Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(8):1337-1348. doi:10.7150/jca.23162 This issue Cite
Research Paper
A Real-World Data Study to Evaluate Treatment Patterns, Clinical Characteristics and Survival Outcomes for First- and Second-Line Treatment in Locally Advanced and Metastatic Urothelial Cancer Patients in Germany
1. Department of Urology, Medical Faculty, Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany
2. School of Medicine and Health Sciences, University Hospital for Urology, Carl von Ossietzky University Oldenburg, Germany
3. Genentech, South San Francisco, CA, USA
4. Roche, Basel, Switzerland
5. Real World Insights, QuintilesIMS, Frankfurt, Germany
6. Department of Urology, University Hospital Schleswig-Holstein, Lübeck, Germany
*Dr. Günter Niegisch and Dr. Holger Gerullis contributed equally to this manuscript
Received 2017-10-4; Accepted 2017-12-30; Published 2018-3-29
Abstract
Background: Worldwide, urothelial carcinoma (UC) is a common cause of morbidity and mortality. In particular, the incidence of bladder cancer varies widely across Europe; Germany has the ninth highest international age-standardized incidence. For advanced UC or metastatic UC (mUC), platinum-based combination chemotherapy is the standard first-line (1L) treatment; however, there is wide heterogeneity of second-line (2L) treatments, ranging from vinflunine in parts of Europe to taxanes and other agents elsewhere in Europe, in the United States and globally. Limited data exist on treatment patterns and outcomes in patients with advanced UC or mUC in the routine clinical setting in Germany. The objective of this study was to describe clinical characteristics, treatment patterns and subsequent outcomes in this setting.
Methods: This retrospective observational cohort analysis evaluated 1L and 2L treatment patterns and overall survival (OS) in patients aged ≥18 years with advanced UC or mUC (T4b, N2-3 and/or M1) at office-based urology and academic as well as nonacademic urology clinics throughout Germany between 1 November 2009 and 2 June 2016. Data were obtained through the GermanOncology database and additional treatment centers using similar electronic case report forms.
Results: Among the 435 patients included in the analysis, 435 received 1L treatment and 125 received 2L treatment. Median age at start of 1L treatment was 69 years, 75% of patients were male, 75% were current or ex-smokers, 15% had hemoglobin <10 g/dL and 44% had creatinine clearance<60 mL/min/1.73; proportions were similar with 2L treatment. Cardiovascular disease was the most frequently reported comorbidity (65%), followed by diabetes (19%). Most patients (77%) received 1L platinum-based combination treatment (most commonly gemcitabine + cisplatin, 83%). Of those treated with 2L treatment, 66% received a single agent (most commonly vinflunine, 71%). Median OS (95% CI) with 1L treatment was 16.1 months (13.7-19.2) overall and 17.7 months (14.4-24.2) with 1L cisplatin + gemcitabine. In the 1L setting, 12-month OS was 61%, 24-month OS was 39% and 36-month OS was 26%. Median (95% CI) OS with 2L treatment was 9.2 months (5.5-11.6) overall and 5.9 months (4.1-12.6) with 2L vinflunine. In the 2L setting, OS rates for the same time periods were 40%, 22% and 8%, respectively. Median (95% CI) progression-free survival was 7 months (6.4-8.1) and 4 months (3.0-4.8), respectively, in the 1L and 2L settings. Objective response rates were 34% in the 1L setting and 14% in the 2L setting. No difference in OS by sex or smoking status was noted. Patients with or without renal impairment had a 12-month OS of 54% or 69%, respectively. OS at 12 months was 63% among patients with an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 to 1 vs 53% among patients with an ECOG PS of ≥2. Cox regression analysis found no difference in OS between vinflunine and other 2L treatments (P = 0.69).
Conclusions: This study provides a contemporary multicenter assessment of real-world treatment patterns and outcomes among palliatively treated patients with UC in Germany. The findings were generally consistent with the poor treatment outcomes observed globally, underscoring the need for effective 1L and 2L treatment for advanced UC or mUC.
Keywords: first-line treatment, German clinical practice, metastatic urothelial carcinoma, second-line treatment, treatment patterns
Introduction
Globally, there were approximately 430,000 new cases of and 165,000 deaths due to bladder cancer in 2012 [1]; it was the ninth most common cancer among both sexes and was the thirteenth most common cause of death due to cancer [1]. The incidence and prevalence of bladder cancer varies widely across European countries [1, 2]. In Germany, urothelial carcinoma (UC) constitutes one of the most common cancers [3]. Based on 2012 data, Germany had the ninth highest international age-standardized incidence of bladder cancer in men and women [4]. For bladder cancer, of which UC accounts for >90% of cases, an incidence of 28,910 newly diagnosed cases per year was reported in Germany, of which 15,400 were invasive [1, 4]. In Germany, non-UCs of the urinary bladder are infrequent (<10%) [5, 6]. Similarly, UC of the upper urinary tract, ureters and renal pelvis accounts for <10% of urothelial bladder cancers [7-9], with 2770 incident cases per year in Germany, in both sexes [10].
The clinical course of patients with UC varies widely, with approximately two-thirds of patients experiencing an uncomplicated clinical course due to a relatively low risk of invasion and metastasis. However, the remaining patients face an aggressive disease with often rapid local and systemic progression [1, 11]. As many as 50% to 70% of non-muscle-invasive bladder cancers recur, and approximately 10% to 20% progress to muscle-invasive disease [1, 12] that may portend a worse prognosis. For instance, according to the large US-based Surveillance, Epidemiology, and End Results Program of the National Cancer Institute, approximately 34% of bladder cancers are localized and associated with a 5-year survival of approximately 70%. Comparatively, patients with regional (7% of diagnoses) or distant (4% of diagnoses) spread have 5-year survival rates of 35% and 5%, respectively [13].
In the palliative setting, cisplatin-based combination chemotherapy is the standard first-line (1L) treatment [11], and median overall survival (mOS) with such regimens is 13 to 16 months [11, 14-16]. However, cisplatin eligibility remains a limiting factor in elderly patients because renal dysfunction (creatinine clearance [CrCL] <60 mL/min), poor performance status (Eastern Cooperative Oncology Group performance status [ECOG PS] of ≥2) and comorbidities (eg, New York Heart Association Class III heart failure, grade ≥2 hearing loss, grade ≥2 neuropathy) exclude many patients (≈50%) from recommended 1L treatment [17-20]. Treatment of patients who progress after 1L treatment remains a challenge, because second-line (2L) treatment options are limited. Current practice guidelines in Europe recommend vinflunine as 2L treatment [11, 17]; however, 2L treatment of mUC varies geographically because there is no global standard of care for 2L treatment. Vinflunine was approved by the European Medicines Agency in 2009 but is not used throughout all of Europe [21]. Support for vinflunine in the 2L setting was based on the results from a phase 3 study showing that vinflunine + best supportive treatment resulted in an overall response rate of 8.6% compared with 0% with best supportive treatment alone [22]. Although no survival advantage was found in the intent-to-treat population (n = 370), improvement in mOS favored vinflunine (4 vs 7 months) in the eligible population (n = 357, which excluded 13 intent-to-treat patients who had ≥1 major protocol violation at baseline; P = 0.036) [22]. In countries where vinflunine is not approved, including the United States, taxanes or taxane-based combinations are considered an appropriate alternative [11, 23, 24].
Data are limited on treatment patterns and outcomes in patients with advanced UC or metastatic UC (mUC) in the routine clinical setting in Germany. The primary objective of this retrospective, observational cohort analysis was to describe treatment patterns and survival outcomes among patients with a diagnosis of incurable locally advanced UC or mUC treated with 1L and 2L systemic chemotherapy in the palliative setting in Germany.
Materials and Methods
Study design
This noninterventional, retrospective analysis evaluated the 1L and 2L treatments and survival outcomes of patients with locally advanced UC or mUC in the real-world clinical practice setting in Germany. The study protocol (MO39086) was approved by the ethics committee at the Medical Faculty of the Heinrich-Heine-University Düsseldorf (study number 5566). The protocol was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Contributing databases
Data for this study were obtained through 2 sources, and the overall study and observation periods are outlined in Figure S1. The GermanOncology (GO) network contributed data from office-based urology clinics, distributed across Germany, which collect patient characteristics, treatment data and outcome data using a standardized electronic case report form (eCRF). The GO database began in January 2012 and currently includes patient clinical characteristics, chemotherapy and radiotherapy treatment regimens, comorbid conditions and supportive therapy use from >12,500 patients being treated for cancer. To reach the appropriate sample size of patients with advanced or mUC, 25 additional treatment centers were recruited to contribute patient data by means of a modified eCRF. This modified eCRF extended the GO eCRF to enable more tailored and detailed data collection by including prognostic variables (eg, PS, hemoglobin, CrCl and site of metastases). For the newly accrued patients from these additional data sources, only data from up to June 2, 2016 (submission of protocol to institutional review boards) were considered in accordance with the requirements of the ethical permission obtained for the study.
Patient population and definitions
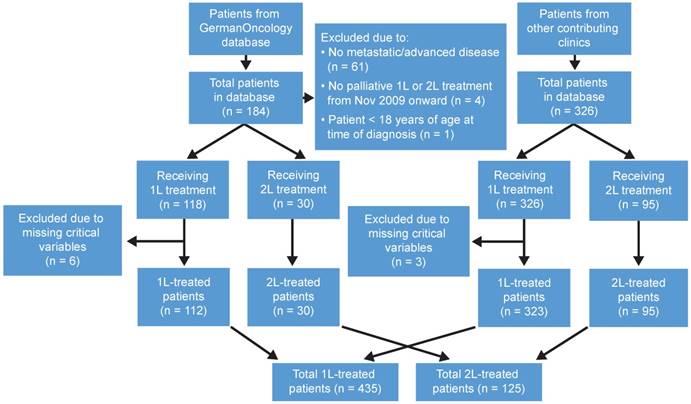
Eligible patients included adults aged ≥18 years with a diagnosis of locally advanced (transitional cell) UC of the upper and lower urinary tracts, defined as T4b, N(any), M0, T(any), N2-3, M0 or metastatic cancer defined as T(any), N(any), M1 (with clinical, radiological or pathological confirmation of metastasis) who received palliative 1L or 2L treatment starting on or after November 1, 2009 (Figure S1). Patients were excluded if their medical records were missing data on critical variables (Figure 1). Critical variables included age, sex, stage at study entry, line of therapy, start date of first or second line of therapy, end date of 1L or 2L treatment and therapeutic agents. Patients included in this registry were eligible for 1L, 2L or both cohorts depending on the treatments (lines of therapy) received. Patients in the 2L cohort, if data were complete for their 1L treatment, were also included in the 1L analysis if otherwise eligible for that analysis. Patients with nonmuscle invasive bladder cancer were not included.
Treatment and outcome definitions
For 1L treatment, the index date for study participants was the start date of the 1L systemic treatment. For 2L treatment, the index date was the date when the 2L chemotherapeutic treatment for recurrence after previous treatments/treatment failure of 1L treatment was initiated (Figure S1). Treatment response was assessed by the treating physician. The objective response rate was defined as the number of patients with a complete or partial response divided by the total number of patients.
Statistical analysis
Results for treatment patterns and clinical characteristics were reported as the numbers and percentages of patients, and descriptive analyses including means and SDs or 95% CIs and/or medians and interquartile ranges (Q1-Q3) were used for continuous variables. The numbers and percentages of patients were used for categorical variables. All statistical analyses were conducted using SAS 9.4.
Survival analyses
OS was defined as the interval between the index date of 1L treatment or 2L treatment and the date of death; patients lost to follow-up or alive at the end of the study period were censored at the last available date known to be alive. The mOS was estimated in months using the Kaplan-Meier method with 95% CIs. Milestone survival rates at 6, 12, 24 and 36 months were calculated with 95% CIs around the estimates using the Life Table (actuarial) method.
Cox regression analysis was used to compare outcomes in patients receiving vinflunine with other 2L treatments, adjusted for hypothesized prognostic factors based on the vinflunine phase 3 study (ECOG PS [>0 vs 0], liver metastases [yes vs no], hemoglobin level [<10 vs ≥10 g/dL]), age at index (≥68 vs <68 years) and sex [25]. Patients with missing covariates in the Cox regression were excluded from the regression analysis. All prognostic factors were included in the model at the same time. However, because information on hemoglobin level was not available for patients from the GO database, survival differences between vinflunine vs any other non-vinflunine-containing therapy using the Bellmunt model could only be assessed in a subset of the total 2L-treated patient population.
Progression-free survival (PFS) was measured from the index date to the date of progression or death due to any cause or initiation of new regimen, censoring patients who were still alive and did not progress at the last visit date. Median PFS (mPFS) was estimated, with 95% CIs, in months using the Kaplan-Meier method.
Results
Sites and geographic representation
Care settings included office-based sites (1L: 229 patients; 2L: 61 patients), nonacademic clinics (1L: 70 patients; 2L: 8 patients) and academic clinics (1L: 136 patients; 2L: 56 patients). Contributing centers covered most of Germany in a manner reflecting the distribution of the population. As such, most patients came from North Rhine-Westphalia (n = 183, 42%), followed by Bavaria (n = 42, 10%) and Berlin (n = 63, 15%), which are all densely populated federal states. A large hospital in Berlin provided coverage to patients in nearby Mecklenburg-Vorpommern and Brandenburg. Less densely populated states such as Saxony-Anhalt (n = 19, 4%) or Saxony (n = 5, 1%) had a lower contribution of patients, and federal states that were not covered mainly reflect low-populated states such as Saarland or Thuringia.
Patient population and baseline characteristics
Of the 444 eligible patients, 9 were excluded for missing data on critical variables during 1L treatment. Overall, 435 patients were included in the 1L analysis, of which 125 were also included in the analysis of 2L treatment. Details of patient attrition by data source are shown in Figure 1. In general, of the 184 patients with UC extracted from the GO database, 118 met the inclusion criteria—112 of whom received 1L chemotherapy and 30 of whom received 2L chemotherapy. Of the 326 patients obtained from the other contributing centers, all met the inclusion criteria—of whom 323 received 1L chemotherapy and 95 received 2L chemotherapy (Figure 1).
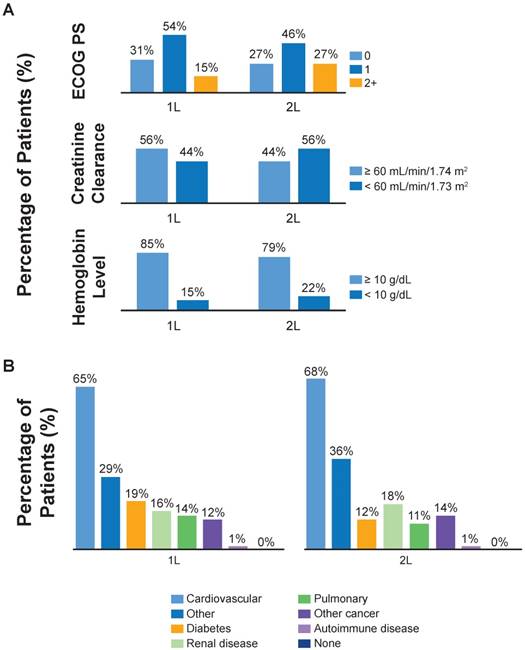
In patients treated with 1L treatment, the mean age was 66 years at the time of primary diagnosis and 67.4 years at the time of 1L treatment (Table 1). Patients treated with 2L chemotherapy had a mean age of 64.3 years at the time of initial diagnosis and of 66.6 years at the time of 2L index date. The majority of 1L- and 2L-treated patients were male, were current or former smokers and had metastatic (M1) disease (Table 1). A large number of patients had missing baseline smoking status (n = 225, 52%), CrCl levels (n = 183, 42%) and hemoglobin levels (n = 182, 42%). The most common distant metastatic sites in 1L-treated patients were the liver (42%) and other visceral organs (38%). In those who received 2L treatment, the most common metastatic sites also included the liver (32%), other visceral organs (30%) and bone (30%). A higher percentage of 2L-treated than 1L-treated patients had an ECOG PS of ≥2, CrCl <60 mL/min and hemoglobin <10 mg/dL (Figure 2A). The most common comorbidities were cardiovascular disease, diabetes and renal insufficiency, which were similar in frequency between the 1L and 2L treatment groups (Figure 2B, Table 1).
Treatment patterns
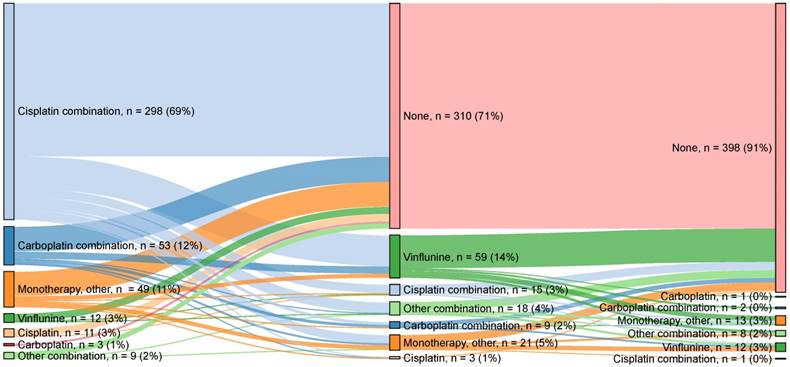
The median time from primary diagnosis to any 1L palliative treatment was 4.9 months, and the median time from diagnosis to any 2L palliative treatment was 7.5 months. A graphic illustration of treatment flow across 1L, 2L and 3L treatments is provided in Figure 3. Approximately 77% (n = 334) of 1L-treated patients received platinum-based combination treatment. The most common 1L combination treatment was gemcitabine + cisplatin (n = 276, 83%), followed by gemcitabine + carboplatin (n = 47, 14%). A minority of patients (n = 75, 17.2%) were treated with 1L single-agent treatment. Gemcitabine (n = 38, 51%) was the most common 1L single agent, followed by vinflunine (n = 12, 16%) and cisplatin (n = 11, 15%). In this analysis, approximately 29% (n = 125) of 1L-treated patients received 2L treatment, and 9% (n = 37) received 3L treatment (Figure 3). However, subsequent treatment data may not be fully captured due to ongoing treatment, progression or death, as discussed below. Of the 2L-treated patients, 66% (n = 83) received single-agent treatment. The most common was vinflunine (n = 59, 71%), followed by gemcitabine (n = 7, 8%) and paclitaxel (n = 5, 6%). Approximately one-third (n = 40, 32%) of 2L-treated patients received combination treatment, and the most common combinations were gemcitabine + cisplatin (n = 15, 37%), gemcitabine + paclitaxel (n = 15, 37%) and gemcitabine + carboplatin (n = 8, 20%).
Patient attrition. Schematic flow diagram indicating study databases, reasons for exclusion and analysis populations. 1L: first line; 2L: second line.
Baseline characteristics and comorbidities by line of therapy. (A) Eastern Cooperative Oncology Group performance status (ECOG PS), creatinine clearance and hemoglobin levels by line of therapy. Percentages of patients with missing baseline ECOG PS, creatinine clearance and hemoglobin were 7%, 42% and 42%, respectively. (B) Common comorbidities by line of therapy.
Baseline characteristics by line of therapy and most common 1L and 2L treatments
| Characteristic | Patients With 1L Treatment | 1L Cisplatin+ Gemcitabinea | Patients With 2L Treatment | 2L Vinflunineb |
|---|---|---|---|---|
| Patient count at start, n | 435 | 276 | 125 | 59 |
| Age at primary diagnosis, yearsc | ||||
| Mean (SD) | 66.0 (10) | 64.9 (9) | 64.3 (10) | 65.1 (10) |
| Median | 67 | 66 | 65 | 67 |
| Age at index date, yearsd | ||||
| Mean (SD) | 67.4 (10) | 66.2 (9) | 66.6 (10) | 66.6 (10) |
| Median | 69 | 67 | 69 | 69 |
| Baseline characteristic, n (%) | ||||
| Sex | ||||
| Male | 326 (75) | 204 (74) | 87 (70) | 40 (68) |
| Female | 109 (25) | 72 (26) | 38 (30) | 19 (32) |
| Smoking status | ||||
| Never | 53 (25) | 27 (18) | 18 (32) | 9 (35) |
| Current/former | 157 (75) | 122 (82) | 39 (68) | 17 (65) |
| Missing | 225 (52) | 127 (46) | 68 (54) | 33 (56) |
| Tumor stage at study entry | ||||
| IV (only IV is permitted, if available) | 363 (100) | 260 (100) | 103 (100) | 46 (100) |
| Missing | 72 (17) | 16 (6) | 22 (18) | 13 (22) |
| Primary tumor grade | ||||
| Low grade | 299 (931) | 235 (96) | 88 9 (94) | 40 (98) |
| High grade | 22 (7) | 9 (4) | 6 (6) | 1 (2) |
| Missing | 114 (26) | 32 (12) | 31 (25) | 18 (31) |
| Metastatic status at study entrye | ||||
| M0 | 142 (39) | 106 (41) | 32 (31) | 16 (35) |
| M1 primary | 136 (37) | 97 (37) | 33 (32) | 16 (35) |
| M1 recurrent | 89 (24) | 58 (22) | 39 (38) | 14 (30) |
| Missing | 68 (16) | 15 (5) | 21 (17) | 13 (22) |
| Extent of disease at study entryf | ||||
| Locally advanced, moderate lymph node involvementf | 9 (2) | 8 (3) | 4 (3) | 2 (3) |
| Bladder confined, extensive lymph node involvementf | 133 (31) | 98 (36) | 28 (22) | 14 (24) |
| Location of distant metastasis (if M1)e | ||||
| Liver | 56 (42) | 44 (48) | 17 (34) | 8 (38) |
| Other visceralg | 50 (38) | 31 (34) | 15 (30) | 7 (33) |
| Bone | 41 (31) | 30 (33) | 15 (30) | 5 (24) |
| Other nonvisceralg | 33 (25) | 20 (22) | 14 (28) | 5 (24) |
| Missing | 93 (41) | 63 (41) | 22 (31) | 9 (30) |
| ECOG PS | ||||
| 0 | 125 (31) | 96 (36) | 31 (27) | 18 (33) |
| 1 | 218 (54) | 141 (52) | 54 (46) | 18 (33) |
| 2+ | 62 (15) | 32 (12) | 32 (27) | 18 (33) |
| Missing | 30 (7) | 7 (3) | 8 (6) | 5 (8) |
| Hemoglobin level | ||||
| < 10 g/dL | 39 (15) | 29 (15) | 17 (22) | 6 (17) |
| ≥ 10 g/dL | 214 (85) | 161 (85) | 62 (78) | 29 (83) |
| Missing | 182 (42) | 86 (31) | 46 (37) | 24 (41) |
| CrCl | ||||
| < 60 mL/min/1.73 m2 | 110 (44) | 71 (38) | 45 (56) | 22 (61) |
| ≥ 60 mL/min/1.73 m2 | 142 (56) | 118 (62) | 35 (44) | 14 (39) |
| Missing | 183 (42) | 87 (32) | 45 (36) | 2 (39) |
| Comorbiditiese,h | ||||
| Pulmonary | 46 (14) | 26 (12) | 10 (11) | 5 (11) |
| Cardiovascular | 222 (65) | 128 (60) | 64 (68) | 32 (70) |
| Diabetes | 66 (19) | 49 (23) | 11 (12) | 6 (13) |
| Renal disease | 53 (16) | 23 (11) | 17 (18) | 8 (17) |
| Autoimmune diseasei | 4 (1) | 4 (2) | 1 (1) | 0 (0.0) |
| Other | 100 (29) | 59 (27) | 34 (36) | 16 (35) |
| Other cancer | 41 (12) | 21 (10) | 13 (14) | 8 (17) |
| Missing | 93 (21) | 61 (22) | 31 (25) | 13 (22) |
| Insurance type | ||||
| Private | 48 (11) | 29 (11) | 20 (16) | 7 (12) |
| Public | 387 (89) | 247 (90) | 105 (84) | 52 (88) |
1L: first line; 2L: second line; CrCl: creatinine clearance; ECOG PS: Eastern Cooperative Oncology Group performance status.
a Most common 1L treatment. b Most common 2L treatment.
c Primary diagnosis was the initial diagnosis of urothelial carcinoma, independent of stage. d For 1L treatment, the index date was the start date of the 1L systemic treatment. For 2L treatment, the index date was the date when the 2L chemotherapeutic treatment for recurrence after previous treatments/treatment failure of 1L treatment was initiated. e Values within categories do not necessarily add up to 100% due to non-mutually exclusive groups and/or missing values, which do not contribute to the denominators used for percentages.
f For patients with non-M1 or non-missing status. Locally advanced disease with moderate lymph node involvement refers to Tb, N ≤1, M0 (incudes Nx or Mx, if T4b confirmed). Bladder-confined disease with extensive lymph node involvement refers to T4b, N2-3 M0 (includes Tx or Mx if N2-3 confirmed)
g Other metastatic sites, including the lung, peritoneum, non-visceral: brain, skin and otdher sites. h Variable not collected by GermanOncology; total N = 112 for 1L and 30 for 2L. i Including ≥1 of the following diseases: myasthenia gravis, myositis, autoimmune hepatitis, systemic lupus erythematosus, rheumatoid arthritis, inflammatory bowel disease, vascular thrombosis associated with anti-phospholipid syndrome, Wegener granulomatosis, Sjögren syndrome, Guillain-Barré syndrome, multiple sclerosis, vasculitis or glomerulonephritis.
Treatment patterns across lines of therapy. Sankey diagram of treatments received. Possible reasons for not receiving second-line treatment include death, still receiving first-line treatment, clinical factors and patient preference.
Treatment outcomes
OS by treatment type
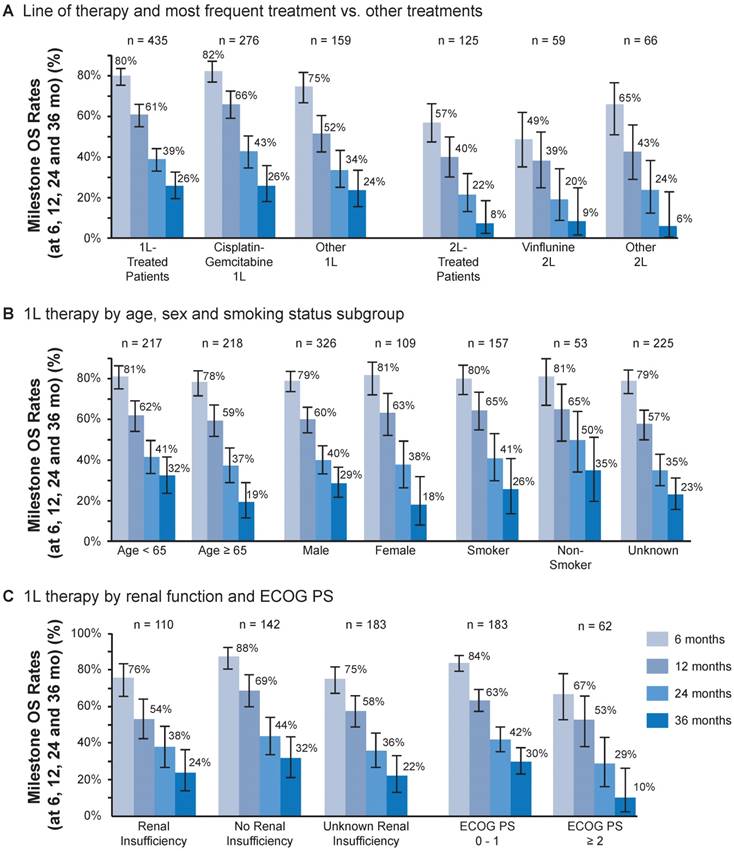
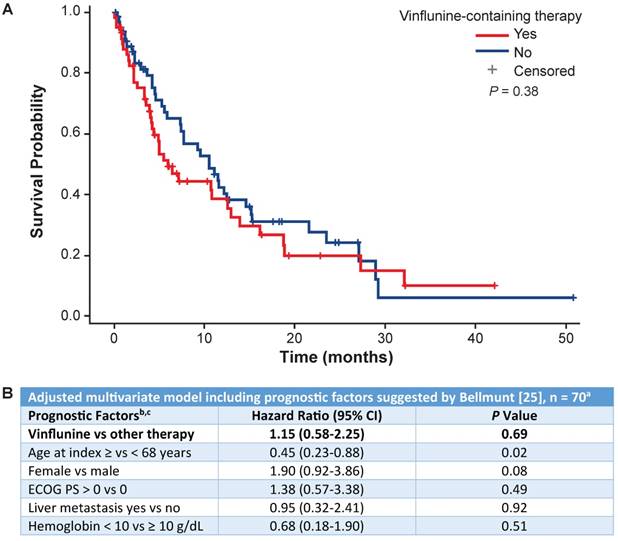
OS by line of treatment and most common vs other treatments is shown in Figure 4A. Of the patients who received 1L treatment, a total of 206 (47%) deaths occurred, and a total of 229 (53%) were censored. Of the patients who received 2L treatment, a total of 79 (63%) deaths occurred and 46 (37%) were censored. The mOS (95% CI) with 1L treatment overall was 16.1 months (13.7-19.2) and with cisplatin + gemcitabine was 17.7 months (14.4-24.2) (Table 2). In the 1L setting, OS rates at 6, 12, 24 and 36 months were 80%, 61%, 39% and 26%, respectively (Figure 4A). The mOS (95% CI) with 2L treatment overall was 9.2 months (5.5-11.6) and 5.9 months (4.1-12.6) with 2L vinflunine (Table 2). In the 2L setting, OS rates at the same time periods were 57%, 40%, 22% and 8%, respectively. Survival rates with the 1L combination of gemcitabine + cisplatin at 6, 12, 24 and 36 months were 82%, 66%, 43% and 26%, respectively. OS was numerically higher for the combination of gemcitabine + cisplatin at 6 and 12 months compared with other 1L treatments. This difference became less apparent at 24 and 36 months. OS with 2L treatment with vinflunine was similar to that with other 2L agents at all time points. OS rates with vinflunine at 6, 12, 24 and 36 months were 49%, 39%, 20% and 9%, respectively, in the 2L setting (Figure 4A). Cox multivariate regression analysis found no difference in survival between vinflunine and other 2L treatments (adjusted P value = 0.69) (Figure 5A).
OS by clinical characteristics
OS in the 1L setting was similar by age (<65 or ≥65 years) at 6, 12 and 24 months and was numerically higher at 36 months in favor of patients aged <65 years (Figure 4B). OS at 6, 12, 24 and 36 months was similar by sex and smoking status (Figure 4B). In 1L-treated patients, those without renal insufficiency had numerically higher OS at 6 and 12 months but not thereafter. In 1L-treated patients, survival appeared higher in patients with an ECOG PS of 0 to 1 compared with an ECOG PS of ≥2 (Figure 4C). No difference in survival in the 2L setting was found based on Bellmunt risk factors [25] (Figure 5B).
Treatment response and progression by line of therapy
Key measures of response and progression by line of therapy are shown in Table 2. Objective responses occurred in 34% of patients treated with any 1L treatment, 35% of patients treated with 1L cisplatin + gemcitabine, 13% of 2L-treated patients and 5% of patients treated with 2L vinflunine (Table 2). The mPFS (95% CI) values in patients treated with any 1L treatment, 1L cisplatin + gemcitabine, any 2L treatment and 2L vinflunine were 7 months (6.4-8.1), 7 months (6.4-9.1), 4 months (3.0-4.8) and 3 months (2.5-4.2), respectively. Of the patients who received 1L treatment, a total of 228 (66%) PFS events (death or progression) occurred, and 147 (34%) were censored. In the patients who received 2L treatment, a total of 99 (79%) PFS events (death or progression) occurred—26 (21%) of which were censored. Early disease progression within 6 and 12 months of start of treatment occurred in 26% (n = 115) and 43% (n = 186) of patients treated in the 1L setting and in 43% (n = 54) and 55% (n = 69) of patients treated with 2L chemotherapy, respectively (Table 2).
First-line (1L) treatment outcomes. (A) Milestone overall survival (OS) at 6, 12, 24 and 36 months by line of therapy and most frequent vs other treatments. (B) Milestone OS at 6, 12, 24 and 36 months for 1L treatment by age, sex and subgroups of smoking status. (C) Milestone OS at 6, 12, 24 and 36 months for 1L treatment by renal function and Eastern Cooperative Oncology Group performance status (ECOG PS). Bars show 95% CIs.
Clinical outcomes by line of therapy
| 1L-Treated Patients (n = 435) | 1L Cisplatin + Gemcitabinea (n = 276) | 2L-Treated Patients (n = 125) | 2L Vinflunineb (n = 59) | |
|---|---|---|---|---|
| PFS, median (95% CI), months | 7.2 (6.4-8.1) | 7.6 (6.4-9.1) | 4.0 (3.0-4.8) | 3.1 (2.5-4.2) |
| OS, median (95% CI), months | 16.1 (13.7-19.2) | 17.7 (14.4-24.2) | 9.2 (5.5-11.6) | 5.9 (4.1-12.6) |
| Objective response, n (%)c | 117 (34) | 86 (35) | 15 (14%) | 3 (6) |
| Best overall response rate, n (%)d | ||||
| Complete response | 38 (11) | 29 (12) | 2 (2) | 0 (0.0) |
| Partial response | 79 (22) | 57 (23) | 13 (12) | 3 (6) |
| Stable disease | 89 (26) | 62 (25) | 29 (26) | 16 (30) |
| Progressive disease | 104 (30) | 76 (31) | 45 (41) | 26 (48) |
| Not clearly definable | 38 (11) | 21 (9) | 22 (20) | 9 (17) |
| Missing or unknown | 87 (20) | 31 (11) | 14 (11) | 5 (9) |
| Early disease progression, n (%) | ||||
| 6 months | 115 (26) | 78 (28) | 54 (43) | 28 (47) |
| 12 months | 186 (43) | 131 (47) | 69 (55) | 34 (58) |
a Most common first-line treatment.
b Most common second-line treatment.
c Defined as the number of patients who achieved a complete or partial response (with percentages based on the total number of patients).
Cox regression analysis comparing overall survival with second-line vinflunine vs other second-line treatments. (A) Overall survival analysis comparing second-line vinflunine-treated vs other treatments. (B) Cox regression analysis using the Bellmunt prognostic model for vinflunine vs other second-line treatments. a 70 patients remained after all patients with missing covariates were excluded. b The model was stratified by center type. c The reference groups for each factor were as follows: other therapy, age <68 years, male sex, ECOG = 0, no liver metastasis, hemoglobin ≥10 g/dL. ECOG PS: Eastern Cooperative Oncology Group performance status.
Discussion
In this analysis, baseline characteristics and demographics of patients with advanced UC or mUC in Germany were consistent with expectations (>65 years of age, mostly male and current or former smoker) [11, 13, 17]. The 1L treatment regimens were also consistent with international guideline recommendations, as the majority of patients were treated with 1L cisplatin-based combination treatment (71%) [11, 17].
Furthermore, the remaining patients were preferably treated with carboplatin-based combinations, most commonly carboplatin + gemcitabine. Although high estimated rates of cisplatin ineligibility (≈50%) have been reported [26], it is interesting to note that in our study the combination of gemcitabine + cisplatin was the most common 1L treatment among patients with an ECOG PS of ≥2 and renal insufficiency (CrCL <60 mL/min) (76% and 79%). Patients with these characteristics could be considered cisplatin ineligible [18-20] and could be excluded from randomized clinical trials, suggesting different treatment patterns between randomized clinical trials and those in the real-world practice setting. Furthermore, the mOS in patients in this subgroup was worse than that in their healthier counterparts, reflecting the great medical need for these underrepresented patients (ECOG PS of 0 to 1 and ≥2 = 17.8 and 12.6 months, respectively; CrCl ≥ and <60 mL/min: 19.2 and 12.8 months respectively; and no liver metastases and liver metastases: 14.9 and 11.0 months, respectively). Indeed, the high percentage of cisplatin-treated patients may be due to the common practice of administering cisplatin-based treatment to those technically ineligible given the superiority of cisplatin-based combination therapy in this setting [27].
In our study, the mPFS and mOS in all patients who received 1L treatment were 7.2 and 16.1 months, respectively, which is comparable with data from randomized clinical trials among patients with locally advanced UC or mUC (eg, mPFS of 8 months and mOS of 13 of 16 months [15, 16]. Less than one-third (29%) of 1L-treated patients went on to receive 2L treatment. The 1L-treated patients who did not receive 2L chemotherapy (71%) may have experienced stable disease that did not require additional lines of therapy, may have experienced progressive disease and refused treatment, may have died before 2L chemotherapy or may have not received 2L chemotherapy for unknown reasons. Patients in our study who received any 2L treatment had an mPFS and mOS of 4 and 9 months, respectively—in line with previously reported randomized data with taxanes and vinflunine: mPFS of 2 to 3 months and mOS of 6 to 7 months [22, 28]. In addition, our real-world results from Germany show the overall 2L mOS (≈9 mo) is in line with the ≈8-mo mOS demonstrated in a US population-based study previously reported [29].
The most common 2L treatment in our study was single-agent vinflunine (71%). This finding is consistent with current European guideline recommendations to offer vinflunine to patients progressing after platinum-based combination chemotherapy for metastatic disease [11]. In our study, the mOS (95% CI) among patients treated with 2L vinflunine (n = 59) was 5.9 months (4.1-12.6), shorter than that in those treated with any 2L agents (9.2 months; 5.5-11.6; n = 125). The mPFS was similar between the 2 groups: 3.1 and 4.0 months, respectively. These findings are consistent with those of contemporary studies that showed that mOS ranged from 5 to 10 months and mPFS from 2 to 6 months in patients with advanced UC treated with vinflunine [22, 30-34]. However, multivariate Cox regression analysis found no significant difference in OS between vinflunine and other 2L treatment regimens. However, results should be interpreted with caution due to small sample sizes. This finding agrees with data from a recent phase 3 trial comparing the checkpoint inhibitor pembrolizumab with the standard 2L chemotherapies vinflunine, docetaxel or paclitaxel. In this trial, the efficacy of standard 2L chemotherapeutic drugs was similar as well in 2L-treated patients [35]. However, results from a phase 3 trial comparing atezolizumab versus chemotherapy in patients with locally advanced or mUC who progressed after platinum-based chemotherapy found that survival with vinflunine was better than hypothesized compared with taxane-based treatment [36].
This study had several strengths and limitations. The results presented here are descriptive, and any comparisons of outcomes should be interpreted with caution because the patients were treated in routine clinical practice and not randomized to treatment arms; thus, unmeasured confounding variables may exist. The practice patterns described here may be applicable to Germany but not generalizable to practice patterns outside of Germany. Differences in treatment patterns may exist between office and academic care settings, but were not differentiable in this analysis. No detailed safety data was available as this study focused on treatment patterns and efficacy outcomes. The sample sizes of the subgroups were small, so limited statistical analyses were conducted. This study described several outcomes, including objective response rate, treatment response, PFS and OS, which is unusual for observational research studies. However, a large number of patients had missing baseline characteristics that may have affected outcome estimates (OS and PFS) within a specific subgroup as well as estimates of best overall response. Data collection relied on eCRF and responses of physicians and their staff, who transferred information from patient medical records; not only could there have been bias in the selection of patient records from which to transcribe the data, but there could have been errors introduced when the eCRFs were completed.
Overall, the results of this and previous studies of advanced UC and mUC show that outcomes are generally poor, because progression after 1L failure is common and portends a worse prognosis. Effective treatment options in 1L and 2L settings are scarce, and many patients do not receive 2L treatment. These results underscore the large unmet need for new therapeutic options for patients who progress after 1L treatment. Therefore, a better understanding of the real-world patterns of chemotherapy use and its impact on survival is warranted due to the emergence of cancer immunotherapy in the 1L and 2L settings [35, 37-41].
Supplementary Material
Supplementary figure S1.
Acknowledgements
We thank Ling-I Hsu and Irmarie Reyes-Rivera for their contributions to the study. We thank the recruiting physicians and their teams for their efforts with the data collection: Dr. Dimitri Barski, Urologische Klinik, Lukaskrankenhaus Neuss, Neuss; Dr. med. Katrin Bothe, Klinik für Urologie und Kinderurologie, Universitätsklinikum Schleswig-Holstein, Campus Kiel, Kiel; PD Dr. med. Gunhild von Amsberg, Zentrum für Onkologie, Medizinische Klinik und Poliklinik, Universitätsklinikum Hamburg-Eppendorf, Hamburg; PD Dr. med. Jan Lehmann, Urologische Gemeinschaftspraxis Prüner Gang, Kiel; Prof. Dr. Stefan Hautmann, Klinik für Urologie, Klinikum Lüdenscheid, Lüdenscheid; Prof. Dr. Christian Schwentner, Klinik für Urologie, Diakonie-Klinikum Stuttgart, Stuttgart; Prof. Dr. med. Axel Merseburger, Klinik für Urologie, Universitätsklinikum Schleswig-Holstein, Campus Lübeck, Lübeck; Prof. Dr. med. Axel Hegele, Klinik für Urologie und Kinderurologie, Philipps-Universität Marburg, Marburg; Dr. med. Armin Leitenberger, Klinik für Urologie, Klinikum Wolfsburg, Wolfsburg; Dr. med. Thomas Kretz, Überörtliche Urologische Gemeinschaftspraxis Heinsberg/Hückelhoven, Betriebsstätte Heinsberg, Heinsberg; Dr. med. Wolfgang Rulf, Urologie Neandertal, Ortsübergreifende Gemeinschaftspraxis für Urologie, Praxis Erkrath-Hochdahl, Erkrath; PD Dr. med. Henrik Suttmann, Urologikum Hamburg, Hamburg; Dr. med. Matthias Schulze, Praxis Markkleeberg, Markkleeberg; Dr. med. Thomas Pulte, Urologische Praxis am Wasserturm, Würselen; Dr. med. Jörg Klier, Urologische Partnerschaft Köln, Köln; Dr. med. Eva Hellmis, Urologicum Duisburg, Duisburg; Dr. med Hans-Jürgen Hurtz, Gemeinschaftspraxis und Tagesklinik, Hämatologie/Onkologie/Gastroenterologie, Halle (Saale); Dr. Clemens Schulte, Gemeinschaftspraxis für Hämatologie und Onkologie, Dortmund. This study was sponsored by F. Hoffmann-La Roche, Ltd. Medical writing assistance for this manuscript was provided by Eric Berlin, MD, of Health Interactions, Inc, and funded by F. Hoffmann-La Roche, Ltd.
Competing Interests
Güenter Niegisch has served in a consulting or advisory role with Roche Pharma AG, has received research funding from 4SC and has received travel and accommodations from Pfizer. Shih-Wen Lin and Julie Pavlova are employees of Genentech and Roche and have stock ownership.
References
1. International Agency for Research on Cancer. World Cancer Report 2014. http://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-2014
2. Burger M, Catto JW, Dalbagni G. et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63(2):234-241
3. International Agency for Research on Cancer. Country factsheets: Germany. 2012. http://eco.iarc.fr/eucan/Country.aspx?ISOCountryCd=276
4. Cancer in Germany. Cancer in Germany 2011/2012. http://www.krebsdaten.de/Krebs/EN/Content/Publications/Cancer_in_Germany/cancer_in_germany_node.html
5. Chalasani V, Chin JL, Izawa JI. Histologic variants of urothelial bladder cancer and nonurothelial histology in bladder cancer. Can Urol Assoc J. 2009;3(6 Suppl 4):S193-8
6. Klaile Y, Schlack K, Boegemann M, Steinestel J, Schrader AJ, Krabbe LM. Variant histology in bladder cancer: how it should change the management in non-muscle invasive and muscle invasive disease? Transl Androl Urol. 2016;5(5):692-701
7. Rouprêt M, Babjuk M, Böhle A, Burger M, Compérat E. European Association of Urology Guidelines on Urothelial Carcinomas of the Upper Urinary Tract. 2015. http://uroweb.org/wp-content/uploads/06-UTUC_druk_LR.pdf
8. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29
9. Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol. 2000;164(5):1523-1525
10. Cancer in Germany. Cancer in Germany. 2016. http://www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebsgeschehen/Krebsgeschehen_download.pdf?__blob=publicationFile
11. Witjes AJ, Lebret T, Comperat EM. et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur Urol. 2017;71(3):462-475
12. Isharwal S, Konety B. Non-muscle invasive bladder cancer risk stratification. Indian J Urol. 2015;31(4):289-296
13. National Cancer Institute. SEER Cancer Stat Fact Sheets: bladder cancer. 2017. https://seer.cancer.gov/statfacts/html/urinb.html
14. Loehrer P.J.Sr, Einhorn LH, Elson PJ. et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol. 1992;10(7):1066-1073
15. von der Maase H, Sengelov L, Roberts JT. et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23(21):4602-4608
16. Bellmunt J, von der Maase H, Mead GM. et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J Clin Oncol. 2012;30(10):1107-1113
17. Bellmunt J, Orsola A, Leow JJ. et al. Bladder cancer: ESMO practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii40-8
18. Galsky MD, Hahn NM, Rosenberg J. et al. Treatment of patients with metastatic urothelial cancer "unfit" for cisplatin-based chemotherapy. J Clin Oncol. 2011;29(17):2432-2438
19. Sonpavde G, Galsky MD, Vogelzang NJ. First-line systemic therapy trials for advanced transitional-cell carcinoma of the urothelium: should we stop separating cisplatin-eligible and -ineligible patients? J Clin Oncol. 2010;28(25e):441-2 author reply e443-4
20. Houede N, Locker G, Lucas C. et al. Epicure: a European epidemiological study of patients with an advanced or metastatic Urothelial Carcinoma (UC) having progressed to a platinum-based chemotherapy. BMC Cancer. 2016;16(1):752
21. JAVLOR (vinflunine). [summary of product characteristics]. South San Francisco, CA: Genentech, Inc. 2016
22. Bellmunt J, Theodore C, Demkov T. et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol. 2009;27(27):4454-4461
23. Sonpavde G, Pond GR, Choueiri TK. et al. Single-agent taxane versus taxane-containing combination chemotherapy as salvage therapy for advanced urothelial carcinoma. Eur Urol. 2016;69(4):634-641
24. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: bladder cancer. V5.2017. 2017. https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf
25. Bellmunt J, Choueiri TK, Fougeray R. et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol. 2010;28(11):1850-1855
26. Bellmunt J, Mottet N, De Santis M. Urothelial carcinoma management in elderly or unfit patients. EJC Suppl. 2016;14(1):1-20
27. Bamias A, Tzannis K, Harshman LC. et al. Impact of contemporary patterns of chemotherapy utilization on survival in patients with advanced cancer of the urinary tract: a retrospective international study of invasive/advanced cancer of the urothelium (RISC). Ann Oncol. 2017 [Epub ahead of print]
28. Choueiri TK, Ross RW, Jacobus S. et al. Double-blind, randomized trial of docetaxel plus vandetanib versus docetaxel plus placebo in platinum-pretreated metastatic urothelial cancer. J Clin Oncol. 2012;30(5):507-512
29. Pal SK, Galsky MD, Lin S. et al. Second-line metastatic urothelial carcinoma treatment and survival in real-world patients in the United States. Ann Oncol. 2016;27(6):266-295
30. Hegele A, Goebell P, Matz U, Neuhaus T. Monotherapy with intravenous vinflunine in patients with advanced or metastatic urothelial cancer after failure of a platinum-containing regimen: a retrospective analysis of German routine data. Urol Int. 2014;92(2):174-179
31. Retz M, de Geeter P, Goebell PJ, Matz U, de Schultz W, Hegele A. Vinflunine in routine clinical practice for the treatment of advanced or metastatic urothelial cell carcinoma - data from a prospective, multicenter experience. BMC Cancer. 2015;15:455-460
32. Garcia-Donas J, Font A, Perez-Valderrama B. et al. Maintenance therapy with vinflunine plus best supportive care versus best supportive care alone in patients with advanced urothelial carcinoma with a response after first-line chemotherapy (MAJA; SOGUG 2011/02): a multicentre, randomised, controlled, open-label, phase 2 trial. Lancet Oncol. 2017;18(5):672-681
33. Medioni J, Di Palma M, Guillot A, Spaeth D, Theodore C. Efficacy and safety of vinflunine for advanced or metastatic urothelial carcinoma in routine practice based on the French multi-centre CURVE study. BMC Cancer. 2016;16:217-225
34. Castellano D, Puente J, de Velasco G. et al. Safety and effectiveness of vinflunine in patients with metastatic transitional cell carcinoma of the urothelial tract after failure of one platinum-based systemic therapy in clinical practice. BMC Cancer. 2014;14:779-287
35. Bellmunt J, de Wit R, Vaughn DJ. et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015-1026
36. Powles T, Loriot Y, Durán I. et al. IMvigor211: a phase III randomized study examining atezolizumab versus chemotherapy for platinum-treated advanced urothelial carcinoma. Presented at EACR-AACR-SIC Special Conference [abstract 606]. 2017 Florence, Italy
37. Rosenberg JE, Hoffman-Censits J, Powles T. et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909-1920
38. Balar AV, Galsky MD, Rosenberg JE. et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67-76
39. Sharma P, Retz M, Siefker-Radtke A. et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312-322
40. Apolo AB, Infante JR, Balmanoukian A. et al. Avelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase Ib study. J Clin Oncol. 2017;35:2117-2124
41. Massard C, Gordon MS, Sharma S. et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol. 2016;34(26):3119-3125
Author contact
![]() Corresponding author: E-mail: Guenter.Niegischuni-duesseldorf.de
Corresponding author: E-mail: Guenter.Niegischuni-duesseldorf.de

 Global reach, higher impact
Global reach, higher impact