Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(4):657-664. doi:10.7150/jca.16200 This issue Cite
Research Paper
The Prognostic Impact of the Carcinoembryonic Antigen in Ampullary Cancer - A Retrospective Single Center Study
1. Institute for Surgical Pathology, Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Germany;
2. Clinic for General and Visceral Surgery, University of Freiburg, Faculty of Medicine, University of Freiburg, Germany;
3. Clinic for Surgery, University Clinic Schleswig-Holstein Campus Lübeck, Germany;
4. Comprehensive Cancer Center Freiburg, Medical Center - University of Freiburg, Germany;
5. German Cancer Consortium (DKTK) and German Cancer Research Center (DKFZ), Heidelberg, Germany;
6. Institute of Molecular Medicine and Cell Research, University of Freiburg, Freiburg, Germany;
7. BIOSS Centre for Biological Signaling Studies, University of Freiburg, Freiburg, Germany.
8. Division of Cardiac Surgery, University Hospital Basel, Basel, Switzerland.
*Ulrich Wellner and Peter Bronsert share last Authorship.
Received 2016-5-17; Accepted 2016-9-4; Published 2017-2-25
Abstract
Background: Carcinoembryonic antigen cell adhesion molecule (CEA) is a commonly immunohistochemically used antibody in pathological routine diagnostics with an overexpression in different cancers. We aimed to examine the immunohistochemically detectable CEA level in ampullary cancer and to correlate it with clinico-pathological data.
Methods: Shot-gun proteomics revealed CEA in undifferentiated ampullary cancer cell lines. Next, tumor tissue of 40 ampullary cancers of a retrospective single center cohort of 40 patients was stained immunohistochemically for CEA; CEA expression was determined and correlated with clinico-pathological data.
Results: Thirty-six patient specimens were included in statistical analysis. CEA expression and lymph node ratio (LNR) were the only independent predictors of overall survival in multivariate analysis.
Conclusion: To our knowledge, cell line and patient cohorts are the largest and characterized cohorts examined for CEA so far. Hereby, CEA expression in ampullary cancer cells permits an estimation of outcome and suggests an opportunity for individualized CEA-directed therapy. Further trials with larger cohorts are needed to verify our results and to integrate CEA immunohistochemistry into clinical routine.
Keywords: CEA, ampullary cancer, carcinoembryonic antigen.
Introduction
The Ampulla of Vater is a complex anatomic structure formed by the confluence of the pancreatic duct, the common bile duct and duodenal mucosa [1, 2]. Tumors arising in this region show a mixture of histophathological patterns including intestinal, pancreaticobiliary or mixed differentiation [3-5]. Among all gastrointestinal neoplasms, carcinomas of the Ampulla of Vater (AMPAC) are diagnosed in about 0.5 % of cases [6]. Prognostic factors for overall survival include nodal status [7], resection margin status (R), pancreatic head infiltration [8] and tumor size [9]. The family of the CEAs was discovered in 1965 by Gold et al. [10]. Physiologically they are expressed in fetal gut, liver, and pancreas between the second and sixth months of gestation, with intriguing re-appearance in cell dedifferentiation [11]. In the process of cell differentiation, members of the CEAs are down-regulated and their physiological expression is confined to the apical region of epithelial cells in most parts of the gastrointestinal tract [12]. Many human tumor tissues display overexpression of CEA (e.g., stomach, colon, rectum, pancreas, lung and cervix) [13] and some tumors show elevated CEA serum levels. For example in pancreatic cancer, a combination of serum levels of CEA and CA19-9 allows specific diagnosis [14]. In gastric cancer, high CEA serum levels are associated with poor prognosis [15]. Furthermore in non-small cell lung cancer (NSCLC) elevated CEA levels are associated with circulating tumor cells [16].
Cell-surface localization of CEA is mediated by a carboxy-terminal glycosylphosphatidylinositol (GPI) anchor [17]. At present the expression of CEA is frequently used for the immunohistochemical evaluation of normal and malignant tumorous tissue. However, increasing evidence supports that CEA is also functionally involved in tumor biology. In general, CEA is postulated to play an important regulatory role in apoptosis [18]. It plays an important role in mediating cell-cell/extracellular matrix contacts and thus inhibits anoikis, which is an apoptosis subtype that is triggered by perturbed cell-matrix interactions [19].
Based on the continuing interest in CEA, we have investigated CEA expression in an AMPAC patient cohort with detailed clinico-pathological annotation.
Materials and Methods
Ethics statement
The analyses were performed according to the guidelines of the Declaration of Helsinki. The study was approved by the Ethics Committee of the Medical University Freiburg, (ref 13/11). Before study inclusion, patient data were anonymized.
Patients and tumor tissue
Patients, who were primarily treated by surgery for AMPAC between 2007 and 2011 at the Clinic for General and Visceral Surgery, Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Germany, were included in the study cohort. No perioperative deaths have been observed. Histopathological workup was performed at the Institute for Surgical Pathology, Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Germany. For the current study, all histological samples from the tumor were revalidated independently by two experienced pathologists (PB, ST). Postoperative adjuvant Gemcitabine based therapy was conducted, if patients were resected R1 or AJCC/UICC Stage Grouping was 2a or higher. Clinical data from the database of the Clinic for General and Visceral Surgery, Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Germany, were reviewed by BK and JH for correctness. 40 patients were initially included. For the study one patient fell out due to inadequate tumor material, three patients (resection margin positive (R1)) were excluded due to the little case number.
Standard workup - resection specimens
For all AMPAC specimens included into this study, a standardized workup for gross examination was performed as described previously [20]. Briefly, all specimens were transferred for frozen section to the Institute of Surgical Pathology, Medical Center- University of Freiburg, Germany; and prior the examination by experienced pathologists tumor masses were measured; staging specific parameters (e.g. tumorsize, histological WHO type, tumor grade, UICC classification (pTNM)), status of the resection margins, presence or absence of lymphangiosis or hemangiosis carcinomatosa and perineural invasion were documented.
Proteomics
Mass spectrometry based proteomic analysis of the AMPAC cell line SNU478 was previously reported [21]. For the present work, we additionally determined relative protein expression levels using the iBAQ method [22].
Histological Subtype
According to Albores-Saavedra et al. [23] the tumors were classified into adenocarcinoma with intestinal-type, mixed-type, pancreaticobiliary-type and undifferentiated growth pattern.
CEA immunohistochemistry and evaluation
For immunohistochemical analysis the histological slides were pretreated for 15 minutes with Dako PTLink with EnVision™ FLEX Target Retrieval Solution, High pH (Dako DM827). Thereafter followed a five minutes treatment with EnVision™ FLEX Peroxidase-Blocking Reagent (Dako SM801) and incubation with ready-to-use primary antibody (Carcinoembryonic Antigen (CEA) Clone II-7 (Dako IR622) for 20 minutes. Visualization was done with HRP-conjugated secondary antibody and DAB chromogen according to the manufacturer's instructions (EnVision™ FLEX /HRP (Dako SM802) and EnVision™ FLEX DAB+ Chromogen (Dako DM827) 1/51 in EnVision™ FLEX Substrate Buffer (Dako SM803)). Sections were counterstained with hemalaun for one minute, dehydrated in an ascending alcohol concentration and covered with Xylol and Coverslipping Film (Tissue-TekR 4770).
CEA expression was quantified by expression intensity (0 to 3) and percentage of CEA-positive tumor cells in vision fields of 200 fold magnifications by two experienced pathologists, blinded for patient data and clinical outcome. For semi-quantitative analyses, CEA expression intensity and expression percentage were multiplied and normalized according to the overall mean.
Statistical analysis
For statistical calculations IBM SPSS Statistics Version 21 (IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.) was used. Survival data was analyzed according to the Kaplan-Meier method and Logrank test. For univariate analyses Spearman Chi squared and Kruskal-Wallis tests were used. For multivariate significance, clinico-pathological predictors were tested in a Cox proportional hazards model. Significance level was set to p=0.05. All statistical tests were performed two-sided.
Results
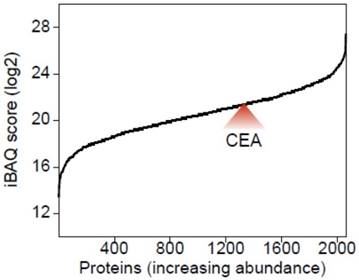
Proteomic Analysis Indicates Abundant CEA Expression in an Ampullary Cancer Cell Line
We have recently investigated the proteome composition of five different AMPAC cell lines, namely AMP7, AVC1, RCB1280, SNU869 and SNU478 to better understand their suitability as in vitro model systems for the investigation of AMPAC [21]. For the present work, we employed the iBAQ method [22] for all five aforementioned cell lines to determine relative protein abundances. As highlighted in Fig. 1, CEA was only identified in SNU478 cell and is among the top 50 % of all identified proteins ranked according to their abundance. This finding further substantiates AMPAC-associated expression of CEAs and characterizes SNU478 cells as a potential in vitro model system for putative investigations on functional roles of CEAs. The elevated expression levels of CEAs in SNU478 correspond to its partially dedifferentiated status.
Baseline parameters
36 patients with AMPAC were included. Mean age was 64 years. Patients received a pylorus preserving pancreaticoduodenectomy (PPPD), a Whipple operation or a total pancreatectomy. Mean tumor size was 20 mm. According to the current UICC [24] / AJCC [25]-Classification patients were staged as T1, T2, T3 and T4 for tumor extent, N0 and N1 for local nodal status and M0 and M1 for distant metastases. Furthermore, patients were grouped into UICC/AJJC into Stage IA (pT1, pN0), IB (pT2, pN0, pM0), IIA (pT3, pN0, pM0), IIB (pT1-3, pN1, pM0), III (pT4, pN0/1, pM0) and IV (pT1-4, pN0/1, pM1). Most tumors were moderately differentiated, some tumors were poorly differentiated and respectively one tumor was well and one undifferentiated. More details are presented in Table 1.
CEA expression level in the SNU478 AMPAC cell line across all identified proteins. Using the MaxQuant implemented iBAQ score, the average abundance (log2 transformed) of all proteins was plotted from the least to the most abundant protein.
Histological subtyping
Using conventional histology, 18 tumors (50.0 %) with an intestinal-type, two tumors (5.6 %) with a mixed-type, 12 tumors (33.3 %) with a pancreaticobiliary-type and four tumors (11.2 %) with an undifferentiated growth pattern were identified.
CEA immunohistochemistry
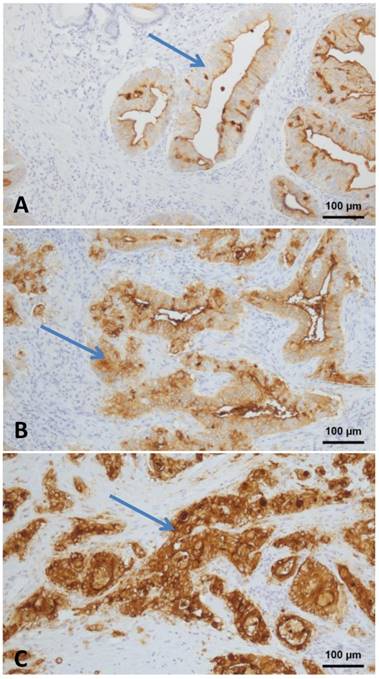
CEA expression was analyzed in 36 patients with AMPAC. A completely negative reaction for CEA was not observed in any tumor. Weak staining intensity (Fig. 2A) was seen in 14 tumors (39.2 %), moderate staining intensity (Fig. 2B) in 14 tumors (39.2 %) and strong staining intensity (Fig. 2C) in eight tumors (22.4 %). Quantitatively, the tumor with the lowest CEA positivity expressed CEA in 5% of all tumor cells. The highest detected percentage of CEA positive tumor cells was 95 %. All tumors demonstrated a mixed cytoplasmatic and membranous staining. A nuclear CEA expression was not detectable.
Multivariate analysis (included basement parameters): CEA ratio and LNR as multivariate prognostic relevant parameters of ampullary cancer (NR - not reached; NI - not included; e - excluded; HR - Hazard Ratio, CI - Confidence Interval).
| Parameters | Condition | n | Events (deaths) | Mean survival (month) | Log Rank p | Cox p | HR |
|---|---|---|---|---|---|---|---|
| All patients | 36 | 10 | 73 | ||||
| Age | < mean | 18 | 6 | 80 | 0.499 | NI | |
| > mean | 18 | 4 | 78 | ||||
| Sex | female | 16 | 4 | 77 | 0.969 | NI | |
| male | 20 | 6 | 81 | ||||
| Operation | PPPD | 31 | 7 | 86 | 0.078 | e | |
| Whipple | 4 | 2 | 54 | ||||
| Total PE | 1 | 1 | 22 | ||||
| T-Group | T 1/2 | 19 | 5 | 84 | 0.483 | NI | |
| T 3/4 | 17 | 5 | 70 | ||||
| N-Status | N0 | 15 | 2 | 97 | 0.105 | e | |
| N1 | 21 | 8 | 67 | ||||
| LNR | < mean | 21 | 2 | 103 | 0.003 | 0.004 | 7.766 (CI 1.630 - 37.012) |
| > mean | 15 | 8 | 50 | ||||
| M | M0 | 34 | 9 | 81 | 0.303 | NI | |
| M1 | 2 | 1 | 25 | ||||
| L | L0 | 20 | 3 | 98 | 0.030 | e | |
| L1 | 16 | 7 | 53 | ||||
| V | V0 | 34 | 9 | 80 | 0.618 | NI | |
| V1 | 2 | 1 | 61 | ||||
| Pn | Pn0 | 24 | 6 | 86 | 0.276 | NI | |
| Pn1 | 12 | 4 | 34 | ||||
| G | low | 25 | 5 | 91 | 0.150 | e | |
| high | 11 | 5 | 59 | ||||
| AJCC Stage Group | Stage 1A | 2 | 0 | NR | 0.763 | NI | |
| Stage 1B | 10 | 2 | NR | ||||
| Stage 2A | 1 | 0 | NR | ||||
| Stage 2B | 17 | 6 | NR | ||||
| Stage 3 | 4 | 1 | 38 | ||||
| Stage 4 | 2 | 1 | 7 | ||||
| Tumorsize | < mean | 16 | 4 | 85 | 0.67 | e | |
| > mean | 18 | 6 | 70 | ||||
| Subtype | Intestinal | 18 | 3 | 90 | 0.331 | NI | |
| Mixed | 2 | 1 | 74 | ||||
| PB | 12 | 5 | 60 | ||||
| Undiff. | 4 | 1 | 53 | ||||
| Subtype- Group | Intestinal | 18 | 3 | 90 | 0.123 | e | |
| Non-Intest | 18 | 7 | 67 | ||||
| CEA intensity | low | 14 | 1 | 82 | <0.001 | e | |
| medium | 14 | 5 | 76 | ||||
| high | 8 | 4 | 23 | ||||
| CEA %-intensity | < mean | 19 | 2 | 94 | 0.009 | 0.018 | 5.280 (CI 1.114 - 25.023) |
| > mean | 17 | 8 | 57 |
N-Status: nodal status; LNR: lymph node ratio; M: distant metastasis; L: lymphangiosis carcinomatosa; V: haemangiosis carcinomatosa; Pn: perineural invasion; G: grading.
Univariate analyses
Classical pathological parameters including LNR (p=0.003) and lymphangiosis carcinomatosa (p=0.03) demonstrated a significant correlation with overall survival in univariate analysis. Grouped histological tumor differentiation (intestinal vs. non-intestinal) (p=0.123), surgical intervention (p=0.078), N- (N0 vs. N1) (p=0.105) stage classification revealed statistical trends for survival. The remaining parameters patient´s age and gender, tumor size, T- Group (T1/2 vs. 3/4), AJCC Stage Group (I-IV) and histological tumor differentiation (intestinal, mixed, pancreaticobiliary and undifferentiated) had no statistical effect on overall survival.
The mean of the CEA expression intensity and CEA expression product (percentage multiplied with intensity thereof the mean) were significant predictors of survival (p= < 0.001 and p= 0.009).
To analyze the CEA expression pattern in the different histologic subtypes of AMPAC, two-sided Chi squared and Kruskal-Wallis tests were performed (Table 2). Hereby only statistical trends were identified for CEA expression in the pancreatobiliary type (83% moderate to high staining intensity) compared to intestinal type AMPAC (89% weak to moderate staining intensity).
CEA Expression in ampullary cancer: A - weak staining intensity; B - moderate staining intensity; C - strong staining intensity (Arrow: positive tumor cells). All images taken at 100 fold magnification from ampullary cancer specimen.
CEA expression pattern in histological subtypes of ampullary adenocarcinoma. p values derived from two-sided Chi squared and Kruskal-Wallis test.
| histologic subtype | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| intestinal | mixed | pancreato-biliary | poorly differentiated | p | ||||||
| n / median | % / range | n / median | % / range | n / median | % / range | n / median | % / range | |||
| n | 18 | 2 | 12 | 4 | - | |||||
| CEA percent | 45 | 5-95 | 60 | 60-60 | 60 | 5-85 | 63 | 15-95 | 0.930 | |
| CEA intensity | negative | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0.082 |
| weak | 10 | 56% | 0 | 0% | 2 | 17% | 2 | 50% | ||
| moderate | 6 | 33% | 2 | 100% | 6 | 50% | 0 | 0% | ||
| strong | 2 | 11% | 0 | 0% | 4 | 33% | 2 | 50% | ||
| CEA product | high | 12 | 67% | 0 | 0% | 5 | 42% | 2 | 50% | 0.237 |
| low | 6 | 33% | 2 | 100% | 7 | 58% | 2 | 50% | ||
N-Status: nodal status; LNR: lymph node ratio; M: distant metastasis; L: lymphangiosis carcinomatosa; V: haemangiosis carcinomatosa; Pn: perineural invasion; G: grading.
Multivariate analysis
For multivariate survival analysis, all variables displaying significant correlations and trends (p < 0.15) were included in a Cox proportional hazards model with forward selection and backward elimination. Only CEA expression product (p=0.018) and LNR (p=0.004) were independent predictors of survival after resection. In backward elimination only CEA expression intensity persisted. More details are presented in Table 1.
Discussion
Numerous studies concentrate on parameters influencing the outcome of AMPAC. We identified expression level of CEA and LNR as independent prognostic factors for overall survival in patients with AMPAC.
Comparable to our results, Tol et al. recently presented LNR as an independent prognostic factor in AMPAC [26]. Kohler et al. had previously added histological tumor subtype, local tumor spread and lymph node metastases as independent prognostic factors [27]. Our univariate analysis has revealed similar results, in addition to the histological subtype (intestinal versus non-intestinal, previously published [20]) we found that LNR and CEA are significant survival predictors in univariate analysis. Nevertheless, no statistical significances but trends between CEA and the histological subtype revealing a higher CEA expression in the pancreatibiliary subtype in AMPAC was noted and is in concordance with reduced survival. Interestingly, in multivariate analysis only CEA expression and LNR were independent. Contrary to our results, Lowe et al. had postulated perineural invasion as a more significant prognostic factor regarding survival time than histological subtype [28]. Schueneman et al. had found pancreaticobiliary subtype, perineural infiltration and patient age to be independently correlated with overall survival in a cohort of 154 AMPAC patients [29]. For periampullary carcinomas as a whole, Westgaard et al. had demonstrated that histological subtype is an independent prognostic factor [30], supporting our results that a pancreaticobiliary differentiation predicts poor prognosis.
For pathologists, CEA is a well-established and frequently used immunohistochemical antibody in routine diagnostics. Many human solid cancers overexpress CEA (e.g., stomach, colon, rectum, pancreas, lung and cervix) [13]. Consequently, the suitability of CEA for the immunopathological evaluation of tumors is controversially discussed. Alapat et al. found in medullary thyroid carcinoma a positive immunohistochemical staining for CEA combined with normal CEA serum levels [31]. In colorectal carcinoma the CEA expression correlates especially in combination with elevated serum CEA levels significantly with patients overall survival [32]. In pancreatic and AMPAC Blackman et al. revealed that CEA expression showed strong cytoplasmic positmpivity while in normal and adenomatous tissues they identified CEA positivity mostly along glycocalyceal cell borders [33]. Furthermore in NSCLC could be shown in 2012 that tumor CEA level was confirmed to be an independent prognostic factor in multivariate analysis [34].
In a study with 23 carcinomas (15 duodenal adenocarcinomas and eight AMPAC), Zhu et al. had previously analyzed the impact of CEA, EMA, p53 and TGF-alpha expression regarding patient survival time, tumor stage or histological grade without statistically significant correlation [35]. They have analyzed a number of eight AMPAC regarding immunohistochemical CEA expression, so we speculate that their number of sample was too small to reach statistical significance. In a larger cohort comprising 24 patients, Kamisawa et al. were able to prove the impact of CEA on survival in univariate but not in multivariate analyses [36]. Nevertheless, the findings of Kamisawa et al. further support our results. At this point it should be highlighted, that our cohort comprises 36 ampullary cancers, which is to our knowledge the largest ampullary carcinoma cohort analysed regarding CEA expression in literature thus far. There are many studies regarding CEA serum levels in patients as a tumor marker in different solid cancers [37, 38]. In this context Kim et al. found in their analyses of 104 ampullary cancers a multivariate significant influence of a CEA serum level > 5 ng/ml and disease recurrence. In univariate analyses especially the CEA serum level of > 5 ng/ml of the intestinal subtype was adversely correlated with disease free survival. [39]. But immunhistochemical analyses and correlation with clinico-pathological data are rare. Batge et al. postulate in 1986 that pancreatic “duct type” carcinomas, in contrast to “non duct type” tumors and “normal ducts”, are distinguished by the presence of a CEA related epitope [40]. In 1991 Yamaguchi et al analysed CEA expression in pancreatoduodenal carcinomas but were not able to demonstrate a prognostic relevance [41]. Recently published data for pancreatic cancer demonstrated a positive correlation between CEA overexpression and lymph node status as well as distant metastases and showed a decreased overall survival in univariate analysis. Nevertheless, in multivariate analyses, CEA also failed to reach statistical significance [42]. Obviously our immunohistological study had to deal with the difficulty of tumor heterogeneity and sampling error like other studies. Because of our standardized gross examination and the product of the percentage of positive tumor cells and the staining intensity used for our analyses, a possible bias was minimized.
Biologically, a possible explanation for the positive correlation between CEA expression and tumor aggressiveness was postulated by Ilantzis et al. Their results support the model of a direct influence of CEA onto colon carcinogenesis by inhibiting colonocyte differentiation [43]. Ordoñez et al. showed a prolonged survival of colonocytes without cell-basement membrane adhesion in case of CEA overexpression, compared to mature colonocytes [44]. Furthermore Ilantzis et al. showed that deregulated overexpression of CEA blocks cellular polarization, disrupts tissue architecture and blocks differentiation in cell lines and in vivo [45]. Under physiological circumstances, detached cells undergo anoikis, which can be prohibited by CEA overexpression [19].
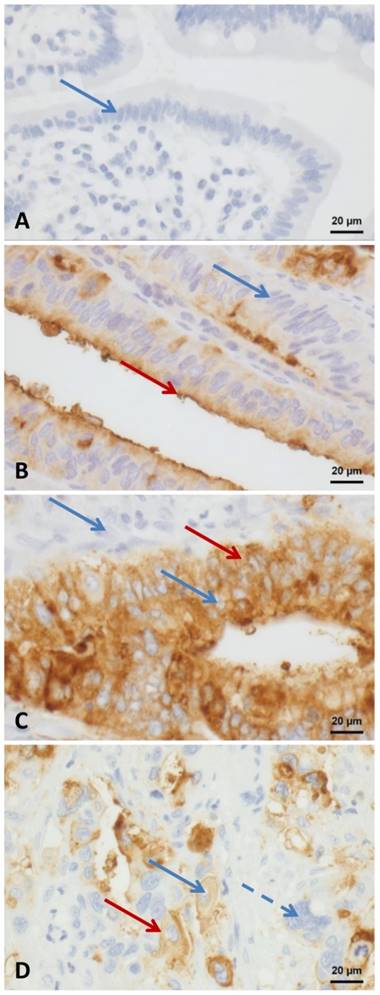
CEA Expression A: negative control - duodenal mucosa; B: tumor with a predominantly apical positivity - red arrow apical positivity; C: tumor with a predominantly cytoplasmatic positivity - red arrow cytoplasmatic positivity; D positive and negative tumor cells - blue arrow dotted: negative tumor cell, Red arrow: membranous and cytoplasmatic positivity; A-D blue arrows negative nuclei. All images taken at 400 fold magnification.
Furthermore, our results have the capability gaining therapeutically relevant importance because of the bispecific T-cell engager MEDI-565 (MT111). The CEA/CD3-Bispecific Antibody MEDI-565 (MT111) binds CEA positive tumor cells and develops cytotoxicity against these tumor cells in vitro. In vivo, MT111 inhibits growing of colon carcinoma, which was recently supported by a clinical phase I study [46]. Thereby, CEA could be a potential target for MT111 and lengthen patient survival time of patients suffering from ampullary carcinoma with CEA overexpression. Unfortunately CEA serum levels of the patients from the cohort were not available in this study. Hence, comparing CEA serum levels to the immunohistochemically detected CEA expression in the tumor tissue was not possible.
Conclusion
Our findings highlight CEA as a multivariate significant prognosticator in a group of ampullary carcinomas. Although AMPAC typically feature a favorable prognosis because of their early clinical symptoms compared to other pancreatic tumors, the CEA expression may be of value for the detection of cases with a relatively poor prognosis and a specific individual therapeutic need and option. The low incidence of ampullary cancer and the consecutive small patient cohort, even in a high-throughput medical center, is a legitimate limitation. Multicentric prospective clinical trials comprising larger cohorts are the logical consequence for verifying our results and to integrate CEA into clinical routine diagnostics.
Acknowledgements
O.S. is supported by grants of the Deutsche Forschungsgemeinschaft (DFG) (SCHI 871/2 and SCHI 871/5, SCHI 871/6, GR 1748/6, and INST 39/900-1) and the SFB850 (Project B8), a starting grant of the European Research Council (Programme “Ideas” - Call identifier: ERC-2011- StG 282111-ProteaSys), and the Excellence Initiative of the German Federal and State Governments (EXC 294, BIOSS).
Z.W.L is funded by Marie Curie IIF fellowship (Call Identifier: PIIF-GA-2012-329622 GlycoMarker).
The article processing charge was funded by the German Research Foundation (DFG) and the Albert Ludwigs University Freiburg in the funding programme Open Access Publishing.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Gassler N, Knuchel R. [Tumors of Vater's ampulla]. Der Pathologe. 2012;33(1):17-23
2. Doerr W, Seifert G. Spezielle pathologische Anatomie, Bd 10, 2 Aufl. Pathologie der Leber und Gallenwege. Springer, Berlin, Heidelberg, New York. 2000:1219-1257
3. Morini S, Perrone G, Borzomati D, Vincenzi B, Rabitti C, Righi D, Castri F, Manazza AD, Santini D, Tonini G. et al. Carcinoma of the ampulla of Vater: morphological and immunophenotypical classification predicts overall survival. Pancreas. 2013;42(1):60-66
4. Kimura W, Futakawa N, Yamagata S, Wada Y, Kuroda A, Muto T, Esaki Y. Different clinicopathologic findings in two histologic types of carcinoma of papilla of Vater. Japanese journal of cancer research: Gann. 1994;85(2):161-166
5. Schirmacher P, Buchler MW. Ampullary adenocarcinoma - differentiation matters. BMC cancer. 2008;8:251
6. Albores-Saavedra J, Schwartz AM, Batich K, Henson DE. Cancers of the ampulla of vater: demographics, morphology, and survival based on 5,625 cases from the SEER program. Journal of surgical oncology. 2009;100(7):598-605
7. Barton RM, Copeland EM 3rd. Carcinoma of the ampulla of Vater. Surgery, gynecology & obstetrics. 1983;156(3):297-301
8. Beger HG, Treitschke F, Gansauge F, Harada N, Hiki N, Mattfeldt T. Tumor of the ampulla of Vater: experience with local or radical resection in 171 consecutively treated patients. Arch Surg. 1999;134(5):526-532
9. Klempnauer J, Ridder GJ, Maschek H, Pichlmayr R. Carcinoma of the ampulla of vater: determinants of long-term survival in 94 resected patients. HPB surgery: a world journal of hepatic, pancreatic and biliary surgery. 1998;11(1):1-11
10. Gold P, Goldenberg NA. The Carcinoembryonic Antigen (CEA): Past, Present, and Future. Perspectives in Colon and Rectal Surgery. 1996:9 (2)
11. Gold P, Freedman SO. Specific carcinoembryonic antigens of the human digestive system. The Journal of experimental medicine. 1965;122(3):467-481
12. Benchimol S, Fuks A, Jothy S, Beauchemin N, Shirota K, Stanners CP. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell. 1989;57(2):327-334
13. Goldenberg DM, Sharkey RM, Primus FJ. Carcinoembryonic antigen in histopathology: immunoperoxidase staining of conventional tissue sections. Journal of the National Cancer Institute. 1976;57(1):11-22
14. Steinberg WM, Gelfand R, Anderson KK, Glenn J, Kurtzman SH, Sindelar WF, Toskes PP. Comparison of the sensitivity and specificity of the CA19-9 and carcinoembryonic antigen assays in detecting cancer of the pancreas. Gastroenterology. 1986;90(2):343-349
15. Deng K, Yang L, Hu B, Wu H, Zhu H, Tang C. The Prognostic Significance of Pretreatment Serum CEA Levels in Gastric Cancer: A Meta-Analysis Including 14651 Patients. PloS one. 2015;10(4):e0124151
16. Chen X, Wang X, He H, Liu Z, Hu JF, Li W. Combination of circulating tumor cells with serum carcinoembryonic antigen enhances clinical prediction of non-small cell lung cancer. PloS one. 2015;10(5):e0126276
17. http://www.carcinoembryonic-antigen.de/human/index.html
18. Soeth E, Wirth T, List HJ, Kumbhani S, Petersen A, Neumaier M, Czubayko F, Juhl H. Controlled ribozyme targeting demonstrates an antiapoptotic effect of carcinoembryonic antigen in HT29 colon cancer cells. Clinical cancer research: an official journal of the American Association for Cancer Research. 2001;7(7):2022-2030
19. Camacho-Leal P, Stanners CP. The human carcinoembryonic antigen (CEA) GPI anchor mediates anoikis inhibition by inactivation of the intrinsic death pathway. Oncogene. 2008;27(11):1545-1553
20. Bronsert P, Kohler I, Werner M, Makowiec F, Kuesters S, Hoeppner J, Hopt UT, Keck T, Bausch D, Wellner UF. Intestinal-type of differentiation predicts favourable overall survival: confirmatory clinicopathological analysis of 198 periampullary adenocarcinomas of pancreatic, biliary, ampullary and duodenal origin. BMC cancer. 2013;13:428
21. Lai ZW, Bolm L, Fuellgraf H, Biniossek ML, Makowiec F, Hopt UT, Werner M, Keck T, Bausch D, Sorio C. et al. Characterization of various cell lines from different ampullary cancer subtypes and cancer associated fibroblast-mediated responses. BMC cancer. 2016;16(1):195
22. Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M. Global quantification of mammalian gene expression control. Nature. 2011;473(7347):337-342
23. Albores-Saavedra JH, Henson DE, Klimstra DS. Tumors of the gall bladder, extrahepatic bile duct and ampulla of vater. In Atlas of Tumor Pathology. Washington DC: Armed Forces Institute of Pathology. 2000
24. Wittekind C, Meyer H-J. TNM Classification of Malignant Tumours, 7th Edition, vol. 7. 2009
25. Edge S. et al. AJCC Cancer Staging Manual, vol 7th. 2010.
26. Tol JA, Brosens LA, van Dieren S, van Gulik TM, Busch OR, Besselink MG, Gouma DJ. Impact of lymph node ratio on survival in patients with pancreatic and periampullary cancer. The British journal of surgery. 2015;102(3):237-245
27. Kohler I, Jacob D, Budzies J, Lehmann A, Weichert W, Schulz S, Neuhaus P, Rocken C. Phenotypic and genotypic characterization of carcinomas of the papilla of Vater has prognostic and putative therapeutic implications. American journal of clinical pathology. 2011;135(2):202-211
28. Lowe MC, Coban I, Adsay NV, Sarmiento JM, Chu CK, Staley CA, Galloway JR, Kooby DA. Important prognostic factors in adenocarcinoma of the ampulla of Vater. The American surgeon. 2009;75(9):754-760 discussion 761
29. Schueneman A, Goggins M, Ensor J, Saka B, Neishaboori N, Lee S, Maitra A, Varadhachary G, Rezaee N, Wolfgang C. et al. Validation of histomolecular classification utilizing histological subtype, MUC1, and CDX2 for prognostication of resected ampullary adenocarcinoma. British journal of cancer. 2015;113(1):64-68
30. Westgaard A, Tafjord S, Farstad IN, Cvancarova M, Eide TJ, Mathisen O, Clausen OP, Gladhaug IP. Pancreatobiliary versus intestinal histologic type of differentiation is an independent prognostic factor in resected periampullary adenocarcinoma. BMC cancer. 2008;8:170
31. Alapat DV, Ain KB, Sloan DA, Monaghan KG, Karabakhtsian RG. Disparity between tissue and serum calcitonin and carcinoembryonic antigen in a patient with medullary thyroid carcinoma. Endocrine. 2011;39(2):148-152
32. Cunningham L, Stocking B, Halter SA, Kalemeris G. Immunoperoxidase staining of carcinoembryonic antigen as a prognostic indicator in colorectal carcinoma. Diseases of the colon and rectum. 1986;29(2):111-116
33. Blackman E, Nash SV. Diagnosis of duodenal and ampullary epithelial neoplasms by endoscopic biopsy: a clinicopathologic and immunohistochemical study. Human pathology. 1985;16(9):901-910
34. Wang J, Ma Y, Zhu ZH, Situ DR, Hu Y, Rong TH. Expression and prognostic relevance of tumor carcinoembryonic antigen in stage IB non-small cell lung cancer. Journal of thoracic disease. 2012;4(5):490-496
35. Zhu L, Kim K, Domenico DR, Appert HE, Howard JM. Adenocarcinoma of duodenum and ampulla of Vater: clinicopathology study and expression of p53, c-neu, TGF-alpha, CEA, and EMA. Journal of surgical oncology. 1996;61(2):100-105
36. Kamisawa T, Fukayama M, Koike M, Tabata I, Egawa N, Isawa T, Okamoto A, Hayashi Y. Carcinoma of the ampulla of Vater: expression of cancer-associated antigens inversely correlated with prognosis. The American journal of gastroenterology. 1988;83(10):1118-1123
37. Vasiliades G, Kopanakis N, Vasiloglou M, Zografos G, Margaris H, Masselou K, Kokosi E, Liakakos T. Role of the hematopoietic cytokines SCF, IL-3, GM-CSF and M-CSF in the diagnosis of pancreatic and ampullary cancer. The International journal of biological markers. 2012;27(3):e186-194
38. Chen Y, Gao SG, Chen JM, Wang GP, Wang ZF, Zhou B, Jin CH, Yang YT, Feng XS. Serum CA242, CA199, CA125, CEA, and TSGF are Biomarkers for the Efficacy and Prognosis of Cryoablation in Pancreatic Cancer Patients. Cell biochemistry and biophysics. 2014
39. Kim WS1, Choi DW, Choi SH, Heo JS, You DD, Lee HG. Clinical significance of pathologic subtype in curatively resected ampulla of vater cancer. J Surg Oncol. 2012;105(3):266-72
40. Batge B, Bosslet K, Sedlacek HH, Kern HF, Kloppel G. Monoclonal antibodies against CEA-related components discriminate between pancreatic duct type carcinomas and nonneoplastic duct lesions as well as nonduct type neoplasias. Virchows Archiv A, Pathological anatomy and histopathology. 1986;408(4):361-374
41. Yamaguchi K, Enjoji M, Tsuneyoshi M. Pancreatoduodenal carcinoma: a clinicopathologic study of 304 patients and immunohistochemical observation for CEA and CA19-9. Journal of surgical oncology. 1991;47(3):148-154
42. Gebauer F, Wicklein D, Horst J, Sundermann P, Maar H, Streichert T, Tachezy M, Izbicki JR, Bockhorn M, Schumacher U. Carcinoembryonic antigen-related cell adhesion molecules (CEACAM) 1, 5 and 6 as biomarkers in pancreatic cancer. PloS one. 2014;9(11):e113023
43. Ilantzis C, Jothy S, Alpert LC, Draber P, Stanners CP. Cell-surface levels of human carcinoembryonic antigen are inversely correlated with colonocyte differentiation in colon carcinogenesis. Laboratory investigation; a journal of technical methods and pathology. 1997;76(5):703-716
44. Ordonez C, Screaton RA, Ilantzis C, Stanners CP. Human carcinoembryonic antigen functions as a general inhibitor of anoikis. Cancer research. 2000;60(13):3419-3424
45. Ilantzis C, DeMarte L, Screaton RA, Stanners CP. Deregulated expression of the human tumor marker CEA and CEA family member CEACAM6 disrupts tissue architecture and blocks colonocyte differentiation. Neoplasia. 2002;4(2):151-163
46. Huehls AM, Coupet TA, Sentman CL. Bispecific T-cell engagers for cancer immunotherapy. Immunology and cell biology. 2015;93(3):290-296
Author contact
![]() Corresponding author: Hannah Fuellgraf: Breisacher Str. 115a, 79106 Freiburg, Germany; email: hannah.fuellgrafde
Corresponding author: Hannah Fuellgraf: Breisacher Str. 115a, 79106 Freiburg, Germany; email: hannah.fuellgrafde

 Global reach, higher impact
Global reach, higher impact