Impact Factor
ISSN: 1837-9664
J Cancer 2025; 16(7):2261-2274. doi:10.7150/jca.108194 This issue Cite
Review
Nano-strategies for Targeting Tumor-Associated Macrophages in Cancer immunotherapy
1. Department of General Surgery, The Second Affiliated Hospital of Soochow University, Suzhou 215004, P. R. China.
2. Key Laboratory of Radiation Medicine and Protection, School for Radiological and Interdisciplinary Sciences (RAD-X), Collaborative Innovation Center of Radiation Medicine of Jiangsu Higher Education Institutions, Soochow University, Suzhou 215123, P. R. China.
3. Department of Thoralic Surgery, Suzhou Municipal Hospital Institution, Suzhou 215000, P. R. China.
4. Department of Thoralic Surgery, Dushu Lake Hospital Affiliated to Soochow University, Suzhou, 215123, P. R. China.
Abstract:
Received 2024-12-4; Accepted 2025-2-12; Published 2025-3-31
Abstract
Tumor-associated macrophages (TAMs) are one type of the most abundant immune cells within tumor, resulting in immunosuppresive tumor microenvironment and tumor resistance to immunotherapy. Thus, targeting TAMs is a promising therapeutic strategy for boosting cancer immunotherapy. This study provides an overview of current therapeutic strategies targeting TAMs, which focus on blocking the recruitment of TAMs by tumors, regulating the polarization of TAMs, and directly eliminating TAMs using various nanodrugs, especially with a new categorization based on the specific signaling pathways, such as NF-κB, HIF-1α, ROS, STAT, JNK, PI3K, and Notch involved in their regulatory mechanism. The latest developments of nanodrugs modulating these pathways are discussed in determining the polarization of TAMs and their role in the tumor microenvironment. Despite the challenges in clinical translation and the complexity of nanodrug synthesis, the potential of nanodrugs in enhancing the effectiveness of cancer immunotherapy is worthy of expecting.
Keywords: Cancer immunotherapy, Tumor-associated macrophages, Nanodrugs, M1 polarization, Signaling pathways
Introduction
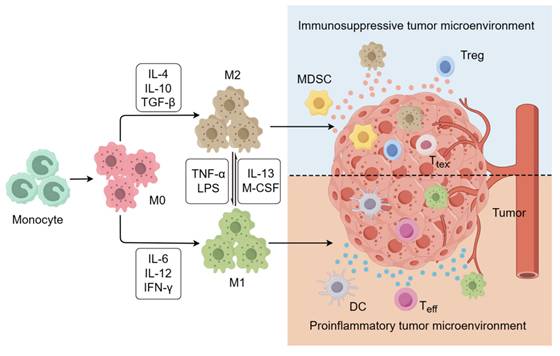
Cancer immunotherapy can be designed to harness the innate and adaptive immune systems to attack tumor cells, ultimately improving the survival outcomes of cancer patients, particularly those in advanced stages. This approach has emerged as a focal point in both basic and clinical cancer research [1]. Although clinical trials have shown excellent results, several key challenges still need to be addressed, including low response rates, durability of response, immune-related adverse events, and atypical clinical responses [2]. Recent investigations have highlighted the pivotal role of tumor-associated macrophages (TAMs) in mediating resistance to immunotherapy, given their prominence as key immunosuppressive cells within the tumor microenviron-ment (TME) [3]. TAMs derived from circulating monocytes and recruited to the tumor site dynamically shape the TME, fostering conditions conducive to tumor growth and progression [4]. Macrophages are divided into two main subsets based on their phenotype and function: classically activated M1 macrophages and alternatively activated M2 macrophages (Figure 1). M1 macrophages typically contribute to anti-tumor immunity by engaging in antigen presentation and producing proinflammatory cytokines like interleukin-6 (IL-6), interleukin-12 (IL-12), and interleukin-1 (IL-1). M2 macro-phages promote tumor growth by secreting a series of anti-inflammatory cytokines such as interleukin-4 (IL-4), interleukin-10 (IL-10), and transforming growth factor beta (TGF-β) [5]. Macrophages infiltrating the tumor site account for approximately 50% of immune cells in the TME [6]. Within the TME, the polarization of tumor infiltrating TAMs can be influenced by various factors, ultimately adopting an M2-like phenotype owing to conditions such as hypoxia, oxidative stress, and lactate accumulation [7]. These M2-like TAMs secrete differential immunosuppressive cytokines that can enhance the recruitment of other immunosuppressive cells, such as regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), while also contributing to the conversion of effector T cells (Teff) to exhausted T cells (Ttex), ultimately fueling tumor proliferation, angiogenesis, and metastasis [8, 9]. This close association with tumor prognosis highlights the importance of targeting TAMs to enhance the effectiveness of the immune system in combating tumors [10, 11].
However, traditional drugs targeting TAMs have some limitations, including inadequate specificity, notable side effects, and potential for drug resistance [12]. In this context, nanodrugs may partially address these issues by improving drug stability, solubility, and bioavailability [13, 14]. Additionally, nanodrugs can be rationally designed to possess targeted delivery capabilities through passive or active targeting, thereby minimizing the off-target effects. Numerous novel nanodrugs have been developed to enhance cancer immunotherapy by targeting TAMs, showing promising preclinical and clinical results [15, 16]. This paper reviews nanodrugs that target TAMs and discusses the underlying challenges in their development.
Therapeutic strategies for targeting TAMs
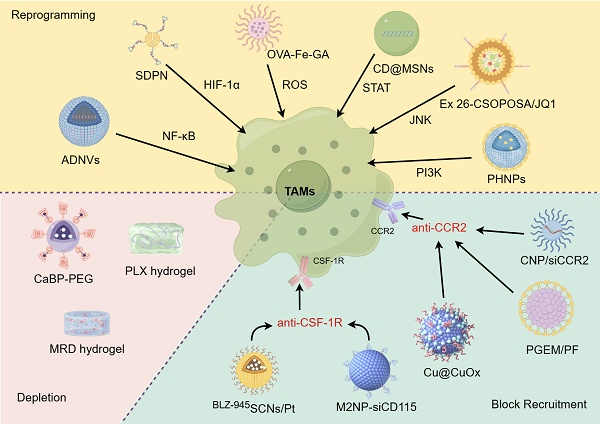
TAMs play a critical role in promoting tumor growth and resistance to treatment in various cancer types. These discoveries lay a strong groundwork for targeting these cells and their precursors to enhance patient outcomes [17]. Current therapeutic strategies for targeting TAMs using nanodrugs can be broadly divided into three approaches: blocking TAMs recruitment to the TME, depleting and/or suppressing TAMs, and reprogramming TAMs polarization to the M1 phenotype (Figure 2) [18-20].
Blocking tumor recruitment of TAMs
Monocytes/macrophages are recruited to the TME by various factors secreted by tumor cells and tumor stromal cells. Once infiltrated, these monocytes/macrophages are educated by the TME and differentiate into TAMs, serving as a continuous supply of TAMs. Therefore, targeting key factors involved in monocyte/macrophage recruitment is crucial. Among these factors, colony stimulating factor 1(CSF-1) plays a crucial role in the recruitment of monocytes to the TME [21-24]. CSF-1 interacts with the colony stimulating factor 1 receptor (CSF-1R) on monocytes, leading to their polarization into TAMs [25-27]. Monocyte chemoattractants, such as C-C motif chemokine ligand 2 (CCL2) and its homologous receptor C-C chemokine receptor type 2 (CCR2), are targeted for the treatment of solid tumors and hematological malignancies using monoclonal antibodies and receptor antagonists. Many antibodies and small molecule drugs targeting the CCL2/CCR2 axis have entered clinical trial stages. However, most of them have not achieved the desired efficacy, such as carumab [28]. Nanomaterials offer a potential solution for enhancing drug efficacy through targeted delivery and sustained release.
The polarization of macrophages. M0 macrophages can differentiate into either M1 or M2 macrophages when stimulated by different cytokines. M1 macro-phages are known for their involvement in inflammatory responses and have both proinflammatory and anti-tumor effects. In contrast, M2 macrophages are commonly found in the immunosuppresive microenvironment, where they exert an anti-inflammatory effect and contribute to tumor growth and metastasis.
Current therapeutic strategies for targeting TAMs with nanodrugs. Through blocking tumor recruitment of TAMs, depleting/suppressing TAMs, and reprogramming TAMs, the goal is to reshape the TME and enhance the immune response against cancer. Nanodrugs have the potential to precisely deliver and release therapeutic agents, offering a promising approach to enhance the effectiveness of TAM-targeting immunotherapies while minimizing side effects.
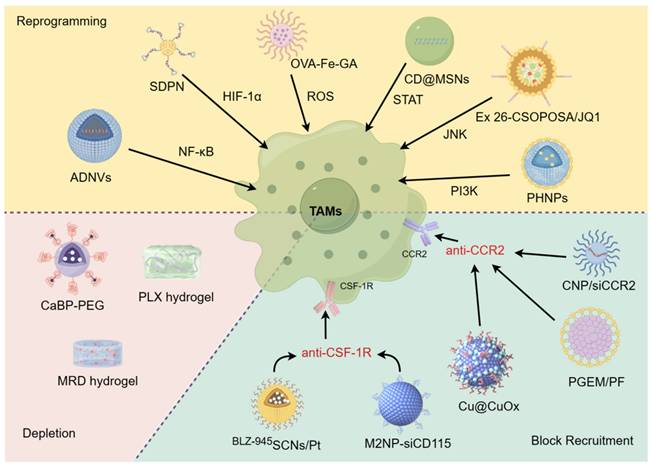
Zhang et al. developed CCR2-targeted small copper nanoparticles (Cu@CuOx) that can inhibit TAMs recruitment and deliver the chemotherapeutic drug gemcitabine to treat pancreatic ductal adenocarcinoma (PDAC). By covalently conjugating the CCR2-targeting peptide ECL1i and gemcitabine to nanoparticles, Cu@CuOx nanoparticles ingeniously achieves precise targeting of tumor cells and effective drug delivery. These nanoparticles also enabled PET imaging owing to their 64Cu radiolabeling. Systemic administration of gemcitabine-loaded Cu@CuOx in a PDAC xenograft mouse model significantly reduced tumor growth and extended survival [29]. Möckel et al. studied the effect of the surface charge of polymer nanoparticles on the biodistribution of monocytes for targeted delivery. They discovered that positively charged nanoparticles accumulated better in monocytes, and after carrying siCCR2 (CNP/siCCR2), they could significantly improve anti-tumor outcomes by modifying the TME in a mouse breast cancer model [30]. Wan et al. incorporated the CCR2 antagonist PF-6309 (PF) into a polymeric micelle system based on gemcitabine (PGEM). The PGEM/PF formulation effectively reversed CCL2/CCL7-mediated immune suppression and significantly induced potent anti-tumor immunity to reduce the pancreatic tumor burden. This study paves a new avenue for the rational design of nanomedicine based on bioinformatics and computational modeling [31]. In addition, Shen et al. created a dual-targeting immunostimulatory nanocarrier (BLZ-945SCNs/Pt) for chemoimmunotherapy that simultaneously addresses TAMs and tumor cells. This nanocarrier was designed to release a platinum (Pt) prodrug and a CSF-1R inhibitor (BLZ-945) into the prevascular areas of tumors. The BLZ-945SCNs/Pt undergo supersensitive structural collapse in the prevascular regions of tumor tissues, simultaneously releasing Pt-prodrug-conjugated small particles and BLZ-945. The released BLZ-945 can be preferentially taken up by TAMs, leading to the depletion of TAMs from tumor tissues. Meanwhile, the small particles carrying Pt-prodrug can penetrate deeply into the tumor and intracellularly specific release drug to kill more cancer cells. This pH-sensitive co-delivery nanocarrier not only induces apoptosis of tumor cells but also modulates the tumor immune microenvironment, ultimately enhancing the antitumor effect of CD8+ cytotoxic T cells through the depletion of TAMs [32]. Taken together, the interception of TAM recruitment represents a pivotal strategy for altering the polarization of TAMs toward an immunesuppressive phenotype, offering significant promise for future therapeutic development.
Depleting and/or suppressing TAMs
Multiple preclinical and clinical studies have shown that reducing the number of TAMs can inhibit tumor progression and improve treatment outcomes [33-38]. Because TAMs are primarily derived from circulating monocytes, depleting monocytes is also another way to reduce the number of TAMs. Some targeted drugs have been able to deplete TAM populations or inhibit TAM activity to some extent. Nevertheless, the primary obstacles that remain are the lack of specificity, high toxicity, and limited efficacy [39].
Recently, Zhang et al. developed an ATP-supersensitive nanogel (BBLZ-945@PAC-PTX) that sequentially eliminates these barriers. Upon reaching the tumor, it collapses in response to ATP, releasing BBLZ-945 to deplete TAMs. The shrunk nanogel (PAC-PTX) penetrates deeper, blocking CXCR4 to reduce immunosuppressive cells and internalizing into tumor cells for killing and T cell priming. This "dominolike" strategy enhances immune cell infiltration and boosts immune response, providing a high-responsive chemoimmunotherapy option [40]. Zhou et al. developed an engineered biomimetic nano-red blood cell system, denoted as V(Hb)@DOX, which targets the depletion of M2-type TAMs, alleviates tumor hypoxia, and holds significant potential for reprogramming the TME and enhancing the efficacy of chemoimmunotherapy [41]. Similarly, Li et al. developed a biocompatible alginate-based hydrogel designed for localized pexidartinib (PLX) release to effectively deplete TAMs. This strategy not only promotes T cell infiltration, but also synergies with platelet activation post-surgery to enhance the release of aPD-1, thereby boosting the immunotherapeutic efficacy against tumor recurrence [42]. Additionally, the clinical drug bisphosphonates, known for their ability to deplete TAMs [43], were utilized by Liu et al. to prepare CaBP-PEG nanoparticles via a mineralization method using a poly(polyethylene glycol) (PEG) coating. CaBP-PEG nanoparticles composed of Ca2+ and bisphosphonates are highly biocompatible and multifunctional. Through innovative chelator-free radiolabeling chemistry, the authors successfully developed CaBP(99mTc)-PEG and CaBP(32P)-PEG for SPECT imaging guided enhanced cancer radioisotope therapy, and demonstrating its potential and clinical translation value in cancer treatment [44]. Nevertheless, while reducing TAMs can weaken their role in promoting tumor growth, the immunosuppressive environment of TME frequently hinders the success of treatment approaches. In this regard, rather than eliminating TAMs, reprogramming them could be a more beneficial approach to counteract the immunosuppressive TME and improve the effectiveness of drug therapies.
Reprogramming TAMs into M1-type macrophages
Currently, there is significant interest in reprogramming TAMs by activating specific signaling pathways associated with macrophages, which can induce polarization towards an anti-tumor M1 phenotype. In TAMs, various signaling pathways are involved in the regulation of TAM polarization, such as nuclear factor-κB (NF-κB), hypoxia inducible factor-1 alpha (HIF-1α), reactive oxygen species (ROS), signal transducer and activator of transcription (STAT), c-Jun NH2-terminal kinase (JNK), phosphatidylinositol 3-kinase (PI3K), and Notch etc (Figure 3) [45, 46]. For instance, activation of the NF-κB signaling pathway typically promotes TAMs polarization towards the M1 direction, while other pathways such as HIF-1α might be closely associated with M2-like TAMs [47]. Therefore, targeting these signaling pathways in TAMs holds promise for phenotypic reprogramming [48].
Preclinical studies have shown that nanomaterial-mediated reprogramming of TAMs exhibits significant anti-tumor efficacy [49-51]. These nanomaterials not only modify the phenotype of TAMs but also activate T cells and other immune cells, thereby bolstering the overall immune system's assault on the tumor. The success of this approach hinges on the meticulous design of nanomaterials, encompassing factors such as size, shape, surface characteristics, and payload selection, all of which impact their distribution, targeting, and biological effects in the body. Subsequently, our focus shifts to a review of nanodrugs targeting specific signaling pathways involved in TAM polarization (Table 1).
NF-κB pathway
The NF-κB signaling pathway is a crucial pathway involved in immunity, stress response, cell apoptosis, and differentiation, and it plays a key role in the development of various diseases, including inflammatory infections, autoimmune and metabolic disorders, and cancer [52, 53]. This signaling pathway also exists in TAMs and is crucial in determining their polarization. Inhibition of NF-κB signaling pathway-mediated immune suppression related to M2 macrophages can hinder the metastasis of breast cancer cell [54]. Furthermore, the involvement of the NF-κB signaling pathway can be observed in almost all fundamental characteristics of cancer [55].
Cellular signaling networks in TAMs. The cellular signaling networks within TAMs are vital for regulating their polarization. Signaling pathways such as NF-κB, HIF-1α, ROS, STAT, JNK, PI3K, and Notch play a critical role in determining the direction of TAM polarization. These pathways can function independently or interact with each other to influence TAM polarization. Consequently, they present potential targets for nanodrugs in cancer immunotherapy focused on TAMs.
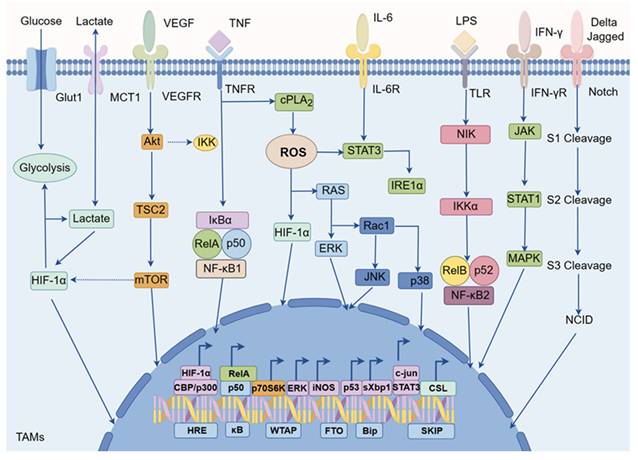
Nanodrugs targeting the signaling pathways in TAMs to promote M1 polarization.
| Pathways | Nanodrugs | Biological effect | Ref. |
|---|---|---|---|
| NF-κB | Artemisia-derived nanovesicles (ADNVs) | Carrying plant-derived mitochondrial DNA to activate cGAS-STING/NF-κB pathway | 58 |
| Nanomicelle dissolving microneedle (DMN) | Loading cIAP inhibitor LCL-161 and R848 agonist to activate NF-κB pathway | 60 | |
| Silica nanoparticles (SNs) | Activating the mechanosensitive protein Piezo1, leading to calcium ion influx, NF-κB pathway activation | 61 | |
| Ginsenoside nanoparticles (GDNPs) | Containing ceramide lipids and proteins from ginseng to activate NF-κB pathway | 62 | |
| Poly(I:C) functionalized ferumoxytol (FMT) nanoparticles (FP-NPs) | Combining FMT and poly(I:C) to activate NF-κB pathway | 63 | |
| Mannose-modified PLGA (MAN-PLGA-N) | Using precision nanoparticle-based ROS photogeneration to activate NF-κB pathway | 64 | |
| HIF-1α | Sophorolipid-associated membrane-biomimetic choline phosphate-poly(lactic-co-glycolic) acid hybrid nanoparticle (SDPN) | Delivering luteolin and silibinin act synergistically on STAT3 and HIF-1α pathway | 69 |
| CL4H6 lipid nanoparticle (CL4H6-LNP) | Delivering HIF-1α siRNA to silence HIF-1α pathwaty | 70 | |
| ROS | Ovalbumin- Fe-gallic acid (OFG) nanoparticles | Emulating the NOX2 enzyme's sequential ROS generation process | 81 |
| Nanoliposome C6-Ceramide (LipC6) | LipC6 injection significantly regulate IRF1, 2, 3, 6 and activate ROS production | 82 | |
| Fe3O4 nanoparticle | Inducing ferroptosis by activating the generation of ROS | 83 | |
| Platinum(IV)/chloroquine/ perfluorohexane nanoparticle - anti-PD-L1 peptide (Pt(IV)/CQ/PFH NPs-DPPA-1) | Reprogramming the metabolic pathway and activating ROS | 84 | |
| STAT | Carbon nanodot@mesoporous silica nanoparticles (CD@MSNs) | Stimulating NK cells to secrete IFN-γ that activate STAT1 | 94 |
| Paclitaxel-loaded ginsenoside Rg3 liposomes (Rg3-PTX-LPs) | Inhibiting IL-6/STAT3/p-STAT3 pathway activation | 95 | |
| Two-dimensional carbon-based nanomaterials-graphdiyne oxide nanosheets (GDYO) | Interacting with an intracellular protein corona consisting of STAT3 to inhibit STAT3 activation | 96 | |
| DPPA-1 peptide@oleanolic acid (DPAM@OA) | Elevating the expressions of VACM-1 and ICAM-1 and reducing the level of p-STAT3 | 97 | |
| JNK | Sphingosine 1-phosphate receptor 1 antagonist Ex 26/ JQ1 (Ex 26-CSOPOSA/JQ1) | Delivering the oncogene c-Myc inhibitor JQ1 to inhibiting JNK pathway | 107 |
| PI3K | Gemcitabine-dendritic poly-lysine@ polycaprolactone-polyethylene glycol micelles/Wtmn (GD@PP/Wtmn) | Loading PI3K inhibitor wortmannin(Wtmn) to TAMs | 118 |
| M2pep-mixed micelle/BEZ 235/siRNA (M2pep-MM/BEZ/siRNA) | Encapsulating BEZ 235 and CSF-1R siRNA to block of PI3k-γ and CSF-1R | 119 | |
| Porous hollow iron oxide nanoparticles@DPA-S-S-bovine serum albumin-mannose@3-methyladenine (PHNPs@DPA-S-S-BSA-MA@3-MA) | loading a P13K-γ small molecule inhibitor and further modified by mannose to target TAMs | 120 |
Schematic illustration of the interaction between nanobiointerfaces and TAM repolarization in situ by SNs elasticity. The stiff and soft SNs interact with the macrophage cell membrane, and the soft SNs activate Piezo1 through slight plasma membrane deformation, further inducing Ca2+ influx and activating the NF-κB pathway. The soft SNs can penetrate the intratumoral hypoxic regions and reprogram TAMs in situ (Copyright © 2024 American Chemical Society).
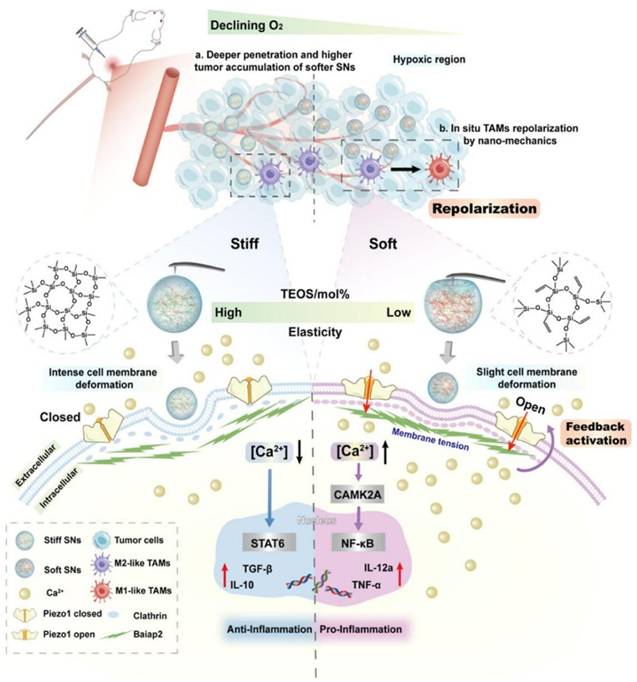
Nanodrugs employ two primary strategies to modulate the NF-κB signaling pathway in TAMs: 1) acting as nanocarriers to encapsulate NF-κB pathway inhibitors, which allows for targeted regulation of TAMs, and 2) directly influencing the NF-κB signaling pathway through the intrinsic properties of the nanodrugs themselves [56, 57]. Liu et al. isolated Artemisia-derived nanovesicles (ADNVs) from Artemisia annua, which are naturally occurring exosome-like nanoparticles that can carry plant-derived mitochondrial DNA (mtDNA). This mtDNA within ADNVs activates the NF-κB pathway, leading to the reprogramming of TAMs into anti-tumor M1 phenotypes, thus offering a novel approach to cancer therapy [58]. Chen et al. designed an ingenious in situ self-assembled nanomicelle dissolving microneedle (DMN) patch for intralesional delivery of immunogenic cell death-inducer (IR780) and autophagy inhibitor (chloroquine, CQ) co-encapsulated micelles (C/I-Mil), which can act as an im-mune modulator to remodel TAMs toward the M1 phenotype by activating NF-κB [59]. Fredrich et al. developed a kind of glycated nanoparticles targeted to myeloid cells, containing three therapeutic payloads to modulate the interaction of TAMs and tumor cells, which targeted the toll-like receptor (TLR), NF-κB, and Janus Kinase (JAK) signaling pathways to drive TAMs towards a distinct anti-tumor phenotype characterized by increased IL-12 production and T cell activation [60]. In addition, Yang et al. explored the biological effects of elastic silica nanoparticles (SNs) with variational elasticity Young's moduli ranging from 81 to 837 MPa on macrophages, showing that soft SNs can repolarize TAMs to M1. The elasticity of SNs affects cell endocytosis, membrane tension, curvature protein Baiap2, and cytoskeleton. Moreover, they can activate the mechanosensitive protein Piezo1 leading to calcium ion influx, NF-κB pathway activation, and inflammatory response (Figure 4). The approach of directly targeting macrophages using the material itself is relatively rare. As a framework, this kind of material can further enhance the targeting and repolarization of TAMs in addition to drug delivery [61]. In another study, Cao et al. identified ginsenoside nanopar-ticles (GDNPs), a new class of extracellular vesicle-like particles from ginseng, which served as an immunomodulatory agent capable of altering macrophage polarization from the M2 to M1 phenotype. Their study showed that GDNPs, through components such as ceramide lipids and proteins, potentially activate Toll-like receptor 4 (TLR4), leading to a significant reduction in melanoma growth in mice and an increase in M1 macrophages within tumor tissues [62]. Zhao et al. investigated the anticancer potential of macrophages activated by combining ferumoxytol (FMT) with TLR3 agonist poly(I:C) or poly(I:C)-functionalized FMT nanoparticles (FP-NPs). They demonstrated that FP-NPs did not affect the viability of B16F10 cells but selectively inhibited tumor growth by shifting TAMs to the M1 phenotype via the NF-κB signaling pathway [63]. Furthermore, Shi et al. successfully reprogrammed TAMs to an anti-tumor M1 phenotype using a photo-generation technology based on mannose-modified PLGA, in which co-encapsulated ICG and TiO2 with NH4HCO3 (denoted as MAN-PLGA-N). MAN-PLGA-N modulates the NF-κB signaling pathway in TAMs. This approach demonstrated superior efficiency, surpassing that of LPS stimulation, representing significant progress in the development of novel cancer therapeutic approaches [64].
These studies highlight the innovative strategies employed by nanodrugs to modulate the NF-κB signaling pathway in TAMs, offering new insights into cancer immunotherapy. The use of natural and synthetic nanomaterials to directly target and repolarize TAMs represents a significant advancement, which not only enhances the delivery of therapeutic agents but also directly influences the tumor microenvironment. However, the translation of these strategies to clinical settings will require further research to address issues such as biocompatibility, toxicity, and the long-term effects of nanomaterials in the body. Additionally, the development of more sophisticated targeting ligands and delivery systems will be crucial to ensure the specificity and effectiveness of these nanodrugs in human trials.
HIF-1α pathway
Studies have shown that the expression of HIF-1α is primarily induced by the complex tumor microenvironment, including conditions such as intratumoral hypoxia and elevated lactate levels, which subsequently trigger a series of responses [55, 65]. The increased expression of HIF-1α contributes to the recruitment of TAMs and the polarization of M2-like TAMs, resulting in enhanced tumor proliferation, migration, invasion, angiogenesis, and drug resistance [66]. HIF-1α accumulates in the nucleus and binds to short DNA sequences called hypoxia response elements (HREs), which are located near oxygen-sensitive genes such as vascular endothelial growth factor (VEGF) [67]. The expression of these genes demonstrates why macrophages can express VEGF in avascular and hypoxic areas, explaining the mechanism behind angiogenesis in a low-oxygen, low-pH microenvironment [68].
Recently, various nanodrugs have been developed to regulate macrophage polarization in the TME by targeting HIF-1α. Gu et al. developed a hybrid nanoparticle called sophorolipid-associated membrane-biomimetic choline phos-phate-poly(lactic-co-glycolic) acid (SDPN). These nanoparticles are associated with sophorolipids, which provide colloidal stability and rapid diffusion in mucus, resulting in improved endocytosis driven by the interaction between dipalmitoyl choline phosphate and phosphatidyl choline, as well as optimized membrane fluidity and rigidity of SDPN. When loaded with luteolin and silibinin, SDPN regulated the conversion of M2 TAMs into the M1 phenotype and reduced the proportion of the M2 phenotype through co-action on STAT3 and HIF-1α [69]. Additionally, Shobaki et al. employed a lipid nanoparticle (LNP) formulation to target and deliver siSTAT3/siHIF-1α to TAMs. Using the optimized siRNA-loaded CL4H6-LNPs to target TAMs, they achieved an an-titumor therapeutic effect in a human tumor xenograft model through the silencing of signal STAT3 and HIF-1α, thereby increasing the number of M1-type macrophages [70]. Visibly, these nanodrugs can modulate the HIF-1α pathway to repolarize TAMs from pro-tumor M2 to anti-tumor M1 phenotypes, thus boosting the immune response against cancer. Sophisticated nanoparticle formulations like SDPN and LNPs enhance drug delivery and efficacy. These strategies offer new cancer treatment avenues and underscore the importance of targeting the tumor microenvironment to amplify immunotherapy effectiveness.
ROS pathway
ROS are typically generated as byproducts of oxygen consumption and cellular metabolism through the partial reduction of oxygen [71]. Under oxidative stress conditions, excessive ROS can damage cellular proteins, lipids, and DNA, leading to fatal cell injuries and various pathologies, such as aging, cancer, neuro-degenerative diseases, cardiovascular diseases, and diabetes [72, 73]. While ROS are often viewed as harmful to cells, they also play crucial roles in regulating signal transduction pathways [74, 75] and gene expression [76]. When addressing hypoxia within TME, the presence of oxygen can trigger the generation of free radicals via the ROS signaling pathway, influencing the polarization and function of TAMs. Notably, ROS are interconnected with several signaling pathways such as JNK, STAT3, HIF-1α, and PI3K [77, 78]. For instance, in non-small cell lung cancer (NSCLC), tumor-derived nicotinamide adenine dinucleotide phosphate (NADPH) oxidase recruits M2-TAMs through ROS/PI3K-dependent production of various cytokines, thereby facilitating the growth of NSCLC cells [79].
Numerous nanodrugs have been developed to target ROS in biomedicine field. Most of nanodrugs are developed with the aim of addressing hypoxia in the TME, leading to the activation of the ROS signaling pathway [80]. Inspired by the role of NADPH oxi-dase 2 (NOX2), Zhang et al. developed a novel nanosystem named OVA-Fe-GA (OFG), which integrates ovalbumin (OVA) and a network composed of Fe-gallic acid (GA) to emulate the ROS generation process of the NOX2 enzyme (O2 to O2•-, H2O2 and •OH). OFG to reprograme TAMs towards the M1 phenotype and significantly enhances their antigen cross-presentation in mice carrying B16-OVA tumors [81]. Li et al. developed a nanolipid-based delivery system for C6-ceramide (LipC6), which reduced the number of TAMs and their ROS production. LipC6 was found to induce the differentiation of TAMs into the M1 phenotype, leading to a decrease in immunosuppression and enhancement of CD8+ T activity [82]. Moreover, during a study on the molecular mechanisms of Fe3O4 nanoparticle-induced ferroptosis in macrophages and polarization to-wards the M1 phenotype, Shi et al. discovered that both macrophage ferroptosis and TAMs polarization are characterized by high levels of ROS expression [83]. Recently, Yang et al. developed an oxygen-consumption-responsive nano-ultrasonic contrast agent, Pt(IV)/CQ/PFH NPs-DPPA-1, based on the ROS signaling pathway. This agent reprograms the metabolism of immature dendritic cells (iDCs) and TAMs to enhance the ratio of mature dendritic cells (mDCs) and proinflammatory M1 cells [84]. Taken together, these nanodrugs can achieve precise treatment of tumors and regulation of the TME by targeting the ROS signaling pathway in TAMs. Given the complexity of ROS, further research and development will contribute to optimizing the design and utilization of nanodrugs to improve ROS specificity and maximize their potential in ROS-regulated therapies.
STAT pathway
STAT proteins belong to a cytoplasmic transcription factor family characterized by a conserved overall structure and modular domains [85]. Among these, STAT3 and STAT1 play pivotal roles in macrophage polarization. The expression of STAT1 protein is primarily induced by IFN-γ activation, as it acts as a downstream effector of IFN-γ signaling [86]. In TAMs, IFN-γ has been shown to enhance the M1/M2 ratio by down-regulating miR-3473b [87]. On the other hand, STAT3 is implicated in various biological processes, including cell proliferation, survival, differentiation, and angiogenesis [88, 89]. However, the STAT3 signaling pathway tends to promote macrophage polarization towards the M2 phenotype [90]. Recent research has highlighted that STAT3 activation contributes to tumor progression by upregulating protease expression in TAMs [91]. Subsequent experiments revealed that conditioned medium from tumor cells triggers inositol-requiring enzyme-1 (IRE-1) signaling, leading to TAM polarization through concurrent activation of STAT3 and production of synaptotagmin-binding protein 1 (sXBP1) [92, 93]. Consequently, the activation of the STAT3 signaling pathway is intricately linked to TAM behavior.
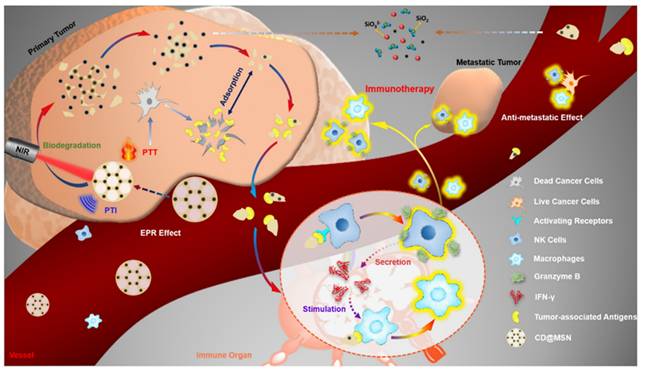
Nanodrugs have emerged as promising tools for regulating the polarization state of TAMs in the TME through modulation of the STAT signaling pathway. Qian et al. employed a hydrogen-bonding/electrostatically assisted co-assembly strategy to uniformly integrate carbon nanodots (CDs) into a mesoporous silica framework (CD@MSNs). Combined with photothermal therapy, CD@MSNs can stimulate NK cells to secrete IFN-γ, which might activate STAT1, promote the polarization of TAMs towards the M1 phenotype, and achieve immune-mediated suppression of tumor metastasis (Figure 5). Although CD@MSNs does not directly bind to targets on tumor-associated macrophages, it instead promotes the secretion of anti-tumor cytokines by other immune cells, thereby achieving the effect of targeting macrophages. This approach not only targets TAMs effectively but also leverages the body's immune system to fight cancer, providing a more comprehensive and robust treatment strategy. [94]. Moreover, the unique (ginsenoside Rg3- paclitaxel- Liposomes) Rg3-PTX-LPs, loaded with paclitaxel (PTX), were prepared using the thin-film hydra-tion method described by Zhu et al. The TME remodeling mechanism of Rg3-PTX-LPs included the suppression of IL-6/STAT3/p-STAT3 pathway activation to polarize pro-tumor M2 macrophages into an anti-tumor M1 phenotype, inhibition of MDSCs, reduction of tumor-associated fibroblasts (TAFs), and collagen fibers in the TME, ultimately promoting tumor cell apoptosis [95]. Guo et al. engineered two-dimensional carbon-based nanomaterials, specifically graphdiyne oxide (GDYO) nanosheets, to interact with an intracellular protein corona comprising STAT3. This interaction induces the expression of an anti-tumor phenotype in TAMs [96]. Furthermore, based on this signaling pathway, Xu et al. developed a nano-immuno-synergist (DPAM@OA) to restore inactivated macrophages in the ma-lignant glioma microenvironment [97]. Therefore, these findings indicate that nanodrugs can induce the conversion of M2-like TAMs to M1 macrophages by regulating the STAT1/3 signaling pathway, thereby promoting anti-tumor immune responses and inhibiting the growth and metastasis of tumor cells.
JNK pathway
The JNK signaling pathway is a subgroup of the mitogen-activated protein kinase (MAPK) family, which is primarily activated by cytokines and environmental stress [98-100]. Functional outcomes were different according to the specific JNK activation. In normal cells, JNKs can phosphorylate and regulate transcription factors such as c-Jun N-terminal Kinase 1 (JNK1), activating transcription factor 2 (ATF2), ETS domain-containing protein Elk-1 (ELK-1), tumor protein 53 (p53), recombinant c-Myc binding protein (MYCBP), and other non-transcription factors such as the Bcl family, in response to various extracellular stimuli [101-103]. JNKs are considered to be either positive or negative regulators of cancer and are associated with cell survival, apoptosis, malignant transformation, and tumor development, depending on the cell type and lineage being studied [104]. For instance, Recombinant thrombospondin 1(THBS1) derived from oral squamous cell carcinoma exosomes participates in the polarization of macrophages to an M1-like phenotype through p38, Akt, and SAPK/JNK signaling in the early phase [105]. Additionally, dioscin elicits anti-tumor immunity by inhibiting M2 macrophage polarization through the JNK and STAT3 pathways in lung cancer [106]. Currently, there is little research on nanotherapy targeting the JNK signaling pathway in TAMs. Most nanodrugs are used as carriers to encapsulate small-molecule drugs to modulate target genes. For instance, the sphingosine 1-phosphate receptor 1 (S1PR1) antagonist Ex26, modified by Zhou et al., was used to deliver the oncogene c-Myc inhibitor JQ1 via S1PR1 to TAMs. This approach effectively inhibits tumor-derived exosome-induced M2 polarization through the JNK signaling pathway [107]. We believe that targeting the JNK signaling pathway in TAMs represents a promising therapeutic strategy for reprogramming TAMs, and further exploration is needed to achieve a reliable and strong therapeutic effect.
Schematic diagram of in vivo delivery process of framework swelling-triggered biodegradable CD@MSN and its application for photothermal imaging-guided tumor-targeted PTT cooperated with debris-mediated immunotherapy against tumor metastasis (Copyright © 2019 American Chemical Society).
PI3K pathway
PI3K is a member of the lipid kinase family [108] and is first discovered 30 years ago [109]. It has been classified into three types (I, II, III) in mammals, with particular attention given to class I PI3K in cancer research [110-112]. PI3K activation primarily involves binding to substrates near the inner side of the plasma membrane [113, 114]. Studies have shown that the PI3K/Akt signaling pathway plays an important role in macrophage activation and gene expression. The impact of Akt kinase on macrophage polarization varies, as Akt1 gene knockout (Akt1-/-) leads to M1 polarization, whereas Akt2-/- results in M2 polarization [115, 116]. Furthermore, exosome-derived miRNAs from M2 macrophages can inhibit glioblastoma cell migration and invasion through the PI3K/AKT/mTOR signaling pathway [117].
To achieve dual targeting of TAMs and tumor cells in pancreatic cancer, Li et al. used a TME-responsive micellar system to co-load gemcitabine (GEM) and the PI3K inhibitor wortmannin (Wtmn). Specifically, GEM was covalently linked to dendritic poly-lysine DGL (GD) nanoparticles, which were then connected via a cathepsin B (CTSB) substrate peptide to polycaprolactone-polyethylene glycol micelles encapsu-lating Wtmn (PP/Wtmn) to form raspberry-like GD@PP/Wtmn micelles. Inhibiting the PI3K pathway repolarizes M2-like TAMs into M1-like TAMs, activating the anti-tumor immune response and synergizing with GEM to further suppress tumor growth [118]. Li et al. developed a nanomicelle targeting M2 TAMs, which is capable of co-delivering the PI3K-γ inhibitor NVP-BEZ 235 and CSF-1R-siRNA for specific TAMs reprogramming and activation of anti-tumor immune responses. The formulated nanomicelles showed improved targeting efficiency towards M2 TAMs both in vitro and in vivo. Additionally, compared to single pathway blockade, the dual blockade of PI3K-γ and CSF-1R has demonstrated enhanced remodeling effects on TAMs by reducing the number of M2 TAMs and increasing that of M1 TAMs, further suppressing the infiltration of MDSCs [119]. Furthermore, Li et al. synthesized porous hollow iron oxide nanoparticles (PHNPs) for loading a PI3K-γ small-molecule inhibitor (3-methyladenine, 3-MA) and further modified them with mannose for TAM targeting. The delivery system PHNPs@DPA-S-S-BSA-MA@3-MA exhibited excellent specificity. The combined action of PHNPs and 3-MA activates the inflammatory factor NF-κB p65 in macro-phages, synergistically switching TAMs into pro-inflammatory M1-type macrophages [120]. Therefore, although there are few nanodrugs targeting this pathway, combining a nanosystem with a PI3K inhibitor is a promising approach to overcome the short-comings of traditional drugs.
Notch pathway
The Notch signaling pathway consists of four main components: Notch receptors, Notch ligands, CSL (CBF-1, Suppressor of hairless, Lag) DNA-binding proteins, and downstream target genes. The Notch signaling pathway regulates many aspects of tumor initiation and progression [121], in which the DNA-binding protein RBP-J/CBF1 mediates the main transcriptional signal of Notch receptors [122-124], activating macrophages to exhibit stronger tumor-suppressive effects [125, 126]. Notch cleaves its transcriptionally active intracellular domain through a three-step proteolytic cleavage, releasing it to bind with the transcription factor CSL and regulate downstream gene expression. Deletion of CSL in monocytic lineages blocks TAMs differentiation and TAM-mediated immunosuppressive functions [127]. Notch is highly expressed in drug-resistant breast cancer, leading to the differentiation of TAMs into an M2 phenotype and acquiring drug resistance [128]. To date, there are no available studies regarding the application of nanodrugs that directly target the Notch signaling pathway. However, Scheurlen et al. proposed that integrating Notch-activating ligands (such as Dll1) and/or Notch1 overexpression plasmids into mannose-modified nanoparticles could selectively regulate the Notch signaling pathway in TAMs [129]. Further research into nanodrugs that modulate Notch signaling in TAMs could pave the way for innovative cancer treatments.
Conclusions and future perspectives
Macrophages possess high plasticity and show abundant infiltration in various pathological tissues, such as tumors, inflammation, rheumatoid arthritis, and parasitic infections. These are closely associated with the occurrence, development, and complications of the disease. Under the influence of a complex TME, macrophages infiltrating the tumor, which we defined as TAMs, undergo M2-like polarization and pro-mote tumor progression. To exert a cytotoxic effect on tumors, it is important to target TAMs, as well as a mainstream immunotherapy strategy for tumor treatment. With the development of nanodrugs, the efficacy of traditional targeted TAMs drugs can be improved, particularly synergizing with other treatments. However, the biggest challenge for nanodrugs lies in their future clinical translation. Although many nanodrugs have shown promising results in preclinical studies, there are still many issues regarding their clinical efficacy and safety that need to be addressed. Since nanoparticles can accumulate in non-target tissues to bring potential toxicity or trigger immune responses, resulting in insufficient treatment efficacy and severe adverse effects, the biocompatibility and safety of nanodrugs are major concerns. Biodistribution and targeting efficiency are also critical issues, which include non-specific uptake by non-target cells and limited penetration into dense tumor tissues. The complex synthesis and quality control of nanoparticles make large-scale production to be challenging and costly, due to the ensuring consistency in particle size, shape, and surface properties. For regulatory and clinical trial challenges, they include the lack of established guidelines for safety and efficacy evaluation, as well as the complexity of trial design to account for the unique pharmacokinetics of nanoparticles. Additionally, the high cost of development and production may limit the accessibility of nanodrugs, especially in low-resource settings. In summary, although the path to clinical translation is fraught with challenges, nanodrugs offer significant potential for enhancing cancer immunotherapy by targeting TAMs. Addressing these challenges will require interdisciplinary efforts, including advances in materials science, pharmacology, and clinical research. Once overcoming these hurdles, nanodrugs have the potential to revolutionize cancer treatment and improve patient outcomes.
Acknowledgements
Funding
This research was funded by the Natural Science foundation of the Jiangsu High Education Institutions of China (No. 24KJB310016), Interdisciplinary Basic Frontier Innovation Program of Suzhou Medical College of Soochow University (No. YXY2304036), the Open Project Program of the State Key Laboratory of Radiation Medicine and Protection (No. GZK12023025), the Science and Technology Project from Suzhou City (No. KJXW2022024) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author contributions
Conceptualization, writing original draft preparation, Q.L.; visualization, R.H. and H.X.; supervision, project administration, funding acquisition, writing review and editing, J.X., Y.W. and X.C. All authors have read and agreed to the published version of the manuscript.
Data availability statement
All data appear in the manuscript. For further inquiries, please contact the first author or corresponding author.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Zemek RM, Anagnostou V, Pires da Silva I, Long GV, Lesterhuis WJ. Exploiting temporal aspects of cancer immunotherapy. Nat Rev Cancer. 2024;24:480-97
2. Yang K, Halima A, Chan TA. Antigen presentation in cancer - mechanisms and clinical implications for immunotherapy. Nat Rev Clin Oncol. 2023;20:604-23
3. Nasir I, McGuinness C, Poh AR, Ernst M, Darcy PK, Britt KL. Tumor macrophage functional heterogeneity can inform the development of novel cancer therapies. Trends Immunol. 2023;44:971-85
4. Zhang X, Ji L, Li MO. Control of tumor-associated macrophage responses by nutrient acquisition and metabolism. Immunity. 2023;56:14-31
5. Gunassekaran GR, Poongkavithai Vadevoo SM, Baek M-C, Lee B. M1 macrophage exosomes engineered to foster M1 polarization and target the IL-4 receptor inhibit tumor growth by reprogramming tumor-associated macrophages into M1-like macrophages. Biomaterials. 2021;278:121137
6. De Meyer GRY, Zurek M, Puylaert P, Martinet W. Programmed death of macrophages in atherosclerosis: mechanisms and therapeutic targets. Nat Rev Cardiol. 2024;21:312-25
7. Liu H, Pan M, Liu M, Zeng L, Li Y, Huang Z. et al. Lactate: a rising star in tumors and inflammation. Front Immunol. 2024;15:1496390
8. Kumagai S, Koyama S, Itahashi K, Tanegashima T, Lin YT, Togashi Y. et al. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell. 2022;40:201-18 e9
9. Cheng K, Cai N, Zhu J, Yang X, Liang H, Zhang W. Tumor-associated macrophages in liver cancer: From mechanisms to therapy. Cancer Commun (Lond). 2022;42:1112-40
10. Abdin SM, Paasch D, Morgan M, Lachmann N. CARs and beyond: tailoring macrophage-based cell therapeutics to combat solid malignancies. J Immunother Cancer. 2021;9:e002741
11. Kumari N, Choi SH. Tumor-associated macrophages in cancer: recent advancements in cancer nanoimmunotherapies. J Exp Clin Cancer Res. 2022;41:68
12. Li S-L, Hou H-Y, Chu X, Zhu Y-Y, Zhang Y-J, Duan M-D. et al. Nanomaterials-Involved Tumor-Associated Macrophages' Reprogramming for Antitumor Therapy. ACS Nano. 2024;18:7769-95
13. Jin Y, Huang Y, Ren H, Huang H, Lai C, Wang W. et al. Nano-enhanced immunotherapy: Targeting the immunosuppressive tumor microenvironment. Biomaterials. 2024;305:122463
14. Dai J, Ashrafizadeh M, Aref AR, Sethi G, Ertas YN. Peptide-functionalized, -assembled and -loaded nanoparticles in cancer therapy. Drug Discov Today. 2024;29:103981
15. Zhang Y, Han G, Gu J, Chen Z, Wu J. Role of tumor-associated macrophages in hepatocellular carcinoma: impact, mechanism, and therapy. Front Immunol. 2024;15:1429812
16. Kumthekar P, Ko CH, Paunesku T, Dixit K, Sonabend AM, Bloch O. et al. A first-in-human phase 0 clinical study of RNA interference-based spherical nucleic acids in patients with recurrent glioblastoma. Sci Transl Med. 2021;13:eabb3945
17. Zhang Q, Sioud M. Tumor-Associated Macrophage Subsets: Shaping Polarization and Targeting. Int J Mol Sci. 2023;24:7493
18. Cassetta L, Pollard JW. A timeline of tumour-associated macrophage biology. Nat Rev Cancer. 2023;23:238-57
19. Xiang X, Wang J, Lu D, Xu X. Targeting tumor-associated macrophages to synergize tumor immunotherapy. Signal Transduction and Targeted Therapy. 2021;6:75
20. Pan Y, Yu Y, Wang X, Zhang T. Tumor-Associated Macrophages in Tumor Immunity. Front Immunol. 2020;11:583084
21. Zhou Y, Qian M, Li J, Ruan L, Wang Y, Cai C. et al. The role of tumor-associated macrophages in lung cancer: From mechanism to small molecule therapy. Biomed Pharmacother. 2024;170:116014
22. Wen J, Wang S, Guo R, Liu D. CSF1R inhibitors are emerging immunotherapeutic drugs for cancer treatment. Eur J Med Chem. 2023;245:114884
23. Murrey MW, Ng IT, Pixley FJ. The role of macrophage migratory behavior in development, homeostasis and tumor invasion. Front Immunol. 2024;15:1480084
24. Wen Z, Sun H, Zhang Z, Zheng Y, Zheng S, Bin J. et al. High baseline tumor burden-associated macrophages promote an immunosuppressive microenvironment and reduce the efficacy of immune checkpoint inhibitors through the IGFBP2-STAT3-PD-L1 pathway. Cancer Commun (Lond). 2023;43:562-81
25. Mehta AK, Cheney EM, Hartl CA, Pantelidou C, Oliwa M, Castrillon JA. et al. Targeting immunosuppressive macrophages overcomes PARP inhibitor resistance in BRCA1-associated triple-negative breast cancer. Nat Cancer. 2021;2:66-82
26. Akkari L, Bowman RL, Tessier J, Klemm F, Handgraaf SM, de Groot M. et al. Dynamic changes in glioma macrophage populations after radiotherapy reveal CSF-1R inhibition as a strategy to overcome resistance. Sci Transl Med. 2020;12:eaaw7843
27. Chang Y-W, Hsiao H-W, Chen J-P, Tzeng S-F, Tsai C-H, Wu C-Y. et al. A CSF-1R-blocking antibody/IL-10 fusion protein increases anti-tumor immunity by effectuating tumor-resident CD8+ T cells. Cell Rep Med. 2023;4:101154
28. Brana I, Calles A, LoRusso PM, Yee LK, Puchalski TA, Seetharam S. et al. Carlumab, an anti-C-C chemokine ligand 2 monoclonal antibody, in combination with four chemotherapy regimens for the treatment of patients with solid tumors: an open-label, multicenter phase 1b study. Target Oncol. 2015;10:111-23
29. Zhang X, Detering L, Sultan D, Luehmann H, Li L, Heo GS. et al. CC Chemokine Receptor 2-Targeting Copper Nanoparticles for Positron Emission Tomography-Guided Delivery of Gemcitabine for Pancreatic Ductal Adenocarcinoma. ACS Nano. 2021;15:1186-98
30. Möckel D, Bartneck M, Niemietz P, Wagner M, Ehling J, Rama E. et al. CCL2 chemokine inhibition primes the tumor vasculature for improved nanomedicine delivery and efficacy. J Control Release. 2023;365:358-68
31. Wan Z, Huang H, West RE, Zhang M, Zhang B, Cai X. et al. Overcoming pancreatic cancer immune resistance by codelivery of CCR2 antagonist using a STING-activating gemcitabine-based nanocarrier. Mater Today (Kidlington). 2023;62:33-50
32. Shen S, Li H-J, Chen K-G, Wang Y-C, Yang X-Z, Lian Z-X. et al. Spatial Targeting of Tumor-Associated Macrophages and Tumor Cells with a pH-Sensitive Cluster Nanocarrier for Cancer Chemoimmunotherapy. Nano Letters. 2017;17:3822-9
33. LaMarche NM, Hegde S, Park MD, Maier BB, Troncoso L, Le Berichel J. et al. An IL-4 signalling axis in bone marrow drives pro-tumorigenic myelopoiesis. Nature. 2024;625:166-74
34. Beckermann KE, Patnaik A, Winer I, Tan W, Bashir B, Kyriakopoulos CE. et al. A phase 1b open-label study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of py314 in combination with pembrolizumab in patients with advanced renal cell carcinoma. Invest New Drugs. 2024;42:179-84
35. Haston S, Gonzalez-Gualda E, Morsli S, Ge J, Reen V, Calderwood A. et al. Clearance of senescent macrophages ameliorates tumorigenesis in KRAS-driven lung cancer. Cancer Cell. 2023;41:1242-1260.e6
36. Di Carlo SE, Raffenne J, Varet H, Ode A, Granados DC, Stein M. et al. Depletion of slow-cycling PDGFRα+ADAM12+ mesenchymal cells promotes antitumor immunity by restricting macrophage efferocytosis. Nature Immunology. 2023;24:1867-78
37. Do MH, Shi W, Ji L, Ladewig E, Zhang X, Srivastava RM. et al. Reprogramming tumor-associated macrophages to outcompete endovascular endothelial progenitor cells and suppress tumor neoangiogenesis. Immunity. 2023;56:2555-2569.e5
38. Wang Y-N, Wang Y-Y, Wang J, Bai W-J, Miao N-J, Wang J. Vinblastine resets tumor-associated macrophages toward M1 phenotype and promotes antitumor immune response. Journal For Immunotherapy of Cancer. 2023;11:e007253
39. Zhao C, Pang X, Yang Z, Wang S, Deng H, Chen X. Nanomaterials targeting tumor associated macrophages for cancer immunotherapy. J Control Release. 2022;341:272-84
40. Zhang F, Dong J, Huang K, Duan B, Li C, Yang R. et al. "Dominolike" Barriers Elimination with an Intratumoral Adenosine-Triphosphate-Supersensitive Nanogel to Enhance Cancer Chemoimmunotherapy. ACS Nano. 2023;17:18805-17
41. Jin H, Wan C, Zou Z, Zhao G, Zhang L, Geng Y. et al. Tumor Ablation and Therapeutic Immunity Induction by an Injectable Peptide Hydrogel. ACS Nano. 2018;12:3295-310
42. Li Z, Ding Y, Liu J, Wang J, Mo F, Wang Y. et al. Depletion of tumor associated macrophages enhances local and systemic platelet-mediated anti-PD-1 delivery for post-surgery tumor recurrence treatment. Nat Commun. 2022;13:1845
43. Junankar S, Shay G, Jurczyluk J, Ali N, Down J, Pocock N. et al. Real-time intravital imaging establishes tumor-associated macrophages as the extraskeletal target of bisphosphonate action in cancer. Cancer Discov. 2015;5:35-42
44. Tian L, Yi X, Dong Z, Xu J, Liang C, Chao Y. et al. Calcium Bisphosphonate Nanoparticles with Chelator-Free Radiolabeling to Deplete Tumor-Associated Macrophages for Enhanced Cancer Radioisotope Therapy. ACS Nano. 2018;12:11541-51
45. Shu Y, Cheng P. Targeting tumor-associated macrophages for cancer immunotherapy. Biochim Biophys Acta Rev Cancer. 2020;1874:188434
46. Chen S, Saeed AFUH, Liu Q, Jiang Q, Xu H, Xiao GG. et al. Macrophages in immunoregulation and therapeutics. Signal Transduction and Targeted Therapy. 2023;8:207
47. Dorrington MG, Fraser IDC. NF-κB Signaling in Macrophages: Dynamics, Crosstalk, and Signal Integration. Front Immunol. 2019;10:705
48. Kloosterman DJ, Akkari L. Macrophages at the interface of the co-evolving cancer ecosystem. Cell. 2023;186:1627-51
49. Liu L, He H, Liang R, Yi H, Meng X, Chen Z. et al. ROS-Inducing Micelles Sensitize Tumor-Associated Macrophages to TLR3 Stimulation for Potent Immunotherapy. Biomacromolecules. 2018;19:2146-55
50. Menjivar RE, Nwosu ZC, Du W, Donahue KL, Hong HS, Espinoza C. et al. Arginase 1 is a key driver of immune suppression in pancreatic cancer. Elife. 2023;12:e80721
51. Xiao H, Guo Y, Li B, Li X, Wang Y, Han S. et al. M2-Like Tumor-Associated Macrophage-Targeted Codelivery of STAT6 Inhibitor and IKKβ siRNA Induces M2-to-M1 Repolarization for Cancer Immunotherapy with Low Immune Side Effects. ACS Cent Sci. 2020;6:1208-22
52. Guo Q, Jin Y, Chen X, Ye X, Shen X, Lin M. et al. NF-κB in biology and targeted therapy: new insights and translational implications. Signal Transduction and Targeted Therapy. 2024;9:53
53. Capece D, Verzella D, Flati I, Arboretto P, Cornice J, Franzoso G. NF-κB: blending metabolism, immunity, and inflammation. Trends Immunol. 2022;43:757-75
54. Zhang M, Liu ZZ, Aoshima K, Cai WL, Sun H, Xu T. et al. CECR2 drives breast cancer metastasis by promoting NF-κB signaling and macrophage-mediated immune suppression. Sci Transl Med. 2022;14:eabf5473
55. Di Cara F, Savary S, Kovacs WJ, Kim P, Rachubinski RA. The peroxisome: an up-and-coming organelle in immunometabolism. Trends Cell Biol. 2023;33:70-86
56. Ahmad A, Rashid S, Chaudhary AA, Alawam AS, Alghonaim MI, Raza SS. et al. Nanomedicine as potential cancer therapy via targeting dysregulated transcription factors. Semin Cancer Biol. 2023;89:38-60
57. Zhang Y, Lu J, Li H, Song H. Advances in dietary polysaccharides as hypoglycemic agents: mechanisms, structural characteristics, and innovative applications. Crit Rev Food Sci Nutr. 2025;65:1383-1403
58. Liu J, Xiang J, Jin C, Ye L, Wang L, Gao Y. et al. Medicinal plant-derived mtDNA via nanovesicles induces the cGAS-STING pathway to remold tumor-associated macrophages for tumor regression. Journal of Nanobiotechnology. 2023;21:78
59. Chen M, Yang D, Sun Y, Liu T, Wang W, Fu J. et al. In Situ Self-Assembly Nanomicelle Microneedles for Enhanced Photoimmunotherapy via Autophagy Regulation Strategy. ACS Nano. 2021;15:3387-401
60. Fredrich IR, Halabi EA, Kohler RH, Ge X, Garris CS, Weissleder R. Highly Active Myeloid Therapy for Cancer. ACS Nano. 2023;17:20666-79
61. Yang Z, Zhao Y, Zhang X, Huang L, Wang K, Sun J. et al. Nano-mechanical Immunoengineering: Nanoparticle Elasticity Reprograms Tumor-Associated Macrophages via Piezo1. ACS Nano. 2024;18:21221-35
62. Cao M, Yan H, Han X, Weng L, Wei Q, Sun X. et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. Journal For Immunotherapy of Cancer. 2019;7:326
63. Zhao J, Zhang Z, Xue Y, Wang G, Cheng Y, Pan Y. et al. Anti-tumor macrophages activated by ferumoxytol combined or surface-functionalized with the TLR3 agonist poly (I: C) promote melanoma regression. Theranostics. 2018;8:6307-21
64. Shi C, Liu T, Guo Z, Zhuang R, Zhang X, Chen X. Reprogramming Tumor-Associated Macrophages by Nanoparticle-Based Reactive Oxygen Species Photogeneration. Nano Letters. 2018;18:7330-42
65. Janbandhu V, Tallapragada V, Patrick R, Li Y, Abeygunawardena D, Humphreys DT. et al. Hif-1a suppresses ROS-induced proliferation of cardiac fibroblasts following myocardial infarction. Cell Stem Cell. 2022;29:281-297.e12
66. Gu W, Gong L, Wu X, Yao X. Hypoxic TAM-derived exosomal miR-155-5p promotes RCC progression through HuR-dependent IGF1R/AKT/PI3K pathway. Cell Death Discov. 2021;7:147
67. Zhang D, Tian X, Wang Y, Liu F, Zhang J, Wang H. et al. Polyphyllin I ameliorates gefitinib resistance and inhibits the VEGF/VEGFR2/p38 pathway by targeting HIF-1a in lung adenocarcinoma. Phytomedicine. 2024;129:155690
68. Deng X, Song Q, Zhang Y, Liu W, Hu H, Zhang Y. Tumour microenvironment-responsive nanoplatform based on biodegradable liposome-coated hollow MnO2 for synergistically enhanced chemotherapy and photodynamic therapy. J Drug Target. 2022;30:334-47
69. Gu X, Zhang R, Sun Y, Ai X, Wang Y, Lyu Y. et al. Oral membrane-biomimetic nanoparticles for enhanced endocytosis and regulation of tumor-associated macrophage. Journal of Nanobiotechnology. 2023;21:206
70. Shobaki N, Sato Y, Suzuki Y, Okabe N, Harashima H. Manipulating the function of tumor-associated macrophages by siRNA-loaded lipid nanoparticles for cancer immunotherapy. J Control Release. 2020;325:235-48
71. Cheung EC, Vousden KH. The role of ROS in tumour development and progression. Nat Rev Cancer. 2022;22:280-97
72. Liu J, Han X, Zhang T, Tian K, Li Z, Luo F. Reactive oxygen species (ROS) scavenging biomaterials for anti-inflammatory diseases: from mechanism to therapy. Journal of Hematology & Oncology. 2023;16:116
73. Yang Y-C, Zhu Y, Sun S-J, Zhao C-J, Bai Y, Wang J. et al. ROS regulation in gliomas: implications for treatment strategies. Front Immunol. 2023;14:1259797
74. Yang S, Lian G. ROS and diseases: role in metabolism and energy supply. Mol Cell Biochem. 2020;467:1-12
75. Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020;21:363-83
76. Lambertini M, Blondeaux E, Agostinetto E, Hamy A-S, Kim HJ, Di Meglio A. et al. Pregnancy After Breast Cancer in Young BRCA Carriers: An International Hospital-Based Cohort Study. JAMA. 2024;331:49-59
77. Zhang J, Fan J, Zeng X, Nie M, Luan J, Wang Y. et al. Hedgehog signaling in gastrointestinal carcinogenesis and the gastrointestinal tumor microenvironment. Acta Pharm Sin B. 2021;11:609-20
78. Yang M, Li J, Gu P, Fan X. The application of nanoparticles in cancer immunotherapy: Targeting tumor microenvironment. Bioactive Materials. 2021;6:1973-87
79. Zhang J, Li H, Wu Q, Chen Y, Deng Y, Yang Z. et al. Tumoral NOX4 recruits M2 tumor-associated macrophages via ROS/PI3K signaling-dependent various cytokine production to promote NSCLC growth. Redox Biology. 2019;22:101116
80. Raju GSR, Pavitra E, Varaprasad GL, Bandaru SS, Nagaraju GP, Farran B. et al. Nanoparticles mediated tumor microenvironment modulation: current advances and applications. Journal of Nanobiotechnology. 2022;20:274
81. Zhang T, Yin W, Zhao Y, Huang L, Gu J, Zang J. et al. NOX2 Enzyme Mimicking Nano-Networks Regulate Tumor-Associated Macrophages to Initiate Both Innate and Adaptive Immune Effects. Adv Healthc Mater. 2024;13:e2302387
82. Li G, Liu D, Kimchi ET, Kaifi JT, Qi X, Manjunath Y. et al. Nanoliposome C6-Ceramide Increases the Anti-tumor Immune Response and Slows Growth of Liver Tumors in Mice. Gastroenterology. 2018;154:1024-1036.e9
83. Wu C, Shen Z, Lu Y, Sun F, Shi H. p53 Promotes Ferroptosis in Macrophages Treated with Fe3O4 Nanoparticles. ACS Appl Mater Interfaces. 2022;14:42791-803
84. Yang X, Zhao M, Wu Z, Chen C, Zhang Y, Wang L. et al. Nano-ultrasonic Contrast Agent for Chemoimmunotherapy of Breast Cancer by Immune Metabolism Reprogramming and Tumor Autophagy. ACS Nano. 2022;16:3417-31
85. Li Y-J, Zhang C, Martincuks A, Herrmann A, Yu H. STAT proteins in cancer: orchestration of metabolism. Nat Rev Cancer. 2023;23:115-34
86. Ghosh C, Kakar R, Hoyle RG, Liu Z, Guo C, Li J. et al. Type I gamma phosphatidylinositol phosphate 5-kinase i5 controls cell sensitivity to interferon. Dev Cell. 2024;59:1028-1042.e5
87. Wu C, Xue Y, Wang P, Lin L, Liu Q, Li N. et al. IFN-γ primes macrophage activation by increasing phosphatase and tensin homolog via downregulation of miR-3473b. J Immunol. 2014;193:3036-44
88. Hanlon MM, Rakovich T, Cunningham CC, Ansboro S, Veale DJ, Fearon U. et al. STAT3 Mediates the Differential Effects of Oncostatin M and TNFα on RA Synovial Fibroblast and Endothelial Cell Function. Front Immunol. 2019;10:2056
89. Hu X, Li J, Fu M, Zhao X, Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduction and Targeted Therapy. 2021;6:402
90. Ma S, Sun B, Duan S, Han J, Barr T, Zhang J. et al. YTHDF2 orchestrates tumor-associated macrophage reprogramming and controls antitumor immunity through CD8+ T cells. Nature Immunology. 2023;24:255-66
91. Zhai K, Huang Z, Huang Q, Tao W, Fang X, Zhang A. et al. Pharmacological inhibition of BACE1 suppresses glioblastoma growth by stimulating macrophage phagocytosis of tumor cells. Nat Cancer. 2021;2:1136-51
92. Di Conza G, Tsai CH, Gallart-Ayala H, Yu YR, Franco F, Zaffalon L. et al. Tumor-induced reshuffling of lipid composition on the endoplasmic reticulum membrane sustains macrophage survival and pro-tumorigenic activity. Nat Immunol. 2021;22:1403-15
93. Ren F, Zhu W, Yang S, Zhang C, Hou Y, Li R. et al. Coumarin-Based Fluorescent Inhibitors for Photocontrollable Bioactivation. Mol Pharm. 2023;20:3223-33
94. Qian M, Chen L, Du Y, Jiang H, Huo T, Yang Y. et al. Biodegradable Mesoporous Silica Achieved via Carbon Nanodots-Incorporated Framework Swelling for Debris-Mediated Photothermal Synergistic Immunotherapy. Nano Letters. 2019;19:8409-17
95. Zhu Y, Wang A, Zhang S, Kim J, Xia J, Zhang F. et al. Paclitaxel-loaded ginsenoside Rg3 liposomes for drug-resistant cancer therapy by dual targeting of the tumor microenvironment and cancer cells. J Adv Res. 2023;49:159-73
96. Guo M, Zhao L, Liu J, Wang X, Yao H, Chang X. et al. The Underlying Function and Structural Organization of the Intracellular Protein Corona on Graphdiyne Oxide Nanosheet for Local Immunomodulation. Nano Letters. 2021;21:6005-13
97. Xu M, Cheng Y, Meng R, Yang P, Chen J, Qiao Z. et al. Enhancement of Microglia Functions by Developed Nano-Immuno-Synergist to Ameliorate Immunodeficiency for Malignant Glioma Treatment. Adv Healthc Mater. 2023;12:e2301861
98. Fang Z, Xu H, Duan J, Ruan B, Liu J, Song P. et al. Short-term tamoxifen administration improves hepatic steatosis and glucose intolerance through JNK/MAPK in mice. Signal Transduction and Targeted Therapy. 2023;8:94
99. Gadina M, Le MT, Schwartz DM, Silvennoinen O, Nakayamada S, Yamaoka K. et al. Janus kinases to jakinibs: from basic insights to clinical practice. Rheumatology (Oxford). 2019;58:i4-i16
100. Morris R, Kershaw NJ, Babon JJ. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018;27:1984-2009
101. Guo H, Lei H, Zhang B-G, Xu Z-C, Dong C, Hao Y-Q. c-Jun NH2-terminal kinase (JNK)/stress-activated protein kinase-associated protein 1 is a critical regulator for arthritis progression by meditating inflammation in mice model. Int Immunopharmacol. 2020;81:106272
102. Yuan Y, Wu H, Shuai B, Liu C, Zhu F, Gao F. et al. Mechanism of HSP90 Inhibitor in the Treatment of DSS-induced Colitis in Mice by Inhibiting MAPK Pathway and Synergistic Effect of Compound Sophorae Decoction. Curr Pharm Des. 2022;28:3456-68
103. Das A. The emerging role of microplastics in systemic toxicity: Involvement of reactive oxygen species (ROS). The Science of the Total Environment. 2023;895:165076
104. Cui C, Zhang H, Yang C, Yin M, Teng X, Yang M. et al. Inhibition of JNK Signaling Overcomes Cancer-Associated Fibroblast-Mediated Immunosuppression and Enhances the Efficacy of Immunotherapy in Bladder Cancer. Cancer Res. 2024;84:4199-213
105. Xiao M, Zhang J, Chen W, Chen W. M1-like tumor-associated macrophages activated by exosome-transferred THBS1 promote malignant migration in oral squamous cell carcinoma. J Exp Clin Cancer Res. 2018;37:143
106. Cui L, Yang G, Ye J, Yao Y, Lu G, Chen J. et al. Dioscin elicits anti-tumour immunity by inhibiting macrophage M2 polarization via JNK and STAT3 pathways in lung cancer. J Cell Mol Med. 2020;24:9217-30
107. Zhou X, Hong Y, Liu Y, Wang L, Liu X, Li Y. et al. Intervening in hnRNPA2B1-mediated exosomal transfer of tumor-suppressive miR-184-3p for tumor microenvironment regulation and cancer therapy. Journal of Nanobiotechnology. 2023;21:422
108. Su WY, Tian LY, Guo LP, Huang LQ, Gao WY. PI3K signaling-regulated metabolic reprogramming: From mechanism to application. Biochim Biophys Acta Rev Cancer. 2023;1878:188952
109. Arafeh R, Samuels Y. PIK3CA in cancer: The past 30 years. Semin Cancer Biol. 2019;59:36-49
110. Xue C, Li G, Lu J, Li L. Crosstalk between circRNAs and the PI3K/AKT signaling pathway in cancer progression. Signal Transduct Target Ther. 2021;6:400
111. El Motiam A, de la Cruz-Herrera CF, Vidal S, Seoane R, Baz-Martínez M, Bouzaher YH. et al. SUMOylation modulates the stability and function of PI3K-p110β. Cell Mol Life Sci. 2021;78:4053-65
112. Fontana F, Giannitti G, Marchesi S, Limonta P. The PI3K/Akt Pathway and Glucose Metabolism: A Dangerous Liaison in Cancer. International Journal of Biological Sciences. 2024;20:3113-25
113. Cao J, Tao C, Qin X, Wu K, Yang H, Liu C. et al. PI3K-Akt-SGF1-Dimm pathway mediates the nutritional regulation of silk protein synthesis in Bombyx mori. Int J Biol Macromol. 2024;278:134650
114. Tamaskovic R, Schwill M, Nagy-Davidescu G, Jost C, Schaefer DC, Verdurmen WPR. et al. Intermolecular biparatopic trapping of ErbB2 prevents compensatory activation of PI3K/AKT via RAS-p110 crosstalk. Nat Commun. 2016;7:11672
115. Zhao S, Mi Y, Guan B, Zheng B, Wei P, Gu Y. et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. Journal of Hematology & Oncology. 2020;13:156
116. Zhao S-J, Kong F-Q, Jie J, Li Q, Liu H, Xu A-D. et al. Macrophage MSR1 promotes BMSC osteogenic differentiation and M2-like polarization by activating PI3K/AKT/GSK3β/β-catenin pathway. Theranostics. 2020;10:17-35
117. Yao J, Wang Z, Cheng Y, Ma C, Zhong Y, Xiao Y. et al. M2 macrophage-derived exosomal microRNAs inhibit cell migration and invasion in gliomas through PI3K/AKT/mTOR signaling pathway. J Transl Med. 2021;19:99
118. Li T, Chen D, Liu H, Tao Y, He X, Zang S. et al. Spatially targeting and regulating tumor-associated macrophages using a raspberry-like micellar system sensitizes pancreatic cancer chemoimmunotherapy. Nanoscale. 2022;14:13098-112
119. Li M, Li M, Yang Y, Liu Y, Xie H, Yu Q. et al. Remodeling tumor immune microenvironment via targeted blockade of PI3K-γ and CSF-1/CSF-1R pathways in tumor associated macrophages for pancreatic cancer therapy. J Control Release. 2020;321:23-35
120. Li K, Lu L, Xue C, Liu J, He Y, Zhou J. et al. Polarization of tumor-associated macrophage phenotype via porous hollow iron nanoparticles for tumor immunotherapy in vivo. Nanoscale. 2020;12:130-44
121. Meurette O, Mehlen P. Notch Signaling in the Tumor Microenvironment. Cancer Cell. 2018;34:536-48
122. Tabaja N, Yuan Z, Oswald F, Kovall RA. Structure-function analysis of RBP-J-interacting and tubulin-associated (RITA) reveals regions critical for repression of Notch target genes. The Journal of Biological Chemistry. 2017;292:10549-63
123. Jiang Y, Wang Y, Chen G, Sun F, Wu Q, Huang Q. et al. Nicotinamide metabolism face-off between macrophages and fibroblasts manipulates the microenvironment in gastric cancer. Cell Metabolism. 2024;36:1806-1822.e11
124. He F, Li W-N, Li X-X, Yue K-Y, Duan J-L, Ruan B. et al. Exosome-mediated delivery of RBP-J decoy oligodeoxynucleotides ameliorates hepatic fibrosis in mice. Theranostics. 2022;12:1816-28
125. Song P, Duan J-L, Ding J, Liu J-J, Fang Z-Q, Xu H. et al. Cellular senescence primes liver fibrosis regression through Notch-EZH2. MedComm (2020). 2023;4:e346
126. Zhao J-L, Ye Y-C, Gao C-C, Wang L, Ren K-X, Jiang R. et al. Notch-mediated lactate metabolism regulates MDSC development through the Hes1/MCT2/c-Jun axis. Cell Reports. 2022;38:110451
127. Yang F, Chen Q, Yang M, Maguire EM, Yu X, He S. et al. Macrophage-derived MMP-8 determines smooth muscle cell differentiation from adventitia stem/progenitor cells and promotes neointima hyperplasia. Cardiovasc Res. 2020;116:211-25
128. Liu H, Wang J, Zhang M, Xuan Q, Wang Z, Lian X. et al. Jagged1 promotes aromatase inhibitor resistance by modulating tumor-associated macrophage differentiation in breast cancer patients. Breast Cancer Res Treat. 2017;166:95-107
129. Scheurlen KM, Chariker JH, Kanaan Z, Littlefield AB, George JB, Seraphine C. et al. The NOTCH4-GATA4-IRG1 axis as a novel target in early-onset colorectal cancer. Cytokine Growth Factor Rev. 2022;67:25-34
Author contact
![]() Corresponding author: *Correspondence: 18862111447com (J.X.); Wuyoyocom (Y.W.) xjchengedu.cn (X.C.)
Corresponding author: *Correspondence: 18862111447com (J.X.); Wuyoyocom (Y.W.) xjchengedu.cn (X.C.)

 Global reach, higher impact
Global reach, higher impact