No significant difference in the body weight was observed among the three groups (Figure 6A). However, tumor volume and weight were significantly increased in the HCT116R group compared with the WT group, with YAP knockdown reversing this trend (Figure 6B-D). The TUNEL assay confirmed that YAP knockdown significantly increased 5-FU-induced apoptosis in subcutaneous tumors (Figure 6E-F). Likewise, the IHC staining of subcutaneous tumors revealed increased PCNA and Ki67 positive cells in the HCT116R-KD group compared with the HCT116R group (Figure 6G-H). These findings indicated that YAP downregulation could reverse CRC cell chemoresistance in vivo.
Enrichment analysis of YAP functions in CRC. The top 50 genes positively (A) and negatively (B) associated with YAP in CRC are shown. GO enrichment analysis (C) illustrates the correlation of identified genes with biological processes (BP), cellular components (CC), and molecular functions (MF). KEGG analysis (D) indicates YAP involvement in various regulatory pathways.
Role of YAP in the chemoresistance of CRC cells. (A-B) Western blot analysis of YAP expression in LOVO, SW620, HCT116, SW480, and HT29 cell lines. (C) Viability of HCT116 and SW480 cells in WT and chemoresistant cells. (D) Western blot analysis of YAP expression in HCT116R and SW480R cells. (E) Western blot analysis of YAP expression in the NC and KD groups. (F) Viability of HCT116R and SW480R cells in the NC and KD groups. (G) EdU assay was used to measure the proliferation of chemoresistant cells in the NC and KD groups. (H) Colony formation assay was utilized to measure the proliferation of chemoresistant cells in the NC and KD groups. (I) Flow cytometry analysis was performed to analyze the apoptosis of chemoresistant cells in the NC and KD groups.
YAP regulates 5-FU resistance through GLUT3 in CRC cells
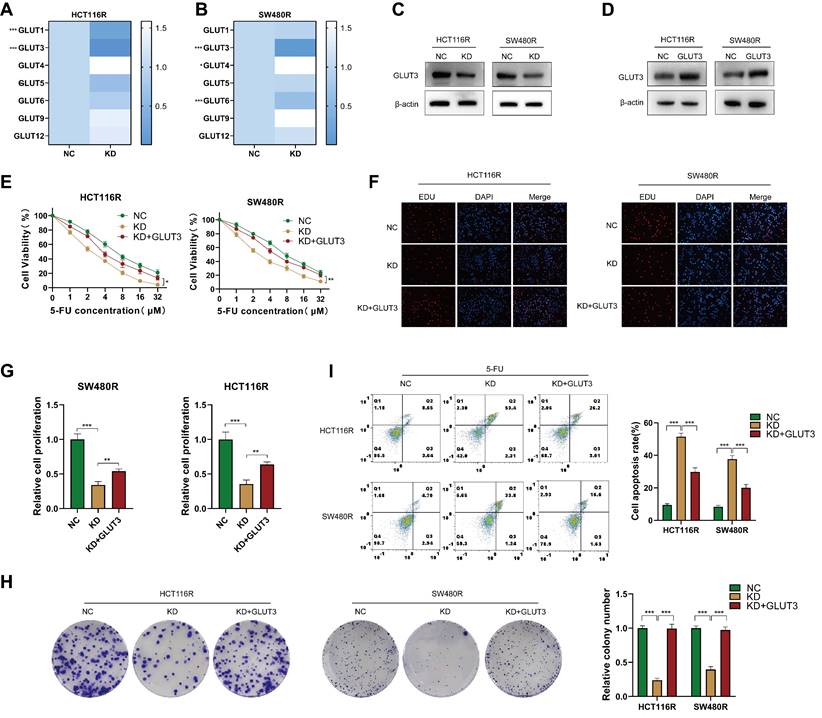
The abnormal expression of GLUT has been shown to affect chemotherapy by regulating glycolysis [24, 25]. The examination of GLUT family mRNA levels in chemoresistant CRC cells after YAP downregulation revealed that GLUT3 was the most significantly decreased member in both HCT116R-KD and SW480R-KD cells (Figure 7A-B). Meanwhile, GLUT2, GLUT7, GLUT8, GLUT10, and GLUT11 were undetected. Western blot confirmed the role of YAP in promoting GLUT3 expression at the protein level (Figure 7C). Thus, it was hypothesized that the effect of YAP on CRC cell chemoresistance is GLUT3-dependent. To confirm this, a GLUT3 stably overexpressing cell line was generated in chemoresistant CRC cells; the overexpression was verified using western blot (Figure 7D). The CCK-8, EdU, colony formation, and flow cytometry assays demonstrated that YAP knockdown increased the sensitivity of chemoresistant CRC cells to 5-FU, which was reversed by the overexpression of GLUT3 (Figure 7E-I). These findings suggested that YAP promotes CRC cell chemoresistance through GLUT3-mediated glycolytic reprogramming.
The effect of YAP in CRC cells glycolysis. (A) GSEA of gene sets for glycolysis signaling pathway. (B-E) ATP production (B), lactate production (C), relative glucose uptake rate (D), and pyruvate production (E) of chemoresistant cells in the NC and KD group. (F) Western blot analysis of HK2, ENO1, LDHA, and PGAM1 expression in chemoresistant cells. (G) Quantification of the expression of the abovementioned proteins.
YAP promotes CRC cell chemoresistance through mTOR/GLUT3 pathway
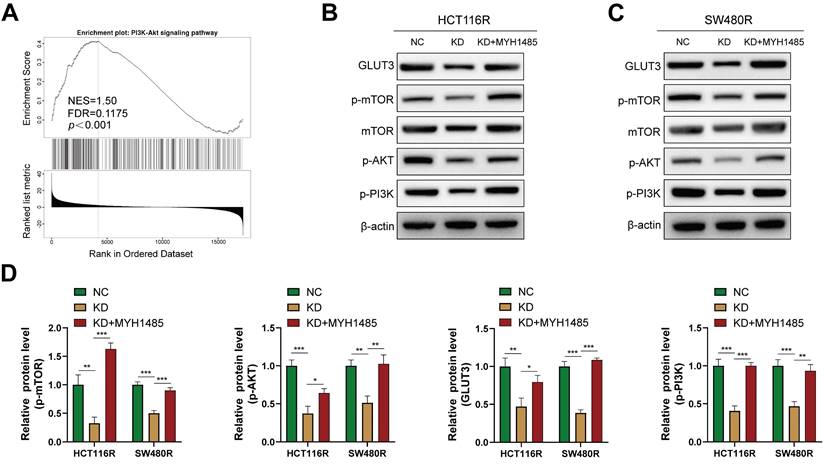
The activation of the mTOR signaling pathway is associated with glycolysis and drug resistance in various cancers [26, 27]. In this context, previous studies have reported that YAP can activate the PI3K/AKT/mTOR pathway [28]. However, the relationship between mTOR and GLUT3 in CRC remains unclear. GSEA performed on the aforementioned YAP-related genes revealed a significant correlation between YAP and the mTOR pathway (Figure 8A). Thus, it was hypothesized that YAP promotes GLUT3 expression by activating the mTOR pathway, thereby enhancing CRC cell chemoresistance. Western blot results indicated that the inhibition of YAP in chemoresistant CRC cells decreased the expression of p-mTOR, p-PI3K, and p-AKT, which are key proteins in the mTOR pathway, without significantly altering the total mTOR expression (Figure 8B-D). However, treatment with MHY1485, an mTOR pathway activator, increased GLUT3 expression, a response suppressed by YAP downregulation (Figure 8B-D). These findings suggested that YAP promotes GLUT3 expression by activating the mTOR pathway in CRC cells, thereby augmenting CRC chemoresistance.
Discussion
For most patients with advanced CRC, surgery combined with adjuvant chemoradiotherapy has become the standard treatment [29-31], as this combination can significantly improve the recurrence rate and prognosis in these patients [32-35]. However, the development of chemoresistance has become a major obstacle in the progress of CRC treatment [36]. In the present study, YAP was identified as an oncogene that promoted chemoresistance in CRC cells both in vitro and in vivo. Therefore, it may be used as a diagnostic and prognostic marker of CRC.
Effect of YAP knockdown on the chemoresistance of CRC cells in vivo. (A) Mouse body weight in the WT, HCT116R, and HCT116R-KD groups. (B) Representative images of subcutaneous tumors. (C) Tumor volume in the WT, HCT116R, and HCT116R-KD groups. (D) Analysis of tumor weight. (E-F) TUNEL assay was performed to measure the apoptosis level in tumors. (G-H) IHC staining of PCNA and Ki67 in subcutaneous tumors.
YAP, a key downstream effector of the Hippo pathway, has been implicated in promoting proliferation and metastasis in various cancers [37-40]. Indeed, some recent studies have shown that YAP is highly expressed in various cancer tissues. For example, Pei et al. showed that YAP expression was higher in cholangiocarcinoma tissues than in normal bile duct tissues and that patients with high YAP expression exhibited poor prognosis [41]. In the context of CRC, the present study reaffirmed YAP overexpression in CRC tissues, which correlated with poor patient prognosis. Of note, YAP expression was as an independent risk factor of CRC prognosis and significantly predicted 3- and 5-year survival rates in patients with CRC. These findings are in line with existing research suggesting elevated YAP expression in CRC tissues and its association with unfavorable patient outcomes. For instance, Wang et al. detected higher YAP expression in CRC tumor tissues than in normal intestinal tissues, and the higher expression correlated with poor patient prognosis [42]. These observations are consistent with the present study findings.
Role of the YAP/GLUT3 axis in CRC chemoresistance. (A-B) qPCR assay was used to measure the expression of GLUTs in chemoresistant cells in the NC and KD groups. (C) Western blot was performed to assess GLUT3 expression in the NC and KD groups. (D) Western blot was used to measure GLUT3 expression in the NC and GLUT3 groups. (E) Viability of HCT116R and SW480R cells in the NC, KD, and KD + GLUT3 groups. (F-G) EdU assay was employed to measure the proliferation of chemoresistant cells in the abovementioned groups. (H) Colony formation assay was performed to measure the proliferation of chemoresistant cells in the abovementioned groups. (I) Flow cytometry was used to analyze apoptosis in chemoresistant cells in the abovementioned groups.
Effect of YAP on the mTOR pathway (A) GSEA of gene sets for the PI3K/AKT signaling pathway. (B-C) Western blot analysis of GLUT3, p-mTOR, mTOR, p-AKT, and p-PI3K expression in chemoresistant cells. (D) Quantification of the expression of the abovementioned proteins.
Although the mechanisms of chemoresistance in cancer cells remain unclear, YAP has been shown to promote chemoresistance in certain cancers. In breast cancer cells, YAP interacts with SRGN to regulate HDAC2 expression, thereby promoting breast cancer cell resistance to multiple drugs [40]. Likewise, Zhou et al. reported abnormal YAP expression in chemoresistant hepatocellular carcinoma (HCC) cells, displaying its ability to induce drug resistance through the mTOR pathway [43]. The functional enrichment analysis unveiled the association of YAP-related genes with the DNA replication and mTOR pathways, indicative of the potential involvement of YAP in CRC cell chemoresistance. Of note, in vitro and in vivo experiments substantiated the prospect of YAP inhibition in enhancing the efficacy of 5-FU on CRC cells, thereby positioning YAP as a promising therapeutic target for CRC.
KEGG enrichment analysis in the former part revealed that YAP was highly associated with the glycolysis pathway. The reprogramming of glucose metabolism is an important hallmark of malignancy, as it can provide energy for tumor cell proliferation [44]. This feature may be exploited to improve cancer therapy via the inhibition of glucose metabolism [45]. GLUT3, a member of the GLUT family, is highly expressed on various malignant tumor tissues, including CRC [14, 46]. In the current study, YAP downregulation in chemoresistant CRC cells suppressed the glycolysis level as well as the expression of key proteins in the glycolysis pathway. Mechanistically, GLUT3 expression was significantly reduced in YAP-knockdown chemoresistant CRC cells. In addition, rescue experiments confirmed that YAP could promote CRC cell chemoresistance through GLUT3. Consistent with these findings, YAP has been shown to regulate FOXC2-induced glycolysis by upregulating HK2 expression in nasopharyngeal carcinoma [47]. Likewise, in CRC, the YAP/GLUT3 axis could activate the metabolic reprogramming of CRC cells to promote tumor metastasis, indicating that YAP can regulate tumor malignancy and glucose metabolism in CRC [48].
The activation of the mTOR signaling pathway is the key to the promotion of cell growth, and its abnormal expression is often associated with several cancers [49-51]. mTOR, a mediator of PI3K signaling pathway, is activated by the upstream PI3K/AKT [52], and the mTOR pathway is closely related to the development of chemoresistance. For example, studies have shown that the mTOR pathway is abnormally activated in oxaliplatin-treated CRC cells, and in vitro experiments have confirmed that the combination of oxaliplatin and mTOR inhibitors exhibits a positive synergistic effect [53]. Based on GSEA, it was speculated that YAP is closely related to the mTOR pathway in CRC. To test this hypothesis, the expression of YAP was knocked down in CRC cells, which revealed that the expression of p-mTOR, p-PI3K, and p-AKT, which are key proteins in the mTOR pathway, was decreased while the total mTOR expression level did not change significantly. The mTOR agonist MYH1485 used to activate the mTOR pathway in CRC cells showed that the expression level of GLUT3 was upregulated, thereby validating the positive promotion effect of the mTOR pathway on GLUT3. In HCC cells, the inhibition of the mTOR pathway impeded the expression of GLUT1/3 and promoted the radiosensitivity of HCC cells [54]. This is line with the present study finding that mTOR regulates GLUT3 expression.
Taken together, the current study results suggested YAP as a potential diagnostic and prognostic marker of CRC. In addition, the study uncovered the role of YAP in promoting chemoresistance in CRC cells. The involvement of YAP in glycolytic reprogramming, GLUT3 modulation, and mTOR pathway activation expands the understanding of the molecular mechanisms underlying CRC progression and resistance. Thus, targeting YAP may be a promising avenue for enhancing the efficacy of chemotherapy for CRC. The study findings offered valuable insights for future therapeutic strategies in CRC.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
Funding
This work was supported by Suzhou Science and Education Promoting Health Youth Science and Technology Project (grant no. KJXW2021002) and the National Natural Science Foundation of China (grant no. 82273231).
Author contributions
QXX, ZSJ, ZY, and ZYY: acquisition and verification of data, analysis and interpretation of the data, and drafting of the manuscript; JWG, RYZ, HHL, and HGR: analysis and interpretation of the data; BC, BW, and LHJ: funding acquisition, study design, and study supervision. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (2020076). Informed consent was obtained from all enrolled patients. All animal experiments were approved by the Ethics Committee of the First Affiliated Hospital of Soochow University and were performed in accordance with the First Affiliated Hospital of Soochow University's Guidelines for Animal Care and Use.
Data availability statement
The raw data analyzed in this study are available from the corresponding author on reasonable request.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-49
2. Zacharakis M, Xynos ID, Lazaris A, Smaro T, Kosmas C, Dokou A. et al. Predictors of survival in stage IV metastatic colorectal cancer. Anticancer Res. 2010;30:653-60
3. Vasan N, Baselga J, Hyman DM. A view on drug resistance in cancer. Nature. 2019;575:299-309
4. Fu M, Hu Y, Lan T, Guan KL, Luo T, Luo M. The Hippo signalling pathway and its implications in human health and diseases. Signal Transduct Target Ther. 2022;7:376
5. Kim MK, Jang JW, Bae SC. DNA binding partners of YAP/TAZ. BMB Rep. 2018;51:126-33
6. Hansen CG, Moroishi T, Guan KL. YAP and TAZ: a nexus for Hippo signaling and beyond. Trends Cell Biol. 2015;25:499-513
7. Santoro R, Zanotto M, Carbone C, Piro G, Tortora G, Melisi D. MEKK3 Sustains EMT and Stemness in Pancreatic Cancer by Regulating YAP and TAZ Transcriptional Activity. Anticancer Res. 2018;38:1937-46
8. Qu Y, Zhang L, Wang J, Chen P, Jia Y, Wang C. et al. Yes-associated protein (YAP) predicts poor prognosis and regulates progression of esophageal squamous cell cancer through epithelial-mesenchymal transition. Exp Ther Med. 2019;18:2993-3001
9. Wu Q, Guo J, Liu Y, Zheng Q, Li X, Wu C. et al. YAP drives fate conversion and chemoresistance of small cell lung cancer. Sci Adv. 2021;7:eabg1850
10. Qin X, Lv X, Li P, Yang R, Xia Q, Chen Y. et al. Matrix stiffness modulates ILK-mediated YAP activation to control the drug resistance of breast cancer cells. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165625
11. Jiang L, Zhang J, Xu Q, Wang B, Yao Y, Sun L. et al. YAP promotes the proliferation and migration of colorectal cancer cells through the Glut3/AMPK signaling pathway. Oncol Lett. 2021;21:312
12. Wright EM. Glucose transport families SLC5 and SLC50. Mol Aspects Med. 2013;34:183-96
13. Chai YJ, Yi JW, Oh SW, Kim YA, Yi KH, Kim JH. et al. Upregulation of SLC2 (GLUT) family genes is related to poor survival outcomes in papillary thyroid carcinoma: Analysis of data from The Cancer Genome Atlas. Surgery. 2017;161:188-94
14. Bakht MK, Lovnicki JM, Tubman J, Stringer KF, Chiaramonte J, Reynolds MR. et al. Differential Expression of Glucose Transporters and Hexokinases in Prostate Cancer with a Neuroendocrine Gene Signature: A Mechanistic Perspective for (18)F-FDG Imaging of PSMA-Suppressed Tumors. J Nucl Med. 2020;61:904-10
15. Yang M, Guo Y, Liu X, Liu N. HMGA1 Promotes Hepatic Metastasis of Colorectal Cancer by Inducing Expression of Glucose Transporter 3 (GLUT3). Med Sci Monit. 2020;26:e924975
16. Wang Z, Goto Y, Allevato MM, Wu VH, Saddawi-Konefka R, Gilardi M. et al. Disruption of the HER3-PI3K-mTOR oncogenic signaling axis and PD-1 blockade as a multimodal precision immunotherapy in head and neck cancer. Nat Commun. 2021;12:2383
17. Gao W, Guo H, Niu M, Zheng X, Zhang Y, Xue X. et al. circPARD3 drives malignant progression and chemoresistance of laryngeal squamous cell carcinoma by inhibiting autophagy through the PRKCI-Akt-mTOR pathway. Mol Cancer. 2020;19:166
18. Loong JH, Wong TL, Tong M, Sharma R, Zhou L, Ng KY. et al. Glucose deprivation-induced aberrant FUT1-mediated fucosylation drives cancer stemness in hepatocellular carcinoma. J Clin Invest. 2021;131(11):e143377
19. Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F. et al. Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373:1814-23
20. Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S. et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373:1803-13
21. Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E. et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387:968-77
22. Zha X, Hu Z, Ji S, Jin F, Jiang K, Li C. et al. NFκB up-regulation of glucose transporter 3 is essential for hyperactive mammalian target of rapamycin-induced aerobic glycolysis and tumor growth. Cancer Lett. 2015;359:97-106
23. Cipponi A, Goode DL, Bedo J, McCabe MJ, Pajic M, Croucher DR. et al. MTOR signaling orchestrates stress-induced mutagenesis, facilitating adaptive evolution in cancer. Science. 2020;368:1127-31
24. Sawayama H, Ogata Y, Ishimoto T, Mima K, Hiyoshi Y, Iwatsuki M. et al. Glucose transporter 1 regulates the proliferation and cisplatin sensitivity of esophageal cancer. Cancer Sci. 2019;110:1705-14
25. Watanabe M, Naraba H, Sakyo T, Kitagawa T. DNA damage-induced modulation of GLUT3 expression is mediated through p53-independent extracellular signal-regulated kinase signaling in HeLa cells. Mol Cancer Res. 2010;8:1547-57
26. Feng M, Xiong G, Cao Z, Yang G, Zheng S, Qiu J. et al. LAT2 regulates glutamine-dependent mTOR activation to promote glycolysis and chemoresistance in pancreatic cancer. J Exp Clin Cancer Res. 2018;37:274
27. Weng ML, Chen WK, Chen XY, Lu H, Sun ZR, Yu Q. et al. Fasting inhibits aerobic glycolysis and proliferation in colorectal cancer via the Fdft1-mediated AKT/mTOR/HIF1α pathway suppression. Nat Commun. 2020;11:1869
28. Tumaneng K, Schlegelmilch K, Russell RC, Yimlamai D, Basnet H, Mahadevan N. et al. YAP mediates crosstalk between the Hippo and PI(3)K-TOR pathways by suppressing PTEN via miR-29. Nat Cell Biol. 2012;14:1322-9
29. Kao PS, Chang SC, Wang LW, Lee RC, Liang WY, Lin TC. et al. The impact of preoperative chemoradiotherapy on advanced low rectal cancer. J Surg Oncol. 2010;102:771-7
30. Zha S, Li T, Zheng Q, Li L. Whether Patients With Stage Ⅱ/Ⅲ Colorectal Cancer Benefit From Adjuvant Chemotherapy: A Modeling Analysis of Literature Aggregate Data. Front Pharmacol. 2022;13:826785
31. Feeney G, Sehgal R, Sheehan M, Hogan A, Regan M, Joyce M. et al. Neoadjuvant radiotherapy for rectal cancer management. World J Gastroenterol. 2019;25:4850-69
32. Papaccio F, Roselló S, Huerta M, Gambardella V, Tarazona N, Fleitas T. et al. Neoadjuvant Chemotherapy in Locally Advanced Rectal Cancer. Cancers (Basel). 2020;12(12):3611
33. Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM. et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:29-42
34. Conroy T, Bosset JF, Etienne PL, Rio E, François É, Mesgouez-Nebout N. et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:702-15
35. Roselló S, Papaccio F, Roda D, Tarazona N, Cervantes A. The role of chemotherapy in localized and locally advanced rectal cancer: A systematic revision. Cancer Treat Rev. 2018;63:156-71
36. Marine JC, Dawson SJ, Dawson MA. Non-genetic mechanisms of therapeutic resistance in cancer. Nat Rev Cancer. 2020;20:743-56
37. Kang W, Tong JH, Chan AW, Lee TL, Lung RW, Leung PP. et al. Yes-associated protein 1 exhibits oncogenic property in gastric cancer and its nuclear accumulation associates with poor prognosis. Clin Cancer Res. 2011;17:2130-9
38. Lu T, Sun L, Zhu X. Yes-associated protein enhances proliferation and attenuates sensitivity to cisplatin in human gastric cancer cells. Biomed Pharmacother. 2018;105:1269-75
39. Wang C, Cheng L, Song S, Wu S, Sun G. Gli1 interacts with YAP1 to promote tumorigenesis in esophageal squamous cell carcinoma. J Cell Physiol. 2020;235:8224-35
40. Zhang Z, Qiu N, Yin J, Zhang J, Liu H, Guo W. et al. SRGN crosstalks with YAP to maintain chemoresistance and stemness in breast cancer cells by modulating HDAC2 expression. Theranostics. 2020;10:4290-307
41. Pei T, Li Y, Wang J, Wang H, Liang Y, Shi H. et al. YAP is a critical oncogene in human cholangiocarcinoma. Oncotarget. 2015;6:17206-20
42. Wang L, Shi S, Guo Z, Zhang X, Han S, Yang A. et al. Overexpression of YAP and TAZ is an independent predictor of prognosis in colorectal cancer and related to the proliferation and metastasis of colon cancer cells. PLoS One. 2013;8:e65539
43. Zhou Y, Wang Y, Zhou W, Chen T, Wu Q, Chutturghoon VK. et al. YAP promotes multi-drug resistance and inhibits autophagy-related cell death in hepatocellular carcinoma via the RAC1-ROS-mTOR pathway. Cancer Cell Int. 2019;19:179
44. Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol. 2015;17:351-9
45. Ganapathy-Kanniappan S. Molecular intricacies of aerobic glycolysis in cancer: current insights into the classic metabolic phenotype. Crit Rev Biochem Mol Biol. 2018;53:667-82
46. Nowak N, Kulma A, Gutowicz J. Up-regulation of Key Glycolysis Proteins in Cancer Development. Open Life Sci. 2018;13:569-81
47. Song L, Tang H, Liao W, Luo X, Li Y, Chen T. et al. FOXC2 positively regulates YAP signaling and promotes the glycolysis of nasopharyngeal carcinoma. Exp Cell Res. 2017;357:17-24
48. Kuo CC, Ling HH, Chiang MC, Chung CH, Lee WY, Chu CY. et al. Metastatic Colorectal Cancer Rewrites Metabolic Program Through a Glut3-YAP-dependent Signaling Circuit. Theranostics. 2019;9:2526-40
49. Li H, Prever L, Hirsch E, Gulluni F. Targeting PI3K/AKT/mTOR Signaling Pathway in Breast Cancer. Cancers (Basel). 2021;13(14):3517
50. Cosialls E, Pacreau E, Duruel C, Ceccacci S, Elhage R, Desterke C. et al. mTOR inhibition suppresses salinomycin-induced ferroptosis in breast cancer stem cells by ironing out mitochondrial dysfunctions. Cell Death Dis. 2023;14:744
51. Wang T, Chen S, Wang Z, Li S, Fei X, Wang T. et al. KIRREL promotes the proliferation of gastric cancer cells and angiogenesis through the PI3K/AKT/mTOR pathway. J Cell Mol Med. 2023;28(1):e18020
52. Pungsrinont T, Kallenbach J, Baniahmad A. Role of PI3K-AKT-mTOR Pathway as a Pro-Survival Signaling and Resistance-Mediating Mechanism to Therapy of Prostate Cancer. Int J Mol Sci. 2021;22(20):11088
53. Lu M, Zessin AS, Glover W, Hsu DS. Activation of the mTOR Pathway by Oxaliplatin in the Treatment of Colorectal Cancer Liver Metastasis. PLoS One. 2017;12:e0169439
54. Huang H, Xue J, Xie M-L, Xie T. Osthole inhibits GSK-3β/AMPK/mTOR pathway-controlled glycolysis and increases radiosensitivity of subcutaneous transplanted hepatocellular carcinoma in nude mice. Strahlenther Onkol. 2024;200(5):444-452
Author contact
![]() Corresponding author: Dr Linhua Jiang, Department of General Surgery, The First Affiliated Hospital of Soochow University, Suzhou, 215006, Jiangsu, China. Email: jianglinhuaaaacom; Dr Bo Wei, Deputy Director of Department of General Surgery, Institute of General Surgery/Department of General Surgery, First Medical Center, Chinese PLA General Hospital, Beijing 100853, China. Email: weibocom.cn; Dr Bo Cao, Department of General Surgery, Institute of General Surgery/Department of General Surgery, First Medical Center, Chinese PLA General Hospital, Beijing 100853, China. Email: caobo_darkcom.
Corresponding author: Dr Linhua Jiang, Department of General Surgery, The First Affiliated Hospital of Soochow University, Suzhou, 215006, Jiangsu, China. Email: jianglinhuaaaacom; Dr Bo Wei, Deputy Director of Department of General Surgery, Institute of General Surgery/Department of General Surgery, First Medical Center, Chinese PLA General Hospital, Beijing 100853, China. Email: weibocom.cn; Dr Bo Cao, Department of General Surgery, Institute of General Surgery/Department of General Surgery, First Medical Center, Chinese PLA General Hospital, Beijing 100853, China. Email: caobo_darkcom.

 Global reach, higher impact
Global reach, higher impact