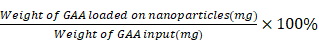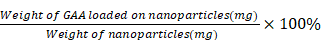Impact Factor
ISSN: 1837-9664
J Cancer 2025; 16(4):1101-1117. doi:10.7150/jca.98452 This issue Cite
Research Paper
LDH Isoenzyme and GAA-BSA Nanoparticles: A Novel Therapy Approach for Proneural Subtype Glioblastoma Multiforme
1. Hematological Disease Institute of Jiangsu University, Affiliated Hospital of Jiangsu University, Zhenjiang, China, 212001.
2. Center of Clinical Laboratory, Dushu Lake Hospital Affiliated to Soochow University, Medical Center of Soochow University, Suzhou Dushu Lake Hospital, Suzhou, Jiangsu, China, 215213.
3. Department of Pharmaceutics, School of Pharmacy, Jiangsu University, Zhenjiang, China.
† Mengting Yang and Xiu Han contributed equally to this work and should be considered co-first authors.
Received 2024-5-14; Accepted 2024-12-16; Published 2025-1-6
Abstract
Glioblastoma multiforme (GBM), whose pathogenesis involves proneural-to-mesenchymal transition (PMT), is the most malignant type of glioma and is associated with a bleak prognosis. Lactate dehydrogenase (LDH) comprises two major subunits, LDHA and LDHB, which can assemble into five different isoenzymes (LDH1-5). However, the role of LDH isoenzyme and its subunits in different GBM subtypes is largely unknown. Our findings reveal that LDHA and LDHB subunits correlated with mesenchymal and proneural subtype classification, and have prognostic and clinical significance in GBM patients. Moreover, it is demonstrated that LDH5, characterized by high LDHA and low LDHB levels, is highly expressed in mesenchymal subtype GBM cells and promotes proliferation, migration, and PMT. Conversely, proneural subtype GBM cells exhibited LDH1 dominance, and low LDHA and high LDHB levels. Notably, LDH1 played a pivotal role in the proliferation, migration, and PMT of proneural glioma cells. For treatment of proneural subtype GBM, gossypol-acetic acid (GAA)-bovine serum albumin (BSA) nanoparticles (GAA-BSA NPs) were developed to ameliorate PMT by targeting LDH1. These nanoparticles effectively suppress proneural subtype tumor growth both in vitro and in vivo, surpassing their efficacy against the mesenchymal subtype. The results offer several novel insights into the role of LDH isoenzyme in subtype classification between mesenchymal and proneural GBM and provide a promising therapeutic approach for proneural subtype GBM.
Keywords: glioblastoma multiform, lactate dehydrogenase, subtype classification, proneural-to-mesenchymal transition, nanoparticles
Introduction
Glioblastoma multiforme (GBM), classified as a World Health Organization grade IV tumor, is extremely malignant and has a high prevalence rate, with a grim clinical prognosis among adults [1]. Despite the availability of comprehensive treatment strategies encompassing surgical intervention, ionizing radiation, and chemotherapy with the alkylating agent temozolomide, the median survival of patients with GBM following diagnosis rarely exceeds 1-year [2, 3]. Distinguished by their gene expression profiles, GBMs can be categorized into four distinct subtypes, namely, classical, proneural, neural, and mesenchymal [4, 5]. During the progression of GBM, the mesenchymal subtype originates from the proneural subtype through a process known as proneural-to-mesenchymal transition (PMT) [6]. This transition is associated with tumor microenvironment, metabolic molecular and treatment-related factors to tumor recurrent and therapy [7].
Lactate dehydrogenase (LDH) is the core enzyme involved in catalyzing the formation of lactate through glycolysis; this process, known as the Warburg effect, is a hallmark of metabolic alteration in cancer cells [8]. The LDH isoenzyme is composed of two subunits (LDHA and LDHB), forming a tetramer structure that produces five key isoforms, namely, LDH1(4B), LDH2(3B1A), LDH3(2B2A), LDH4(3B1A), and LDH5(4A) [9, 10]. LDH isoenzyme shows variations in distribution among various tissues in the human body and between malignant tissue samples, indicating it can serve as a vital molecular marker for cancer diagnosis [11]. Although the effects of LDH isoenzyme on GBM growth and invasiveness have been studied [12], its role and potential as a therapeutic target in PMT warrants further investigation.
Although many effective LDHA/B inhibitors that target LDH isoenzyme have been developed by pharmaceutical companies and academic institutions, these inhibitors have not been applied in the clinic for various reasons such as poor pharmacokinetic properties and low in vivo efficacy [13, 14]. Gossypol-acetic acid (GAA), a dehydrogenase inhibitor, has demonstrated the capacity to interfere with mitochondrial metabolism, induce autophagic cell death, and promote tumor cell apoptosis [15]. However, clinical studies in patients with glioma have shown a low response rate to treatment with GAA [16], although the reasons responsible for this outcome remain unclear. Unique tumor microenvironment characterized by hypoxia and high ferric ions (Fe3+) levels features often encountered in gliomas, and iron chelators can change the metabolism of tumor cells by reducing the intake of Fe3+ [17, 18]. Studies have shown that GAA tends to bind with iron in hemoglobin to form gossypol-iron complexes, which in turn inhibit iron absorption[19]. Within this context, we hypothesized that tumor microenvironment's high Fe3+ levels may influence the efficacy of GAA in tumor treatment. Owing to its exceptional biocompatibility and biodegradability, bovine serum albumin (BSA) is commonly utilized as a drug carrier [20]. Additionally, BSA nanoparticles (NPs) are soluble in water, stable, and capable of gradually releasing hydrophobic drugs [21]. These attributes render them promising tools for the treatment of GBM [22].
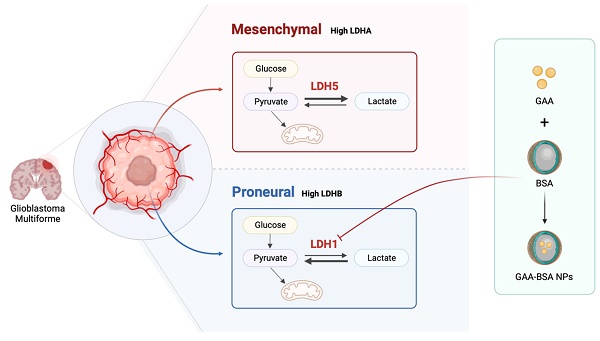
In the present study, we investigated the different roles of the LDH isoenzyme (LDH1 and LDH5) within the proneural and mesenchymal GBM subtypes, respectively, and examined their key roles in the PMT process. Furthermore, we developed a strategy targeting LDH1 through encapsulating GAA with BSA to generate NPs that could be used in the treatment of proneural subtype GBM. Our results offer novel insights into the role of LDH isoenzyme in subtype classification between mesenchymal and proneural GBM and provide a promising treatment approach for proneural subtype GBM.
Materials and methods
Data source
RNA-seq transcriptome data and clinical data (survival time and survival status) of patients with glioblastoma were collected from The Cancer Genome Atlas (TCGA) database (http://cancergenome.nih.gov/) and The Chinese Glioma Genome Atlas (CGGA) database (http://www.cgga.org.cn/). Verhaak glioblastoma mesenchymal and proneural gene signatures were obtained from the Molecular Signatures Database (https://www.gsea-msigdb.org/gsea/index.jsp). The TCGA data set downloaded was consisting of 599 cases, with 369 males and 230 females. Among these, primary clinical data (including age, pathology, and survival) is available for 596 cases, while secondary clinical data (including treatment information) is available for 550 cases. The data in the TCGA database mainly comprises primary GBM cases, where primary GBM patients have no antecedent history of lower-grade disease, whereas secondary GBM arises from previously diagnosed lower-grade gliomas[23]. The samples of Verhaak database were collected and processed through the TCGA Biospecimens Core Resource, 200 GBMs and 2 normal samples were selected within specific standards[24]. All the subjects of CGGA were consistently diagnosed with glioma and were then further classified according to the 2007/2016 WHO classification system, clinical data, including basic clinical information, survival, and therapy information were available[25].
Survival analysis
Kaplan-Meier plots were used to assess the relationship between LDHA and LDHB expression and prognosis (OS) of cancers. Proportional-hazards hypothesis testing and fitted survival regression were performed with the survival package (version 3.3.1), and the results were visualized with the survminer package and the ggplot2 (version 3.3.6) package. The Log-rank test was used in the hypothesis test, and p < 0.05 is considered statistically significant.
Clinical significance and prognosis analysis
Forest map was used for further clinical significance analysis of LDH in GBM. The survival package (version 3.3.1) was used for proportional hazards hypothesis testing and Cox regression analysis. The survival package was used for proportional hazards hypothesis testing and Cox regression analysis. Forest map visualization was performed using ggplot2 (version 3.3.6).
Cell lines and cell culture
The human glioma cell lines SW1783, LN229, U87MG, and U251MG were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were cultured at 37°C under normoxic conditions (21% O2 and 5% CO2) or hypoxic conditions (1% O2, 5% CO2, and 94% N2), and maintained in Dulbecco's modified Eagle's medium (DMEM; Meilun, Dalian, China) supplemented with 10% fetal bovine serum (FBS; Gibco, Carlsbad, CA, USA) for up to 3 months after resuscitation from frozen stocks, with fewer than 20 passages. Short tandem repeat profiling was used to authenticate the cell lines.
Plasmid construction, transfection
The short hairpin sh-EGFP, sh-LDHA, and sh-LDHB plasmids were previously constructed in our laboratory. The p3×FLAG Myc-CMV™14 expression vector and pLKO.1-puro or pLKOTet-ON-puro vectors were purchased from Sigma-Aldrich (San Francisco, CA, USA). Cells were seeded in six-well plates to reach 70-90% confluency before transfection. The next day, transfection was performed according to the instructions provided by the manufacturer, utilizing Lipofectamine® 2000 transfection reagent (Invitrogen, Carlsbad, CA, USA). Following an additional 48 h of incubation, the cells were analyzed using various techniques described below. Each test was repeated in triplicate.
RNA extraction, reverse transcription, and quantitative real-time PCR
Total RNA was extracted using RNAiso Plus (Vazyme, Nanjing, China). Reverse transcription was performed using a RevertAid First Strand cDNA Synthesis Kit (Vazyme, Nanjing, China) according to the instructions provided by the manufacturer. Quantitative real-time PCR was performed in triplicate in 20 μL reactions with a SYBR GREEN PCR Kit (Vazyme, Nanjing, China). The 2-ΔΔCT method, with β-actin serving as an endogenous control, was used to calculate the relative mRNA expression. The sequences of primers used are provided in Table 1. All experiments were conducted in biological triplicates.
Western blotting analysis
Before treatment with radioimmunoprecipitation assay lysis buffer at 4°C for 10 min, the cultured cells were rinsed with cold phosphate-buffered saline (PBS). The mixture was then heated at 100°C for 10 min and centrifuged at 4°C at 12,000 × g min-1 for 10 min. The obtained total protein was subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (~20 μg of protein per lane). Subsequently, the proteins were transferred onto a polyvinylidene difluoride membrane, which was blocked with 5% BSA for 1 h at room temperature. Thereafter, the membrane was incubated with primary antibodies overnight at 4°C, followed by incubation at 1 h at room temperature with secondary antibodies. The following primary antibodies were used at dilutions recommended by the manufacturers: anti-LDHA (sc-137243; SCBT, Santa Cruz, CA, USA), anti-LDHB (sc-100775; SCBT, Santa Cruz, CA, USA), anti-oligodendrocyte transcription factor 2 (anti-OLIG2; sc-293163; SCBT, Santa Cruz, CA, USA), anti-vimentin (anti-VIM; 5741; Cell Signaling Technology, Danvers, MA, USA), and anti-β-Tubulin (MA5-11732; Thermo Fisher Scientific, MA, USA). Goat anti-rabbit (sc-2004; SCBT, Santa Cruz, CA, USA) and goat anti-mouse secondary antibodies (sc-2005; SCBT, Santa Cruz, CA, USA) conjugated with horseradish peroxidase were used for detection. Visualization was performed using an enhanced chemiluminescence substrate. β-Tubulin served as a loading control. All experiments were conducted in biological triplicates. Protein expression was quantitatively analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Sequences of primers used in qRT-PCR
| Forward sequences | Reverse sequences | |
|---|---|---|
| LDHA | 5′-CCAACATGGCAGCCTTTTCC-3′ | 5′-TCACGTTACGCTGGACCAAA-3′ |
| LDHB | 5′-TGACTTTGTCTTCTCCGCACG -3′ | 5′-TCAGCCAGAGACTTTCCCAGA-3′ |
| β-actin | 5′-CACCATTGGCAATGAGCGGTTC-3′ | 5′-AGGTCTTTGCGGATGTCCACGT-3′ |
Native gel electrophoresis for the separation of LDH isoenzymes
Separation of LDH isoenzymes through native gel electrophoresis was conducted using precast 6.5% polyacrylamide slab gels. Before treatment with radioimmunoprecipitation assay lysis buffer at 4°C for 30 min, cultured cells and mouse brain tissues were rinsed with cold PBS. The sample wells were rinsed with running buffer, and the gels were positioned in the electrophoretic unit. Subsequently, the gel was placed in a developing chamber containing the developer solution (H2O 18.4 mL, 1 M Tris 4 mL, tetrazolium-blue 12 mL, phenazine methosulphate 4 mL, Na-lactate 4 mL, and nicotinamide adenine dinucleotide [NAD] 1.3 mL) followed by incubation at 37°C for 30 min to initiate a color reaction. Finally, the gel was rinsed with water.
Cell Counting Kit-8 (CCK-8) assay
The measurement of viable cell mass was performed using a CCK-8 (Vazyme) according to the instructions provided by the manufacturer. Briefly, cells were seeded in a 96-well plate (1×103 cells/well) and incubated at 37°C with 5% CO2. On days 1-6, CCK-8 solution (10 μL) was added to each well, and cells were incubated at 37°C for 2 h. Next, the absorbance was determined at 450 nm wavelength. Cytotoxicity was evaluated by CCK-8 assay according to the instructions provided by the manufacturer. Briefly, cells were seeded in 96-well flat-bottomed plates (5×103 cells/well) and subjected to the following treatments: 1) 50 μmol/L 0.2 M in 2-methyl tetrahydrofuran (Fecl3; 710857; Sigma-Aldrich), 2) different concentrations of GAA (A506218-0001; Sangon Biotech, Shanghai, China), and 3) different concentrations of GAA-BSA NPs.
Colony formation assay
Transfected glioma cells were harvested and resuspended in medium. Next, cells were transferred to a six-well plate (500, 1×103, and 2×103 cells per well) for 10-14 days until large colonies were visible. Colonies were fixed and stained with 0.05% crystal violet for 30 min. The number of colonies was counted.
Transwell migration assays
Transfected cells were collected, resuspended in serum-free medium, and transferred to Transwell upper chamber with permeable inserts featuring membranes with 8 μm pores (1×104 cells per well). The lower chamber was filled with culture medium with 10% FBS for 10 h. The cells on the upper chamber were scraped and washed with PBS, and stained with 4% paraformaldehyde for 30 min. Finally, cells were counted in five independent fields and the average number of cells per field was used to produce the graphs. Each test was repeated in triplicate.
Tissue microarray immunohistochemistry
Tissue sections were air-dried, deparaffinized, and rehydrated. Endogenous peroxidase activity was blocked with H2O2 (3%) for 10 min. Antigen retrieval was performed with citrate buffer in a pressure cooker for 10 min. A 5% serum-free protein block was applied for 20 min to block non-specific antibody binding. Subsequently, slides were incubated overnight at 4°C with the primary antibody anti-LDHA (sc-137243; SCBT, Santa Cruz, CA, USA), anti-LDHB (sc-100775; SCBT, Santa Cruz, CA, USA), anti-YKL-40 (BS6564; Bioworld Technology, Bloomington, USA) and anti-vimentin (anti-VIM; 5741; Cell Signaling Technology, Danvers, MA, USA), followed by incubation at room temperature for 30 min with a species-specific secondary antibody (SA1020; BOSTER, Wuhan, China). For negative controls, the primary antibody was omitted and replaced with negative immunoglobulin G. The reaction was developed using 3,3'-diaminobenzidine (AR1022; BOSTER, Wuhan, China), and slides were counterstained with hematoxylin. Immunostaining intensity and reactivity were assessed using CaseViewer after scanning, employing a digital microscope application at ×40 magnification.
The tissue microarray was procured from Easy Biotrade (Nanjing, China). Human glioma samples employed in this study were validated by a pathologist based on the World Health Organization criteria. This tissue microarray consists of 60 clinical samples, all of which were glioblastoma (Grade IV glioma) cases. Among these, 39 were male and 21 were female, with an age range of 20 to 78 years. A heatmap was generated from these data, and conditional formatting in MS Excel (Microsoft Corp., Redmond, WA, USA) was used to produce colored heatmaps.
Molecular docking and site prediction
The three-dimensional structure of LDH1 was evaluated according to the probability density function or Discrete Optimized Protein Energy fraction of the statistical potential energy of atoms. Based on the optimal three-dimensional structure of LDH1, Glide software for molecular docking was used to screen small-molecule compounds from databases (such as ChemDiv, ChemBridge, and SPECS) with different precision scoring functions (high-throughput virtual screening, standard precision, and extra precision) and analyze the binding sites.
Synthesis of GAA-BSA NPs
GAA-BSA NPs were synthesized using the previously reported desolvation method [26]. Briefly, 30 mg of BSA (4240GR100; BioFroxx Hangzhou, China) in 1 mL of pure water was adjusted to pH 8.2 using sodium hydroxide (NaOH; DWH750; Hushi, Shanghai, China). Next, we dissolved 1 mg of GAA (A506218-0001; Sangon Biotech) in 3 mL of ethanol and added it dropwise to the BSA solution at a rate of 1 mL min-1 while stirring using a magnetic stirrer at 700 rpm and a temperature of 37°C. The NPs were formed after stirring for 12 h, during which 8% glutaraldehyde solution was added for cross-linking. The NP suspension was purified by ultracentrifugation at 12,000 rpm for 30 min, and subsequently redispersed in pure water under sonication. Finally, GAA-BSA NPs were obtained by freeze-drying.
Size distribution, polydispersity, and zeta potential measurement
The particle size, polydispersity index, and zeta potential were determined at 25°C using a Zetasizer (Nano ZS; Malvern, UK). All measurements were performed in triplicate.
Morphological analysis
For morphological study, the GAA-BSA NP powder was evenly scattered on the sample table with a toothpick and sprayed with gold. Subsequently, the NPs were observed using a scanning electron microscope (Scio2 HiVac; FEI, USA). To further investigate the internal structure of GAA-BSA NPs, the suspension was dropped on a copper grating and the samples were observed using a transmission electron microscope (Tecnai 12; FEI).
Determination of drug-loading efficiency (DLE) and drug-loading capacity (DLC)
To calculate the DLE and DLC, 1.003 mg of GAA-BSA NP powder was precisely weighed, dissolved in 1 mL of pure water, and made up to a volume of 10 mL with methanol. After stirring and centrifugation, the content of GAA in the supernatant was measured using high-performance liquid chromatography. The analysis was performed on a VP-ODS C18 column (250 mm×4.6 mm×5 µm; SHIMADZU, Tokyo, Japan) at 35°C. The mobile phase was a mixture of potassium dihydrogen phosphate and acetonitrile in a volume ratio of 50:50 (volume/volume). The detection wavelength was set at 247 nm. DLE and DLC were calculated using the following equations:
DLE =
DLC =
In vitro drug release tests
In vitro release of GAA-BSA NPs was studied using a dynamic dialysis method. Specifically, GAA-BSA NPs (containing 0.2 mg of GAA) and 0.2 mg GAA were dissolved in 1 mL of PBS (with 3% dimethyl sulfoxide) and transferred to dialysis bags with a molecular weight cut-off of 3,500. Next, the bags were submerged in 50 mL of 0.1 M PBS (pH 7.4) and maintained at 37°C with shaking at a speed of 120 rpm. At predetermined time intervals (from 30 min to 96 h), 1 mL of the medium was withdrawn and replenished with an equal volume of fresh medium. The samples were diluted with methanol and subjected to high-performance liquid chromatography analysis after centrifugation. Each measurement was performed in triplicate. Animal studies were approved by the Ethics Committee of Jiangsu University (approval number: 2021102901) and complied with the ARRIVE guidelines and the National Research Council's Guide for the Care and Use of Laboratory Animals.
Cellular binding and internalization
The GAA-BSA NPs were labelled with fluorescein isothiocyanate (FITC; F7250; Merck, Darmstadt, Germany; excitation wavelength: 488 nm, emission wavelength: 517 nm) (GAA-BSA-FITC NPs). U87MG and U251MG cells (0.5 mL, 1 × 105 cells mL-1) were seeded in a confocal dish and incubated in 1 mL of DMEM containing 10% FBS for 12 h. Thereafter, 100 μg mL-1 of GAA-BSA-FITC NPs were added into the culture medium and co-cultured with cells at 37°C for 4 h. Following incubation, the cells were fixed with 4% paraformaldehyde for 15 min. Thereafter, the cells were washed three times with PBS and stained with 4,6-diamidino-2-phenylindole (DAPI; 10236276001; Merck) at room temperature. The binding and internalization behavior of the nanoplatforms within the cells were observed using a confocal microscope (LSM710; Zeiss, Oberkochen, Germany).
Tumor-bearing mouse model
Nude mice (males, 4-6 weeks old) were purchased from CAVENS company (Changzhou, China). Mice were housed under pathogen-free conditions with free access to food and water, at the Animal Center of Jiangsu University (Zhenjiang, China). Animal studies were approved by the Ethics Committee of Jiangsu University (approval number: 2021102901) and complied with the ARRIVE guidelines and National Research Council's Guide for the Care and Use of Laboratory Animals. The mice received an injection of 0.1 mL of U87MG cells (2×105) transfected with sh-EGFP, sh-LDHA, and sh-LDHB plasmids. In addition, the mice received an injection of 0.1 mL of U251MG cells (8×105) transfected with sh-EGFP, sh-LDHA, and sh-LDHB plasmids. Furthermore, the other U87MG- and U251MG-bearing mouse models were established by subcutaneously injecting 0.1 mL of U87MG cells (2×105) and U251MG cells (8×105), respectively, into the BALB/c nude mice. When the tumor volume reached approximately 80 mm3, the tumor-bearing mice were randomized into four groups (n=6 per group). Mice in different groups were administered intraperitoneal injections once every 3 days, for six times in total, as follows: 1) PBS; 2) GAA, 15 mg/kg; 3) GAA-BSA NPs at 15 mg/kg GAA dose; and 4) LDHA inhibitor FX11 (2.5 mg/kg, HY16214; MedChemExpress, Shanghai, China). Tumor size was monitored throughout the treatment period.
Statistical analysis
All statistical analyses were conducted using GraphPad Prism 9 (GraphPad Software Inc., San Diego, CA, USA). Student's t-test was used for comparisons between two groups, and one-way ANOVA analysis of variance was utilized for comparisons involving more than two groups. Data are presented as the mean ± standard deviation of three independent experiments. P-values of <0.05 indicate statistically significant differences (*P value < 0.05; **P value < 0.01; ***P value < 0.001).
Results
LDH subunits are correlated with mesenchymal and proneural subtype classification and have clinical significance in GBM patients
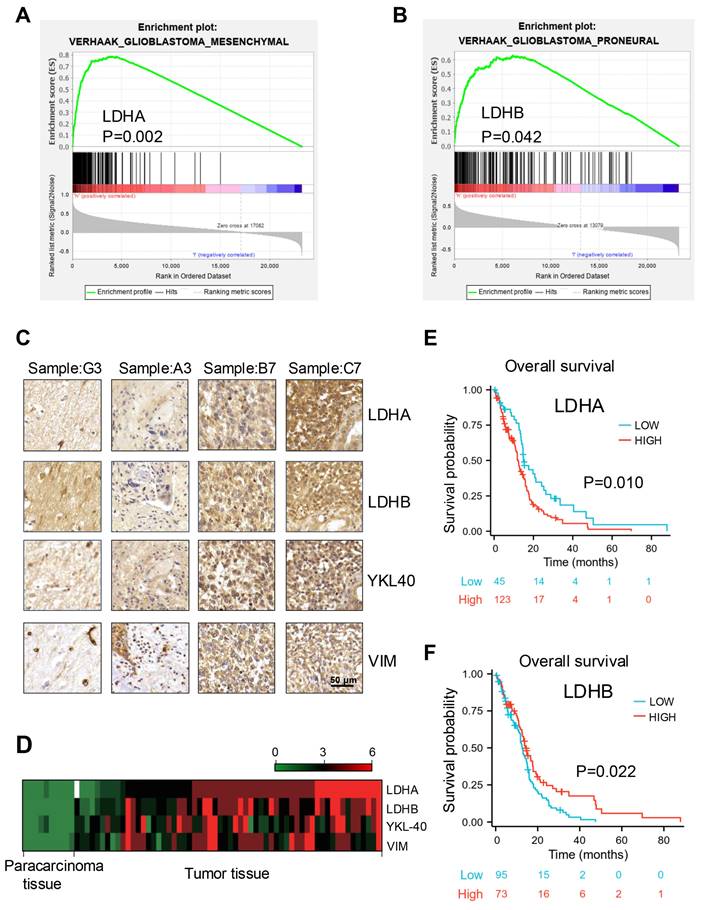
Initially, the relationship between LDH subunits and mesenchymal and proneural molecular subtypes was identified through gene set enrichment analysis (GSEA) using Verhaak gene datasets. The results demonstrated that elevated levels of LDHA were predominantly observed in the mesenchymal subtype, whereas reduced LDHA expression was more frequently associated with the proneural subtype. (Fig. 1A and S1A). Conversely, the expression levels of LDHB exhibited an opposite pattern. (Fig. 1B and S1B). Further, a positive correlation was detected between the expression of mesenchymal markers (YKL-40 and VIM) and LDHA levels by immunohistochemical analysis (Fig. 1C). These findings were further validated by heatmap analysis (Fig. 1D). In addition, we investigated the impact of LDHA and LDHB on GBM prognosis through Kaplan-Meier survival analysis using The Cancer Genome Atlas (TCGA) mRNA database. The results indicated that patients exhibiting high LDHA expression experienced shorter survival periods, while elevated LDHB expression appears to be associated with prolonged survival periods (Fig. 1E and F). After a comprehensive evaluation of histology, grade, gender, and age, univariate and multivariate Cox regression analyses were employed using the Chinese Glioma Genome Atlas (CGGA) database to identify prognostic factors and examine their clinical relevance. (Fig. S1 C-F). These studies identified LDHA and LDHB as potential independent overall survival factors, indicating their potential utility in constructing prognostic calibration curves for predicting GBM outcomes and underscoring their significant clinical relevance.
LDH isoenzymes composed of different subunits determine the classification of glioma cells
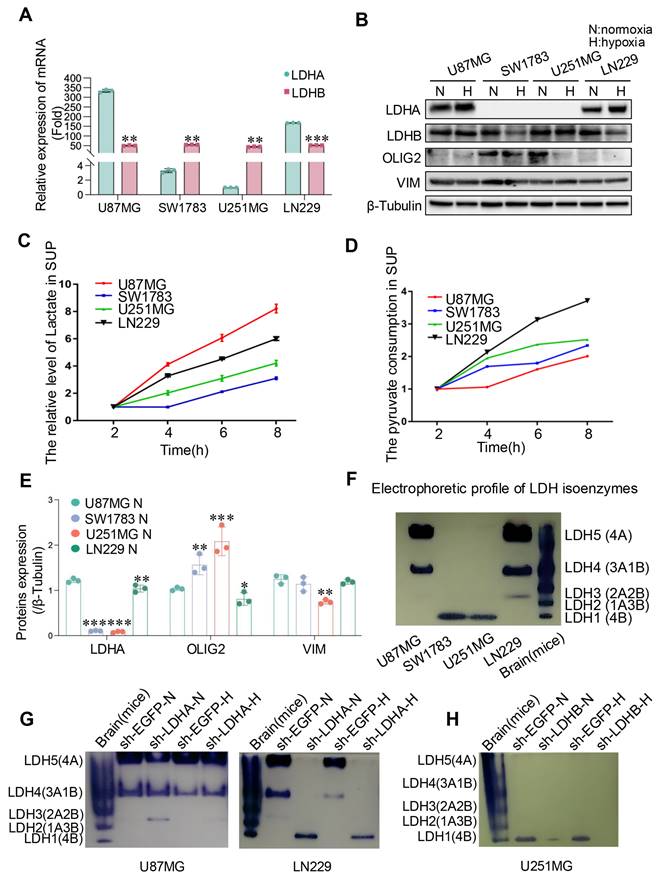
To validate our database-derived analyses, we conducted in vitro experiments across four glioma cell lines. Initially, LDHA and LDHB expression levels were tested. Results indicated a marked upregulation of LDHA at both mRNA and protein levels in LN229 and U87MG cells, whereas LDHB was significantly expressed in U251MG and SW1783 cells at both mRNA and protein levels (Fig. 2A and B), highlighting differential LDH subunit expression across these glioma cell lines.
Considering the pivotal role of LDH in lactate production, we quantified lactate concentrations in the cell culture supernatants and assessed the dynamics of pyruvate consumption. Observations revealed that U87MG and LN229 glioma cells exhibited higher lactate production rates compared to SW1783 and U251MG cells. Notably, U87MG cells produced substantial amounts of lactate while consuming relatively low levels of pyruvate, suggesting an enhanced pyruvate utilization rate (Fig. 2C and D), which may due to variations in their metabolic pathways and enzyme activity[27-29]. To further elucidate the association between LDH subunits and glioblastoma (GBM) phenotypes, we analyzed the expression of OLIG2 (a marker for the proneural phenotype) and VIM (a marker for the mesenchymal phenotype). Consistent with previously observed patterns in GBM specimens, OLIG2 expression was elevated in SW1783 and U251MG cells, while U87MG and LN229 cells exhibited characteristics associated with mesenchymal glioma cells (Fig. 2B and E).
Expression profiles of LDH subunits and their prognostic significance in GBM. (A, B) GSEA based on the Verhaak gene dataset indicated that LDHA and LDHB expression was positively correlated with the mesenchymal (A) and proneural (B) subtype, respectively (P=0.002 and 0.042, respectively). (C) Immunohistochemical detection of protein expression levels of LDHA, LDHB, YKL-40, and VIM in human GBM samples. Scale bar: 50 μm. (D) Heatmap analysis presenting the expression of LDHA, LDHB, YKL-40, and VIM in human GBM samples, based on the results obtained from immunohistochemistry. (E, F) Association of LDHA (E) and LDHB (F) expression with overall survival analysis of patients with GBM (P=0.010 and 0.022, respectively). P-values were determined by one-way ANOVA.
Additionally, native electrophoresis corroborated these findings, revealing that distinct LDH isoenzyme expression profiles were present across the cell lines due to variations in LDH subunit expression. Specifically, LDH5 (4A) was the predominant isoform in LN229 and U87MG cells, whereas LDH1 (4B) was the main isoform in U251MG and SW1783 cells (Fig. 2F). To confirm that the observed isoenzyme patterns were attributable to differential LDH subunit expression, we selectively suppressed LDHA and LDHB using shRNA constructs (sh-LDHA and sh-LDHB, respectively). Knockdown of LDHA and LDHB led to significant reductions in their respective mRNA levels in U87MG, LN229, and U251MG cells relative to control cells (sh-EGFP) (Fig. S2A-C). Furthermore, native electrophoresis demonstrated that LDHA knockdown resulted in diminished LDH5 expression in U87MG and LN229 cells, while LDHB knockdown had minimal impact (Fig. 2G and S3A). Conversely, LDHB inhibition led to decreased LDH1 expression in U251MG cells, whereas LDHA knockdown did not alter the LDH isoenzyme profile (Fig. 2H and S3B). Collectively, these findings indicate that the differential expression of LDH subunits contributes to distinct LDH isoenzyme compositions, which may play a role in defining glioma cell phenotypes.
The expression of LDH isoenzymes in human glioma cells. (A, B) The mRNA (A) and protein (B) expression levels of LDHA and LDHB in human glioma cells were quantified through qRT-PCR and western blotting, respectively. N, normoxic condition; H, hypoxic condition. (C, D) Lactate and pyruvate levels were measured in four human glioma cell lines through spectrophotometry. (E) Quantitative analysis of protein expression levels in (B) was conducted using ImageJ software. (F, G, H) LDH isoenzyme of glioma cells were analyzed using the native gel electrophoresis method, illustrating the normal pattern (F), alterations resulting from LDHA or LDHB knockdown in U87MG, LN229 (G), and U251MG (H) cells. Statistical analysis was conducted using one-way ANOVA; *P<0.05, **P<0.01, and ***P<0.001.
LDH isoenzymes promoted the proliferation and migration of glioma cells and in vivo tumor growth
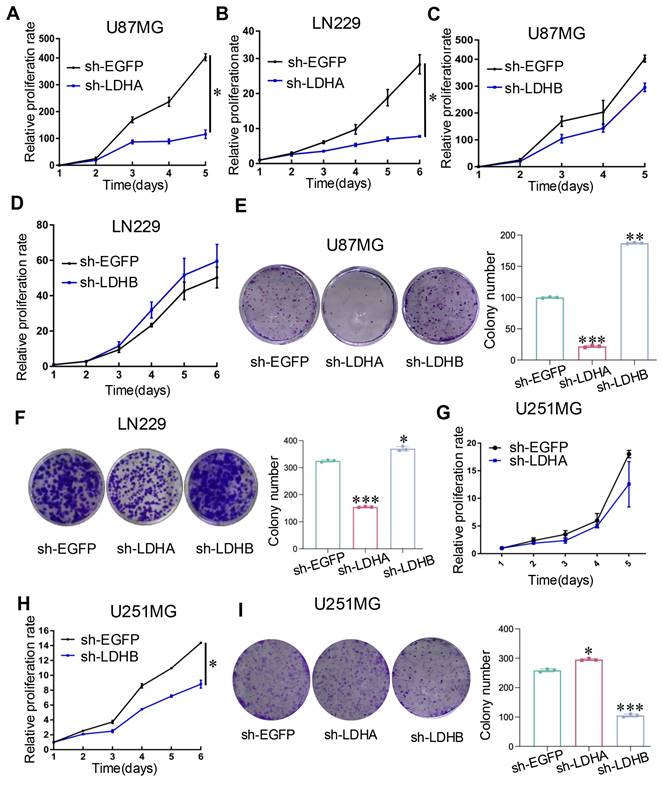
Downregulating the expression of LDHA inhibited the relative proliferation rate of U87MG and LN229 cells in comparison with that in the control group (Fig. 3A and B). Although different outcomes in cell proliferation rates were observed in U87MG and LN229 cells when LDHB was downregulated, there was no statistically significant difference (Fig. 3C and D). The results were further confirmed by colony formation assays (Fig. 3E and F). In contrast, an inverse trend was noted in U251MG cells: LDHB downregulation led to a significant decrease in relative proliferation rate and colony numbers, whereas LDHA downregulation had the opposite effect (Fig. 3G-I).
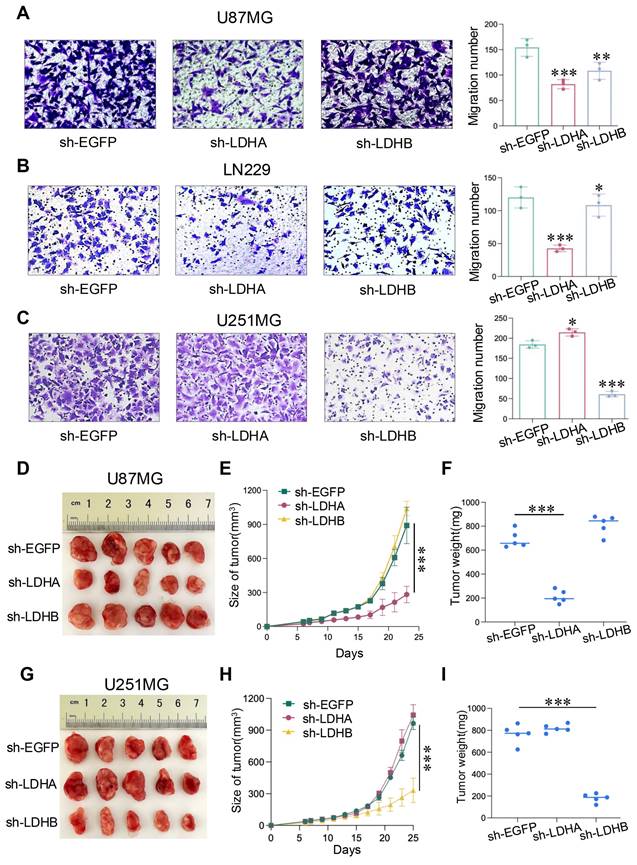
We further investigated the influence of LDH isoenzymes on glioma cell migration. Notably, LDHA knockdown led to a reduction in the number of migrating U87MG and LN229 cells, as evidenced by the Transwell assay, whereas LDHB knockdown exerted the opposite effect (Fig. 4A and B). Conversely, in U251MG cells, sh-LDHB resulted in reduced migratory ability, whereas the number of migrated cells increased in the sh-LDHA group (Fig. 4C). These findings underscore the distinct and critical roles played by LDHA and LDHB in these two glioma subtypes. In summary, LDH5 promotes the proliferation and migration of mesenchymal glioma cells, whereas LDH1 maintains these processes in proneural glioma cells. Thus, LDH isoenzyme differentially regulates the proliferation and migration of mesenchymal and proneural glioma cells.
In vivo studies were conducted by injecting U87MG and U251MG cells into the BALB/c nude mice. Intriguingly, in U87MG group, LDHA knockdown led to decreased tumor growth, whereas LDHB knockdown exerted the opposite effect (Fig. 4D). These results were also observed in terms of tumor volume and weight (Fig. 4E and F). Conversely, in the U251MG group, LDHB knockdown significantly decreased the tumor size, volume, and weight, whereas LDHA knockdown exerted the opposite effect (Fig. 4G-I). Collectively, we found that LDH5 and LDH1 play pivotal roles in the proliferation and migration of glioma cells as well as tumor growth in mesenchymal and proneural subtypes of GBM.
LDH isoenzymes promoted proneural-to-mesenchymal transition (PMT) of glioma cells
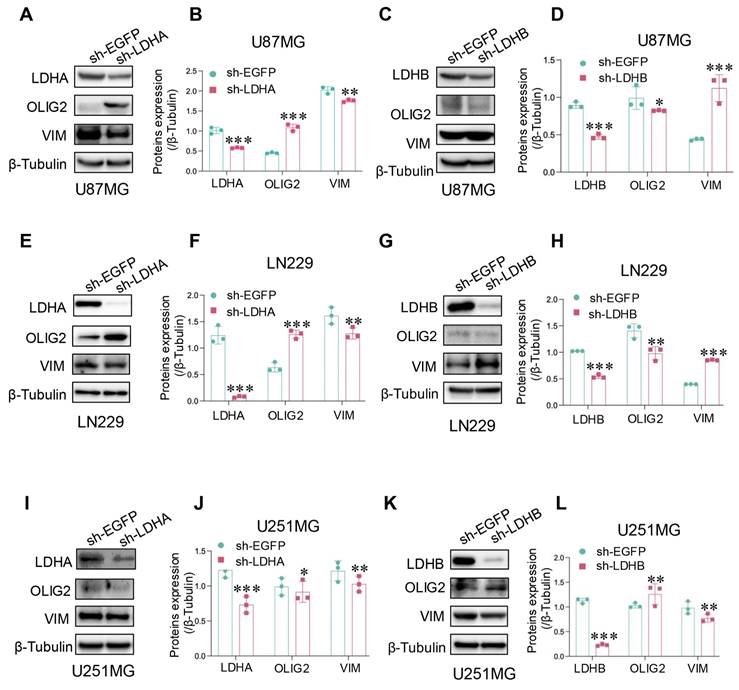
To further investigate the relationship between LDH isoenzymes and PMT in glioma, we analyzed protein expression levels of VIM (mesenchymal marker) and OLIG2 (proneural marker) through western blotting. The results confirmed that LDHA knockdown downregulated mesenchymal marker expression in U87MG cells, while proneural marker expression was upregulated (Fig. 5A and B). However, LDHB knockdown triggered the downregulation of the proneural marker and upregulation of mesenchymal markers (Fig. 5C and D). The corresponding findings in LN229 cells were consistent with these results (Fig. 5E-H), suggesting that LDH5 promotes PMT in mesenchymal glioma cells. Conversely, in U251MG cells, LDHA shRNA had a limited impact on mesenchymal transition, while treatment with LDHB shRNA decreased the expression of mesenchymal markers and increased that of proneural markers (Fig. 5I-L). Thus, LDH1 emerges as a pivotal factor driving PMT in proneural glioma cells. Given that the proneural subtype exhibits a higher propensity for transition into the mesenchymal subtype, it is imperative to intervene at the root of the process by targeting LDH1 in proneural subtype GBM.
Synthesis of GAA-BSA nanoparticles for LDH1 treatment in proneural subtype GBM
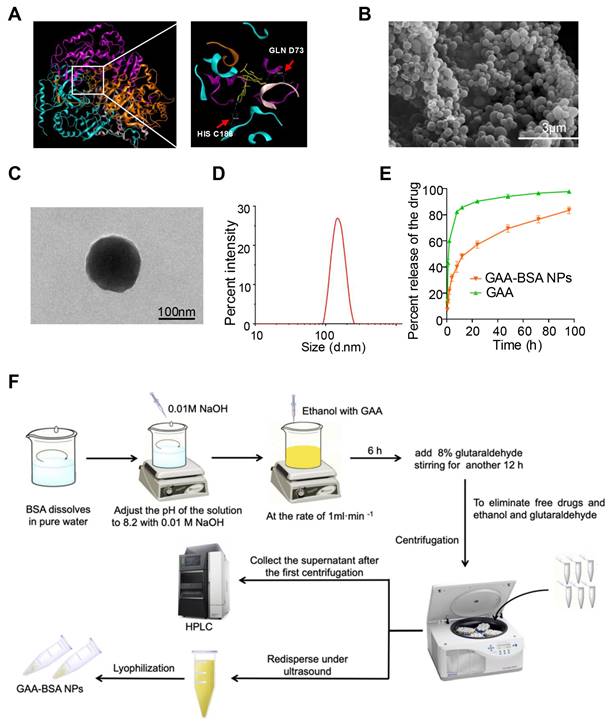
Initially, a computational simulation was conducted to screen 10 drugs, revealing that gossypol-acetic acid (GAA) emerged as the most promising candidate for binding to the LDH1 protein. However, GAA exhibits poor water solubility and is prone to degradation in the tumor microenvironment [30]. Bovine serum albumin (BSA), known for its exceptional biocompatibility and biodegradability, is a commonly utilized drug carrier [31]. Notably, BSA nanoparticles are water-soluble, stable, and capable of slowly releasing hydrophobic drugs [32]. Therefore, to mitigate the impact of the tumor microenvironment, we devised a strategy to encapsulate GAA within BSA protein nanoparticles (NPs), resulting in a novel formulation termed GAA-BSA nanoparticles. The binding sites of GAA on the LDH1 protein were simulated, revealing GLN D73 and HIS C186 as key interaction points (Fig. 6A). These results underscored the potential ability of GAA to target LDH1 protein. Subsequently, GAA-BSA NPs were synthesized and as shown in Fig. 6B and C, scanning electron microscopy (SEM) and transmission electron microscopy (TEM) imaging showed that GAA-BSA NPs were primarily homogenous in size and have a dense core. The average size of the NPs was 147.25±1.56 nm and the zeta potential was -28.87±0.52 mV (Fig. 6D; Table 2), which may prolonged the circulation time in the blood by increasing the blood-brain barrier permeability, targeting the clathrin-mediated endocytic pathway or evading phagocytosis by immune cells [33-36]. Moreover, the NPs displayed a drug-loading efficiency (DLE) of 70.55±1.08%, with a drug-loading capacity (DLC) of 3.10±0.05% (Table 2), which is equivalent to 31.0 μg of GAA per mg of NPs. Drug release from the nanoparticles exhibited a biphasic pattern at pH 7.4, with an initial burst release of 31.78±2.17% within the first 4 h, followed by a gradual release of 83.36±2.0% over the subsequent 90 h (Fig. 6E). Figure 6F depicts a schematic of the preparation of GAA-BSA NPs using the desolvation method.
LDH isoenzymes promoted the proliferation of human glioma cells. (A, B) CCK-8 assay revealed the impact of LDHA knockdown relative to the control in U87MG cells (A) and LN229 cells (B). (C, D) CCK-8 assay revealed the impact of LDHB knockdown in U87MG cells (C) and LN229 cells (D). (E, F) Colony formation assay depicting differences in cell proliferation between U87MG (E) and LN229 (F) cells. (G, H) The proliferative ability of U251MG transfected with sh-EGFP, sh-LDHA (G), or sh-LDHB (H) plasmids determined using CCK-8 assay. (I) Colony formation assay depicting cell proliferation in U251MG cells. Statistical analysis was conducted using one-way ANOVA; *P<0.05, **P<0.01, and ***P<0.001.
LDH isoenzymes promoted the migration of human glioma cells and in vivo tumor growth. (A-C) Transwell migration assay comparing the sh-LDHA or sh-LDHB group with the sh-EGFP group of U87MG (A), LN229 (B), and U251MG (C) cells. (D) Photographs showing U87MG model tumors after treatment. (E) Changes in U87MG tumor volume following different treatments. (F) Mean tumor weights of U87MG tumors. (G) Photographs showing U251MG tumors after treatment. (H) Measurement of U251MG tumor volume at different time points. (I) Mean tumor weights of U251MG tumors. Data are presented as the mean ± standard deviation. Statistical analysis was conducted using one-way ANOVA; *P<0.05, **P<0.01, and ***P<0.001.
Summary of the Characteristics of GAA-BSA NPs; data are shown as mean ± SD (n = 3)
| Formulation | GAA-BSA |
|---|---|
| Particle size (nm) | 147.25 ± 1.56 |
| PDI | 0.118 ± 0.021 |
| Zeta-potential (mV) | -28.87 ± 0.52 |
| DLE (%) | 70.55 ± 1.08 |
| DLC (%) | 3.10 ± 0.05 |
GAA-BSA NPs inhibited proneural subtype GBM growth in vitro and in vivo
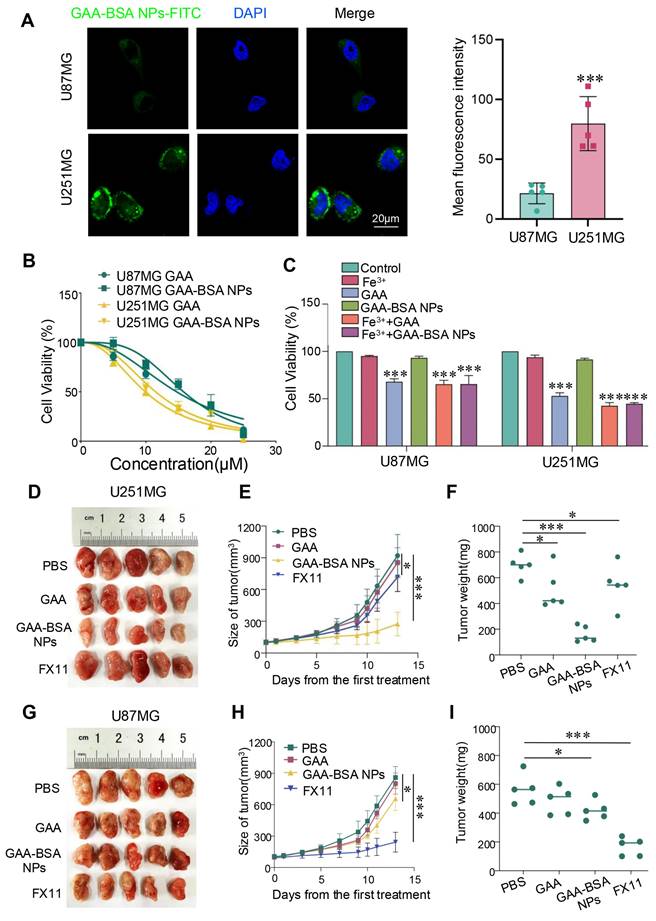
The selective efficacy of GAA-BSA NPs was substantiated by observing cellular uptake in mesenchymal and proneural subtype cells. FITC-conjugated NPs were incubated with U87MG and U251MG cells for 4h. And significantly higher fluorescence intensity was observed in proneural subtype cells than in mesenchymal-type cells. (Fig. 7A). To verify the potential efficacy of GAA-BSA NPs in tumor treatment, in vitro cytotoxicity evaluation by CCK-8 assay was performed. The half-maximal inhibitory concentration (IC50) values of GAA against U87MG and U251MG cell lines were determined to be 14.73 and 9.91 μM, respectively. For GAA-BSA, the IC50 values were 290 μg/mL and 214.6 μg/mL, respectively (the GAA concentration was 15.79 and 11.50 μM, respectively). These results suggest substantial selectivity of GAA-BSA NPs for targeting and eliminating proneural subtype cells (Fig. 7B).
Importantly, this study assessed the potential of GAA-BSA NPs to neutralize the impact of Fe3+ in the tumor microenvironment. The CCK-8 assay results showed that cell viability was significantly reduced in the Fe3++GAA-BSA NPs group compared to the Fe3++GAA group. This observation indicated the capacity of GAA-BSA NPs to enhance cell killing by mitigating the effects of Fe3+ (Fig. 7C). To further assess the therapeutic efficacy of GAA-BSA NPs, we analyzed the tumor size, volume, and weight as indicators of treatment effect in vivo. FX11, an LDHA inhibitor, is utilized to assess the therapeutic sensitivity of mesenchymal and proneural subtype GBM. In proneural type tumor model, tumor size, volume, and weight were markedly reduced after the treatment with GAA-BSA NPs, surpassing the impact of GAA and LDHA inhibitor FX11 (Fig. 7D-F). However, this effect was not observed in the mesenchymal tumor model (Fig. 7G-I). In summary, GAA-BSA NPs may be a promising treatment for targeting proneural subtype GBM. The schematic model is shown in Fig. 8.
LDH isoenzymes promoted the PMT of glioma cells. (A, B) Protein expression of mesenchymal and proneural markers detected after LDHA knockdown in U87MG cells using western blotting and subsequent quantitative analysis (B). (C, D) Protein expression of mesenchymal and proneural markers detected after LDHB knockdown in U87MG cells using western blotting (C) and subsequent quantitative analysis (D). (E, F) Protein expression of mesenchymal and proneural markers detected after LDHA knockdown in LN229 cells, using western blotting (E) and subsequent quantitative analysis (F). (G, H) Protein expression of mesenchymal and proneural markers detected after LDHB knockdown in LN229 cells using western blotting (G) and subsequent quantitative analysis (H). (I-L) Detection of mesenchymal and proneural markers levels after LDHA (I) or LDHB (K) knockdown in U251MG cells, with quantification of the results (J, L). Data are presented as the mean ± standard deviation. Statistical analysis was conducted using one-way ANOVA; *P<0.05, **P<0.01, and ***P<0.001.
Preparation and characterization of GAA-BSA NPs. (A) Computational simulation depicting the binding sites of GAA on LDH1 protein, the arrows indicate the specific binding site GLN D73 and HIS C186. (B) Scanning electron microscopy (SEM) image illustrating GAA-BSA NPs (scale bar: 3 μm). (C) Transmission electron microscopy (TEM) image of GAA-BSA NPs (scale bar: 100 nm). (D) Particle size analysis of GAA-BSA NPs. (E) Comparative evaluation of in vitro drug release profiles between GAA solution and GAA-BSA NP formulation. (F) Schematic drawing of the preparation of GAA-BSA NPs.
Discussion
Although extensive studies have begun to unravel the connection between GBM molecular subtypes and metabolic phenotypes [37], the complex metabolic mechanisms driving subtype classification in GBM remain unclear. This study primarily identified LDH isoenzymes as correlating with mesenchymal and proneural subtype classification and demonstrated prognostic and clinical significance in GBM patients.
LDH is used as a diagnostic biomarker for various tumors and has a long history of application in routine clinical diagnosis [38]. Recently, research attention has shifted to total serum LDH activity, as well as the expression of LDH isoenzymes [39]. Our findings corroborate the notion that LDH isoenzymes might serve as clinically significant molecular markers for distinguishing mesenchymal and proneural GBM subtypes, offering a foundation for more precise molecular typing of GBM. The mesenchymal subtype, which is characterized by heightened aggressiveness and poor prognosis relative to the proneural subtype, can transition from the proneural subtype following radiation therapy and chemotherapy [40]. PMT in GBM is aligned with increased proliferation and migration of cells [41, 42]. This is consistent with previous evidence on the role of LDH isoenzymes in governing cellular growth and migration [43-45]. In this study, we revealed the roles of LDH5 in promoting the proliferation, migration, and PMT of mesenchymal glioma cells, and showed that LDH1 sustains these functions in proneural glioma cells. The present evidence may serve as a cornerstone for developing gene expression-based diagnostic tools for recurrence monitoring and prognosis prediction. We hypothesized that targeting LDH isoenzymes could offer a potential strategy to inhibit this transition from the proneural subtype. This approach may lead to the development of effective therapeutic options against GBM.
The therapeutic efficacy of GAA-BSA NPs for two subtypes of GBM in vitro and in vivo. (A) Visualization of U87MG and U251MG cells treated with GAA-BSA NPs. Cellular nuclei stained with DAPI (blue); intracellular FITC fluorescence (green); and merged images illustrating spatial overlap. Scale bar: 20 μm. (B, C) CCK-8 assay was used to detect the IC50 and cytotoxic potential of GAA and GAA-BSA NPs in U87MG and U251MG glioma cell lines (Fe3+: 50 μM Fecl3, GAA: 10 μM, GAA-BSA: 200 μg/mL including 10 μM GAA). (D) Photographic depiction of U251MG tumor samples post treatment. (E) Measurement of U251MG tumor volume progression over different time points. (F) Average tumor weights of U251MG tumors. (G) Photographs illustrating U87MG model tumors following treatment. (H) Alterations in tumor volume after treatment of U87MG model tumors with various regimens. (I) Mean tumor weights for U87MG model tumors. Mice in the control group were treated with PBS, while those in other groups received free GAA or GAA-BSA NPs, at a dose of 15 mg/kg of GAA; or LDHA inhibitor FX11 at 2.5 mg/kg. All data are presented as the mean ± standard deviation. Statistical analysis was conducted using one-way ANOVA; *P<0.05 and ***P<0.001.
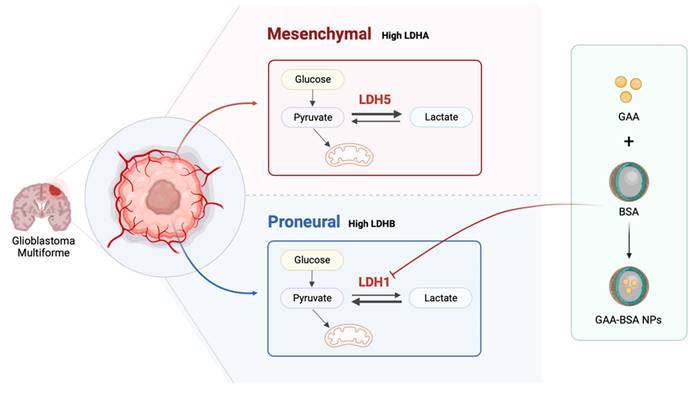
Schematic model showing the role of LDH isoenzyme in subtype classification between mesenchymal and proneural GBM. LDH5 was prominently involved in the mesenchymal subtype, while LDH1 was associated with the proneural subtype. Moreover, LDH1-targeting GAA-BSA NPs were recognized as a potential treatment approach for proneural-subtype GBM. The illustration was created using BioRender (https://www. biorender.com).
Furthermore, targeted therapeutic strategies can be tailored based on LDH isoenzyme expression characteristics. The present investigation of GAA-BSA NPs offers a potentially selective approach to GBM therapy. BSA is a versatile protein system for drug delivery, that is non-toxic, non-immunogenic, biocompatible, and biodegradable [31, 46]. Notably, paclitaxel-loaded human serum albumin NPs (Abraxane) received approval from the US Food and Drug Administration in 2005 for cancer chemotherapy [47, 48]. In this context, we synthesized LDH1-targeting GAA-BSA NPs as a targeted therapeutic modality for proneural subtype GBM. These NPs were shown to effectively suppress proneural subtype tumor growth both in vitro and in vivo, with higher efficacy than against the mesenchymal subtype.
In conclusion, the results offer novel insights into the role of LDH isoenzyme in subtype classification between mesenchymal and proneural GBM and provide a promising treatment approach for proneural-subtype GBM.
Supplementary Material
Supplementary figures.
Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (81772694), China Scholarship Council (CSC) and Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX21_3400).
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Author contributions
Mengting Yang: Investigation, Software, Writing-original draft, Funding acquisition. Xiu Han: Formal analysis, Methodology, Software. Hongxu Li: Methodology, Resources. Fengyi Du: Investigation, Supervision. Chunlai Feng: Conceptualization, Visualization. Aihua Gong: Project administration, Validation, Writing-review & editing, Funding acquisition.
Abbreviations
LDH: lactate dehydrogenase; GAA: gossypol-acetic acid; BSA: bovine serum albumin; PMT: proneural-to-mesenchymal transition; DLC: drug-loading capacity; DLE: drug-loading efficiency; FBS: fetal bovine serum; PBS: phosphate-buffered saline; SEM: scanning electron microscopy; TCGA: the cancer genome atlas.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Miao Y, Liu J, Liu X, Yuan Q, Li H, Zhang Y. et al. Machine learning identification of cuproptosis and necroptosis-associated molecular subtypes to aid in prognosis assessment and immunotherapy response prediction in low-grade glioma. Front Genet. 2022;13:951239
2. Nabian N, Ghalehtaki R, Zeinalizadeh M, Balana C, Jablonska PA. State of the neoadjuvant therapy for glioblastoma multiforme-Where do we stand? Neurooncol Adv. 2024;6:vdae028
3. Wang J, Du Q, Chen J, Liu J, Gu Z, Wang X. et al. Tumor treating fields in glioblastoma: long-term treatment and high compliance as favorable prognostic factors. Front Oncol. 2024;14:1345190
4. Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD. et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157-73
5. Wang Q, Hu B, Hu X, Kim H, Squatrito M, Scarpace L. et al. Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer Cell. 2017;32:42-56 e6
6. Azam Z, To ST, Tannous BA. Mesenchymal Transformation: The Rosetta Stone of Glioblastoma Pathogenesis and Therapy Resistance. Adv Sci (Weinh). 2020;7:2002015
7. Kim Y, Varn FS, Park SH, Yoon BW, Park HR, Lee C. et al. Perspective of mesenchymal transformation in glioblastoma. Acta Neuropathol Commun. 2021;9:50
8. Ding J, Karp JE, Emadi A. Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: Interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer Biomark. 2017;19:353-63
9. Khan AA, Allemailem KS, Alhumaydhi FA, Gowder SJT, Rahmani AH. The Biochemical and Clinical Perspectives of Lactate Dehydrogenase: An Enzyme of Active Metabolism. Endocr Metab Immune Disord Drug Targets. 2020;20:855-68
10. Claps G, Faouzi S, Quidville V, Chehade F, Shen S, Vagner S. et al. The multiple roles of LDH in cancer. Nat Rev Clin Oncol. 2022;19:749-62
11. Petrelli F, Ardito R, Merelli B, Lonati V, Cabiddu M, Seghezzi S. et al. Prognostic and predictive role of elevated lactate dehydrogenase in patients with melanoma treated with immunotherapy and BRAF inhibitors: a systematic review and meta-analysis. Melanoma Res. 2019;29:1-12
12. Guyon J, Fernandez-Moncada I, Larrieu CM, Bouchez CL, Pagano Zottola AC, Galvis J. et al. Lactate dehydrogenases promote glioblastoma growth and invasion via a metabolic symbiosis. EMBO Mol Med. 2022;14:e15343
13. Di Stefano G, Manerba M, Di Ianni L, Fiume L. Lactate dehydrogenase inhibition: exploring possible applications beyond cancer treatment. Future Med Chem. 2016;8:713-25
14. Shibata S, Sogabe S, Miwa M, Fujimoto T, Takakura N, Naotsuka A. et al. Identification of the first highly selective inhibitor of human lactate dehydrogenase B. Sci Rep. 2021;11:21353
15. Cai B, Gong L, Zhu Y, Kong L, Ju X, Li X. et al. Identification of Gossypol Acetate as an Autophagy Modulator with Potent Anti-tumor Effect against Cancer Cells. J Agric Food Chem. 2022;70:2589-99
16. Bushunow P, Reidenberg MM, Wasenko J, Winfield J, Lorenzo B, Lemke S. et al. Gossypol treatment of recurrent adult malignant gliomas. J Neurooncol. 1999;43:79-86
17. Chen Y, Fan Z, Yang Y, Gu C. Iron metabolism and its contribution to cancer (Review). Int J Oncol. 2019;54:1143-54
18. Lin L, Chen H, Zhao R, Zhu M, Nie G. Nanomedicine targets iron metabolism for cancer therapy. Cancer Sci. 2022;113:828-37
19. Mena H, Santos JE, Huber JT, Tarazon M, Calhoun MC. The effects of varying gossypol intake from whole cottonseed and cottonseed meal on lactation and blood parameters in lactating dairy cows. J Dairy Sci. 2004;87:2506-18
20. Wang J, Zhang B. Bovine Serum Albumin as a Versatile Platform for Cancer Imaging and Therapy. Curr Med Chem. 2018;25:2938-53
21. Kouchakzadeh H, Safavi MS, Shojaosadati SA. Efficient delivery of therapeutic agents by using targeted albumin nanoparticles. Adv Protein Chem Struct Biol. 2015;98:121-43
22. Liang F, Zhu L, Wang C, Yang Y, He Z. BSA-MnO(2)-SAL multifunctional nanoparticle-mediated M(1) macrophages polarization for glioblastoma therapy. RSC Adv. 2021;11:35331-41
23. Cancer Genome Atlas Research N. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061-8
24. Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD. et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98-110
25. Zhao Z, Zhang KN, Wang Q, Li G, Zeng F, Zhang Y. et al. Chinese Glioma Genome Atlas (CGGA): A Comprehensive Resource with Functional Genomic Data from Chinese Glioma Patients. Genomics Proteomics Bioinformatics. 2021;19:1-12
26. Huang Y, Hu L, Huang S, Xu W, Wan J, Wang D. et al. Curcumin-loaded galactosylated BSA nanoparticles as targeted drug delivery carriers inhibit hepatocellular carcinoma cell proliferation and migration. Int J Nanomedicine. 2018;13:8309-23
27. Duraj T, Garcia-Romero N, Carrion-Navarro J, Madurga R, Mendivil AO, Prat-Acin R. et al. Beyond the Warburg Effect: Oxidative and Glycolytic Phenotypes Coexist within the Metabolic Heterogeneity of Glioblastoma. Cells. 2021;10:202
28. Renoult O, Laurent-Blond M, Awada H, Oliver L, Joalland N, Croyal M. et al. Metabolic profiling of glioblastoma stem cells reveals pyruvate carboxylase as a critical survival factor and potential therapeutic target. Neuro Oncol. 2024;26:1572-86
29. Wolf A, Agnihotri S, Micallef J, Mukherjee J, Sabha N, Cairns R. et al. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med. 2011;208:313-26
30. Flak DK, Adamski V, Nowaczyk G, Szutkowski K, Synowitz M, Jurga S. et al. AT101-Loaded Cubosomes as an Alternative for Improved Glioblastoma Therapy. Int J Nanomedicine. 2020;15:7415-31
31. Liu Y, Qiao Z, Gao J, Wu F, Sun B, Lian M. et al. Hydroxyapatite-Bovine Serum Albumin-Paclitaxel Nanoparticles for Locoregional Treatment of Osteosarcoma. Adv Healthc Mater. 2021;10:e2000573
32. Sharma P, Aaroe A, Liang J, Puduvalli VK. Tumor microenvironment in glioblastoma: Current and emerging concepts. Neurooncol Adv. 2023;5:vdad009
33. Chen L, Zhao T, Zhao M, Wang W, Sun C, Liu L. et al. Size and charge dual-transformable mesoporous nanoassemblies for enhanced drug delivery and tumor penetration. Chem Sci. 2020;11:2819-27
34. Hornok V, Amin KWK, Kovacs AN, Juhasz A, Katona G, Balogh GT. et al. Increased blood-brain barrier permeability of neuroprotective drug by colloidal serum albumin carriers. Colloids Surf B Biointerfaces. 2022;220:112935
35. Harush-Frenkel O, Debotton N, Benita S, Altschuler Y. Targeting of nanoparticles to the clathrin-mediated endocytic pathway. Biochem Biophys Res Commun. 2007;353:26-32
36. Zhong Q, Zheng C, Yi K, Mintz RL, Lv S, Tao Y. et al. Structural and componential design: new strategies regulating the behavior of lipid-based nanoparticles in vivo. Biomater Sci. 2023;11:4774-88
37. Oizel K, Yang C, Renoult O, Gautier F, Do QN, Joalland N. et al. Glutamine uptake and utilization of human mesenchymal glioblastoma in orthotopic mouse model. Cancer Metab. 2020;8:9
38. Sharma D, Singh M, Rani R. Role of LDH in tumor glycolysis: Regulation of LDHA by small molecules for cancer therapeutics. Semin Cancer Biol. 2022;87:184-95
39. Chen X, Liu L, Kang S, Gnanaprakasam JR, Wang R. The lactate dehydrogenase (LDH) isoenzyme spectrum enables optimally controlling T cell glycolysis and differentiation. Sci Adv. 2023;9:eadd9554
40. Xiao Y, Wang Z, Zhao M, Deng Y, Yang M, Su G. et al. Single-Cell Transcriptomics Revealed Subtype-Specific Tumor Immune Microenvironments in Human Glioblastomas. Front Immunol. 2022;13:914236
41. Segerman A, Niklasson M, Haglund C, Bergstrom T, Jarvius M, Xie Y. et al. Clonal Variation in Drug and Radiation Response among Glioma-Initiating Cells Is Linked to Proneural-Mesenchymal Transition. Cell Rep. 2016;17:2994-3009
42. Chen Z, Wang S, Li HL, Luo H, Wu X, Lu J. et al. FOSL1 promotes proneural-to-mesenchymal transition of glioblastoma stem cells via UBC9/CYLD/NF-kappaB axis. Mol Ther. 2022;30:2568-83
43. Xiong A, Zhang J, Chen Y, Zhang Y, Yang F. Integrated single-cell transcriptomic analyses reveal that GPNMB-high macrophages promote PN-MES transition and impede T cell activation in GBM. EBioMedicine. 2022;83:104239
44. Zhao D, Zou SW, Liu Y, Zhou X, Mo Y, Wang P. et al. Lysine-5 acetylation negatively regulates lactate dehydrogenase A and is decreased in pancreatic cancer. Cancer Cell. 2013;23:464-76
45. Manerba M, Di Ianni L, Govoni M, Roberti M, Recanatini M, Di Stefano G. Lactate dehydrogenase inhibitors can reverse inflammation induced changes in colon cancer cells. Eur J Pharm Sci. 2017;96:37-44
46. Hsu WH, Ku CL, Lai YR, Wang SS, Chou SH, Lin TH. Developing targeted drug delivery carriers for breast cancer using glutathione-sensitive doxorubicin-coupled glycated bovine serum albumin nanoparticles. Int J Biol Macromol. 2023;249:126114
47. Ma P, Mumper RJ. Paclitaxel Nano-Delivery Systems: A Comprehensive Review. J Nanomed Nanotechnol. 2013;4:1000164
48. Sudlow G, Birkett DJ, Wade DN. Spectroscopic techniques in the study of protein binding. A fluorescence technique for the evaluation of the albumin binding and displacement of warfarin and warfarin-alcohol. Clin Exp Pharmacol Physiol. 1975;2:129-40
Author contact
![]() Corresponding author: Aihua Gong, Hematological Disease Institute of Jiangsu University, Affiliated Hospital of Jiangsu University, Zhenjiang, China, 212001. Tel.: +86-13775369530 Email: ahg5edu.cn.
Corresponding author: Aihua Gong, Hematological Disease Institute of Jiangsu University, Affiliated Hospital of Jiangsu University, Zhenjiang, China, 212001. Tel.: +86-13775369530 Email: ahg5edu.cn.

 Global reach, higher impact
Global reach, higher impact