3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(21):5162-5172. doi:10.7150/jca.36300 This issue Cite
Review
Update on current pancreatic treatments: from molecular pathways to treatment
1. 3rd Department of Surgery, “AHEPA” University Hospital, Aristotle University of Thessaloniki, Medical School, Thessaloniki, Greece
2. Anesthesiology Department, “AHEPA” University Hospital, Aristotle University of Thessaloniki, Medical School, Thessaloniki, Greece
3. Department of Pharmacology and Department of Surgery, Faculty of Dentistry, University of Medicine and Pharmacy of Craiova, Craiova, Romania
4. Clinical Pharmacology, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece
5. Surgery Department, University of Timisoara, Romania
6. General Surgery Department, "Dr Carol Davila", University of Medicine and Pharmacy, Bucuresti, Romania
7. University of Medicine and Pharmacy, Craiova, Romania
Received 2019-5-4; Accepted 2019-7-29; Published 2019-8-28
Abstract
Pancreatic cancer is still diagnosed at a late stage although we have novel diagnostic tools. Pancreatic cancer chemotherapy treatment resistance is observed and therefore novel treatments are in need. Anti-cancer stem cell therapy, combination of chemotherapy and/or radiotherapy with immunotherapy, proteins/enzymes and gene therapy are currently under evaluation. Targeted treatment with tyrosine kinase inhibitors is also administered and novel inhibitors are also under evaluation. In the current review we present recent data from our search within the year 2018.
Keywords: pancreatic cancer, gene therapy, stem cells, cancer
Introduction
Pancreatic adenocarcinoma (PAC) is a common malignancy with very poor prognosis with an overall 5-year survival rate of <5% when all stages are combined. It is also known to have resistance to most chemotherapy regimens [1]. Based on current knowledge pre-diagnostic gastrointestinal investigations do not appear to contribute to the poor prognosis of PAC. Currently NICE Guidelines recommend the early use of ultrasound or CT in patients with gastrointestinal symptoms and weight loss. To date we do not have efficient and cost effective screening tests to diagnose this condition at a curable stage and improve survival [2]. Moreover; there is a relationship between molecular heterogeneity and clinical features of pancreatic cancer which remains to date unclear. Different subgroups of pancreatic cancer have been identified, which means that different patients in each subgroup might benefit from individual targeted therapy [3]. Although different treatments can be used such as; chemotherapy, radiotherapy, immunotherapy and molecular targeted therapy over the past few decades; to date surgical resection is still the most powerful therapy to cure PAC. However; due to lack of early signs or symptoms, most patients are diagnosed at a late stage not amenable to surgery [4]. Borderline resectable pancreatic cancer patients treated with neoadjuvant therapy have similar morbidity and survival to their initially resectable pancreatic cancer counterparts [5]. It has been observed that modern neoadjuvant therapy is associated with improved survival. However, rapid development of resistance to gemcitabine is always observed [6]. Extracellular matrix components have an impact on cell migration and invasive behavior of the cancer cells. Collagen has the most evident effect, and provides new insights into the understanding of the intricate interplay between extracellular matrix molecules and cancer cells. It is a tissue target for novel therapeutic targets for PAC treatment [7]. Another type of pancreatic cancer are the pancreatic neuroendocrine neoplasms (PanNENs) which are rare endocrine tumors. It has been observed that they have a different prognosis upon their proliferative state. They are characterized by histopathological grading arising from the endocrine islets with an incidence of 0.2-0.3/100.000 [8, 9]. The histological classification of these tumors is associated with the Ki-67 index and high mitotic count [10].
Based on the recent WHO classification there are three grades; Grade 1 and Grade 2 tumors which are less proliferative and Grade 3 tumors whose prognosis is very poor [10]. Current evidence indicates that neural remodeling and perineural invasion (PNI) of PAC potently increase the risk of cancer relapse, and is considered critical cause of neuropathic pain which affects significantly the quality of life and survival of these patients [11]. It has been observed that a 90% of patients are subjected to intra-pancreatic nerve infiltration by cancer cells, and 69% patients have extra-pancreatic nerve involvement [12, 13]. Pancreatic nerve infiltration is a common pathological characteristic of PAC where the cancer cells invade the surrounding nerves, which damages the nerve ends [14]. Unfortunately to date underlying mechanisms of pancreatic nerve infiltration in PAC are poorly understood. The most important reason for this lack of knowledge is the fact that two third of these patients are diagnosed with pancreatic cancer at a late stage. Therefore, there is an urgent need to develop novel diagnostic tools and therapeutic regimens for pancreatic cancer. In the current article we will provide recent data from 2018 focusing on radiotherapy regimens, molecular targeted treatments, immunotherapy and pancreatic stem cell therapy. Wherever it was necessary we added more data in the text mostly including novel therapies or pathways included in the better understanding of this malignancy.
Molecular Pathways
Immunotherapy
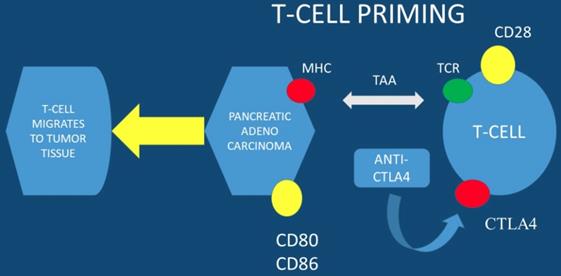
Currently immune checkpoints blockade therapies, such as; anti-programmed death-ligand 1 (anti-PD-L1) and anti- programmed death-ligand 1 (anti-PD-1), present promising anti-tumor efficacy for several types of solid tumors. However, there are no promising results for this therapy in pancreatic cancer with anti-PD-L1 therapy alone. To date the expression level of PD-L1 is correlated with checkpoint immunotherapy efficacy. It has been observed that esophageal ultrasound fine-needle pancreatic core biopsy (FNB) can be used to determine eligibility for immunotherapy. In a previous study at least 3% of malignant pancreas lesions were sensitive to pembrolizumab and more than 8% were sensitive to the family of immune checkpoint inhibitors. Due to the large EUS-FNB tissue samples precision immuno-oncology can be applied to these patients (PD-L1 evaluation is performed in large tissue samples) [15]. The far upstream element-binding protein 1 (FUBP1) is an important transactivator of c-Myc proto-oncogene. FUBP1 has been observed to be overexpressed in pancreatic cancer and is associated with poor prognosis. It has been also observed that FUBP1 promotes tumor cell proliferation and regulates the cancer cell immunity by increasing the PD-L1 expression. FUBP1 is currently investigated as a novel therapeutic modality in order to overcome the immunotherapy resistance in PAC [16]. It has observed that PD1-CD28 fusion protein-transduced CD4+ T cells significantly improves anti-tumoral effect of fusion protein-transduced CD8+ T cells. PD1-CD28 fusion protein-transduces CD4+ T cells and has the potential to overcome the PD-1-PD-L1 immunosuppressive axis in pancreatic cancer [17]. Combined treatment using radio-immunotherapy with gemcitabine (one cycle) significantly suppresses tumor growth and prolongs survival with tolerable toxicity. Moreover; the two-cycle regimen had the highest anti-tumor effect, but was not tolerable. It was also observed that combination of 90Y-labeled 059-053 and gemcitabine is a promising therapeutic option for pancreatic cancer [18]. In the study by Sahin IH et. al. [19] positive results were observed only for the patients with high MSI-H (microsatellite instability) and immunotherapy administration in PAC patients. In another study dendritic cell vaccination was used in order to enhance the FOLFIRINOX regimen and indeed combined treatment significantly increased the lifespan of KIC mice with PAC [20]. Avelumab a monoclonal antibody is now being investigated in a phase III clinical trial in combination with PEGPH20 in chemotherapy resistant pancreatic cancer. PEGPH20 (pegvorhyaluronidase alfa) is the PEGylated version of recombinant human hyaluronidase enzyme, rHuPH20. rHuPH20 temporarily degrades hyaluronan (HA), which is a naturally occurring glycosaminoglycan or chain of natural sugars that is common throughout the body and can accumulate in the tumor microenvironment of certain solid tumor types. PEGylating rHuPH20 increases the plasma half-life of the enzyme, which increases exposure following systemic delivery and enables PEGPH20 to target tumor-associated HA in a unique investigational approach to cancer treatment [21, 22]. PEGPH20 has a half-life of approximately 2 days, thereby enabling systemic activity and sustained duration of action to degrade HA. In many different tumor types tested in murine xenograft models, response to PEGPH20 has been shown to be more robust for tumors characterized by higher HA expression. The clinical trial NCT03481920 will close in August 2019 (Figure 1,2).
MHC; major histocompatibility complex, TAA; tumor-associated antigens, CTLA4; cytotoxic T-lymphocyte-associated protein 4
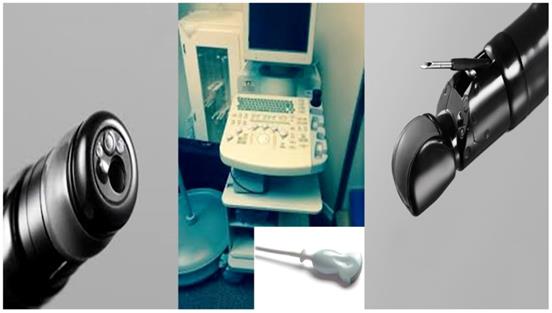
From left to right; Radial esophageal ultrasound, convex probe and convex probe esophageal ultrasound
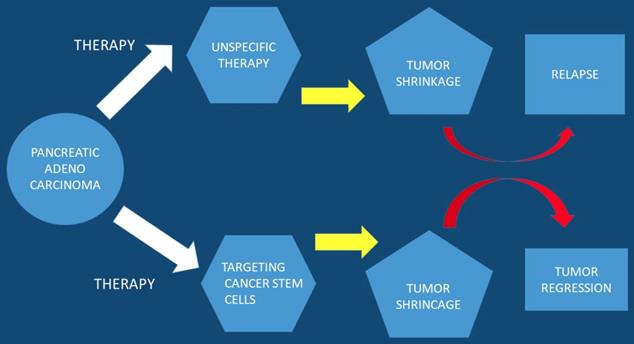
Pancreatic Stem Cells
In current studies it has been observed that a subpopulation of cells exist within tumors, cancer stem cell (CSC), which are capable of self-renewal. Cancer stem cells constitute a small cellular subpopulation within the tumor, however; their resistance to chemotherapy and radiation make them an important therapeutic target for treatment. Cancer stem cells possess a unique metabolic plasticity allowing them to rapidly respond and adapt to microenvironmental changes. Cancer stem cells and their metabolic-epigenetic interplay may constitute a new avenue for therapy specifically targeting cancer stem cells in pancreatic cancer [23]. Furthermore; metabolic processes of cancer stem cells appear attractive, but due to their function and properties development of resistance is likely to occur. Currently a strategy has been developed to specifically target pancreatic cancer stem cells and it is a phase I/II trial. This trial relies on a different approach by turning the body's immune system against this cellular subpopulation [24]. It has been observed that the bioactive sphingolipid induced migration of pancreatic cancer stem cells and signaling was specific to ceramide-1-phosphate. Moreover; pancreatic cancer cells were identified as a rich source of ceramide-1-phosphate. Pancreatic cancer stem cells secrete ceramide-1-phosphate-containing extracellular vesicles as a means of recruiting pancreatic cancer stem cells to sustain tumor growth therefore making ceramide-1-phosphate release a mechanism that could facilitate tumor progression [25]. Furthermore; pancreatic cancer stem cell proliferation has been observed to be strongly inhibited by diethyldithiocarbamate-copper complex loaded into hyaluronic acid decorated liposomes. This type of encapsulation of Cu(DDC)2 complex in HA decorated liposomes has been found to strongly increase the proliferative activity of cancer stem cells [26]. In another study ``Rauwolfia vomitoria`` extract preferentially inhibited pancreatic cancer stem cells [27]. Moreover; ATP Binding Cassette Subfamily G Member 2 which is a non-substrate anticancer agent FL118 has been observed to target drug-resistant cancer stem like cells and overcome treatment resistance of human pancreatic cancer [28]. Another agent the BBI-608 has been administered in advanced cancers with positive results and the clinical trial is still ongoing (NCT01775423) [29] (Figure 3).
Pancreatic stem cells are used as a targeted therapy
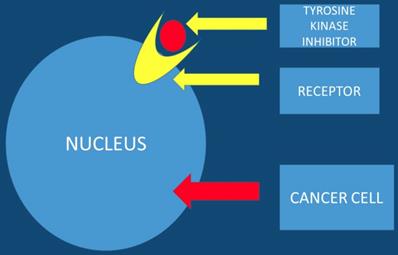
Tyrosine Kinase Inhibitors
Tyrosine kinase inhibitors are known to be promising anticancer agents. However; resistance is observed in several patients treated with tyrosine kinase inhibitors. The acquired resistance is known to be a complex phenomenon that involves different signaling pathways. Therefore there is a need for drug combination therapies that modulate different signaling and growth systems to counter the emergence of resistance. It is necessary to development multi-targeted tyrosine kinase inhibitors and dual small molecule inhibitors that could improve treatment outcomes and lead to more personalized therapeutic approaches for pancreatic patients [30]. Nitroxoline and nelfinavir as single agents or in combination with erlotinib in pancreatic cancer cells can be used [31]. Moreover; the EHMT2-p27 axis is a potential marker to modulate cell response to dual PI3K/mTOR inhibition, which might provide a strategy in personalized therapeutics for pancreatic cancer patients [32]. This study presented data where the combination of proto-oncogene (RAF) and CDK4/6 inhibitors could be a new treatment strategy for K-ras G12R mutant pancreatic cancer [33]. It has been observed that Galunisertib-gemcitabine combination improves overall survival vs. gemcitabine in patients with unresectable pancreatic cancer. To date the use of galunisertib in pancreatic cancer is ongoing in combination with durvalumab (an anti-PD-L1 monoclonal antibody) [34] (Figure 4).
The tyrosine kinase inhibitor blocks the tumor growth
Enzymes and Proteins
It has been observed that mutant p53 prevents the nuclear translocation of the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase. It stabilizes the cytoplasmic localization, and supports glycolysis of cancer cells and inhibits cell death mechanisms by mediating the nuclear enzyme glyceraldehyde-3-phosphate dehydrogenase. The prevention of nuclear localization of enzyme glyceraldehyde-3-phosphate dehydrogenase has been observed to be mediated by both stimulation of AKT and repression of AMPK signaling. Further it is associated with the formation of the SIRT1:GAPDH complex. It has been suggested that mutp53-dependent enhances glycolysis which allows cancer stem cells to acquire sensitivity to anti-glycolytic drugs. Glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase could be a novel personalized therapeutic approach in human cancers carrying mutant TP53 gene [35]. Hmga2 is known to be a prognostic marker which identifies a metastatic cancer cell state in primary PAC. However; Hmga2 does not have any functional impact on PAC progression and therapy efficiency [36]. Integrin b1 is involved in the acquisition of gemcitabine resistance in PAC. Drug-resistance cells from AsPC-1 parent cell line were selected. Results have shown that integrin b1 expression is upregulated in DR-AsPC-1 cells, and integrinb1 knockdown significantly decreases the activity of Cdc42, a target molecule of integrin b1, and p110b expression. It has been observed that knockdown of anyone of integrin b1, Cdc42 and p110b inhibits the activity of PI3K signaling, and sensitizes DR-AsPC-1 cells to gemcitabine. Glutathione-S-transferase pull-down assay has shown that GTP-Cdc42 interacts with p110b. It was observed that integrin b1 promoted gemcitabine resistance in PAC through Cdc42 activation of PI3K p110b signaling. This observation was confirmed by in vivo experiments [37]. In another study the role of miR-429 in modulating PNI in PAC was investigated. It was observed that miR-429 was downregulated in PAC cancer tissues and also decreased in tissues with perineural invasion. In and experiment it was reduced in nine out of ten examined pancreatic cancer cell lines. MiR-429 significantly suppresses cell viability and invasion of the pancreatic cancer cells. An online bioinformatic software predicted that neurotrophin-3 (NT-3)has a potential to target the gene of miR-429. It was showed that neurotrophin-3 mRNA elevated in pancreatic cancer tissues in patients with perineural invasion. It has been observed that MiR-429 upregulation substantially suppresses the neurotrophin-3 mRNA andsecretion in pancreatic cancer cells. An interaction between miR429 and NT-3 has been confirmed with dual luciferase reporter assays. MiR-429 potentially suppresses neurotrophin-3 and alleviated perineural invasion of PAC [38].
Pancreatic Cancer Fibroblasts
Cancer-associated fibroblasts have been observed to be key effector cells in pancreatic ductal adenocarcinoma. They are known to induce tumor growth and progression. Pancreatic stellate cells are the precursors of cancer associated fibroblasts in pancreatic adenocarcinoma that secrete abundant extracellular matrix, cytokines and growth factors. Ηuman relaxin-2 (RLX) which is an endogenous hormone and inhibits pancreatic stem cells differentiation into cancer associated fibroblasts-like myofibroblasts has been evaluated as a therapeutic target. It has been observed that relaxin-2 significantly inhibits tumor growth factor-β and induces pancreatic stem cell differentiation by inhibiting pSmad2 signaling pathway. In specific vitroin primary human pancreatic stem cell, treatment with relaxin-2 dose-dependently inhibited the migration, protein expression of alpha smooth muscle actin, collagen I and contraction. Relaxin-2 has several drawbacks like; poor pharmacokinetics and low systemic vasodilation, which limits its clinical application. A nanoparticle system which chemically conjugates relaxin-2 to superparamagnetic iron oxide nanoparticle (SPION) has been produced to improve its pharmacokinetics. This drug product relaxin-2-spion was observed to be more efficacious compared to free relaxib-2 in vitro. Relaxin-2-spion inhibited tumor growth by itself and also increased the antitumor effect of gemcitabine when injection subcutaneously in a pancreatic tumor stem cell model. In contrast, free relaxin-2 presents no significant antitumor effects. Relaxin-2-spion is an effective antitumor stroma therapy and can be used effectively against pancreatic tumor [39]. Cancer associated fibroblasts are known to be highly chemo resistant. Direct cell-cell contact and high levels of interleukin have been correlated with a high chemo resistance [40].
Proteasome Inhibitors
It is known that inhibition of proteasome activity blocks the degradation of dysfunctional proteins, the result is cancer cell death due to cellular stress. Proteasome inhibitors are a class of anticancer agents, recently; carfilzomib, bortezomib and ixazomib have been FDA-approved to treat multiple myeloma. However, again resistance has been observed to these inhibitors through point mutations in the proteasome ecatalytic subunit. A new proteasome inhibitor was identified aquinolin-chlorobenzothioate, qcbt7, and presents cytotoxicity in a panel of cancer cell lines. QCBT7 is a more stable derivative of quinoline-8-thiol that targets the regulatory subunit. Aquinolin-chlorobenzothioate -7 increases the expression of a set of genes such as; PFKFB4, CHOP, HMOX1 and SLC7A11 at both nascent RNA and protein levels. Moreover; the known proteasome inhibitors mg132 and ixazomib do the same. Aquinolin-chlorobenzothioate -7 induces proteasome inhibition, endoplasmic reticulum stress, hypoxic response, glycolysis and cell death. Importantly, 6-Phosphofructo-2-Kinase/Fructose-2,6-Biphosphatase 4 has been identified as a potential biomarker of proteasome inhibitors that can be used to monitor treatment response in pancreatic cancer [41]. Mutations or copy number abnormalities of genes involved in homologous recombination (HR) have been previously investigated in PAC tissue samples [29]. Pharmacological inhibitors of the enzyme poly ADP ribose polymerase (PARP) are developed for multiple indications; the most important is the treatment of cancer [42]. Today PARP inhibitors appear to improve progression-free survival in women with recurrent platinum-sensitive ovarian cancer [43]. PARPis strategies target HR repair. Moreover; there are multiple approaches are underway to develop biomarkers for identification of patients who will respond to PARP [44-48]. Patients that are cisplatin ineligible may benefit from neoadjuvant use of PARPi combined with carboplatin chemotherapy [49]. Adjuvant use of PARPi in patients with high risk or micro-metastatic PAC could potentially render them disease free or be combined with androgen deprivation in node-positive patients [42].
K-ras
It has been observed that PAC exhibits an oncogenic K-ras mutation rate of ∼90%. Unfortunately to date there is no clinical efficient targeted therapy focused on K-ras mutation PAC. Moreover; gemcitabine resistance is rapidly acquired to these patients. The antibody rt11-i has been created, which directly targets the intracellularly activated GTP-bound form of oncogenic RAS mutants. This antibody significantly sensitizes pancreatic cancer cells to gemcitabine. Furthermore; the co-administration synergistically inhibits angiogenesis, invasion, migration and presents synergistic anticancer activity by inhibiting the RAF/MEK/ERK or PI3K/AKT pathways. Moreover; it was observed that co-treatment inhibits endothelial barrier disruption in tumor vessels, a critical step in vascular leakiness of metastasis, and improves vessel structural stability. The antibody rt11-i synergistically increases the antitumor activity of gemcitabine by inhibiting RAS downstream signaling. In the near future this drug combination could be applied in K-ras positive pancreatic cancer patients [50]. G-quadruplex structure is an important drug target in cancer therapy. Porphyrins: Porphyrin-1(Cobalt containing) and Porphyrin-2 (Palladium containing) are observed to have high affinity towards K-ras-promoter/G-quadruplex. These porphyrins exhibited significant cytotoxicity in human pancreatic ductal carcinoma cell line PANC-1 and MiaPaCa2 and blocked metastasis. The mechanism of action is through inhibition of the epithelial to mesenchymal transition. In vivo studies confirmed both porphyrin compounds to be effective against esophageal adenocarcinoma tumors along with significantly low toxicity against normal Swiss albino mice. It has been observed that these porfirins can reduce the expression of K-ras gene in porphyrin-treated PANC-1, tumor-derived esophageal adenocarcinoma and MiaPaCa2 at both protein and RNA levels. Therefore; a porphyrin-based therapy with G quadruplex DNA ligands at the promoter region of K-ras, can be used as an anticancer treatment strategy [51]. Moreover; Protein kinase C acts through promoting yes-associated protein 1 function to promote the survival of pancreatic cancer cells expressing mu-Kras. This pathway when targeted with YAP1 offers a feasible treatment strategy for developing new therapeutics for treating pancreatic cancer [52].
MiRNAs
It is known that MiRNAs are small, noncoding RNAs post-transcriptionally regulating gene expression. Several miRNAs with altered expression upon proliferation can be used as prognostic biomarkers in pancreatic neuroendocrine neoplasms. The expression of hsa-miR-106b, hsa-miR-21 and hsa-miR-10a have been observed to have a prognostic relevance regarding progression-free and overall survival in patients with pancreatic neuroendocrine neoplasms [53]. Moreover, melatonin and its metabolite N1-acetyl-N2-formyl-5- methoxykynuramine (AFMK) have been observed to enhance chemosensitivity to gemcitabine in pancreatic carcinoma cells (PANC-1). Melatonin and the co-administration of metabolite N1-acetyl-N2-formyl-5-methoxykynuramine can improve the anti-tumor effect of gemcitabine in PANC-1 cells by enhancing the apoptotic pathway [54]. Melittin also induced long non-coding RNA proliferation and migration of pancreatic ductal adenocarcinoma [55]. It was observed that miR-7/MAP3K9 is critically involved in pancreatic cancer progression and that miR-7 could may be a potential target for pancreatic cancer [56]. Moreover; MiR-139-5p transcription is inhibited by EZH2 through up-regulating H3K27me3, thereby downregulation of EZH2 and up-regulation of miR-139-5p impede extracellular matrix and lymph node metastasis in pancreatic cancer. EZH2/miR-139-5p could be a therapeutic strategy for the treatment of pancreatic cancer [57]. The amino acid transporter-targeting gemcitabine prodrug, Gem-Thr, was found to be effective on pancreatic cancer cells with increased pharmacokinetic characteristics than gemcitabine alone [58]. It has been suggested that S-1 protein could be used as first-line chemotherapeutic option for unresectable pancreatic cancer patients aged ≥75 years [59]. SERPINB7 has been identified as the first predictive RNA biomarker for pancreatic cancer. Moreover; patients who expressed SERPINB7 are candidates to receive another treatment than gemcitabine alone [60]. In the study by Shahda S et al. [29] mutations or copy number abnormalities of genes involved in homologous recombination (HR) were investigated in tissue samples. The aim of the study was to describe the HR pathway mutations status and determine their association with treatment response and outcome in patients with PAC. However; no positive association was observed between high scores of homologous recombination deficiency or prolonged survival in patients treated with FOLFIRINOX.
Hypoxia induced factors
There are inhibitors such as the CA9 and APE1/Ref-1that target the hypoxia induced factors and affect pancreatic cancer cell survival [61]. A histone deacetylase (HDAC) inhibitor lmk-235 was observed to lower overall cell viability by inducing apoptosisin in a time and dose dependent manner. Acetylation of histone-H3 increases with higher lmk-235 concentrations. Furthermore; IHC analysis showed that proliferative activity (phosphohistone H3 and Ki-67) decreases upon the highest concentrations of lmk-235. Chromogranin and somatostatin receptor 2 (SSTR2) expression increase on the other hand in a dose-dependent manner. Therefore lmk-235 is a potential therapeutic approach pancreatic neuroendocrine neoplasms [62, 63]. Metarrestin is an effective therapeutic inhibitor and a candidate with a favorable pharmacokinetic profile achieving excellent intra-tumor tissue levels in pancreatic cancer. Pharmacokinetic evaluation of metarrestin in wild-type and Pdx1-Cre;LSL-K-rasG12D/+;Tp53R172H/+ (KPC) mice, showed significant antitumor activity [64].
Radiation
Currently there is a debate of dosimetric analysis of stereotactic rotational versus staticintensity-modulated radiation therapy for PAC. Nine-field intensity-modulated radiation therapy reduces the number of monitoring units and treatment delivery time when compared to volumetric-modulated arc therapy. Volumetric-modulated arc therapy is efficient for locally advanced PAC if combined with SBR [65]. Novel 4D-MRI sequence based on 3D-radial sampling and slab-selective excitation observed that the non-contrast 4D-MRI images showed significantly better contrast when compared to noise ratio for the vessels. The specific sequence is more efficient in identifying both the tumor and boost volume margins for pancreas radiotherapy [66]. Moreover; It was observed that stereotactic body radiotherapy and anti-CD40 are so effective at augmenting T cell priming, that memory CD8 T cell responded that both tumor and self-antigens were induced, resulting in vitiligo in long-term survivors [67]. In another study the SCAD score was evaluated as a method to identify individuals benefiting from re-stereotactic body radiotherapy [68]. PARP inhibitors can be used in combination with chemo- or radiotherapy or as single agents alone [69].
Finally in locally advanced it has been observed that there is a higher effectiveness when chemoradiotherapy is applied simultaneously for locally advanced pancreatic cancer [70]. Regarding borderline resectable patients then it has been observed in a Phase II trial that preoperative FOLFIRINOX followed by individualized chemoradiotherapy in borderline resectable pancreatic cancer results in high rates of R0 resection and prolonged median PFS and median OS, supporting ongoing phase 3 trials [71].
Future perspective
Currently the major issue is to predict the response and to identify additional targets that will improve the efficacy of chemotherapy of pancreatic cancer. In another study it was initiated through a large-scale in vivo and in vitro CRISPR knockout screens in pancreatic ductal adenocarcinoma cells. It was observed that several gene deletion synergistically increased the cytotoxicity of MEK signaling inhibitors. Drugs were created based on in vivo CRISPR screening (DREBIC) method and validated their efficacy using large-scale experimental data from independent experiments. It was observed that comparative analyses demonstrated that DREBIC predicts drug response in cancer cells from a wide range of tissues with high accuracy [72]. There are currently oncolytic viruses for pancreatic cancer (current data and clinical trials) [73]. It is known that oncolytic viruses have been used as a novel class of anti-cancer therapeutics with one virus already receiving United States Food and Drug Administration (FDA) approval (talimogene laherparepvec). It has been observed that these viruses have direct lytic effects on tumor cells as well as immunomodulatory functions which increase the inflammatory cell infiltrates in the tumor microenvironment. However; despite all of the advances this therapeutic modality still remains inefficient. One of the main reasons is the fibrotic tumor stroma and the unique extracellular matrix which creates an environment that promotes tumor growth. An oncolytic virus is a virus that preferentially infects and kills cancer cells. As the infected cancer cells are destroyed by oncolysis, they release new infectious virus particles or virions to help destroy the remaining tumor. Oncolytic viruses are thought not only to cause direct destruction of the tumor cells, but also to stimulate host anti-tumor immune system responses.
A number of viruses including adenovirus, herpes simplex, reovirus, measles, and vaccinia have been clinically tested as oncolytic agents. Most current oncolytic viruses are engineered for tumor selectivity, although there are naturally occurring examples such as reovirus and the senecavirus, resulting in clinical trials [73].
There are underlying pathologic correlations with typical and atypical presentations of pancreatic neuroendocrine neoplasms [74]. Liquid biopsy could be a tool to assess potential biomarkers. However; the method still has setbacks and tissue is still absolutely necessary [75]. Currently the method of liquid biopsy is efficient 60-70% for identifying mutations [76]. Moreover; further prospective trials are needed to define the optimal time-dose-fractionation (radiotherapy) in order to reduce toxicity and improve the palliative outcome. Furthermore; pain control and quality of life should be considered as an endpoint in future trials with local treatment (advanced stage disease [77]. Functionalized MoS2 Nanosheets as Multi-Gene Delivery Vehicles for In Vivo Pancreatic Cancer Therapy have been also constructed to carry gene therapy [78]. In specific the potential of this polymer to encapsulate and enhance drug (5-fluorouricil and bis-(naphthalimidopropyl)-diaminooctane) cytotoxicity in BxPC-3 cells was evaluated. Furthermore; the novel poly(allylamine)-naphthalimide carrier it was observed to amplify the cytotoxic effect with drug treatment after 24 h. The result was reduction of 50% of cancer cell population [79]. Monitoring quality of care for patients with pancreatic cancer was performed with novel quastionaires such as the modified Delphi consensus [80]. There are novel circulating nucleic acids that are associated with outcomes of patients with pancreatic cancer [81]. There is a novel local therapy in a Phase I Study of EUS-guided photodynamic therapy for locally advanced pancreatic cancer [82]. There is currently a debate for evaluation of the prognostic value of the new AJCC 8thedition staging system for patients with pancreatic adenocarcinoma, since there is a need to sub classify stage III?[83]. Currently we have radiofrequency ablation as a treatment option for liver cancer with significant results. On the other hand the use of radiofrequency ablation is relatively new in PAC. Radiofrequency ablation is a promising mechanism to induce antigen-presenting cell infiltration and enhance systemic antitumor T-cell immune response and tumor regression as observed with other solid tumors. Combination of radiofrequency ablation with immunotherapy could represent a novel and promising treatment [84]. To date several factors have been related to the status of para aortic lymph nodes such as; preoperative CA19-9 level, portal vein/superior mesenteric vein invasion, superior mesenteric artery invasion and diameter>1.0cm were 4 independent risk factors to para aortic lymph nodes metastasis. It has been also observed that positive LN8 and LN14 are 2 strong predictors of para aortic lymph nodes metastasis. Therefore a comprehensive analysis covering these risk factors related to metastasis of para aortic lymph nodes should be given before design of future treatment plan whenever involvement of para aortic lymph nodes is suspected [85]. Finally, we have to introduce the older cancer patient to patient activation through counseling, exercise and mobilization [86].
Conclusion
Immune checkpoint inhibitors are a treatment choice for several cancer types and have shown a certain efficacy in the treatment of advanced pancreatic cancer. Therefore could confer long-term survival benefits when used. Combined therapy when possible is certainly more effective and may serve as an alternative option. Further studies should be performed towards combined therapies [87]. Combination systemic therapies or radiotherapy with immune checkpoint inhibitors in pancreatic cancer can overcome resistance to single‑agent checkpoint blockade [88]. Radiotherapy of pancreatic cancer in older patients is feasible and can be used. Radiotherapy can represent a treatment option in pancreatic cancer even in older patients. However; further analyses and prospective trials enrolling older patients are needed to better define the risk/benefit ratio in different treatment settings (e.g performance status, metastatic site) [89]. Association between chronological depressive changes and physical symptoms in postoperative pancreatic cancer patients is important for treatment administration and should be evaluated before treatment administration [90].
Acknowledgements
All authors contributed equally and no financial support was received.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Shrikhande SV, Kleeff J, Kayed H, Keleg S, Reiser C, Giese T. et al. Silencing of X-linked inhibitor of apoptosis (XIAP) decreases gemcitabine resistance of pancreatic cancer cells. Anticancer research. 2006;26:3265-73
2. Apollos JR, Sami S, Prasanth MN, Jeyakumar J, McFadyen AK. Pre-diagnostic delays caused by gastrointestinal investigations do not affect outcomes in pancreatic cancer. Annals of medicine and surgery. 2018;34:66-70 doi:10.1016/j.amsu.2018.07.011
3. Zhang H, Zeng J, Tan Y, Lu L, Sun C, Liang Y. et al. Subgroup analysis reveals molecular heterogeneity and provides potential precise treatment for pancreatic cancers. OncoTargets and therapy. 2018;11:5811-9 doi:10.2147/OTT.S163139
4. Keleg S, Buchler P, Ludwig R, Buchler MW, Friess H. Invasion and metastasis in pancreatic cancer. Molecular cancer. 2003;2:14
5. Bolton NM, Maerz AH, Brown RE, Bansal M, Bolton JS, Conway WC. Multiagent neoadjuvant chemotherapy and tumor response are associated with improved survival in pancreatic cancer. HPB: the official journal of the International Hepato Pancreato Biliary Association. 2018 doi:10.1016/j.hpb.2018.08.013
6. Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D. et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. Jama. 2010;304:1073-81 doi:10.1001/jama.2010.1275
7. Procacci P, Moscheni C, Sartori P, Sommariva M, Gagliano N. Tumor(-)Stroma Cross-Talk in Human Pancreatic Ductal Adenocarcinoma: A Focus on the Effect of the Extracellular Matrix on Tumor Cell Phenotype and Invasive Potential. Cells. 2018:7 doi:10.3390/cells7100158
8. Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Annals of oncology: official journal of the European Society for Medical Oncology. 2008;19:1727-33 doi:10.1093/annonc/mdn351
9. Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE. et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26:3063-72 doi:10.1200/JCO.2007.15.4377
10. Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707-12 doi:10.1097/MPA.0b013e3181ec124e
11. Nigri J, Gironella M, Bressy C, Vila-Navarro E, Roques J, Lac S. et al. PAP/REG3A favors perineural invasion in pancreatic adenocarcinoma and serves as a prognostic marker. Cellular and molecular life sciences: CMLS. 2017;74:4231-43 doi:10.1007/s00018-017-2579-9
12. Xu Q, Wang Z, Chen X, Duan W, Lei J, Zong L. et al. Stromal-derived factor-1alpha/CXCL12-CXCR4 chemotactic pathway promotes perineural invasion in pancreatic cancer. Oncotarget. 2015;6:4717-32 doi:10.18632/oncotarget.3069
13. Nakao A, Harada A, Nonami T, Kaneko T, Takagi H. Clinical significance of carcinoma invasion of the extrapancreatic nerve plexus in pancreatic cancer. Pancreas. 1996;12:357-61
14. Bapat AA, Munoz RM, Von Hoff DD, Han H. Blocking Nerve Growth Factor Signaling Reduces the Neural Invasion Potential of Pancreatic Cancer Cells. PloS one. 2016;11:e0165586. doi:10.1371/journal.pone.0165586
15. Gleeson FC, Levy MJ, Roden AC, Boardman LA, Sinicrope FA, McWilliams RR. et al. EUS fine-needle pancreatic core biopsy can determine eligibility for tumor-agnostic immunotherapy. Endoscopy international open. 2018;6:E1278-E82 doi:10.1055/a-0650-4447
16. Fan P, Ma J, Jin X. Far upstream element-binding protein 1 is up-regulated in pancreatic cancer and modulates immune response by increasing programmed death ligand 1. Biochemical and biophysical research communications. 2018;505:830-6 doi:10.1016/j.bbrc.2018.10.009
17. Rataj F, Kraus FBT, Chaloupka M, Grassmann S, Heise C, Cadilha BL. et al. PD1-CD28 Fusion Protein Enables CD4+ T Cell Help for Adoptive T Cell Therapy in Models of Pancreatic Cancer and Non-hodgkin Lymphoma. Frontiers in immunology. 2018;9:1955. doi:10.3389/fimmu.2018.01955
18. Sugyo A, Tsuji AB, Sudo H, Koizumi M, Ukai Y, Kurosawa G. et al. Efficacy Evaluation of Combination Treatment Using Gemcitabine and Radioimmunotherapy with (90)Y-Labeled Fully Human Anti-CD147 Monoclonal Antibody 059-053 in a BxPC-3 Xenograft Mouse Model of Refractory Pancreatic Cancer. International journal of molecular sciences. 2018:19 doi:10.3390/ijms19102979
19. Sahin IH, Askan G, Hu ZI, O'Reilly EM. Immunotherapy in pancreatic ductal adenocarcinoma: an emerging entity? Annals of oncology: official journal of the European Society for Medical Oncology. 2017;28:2950-61 doi:10.1093/annonc/mdx503
20. Collignon A, Silvy F, Robert S, Trad M, Germain S, Nigri J. et al. Dendritic cell-based vaccination: powerful resources of immature dendritic cells against pancreatic adenocarcinoma. Oncoimmunology. 2018;7:e1504727. doi:10.1080/2162402X.2018.1504727
21. Kultti A, Li X, Jiang P, Thompson CB, Frost GI, Shepard HM. Therapeutic targeting of hyaluronan in the tumor stroma. Cancers. 2012;4:873-903 doi:10.3390/cancers4030873
22. Whatcott CJ, Diep CH, Jiang P, Watanabe A, LoBello J, Sima C. et al. Desmoplasia in Primary Tumors and Metastatic Lesions of Pancreatic Cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2015;21:3561-8 doi:10.1158/1078-0432.CCR-14-1051
23. Perusina Lanfranca M, Thompson JK, Bednar F, Halbrook C, Lyssiotis C, Levi B. et al. Metabolism and epigenetics of pancreatic cancer stem cells. Seminars in cancer biology. 2018 doi:10.1016/j.semcancer.2018.09.008
24. Marcucci F, Rumio C, Lefoulon F. Anti-Cancer Stem-like Cell Compounds in Clinical Development - An Overview and Critical Appraisal. Frontiers in oncology. 2016;6:115. doi:10.3389/fonc.2016.00115
25. Kuc N, Doermann A, Shirey C, Lee DD, Lowe CW, Awasthi N. et al. Pancreatic ductal adenocarcinoma cell secreted extracellular vesicles containing ceramide-1-phosphate promote pancreatic cancer stem cell motility. Biochemical pharmacology. 2018;156:458-66 doi:10.1016/j.bcp.2018.09.017
26. Marengo A, Forciniti S, Dando I, Dalla Pozza E, Stella B, Tsapis N. et al. Pancreatic cancer stem cell proliferation is strongly inhibited by diethyldithiocarbamate-copper complex loaded into hyaluronic acid decorated liposomes. Biochimica et biophysica acta General subjects. 2018;1863:61-72 doi:10.1016/j.bbagen.2018.09.018
27. Dong R, Chen P, Chen Q. Inhibition of pancreatic cancer stem cells by Rauwolfia vomitoria extract. Oncology reports. 2018 doi:10.3892/or.2018.6713
28. Ling X, Wu W, Fan C, Xu C, Liao J, Rich LJ. et al. An ABCG2 non-substrate anticancer agent FL118 targets drug-resistant cancer stem-like cells and overcomes treatment resistance of human pancreatic cancer. Journal of experimental & clinical cancer research: CR. 2018;37:240. doi:10.1186/s13046-018-0899-8
29. A Phase I Clinical Study of BBI608 in Adult Patients With Advanced Malignancies.
30. Lakkakula B, Farran B, Lakkakula S, Peela S, Yarla NS, Bramhachari PV. et al. Small molecule tyrosine kinase inhibitors and pancreatic cancer-Trials and troubles. Seminars in cancer biology. 2018 doi:10.1016/j.semcancer.2018.09.011
31. Veschi S, De Lellis L, Florio R, Lanuti P, Massucci A, Tinari N. et al. Effects of repurposed drug candidates nitroxoline and nelfinavir as single agents or in combination with erlotinib in pancreatic cancer cells. Journal of experimental & clinical cancer research: CR. 2018;37:236. doi:10.1186/s13046-018-0904-2
32. Tian YF, Wang HC, Luo CW, Hung WC, Lin YH, Chen TY. et al. Preprogramming therapeutic response of PI3K/mTOR dual inhibitor via the regulation of EHMT2 and p27 in pancreatic cancer. American journal of cancer research. 2018;8:1812-22
33. Lee T, Kim K, Lee J, Park SH, Park YS, Lim HY. et al. Antitumor activity of sorafenib plus CDK4/6 inhibitor in pancreatic patient derived cell with KRAS mutation. Journal of Cancer. 2018;9:3394-9 doi:10.7150/jca.26068
34. Melisi D, Garcia-Carbonero R, Macarulla T, Pezet D, Deplanque G, Fuchs M. et al. Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. British journal of cancer. 2018 doi:10.1038/s41416-018-0246-z
35. Butera G, Pacchiana R, Mullappilly N, Margiotta M, Bruno S, Conti P. et al. Mutant p53 prevents GAPDH nuclear translocation in pancreatic cancer cells favoring glycolysis and 2-deoxyglucose sensitivity. Biochimica et biophysica acta Molecular cell research. 2018;1865:1914-23 doi:10.1016/j.bbamcr.2018.10.005
36. Chiou SH, Dorsch M, Kusch E, Naranjo S, Kozak MM, Koong AC. et al. Hmga2 is dispensable for pancreatic cancer development, metastasis, and therapy resistance. Scientific reports. 2018;8:14008. doi:10.1038/s41598-018-32159-x
37. Yang D, Tang Y, Fu H, Xu J, Hu Z, Zhang Y. et al. Integrin beta1 promotes gemcitabine resistance in pancreatic cancer through Cdc42 activation of PI3K p110beta signaling. Biochemical and biophysical research communications. 2018;505:215-21 doi:10.1016/j.bbrc.2018.09.061
38. Liu D, Song L, Dai Z, Guan H, Kang H, Zhang Y. et al. MiR-429 suppresses neurotrophin-3 to alleviate perineural invasion of pancreatic cancer. Biochemical and biophysical research communications. 2018 doi:10.1016/j.bbrc.2018.09.147
39. Mardhian DF, Storm G, Bansal R, Prakash J. Nano-targeted relaxin impairs fibrosis and tumor growth in pancreatic cancer and improves the efficacy of gemcitabine in vivo. Journal of controlled release: official journal of the Controlled Release Society. 2018;290:1-10 doi:10.1016/j.jconrel.2018.09.031
40. Neumann CCM, von Horschelmann E, Reutzel-Selke A, Seidel E, Sauer IM, Pratschke J. et al. Tumor-stromal cross-talk modulating the therapeutic response in pancreatic cancer. Hepatobiliary & pancreatic diseases international: HBPD INT. 2018;17:461-72 doi:10.1016/j.hbpd.2018.09.004
41. Hu S, Jin Y, Liu Y, Ljungman M, Neamati N. Synthesis and mechanistic studies of quinolin-chlorobenzothioate derivatives with proteasome inhibitory activity in pancreatic cancer cell lines. European journal of medicinal chemistry. 2018;158:884-95 doi:10.1016/j.ejmech.2018.09.037
42. Rimar KJ, Tran PT, Matulewicz RS, Hussain M, Meeks JJ. The emerging role of homologous recombination repair and PARP inhibitors in genitourinary malignancies. Cancer. 2017;123:1912-24 doi:10.1002/cncr.30631
43. Graziani G, Szabo C. Clinical perspectives of PARP inhibitors. Pharmacological research. 2005;52:109-18 doi:10.1016/j.phrs.2005.02.013
44. Larsen MJ, Kruse TA, Tan Q, Laenkholm AV, Bak M, Lykkesfeldt AE. et al. Classifications within molecular subtypes enables identification of BRCA1/BRCA2 mutation carriers by RNA tumor profiling. PloS one. 2013;8:e64268. doi:10.1371/journal.pone.0064268
45. Mukhopadhyay A, Elattar A, Cerbinskaite A, Wilkinson SJ, Drew Y, Kyle S. et al. Development of a functional assay for homologous recombination status in primary cultures of epithelial ovarian tumor and correlation with sensitivity to poly(ADP-ribose) polymerase inhibitors. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16:2344-51 doi:10.1158/1078-0432.CCR-09-2758
46. Lee JM, Gordon N, Trepel JB, Lee MJ, Yu M, Kohn EC. Development of a multiparameter flow cytometric assay as a potential biomarker for homologous recombination deficiency in women with high-grade serous ovarian cancer. Journal of translational medicine. 2015;13:239. doi:10.1186/s12967-015-0604-z
47. Timms KM, Abkevich V, Hughes E, Neff C, Reid J, Morris B. et al. Association of BRCA1/2 defects with genomic scores predictive of DNA damage repair deficiency among breast cancer subtypes. Breast Cancer Res. 2014;16:475. doi:10.1186/s13058-014-0475-x
48. Telli ML, Timms KM, Reid J, Hennessy B, Mills GB, Jensen KC. et al. Homologous Recombination Deficiency (HRD) Score Predicts Response to Platinum-Containing Neoadjuvant Chemotherapy in Patients with Triple-Negative Breast Cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016;22:3764-73 doi:10.1158/1078-0432.CCR-15-2477
49. Tilki D, Svatek RS, Novara G, Seitz M, Godoy G, Karakiewicz PI. et al. Stage pT0 at radical cystectomy confers improved survival: an international study of 4,430 patients. J Urol. 2010;184:888-94 doi:10.1016/j.juro.2010.04.081
50. Kang YW, Lee JE, Jung KH, Son MK, Shin SM, Kim SJ. et al. KRAS targeting antibody synergizes anti-cancer activity of gemcitabine against pancreatic cancer. Cancer letters. 2018;438:174-86 doi:10.1016/j.canlet.2018.09.013
51. Pattanayak R, Barua A, Das A, Chatterjee T, Pathak A, Choudhury P. et al. Porphyrins to restrict progression of pancreatic cancer by stabilizing KRAS G-quadruplex: In silico, in vitro and in vivo validation of anticancer strategy. European journal of pharmaceutical sciences: official journal of the European Federation for Pharmaceutical Sciences. 2018;125:39-53 doi:10.1016/j.ejps.2018.09.011
52. Wang P, Wei D, Zhang H, Chen J, Zhang D, Ganapathy S. et al. PKCiota and YAP1 are crucial in promoting pancreatic tumorigenesis. Oncotarget. 2018;9:32736-50 doi:10.18632/oncotarget.25127
53. Grolmusz VK, Kovesdi A, Borks K, Igaz P, Patocs A. Prognostic relevance of proliferation-related miRNAs in pancreatic neuroendocrine neoplasms. European journal of endocrinology. 2018;179:219-28 doi:10.1530/EJE-18-0305
54. Leja-Szpak A, Nawrot-Porabka K, Goralska M, Jastrzebska M, Link-Lenczowski P, Bonior J. et al. Melatonin and its metabolite N1-acetyl-N2-formyl-5-methoxykynuramine (afmk) enhance chemosensitivity to gemcitabine in pancreatic carcinoma cells (PANC-1). Pharmacological reports: PR. 2018;70:1079-88 doi:10.1016/j.pharep.2018.05.007
55. Wang X, Li H, Lu X, Wen C, Huo Z, Shi M. et al. Melittin-induced long non-coding RNA NONHSAT105177 inhibits proliferation and migration of pancreatic ductal adenocarcinoma. Cell death & disease. 2018;9:940. doi:10.1038/s41419-018-0965-3
56. Xia J, Cao T, Ma C, Shi Y, Sun Y, Wang ZP. et al. miR-7 Suppresses Tumor Progression by Directly Targeting MAP3K9 in Pancreatic Cancer. Molecular therapy Nucleic acids. 2018;13:121-32 doi:10.1016/j.omtn.2018.08.012
57. Ma J, Zhang J, Weng YC, Wang JC. EZH2-Mediated microRNA-139-5p Regulates Epithelial-Mesenchymal Transition and Lymph Node Metastasis of Pancreatic Cancer. Molecules and cells. 2018;41:868-80 doi:10.14348/molcells.2018.0109
58. Hong S, Fang Z, Jung HY, Yoon JH, Hong SS, Maeng HJ. Synthesis of Gemcitabine-Threonine Amide Prodrug Effective on Pancreatic Cancer Cells with Improved Pharmacokinetic Properties. Molecules. 2018:23 doi:10.3390/molecules23102608
59. Harano Y, Babazono A, Fujita T, Jiang P. Efficacy of S-1 monotherapy for older patients with unresectable pancreatic cancer: A retrospective cohort study. Journal of geriatric oncology. 2018 doi:10.1016/j.jgo.2018.09.004
60. Bianconi D, Herac M, Spies D, Kieler M, Brettner R, Unseld M. et al. SERPINB7 Expression Predicts Poor Pancreatic Cancer Survival Upon Gemcitabine Treatment. Translational oncology. 2018;12:15-23 doi:10.1016/j.tranon.2018.08.019
61. Logsdon DP, Shah F, Carta F, Supuran CT, Kamocka M, Jacobsen MH. et al. Blocking HIF signaling via novel inhibitors of CA9 and APE1/Ref-1 dramatically affects pancreatic cancer cell survival. Scientific reports. 2018;8:13759. doi:10.1038/s41598-018-32034-9
62. Wanek J, Gaisberger M, Beyreis M, Mayr C, Helm K, Primavesi F. et al. Pharmacological Inhibition of Class IIA HDACs by LMK-235 in Pancreatic Neuroendocrine Tumor Cells. International journal of molecular sciences. 2018:19 doi:10.3390/ijms19103128
63. Nikbakht Dastjerdi MP, Azarnezhad AP, Hashemibeni BP, Salehi MP, Kazemi MP, Babazadeh ZP. An Effective Concentration of 5-Aza-CdR to Induce Cell Death and Apoptosis in Human Pancreatic Cancer Cell Line through Reactivating RASSF1A and Up-Regulation of Bax Genes. Iranian journal of medical sciences. 2018;43:533-40
64. Vilimas T, Wang AQ, Patnaik S, Hughes EA, Singleton MD, Knotts Z. et al. Pharmacokinetic evaluation of the PNC disassembler metarrestin in wild-type and Pdx1-Cre;LSL-Kras(G12D/+);Tp53(R172H/+) (KPC) mice, a genetically engineered model of pancreatic cancer. Cancer chemotherapy and pharmacology. 2018 doi:10.1007/s00280-018-3699-0
65. Cho I, Park JW, Cho B, Kwak J, Yoon SM, Nesseler JP. et al. Dosimetric analysis of stereotactic rotational versus static intensity-modulated radiation therapy for pancreatic cancer. Cancer radiotherapie: journal de la Societe francaise de radiotherapie oncologique. 2018 doi:10.1016/j.canrad.2018.01.007
66. Yang W, Fan Z, Deng Z, Pang J, Bi X, Fraass BA. et al. Novel 4D-MRI of tumor infiltrating vasculature: characterizing tumor and vessel volume motion for selective boost volume definition in pancreatic radiotherapy. Radiation oncology. 2018;13:191. doi:10.1186/s13014-018-1139-2
67. Yasmin-Karim S, Bruck PT, Moreau M, Kunjachan S, Chen GZ, Kumar R. et al. Radiation and Local Anti-CD40 Generate an Effective in situ Vaccine in Preclinical Models of Pancreatic Cancer. Frontiers in immunology. 2018;9:2030. doi:10.3389/fimmu.2018.02030
68. Zhu X, Li F, Ju X, Shen Y, Cao Y, Cao F. et al. Prediction of overall survival after re-irradiation with stereotactic body radiation therapy for pancreatic cancer with a novel prognostic model (the SCAD score). Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2018 doi:10.1016/j.radonc.2018.08.012
69. Shall S, Gaymes T, Farzaneh F, Curtin NJ, Mufti GJ. The Use of PARP Inhibitors in Cancer Therapy: Use as Adjuvant with Chemotherapy or Radiotherapy, Use as a Single Agent in Susceptible Patients, and Techniques Used to Identify Susceptible Patients. Methods in molecular biology. 2017;1608:343-70 doi:10.1007/978-1-4939-6993-7_23
70. Oh ES, Kim TH, Woo SM, Lee WJ, Lee JH, Youn SH. et al. Effectiveness and feasibility of concurrent chemoradiotherapy using simultaneous integrated boost-intensity modulated radiotherapy with and without induction chemotherapy for locally advanced pancreatic cancer. Radiation oncology journal. 2018;36:200-9 doi:10.3857/roj.2018.00073
71. Murphy JE, Wo JY, Ryan DP, Jiang W, Yeap BY, Drapek LC. et al. Total Neoadjuvant Therapy With FOLFIRINOX Followed by Individualized Chemoradiotherapy for Borderline Resectable Pancreatic Adenocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2018;4:963-9 doi:10.1001/jamaoncol.2018.0329
72. Szlachta K, Kuscu C, Tufan T, Adair SJ, Shang S, Michaels AD. et al. CRISPR knockout screening identifies combinatorial drug targets in pancreatic cancer and models cellular drug response. Nature communications. 2018;9:4275. doi:10.1038/s41467-018-06676-2
73. Eissa IR, Bustos-Villalobos I, Ichinose T, Matsumura S, Naoe Y, Miyajima N. et al. The Current Status and Future Prospects of Oncolytic Viruses in Clinical Trials against Melanoma, Glioma, Pancreatic, and Breast Cancers. Cancers. 2018:10 doi:10.3390/cancers10100356
74. Ciaravino V, De Robertis R, Tinazzi Martini P, Cardobi N, Cingarlini S, Amodio A. et al. Imaging presentation of pancreatic neuroendocrine neoplasms. Insights into imaging. 2018 doi:10.1007/s13244-018-0658-6
75. Qi ZH, Xu HX, Zhang SR, Xu JZ, Li S, Gao HL. et al. The Significance of Liquid Biopsy in Pancreatic Cancer. Journal of Cancer. 2018;9:3417-26 doi:10.7150/jca.24591
76. Uchoa Guimaraes CT, Ferreira Martins NN, Cristina da Silva Oliveira K, Almeida CM, Pinheiro TM, Gigek CO. et al. Liquid biopsy provides new insights into gastric cancer. Oncotarget. 2018;9:15144-56 doi:10.18632/oncotarget.24540
77. Buwenge M, Macchia G, Arcelli A, Frakulli R, Fuccio L, Guerri S. et al. Stereotactic radiotherapy of pancreatic cancer: a systematic review on pain relief. Journal of pain research. 2018;11:2169-78 doi:10.2147/JPR.S167994
78. Yin F, Anderson T, Panwar N, Zhang K, Tjin SC, Ng BK. et al. Functionalized MoS2 Nanosheets as Multi-Gene Delivery Vehicles for In Vivo Pancreatic Cancer Therapy. Nanotheranostics. 2018;2:371-86 doi:10.7150/ntno.27308
79. Alsuraifi A, Lin PKT, Curtis A, Lamprou DA, Hoskins C. A Novel PAA Derivative with Enhanced Drug Efficacy in Pancreatic Cancer Cell Lines. Pharmaceuticals. 2018:11 doi:10.3390/ph11040091
80. Maharaj AD, Ioannou L, Croagh D, Zalcberg J, Neale RE, Goldstein D. et al. Monitoring quality of care for patients with pancreatic cancer: a modified Delphi consensus. HPB: the official journal of the International Hepato Pancreato Biliary Association. 2018 doi:10.1016/j.hpb.2018.08.016
81. Bernard V, Kim DU, San Lucas FA, Castillo J, Allenson K, Mulu FC. et al. Circulating Nucleic Acids Associate with Outcomes of Patients with Pancreatic Cancer. Gastroenterology. 2018 doi:10.1053/j.gastro.2018.09.022
82. DeWitt JM, Sandrasegaran K, O'Neil B, House MG, Zyromski NJ, Sehdev A. et al. Phase I Study of EUS-guided Photodynamic Therapy for Locally Advanced Pancreatic Cancer. Gastrointestinal endoscopy. 2018 doi:10.1016/j.gie.2018.09.007
83. Song M, Yoon SB, Lee IS, Hong TH, Choi HJ, Choi MH. et al. Evaluation of the prognostic value of the new AJCC 8th edition staging system for patients with pancreatic adenocarcinoma; a need to subclassify stage III? European journal of cancer. 2018;104:62-9 doi:10.1016/j.ejca.2018.08.027
84. Reccia I, Kumar J, Habib N, Sodergren M. The use of radiofrequency ablation in pancreatic cancer in the midst of the dawn of immuno-oncology. Medical oncology. 2018;35:151. doi:10.1007/s12032-018-1209-1
85. Zhang X, Zhang J, Fan H, Liu Y, He Q. Risk factors related to metastasis of para-aortic lymph nodes in pancreatic ductal adenocarcinoma: A retrospective observational study. Medicine. 2018;97:e12370. doi:10.1097/MD.0000000000012370
86. Mikkelsen MK, Lund CM, Vinther A, Tolver A, Ragle AM, Johansen JS. et al. Engaging the older cancer patient; Patient Activation through Counseling, Exercise and Mobilization - Pancreatic, Biliary tract and Lung cancer (PACE-Mobil-PBL) - study protocol of a randomized controlled trial. BMC cancer. 2018;18:934. doi:10.1186/s12885-018-4835-2
87. Sun D, Ma J, Wang J, Zhang F, Wang L, Zhang S. et al. Clinical observation of immune checkpoint inhibitors in the treatment of advanced pancreatic cancer: a real-world study in Chinese cohort. Therapeutics and clinical risk management. 2018;14:1691-700 doi:10.2147/TCRM.S173041
88. Gong J, Hendifar A, Tuli R, Chuang J, Cho M, Chung V. et al. Combination systemic therapies with immune checkpoint inhibitors in pancreatic cancer: overcoming resistance to single-agent checkpoint blockade. Clinical and translational medicine. 2018;7:32. doi:10.1186/s40169-018-0210-9
89. Ciabatti S, Cammelli S, Frakulli R, Arcelli A, Macchia G, Deodato F. et al. Radiotherapy of pancreatic cancer in older patients: A systematic review. Journal of geriatric oncology. 2018 doi:10.1016/j.jgo.2018.09.007
90. Sato N, Hasegawa Y, Saito A, Motoi F, Ariake K, Katayose Y. et al. Association between chronological depressive changes and physical symptoms in postoperative pancreatic cancer patients. BioPsychoSocial medicine. 2018;12:13. doi:10.1186/s13030-018-0132-1
Author contact
![]() Corresponding author: Paul Zarogoulidis, M.D, Ph.D, 3rd Department of Surgery, ``AHEPA`` University Hospital, Aristotle University of Thessaloniki, Medical School, Thessaloniki, Greece. Mobile: 00306977271974; E-mail: pzarogcom
Corresponding author: Paul Zarogoulidis, M.D, Ph.D, 3rd Department of Surgery, ``AHEPA`` University Hospital, Aristotle University of Thessaloniki, Medical School, Thessaloniki, Greece. Mobile: 00306977271974; E-mail: pzarogcom

 Global reach, higher impact
Global reach, higher impact