Impact Factor
ISSN: 1837-9664
J Cancer 2014; 5(2):143-150. doi:10.7150/jca.7439 This issue Cite
Research Paper
Evaluation of Antioxidant and Antiangiogenic Properties of Caesalpinia Echinata Extracts
1. Departamento de Farmácia, Universidade Federal de Pernambuco. Avenida Professor Moraes Rêgo, s/n Cidade Universitária, Recife, PE, 50670-420 Pernambuco, Brasil.
2. Departamento de morfologia e fisiologia animal, Universidade Federal Rural de Pernambuco. Rua Dom Manoel de Medeiros, S/N, Dois Irmãos, Recife, PE, 52171-900 Pernambuco, Brasil.
3. Departamento de Bioquímica, Universidade Federal de Pernambuco. Avenida Professor Moraes Rêgo, s/n Cidade Universitária, Recife, PE, 50670-420 Pernambuco, Brasil.
4. Departamento de morfologia e fisiologia animal, Universidade Federal Rural de Pernambuco. Rua Dom Manoel de Medeiros, S/N, Dois Irmãos, Recife, PE, 52171-900 Pernambuco, Brasil.
5. Departamento de imunopatologia Keiso Asami (LIKA), Federal de Pernambuco. Avenida Professor Moraes Rêgo, s/n Cidade Universitária, Recife, PE, 50670-420 Pernambuco, Brasil.
6. Centro Brasileiro de Pesquisas Físicas, Rua Dr. Xavier Sigaud, 150, Urca, RJ, 22290-180 Rio de Janeiro, Brasil.
7. Departamento de Farmácia, Universidade Federal de Pernambuco. Avenida Professor Moraes Rêgo, s/n Cidade Universitária, Recife, PE, 50670-420 Pernambuco, Brasil.
Received 2013-8-17; Accepted 2013-11-16; Published 2014-1-23
Abstract
Natural products contain important combinations of ingredients, which may to some extent help to modulate the effects produced by oxidation substrates in biological systems. It is known that substances capable of modulating the action of these oxidants on tissue may be important allies in the control of neovascularization in pathological processes. The aim of this study was to evaluate the antioxidant and antiangiogenic properties of an ethanol extract of Caesalpinia echinata. The evaluation of antioxidant properties was tested using two methods (DPPH inhibition and sequestration of nitric oxide). The antiangiogenic properties were evaluated using the inflammatory angiogenesis model in the corneas of rats. The extract of C. echinata demonstrated a high capacity to inhibit free radicals, with IC50 equal to 42.404 µg/mL for the DPPH test and 234.2 µg/mL for nitric oxide. Moreover, it showed itself capable of inhibiting the inflammatory angiogenic response by 77.49%. These data suggest that biochemical components belonging to the extract of C. echinata interfere in mechanisms that control the angiogenic process, mediated by substrates belonging to the arachidonic acid cascade, although the data described above also suggest that the NO buffer may contribute to some extent to the reduction in the angiogenic response.
Keywords: antioxidant, angiogenesis, Caesalpinia echinata, natural products
INTRODUCTION
Angiogenesis is a complex multi-stage process that leads to the formation of new blood vessels from pre-existing capillaries. It is essential for various physiological processes, such as the growth and development of organs, capillary growth, the reproductive cycle and the healing of wounds. On the other hand, it may also contribute to the development of various pathological processes, such as rheumatoid arthritis, diabetic retinopathy, and the growth and metastasis of tumors [1, 2].
The creation of new blood vessels is regulated by a delicate equilibrium between signaling molecules of a pro- and anti-angiogenic nature. After activation of the endothelial cells by a pro-angiogenic stimulus, the new vessels are formed by a series of complex morphological and biochemical events in various stages. As a result, one finds events such as the degradation of the base membrane and remodeling of the extracellular matrix, where the action of proteases secreted by the endothelial cells brings about important changes in the cell-adhesion mechanisms, culminating in migration and proliferation of endothelial cells and the formation of the endothelial capillary tube [3, 4, 5].
Various studies suggest that vascular endothelial growth factor (VEGF) is the main mediator of the onset of angiogenesis and that this cytosine is capable of inducing vasodilation by producing nitric oxide (NO), and also of increasing the permeability of endothelial cells, or even stimulating the proliferation, survival, migration and differentiation of these cells (CE) [4, 6, 7].
Recent research has shown that vascular cells can produce reactive oxygen species (ROS) through NADPH oxidase. In fact, free radicals may play an ambiguous role in neovasularization, if, on the one hand, high concentrations of ROS causing oxidative stress, lead to apoptosis, while low levels function as signaling molecules mediating the proliferation and migration of the CEs, which may help to spread angiogenesis in vivo [8, 9]. According to Urao [8] ROS levels may be determined by the mechanisms that control the production of oxidant species associated with the activation of enzyme systems that perform an antioxidant function.
Understanding the role of ROS in triggering the formation of new blood vessels may enable these components to be classified as potential targets for treatment of angiogenesis-dependent diseases, leading us to infer that substances capable of modulating the action of these oxidants on tissue could be important allies in the control of neovascularization in pathological processes [4, 10].
On the other hand, it is known that many natural products contain important combinations of ingredients that may to some extent help to modulate the effects produced by oxidation substrates in biological systems.
The wood of Caesalpinia echinata Lam. (Fabaceae), commonly known as Pau-brasil, has thus been reported to contain a wide range of polyphenols, in particular flavonoids (brazilin and its derivative brazilein), in addition to lignins and lower concentrations of tannins and coumarins [11, 12, 13]. In view of this, it may be that this broad and diverse range of polyphenols, with known antioxidant properties [14], plays an important role in the modulation of expanding angiogenic systems, as, for example, in the local circulation of blood in the corneas of animals stimulated by irritant substances.
It is worth noting that different parts of pau-brasil are commonly used as adstringents, healing agents, oral analgesics and tonics, with the bark of the trunk also being used to treat diarrhea and dysentery and to strengthen the gums [15, 16, 17]. Studies have also shown that this species also has properties of medical interest, in particular antimicrobial, antifungal, anti-inflammatory, anti-nociceptive and antitumor properties [11, 18, 19, 20, 21, 22] (FREIRE, 2004; SOUZA, et al., 2004; GRANGEIRO, 2009; SHEN, et al., 2007; YEN, et al., 2010).
In view of this, the main aim of the present study was to evaluate the antioxidant and antiangiogenic properties of the ethanol extract of Caesalpinia echinata Lam, in a model of inflammatory angiogenesis in the corneas of albino Swiss Wistar rats.
METHODOLOGY
Ethanol extract of Caesalpinia echinata
The botanical material (wood) of C. echinata was harvested in December 2008, in the municipality of São Lourenço da Mata, Pernambuco, Brazil. The exsiccate of these specimens was identified by a botanist (Marlene Barbosa) and duly catalogued and stored in the Geraldo Mariz herbarium, at the Federal University of Pernambuco, with the identification tag 41,764.
After proper cleaning and processing of the material, the extract was obtained by shredding the wood of Caesalpinia echinata Lam. and marinating it in 98.2° GL ethanol for 72 hours, for subsequent desiccation in a Heizbed OB type evaporator, Heidolph 30-180C 1300W. The resulting material was re-suspended in 0.9% saline solution for future use.
Animals
Rattus norvegicus var. albino Wistar rats weighing around 180g were used. The animals were kept in polypropylene cages, in a 12h light/dark cycle at a temperature of (22 ± 2°C). Feeding was in the form of “pellets” and water ad libitum. Before division into groups, the animals underwent a clinical evaluation, the exclusion criterion being the presence of negative clinical signs or eye diseases.
The experimental protocol was approved by the Animal Experiments Ethics Committee of the UFPE, process n° 23076.056746/2012.
Antioxidant activity of the DPPH free radical
Antioxidant activity was determined by the sequestration reactions of the stable DPPH• radical (2,2-diphenyl-1-picryl-hydrazyl), for molecular components present in the extract of C. echiinata according to the methodology suggested by Blois [23].
A 20 mg/mL methanol solution of DPPH• was prepared, so as to present absorbance of 517 nm between 0.6 and 0.7. An alíquot of 250 µL of this solution was mixed with 40 µL of different concentrations of extract. Thirty minutes later, the absorbance was measured at 517 nm. Gallic acid was used as the reference compound for this assay and tests conducted in triplicate.
Analysis of Nitric Oxide (NO) Sequestration in vitro
Nitric oxide was produced from sodium nitroprusside and quantified using the Griess reaction following the method described by Marcocci et al [24]. Sodium nitroprusside, in a pH 7.4 water solution, spontaneously generates nitric oxide, which in turn interacts with oxygen to form nitrite ions, which can be measured using the Griess reagent. The molecular extracts of nitric oxide compete with oxygen, reducing the production of nitrite ions. For the experiment, 10 mM sodium nitroprusside in a phosphate buffer (PBS - pH 7.4) as added to different preparations at concentrations of 10, 25, 50, 100 and 200µg/mL of extract of C. echinata, both incubated at 25º C for 150 min. Then, 0.5 mL of Griess reagent (1% sulfanilamide, 2% H3PO4 and 0.1% naphthyl ethylenediamine dihydrochloride) were added to each sample. The absorbance of the chromophore formed was read at 540 nm, using gallic acid as the positive control.
The results were expressed in terms of minimum inhibitory concentration (Ic), with the percentage inhibition given by the formula I% = [(Ac - As)/ Ac] . 100 where Ac is the absorbance of the control and As the absorbance in the presence of the extract. A relation was established between the concentrations of the extract and the Ic given by regression analysis, using a Prism Grafic Pad Version 5.0 spreadsheet.
MTT assay
Cell viability was evaluated using the MTT assay which measures the metabolic conversion of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich) salt to the colored formazan dye. Vero cells (1×105/mL) were incubated in a 96-well plate in quadruplicate for 24 h. Cells were then treated with extracts (10, 25, 50, 100, 200 μg/mL) for 24 h. At the end of the incubation, the medium was removed and a MTT solution (5 mg/mL in RPMI) was added to the cells that were further incubated for 3 h. Afterwards the medium was removed and the intracellular formed formazan product was dissolved in DMSO. The optical density (OD) was measured at 595 nm in a microplate reader (Benchmark plus, Bio-Rad, Califórnia, EUA). Cell viability was calculated in comparison to the OD obtained by control cell, considered as 100%.
Protective effects against H2O2-induced damage
Vero cells (1×105/mL) were incubated in a 96-well plate in quadruplicate for 24 h. Cells were then treated with extracts (10, 25, 50, 100, 200 μg/mL) for 30 min and subsequently with H2O2 (1 mM) for 24 h. At the end of the incubation, the medium was removed and a MTT solution (5 mg/mL in RPMI) was added to the cells that were further incubated for 3 h. Afterwards the medium was removed and the intracellular formed formazan product was dissolved in DMSO. The optical density (OD) was measured at 595 nm in a microplate reader (Benchmark plus, Bio-Rad, Califórnia, EUA). Cell viability was calculated in comparison to the OD obtained by control cell, considered as 100%.
Antiangiogenic Activity
For evaluation of antiangiogenic activity, Wistar rats were divided into two groups, one treatment group (n=9) and one control (n = 8). After a five-day acclimatization period, these animals were anesthetized with a combination of xylazine (50mg/Kg) and ketamine (50mg/Kg) (im), complemented when handling the animals with instillation into the conjunctival sac, of two drops of 0.5% proxymetacaine hydrochloride local anesthetic. Once anesthetized, each animal underwent a procedure to pull back the eyelids, exposing almost the whole surface of the cornea and the superior limbus region. Each animal was thus cauterized in the upper edge of the right cornea, with the aid of 16X microscopic surgery. A 3 mm diameter circular paper filter, previously soaked in a solution of sodium hydroxide (NaOH) 1M for 1 min was used for this. The paper filter was then placed about 1 mm from the corneo-conjunctival limbus, and left there for 30s. Shortly thereafter, the eye was rinsed with 10ml of 0.9% saline solution, to remove excess NaOH. This technique produced a circular, homogenous, well-defined cauterization site of about 3.5 mm in diameter [25].
After 18 hours, the animals received specific treatment with a single dose of 10µL alcohol extract solution plus Tween 80, at a final concentration of 100mg/mL, if in the treatment group. The animals in the control group received 10µL of saline solution. After 18 days, the animals were anesthetized according to the anesthesiological protocol described above, and, using a digital camera attached to the eyepiece of the surgical microscope, images of the area of the eye treated were obtained, with magnification of 16X.
The images were transferred to a computer database and made available in Microsoft Paint format for enlargement and evaluation of the density of vessels in a circular area arranged in front of the pupils. The surface area A was obtained using the formula A = 4/3πr3, where r is the radius.
RESULTS AND DISCUSSION
Antioxidant activity of the DPPH free radical
Oxidative processes form an essential part of biochemical mechanisms related to functions such as cell respiration and has important relations to other aspects of metabolism. Different types of free radical can thus be produced naturally in the organism. In view of their abundance and application to different aspects of the functioning of the organism, the most commonly studied types of possible physiologically administered radicals have been reactive oxygen species (ROS).
Many researchers have observed that, in low concentrations, reactive oxygen species (ROS) appear to have beneficial functions related to clear physiological responses, such as defense against infections agents, cell growth regulation, intercellular and intracellular signaling, and synthesis of important biological substances, such as steroidal hormones [26]. However, an excess of these appears to be associated with the development of various diseases, such as those related to oxidative stress, including cancer, cardiovascular disease, cataracts, a compromised immune system and brain dysfunctions [27, 28].
Under many circumstances, the mechanisms available to certain types of organism for controlling free radicals, even against ROS, are not efficient within a certain time limit. This leads to the introduction so-called antioxidant agents, which are compounds that, even at relatively low concentrations, protect the biological system from the harmful effects of the oxidation process. Normally, exhibit an extensive capacity to stabilize these kinds of radical before they can cause cell damage [26, 29].
The 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical is normally used to evaluate the ability of antioxidant compounds to sequester free radicals. DPPH• is a stable radical that accepts the transfer of one electron from an antioxidant compound and, when undergoing electron reduction, loses its purple color, thereby affecting the absorbance of the medium [30, 31, 32].
In the present study, EECe performed satisfactorily in terms of capturing the organic DPPH radical, with an IC50 of 42.404 µg/mL, which was better than the performance of gallic acid (IC50 = 165.070 µg/mL), with a percentage difference of 74.3%.
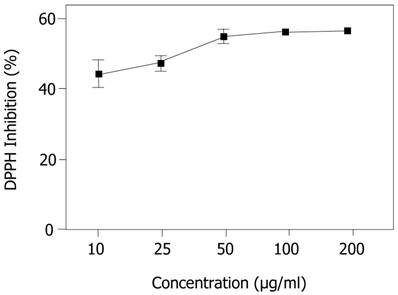
Figure 1 shows the performance of the extract at different concentrations (10, 25, 50, 100 and 200 µg/mL) in terms of stabilization of radicals, where it can be seen that the antioxidant response improves with increased concentration, creating a dose-dependent effect in the antioxidant activity of the extract with regard to the DPPH radical, indicating that, in general, a substrate that exhibits great potential to sequester free radicals has a low IC50. Figure 1 shows that, from 50 µg/mL onwards, the percentage inhibition of the DPPH radical tends to be constant, suggesting a saturation point, under the experimental conditions used for these assays.
Inhibition of 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical by ethanol extract of Caesalpinia echinata. Significant values ± S.D. (n= 3)
In our previous studies of the phytochemical composition of C. echinata we discovered the presence of polyphenols (flavonoids, tannins and coumarins) [11]. Fukushima & Fuzeto [12] have reported the presence of lignins and both results accord with the report made by Rezende et al. [33], who point to phenol derivatives C6-C3 as the most commonly observed metabolites in species of the genus Caesalpinia.
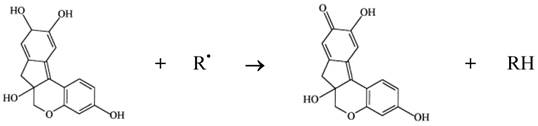
Phenol compounds are normally able to interfere in a series of physiological events in animals, including those relating to oxidation processes [14]. It is worth noting that various authors have suggested that brazilin, a homoflavonoid, is the main pharmacological product of interest in the wood of pau-brasil. It should be added that brazilin can be transformed into brazilein on contact with air or light, owing to an oxidation process in which the hydroxyl of brazilin is converted into a carbonyl group [13]. Both brazilin and brazilein have important antioxidant properties [34, 35]. The mechanism of action involved in the antioxidant activity of brazilin must be connected to its ability to oxidize in the presence of radical (e.g. reactive species of oxygen and nitrogen), resulting in a more stable, less-reactive radical. This happens due to the high reactivity of hydroxyl group of the flavonoids, able to inactivate the radicals, according to the diagram in Figure 2.
Antioxidant activity of brazilin
Analysis of in vitro sequestration of nitric oxide (NO)
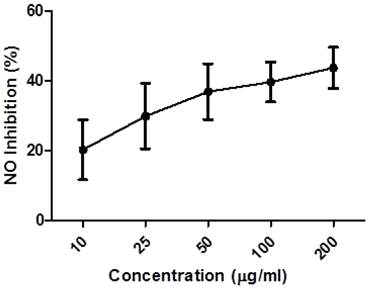
Endogenous nitric oxide (NO) is mainly produced from its precursor, the amino acid L-arginine, in the vascular endothelium, by phagocytes and some kinds of brain cell, performing a variety of roles in various physiological systems [36, 37]. However, in excess, it may be associated with the genesis of various diseases [38, 39]. These studies evaluated the capacity of the ethanol extract of Caesalpinia echinata (EECe) to inhibit “in vitro” production of nitric oxide through the mediation of sodium nitroprusside. Figure 3 leads us to infer that different concentrations of EECe (10, 25, 50, 100 and 200 µg/mL) inhibited production of the NO radical in a dose-dependent manner. The IC50 was 234,200 µg/mL, with the same type of analysis applied to the gallic acid standard giving a result of IC50= 174.560 µg/mL. These data suggest that EECe has an inhibitory effect of only 25.49% in relation to gallic acid.
It is interesting to note here that the inhibitory effect on the NO radical obtained was practically the inverse of the inhibitory effect achieved from the evaluation of DPPH, as the data in Table 1 show. A careful look at the IC50 figures for C. echinata shows that this parameter was 81.9% greater than NO, revealing a greater impact on the identification and capture of this type of radical.
In fact, at least three types of enzyme systems of the nitric oxide synthase—NOS—type can be associated with the production of NO and L-citrulline, depending on the availability of oxygen and nicotinamide dinucleotide phosphate (NADPH) as cofactors, in addition to the L-arginine substrate. Two of these systems depend on Ca++ and these are called constitutive with regard to neuronal (NO-1 or nNOS) or endothelial (NO-3 or eNOS) systems, while one is independent of Ca++ (NOS-2 or iNOS), known as induced, and associated with cell types such as macrophages, microglia, and others that are responsible for mediating inflammation, such as lipopolysaccharides in bacterial membranes, endotoxins and cytokines such as IL-1β, TNF-α and INF-γ [40, 41].
There are many disorders of the mechanisms regulating NO production, ranging from modifications of the G-protein-coupled receptors in membranes, brought on by saturation of H+ type ions in cases of ischemia, or a fall in levels of Mg2+ cytoplasm associated with metabolic disorders [42]. In cases of sepsis or tissue destruction, overproduction of NO may be mediated by macrophages, leading to generalized vasodilation and serious cardiovascular ramifications [43]. The results achieved in the present study suggest that extract of C. echinata may help to reduce the number of NO radicals in patients with organic disorders that make it possible to produce this type of radical, in a dose-dependent manner.
IC50 concentration values that inhibit 50% of the radical in question in μg/mL, used in methods for sequestering the DPPH radical and nitric oxide (NO), comparing the results with the gallic acid standard.
| Samples | DPPH (IC50) μg/mL | NO (IC50) μg/mL |
|---|---|---|
| Caesalpinia echinata | 42.404 | 234.200 |
| Gallic Acid | 165.070 | 174.560 |
Inhibition of NO radical by the ethanol extract of Caesalpinia echinata. Significant values ± S.D. (n= 3)
Jayakumar & Kanthimathi [44] note that nitric oxide is a free radical involved in the pathogenesis of cancer, as its presence changes the mechanisms associated with the vascularization dynamics of tumors and metastases. In fact, many studies indicate that nitric oxide plays a part in neo-vascularization, since its release is normally associated with events linked to the angiogenic cascade [45, 46, 47].
As noted above, the presence of polyphenol compounds in C. echinata wood may be associated with its capacity to inhibit free radicals. Research on antioxidant substances has shown that these kinds of compounds are capable of reducing inflammatory edema, inhibiting angiogenesis and the proliferation of tumor cells [44, 47, 48, 49].
In other studies carried out by this work group, it was shown that the ethanol extract of C. echinata was capable of reducing edema induced by carrageenan in rats' paws, and inhibiting the growth of Ehrlich's carcinoma and 180 sarcoma in “in vivo” assays [11].
MTT Test
The natural products have been an important source of antineoplastic components. It is known that many plants have significant antitumor properties [50]. C. echinata has demonstrated in previous studies a marked antitumoral effect in vivo, against the strains Ehrlich Carcinoma and Sarcoma 180. Knowing the possible cytotoxic potential of this vegetable we held a capacity assessment of cytotoxic ethanolic extract of C. echinata ahead the lineage of Vero cells.
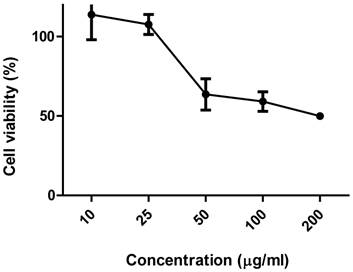
Figure 4 shows the activity of ethanolic extract of C. echinata about the viability of the culture of Vero cells. The toxic concentrations for 50% of the cells (CC50), determined using the MTT dye, was 168.4483 µg/mL (0.17 mg/mL). Here, it is interesting to note that the cytotoxic response intensifies according as increases the concentration of EE of C. echinata.
Viability of culture of VERO cells subjected to different concentrations of the ethanolic extract of Caesalpinia echinata.
Extracts of vegetable origin which contains principles antioxidants usually present potential cytotoxic and antitumor activity in experimental animals [51]. There are no other studies regarding the cytotoxic activity of C. echinata, however, this activity has already been observed in other species of the genus Caesalpinia, being this activity related to the presence of homoisoflavonoides, diterpenoides, as well as other polyphenolic compounds present in the chemical composition of wood; these plants [51, 52].
Protective effects against H2O2-induced damage
In this experiment, a fibroblast cell culture established from the renal cells of monkey Cercopithecus aethiops (African green monkey) was used to investigate the protective properties of EE of C. echinata against oxidative stress induced by H2O2.
The cells pre-treated with EE of C. echinata showed significant increase in viability when compared with cells exposed only to hydrogen peroxide, especially in concentrations of 50 µg/mL (12.5 ± 4.63 % ), 100 µg/mL (16.93 ± 3.52 %) and 200 µg/mL (46.52 ± 6.79 % ). The highest concentration tested it was observed that 46.5% of the cells pre-treated with EE of C. echinata survived exposure to oxidizing agent H2O2.
Our data demonstrate that, in in vitro conditions, the EE of C. echinata 200 μg/mL exerts an important protective action against the oxidative stress generated by H2O2. As discussed earlier, this result is possibly due to the antioxidant activity observed in this extract, which is probably related to the ability of polyphenolic compounds in act with antioxidant.
Antiangiogenic Activity
Angiogenesis has been described as one of the main events in the process of tumor growth, interfering in the mechanisms involved in the proliferation of metastases. Apart from cancer, other pathological conditions also persistently stimulate angiogenesis, such as diabetic retinopathy, hemangioma, arthritis, psoriasis and atherosclerosis. In view of this, inhibition of angiogenesis has been proposed as a strategy for combating cancer and other angiogenesis-dependent diseases.
The capacity to inhibit angiogenesis mediated by extract of C. echinata was evaluated using a model of inflammatory angiogenesis in the corneas of rats, according to the methodology described by Fechine-Jamacaru; Fechine Júnior; Moraes Filho [25], adapted for Wistar rats.
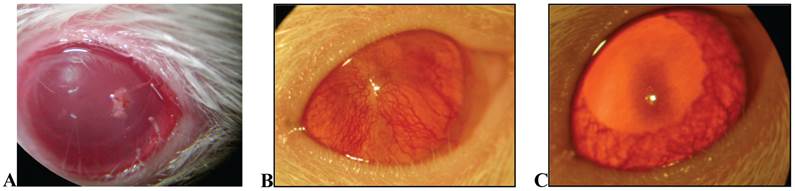
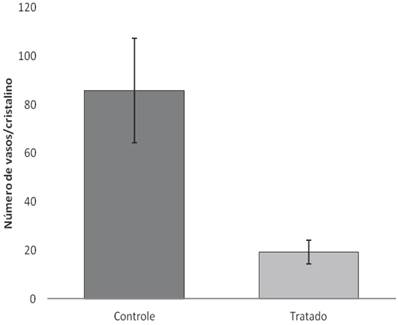
After 18 days of treatment with topical application of EECe (100mg/mL) at a dose of 10 µL/eye, it was observed that C. echinata inhibits the inflammatory angiogenic response caused by cauterization of rats' corneas with NaOH (1M), giving rise to a substantial reduction in the number of new capillaries (Figs. 5A, B and C), showing inhibitory activity (Figure 6) of 77.49%.
These data suggest that biochemical components belonging to the extract of C. echinata interfere with mechanisms that effect the angiogenic process, mediated by substrates belonging to the arachidonic acid cascade, although the data described above also suggest that NO buffer may contribute in some way to reducing the angiogenic response.
CONCLUSION
By way of conclusion, the results obtained in the present study have shown that the extract of C. echinata wood is capable of inhibiting the oxidant activity of DPPH and NO radicals in in vitro assays. In addition to presenting protective action against oxidative stress H2O2-induced. Associated with its antioxidant capacity, the extract also reduced the inflammatory angiogenic response in the corneas of rats.
We hope than further studies can be developed, with more specific experimental models, to shed more detailed light on the biochemical agents and the main mechanisms associated with the action of C. echinata in mediating vascular events, especially those associated with angiogenesis in cancer.
A) Overview of crystalline rats stimulated with NaOH (at the time of cauterizing); B) View of crystal in Wistar rat stimulated with NaOH, not treated (control); C) crystal in Wistar rat treated with EECe.
Number of vessels counted on the surface of the crystal in Wistar rats treated with EECe.
Competing Interests
The authors have declared that no competing interest exists.
References
1. González RP, Leyva A, Melo RAB, Moreira RDM, Pessoa C, Farias R F, Moraes MO. Método para o estudo in vivo da angiogênese: indução de neovascularização na córnea de coelho. Acta Cir. Bras. 2000;15:00-00
2. Damico FM. Angiogênese e doenças da retina. Arquivos Brasileiros de Oftalmologia. 2007;70:547-53
3. Castro PR. Cinética da angiogênese inflamatória induzida por implante de esponja na musculatura abdominal em camundongos. 2012 [Dissertação de mestrado]. Belo Horizonte: Universidade Federal de Minas Gerais.
4. Ushio-Fukai M. Redox signaling in angiogenesis: Role of NADPH oxidase. Cardiov. Res. 2006;71:226-35
5. Dias PF, Ribeiro-do-Valle RM, Maraschin RP, Maraschim M. Novos moduladores da formação de vasos sanguíneos. Biotecnologia Ciência & Desenvolvimento. 2002 nº 25
6. Capp C, Zennig N, Wajner S, Maia A.L. Papel do fator de crescimento endotelial vascular nos carcinomas de tireoide. Revista HCPA. 2009;29:51-59
7. Duarte M, Filho AL, Schmitt FC. “Angiogenesis, haemostasis and cancer: new paradigms and old concerns”. Bras Patol Med Lab. 2007;43:441-449
8. Urao N, Sudhahar V, Kim S-J. et al. Critical Role of Endothelial Hydrogen Peroxide in Post-Ischemic Neovascularization. PLoS ONE. 2013;8:1-12
9. Łuczak K, Balcerczyk A, Soszyński M, Bartosz G. Low concentration of oxidant and nitric oxide donors stimulate proliferation of human endothelial cells in vitro. Cell Biol Int. 2004;28:483-486
10. Polytarchou C, Papadimitriou E. “Antioxidants Inhibit Angiogenesis in Vivo through Down-regulation of nitric oxide synthase Expression and Activity. Free Radic Res. 2004;38:501-8
11. SILVA ECB. Avaliação Biológica de Caesalpinia echinata Lam. Usos e Riscos. ]Dissertação de Mestrado]. Recife: Universidade Federal de Pernambuco, Faculdade de Ciências Farmacêuticas, 114p. 2006
12. Fukushima RS, Fuzeto A. Quantificação do teor de lignina na madeira do pau-brasil (Caesalpinia echinata Lam.) através de três procedimentos analíticos. Simpósio Pau-brasil: ciência e arte, 2003, São Paulo, SP. Anais (CD-ROM). 2003
13. Oliveira LFC, Edwards HGM, Velozo ES, Nesbitt M. Vibrational spectroscopic study of brazilin and brazilein, the main constituents of brazilwood from Brazil. Vibrational Spectroscopy. 2002;28:143-249
14. Balasundram N, Sundram K, Samman S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chemistry. 2006;99:91-203
15. Silva RC. Plantas medicinais na saúde bucal. Vitória: Artgraf. 2001:136 p
16. Xavier MN, Ramos INC, Xavier Filho L. A Fitoterapia no Combate as Afecções Bucais, Editora idéia, 1995.
17. Ramalho RS. “Pau-brasil (Caesalpinia echinata Lam.)”.Viçosa: Universidade Federal de Viçosa, 1978. 11p.
18. Freire MLLB. Avaliação da atividade antitumoral e antibacteriana do extrato etanólico da Caesalpinia echinata Lam [Monografia de Conclusão de Curso]”. Recife: Departamento de Antibióticos, Universidade Federal de Pernambuco, 25p. 2004
19. Souza R.T.D, et al. Caracterização da presença de ácido daniélico em extratos de ramos de pau-brasil com atividade fungitóxica. http://www.botanicasp.org.br/pau_brasil/resumos/29.htm
20. Grangeiro A.R.S. Avaliação Biológica do potencial toxicológico e farmacológico das folhas de Caesalpinia echinata Lam. [Dissertação de Mestrado]”. Recife: Faculdade de Ciências Farmacêuticas, Universidade Federal de Pernambuco, 42p. 2009
21. Shen J, Zhang H, Lin H, Su H, Xing D, Du L. Brazilein protects the brain against focal cerebral ischemia reperfusion injury correlating to inflammatory response suppression”. European Journal of Pharmacology. 2007;558:88-95
22. Yen C-T, Nakagawa-Goto K, Hwang T.L. et al. Antitumor agents. 271: Total synthesis and evaluation of brazilein and analogs as anti-inflammatory and cytotoxic agents”. Bioorg. Med. Chem. Lett. 2010;20:1037-1039
23. Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199-1200
24. Marcocci L, Maguire JJ, Droy MT. The nitric oxide scavenging properties of Gingo biloba extract EGb 761. Biochem. Biophys. Res. Commun. 1994;15:748-755
25. Fechine-Jamacaru FV, Fechine Júnior JU, Moraes Filho MO. Modelo de angiogênese inflamatória em córnea de coelho induzida pela cauterização alcalina pontual. Acta Cirúrgica Brasileira. 2005;20:64-73
26. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44-84
27. Souza TM, Severi JA, Silva VYA, Santos E, Pietro RCLR. Bioprospecção de atividade antioxidante e antimicrobiana da casca de Stryphnodendron adstringens (Mart.) Coville (Leguminosae-Mimosoidae). Rev. Ciênc. Farm. Básica Apl. 2007;28:221-226
28. Barreiros ALBS, Barreiros ML, David JM, David JP, Queiroz LP. Atividade antioxidante de substâncias presentes em Dioclea violacea e Erythroxylum nummularia. Revista Brasileira de Farmacognosia. 2003;13:08-11
29. Naik SR. Antioxidants and their role in biological functions: An overview. Indian drugs. 2003;40:501-516
30. Roesler R, Malta L.G, Carrasco L.C, Holanda R.B, Sousa CAS, Pastore GM. Antioxidant activity of cerrado fruits. Ciênc. Tecnol. Aliment. 2007;27:53-60
31. Kumaran A, Karunakaran RJ. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. L.W.T. 2007;40:344-352
32. Duarte-Almeida JM, Santos RJ, Genovese MI, Lajolo FM. Avaliação da atividade antioxidante utilizando sistema β-caroteno/ácido linoléico e método de sequestro de radicais DPPH•. Ciênc. Tecnol. Aliment. 2006;26:446-452
33. Rezende CM, Corrêa VFS, Costa AVM, Castro BCS, Alves RJV. Constituintes Químicos Voláteis das Flores e Folhas do Pau-Brasil (Caesalpinia echinata, Lam.). Química Nova. 2004;27:414-416
34. Hu J, Yan X, Wang W, Wu H, Hua L, Du L. Antioxidnat activity in vitro of three constituents from Caesalpinia sappan L. Tsinghua Science and Technology. 2008;13:474-479
35. Liang C.H, Chan L.P, Chou T.H, Chiang F.Y, Yen C.M, Chen P.J, Ding H.Y, Lin R.J. Brazilein from Caesalpinia sappan L. Antioxidant Inhibits Adipocyte Differentiation and Induces Apoptosis through Caspase-3 Activity and Anthelmintic Activities against Hymenolepis nana and Anisakis simplex. Evidence-Based Complementary and Alternative Medicine. 2013;2013:2013
36. Rosselli M, Keller PJ, Dubey RK. Role of nitric oxide in the biology, physiology and pathophysiology of reproduction. Human Reproduction Update. 1998;4:3-24
37. Saravana PS, Renukadevi KP. Methods for Determination of Antioxidant Capacity, Total Flavonols and Flavonoids in Chlamydomonas reinhardtii (CC-124). J. Pharm. Res. 2011;4:2384-2387
38. Pacher P, Beckman JS, Liaudet L. Nitric Oxide and Peroxynitrite in Health and Disease”. Physiol Rev. 2007;87:315-424
39. Dai X, Faber JE. Endothelial Nitric Oxide Synthase Deficiency Causes Collateral Vessel Rarefaction and Impairs Activation of a Cell Cycle Gene Network During Arteriogenesis. Circul. Res. 2010;106:1870-1881
40. Bodgan C. Nitric oxide and the immune response. Nat. Immunol. 2001;2:907-916
41. Luiking YC, Poeze M, Ramsay G, Deutz NE. Reduced citrulline production in sepsis is related to diminished de novo arginine and nitric oxide production. Am J Clin Nutr. 2009;89:142-152
42. Volpe MA, Carneiro JJ, Magna LA, Viaro F, Origuela EAL, Evora PRB. Disfunção endotelial após isquemia global e reperfusão em cirurgia cardíaca com circulação extracorpórea: estudo do papel do magnésio em artérias coronarianas caninas. Rev Bras Cir Cardiovasc. 2002;17:187-200
43. Luiking YV, Deutz NEP. Isotopic investigation of nitric oxide metabolism in disease. Curr. Opin. Clin. Nutr. Metab. Care. 2003;6:103-108
44. Jayakumar R, Kanthimathi MS. Inhibitory effects of fruit extracts on nitric oxide-induced proliferation in MCF-7 cells. Food Chem. 2011;126:956-960
45. Ziche M, Morbidelli L, Masini E, Amerini S. et al. Nitric Oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J. Clin. Invest. 1994;94:2036-2044
46. Gallo O, Masini E, Morbidelli L, Franchi A, Fini-Storchi I, Vergari W.A, Ziche M. Role of Nitric Oxide in angiogenesis and tumor progression in head and neck cancer. J. Natl Cancer Inst. 1998;90:588-596
47. Ziche M, Morbidelli L. Nitric Oxide and Angiogenesis. J. Neuro-Oncology. 2000;50:39-148
48. Bennett LL, Rojas S, Seefeldt T. Role of Antioxidants in the Prevention of Cancer. Journal of Experimental & Clinical Medicine. 2012;4:215-222
49. Hofseth LJ. Nitric oxide as a target of complementary and alternative medicines to prevent and treat inflammation and câncer. C. Lett. 2008;268:10-30
50. Nussenbaum F, Herman IM. Tumor Angiogenesis: Insights and Innovations. J. Onc. 2010;2010:1-24
51. Hoff PM, Machado KK. Role of angiogenesis in the pathogenesis of cancer. Cancer Treatment Reviews. 2012;38:825-833
52. Quesada AR, Muñoz-Chápuli R, Medina MA. Anti-angiogenic drugs: from bench to clinical trials. Medicinal Research Reviews. 2006;26:483-530
Author contact
![]() Corresponding author: gersonpaiva1974com
Corresponding author: gersonpaiva1974com

 Global reach, higher impact
Global reach, higher impact