3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(3):665-671. doi:10.7150/jca.28528 This issue Cite
Research Paper
Transarterial Chemoembolization related to Good Survival for Selected Patients with advanced Hepatocellular Carcinoma
1. Department of Hepatobiliary Oncology, Cancer Centre, Sun Yat-sen University, Guangzhou, China
2. Collaborative Innovation Centre for Cancer Medicine, Cancer Centre, Sun Yat-sen University, Guangzhou, China
3. Sate Key Laboratory of Oncology in South China, Guangzhou, China
4. Department of Radiology, Sun Yat-sen University Cancer Centre, Guangzhou, China
5. Department of Oncology, Shanghai Medical College, Fudan University, Shanghai 200032, China
* Conributed equally to this work.
Received 2018-7-15; Accepted 2018-10-3; Published 2019-1-1
Abstract
Background & aims: It remains controversial whether patients with advanced-stage hepatocellular carcinoma could be benefit from transarterial chemoembolization (TACE) treatment. The purpose of the present study is to identify predictors of survival following TACE in patients with advanced HCC.
Methods: Overall, 303 patients with Barcelona Clinic Liver Cancer (BCLC) stage C HCC who were first treated with TACE from Sun Yat-sen University Cancer Centre between January 2009 and December 2013 were reviewed and enrolled in this study. We carried out Kaplan-Meier and Cox proportional hazard model analyses of prognostic factors.
Results: The median survival of the whole cohort was 8.4 months. Multivariable Cox regression analyses confirmed that four risk factors, high serum levels of gamma-glutamyl transpeptidase (GGT), C-reactive protein (CRP), alkaline phosphatase (ALP) and presence of portal vein tumour thrombosis (PVTT), were independent prognostic factors for overall survival. The expected median survival among patients with 0-1 and 2-4 risk factors were 18.1 (95% CI: 15.5-20.7) and 6.8 (95% CI: 5.8-7.8) months, respectively. Objective tumor response among patients with 0-1 and 2-4 risk factors were 38.9% and 17.3%, respectively.
Conclusion: We found four risk factors were associated with dismal overall survival for advanced HCC patients: serum GGT level, serum CRP, serum ALP and presence of PVTT. TACE may be recommended for patients with advanced HCC with 0-1 risk factors due to the favourable prognosis.
Keywords: Advanced hepatocellular, Transarterial chemoembolization, Prognosis
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the second leading cause of cancer mortality worldwide, resulting in more than 700,000 deaths annually.(1) A majority of patients with HCC are diagnosed at an intermediate to advanced stage when the chances of curative treatments are limited.(2) For these patients, palliative treatments including transarterial chemoembolization (TACE) and sorafenib have been widely used. According to the Barcelona Clinic Liver Cancer (BCLC) staging classification, adopted by the American Association for the Study of Liver Diseases (AASLD)/European Association for the Study of the Liver (EASL) guidelines, (2, 3) TACE is recommended for intermediate-stage patients (BCLC stage B) to achieve local control and palliation. Patients with advanced-stage (BCLC stage C) disease have a dismal prognosis, with an expected median survival of 6 months; sorafenib, a multitargeted kinase inhibitor, has been recommended as the standard treatment for these patients.(4) Sorafenib treatment efficacy is considered modest (median survival 10.7 months with sorafenib vs 7.9 months without)(4) and is costly. Therefore, the use of sorafenib as the first-line treatment for advanced HCC is uncommon in real life, despite the high number of patients diagnosed with BCLC stage C disease.(5, 6) Rather than sorafenib, TACE and resection are the most frequent first-line treatments for patients with stage C disease in clinical practice according to recently communicated data in real-life clinical practice of HCC management.(5)
HCC with portal vein invasion is considered to be a theoretical contraindication to TACE. However, many studies have suggested that TACE could be safely and feasibly performed in HCC patients with portal vein invasion.(7-9) There is no consensus on whether BCLC stage C patients can benefit from treatment with TACE. The heterogeneity in the BCLC stage C group is associated with a wide range of prognoses and survival outcomes. Therefore, a clinical decision to use TACE in BCLC-stage C patients may well be based on the clinical characteristics that are relevant for the outcome rather than those simply depicted with BCLC staging. The purpose of our present study was to identify clinically useful factors that can predict prognosis after TACE in patients with advanced HCC.
Materials and methods
Patients and design
This study was approved by the institutional review boards of the participating hospitals. Written informed consent was obtained from all patients. In total, 303 consecutive patients with advanced HCC who underwent TACE as an initial treatment were recruited in this retrospective study from the Sun Yat-sen University (SYSU) Cancer Centre between January 2009 and December 2013. The inclusion criteria were (1) HCC patients with BCLC stage C disease, (2) Child-Pugh A or B liver function, and (3) HCC with no previous treatment. Patients with Child-Pugh class C liver function, BCLC stage A/B disease, ECOG performance status 3-4 or no follow-up data were excluded from this study.
The diagnosis of HCC was based on the criteria used by the European Association for the Study of the Liver and European Organisation for Research and Treatment of Cancer (EASL-EORTC)(3) as follows: presence of one of the typical imaging hallmarks of HCC (hypervascular in the arterial phase with washout in the portal venous or delayed phases) or a cytological/histological diagnosis of HCC.
TACE
TACE was performed using the techniques described in our previous report.(10) Depending on the size of, location of, and arterial supply to the tumour, the tip of the catheter was advanced into the segmental artery or specific tumour-feeding artery. Chemoembolization was performed using epirubicin, lobaplatin and mitomycin C were mixed in 9 ml of water-soluble contrast medium and 1 ml of sterile water for injection. Pure lipiodol or gelatin sponge particles (500-1,000 μm) were then injected if the chemoembolized artery territory did not show stagnant flow. The dose of anticancer agent-lipiodol emulsion and the pieces of embolic materials used for TACE were determined based on the tumor size, extension of the lesions and tumor blood supply.
Repeated TACE was performed once every 8-12 weeks until one of the following end points was reached: (1) complete devascularization of the tumour; (2) technical impossibility to embolize the residual tumour, for example, tumour only supplied by extrahepatic collateral arteries; (3) development of contraindications to TACE; or (4) total resection or ablation of tumour by subsequent surgery or local ablation. Following these criteria and depending on survival, 178 patients (58.7%) received TACE only once, 86 patients (28.4%) underwent TACE twice, 27 patients (8.9%) underwent TACE thrice, 10 patients (3.3%) underwent TACE four times or more, and 2 patients (0.7%) underwent TACE nine times. TACE-related death was designated death with 30 days after the initial therapy.
Tumor Response Evaluation after TACE
The evaluation of the response to TACE within three months (after the first or second TACE session) was assessed by expert abdominal radiologists according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST).(11) The patients with a complete response (CR) or a partial response (PR) were grouped as having an objective response to TACE.
Follow-up
The patients were followed-up by dynamic CT or MRI 4 weeks after treatment and then once every 3-4 months thereafter. During each of these follow-up visits, a detailed history was collected, and a complete physical examination was carried out. Blood tests, including serum liver function tests and alpha fetoprotein (AFP) level analysis, were also performed. The endpoint of the current study was OS, calculated from the initial date of TACE treatment until death from any cause or until the last follow-up.
Statistical analysis
Analyses were performed with IBM SPSS (V.18.0; SPSS) and GraphPad PRISM (V.6.0, La Jolla, CA, USA) software. The optimal cut-off point for pre-TACE serum markers was determined using the X-tile (V.3.6.1 Yale University, New Haven, CT, USA) program.(12) For continuous variables, the data are expressed as the mean ± standard error of the mean. Categorical variables were analysed using Fisher's exact test, and continuous variables were compared using unpaired Student's t-tests. Patient survival curves were calculated using the Kaplan-Meier method and analysed using the log-rank test. Prognostic factors were examined by univariate and multivariate analyses using the Cox proportional hazards model. P < 0.05 was considered statistically significant.
The baseline characteristics of the Hepatocellular carcinoma patients (n=303).
| Variables | Value |
|---|---|
| Age, y, median (range) | 50 (23-77) |
| Gender (male/female) | 274/29 (90.4%/9.6%) |
| AFP, ng/ml, median (range) | 1145.0 (1.15-121000.0) |
| Hepatitis B (yes/no) | 267/36 (88.1%/11.9%) |
| WBC, 10E9/L, median (range) | 6.8 (2.9-15.1) |
| HB, g/L, median (range) | 141.6 (65.0-195.0) |
| PLT, 10E9/L, median (range) | 185.0 (49.5-604.0) |
| ALT, U/L, median (range) | 46.7 (4.2-647.3) |
| AST, U/L, median (range) | 62.0 (19.2-811.3) |
| ALB, g/L, median (range) | 39.5 (28.2-51.6) |
| TBIL, µmol/L, median (range) | 15.9 (3.9-76.7) |
| GGT, U/L, median (range) | 170.5 (21.0-1741.1) |
| LDH, U/L, median (range) | 243.7 (110.3-4248.7) |
| ALP, U/L, median (range) | 136.0 (49.7-689.6) |
| CRP, mg/L, median (range) | 13.79 (0.19-284.45) |
| PT, sec, median (range) | 12.5 (9.7-17.1) |
| Child-Pugh classification (A/B) | 287/16 (94.7%/5.3%) |
| Ascites (yes/no) | 24/279 (7.9%/92.1%) |
| Tumor size, cm, median (range) | 9.5 (2.1-20.0) |
| Tumor number (1/>1) | 118/185 (38.9%/61.1%) |
| PVTT (yes/no) | 233/70 (76.9%/23.1%) |
| Extrahepatic metastasis (yes/no) | 125/178 (58.7%/41.3%) |
Abbreviations: AFP, alpha-fetoprotein; WBC, white blood cell; HB, hemoglobin; PLT, platelet; AST, aspartate aminotransferase; ALT, alanine transaminase; TBIL, total bilirubin; ALB, albumin; GGT, gama-glutamyl transpeptidase; LDH, lactate dehydrogenase; ALP, alkaline phosphatase; CRP, C-reaction protein; PT, prothrombin time; PVTT, portal vein tumor thrombosis.
Results
Baseline characteristics
A total of 303 consecutive patients who met our criteria were included in this study. Patient baseline characteristics are summarized in Table 1. The cohort included 274 males (90.4%) and 29 females (9.6%) with a median age of 53 years (range 23-77 years). Hepatitis virus B was the predominant cause of HCC (88.1%). The majority of our patients had a good liver functional reserve of Child-Pugh A grade (94.7%). Of the 303 patients who were examined during the follow-up period, 275 patients (90.8%) died. The median OS was 8.4 months (range 1.07-65.30) in this study. The 1- and 3-year survival was 36.0% and 1.3%, respectively.
Univariate and multivariate Cox regression analyses
In previous studies, the cut-off values of clinical factors cover the whole clinical spectrum of HCC and are not ideally suited to patients with advanced HCC. Moreover, no studies to date have specifically focused on patients with advanced HCC. Therefore, we employed the X-tile program to determine the optimal cut-off value for pre-TACE serum factors and explored their prognostic effects in a large cohort of patients with advanced HCC. The optimal cut-off values of clinical factors are shown in Table 2.
Univariable and Multivariable analysis of prognostic factors in Hepatocellular carcinoma patients. (n=303)
| Overall Survival | |||
|---|---|---|---|
| Variables | Univariable value | Multivariable Analysis | |
| HR (95% CI) | p value | ||
| Age, y (≥50 vs <50) | 0.914 | ||
| Gender (Male vs Female) | 0.708 | ||
| HbsAg (Yes/No) | 0.683 | ||
| AFP, ng/ml, (≥400 vs <400) | 0.019 | n.a. | |
| AST, UL, (>60 vs ≤60) | 0.008 | n.a. | |
| ALT, U/L, (>50 vs ≤50) | 0.474 | ||
| GGT, U/L, (>200 vs ≤200) | <0.001 | 1.742 (1.333-2.276) | <0.001 |
| LDH, U/L, (>250 vs ≤250) | 0.054 | ||
| ALP, U/L, (>130 vs ≤130) | <0.001 | 1.323 (1.012-1.730) | 0.041 |
| CRP, mg/L, (>5.0 vs ≤5 ) | <0.001 | 1.526 (1.145-2.036) | 0.004 |
| TBIL, umol/L, (>25 vs ≤25) | 0.022 | n.a. | |
| ALB, g/L, (>35 vs ≤35) | 0.715 | ||
| PT, sec, (>14.0 vs ≤14.0) | 0.275 | ||
| Child-Pugh classification(B vs A) | 0.821 | ||
| Ascites (yes/no) | 0.061 | ||
| Tumor size, cm, (≥7.0 vs <7.0) | 0.052 | n.a. | |
| Tumor number (>1 vs 1) | 0.593 | ||
| PVTT (yes/no) | 0.002 | 1.418 (1.058-1.900) | 0.019 |
| Extrahepatic metastasis (yes/no) | 0.068 | n.a. | |
Abbreviations: AFP, alpha-fetoprotein; AST, aspartate aminotransferase; ALT, alanine transaminase; GGT, gamma-glutamyl transpeptidase; LDH, lactate dehydrogenase; ALP, alkaline phosphatase; CRP, C-reactive protein; TBIL, total bilirubin; ALB, albumin; PT, prothrombin time; PVTT, Portal vein tumor thrombosis; n.a, not applicable.
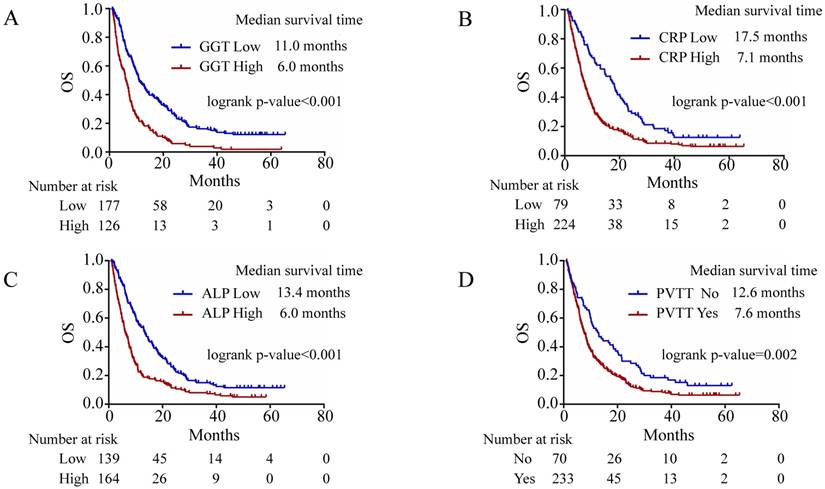
The univariate and multivariate analyses of prognostic factors for OS are shown in Table 2. In univariate analysis, levels of AFP, aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), C-reactive protein (CRP), and total bilirubin (TBIL) and portal vein tumour thrombosis (PVTT) were associated with OS (all p < 0.05). In multivariate analysis, significantly independent prognostic factors of survival were GGT (> 200 U/L), CRP (> 5.0 mg/L), ALP (> 130 U/L) and the presence of PVTT (Table 2). The Kaplan-Meier OS curves for these four factors are shown in Fig. 1. The median survival of patients with high serum GGT level (> 200 U/L) and low serum GGT level (≤ 200 U/L) was 6.0 months and 11.0 months, respectively (p < 0.001) (Fig. 1A). The median survival of HCC patients with high serum CRP (> 5.0 mg/L) and low serum CRP (≤ 5 mg/L) levels treated with TACE was 7.1 and 17.5 months (p < 0.001), respectively (Fig. 1B). The median survival according to serum ALP level was 6.0 months and 13.4 months in the ALP-high group and the ALP-low group, respectively (p < 0.001) (Fig. 1C). The median survival of HCC patients with and without PVTT was 7.6 and 12.6 months (p = 0.002), respectively (Fig. 1D).
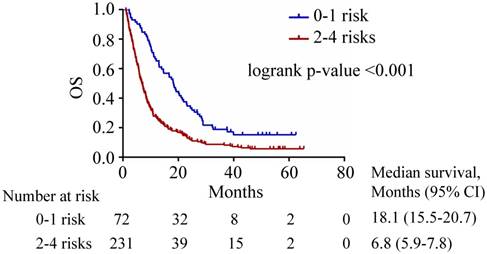
The Kaplan-Meier analysis of patient survival according the number of risk factors is summarized in Table 3. The patients were further classified into two groups according to their numbers of risk factors: 0-1 risk factors and 2-4 risk factors. The patients with 0-1 risk factors may be benefit from TACE treatment and their median survival was 18.1 months (95% CI 15.5-20.7 months) (Fig 2), which was longer than the median survival of 8.4 months in all patients. In contrast, patients with 2-4 risk factors were associated with a dismal median OS of 6.8 months (95% CI 5.9-7.8 months) (Fig 2).
Tumor response
The tumor responses of patients in the study are shown in Table 4. Because this study was retrospective, 43 (14.2%) patients were unable to be evaluated for tumor response due to absent images at the time for retrospective evaluation. Objective response rates were 38.9% and 17.3% for 0-1 risk factors and 2-4 risk factors groups, respectively (Table 4).
Median survival according to number of risk factors.
| Number of risk factors | Number of patients (N, %) | Kaplan-Meier analysis | |
|---|---|---|---|
| Median survival | 95% Confidence interval | ||
| 0 | 14 (4.6) | 21.4 | 7.4-35.5 |
| 1 | 58 (19.1) | 14.7 | 8.4-21.0 |
| 2 | 84 (27.7) | 8.6 | 5.9-11.4 |
| 3 | 67 (22.2) | 8.3 | 5.9-10.8 |
| 4 | 80 (26.4) | 5.5 | 4.2-6.9 |
Tumor response for each group.
| Outcome, n (%) | Number of risk factors | P value | |
|---|---|---|---|
| 0-1 risk factors (n=72) | 2-4 risk factors (n= 231) | ||
| Tumor response evaluation | |||
| Objective response | 28 (38.9) | 40 (17.3) | < 0.001 |
| Complete response | 4 (5.6) | 1 (0.4) | |
| Partial response | 24 (33.3) | 39 (16.9) | |
| Stable disease | 11 (15.3) | 61 (26.4) | 0.053 |
| Progressive disease | 21 (29.2) | 99 (42.9) | 0.038 |
| Not evaluated | 12 (16.7) | 31 (13.4) | 0.491 |
P value was calculated by a two-sided χ2 test.
Kaplan-Meier survival curves for OS after TACE treatment in HCC patients. Kaplan-Meier analysis for OS according to the four most significant predictors. (A) Kaplan-Meier analysis was conducted based on serum GGT levels (p < 0.001). (B) Kaplan-Meier analysis was conducted based on serum CRP levels (p < 0.001). (C) Kaplan-Meier analysis was conducted based on serum ALP levels (p < 0.001). (D) Kaplan-Meier analysis was conducted according to PVTT (p = 0.002). Abbreviations: GGT, gamma-glutamyl transpeptidase; CRP, C-reaction protein; ALP, alkaline phosphatase; PVTT, portal vein tumour thrombosis.
Kaplan-Meier curves stratified according to risk factors. The median survival periods for patients with 0-1 (blue line) and 2-4 risks (red line) were 18.1 and 6.8 months, respectively.
Treatment Administration after TACE
The study TACE sessions and subsequent treatments administered in each group are listed in Table 5. There was a statistically significant difference in the number of subsequent hepatic resection treatment sessions between 0-1 risk factors and 2-4 risk factors groups (P < 0.001). Meanwhile, there was a statistically significant difference in the number of subsequent TACE treatment sessions between 0-1 risk factors and 2-4 risk factors groups (P < 0.001). However, there was no statistically significant difference in the number of subsequent sorafenib treatment sessions between the two groups (P = 0.540).
Number of patients who received subsequent treatments for each group.
| Types of Treatment, n (%) | Number of risk factors | ||
|---|---|---|---|
| 0-1 risk factors (n=72) | 2-4 risk factors (n=231) | *p value | |
| Study TACE sessions | 146 | 348 | |
| Subsequent treatments | |||
| Hepatic resection | 12 (16.7) | 10 (4.3) | <0.001 |
| Radiofrequency ablation | 9 (12.5) | 18 (7.8) | 0.221 |
| TACE | 44 (61.1) | 81 (35.1) | <0.001 |
| Sorafenib | 9 (12.5) | 23 (10.0) | 0.540 |
* P value was calculated by a two-sided χ2 test.
Discussion
To date, several published scoring systems, such as ART(13), STATE/START(14), ABCR(15) and HAP(16), have been used to predict outcome in intermediate stage HCC patients undergoing TACE. However, few studies have reported the prognostic factors associated with outcomes following TACE therapy for patients with advanced HCC. Therefore, our present study mainly focused on survival outcomes and prognostic factors associated with patient survival to determine which advanced HCC patients would benefit most from TACE. TACE was considered a risky option for HCC patients with advanced PVTT in many countries. However, previous studies have found that there are no significant differences in 30-day mortality rates between groups of patients undergoing TACE and those treated conservatively among HCC patients with PVTT, suggesting that the TACE regimen is safe for these patients.(8, 17)
In our present study series, we demonstrated that four risk factors, high pre-treatment GGT, ALP, and CRP levels and existence of PVTT, are associated with unfavourable prognosis after TACE treatment in patients with advanced HCC. Moreover, the prognosis varied quite substantially according to the number of risk factors, with median survival periods ranging from 5.5 months to 21.4 months. This means that not all patients with advanced HCC could benefit from TACE treatment. We suggest from these findings that further prognostic classification according to the number of risk factors may be important because it will facilitate the suitable management of patients with advanced HCC. In the present study also, we observed median survivals of 18.1 (95% CI: 15.5-20.7) and 6.8 (95% CI: 5.8-7.8) months in the 0-1 and 2-4 risk factor groups, respectively. Based on the survival results, TACE is most recommended in patients with advanced HCC if they only have 0 or 1 risk factors. However, patients with 2-4 risk factors (median survival 6.8 months), TACE is not recommended for these patients and other options should be considered.
The subsequent treatments for patients who had stopped study TACE might influence the outcome of patients. Also, the subsequent treatment in patients with 0-1 risk group was more active than patients with 2-4 risk factors group. However, this can be explained by the better treatment response in the former because the subsequent treatments depended mostly on tumor burden and liver function.
The risk factors included in our study are consistent with those of previous studies. GGT is a ubiquitous epithelial enzyme(18) that catalyses the transfer of gamma-glutamyl functional groups from molecules such as glutathione to an acceptor that may be an amino acid, a peptide or water (forming glutamate).(19, 20) GGT has frequently emerged as a significant risk factor in other studies.(21, 22) Consistent with the findings of Ju-Bo Zhang et al., elevated serum GGT levels were associated with an increased risk of death in HCC patients treated with TACE. ALP is an enzyme that is widely distributed in human liver, bone, intestine, and kidney tissues and has been identified as a prognostic factor in other studies.(23, 24) Serum ALP levels are usually elevated in patients with liver diseases and thus may reflect the status of liver injury. (25) In addition, ALP has already been included in the Chinese University Prognostic Index, an HCC staging system that assigns a score of 3 when ALP is > 200 IU/L, indicating the potential roles of ALP in predicting the prognosis of HCC patients.(26) CRP is synthesized by the liver in response to interleukin-6 released by macrophages and adipocytes.(27, 28) CRP is used mainly as a marker of inflammation. CRP has been reported to be a useful prognostic factor in patients with HCC independent of treatment such as resection, sorafenib, orthotopic liver transplantation and TACE.(29-34) In HCC patients with BCLC stage-B, the selection for TACE treatment score and the assessment for retreatment with TACE score are include the serum CRP levels.(14) However, in previous studies, the cut-off values of these factors cover the whole clinical spectrum of HCC and are not ideally suited to patients with advanced HCC. Therefore, we employed the X-tile program to determine the optimal cut-off value for these factors.
However, there are some limitations in our present study. First, selection bias may exist due to the retrospective nature of this study and because all the patients were enrolled from single centre. Second, as this was a single-arm study, further studies are warranted to compare the effects of TACE with those of sorafenib or radioembolization in patients with advanced HCC. Lastly, the mechanism underlying the association between clinical serum factors (GGT, ALP, and CRP) and the prognosis for advanced HCC remains largely unknown.
In conclusion, TACE provides acceptable tolerability and favorable survival benefits for selected patients with advanced HCC. We found that four risk factors were associated with shortening the length of patient survival after TACE, including elevated serum GGT levels, serum CRP levels, serum ALP levels and existence of PVTT. TACE may be recommended for advanced HCC patients with 0-1 risk factors due to favorable survival outcome, but it is necessary to prospectively validate our results in a larger cohort.
Abbreviations
TACE: transarterial chemoembolization; HCC: hepatocellular carcinoma; BCLC: Barcelona Clinic Liver Cancer; EASL: European Association for the Study of the Liver; AFP: alpha fetoprotein; SYSU: Sun Yat-sen University; WBC: white blood cell; HB: hemoglobin; PLT: platelet; AST: aspartate aminotransferase; ALT: alanine transaminase; ALB: albumin; GGT: gama-glutamyl transpeptidase; LDH: lactate dehydrogenase; ALP: alkaline phosphatase; CRP: C-reaction protein; TBIL: total bilirubin; PT: prothrombin time; PLT: platelet; PVTT: portal vein tumor thrombosis.
Acknowledgements
This work is supported by the National Key R&D Program of China (2017YFA0505803), the National Natural Science Foundation of China (No. 81625017, No.81572385), and the Fundamental Research Funds for the Central Universities of China (No. 16ykjc36).
Authorship
Study concept and design: Yong Le, Jing-Xian Shen and Yong-Fa Zhang.
Acquisition, analysis, or interpretation of data: Yong Le, Jing-Xian Shen, Min-Ke He, Anna Kan, Hai-Long Chen, Zi-Shan Yu, Qi-Jiong Li. Writing and Drafting of the manuscript: Yong Le and Yong-Fa Zhang.
Statistical analysis: Yong Le and Yong-Fa Zhang.Critical revision of the manuscript for important intellectual content: Ming Shi. All authors approved the final submission.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108
2. Bruix J, Sherman M, Practice Guidelines Committee AAftSoLD. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236
3. European Association For The Study Of The L, European Organisation For R, Treatment Of C. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943
4. Palmer DH. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:2498 author reply 2498-2499
5. Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, Kudo M, Johnson P, Wagner S, Orsini LS, Sherman M. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35:2155-2166
6. Park JW, Sherman M, Colombo M, Roberts LR, Schwartz ME, Degos F, Chen PJ, Chen MS, Kudo M, Johnson PJ, Huang BS, Orsini LS. Observations of hepatocellular carcinoma (HCC) management patterns from the global HCC bridge study: First characterization of the full study population. Journal of Clinical Oncology. 2012:30
7. Lee HS, Kim JS, Choi IJ, Chung JW, Park JH, Kim CY. The safety and efficacy of transcatheter arterial chemoembolization in the treatment of patients with hepatocellular carcinoma and main portal vein obstruction. A prospective controlled study. Cancer. 1997;79:2087-2094
8. Chung GE, Lee JH, Kim HY, Hwang SY, Kim JS, Chung JW, Yoon JH, Lee HS, Kim YJ. Transarterial Chemoembolization Can Be Safely Performed in Patients with Hepatocellular Carcinoma Invading the Main Portal Vein and May Improve the Overall Survival. Radiology. 2011;258:627-634
9. Pinter M, Hucke F, Graziadei I, Vogel W, Maieron A, Konigsberg R, Stauber R, Grunberger B, Muller C, Kolblinger C, Peck-Radosavljevic M, Sieghart W. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology. 2012;263:590-599
10. Shi M, Chen JA, Lin XJ, Guo RP, Yuan YF, Chen MS, Zhang YQ, Li JQ. Transarterial chemoembolization as initial treatment for unresectable hepatocellular carcinoma in southern China. World Journal of Gastroenterology. 2010;16:264-269
11. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60
12. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252-7259
13. Hucke F, Sieghart W, Pinter M, Graziadei I, Vogel W, Muller C, Heinzl H, Waneck F, Trauner M, Peck-Radosavljevic M. The ART-strategy: sequential assessment of the ART score predicts outcome of patients with hepatocellular carcinoma re-treated with TACE. J Hepatol. 2014;60:118-126
14. Hucke F, Pinter M, Graziadei I, Bota S, Vogel W, Muller C, Heinzl H, Waneck F, Trauner M, Peck-Radosavljevic M, Sieghart W. How to STATE suitability and START transarterial chemoembolization in patients with intermediate stage hepatocellular carcinoma. J Hepatol. 2014;61:1287-1296
15. Adhoute X, Penaranda G, Naude S, Raoul JL, Perrier H, Bayle O, Monnet O, Beaurain P, Bazin C, Pol B, Folgoc GL, Castellani P, Bronowicki JP, Bourliere M. Retreatment with TACE: the ABCR SCORE, an aid to the decision-making process. J Hepatol. 2015;62:855-862
16. Kadalayil L, Benini R, Pallan L, O'Beirne J, Marelli L, Yu D, Hackshaw A, Fox R, Johnson P, Burroughs AK, Palmer DH, Meyer T. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann Oncol. 2013;24:2565-2570
17. Luo J, Guo RP, Lai EC, Zhang YJ, Lau WY, Chen MS, Shi M. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011;18:413-420
18. Goldberg DM. Structural, functional, and clinical aspects of gamma-glutamyltransferase. CRC Crit Rev Clin Lab Sci. 1980;12:1-58
19. Tate SS, Meister A. gamma-Glutamyl transpeptidase from kidney. Methods Enzymol. 1985;113:400-419
20. Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38:263-355
21. Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and gamma-glutamyltransferase and mortality in the United States population. Gastroenterology. 2009;136:477-485 e411
22. Poynard T, Zourabichvili O, Hilpert G, Naveau S, Poitrine A, Benatar C, Chaput JC. Prognostic value of total serum bilirubin/gamma-glutamyl transpeptidase ratio in cirrhotic patients. Hepatology. 1984;4:324-327
23. Xu XS, Wan Y, Song SD, Chen W, Miao RC, Zhou YY, Zhang LQ, Qu K, Liu SN, Zhang YL, Dong YF, Liu C. Model based on gamma-glutamyltransferase and alkaline phosphatase for hepatocellular carcinoma prognosis. World J Gastroenterol. 2014;20:10944-10952
24. Piras-Straub K, Khairzada K, Gerken G, Saner F, Treckmann J, Paul A, Canbay A, Herzer K. Glutamate dehydrogenase and alkaline phosphatase as very early predictors of hepatocellular carcinoma recurrence after liver transplantation. Digestion. 2015;91:117-127
25. Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med. 2000;342:1266-1271
26. Leung TW, Tang AM, Zee B, Lau WY, Lai PB, Leung KL, Lau JT, Yu SC, Johnson PJ. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760-1769
27. Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol. 2005;288:H2031-2041
28. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805-1812
29. Nakanishi H, Kurosaki M, Tsuchiya K, Yasui Y, Higuchi M, Yoshida T, Komiyama Y, Takaura K, Hayashi T, Kuwabara K, Nakakuki N, Takada H, Ueda M, Tamaki N, Suzuki S, Itakura J, Takahashi Y, Izumi N. Novel Pretreatment Scoring Incorporating C-reactive Protein to Predict Overall Survival in Advanced Hepatocellular Carcinoma with Sorafenib Treatment. Liver Cancer. 2016;5:257-268
30. Chun JM, Kwon HJ, Sohn J, Kim SG, Park JY, Bae HI, Yun YK, Hwang YJ. Prognostic factors after early recurrence in patients who underwent curative resection for hepatocellular carcinoma. J Surg Oncol. 2011;103:148-151
31. Ishizuka M, Kubota K, Kita J, Shimoda M, Kato M, Sawada T. Impact of an inflammation-based prognostic system on patients undergoing surgery for hepatocellular carcinoma: a retrospective study of 398 Japanese patients. Am J Surg. 2012;203:101-106
32. Jang JW, Oh BS, Kwon JH, You CR, Chung KW, Kay CS, Jung HS. Serum interleukin-6 and C-reactive protein as a prognostic indicator in hepatocellular carcinoma. Cytokine. 2012;60:686-693
33. An HJ, Jang JW, Bae SH, Choi JY, Yoon SK, Lee MA, You YK, Kim DG, Jung ES. Serum C-reactive protein is a useful biomarker for predicting outcomes after liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2012;18:1406-1414
34. Sieghart W, Pinter M, Hucke F, Graziadei I, Schoniger-Hekele M, Muller C, Vogel W, Trauner M, Peck-Radosavljevic M. Single determination of C-reactive protein at the time of diagnosis predicts long-term outcome of patients with hepatocellular carcinoma. Hepatology. 2013;57:2224-2234
Author contact
![]() Corresponding author: Ming Shi, M.D. Department of Hepatobiliary Oncology, Cancer Center Sun Yat-sen University 651 Dongfeng East Road; Guangzhou 510060, People's Republic of China. E-mail: shimingedu.cn. Tel: 186-20-87343938. Fax: 186-20-87343585.
Corresponding author: Ming Shi, M.D. Department of Hepatobiliary Oncology, Cancer Center Sun Yat-sen University 651 Dongfeng East Road; Guangzhou 510060, People's Republic of China. E-mail: shimingedu.cn. Tel: 186-20-87343938. Fax: 186-20-87343585.

 Global reach, higher impact
Global reach, higher impact