Impact Factor
ISSN: 1837-9664
J Cancer 2025; 16(4):1127-1136. doi:10.7150/jca.103631 This issue Cite
Review
Implantable Port Catheters versus Peripherally Inserted Central Catheters for Cancer Patients Requiring Chemotherapy: An RCT-Based Meta-Analysis
1. Department of Thoracic Oncology Radiation Therapy, Jiangxi Cancer Hospital & Institute, Nanchang, China, 330029.
2. Department of Thoracic Surgery, The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, China, 330006.
3. Department of Head and Neck Oncology Radiation Therapy, Jiangxi Cancer Hospital & Institute, Nanchang, China, 330029.
Received 2024-9-13; Accepted 2024-12-13; Published 2025-1-6
Abstract
Background: Implantable port catheters (IPCs) and peripherally inserted central catheters (PICCs) are commonly used venous access methods for chemotherapy in cancer patients. However, the question of which is superior remains controversial. This meta-analysis, based on randomized controlled trials (RCTs), systematically compares the safety, cost, and impact on quality of life between these two methods.
Methods: Eligible RCTs comparing IPC and PICC were identified through searches in seven databases. Complications were the primary endpoint, while secondary endpoints included cost, impact on chemotherapy, and quality of life assessments.
Results: Six studies based on five RCTs, including a total of 1,127 patients, were analyzed. Patient data indicated that the PICC group experienced a higher incidence of total complications, thrombosis, deep vein thrombosis, implantation failure, unplanned catheter removal, and local reactions. Conversely, the IPC group had a higher incidence of pocket infection/exit-site infection without septicemia and pain. When considering catheter days, the PICC group again showed a higher incidence of total complications, thrombosis, deep vein thrombosis, implantation failure, unplanned catheter removal, edema, and local reactions. Complication-free survival was better in the IPC group. Although the impact on chemotherapy tended to favor the IPC group, this difference was not statistically significant. The total cost was higher in the IPC group, while the cost per catheter day was similar between the two groups. Quality of life assessments (using EORTC QLQ-C30) revealed similar global health status between the two groups during the post-implantation, mid-treatment, and end-treatment periods. However, the IPC group experienced a smaller decline in global health status post-implantation compared to the PICC group.
Conclusions: Compared to PICC, IPC appears to be a safer and more comfortable intravenous catheterization option for cancer patients undergoing chemotherapy.
Keywords: Implantable port catheters, Peripherally inserted central catheter, Complication, Meta-analysis, Randomized controlled trials
Introduction
Reliable venous access is essential for cancer patients undergoing chemotherapy, with implantable port catheters (IPCs) and peripherally inserted central catheters (PICCs) being the most commonly used options. The choice between these two devices remains a topic of clinical debate due to differences in safety profiles, patient comfort, and complication risks [1,2]. IPCs, surgically implanted under the skin, provide direct access to central veins and are associated with lower rates of catheter-related infections (CRIs) due to less frequent access and a reduced risk of bacterial colonization [3]. Conversely, PICCs, which are inserted through a peripheral vein, are easier to place and remove, making them preferable for patients who require rapid access or have contraindications to surgery [4].
However, PICCs are more prone to infections and venous thromboembolism (VTE), particularly in patients receiving thrombogenic chemotherapy or those with pre-existing hypercoagulable conditions [5]. Studies, including those by Bertoglio et al. and Benvenuti et al., highlight the higher risk of VTE associated with PICCs compared to IPCs, which generally have lower thrombosis rates [4,6]. Despite the increased risks of infection and thrombosis, PICCs are often preferred in settings where lower initial costs and procedural simplicity are prioritized [7].
Patient comfort is another critical factor. IPCs, being fully implanted, are less intrusive and require less frequent maintenance, contributing to higher patient satisfaction and better adherence to treatment [8,9]. Conversely, PICCs, which are visible and require regular care, may lead to increased anxiety and discomfort, potentially impacting the patient's quality of life [10-12].
Previous meta-analyses have often included retrospective studies, which are limited by inherent biases that compromise the reliability of their conclusions. By focusing exclusively on randomized controlled trials (RCTs), our study ensures the highest level of evidence for evaluating the safety, efficacy, and quality of life impacts of these venous access devices.
Materials and methods
Search strategy
RCTs comparing IPC and PICC were systematically searched in the Web of Science, EMBASE, Cochrane Library, PubMed, ScienceDirect, PMC (PubMed Central), and Scopus databases up to December 3, 2024. The MeSH terms used included 'Implantable port catheter' and 'Peripherally inserted central venous catheter'. Additionally, eligible articles were further identified by reviewing references from the retrieved literature. Detailed retrieval strategies are provided in Table S1.
Selection criteria
Inclusion criteria:
(1) Population: Cancer patients requiring chemotherapy.
(2) Intervention and comparison: IPC vs. PICC.
(3) Outcomes: Complications, cost, impact on chemotherapy, and assessment of quality of life (QOF).
(4) Study design: RCTs.
The following articles were excluded: articles without initial data (see data extraction for details), meta-analyses, conference articles, case reports, and reviews. Different articles that focused on the same trial were included if they contained different outcomes; however, when analyzing the same outcome, only the most recent data were used.
Data extraction
Two independent investigators extracted the following data: study characteristics (publication date, country, etc.), participant characteristics (cancer type, age, etc.), complications (thrombosis, infection, etc.), impact on chemotherapy (chemotherapy discontinuation, chemotherapy delay, etc.), cost (total cost, cost per catheter day, etc.), and QOL assessments (global health status, functional scales, etc.). Any disagreements were resolved through re-evaluation.
Outcome assessments
The QOL of patients was assessed at post-implantation, mid-treatment, and end-treatment stages using the EORTC QLQ-C30 and EQ-5D scales [13,14]. Complications were analyzed based on either the number of patients or catheter days. Thrombosis and infection were further categorized and analyzed according to their specific types. Thrombosis, as assessed in our study, included both deep vein thrombosis and catheter-related thrombosis. These conditions are critical complications for catheterized patients as they may lead to catheter occlusion, necessitate catheter removal, and increase the risk of further thromboembolic events. Infections, primarily CRIs, were evaluated in terms of both localized (e.g., pocket infections or exit-site infections) and systemic infections. Localized infections involve bacterial colonization around the catheter insertion site, which can lead to pain and localized inflammation, while systemic infections can progress to bacteremia or sepsis.
Quality assessment for included studies
The quality of the RCTs was assessed using the Jadad scale, which allocates up to 7 points based on randomization, allocation concealment, double blinding, and handling of withdrawals and dropouts, with scores of 4 or higher indicating high quality [15]. The quality of the results was assessed using the GRADE approach [16].
Statistical analysis
Data pooling was analyzed using RevMan 5.3 and STATA 12.0. Continuous variables were analyzed using mean difference (MD), survival outcomes with hazard ratios (HR), and dichotomous variables with pooled risk ratios (RR). All effect sizes were presented with 95% confidence intervals (CI). Heterogeneity was assessed using the I² statistic and χ² test. Significant heterogeneity (I² > 50% or P < 0.1) warranted the use of a random-effects model; otherwise, a fixed-effects model was applied. Funnel plots were used to assess publication bias. Statistical significance was indicated by p < 0.05. (PROSPERO ID: CRD42024583534).
Results
Search results
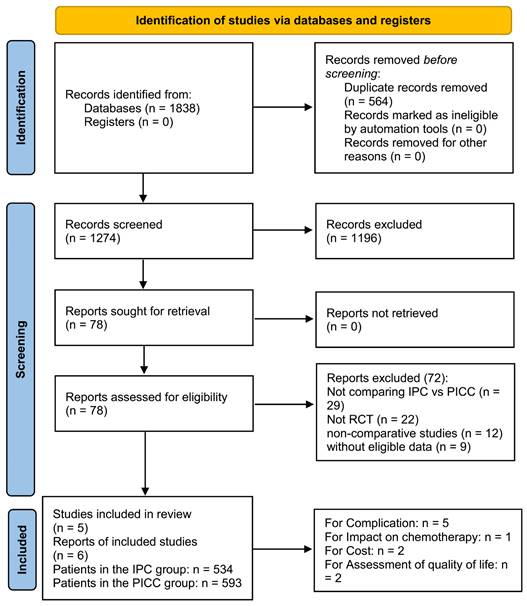
Initially, 1274 studies were identified, and six papers based on five RCTs (IPC group: 534 patients; PICC group: 593 patients) were included in the analysis (Figure 1) [17-22]. Table 1 presents the baseline characteristics. Three studies were conducted in Europe, one in Canada, and one in Australia. All studies were rated as high quality based on the Jadad scale and Cochrane Risk Assessment (Table S2, Figure S1). However, all outcomes were rated as medium to high quality according to the GRADE system (Table S3).
Complications assessment according to patients
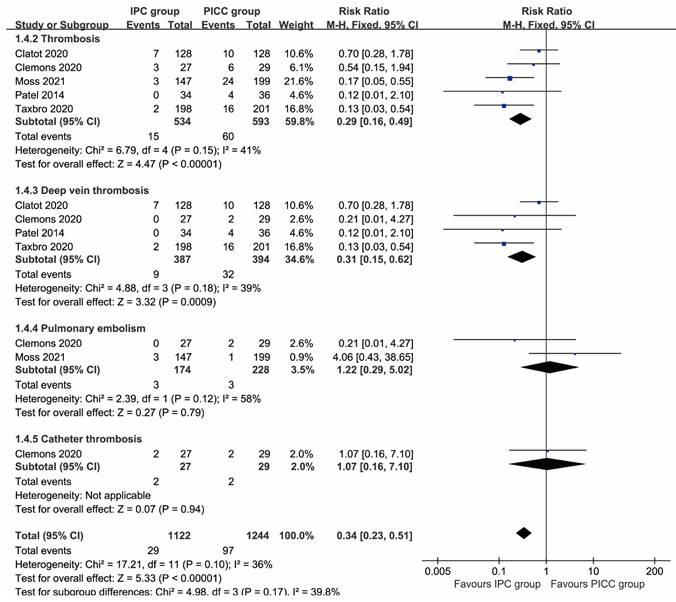
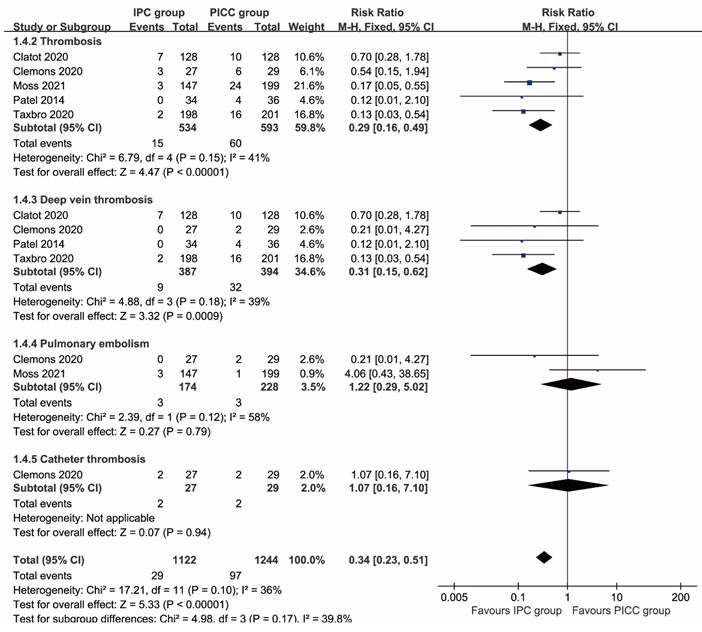
More total complications (RR: 0.51 [0.26, 0.99], P=0.03), including thrombosis (RR: 0.29 [0.16, 0.49], P<0.00001), deep vein thrombosis (RR: 0.31 [0.15, 0.62], P=0.0009), implantation failure (RR: 0.35 [0.15, 0.81], P=0.01), unplanned catheter removal (RR: 0.57 [0.41, 0.78], P=0.0005), and local reactions (RR: 0.14 [0.03, 0.62], P=0.009) were observed in the PICC group. In contrast, more pocket infections/exit-site infections without septicemia (RR: 2.34 [1.17, 4.70], P=0.02) and pain (RR: 4.06 [1.39, 11.87], P=0.01) were found in the IPC group. The rates of pulmonary embolism, catheter thrombosis, infection, sepsis, mechanical complications, catheter disruption, spontaneous catheter migration, partial withdrawal, catheter occlusion, bleeding, and edema were similar between the two groups (Table 2, Figures 2, 3). Complication-free survival was also better in the IPC group (HR: 0.37 [0.25, 0.55], P<0.00001) (Figure S2).
Flow chart.
Baseline characteristics of the included studies.
| Study | ID | Country | Period (year) | Groups | Number of patients | Sex (M/F) | Age (Mean, year) | Cancer type | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Clatot 2020 [17] | NCT02095743 | France | 2014.02-2018.05 | IPC | 128 | 0/128 | 56 | Breast cancer | 6 |
| PICC | 128 | 0/128 | 57.5 | ||||||
| Clemons 2020 [18] | NCT02632435 | Canada | 2016.03-2018.03 | IPC | 27 | 0/27 | 54 | Breast cancer | 8 |
| PICC | 29 | 0/29 | 52 | ||||||
| Moss 2021 [19] | ISRCTN44504648 | UK | 2013.11-2018.02- | IPC | 147 | 66/81 | 61 | Haematological malignancy and Solid tumour | 6 |
| PICC | 199 | 92/107 | 61 | ||||||
| Patel 2014 [20] | - | Australia | 2004.12-2010.01 | IPC | 34 | 19/15 | 60 | Haematological malignancy and Solid tumour | 4 |
| PICC | 36 | 17/19 | 59 | ||||||
| Taxbro 2020 [21], Taxbro 2020 [22] | NCT01971021 | Sweden | 2013.03-2017.02 | IPC | 198 | 83/115 | 65 | Haematological malignancy and Solid tumour | 5 |
| PICC | 201 | 91/110 | 66 |
Abbreviations: ID: Identity document; IPC: Implantable Port Catheter; M/F: Male/Female; PICC: Peripherally Inserted Central Catheter.
Complications assessment according to patients.
| Complications | Studies involved | IPC | PICC | Risk ratio [95% CI] | P-value | |||
|---|---|---|---|---|---|---|---|---|
| Event/total | % | Event/total | % | |||||
| Total | 5 | 175/534 | 32.77% | 269/593 | 45.36% | 0.79 [0.63, 0.98] | 0.03 | |
| Thrombosis | 5 | 15/534 | 2.81% | 60/593 | 10.12% | 0.29 [0.16, 0.49] | < 0.00001 | |
| Deep vein thrombosis | 4 | 9/387 | 2.33% | 32/394 | 8.12% | 0.31 [0.15, 0.62] | 0.0009 | |
| Pulmonary embolism | 2 | 3/174 | 1.72% | 3/228 | 1.32% | 1.22 [0.29, 5.02] | 0.79 | |
| Catheter thrombosis | 1 | 2/27 | 7.41% | 2/29 | 6.90% | 1.07 [0.16, 7.10] | 0.94 | |
| Infection | 3 | 27/309 | 8.74% | 21/363 | 5.79% | 1.63 [0.96, 2.78] | 0.07 | |
| Sepsis | 4 | 13/500 | 2.60% | 12/557 | 2.15% | 1.31 [0.62, 2.77] | 0.48 | |
| Pocket infection/exit-site infection without septicaemia | 4 | 24/500 | 4.80% | 11/557 | 1.97% | 2.34 [1.17, 4.70] | 0.02 | |
| Mecanical complication | 2 | 12/326 | 3.68% | 9/329 | 2.74% | 2.12 [0.15, 29.22] | 0.58 | |
| Implantation failure | 3 | 7/473 | 1.48% | 25/528 | 4.73% | 0.35 [0.15, 0.81] | 0.01 | |
| Catheter disruption | 1 | 1/34 | 2.94% | 2/36 | 5.56% | 0.53 [0.05, 5.57] | 0.6 | |
| Spontaneous catheter migration | 2 | 0/162 | 0.00% | 4/164 | 2.44% | 0.21 [0.02, 1.73] | 0.15 | |
| Unplanned catheter removal | 3 | 42/302 | 13.91% | 95/356 | 26.69% | 0.57 [0.41, 0.78] | 0.0005 | |
| Partial withdrawal | 1 | 0/128 | 0.00% | 1/128 | 0.78% | 0.33 [0.01, 8.11] | 0.5 | |
| Catheter occlusion | 3 | 3/360 | 0.83% | 19/365 | 5.21% | 0.31 [0.02, 3.92] | 0.36 | |
| Bleeding | 1 | 0/128 | 0.00% | 1/128 | 0.78% | 0.33 [0.01, 8.11] | 0.5 | |
| Oedema | 2 | 3/326 | 0.92% | 7/329 | 2.13% | 0.47 [0.13, 1.67] | 0.24 | |
| Local reaction | 1 | 2/128 | 1.56% | 14/128 | 10.94% | 0.14 [0.03, 0.62] | 0.009 | |
| Pain | 2 | 16/162 | 9.88% | 4/164 | 2.44% | 4.06 [1.39, 11.87] | 0.01 | |
Abbreviations: CI: Confidence Interval; IPC: Implantable Port Catheter; PICC: Peripherally Inserted Central Catheter.
Complications assessment according to catheter days
More total complications (RR: 0.39 [0.32, 0.48], P<0.00001), including thrombosis (RR: 0.15 [0.04, 0.54], P<0.00001), deep vein thrombosis (RR: 0.19 [0.03, 1.25], P=0.004), implantation failure (RR: 0.18 [0.08, 0.40], P<0.0001), unplanned catheter removal (RR: 0.25 [0.17, 0.37], P<0.00001), edema (RR: 0.27 [0.08, 0.96], P=0.04), and local reactions (RR: 0.09 [0.02, 0.41], P=0.002) were observed in the PICC group. The rates of pulmonary embolism, infection, sepsis, pocket infection/exit-site infection without septicemia, mechanical complications, spontaneous catheter migration, partial withdrawal, catheter occlusion, bleeding, and pain were similar between the two groups (Table 3).
The impact on chemotherapy
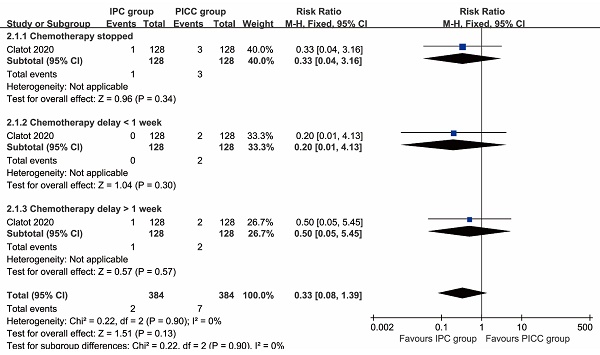
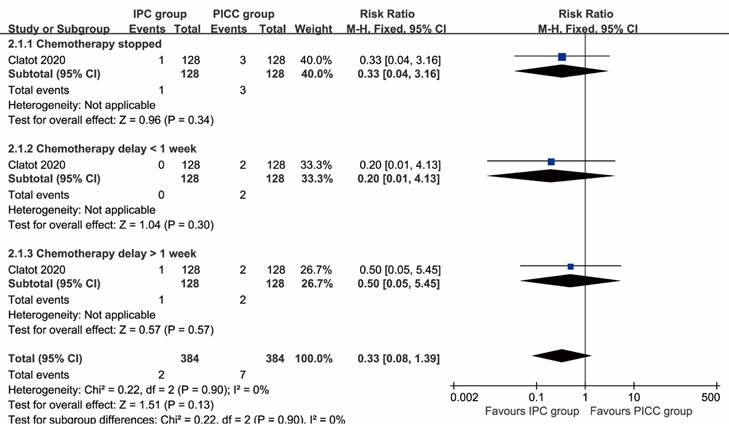
Chemotherapy stopped (RR: 0.33 [0.04, 3.16], P=0.34), chemotherapy delay of less than 1 week (RR: 0.20 [0.01, 4.13], P=0.30), and chemotherapy delay of more than 1 week (RR: 0.50 [0.05, 5.45], P=0.57) all tended to favor the IPC group, although these differences were not statistically significant (Figure 4).
Cost
The total cost (MD: 1665.00 [1595.58, 1734.42] dollars, P<0.00001) was higher in the IPC group, while the cost per catheter-day (MD: 2.82 [-14.27, 19.91] dollars, P=0.75) was similar between the two groups (Figure S3).
Assessment of quality of life
QOL was assessed using the EORTC QLQ-C30. The global health status was similar between the two groups after implantation. During the post-implantation phase, social functioning (MD: -6.20 [-10.19, -2.21], P=0.002) was better in the PICC group. During mid-treatment, emotional functioning (MD: 6.70 [1.43, 11.97], P=0.01) and dyspnoea (MD: 5.70 [0.11, 11.29], P=0.05) were better in the IPC group. During end-treatment, role functioning (MD: -9.30 [-16.14, -2.46], P=0.008) and diarrhoea (MD: -5.80 [-11.41, -0.19], P=0.04) were better in the PICC group (Table 4).
Forest plots of thrombosis (total thrombosis, deep vein thrombosis, pulmonary embolism, and catheter thrombosis) according to patients.
Complications assessment according to catheter days.
| Complications | Studies involved | IPC | PICC | Risk ratio [95% CI] | P-value | |||
|---|---|---|---|---|---|---|---|---|
| Event/total | % | Event/total | % | |||||
| Total | 3 | 148/73127 | 0.202% | 233/43553 | 0.535% | 0.39 [0.32, 0.48] | < 0.00001 | |
| Thrombosis | 3 | 12/73127 | 0.016% | 50/43553 | 0.115% | 0.15 [0.04, 0.54] | < 0.00001 | |
| Deep vein thrombosis | 2 | 9/61227 | 0.015% | 26/36068 | 0.072% | 0.19 [0.03, 1.25] | 0.004 | |
| Pulmonary embolism | 1 | 3/11900 | 0.025% | 1/7485 | 0.013% | 1.89 [0.20, 18.14] | 0.58 | |
| Infection | 2 | 27/29436 | 0.092% | 20/18877 | 0.106% | 0.85 [0.48, 1.52] | 0.59 | |
| Sepsis | 3 | 12/73127 | 0.016% | 10/43553 | 0.023% | 0.74 [0.33, 1.69] | 0.48 | |
| Pocket infection/exit-site infection without septicaemia | 3 | 19/73127 | 0.026% | 10/43553 | 0.023% | 1.10 [0.51, 2.34] | 0.81 | |
| Mecanical complication | 2 | 12/61227 | 0.020% | 9/36068 | 0.025% | 1.30 [0.08, 21.34] | 0.86 | |
| Implantation failure | 3 | 7/73127 | 0.010% | 25/43553 | 0.057% | 0.18 [0.08, 0.40] | < 0.0001 | |
| Spontaneous catheter migration | 1 | 0/17536 | 0.000% | 2/11392 | 0.018% | 0.13 [0.01, 2.71] | 0.19 | |
| Unplanned catheter removal | 2 | 37/29436 | 0.126% | 92/18877 | 0.487% | 0.25 [0.17, 0.37] | < 0.00001 | |
| Partial withdrawal | 1 | 0/17536 | 0.000% | 1/11392 | 0.009% | 0.22 [0.01, 5.32] | 0.35 | |
| Catheter occlusion | 1 | 0/17536 | 0.000% | 1/11392 | 0.009% | 0.22 [0.01, 5.32] | 0.35 | |
| Bleeding | 1 | 0/17536 | 0.000% | 1/11392 | 0.009% | 0.22 [0.01, 5.32] | 0.35 | |
| Oedema | 2 | 3/61227 | 0.005% | 7/36068 | 0.019% | 0.27 [0.08, 0.96] | 0.04 | |
| Local reaction | 1 | 2/17536 | 0.011% | 14/11392 | 0.123% | 0.09 [0.02, 0.41] | 0.002 | |
| Pain | 1 | 12/17536 | 0.068% | 3/11392 | 0.026% | 2.60 [0.73, 9.21] | 0.14 | |
Abbreviations: CI: Confidence Interval; IPC: Implantable Port Catheter; PICC: Peripherally Inserted Central Catheter.
EORTC QLQ-C30 assessment between IPC and PICC during post implantation, mid treatment, and end treatment.
| EORTC QLQ-C30 | Post implantation | Mid treatment | End treatment | |||||
|---|---|---|---|---|---|---|---|---|
| MD [95% CI] | P-value | MD [95% CI] | P-value | MD [95% CI] | P-value | |||
| Global health status | 0.35 [-2.81, 3.52] | 0.83 | 4.80 [-0.04, 9.64] | 0.05 | -3.40 [-8.35, 1.55] | 0.18 | ||
| Functional scales | ||||||||
| Physical functioning | -2.20 [-4.95, 0.55] | 0.12 | -0.50 [-3.87, 2.87] | 0.77 | -1.30 [-5.96, 3.36] | 0.58 | ||
| Role functioning | -2.00 [-6.27, 2.27] | 0.36 | -0.90 [-6.92, 5.12] | 0.77 | -9.30 [-16.14, -2.46] | 0.008 | ||
| Emotional functioning | 0.50 [-4.87, 5.87] | 0.86 | 6.70 [1.43, 11.97] | 0.01 | -3.20 [-8.81, 2.41] | 0.26 | ||
| Cognitive functioning | 0.30 [-3.12, 3.72] | 0.86 | 0.10 [-4.79, 4.99] | 0.97 | -5.80 [-11.00, -0.60] | 0.03 | ||
| Social functioning | -6.20 [-10.19, -2.21] | 0.002 | 0.30 [-4.79, 5.39] | 0.91 | -4.60 [-10.80, 1.60] | 0.15 | ||
| Symptoms scales | ||||||||
| Fatigue | 4.80 [-0.09, 9.69] | 0.05 | -1.70 [-7.47, 4.07] | 0.56 | 3.80 [-2.87, 10.47] | 0.26 | ||
| Nausea and vomiting | -0.20 [-2.40, 2.00] | 0.86 | -2.80 [-8.78, 3.18] | 0.36 | -1.70 [-5.20, 1.80] | 0.34 | ||
| Pain | 2.00 [-2.91, 6.91] | 0.42 | -1.70 [-6.02, 2.62] | 0.44 | 1.10 [-4.83, 7.03] | 0.72 | ||
| Dyspnoea | -3.80 [-8.06, 0.46] | 0.08 | 5.70 [0.11, 11.29] | 0.05 | 6.30 [-0.61, 13.21] | 0.07 | ||
| Insomnia | 1.30 [-6.04, 8.64] | 0.73 | 3.00 [-4.34, 10.34] | 0.42 | -4.30 [-12.26, 3.66] | 0.29 | ||
| Appetite loss | 0.40 [-4.92, 5.72] | 0.88 | 0.80 [-6.82, 8.42] | 0.84 | -5.30 [-12.13, 1.53] | 0.13 | ||
| Constipation | -4.10 [-8.76, 0.56] | 0.08 | -4.00 [-10.18, 2.18] | 0.2 | -3.20 [-10.11, 3.71] | 0.36 | ||
| Diarrhoea | 0.60 [-2.62, 3.82] | 0.72 | 1.20 [-4.78, 7.18] | 0.69 | -5.80 [-11.41, -0.19] | 0.04 | ||
| Financial difficulties | 1.10 [-3.86, 6.06] | 0.66 | -3.80 [-9.76, 2.16] | 0.21 | 0.80 [-5.62, 7.22] | 0.81 | ||
Abbreviations: CI: Confidence Interval; EORTC QLQ-C30: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30; IPC: Implantable Port Catheter; MD: Mean Difference; PICC: Peripherally Inserted Central Catheter.
Forest plots of infection (total infection, sepsis, and pocket infection/exit-site infection without septicaemia) according to patients.
Forest plots of the impact on chemotherapy associated with IPC versus PICC.
Changes in QOL were assessed using the EORTC QLQ-C30 and EQ-5D. After implantation, the IPC group showed better improvements in global health status, physical functioning, role functioning, social functioning, fatigue, nausea and vomiting, appetite loss, constipation, financial difficulties, and health state scores. Conversely, the PICC group demonstrated better improvements in emotional functioning, cognitive functioning, pain, dyspnoea, insomnia, diarrhoea, and index value (Table S4).
Sensitivity analysis
Sensitivity analyses for total complications, thrombosis, and sepsis were performed, demonstrating that excluding any individual study did not affect the reliability of the results (Figure S4).
Publication bias
The analysis of complications according to catheter days, thrombosis, infection, and EORTC QLQ-C30 during the post-implantation phase revealed no evidence of publication bias (Figure S5).
Discussion
The choice between IPC and PICC for chemotherapy in cancer patients remains a significant clinical debate. This controversy stems from the varying risks of complications, patient comfort, and the overall impact on treatment efficacy and quality of life. Given the high stakes of chemotherapy management, understanding which catheterization method offers superior outcomes is essential. Unlike prior meta-analyses that included retrospective studies, our study synthesizes data exclusively from RCTs, ensuring the highest evidence level and minimizing bias. This methodological strength significantly enhances the reliability of our findings and provides a more definitive comparison of IPC and PICC. Notably, our results indicate that IPC may be a safer and more comfortable option, with fewer complications and a potentially more favorable impact on chemotherapy administration, albeit at a higher overall cost.
Our analysis revealed significant differences in the complication profiles of IPC and PICC, favoring the use of IPC in several key aspects. Specifically, the PICC group exhibited a higher incidence of total complications, including thrombosis, deep vein thrombosis, implantation failure, unplanned catheter removal, and local reactions. These findings align with previous studies that have highlighted the increased risk of venous thromboembolism (VTE) associated with PICC, particularly in patients undergoing chemotherapy [23,24]. The higher thrombosis rates in PICC can be attributed to the smaller lumen size and longer catheter length, which contribute to stasis and endothelial injury-key factors in thrombogenesis [25]. Moreover, the ease of PICC placement, often done without fluoroscopic guidance, may result in suboptimal positioning, further increasing the risk of complications [26]. In contrast, IPCs, being surgically implanted under the skin with direct access to central veins, are associated with lower thrombosis rates and fewer CRIs, as they are accessed less frequently and have a reduced risk of bacterial colonization [27,28]. However, the IPC group was not without its complications, showing a higher incidence of pocket infections/exit-site infections and pain. These localized infections, while serious, are typically less life-threatening than the systemic complications more common with PICC [29].
The impact of catheter choice on chemotherapy delivery is critical, as interruptions in treatment can directly affect patient outcomes. Our study found that chemotherapy discontinuation, delays of less than one week, and delays of more than one week all tended to favor the IPC group, though these differences were not statistically significant. This trend suggests that IPC may provide a more reliable venous access route, reducing the likelihood of catheter-related issues that could disrupt chemotherapy [30]. Previous research has shown that IPC, due to their stability and lower maintenance requirements, are less likely to cause treatment interruptions compared to PICC [31]. This reliability is particularly important for patients receiving regimens that are highly thrombogenic or for those with a history of venous access difficulties [32]. Although the differences in our analysis did not reach statistical significance, the consistent trend toward better outcomes with IPC underscores its potential advantage in maintaining uninterrupted chemotherapy, which is crucial for achieving optimal therapeutic results.
QOL is a crucial consideration when evaluating the effectiveness of venous access devices. Our study assessed QOL using the EORTC QLQ-C30 and EQ-5D scales at various stages of treatment and found that global health status was similar between the IPC and PICC groups during post-implantation, mid-treatment, and end-treatment periods. However, the decline in global health status post-implantation was smaller in the IPC group compared to the PICC group, suggesting that IPC may be less disruptive to patients' overall well-being [33]. This finding aligns with previous studies indicating that IPCs, being fully implanted and less intrusive, contribute to higher patient satisfaction and better adherence to treatment [34]. In contrast, PICC, which are visible and require regular maintenance, may lead to increased anxiety and discomfort, negatively affecting the patient's QOL [35]. Additionally, our analysis of specific QOL domains revealed that emotional functioning and dyspnea were better in the IPC group during mid-treatment, further supporting the notion that IPC may offer a more favorable experience for patients undergoing prolonged chemotherapy.
While our study provides important insights, it is not without limitations. First, the inclusion of only a limited number of RCTs may restrict the generalizability of our findings. Despite rigorous selection criteria, the small sample size and heterogeneity of the included studies may have introduced bias. Second, the quality of evidence for some outcomes was rated as medium by the GRADE system, which may affect the robustness of our conclusions. Third, cost analyses were limited by the lack of standardized reporting across studies, making it difficult to draw definitive conclusions about the economic impact of IPC versus PICC. Fourth, the sample size across the included studies varied, which may impact the statistical power and generalizability of the results. Finally, geographical differences and variations in clinical practice may limit the applicability of our conclusions to all patient populations. Differences in catheter management protocols, healthcare infrastructure, and patient demographics across regions could influence complication rates and patient experiences, thus affecting the broader generalizability of IPC and PICC comparisons. Future studies with larger, more diverse populations could provide more robust evidence and help validate our findings in varied clinical contexts.
Conclusions
IPC may be a safer and more comfortable option for cancer patients requiring chemotherapy compared to PICC. IPC is associated with fewer complications and may provide a more reliable venous access route, thereby reducing the risk of chemotherapy interruptions. While both devices had a similar impact on overall quality of life, IPC appeared to be less disruptive to patients' well-being. However, these findings should be interpreted with caution due to the study's limitations, and further high-quality research is needed to confirm these results.
Abbreviations
CI: Confidence Interval; CRIs: Catheter-Related Infections; EORTC QLQ-C30: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30; EQ-5D: EuroQol 5-Dimension Scale; GRADE: Grading of Recommendations Assessment, Development and Evaluation; HR: Hazard ratios; ID: Identity document; IPC: Implantable Port Catheter; MD: Mean Difference; M/F: Male/Female; NOS: Newcastle-Ottawa Scale; PICC: Peripherally Inserted Central Catheter; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; QOL: Quality of Life; RCT: Randomized Controlled Trial; RR: Risk Ratio; VTE: Venous Thromboembolism.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
Funding
This study was supported by Scientific and Technological Projects of Jiangxi Provincial Health Commission (Grant number: SKJP220228121). Role of the Funding: The funding had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Availability of data and material
Data is provided within the manuscript or supplementary information files.
Author contributions
Jianyun Wen had full access to all the data in the manuscript and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Concept and design: Juan Qiu, Shanshan Huang, Pei Wen, Yingxin Jiang, Zan Luo, Wenxiong Zhang, and Jianyun Wen.
Acquisition, analysis, or interpretation of data: Juan Qiu, Shanshan Huang, Pei Wen, Yingxin Jiang, Zan Luo, and Jianyun Wen.
Statistical analysis: Juan Qiu, Shanshan Huang, and Jianyun Wen.
Drafting of the manuscript: Juan Qiu, Shanshan Huang, Pei Wen, and Jianyun Wen.
Critical revision of the manuscript for important intellectual content: Juan Qiu, Shanshan Huang, and Jianyun Wen.
Supervision: Juan Qiu, and Jianyun Wen.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Pinelli F, Barbani F, Defilippo B, Fundarò A, Nella A, Selmi V. et al. Quality of life in women with breast cancer undergoing neoadjuvant chemotherapy: comparison between PICC and PICC-port. Breast Cancer. 2024;31(5):945-954
2. Lu A, Hu M, Qi X, Zhao Y, Huang Y. A retrospective cohort study of implantable venous access port-related and peripherally inserted central catheter-related complications in patients with hematological malignancies in China. SAGE Open Med. 2024;12:20503121241257190
3. He E, Ye K, Zheng H. Clinical effect and safety of venous access ports and peripherally inserted central catheters in patients receiving tumor chemotherapy: a systematic review and meta-analysis. Ann Palliat Med. 2021;10(8):9105-9113
4. Bertoglio S, Cafiero F, Meszaros P, Varaldo E, Blondeaux E, Molinelli C. et al. PICC-PORT totally implantable vascular access device in breast cancer patients undergoing chemotherapy. J Vasc Access. 2020;21(4):460-466
5. Piredda M, Sguanci M, De Maria M, Petrucci G, Usai M, Fiorini J. et al. Nurses' evidence-based knowledge and self-efficacy in venous access device insertion and management: Development and validation of a questionnaire. Nurs Open. 2024;11(7):e2177
6. Benvenuti S, Pintossi C, Porteri E, Gazzaneo M, Bartolini G, Schettino B. et al. Pseudo-tunneling procedure for PICC-ports catheters insertion in patients with small veins at the middle third of the arm. Minerva Surg. 2024 May 29
7. Heggie R, Jaiswal N, McCartney E, Moss J, Menne T, Jones B. et al. Central Venous Access Devices for the Delivery of Systemic Anticancer Therapy: An Economic Evaluation. Value Health. 2024;27(1):7-14
8. Zapletal O, Sirotek L, Coufal O. Venous access in cancer patients. Rozhl Chir. 2019;98(11):427-433
9. Rieger MJ, Schenkel X, Dedic I, Brunn T, Gnannt R, Hofmann M. et al. Complication rates of peripherally inserted central catheters vs implanted ports in patients receiving systemic anticancer therapy: A retrospective cohort study. Int J Cancer. 2023;153(7):1397-1405
10. Rosenthal VD, Yin R, Myatra SN, Memish ZA, Rodrigues C, Kharbanda M. et al. Multinational prospective study of incidence and risk factors for central-line-associated bloodstream infections in 728 intensive care units of 41 Asian, African, Eastern European, Latin American, and Middle Eastern countries over 24 years. Infect Control Hosp Epidemiol. 2023:1-11
11. Rydell H, Huge Y, Eriksson V, Johansson M, Alamdari F, Svensson J. et al. Central Venous Access and the Risk for Thromboembolic Events in Patients Undergoing Neoadjuvant Chemotherapy and Radical Cystectomy for Muscle-Invasive Bladder Cancer. Life (Basel). 2022;12(8):1198
12. Pénichoux J, Rio J, Kammoun L, Vermeulin T, Pepin LF, Camus V. et al. Retrospective analysis of the safety of peripherally inserted catheters versus implanted port catheters during first-line treatment for patients with diffuse large B-cell lymphoma. Eur J Haematol. 2022;109(1):41-49
13. Rogge AA, Liegl G, Snyder C, Rose M, Nolte S; EORTC Quality of Life Group. EORTC QLQ-C30 general population normative data for the United States. Eur J Cancer. 2024;202:114030
14. Shaw C, Longworth L, Bennett B, McEntee-Richardson L, Shaw JW. A Review of the Use of EQ-5D for Clinical Outcome Assessment in Health Technology Assessment, Regulatory Claims, and Published Literature. Patient. 2024;17(3):239-249
15. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ. et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1-12
16. Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380-382
17. Clatot F, Fontanilles M, Lefebvre L, Lequesne J, Veyret C, Alexandru C. et al. Randomised phase II trial evaluating the safety of peripherally inserted catheters versus implanted port catheters during adjuvant chemotherapy in patients with early breast cancer. Eur J Cancer. 2020;126:116-124
18. Clemons M, Stober C, Kehoe A, Bedard D, MacDonald F, Brunet MC. et al. A randomized trial comparing vascular access strategies for patients receiving chemotherapy with trastuzumab for early-stage breast cancer. Support Care Cancer. 2020;28(10):4891-4899
19. Moss JG, Wu O, Bodenham AR, Agarwal R, Menne TF, Jones BL. et al. Central venous access devices for the delivery of systemic anticancer therapy (CAVA): a randomised controlled trial. Lancet. 2021;398(10298):403-415
20. Patel GS, Jain K, Kumar R, Strickland AH, Pellegrini L, Slavotinek J. et al. Comparison of peripherally inserted central venous catheters (PICC) versus subcutaneously implanted port-chamber catheters by complication and cost for patients receiving chemotherapy for non-haematological malignancies. Support Care Cancer. 2014;22(1):121-8
21. Taxbro K, Hammarskjöld F, Thelin B, Lewin F, Hagman H, Hanberger H. et al. Clinical impact of peripherally inserted central catheters vs implanted port catheters in patients with cancer: an open-label, randomised, two-centre trial. Br J Anaesth. 2019;122(6):734-741
22. Taxbro K, Hammarskjöld F, Juhlin D, Hagman H, Bernfort L, Berg S. Cost analysis comparison between peripherally inserted central catheters and implanted chest ports in patients with cancer-A health economic evaluation of the PICCPORT trial. Acta Anaesthesiol Scand. 2020;64(3):385-393
23. Verso M, Agnelli G. Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol. 2003;21(19):3665-75
24. McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med. 2003;348(12):1123-33
25. Chopra V, Anand S, Hickner A. et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382(9889):311-25
26. Amerasekera SS, Jones CM, Patel R. et al. Imaging of the complications of peripherally inserted central venous catheters. Clin Radiol. 2009;64(8):832-40
27. Taxbro K, Hammarskjöld F, Thelin B. et al. Clinical impact of peripherally inserted central catheters vs implanted port catheters in patients with cancer: A randomised controlled study. Br J Cancer. 2019;120(8):811-818
28. Iovino F, Pittiruti M, Buononato M. et al. Central venous access devices in oncology: A review of their use and management in patients receiving antineoplastic treatment. Support Care Cancer. 2015;23(10):3021-9
29. Chopra V, Ratz D, Kuhn L. et al. Peripherally inserted central catheter-related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost. 2014;12(6):847-54
30. Biffi R, Orsi F, Pozzi S. et al. Best choice of central venous insertion site for the prevention of catheter-related complications in adult patients who need cancer therapy: a randomized trial. Ann Oncol. 2009;20(5):935-40
31. Geerts W, Cook D, Selby R. et al. Venous thromboembolism and its prevention in critical care. J Crit Care. 2002;17(2):95-104
32. Khorana AA, Kuderer NM, Culakova E. et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902-7
33. Cotogni P, Pittiruti M, Barbero C. et al. Catheter-related complications in cancer patients on home parenteral nutrition: a prospective study of over 51,000 catheter days. JPEN J Parenter Enteral Nutr. 2013;37(3):375-83
34. Baskin JL, Pui CH, Reiss U. et al. Management of occlusion and thrombosis associated with long-term indwelling central venous catheters. Lancet. 2009;374(9684):159-69
35. Monreal M, Munoz FJ, Parada A. et al. Venous thromboembolism in patients with cancer: a prospective study of its incidence, clinical profile and management. The RIETE Investigators. Eur Heart J. 2004;25(4):321-6
Author contact
![]() Corresponding author: Dr. Jianyun Wen, Department of Head and Neck Oncology Radiation Therapy, Jiangxi Cancer Hospital & Institute, 519 Beijing East Road, Nanchang, China, 330029; E-mail: wenjianyun1985com.
Corresponding author: Dr. Jianyun Wen, Department of Head and Neck Oncology Radiation Therapy, Jiangxi Cancer Hospital & Institute, 519 Beijing East Road, Nanchang, China, 330029; E-mail: wenjianyun1985com.

 Global reach, higher impact
Global reach, higher impact