3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2025; 16(4):1054-1065. doi:10.7150/jca.98852 This issue Cite
Research Paper
Metformin Against Herpes Zoster in Colon Cancer Patients with Type 2 Diabetes: A PSM Analysis
1. Graduate Institute of Business Administration, College of Management, Fu Jen Catholic University, Taipei, Taiwan.
2. Department of Colorectal Surgery, Lo-Hsu Medical Foundation, Lotung Poh-Ai Hospital, Yilan, Taiwan.
3. Artificial Intelligence Development Center, Fu Jen Catholic University, Taipei, Taiwan.
4. Department of Food Nutrition and Health Biotechnology, College of Medical and Health Science, Asia University, Taichung, Taiwan.
5. Big Data Center, Lo-Hsu Medical Foundation, Lotung Poh-Ai Hospital, Yilan, Taiwan.
6. Division of Radiation Oncology, Lo-Hsu Medical Foundation, Lotung Poh-Ai Hospital, Yilan, Taiwan.
7. Department of Healthcare Administration, College of Medical and Health Science, Asia University, Taichung, Taiwan.
8. Cancer Center, Lo-Hsu Medical Foundation, Lotung Poh-Ai Hospital, Yilan, Taiwan.
9. Centers for Regional Anesthesia and Pain Medicine, Taipei Municipal Wan Fang Hospital, Taipei Medical University, Taipei, Taiwan.
10. Department of Management, College of Management, Fo Guang University, Yilan, Taiwan.
†These authors have contributed equally to this study (joint primary authors).
Received 2024-5-24; Accepted 2024-9-10; Published 2025-1-1
Abstract
Background: Herpes zoster is a significant complication in cancer patients, particularly those with compromised immune systems. Previous studies have established the incidence of herpes zoster in gastrointestinal cancer patients, but there is a lack of specific analysis on colorectal cancer patients and the potential preventive role of metformin. This study aims to fill this gap by evaluating metformin's protective effects against herpes zoster in colon cancer patients with type 2 diabetes mellitus (T2DM).
Methods: The study cohort comprised 1,510 T2DM colon adenocarcinoma patients without distant metastasis who received standard treatments from Taiwan Cancer Registry Database. Propensity score matching (PSM) was employed to balance covariates between metformin users and nonusers. Herpes zoster infection risk was assessed using Cox regression models and incidence rate calculations. The dose-dependent effects of metformin were analyzed based on cumulative defined daily doses (cDDD).
Results: Metformin use was associated with a significantly reduced risk of herpes zoster infection (adjusted hazard ratio [aHR].: 0.69, 95% confidence interval [CI].: 0.51 to 0.93). A dose-dependent relationship was observed, with progressively lower aHRs across cDDD quartiles (p for trend < 0.0001). After adjusting for competing mortality risks, the aHR remained significantly lower (aHR: 0.70, 95% CI: 0.51 to 0.65). Metformin users had lower incidence rates and incidence rate ratios (IRR) of herpes zoster infection compared to nonusers (IRR: 0.75, 95% CI: 0.56 to 0.97).
Conclusions: We are the first to demonstrate a dose-dependent protective effect of metformin against herpes zoster in colorectal cancer patients. Our findings indicate that higher doses of metformin correlate with a greater reduction in the risk of herpes zoster.
Keywords: T2DM, Colon Cancer, metformin, Diabetes-associated herpes zoster infection, dose-dependent
Introduction
Colorectal cancer is a significant healthcare concern in Taiwan, with around 15,000 new cases and 6,000 fatalities annually, making it the most prevalent malignancy in the region [1]. The typical age at diagnosis is approximately 66 years for both genders, and survival rates vary based on disease stage, with a five-year survival rate of around 63.0% [1, 2]. As survival prospects improve, the focus shifts towards enhancing patients' quality of life [3], particularly those dealing with cancer-induced immunosuppression. This immunosuppression often renders patients susceptible to herpes virus infections, impacting their quality of life and potentially leading to enduring post-herpetic neuralgia [4]. Notably, a large prospective cohort study in Australia from 2006 to 2016 found that individuals with hematologic or solid cancer had significantly higher rates of herpes zoster infection than those without cancer, emphasizing the relevance of this issue for cancer patients [4].
Colon cancer often comes with a favorable prognosis, involving extended survival assessments over a decade to gauge treatment effectiveness [5, 6]. Therefore, identifying cost-effective methods to reduce herpes zoster infection becomes crucial for enhancing the quality of life in long-term colon cancer survivors and preventing postherpetic neuralgia in this high-risk population. The association between colon cancer and herpes zoster, also known as shingles, is characterized by shared risk factors and a compromised immune system, a consequence of both cancer and its treatments like chemotherapy and radiation therapy [7, 8]. Previous studies have established the incidence of herpes zoster in gastrointestinal cancer patients, but there is a lack of specific analysis on colorectal cancer patients [9, 10]. This immune compromise increases susceptibility to infections and the likelihood of reactivating the varicella-zoster virus (VZV) responsible for herpes zoster [11]. Given that both conditions are more prevalent in older individuals, coinciding with the age group at higher risk for colon cancer diagnosis, vigilance is essential. Emotional stress from cancer and its treatments can further weaken immunity, potentially triggering latent virus reactivation [12]. Additionally, diagnostic challenges may arise as herpes zoster can mimic metastatic colon cancer lesions, leading to diagnostic confusion and treatment delays [13, 14]. Although the association isn't directly causal, individuals with colon cancer should remain vigilant. Herpes zoster in cancer patients can cause severe pain, complications, and treatment interruptions, affecting overall quality of life [15, 16]. Hence, meticulous management and a multidisciplinary approach are imperative to mitigate these complications and enhance patient outcomes.
The exploration of metformin's potential in mitigating herpes zoster infections among diabetic patients delves into a complex interplay involving immunity, chronic illnesses, and pharmaceutical interventions [17]. Following chickenpox or varicella vaccination, the VZV can enter a latent state within sensory ganglia, potentially reactivating to cause herpes zoster, often accompanied by persistent postherpetic neuralgia [18]. Despite the availability of vaccines, the global incidence of varicella and herpes zoster is increasing, particularly among older adults and individuals with cancer or autoimmune diseases, including those with Type 2 diabetes mellitus (T2DM), due to compromised immune function [19, 20]. Metformin, a widely recognized antidiabetic medication, not only regulates blood sugar but also confers various health benefits, such as reducing cardiovascular risk and enhancing the immune system [21-24]. Laboratory studies have indicated that metformin can strengthen T-cell immunity and mitigate inflammation [25]. This has led to the hypothesis that metformin might lower the risk of herpes zoster in individuals with colon cancer undergoing anticancer treatments, prompting a study to compare the incidence of zoster between metformin users and nonusers among colon cancer patients receiving such treatments. Our research aims to uncover metformin's potential protective effects against these conditions, offering valuable insights into their management, particularly in colon cancer patients with diabetes. No prior studies have investigated the potential of metformin to prevent herpes zoster in colorectal cancer patients with T2DM. Our study uniquely highlights this aspect. Previous studies have not specifically analyzed the incidence of herpes zoster in patients with colorectal cancer [9, 10]. Our study is the first to do so.
Materials and Methods
Study population
This population-based cohort investigation harnessed the extensive repository of Taiwan's National Health Insurance Research Database (NHIRD), encompassing a wealth of data spanning disease diagnoses, medical procedures, pharmaceutical prescriptions, demographic particulars, and beneficiary profiles [26-28]. Protecting patient confidentiality, the database incorporates encrypted identifiers, while its integration with the Taiwan Cancer Registry Database (TCRD) and Taiwan's Death Registry furnishes precise insights into cancer management, disease stages, vital status, and causative factors, augmenting the study's veracity and credibility [29-32]. Stringent ethical oversight was observed, with the study protocols securing the imprimatur of the Institutional Review Board of Tzu-Chi Medical Foundation (IRB109-015-B).
Our investigation exclusively targeted patients with colon adenocarcinoma devoid of distant metastases who received standard treatments and were diagnosed with T2DM during the period spanning 2008 to 2018. The established treatment paradigms for stage I-III colon cancer without metastatic spread typically involve a comprehensive approach encompassing surgical intervention, occasionally supplemented with adjuvant therapy. In Taiwan, these therapeutic protocols harmonize with the well-recognized National Comprehensive Cancer Network (NCCN) guidelines [33-36]. The administration of adjuvant chemotherapy is contingent upon several factors, including cancer stage (T3 or higher) and the presence of positive lymph nodes, all serving as risk indicators [36]. This therapeutic strategy is customarily recommended post-surgery to eliminate residual cancer cells that might elude detection yet harbor the potential for recurrence. Two frequently employed chemotherapy regimens are FOLFOX, a combination of 5-fluorouracil, leucovorin, and oxaliplatin, and CAPEOX, which comprises capecitabine and oxaliplatin [36]. Radiation therapy, conversely, does not constitute a standard treatment for colon cancer, except in cases where a notable risk of recurrence looms due to specific factors such as T4 stage infiltration into fixed structures [37].
The study's follow-up period extended until December 31, 2021, and stringent exclusion criteria were meticulously applied: (1) Age restrictions were enforced, excluding individuals both below 20 and above 80 years of age. (2) Thorough data encompassing sex and age were prerequisites for inclusion. (3) Patients afflicted with conditions such as type 1 diabetes, hepatic failure, or undergoing dialysis were systematically excluded. (4) Individuals with a previous diagnosis of herpes zoster before the index date were not factored into the analysis. (5) To eliminate prevalent cases of T2DM, patients diagnosed prior to January 1, 2008, were methodically excluded. (6) Rigorous measures were undertaken to ascertain that metformin use commenced subsequent to the diagnosis of colon cancer, with explicit confirmation that the index date for metformin users postdated the colon cancer diagnosis. Metformin use preceding the cancer diagnosis was rigorously excluded. (7) Sole inclusion encompassed patients who adhered to standard treatments for colon cancer, and (8) individuals who received the zoster vaccine during the follow-up period were meticulously omitted from the study cohort.
Metformin use was meticulously defined as consistent daily administration for the majority of days, with an average dose equivalent to or exceeding 28 cumulative defined daily doses (cDDDs). The index date was meticulously established subsequent to the initial documented use of metformin therapy (≥ 28 cDDDs), ensuring that it unequivocally surpassed the threshold of 28 cDDDs. For metformin nonusers, the index date was harmonized to align with the identical time frame following the diagnosis of colon cancer as their corresponding metformin users. The case group exclusively consisted of T2DM patients who were administered a minimum of 28 cDDDs of metformin subsequent to their colon cancer diagnosis, while the control group comprised individuals who refrained from metformin therapy throughout the entire follow-up period.
Study covariates and propensity score matching
In our study, T2DM colon cancer patients were stratified into distinct age groups at the index date: <60, 60-65, 66-70, and ≥71 years. To mitigate potential confounding variables, we employed Propensity Score Matching (PSM) to achieve balance between metformin users and nonusers. The covariates encompassed a comprehensive set of factors, including age, sex, income level, urbanization level, years of cancer diagnosis, AJCC clinical stage (8th edition), numbers of antidiabetic drugs employed (as a proxy for T2DM severity), utilization of antidiabetic medications, diabetes severity (quantified via adapted Diabetes Complications Severity Index [aDCSI]. scores), presence of coexisting comorbidities, Charlson comorbidity index (CCI) scores, and additional medications (such as statins, aspirin, immunosuppressants, and systemic corticosteroids). PSM was diligently applied to harmonize all covariates between metformin users and nonusers, given their disparate antidiabetic drug usage patterns. Medication use was consistently defined as a minimum of 28 cDDDs post-diagnosis of colon cancer. The index date was stipulated subsequent to the initiation of metformin treatment (≥28 cDDDs), and metformin users and nonusers were matched based on variables collected at this specific index date. Prior to any analytical procedures, we conducted baseline matching between metformin users and nonusers to ensure the equivalence of data at the index date. To prevent redundancy in multivariate analyses, duplicate comorbidities were meticulously excluded from the calculation of CCI scores. Comorbidities were ascertained from diagnostic codes (International Classification of Diseases, Ninth Revision, Clinical Modification, and International Classification of Diseases, Tenth Revision, Clinical Modification) documented in inpatient records or the presence of a minimum of two outpatient visits within a one-year timeframe preceding the index date. Continuous variables were aptly presented with appropriate metrics, such as mean ± standard deviation or median (first quartile and third quartile).
Outcome variables
Our primary objective was to evaluate the risk of herpes zoster infection, closely monitoring occurrences from the index date until either herpes zoster diagnosis or the study's endpoint on December 31, 2021. The diagnosis of herpes zoster was based on clinical presentation and confirmed through medical records coded with the ICD-9-CM code 053 and ICD-10-CM code B02. The lesions were typically located on the skin following the nerve distribution pattern and were not found within the colon tissues, as the clinical manifestation of HZ within internal organs is exceedingly rare and not within the scope of this study. Furthermore, we investigated secondary outcome measures, encompassing the incidence rate (IR) and incidence rate ratio (IRR) of herpes zoster infection within our cohort.
Metformin exposure
Metformin prescriptions in our study were precisely coded using the Anatomical Therapeutic Chemical (ATC) classification system [38], enabling the precise retrieval of pharmaceutical claims data from the NHIRD. To investigate potential dose-response associations, patients were stratified into four subgroups, defined by quartiles (Q) of cDDD. Our statistical models included thorough adjustments for the covariates mentioned above, ensuring a rigorous and comprehensive analysis of the data.
Statistical analysis
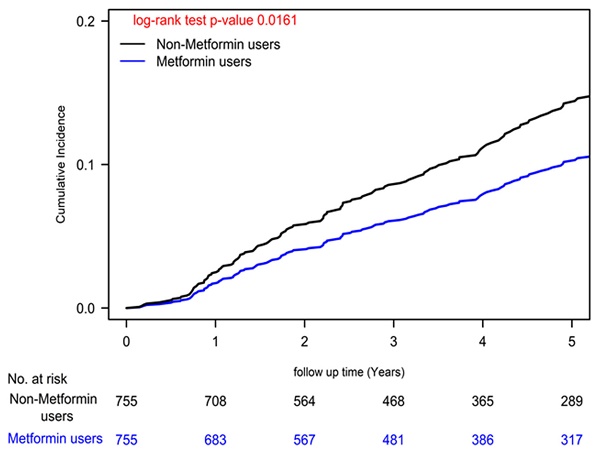
To comprehensively account for potential confounding variables, our Cox regression models underwent meticulous adjustments, encompassing a wide array of covariates, including age, sex, income levels, urbanization degree, years since cancer diagnosis, AJCC clinical stage according to the 8th edition, numbers of antidiabetic drugs used, utilization of antidiabetic medications, aDCSI scores, presence of concurrent comorbidities, CCI scores, and other medications [39]. Furthermore, we employed a time-dependent Cox hazards model to compare the incidence of herpes zoster infection between metformin users and nonusers, with rigorous adjustments for the aforementioned covariates. Recognizing the dynamic nature of metformin prescriptions, we diligently collected metformin usage data every 3 months, enabling precise characterization of metformin status as a time-dependent variable. To address potential biases, we categorized event-free person-years during follow-up periods devoid of metformin use for a minimum of 3 months as unexposed follow-up intervals. Poisson Regression analysis was employed to estimate the IRR of herpes zoster infection, and competing risk analysis was conducted to account for mortality risk. Cumulative incidences of herpes zoster infection were meticulously evaluated using the Kaplan-Meier method, and distinctions between metformin users and nonusers were scrutinized using a stratified log-rank test (Figure 1). Similarly, we estimated the incidence of herpes zoster infection according to various levels of cDDD using the Kaplan-Meier method, with distinctions assessed via a stratified log-rank test (Figure 2). All statistical analyses were executed utilizing SAS software (version 9.4; SAS Institute, Cary, NC, USA), ensuring the robustness and rigor of our data analysis.
Results
Following PSM, our study encompassed a cohort of 1,510 individuals diagnosed with both T2DM and colon adenocarcinoma, devoid of distant metastases, who underwent standard treatments spanning from 2008 to 2018. The average age at the time of T2DM diagnosis mirrored at 65.88 years for both metformin users and nonusers. Notably, a meticulous examination revealed an impeccably balanced distribution of all covariates between the two groups. This equilibrium underscored the triumph of our adjustment procedure in attaining covariate parity, as distinctly evidenced in Table 1, with every absolute standardized mean difference comfortably resting below 0.1, reinforcing the robustness of our analysis [40].
Metformin use and dose-dependent protective effects for herpes zoster infection
Our analysis unveiled a significant reduction in herpes zoster infection risk among colon cancer patients undergoing standard treatments who were also metformin users, as reflected by a noteworthy adjusted hazard ratio (aHR) of 0.69 (95% CI: 0.51 to 0.93; Table 2). This finding was consistently supported by a statistically significant log-rank test result (P=0.0161; Figure 1). Moreover, our Cox regression analysis illuminated a dose-dependent relationship between metformin use and diminishing herpes zoster infection risk. The examination of cDDD of metformin revealed a systematic dose-response pattern, with progressively declining aHRs observed across quartiles (0.10, 0.55, 0.92, and 0.94 for quartiles 4, 3, 2, and 1, respectively) when compared to individuals who had never utilized metformin (P for trend < 0.0001). These compelling findings, visually represented in Figure 2 (P = 0.0001), offer robust evidence for the dose-dependent protective effects of metformin against herpes zoster infection.
Competing risk of mortality
Upon meticulous adjustment for all covariates, as delineated in Table 1, using a dynamic Cox proportional regression model that accounts for the competing risk of mortality (Table 3), we determined that the aHR for herpes zoster infection within our metformin user cohort stood at 0.70 (95% CI: 0.51 to 0.65). Notably, a substantial and dose-dependent reduction in herpes zoster infection risk was evident across various quartiles of cDDD of metformin (P for trend < 0.0001). The aHRs for quartiles 4, 3, 2, and 1 of cDDD were as follows: 0.12 (95% CI: 0.05 to 0.25), 0.58 (95% CI: 0.35 to 0.95), 0.96 (95% CI: 0.87 to 1.13), and 0.97 (95% CI: 0.91 to 1.33), respectively, when contrasted with individuals who had never initiated metformin therapy.
Cumulative Incidences of Herpes Zoster Infection in Patients with Type II Diabetes and Colon Cancer Undergoing Curative Standard Treatments: A Comparison Between Metformin Users and Non-Users.
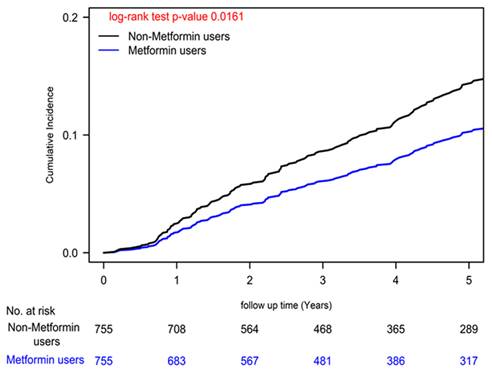
Cumulative Incidences of Herpes Zoster Infection in Patients with Type II Diabetes and Colon Cancer Undergoing Curative Standard Treatments: Varied Metformin Dosages.
Propensity Score-Matched Characteristics of Metformin Users and Non-Users Among Patients with Type II Diabetes and Colon Cancer Undergoing Curative Standard Treatments
| Non-Metformin | Metformin | ASMD | |||
|---|---|---|---|---|---|
| N=755 | N=755 | ||||
| N | % | N | % | ||
| Age (mean ± SD) | 65.88 ± 9.63 | 65.88 ± 9.63 | |||
| Age, median (IQR), y | 69.00 (61.00,76.00) | 69.00 (61.00,76.00) | |||
| Age group, years | 0.0000 | ||||
| <60 | 189 | 25.03% | 189 | 25.03% | |
| 60-65 | 146 | 19.34% | 146 | 19.34% | |
| 66-70 | 134 | 17.75% | 134 | 17.75% | |
| ≥71 | 286 | 37.88% | 286 | 37.88% | |
| Sex | 0.0242 | ||||
| Female | 320 | 42.38% | 329 | 43.58% | |
| Male | 435 | 57.62% | 426 | 56.42% | |
| Income level (NTD) | 0.0000 | ||||
| Low income | 10 | 1.32% | 10 | 1.32% | |
| ≤20 000 | 652 | 86.36% | 652 | 86.36% | |
| 20 001-30 000 | 51 | 6.75% | 51 | 6.75% | |
| 30 001-45 000 | 33 | 4.37% | 33 | 4.37% | |
| >45 000 | 9 | 1.19% | 9 | 1.19% | |
| Urbanization | 0.0084 | ||||
| Rural | 231 | 30.60% | 234 | 30.99% | |
| Urban | 524 | 69.40% | 521 | 69.01% | |
| Years of Cancer Diagnosis | 0.0000 | ||||
| 2008-2011 | 130 | 17.22% | 130 | 17.22% | |
| 2012-2016 | 328 | 43.44% | 328 | 43.44% | |
| 2017-2020 | 297 | 39.34% | 297 | 39.34% | |
| AJCC clinical Stage, 8th edition | 0.0590 | ||||
| I | 237 | 31.39% | 233 | 30.86% | |
| II | 157 | 20.79% | 158 | 20.93% | |
| IIIA | 16 | 2.12% | 23 | 3.05% | |
| IIIB | 106 | 14.04% | 106 | 14.04% | |
| IIIC | 239 | 31.66% | 235 | 31.13% | |
| Types of antidiabetic drugs used (Diabetes severity) | 0.0041 | ||||
| 0 | 314 | 41.59% | 312 | 41.32% | |
| 1 | 185 | 24.50% | 187 | 24.77% | |
| 2 | 160 | 21.19% | 162 | 21.46% | |
| 3 | 69 | 9.12% | 70 | 9.27% | |
| ≥4 | 27 | 3.58% | 24 | 3.18% | |
| Antidiabetic drug | |||||
| Insulin | 94 | 12.45% | 97 | 12.86% | 0.0013 |
| Sulfonylureas | 315 | 41.72% | 311 | 41.19% | 0.0011 |
| SGLT2 inhibitors | 12 | 1.59% | 11 | 1.46% | 0.0009 |
| Alpha-glucosidase inhibitors | 47 | 6.23% | 46 | 6.09% | 0.0012 |
| Thiazolidinediones | 25 | 3.31% | 28 | 3.71% | 0.0007 |
| GLP-1 agonists | 9 | 1.19% | 8 | 1.06% | 0.0016 |
| DPP4 inhibitors | 43 | 5.70% | 47 | 6.23% | 0.0021 |
| Diabetes severity | |||||
| aDCSI Score (mean ± SD) | 1.14 ± 1.56 | 1.14 ± 1.50 | |||
| Median (IQR, Q1-Q3) | 1.00 (0.00,2.00) | 1.00 (0.00,2.00) | |||
| aDCSI Score | |||||
| 0 | 327 | 43.31% | 326 | 43.18% | 0.0008 |
| 1 | 177 | 23.44% | 178 | 23.58% | 0.0003 |
| 2 | 126 | 16.69% | 127 | 16.82% | 0.0009 |
| 3 | 69 | 9.14% | 70 | 9.27% | 0.0002 |
| ≥4 | 56 | 7.42% | 54 | 7.15% | 0.0004 |
| CCI Scores | |||||
| Mean (SD) | 3.46 ± 3.00 | 3.41 ± 3.20 | |||
| Median (Q1-Q3) | 2.00 (0.00,6.00) | 2.00 (1.00,6.00) | |||
| CCI Scores | 0.0780 | ||||
| 0 | 191 | 25.30% | 166 | 21.99% | |
| ≥1 | 564 | 74.70% | 589 | 78.01% | |
| Coexisting comorbidities | |||||
| Hypertension | 571 | 75.63% | 572 | 75.76% | 0.0030 |
| Hyperlipidemia | 395 | 52.32% | 400 | 52.98% | 0.0132 |
| Chronic Obstructive Pulmonary Disease | 205 | 27.15% | 198 | 26.23% | 0.0208 |
| Alcohol-related disorders | 38 | 5.03% | 29 | 3.84% | 0.0578 |
| Coronary artery disease | 273 | 36.16% | 271 | 35.89% | 0.0056 |
| Stroke | 201 | 26.62% | 194 | 25.70% | 0.0209 |
| Heart failure | 75 | 9.93% | 70 | 9.27% | 0.0224 |
| Peripheral vascular disease | 76 | 10.07% | 71 | 9.40% | 0.0226 |
| Chronic kidney disease | 30 | 3.97% | 23 | 3.05% | 0.0500 |
| Depression | 139 | 18.41% | 133 | 17.62% | 0.0206 |
| Anxiety | 66 | 8.74% | 68 | 9.01% | 0.0095 |
| Dementia | 26 | 3.44% | 29 | 3.84% | 0.0214 |
| Psychosis | 59 | 7.81% | 45 | 5.96% | 0.0731 |
| Rheumatoid arthritis | 25 | 3.31% | 23 | 3.05% | 0.0148 |
| Liver Cirrhosis | 257 | 34.04% | 253 | 33.51% | 0.0112 |
| Systemic Lupus Erythematosus | 13 | 1.72% | 17 | 2.25% | 0.0380 |
| Obesity | 240 | 31.79% | 244 | 32.32% | 0.0086 |
| Medication Use | |||||
| Statin | 287 | 38.01% | 294 | 38.94% | 0.0191 |
| Aspirin | 375 | 49.67% | 391 | 51.79% | 0.0424 |
| Immunosuppressant | 5 | 0.66% | 6 | 0.79% | 0.0153 |
| Corticosteroids for systemic use | 626 | 8.91% | 634 | 83.97% | 0.0285 |
| Metformin (cDDD) | |||||
| Never used | 755 | 100.00% | 0 | 0.00% | |
| Q1(153) | 0 | 0.00% | 190 | 25.17% | |
| Q2 (411) | 0 | 0.00% | 188 | 24.90% | |
| Q3 (962) | 0 | 0.00% | 190 | 25.17% | |
| Q4 (> 962) | 0 | 0.00% | 187 | 24.77% | |
| DDD | |||||
| <1 | 755 | 100.00% | 723 | 95.76% | |
| ≥1 | 0 | 0.00% | 32 | 4.24% | |
| Primary Outcome | P value | ||||
| Herpes zoster infection | 104 | 13.77% | 72 | 9.54% | 0.0449 |
Abbreviations: aDCSI, adapted Diabetes Complications Severity Index; DDD, defined daily dose; cDDD, cumulative defined daily dose; DM, diabetes mellitus; T2DM, Type 2 diabetes mellitus; Q, quartile; CCI, Charlson comorbidity index; ASMD, absolute standardized mean difference; N, Numbers; NTD, New Taiwan Dollars; SD, Standard deviation; IQR, interquartile range.
Hazard Ratios for Herpes Zoster Infection in Patients with Type II Diabetes and Colon Cancer Undergoing Curative Standard Treatments, Stratified by Varied Metformin Dosages
| Crude HR (95%CI) | P-value | Adjusted HR (95%CI) | P-value | |||
|---|---|---|---|---|---|---|
| Metformin (ref. Never-users) | ||||||
| Users | 0.75 | (0.56, 1.01) | 0.0557 | 0.69 | (0.51, 0.93) | 0.0161 |
| Metformin DDD (ref. no) | ||||||
| Q1 | 1.25 | (0.83, 3.24) | 0.1762 | 0.94 | (0.84, 3.14) | 0.1023 |
| Q2 | 1.36 | (0.82, 2.40) | 0.2407 | 0.92 | (0.87, 1.35) | 0.0665 |
| Q3 | 0.63 | (0.39, 1.03) | 0.0661 | 0.55 | (0.33, 0.9) | 0.0185 |
| Q4 | 0.12 | (0.05, 0.28) | <0.0001 | 0.10 | (0.04, 0.22) | <0.0001 |
| P for trend | <0.0001 | |||||
Abbreviations: Q, quartile; HR, hazard ratio; CI, confidence interval.
*Adjusted for all covariates shown in Table 1 using a Cox proportional regression model.
IR and IRRs of herpes zoster infection
Within our cohort of individuals with colon cancer and T2DM, we noted that 9.54% of those using metformin (72 individuals) and 13.77% of those not using metformin (104 individuals) experienced herpes zoster infections, marking a statistically significant difference (P=0.0449; Table 1). Metformin users exhibited lower IRs of herpes zoster infection compared to nonusers, as detailed in Table 4. An analysis of the IRRs indicated that metformin users had an IRR (95% CI) of 0.75 (0.56 to 0.97) relative to nonusers.
Competing Risk Analysis of Hazard Ratios for Herpes Zoster Infection Among Patients with Type II Diabetes and Colon Cancer Undergoing Curative Standard Treatments, Stratified by Varied Metformin Dosages
| Crude HR (95%CI ) | P-value | Adjusted HR (95%CI ) | P-value | |||
|---|---|---|---|---|---|---|
| Metformin (ref. Never-users) | ||||||
| Users | 0.75 | (0.56, 1.01) | 0.0557 | 0.70 | (0.51, 0.95) | 0.0210 |
| Metformin DDD (ref. no) | ||||||
| Q1 | 1.25 | (0.83, 3.24) | 0.1762 | 0.97 | (0.91, 1.33) | 0.1527 |
| Q2 | 1.36 | (0.82, 2.40) | 0.2407 | 0.96 | (0.87, 1.13) | 0.1753 |
| Q3 | 0.63 | (0.39, 1.03) | 0.0661 | 0.58 | (0.35, 0.95) | 0.0310 |
| Q4 | 0.12 | (0.05, 0.28) | <0.0001 | 0.12 | (0.05, 0.25) | <0.0001 |
| P for trend | <0.0001 | |||||
Abbreviations: Q, quartile; HR, hazard ratio; CI, confidence interval.
*Adjusted for all covariates shown in Table 1 using a Cox proportional regression model with competing risk of mortality.
Incidence Rate and Incidence Rate Ratio of Herpes Zoster Infection in Patients with Type II Diabetes and Colon Cancer Undergoing Curative Standard Treatments
| Variables | Events | Person-years | IR (10,000 person-years) | IRR | 95% CI for IRR | P |
|---|---|---|---|---|---|---|
| Metformin | ||||||
| Never-users | 104 | 3,617 | 287.53 | Reference | ||
| Users | 72 | 3,793 | 210.17 | 0.75 | (0.56, 0.97) | 0.0434 |
| Metformin | ||||||
| Never-users | 104 | 3,617.0 | 287.53 | Reference | ||
| Q1 | 27 | 517.1 | 580.14 | 1.12 | (0.84, 2.03) | 0.1807 |
| Q2 | 24 | 632.8 | 426.68 | 1.11 | (0.87, 2.27) | 0.1676 |
| Q3 | 16 | 1,039.6 | 182.76 | 0.64 | (0.39, 0.94) | 0.0423 |
| Q4 | 5 | 1,603.7 | 37.41 | 0.13 | (0.06, 0.30) | <0.0001 |
Abbreviations: Q, quartile; IR, incidence rate; IRR, incidence rate ratio; and CI, confidence interval.
Specifically, the IR for herpes zoster infection among metformin users amounted to 210.17 per 10,000 person-years, whereas non-metformin users displayed an IR of 287.53 per 10,000 person-years. When stratified by quartiles of cDDD of metformin, the IRRs (95% CI) for herpes zoster infection in metformin users, compared with nonusers, were as follows: 0.13 (0.06 to 0.30) for quartile 4, 0.64 (0.39 to 0.94) for quartile 3, 1.11 (0.87 to 2.27) for quartile 2, and 1.12 (0.84 to 2.03) for quartile 1.
Discussion
In the realm of devising immunosuppressive strategies for adults, especially those at heightened susceptibility to herpes zoster like solid organ transplant recipients and certain cancer or autoimmune disorder patients, the role of zoster vaccination warrants thoughtful consideration [41-43]. It's worth noting the financial implications associated with these vaccines [44, 45], particularly as they lack coverage under Taiwan's National Health Insurance program. The primary obstacles reported by physicians and patients in relation to vaccination were predominantly financial in nature [44, 45]. Moreover, the imperative for zoster vaccination may not be universally applicable across distinct cancer types, given varying levels of zoster infection risk [46, 47]. Thus, our investigation delivers valuable insights by not only presenting the incidence rate of zoster infection in colon cancer patients undergoing standard treatments but also by calculating the incidence rate ratio for zoster infection (Table 4). Furthermore, we affirm the protective impact of the cost-effective and well-tolerated medication, metformin, in curtailing the risk of zoster infection (Table 2-3). These findings significantly enrich our comprehension of herpes zoster risk management in cancer patients, potentially alleviating concerns regarding vaccination costs and broadening therapeutic horizons. Despite the presence of vaccines and pharmaceutical interventions for herpes zoster, this infection remains a substantial healthcare challenge on a global scale [15]. Notably, our research uncovers a substantial association between metformin usage and a diminished risk of herpes zoster infection. Furthermore, an elevated cumulative metformin dose correlates with a progressively reduced zoster risk (Table 2). These observations suggest that metformin might offer a promising avenue for alleviating the burden of zoster, especially among colon cancer patients with T2DM who are undergoing standard treatments. Of note, our study stands as the inaugural demonstration of a significant reduction in the risk of herpes zoster infection among metformin users in this specific cohort, yielding an aHR of 0.69. This protective effect of metformin exhibits a dose-dependent trend, with consistently lower aHRs across quartiles of cDDD of metformin. Even subsequent to meticulous adjustments for competing mortality risks, the aHR for herpes zoster infection among metformin users maintains its significant reduction at 0.70, underscoring the potential benefits of metformin in mitigating the risk of herpes zoster in colon cancer patients with T2DM. Additionally, metformin users display a lower incidence rate of herpes zoster infection (9.54%) compared to nonusers (13.77%), with an IRR of 0.75, further accentuating the protective role of metformin. Stratified analysis by cDDD quartiles consistently unveils a dose-response relationship.
Metformin holds promise as a means to alleviate the burden of herpes zoster, particularly in colon cancer patients with T2DM undergoing standard treatments (Table 2-4 and Figure 1). This potential is rooted in a multifaceted array of mechanisms. Firstly, Metformin exerts immunomodulatory effects by activating AMP-activated protein kinase, bolstering T-cell immunity [22-24]. This enhanced immune response can lower the risk of Zoster reactivation and mitigate infection severity [22-24]. Secondly, Metformin's anti-inflammatory properties come into play by reducing the secretion of proinflammatory cytokines, thus mitigating the typical inflammatory response triggered by viral infections like Zoster [48-50]. Thirdly, one potential mechanism by which Metformin may reduce the risk of herpes zoster in colon cancer patients with T2DM undergoing standard treatments is its impact on immune modulation [51, 52]. The tumor itself and cancer treatments, such as chemotherapy and radiation therapy, can induce immune suppression [7, 8], rendering patients more vulnerable to infections, including the reactivation of the VZV responsible for Zoster [11]. Additionally, metformin's ability to effectively control blood glucose levels indirectly enhances immune function, as hyperglycemia compromises immune responses [53]. Crucially, the observed dose-dependent effect of metformin on reducing Zoster infection risk implies that a cumulative exposure threshold is necessary for its significant protective impact. Lower doses may not induce substantial immunomodulation, as seen in Q1 and Q2, whereas reaching a certain threshold (Q3 and Q4) is vital to unlock metformin's full potential in mitigating Zoster risk. In essence, metformin's multifaceted influence on immunity, inflammation, and glycemic control collectively positions it as a valuable candidate for reducing the risk of Zoster infection in colon cancer patients with T2DM undergoing standard treatments.
In addition to its protective effects against herpes zoster, metformin offers several direct and indirect clinical benefits for colon cancer patients. Metformin has demonstrated antineoplastic properties, potentially inhibiting cancer cell proliferation and inducing apoptosis in colorectal cancer cells [54], which can enhance overall treatment outcomes. Furthermore, metformin's ability to improve metabolic control is particularly beneficial for colon cancer patients with T2DM, as optimal glycemic control supports overall health and may reduce cancer-related complications [55]. Metformin's anti-inflammatory properties, through the reduction of proinflammatory cytokines, can mitigate inflammation associated with both cancer and its treatment, thereby improving patient well-being [56]. Additionally, metformin's role in immune modulation by activating AMP-activated protein kinase enhances T-cell immunity, which is crucial for patients undergoing immunosuppressive treatments like chemotherapy and radiation therapy [51]. This multifaceted influence of metformin on immunity, inflammation, and glycemic control collectively positions it as a valuable therapeutic agent, not only for reducing the risk of herpes zoster infection but also for potentially lowering the risk of cancer recurrence and improving long-term survival rates in colon cancer patients [51, 54-56]. These benefits underscore the importance of considering metformin as part of a comprehensive management strategy for colon cancer patients with T2DM.
In our study, we harnessed the manifold advantages of PSM, dose-dependent analysis, and time-dependent Cox regression, each serving a unique purpose in our complex real-world investigation. PSM, an invaluable tool in the absence of randomized controlled trials (RCTs) due to ethical considerations [57, 58], played a pivotal role in achieving covariate balance between metformin users and nonusers. Given the impracticality of mandating medication use or vaccination in real-world settings, PSM emulated the random assignment scenario of RCTs within the confines of observational data, bolstering the internal validity of our findings [58]. Notably, Table 1 showcases the meticulous equilibrium achieved in all confounding factors between the case and control groups post PSM, underscoring the robustness of our analytical framework. Dose-dependent analysis unveiled a dose-response relationship between metformin dosage and herpes zoster risk. This nuanced exploration provided critical insights into the optimal metformin dosage required for risk reduction, shedding light on the potential for tailored treatment regimens. Time-dependent Cox regression emerged as a dynamic analytical approach [59], accounting for variations in metformin exposure over the study duration. As metformin use fluctuated throughout the observation period, this method provided a real-time assessment of its influence on herpes zoster risk [59]. It enabled us to categorize individuals based on their evolving metformin exposure status, ensuring a precise representation of the medication's effects over time. To fortify the robustness of our findings, we incorporated competing risk analysis, which factored in the risk of mortality [60-62]. This was especially crucial in a cohort of patients grappling with underlying conditions such as cancer and diabetes, where mortality is a significant consideration. By considering these competing risks, we bolstered the credibility and reliability of our results (Table 3). Taken together, our utilization of PSM, dose-dependent analysis, time-dependent Cox regression, and competing risk analysis collectively fortified the rigor and comprehensiveness of our study. These methodological choices allowed us to derive meaningful conclusions regarding the intricate relationship between metformin use and the risk of herpes zoster infection in colon cancer patients with T2DM undergoing standard treatments, offering valuable insights to the medical community.
Our study reveals that metformin significantly reduces the risk of herpes zoster infection in colon cancer patients with T2DM undergoing standard treatments, with a dose-dependent protective effect. This finding has several clinical implications and applications. Firstly, metformin's ability to lower herpes zoster incidence can improve patient outcomes by preventing complications like postherpetic neuralgia, thus enhancing the quality of life for these patients. Secondly, as a cost-effective and well-tolerated medication, metformin offers a financially viable alternative to vaccination, particularly in resource-limited settings. Integrating metformin into clinical guidelines for managing colon cancer patients with T2DM could optimize both glycemic control and herpes zoster prevention, necessitating collaboration among oncologists, endocrinologists, and primary care providers. The dose-dependent nature of its protective effect also supports a personalized medicine approach, allowing clinicians to tailor therapy based on individual patient risk profiles and tolerance levels. Moreover, this study suggests broader preventive applications of metformin in immunocompromised patients due to its immunomodulatory and anti-inflammatory properties. In summary, our research underscores the potential of metformin as a multifaceted therapeutic agent, enhancing patient outcomes, providing a cost-effective preventive strategy, and supporting personalized medicine, thereby contributing significantly to the holistic management of colon cancer patients with T2DM.Our study emphasizes the potential of metformin in reducing the risk of herpes zoster infection in colon cancer patients with Type 2 Diabetes Mellitus (T2DM) undergoing standard treatments. The protective effect is dose-dependent, suggesting that metformin could be a valuable addition to herpes zoster risk management strategies in this patient population.
Our study boasts several key strengths that enhance its credibility and contribution to the field. It leverages Taiwan's NHIRD and TCRD, providing a robust dataset for a population-based cohort study [29-31, 63]. The integration of these databases with Taiwan's Death Registry enables precise determination of cancer treatments, stages, vital status, and causes of death [29-31, 63]. Notably, our study exclusively focuses on a well-defined cohort of colon adenocarcinoma patients without distant metastasis who received standard treatments, closely aligning with established NCCN guidelines, thereby increasing its specificity and relevance. A significant strength lies in the rigorous application of PSM to balance covariates between metformin users and nonusers. In the absence of RCTs due to ethical constraints, PSM serves as a powerful tool to emulate random assignment within observational data [57, 58], significantly enhancing the internal validity of our findings. This success is evident in the well-balanced distribution of all confounding factors between the two groups (Table 1). Furthermore, our study offers novel insights into the relationship between metformin use and the risk of herpes zoster infection in colon cancer patients with T2DM undergoing standard treatments. It is the first to demonstrate a significant reduction in the risk of herpes zoster infection among metformin users within this specific cohort, revealing a dose-dependent effect that persists even after adjusting for competing mortality risks. These findings present a compelling case for metformin as a potential avenue to alleviate the burden of herpes zoster in this vulnerable population, significantly contributing to our understanding of herpes zoster risk management, particularly in the context of cancer and diabetes care. The innovative approach employed to analyze metformin's protective effects in a real-world, complex clinical scenario underscores the novelty and significance of this study.
Our study, while valuable, comes with several limitations inherent to its retrospective nature and reliance on administrative health data, which may contain errors or missing information. Although we diligently employed PSM to mitigate selection bias, the influence of unmeasured variables, such as lifestyle factors, on both metformin use and herpes zoster risk cannot be entirely ruled out. Despite comprehensive covariate adjustment, concerns regarding the presence of unmeasured confounders persist. The generalizability of our findings is constrained as our study exclusively focuses on colon adenocarcinoma patients with T2DM in Taiwan. Moreover, metformin use was defined solely based on pharmacy claims, without accounting for actual adherence. The termination of the follow-up period in December 2021 raises the potential for missing long-term effects. However, it's worth noting that several cancers were associated with an increased risk of zoster, particularly within the first 2 years after diagnosis [64]. Therefore, the mean follow-up time of 5.44 years for the cohort is deemed sufficient for estimating the protective effects of metformin against herpes zoster in cancer patients. Lastly, our study did not delve into the specific mechanisms underpinning metformin's protective effect against herpes zoster. Despite these limitations, our research significantly contributes by highlighting an association between metformin use and reduced herpes zoster risk within this specific cohort. This association warrants further exploration and confirmation in more extensive and diverse populations and settings, with an emphasis on investigating the underlying mechanisms.
Our population-based cohort study reveals metformin's potential to reduce herpes zoster risk in colon cancer patients with T2DMundergoing standard treatments, with a significant dose-dependent effect as cumulative defined daily doses increase. This suggests metformin, a well-established diabetes medication, could offer an innovative approach to easing herpes zoster's burden in this vulnerable group. While further research is needed to understand the precise mechanisms, our findings underscore metformin's clinical significance in enhancing the well-being of these patients, informing herpes zoster risk management, and highlighting the broader potential of repurposing existing medications to address complex healthcare challenges.
Acknowledgements
We would like to thank the Lo-Hsu Medical Foundation, LotungPoh-Ai Hospital for their financial support of Szu-Yuan Wu's work (Funding Numbers: 11001, 11010, 11013, and 11103). We also appreciate the efforts of all contributors involved in the conception, design, data collection, and analysis of this study. Special thanks to Ming-Chang Li, Ben-Chang Shia, Wan-Ming Chen, and Szu-Yuan Wu for their pivotal roles in this research. Furthermore, we are grateful to all administrative and technical staff for their continuous support.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Health Promotion Administration MoHaW. Taiwan Cancer Registry Annual Report. 2020.
2. Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC. et al. Colorectal cancer statistics, 2020. CA: a cancer journal for clinicians. 2020;70:145-64
3. Marventano S, Forjaz M, Grosso G, Mistretta A, Giorgianni G, Platania A. et al. Health related quality of life in colorectal cancer patients: state of the art. BMC Surg. 2013;13(Suppl 2):S15
4. Qian J, Heywood AE, Karki S, Banks E, Macartney K, Chantrill L. et al. Risk of Herpes Zoster Prior to and Following Cancer Diagnosis and Treatment: A Population-Based Prospective Cohort Study. J Infect Dis. 2019;220:3-11
5. Cardoso R, Guo F, Heisser T, De Schutter H, Van Damme N, Nilbert MC. et al. Overall and stage-specific survival of patients with screen-detected colorectal cancer in European countries: A population-based study in 9 countries. Lancet Reg Health Eur. 2022;21:100458
6. Brouwer NPM, Bos A, Lemmens V, Tanis PJ, Hugen N, Nagtegaal ID. et al. An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients. Int J Cancer. 2018;143:2758-66
7. Rasmussen L, Arvin A. Chemotherapy-induced immunosuppression. Environ Health Perspect. 1982;43:21-5
8. Rotstein S, Blomgren H, Petrini B, Wasserman J, Baral E. Long term effects on the immune system following local radiation therapy for breast cancer. I. Cellular composition of the peripheral blood lymphocyte population. Int J Radiat Oncol Biol Phys. 1985;11:921-5
9. Mahale P, Yanik EL, Engels EA. Herpes Zoster and Risk of Cancer in the Elderly U.S. Population. Cancer Epidemiol Biomarkers Prev. 2016;25:28-35
10. Yamamoto M, Mine H, Akazawa K, Maehara Y, Sugimachi K. Gastrointestinal cancer and herpes zoster in adults. Hepatogastroenterology. 2003;50:1043-6
11. Gershon AA, Breuer J, Cohen JI, Cohrs RJ, Gershon MD, Gilden D. et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;1:15016
12. Zhang L, Pan J, Chen W, Jiang J, Huang J. Chronic stress-induced immune dysregulation in cancer: implications for initiation, progression, metastasis, and treatment. Am J Cancer Res. 2020;10:1294-307
13. Chiang A, Salomon N, Gaikwad R, Kirshner J. A case of cutaneous metastasis mimicking herpes zoster rash. IDCases. 2018;12:167-8
14. Swezey E, Blake R, Alamprese S, Luong T, Angus LDG. Rare presentation of the cutaneous metastasis of colon cancer imitating herpes zoster. J Family Med Prim Care. 2023;12:1003-5
15. Johnson RW, Alvarez-Pasquin MJ, Bijl M, Franco E, Gaillat J, Clara JG. et al. Herpes zoster epidemiology, management, and disease and economic burden in Europe: a multidisciplinary perspective. Ther Adv Vaccines. 2015;3:109-20
16. Tran TN, Ray GT, Horberg MA, Yawn BP, Castillo AL, Saddier P. et al. Complications of herpes zoster in cancer patients. Scand J Infect Dis. 2014;46:528-32
17. Yen FS, Wei JC, Yip HT, Hsu CC, Hwu CM. Metformin use and the risks of herpes zoster and postherpetic neuralgia in patients with type 2 diabetes. J Med Virol. 2023;95:e28278
18. Mueller NH, Gilden DH, Cohrs RJ, Mahalingam R, Nagel MA. Varicella zoster virus infection: clinical features, molecular pathogenesis of disease, and latency. Neurol Clin. 2008;26:675-97 viii
19. Schmid DS, Jumaan AO. Impact of varicella vaccine on varicella-zoster virus dynamics. Clin Microbiol Rev. 2010;23:202-17
20. Papagianni M, Metallidis S, Tziomalos K. Herpes Zoster and Diabetes Mellitus: A Review. Diabetes Ther. 2018;9:545-50
21. Han Y, Xie H, Liu Y, Gao P, Yang X, Shen Z. Effect of metformin on all-cause and cardiovascular mortality in patients with coronary artery diseases: a systematic review and an updated meta-analysis. Cardiovasc Diabetol. 2019;18:96
22. Malik F, Mehdi SF, Ali H, Patel P, Basharat A, Kumar A. et al. Is metformin poised for a second career as an antimicrobial? Diabetes Metab Res Rev. 2018;34:e2975
23. Bramante CT, Ingraham NE, Murray TA, Marmor S, Hovertsen S, Gronski J. et al. Metformin and risk of mortality in patients hospitalised with COVID-19: a retrospective cohort analysis. Lancet Healthy Longev. 2021;2:e34-e41
24. Salvatore T, Pafundi PC, Galiero R, Gjeloshi K, Masini F, Acierno C. et al. Metformin: A Potential Therapeutic Tool for Rheumatologists. Pharmaceuticals (Basel). 2020;13:234
25. Schuiveling M, Vazirpanah N, Radstake T, Zimmermann M, Broen JCA. Metformin, A New Era for an Old Drug in the Treatment of Immune Mediated Disease? Curr Drug Targets. 2018;19:945-59
26. Wen CP, Tsai SP, Chung WS. A 10-year experience with universal health insurance in Taiwan: measuring changes in health and health disparity. Ann Intern Med. 2008;148:258-67
27. Sun M, Chen WM, Wu SY, Zhang J. Dementia risk amongst older adults with hip fracture receiving general anaesthesia or regional anaesthesia: a propensity-score-matched population-based cohort study. Br J Anaesth. 2023;130:305-13
28. Sun M, Chen WM, Wu SY, Zhang J. Dementia risk after major elective surgery based on the route of anaesthesia: A propensity score-matched population-based cohort study. EClinicalMedicine. 2023;55:101727
29. Chen YN, Chiang CW, Tsai YH, Chen WM, Chen M, Shia BC. et al. Effect of Preexisting Sarcopenia on Acute and Late Postoperative Pneumonia Among Patients With Oral Cavity Squamous Cell Carcinoma. J Natl Compr Canc Netw. 2022;20:1299-306.e2
30. Chen WM, Chen M, Hsu JG, Lee TS, Shia BC, Wu SY. Use of Preoperative FDG PET/CT and Survival of Patients with Resectable Non-Small Cell Lung Cancer. Radiology. 2022;305:219-27
31. Zhang J, Lu CY, Chen HM, Wu SY. Neoadjuvant Chemotherapy or Endocrine Therapy for Invasive Ductal Carcinoma of the Breast With High Hormone Receptor Positivity and Human Epidermal Growth Factor Receptor 2 Negativity. JAMA Netw Open. 2021;4:e211785
32. Chen WM, Yu YH, Chen M, Shia BC, Wu SY. Statin Use During Concurrent Chemoradiotherapy With Improved Survival Outcomes in Esophageal Squamous Cell Carcinoma: A Propensity Score-Matched Nationwide Cohort Study. J Thorac Oncol. 2023;18:1082-93
33. Chen YC, Li MC, Yu YH, Lin CM, Wu SY. Chronic Obstructive Pulmonary Disease and Its Acute Exacerbation before Colon Adenocarcinoma Treatment Are Associated with Higher Mortality: A Propensity Score-Matched, Nationwide, Population-Based Cohort Study. Cancers (Basel). 2021;13:4728
34. Zhang J, Yen YC, Qin L, Chang CL, Yuan KS, Wu ATH. et al. Outcomes of Adjuvant Oral versus Intravenous Fluoropyrimidines for High-Risk Stage II or Stage III Colon Adenocarcinoma: A Propensity Score-Matched, Nationwide, Population-Based Cohort Study. J Cancer. 2020;11:4157-65
35. Chen CH, Hsieh MC, Lao WT, Lin EK, Lu YJ, Wu SY. Multidisciplinary team intervention associated with improved survival for patients with colorectal adenocarcinoma with liver or lung metastasis. Am J Cancer Res. 2018;8:1887-98
36. oncology NCpgi. NCCN Clinical practice guidelines in oncology: Colon Cancer. In: (NCCN) NCCN, editor. National Comprehensive Cancer Network (NCCN) Version 22023. April 25, 2023 ed. 94 N Woodhull Rd, Huntington, NY 11743: Harborside Press, LLC; 2023. p. Version 2. 2023
37. Willett CG, Goldberg S, Shellito PC, Grossbard M, Clark J, Fung C. et al. Does postoperative irradiation play a role in the adjuvant therapy of stage T4 colon cancer? Cancer J Sci Am. 1999;5:242-7
38. Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundamental & clinical pharmacology. 2005;19:117-25
39. Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550-60
40. Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150-61
41. Kwon DE, Lee HS, Lee KH, La Y, Han SH, Song YG. Incidence of herpes zoster in adult solid organ transplant recipients: A meta-analysis and comprehensive review. Transpl Infect Dis. 2021;23:e13674
42. Anderson TC, Masters NB, Guo A, Shepersky L, Leidner AJ, Lee GM. et al. Use of recombinant zoster vaccine in immunocompromised adults aged≥ 19 years: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. J American Journal of Transplantation. 2022;22:986-90
43. Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity Mortality Weekly Report: Recommendations Reports. 2008;57:1-30
44. Hurley LP, Lindley MC, Harpaz R, Stokley S, Daley MF, Crane LA. et al. Barriers to the use of herpes zoster vaccine. Ann Intern Med. 2010;152:555-60
45. Hechter RC, Tartof SY, Jacobsen SJ, Smith N, Tseng HF. Trends and disparity in zoster vaccine uptake in a managed care population. Vaccine. 2013;31:4564-8
46. Dagnew AF, Ilhan O, Lee WS, Woszczyk D, Kwak JY, Bowcock S. et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect Dis. 2019;19:988-1000
47. Kimberlin DW, Whitley RJ. Varicella-zoster vaccine for the prevention of herpes zoster. The New England journal of medicine. 2007;356:1338-43
48. Vasamsetti SB, Karnewar S, Kanugula AK, Thatipalli AR, Kumar JM, Kotamraju S. Metformin inhibits monocyte-to-macrophage differentiation via AMPK-mediated inhibition of STAT3 activation: potential role in atherosclerosis. Diabetes. 2015;64:2028-41
49. Bharath LP, Agrawal M, McCambridge G, Nicholas DA, Hasturk H, Liu J. et al. Metformin Enhances Autophagy and Normalizes Mitochondrial Function to Alleviate Aging-Associated Inflammation. Cell Metab. 2020;32:44-55 e6
50. Saenwongsa W, Nithichanon A, Chittaganpitch M, Buayai K, Kewcharoenwong C, Thumrongwilainet B. et al. Metformin-induced suppression of IFN-alpha via mTORC1 signalling following seasonal vaccination is associated with impaired antibody responses in type 2 diabetes. Sci Rep. 2020;10:3229
51. Ma R, Yi B, Riker AI, Xi Y. Metformin and cancer immunity. Acta Pharmacol Sin. 2020;41:1403-9
52. Nojima I, Wada J. Metformin and Its Immune-Mediated Effects in Various Diseases. Int J Mol Sci. 2023;24:755
53. Foretz M, Guigas B, Viollet B. Metformin: update on mechanisms of action and repurposing potential. Nat Rev Endocrinol. 2023;19:460-76
54. Mogavero A, Maiorana MV, Zanutto S, Varinelli L, Bozzi F, Belfiore A. et al. Metformin transiently inhibits colorectal cancer cell proliferation as a result of either AMPK activation or increased ROS production. Sci Rep. 2017;7:15992
55. Tarhini Z, Manceur K, Magne J, Mathonnet M, Jost J, Christou N. The effect of metformin on the survival of colorectal cancer patients with type 2 diabetes mellitus. Sci Rep. 2022;12:12374
56. Bai B, Chen H. Metformin: A Novel Weapon Against Inflammation. Front Pharmacol. 2021;12:622262
57. Dekkers IA, van der Molen AJ. Propensity Score Matching as a Substitute for Randomized Controlled Trials on Acute Kidney Injury After Contrast Media Administration: A Systematic Review. AJR Am J Roentgenol. 2018;211:822-6
58. Lin J, Gamalo-Siebers M, Tiwari R. Propensity score matched augmented controls in randomized clinical trials: A case study. Pharm Stat. 2018;17:629-47
59. Dankner R, Agay N, Olmer L, Murad H, Keinan Boker L, Balicer RD. et al. Metformin Treatment and Cancer Risk: Cox Regression Analysis, With Time-Dependent Covariates, of 320,000 Persons With Incident Diabetes Mellitus. Am J Epidemiol. 2019;188:1794-800
60. Austin PC, Lee DS, Fine JP. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation. 2016;133:601-9
61. Daskivich TJ, Fan KH, Koyama T, Albertsen PC, Goodman M, Hamilton AS. et al. Effect of age, tumor risk, and comorbidity on competing risks for survival in a U.S. population-based cohort of men with prostate cancer. Ann Intern Med. 2013;158:709-17
62. Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170:244-56
63. Chen WM, Yu YH, Chen M, Shia BC, Wu SY. Statin Use During Concurrent Chemoradiotherapy with Improved Survival Outcomes in Esophageal Squamous Cell Carcinoma: A Propensity Score Matched Nationwide Cohort Study. J Thorac Oncol. 2023;18:1082-1093
64. Hansson E, Forbes HJ, Langan SM, Smeeth L, Bhaskaran K. Herpes zoster risk after 21 specific cancers: population-based case-control study. Br J Cancer. 2017;116:1643-51
Author contact
![]() Corresponding authors: Szu-Yuan Wu, MD, MPH, PhD & Ben-Chang Shia, PhD, Professor, College of Medical and Health Science, Asia University, Taichung, Taiwan, Director, Big Data Center, Lo-Hsu Medical Foundation, LotungPoh-Ai Hospital, Yilan, Taiwan; Professor, Division of Radiation Oncology, Department of Medicine, Lo-Hsu Medical Foundation, LotungPoh-Ai Hospital, Yilan, Taiwan; Graduate Institute of Business Administration, College of Management, Fu Jen Catholic University, Taipei, Taiwan; Address: No. 83, Nanchang St., Luodong Township, Yilan County 265, Taiwan; Email: szuyuanwu5399com.
Corresponding authors: Szu-Yuan Wu, MD, MPH, PhD & Ben-Chang Shia, PhD, Professor, College of Medical and Health Science, Asia University, Taichung, Taiwan, Director, Big Data Center, Lo-Hsu Medical Foundation, LotungPoh-Ai Hospital, Yilan, Taiwan; Professor, Division of Radiation Oncology, Department of Medicine, Lo-Hsu Medical Foundation, LotungPoh-Ai Hospital, Yilan, Taiwan; Graduate Institute of Business Administration, College of Management, Fu Jen Catholic University, Taipei, Taiwan; Address: No. 83, Nanchang St., Luodong Township, Yilan County 265, Taiwan; Email: szuyuanwu5399com.

 Global reach, higher impact
Global reach, higher impact