3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2019; 10(21):5234-5243. doi:10.7150/jca.30354 This issue Cite
Research Paper
CD103+ tumor-infiltrating lymphocytes predict favorable prognosis in patients with esophageal squamous cell carcinoma
1. State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou 510060, P. R. China
2. MOE Key Laboratory of Gene Function and Regulation, School of Life Sciences, Sun Yat-sen University, Guangzhou 510275, P. R. China
3. The Key Laboratory of Molecular Biology for High Cancer Incidence Coastal Chaoshan Area, Shantou University Medical College, Shantou 515041, China
*These authors contributed equally to this work.
Received 2018-10-2; Accepted 2019-7-18; Published 2019-8-28
Abstract
As an indispensable factor in preventing the recirculation of tissue lymphocytes to the lymphatic and blood systems, the integrin CD103 has enabled the characterization of lymphocyte populations in non-lymphoid tissues and organs. However, the expression, distribution, and clinical significance of CD103+ tumor-infiltrating lymphocytes (TILs) in esophageal squamous cell carcinoma (ESCC) remain unclear. In the present study, we included tumor and adjacent non-tumor tissue specimens from 198 patients with ESCC who had undergone surgical resection. Immunohistochemistry and immunofluorescence were used to detect CD103+ TIL distribution, as well as the co-expression of CD103 and T cell markers and functional molecules. Kaplan-Meier analysis and the Cox proportional hazards model were used to estimate the prognostic value of CD103+ TILs. The results showed that CD103+ TILs were predominantly located in adjacent non-tumor tissues compared with tumor tissues (P < 0.0001). Immunofluorescence double staining revealed that CD8+ T cells, but not CD4+ T cells, comprised the majority of CD103-expressing cells. Most of these CD103-expressing cells co-expressed CTLA-4 and granzyme B rather than the exhaustion marker PD-1. High density of intratumoral CD103+ TIL is associated with longer overall survival (OS) and disease-free survival (DFS) in both the internal (OS, P = 0.0004 and DFS, P = 0.0002) and external (OS, P = 0.038 and DFS, P = 0.12) cohorts. Multivariate Cox analysis showed the density of CD103+ TILs was an independent positive prognostic factor for OS (hazards ratio [HR] = 0.406; P = 0.0003 in the internal cohort; HR = 0.328, P = 0.01, in the external cohort) and DFS (HR = 0.385; P = 0.0002 in the internal cohort; HR = 0.270, P = 0.003, in the external cohort). Our findings indicate that CD103+ TILs might play an important role in the tumor microenvironment, and intratumoral CD103+ TILs could serve as a promising prognostic marker in ESCC.
Keywords: esophageal squamous cell carcinoma, prognosis, CD103, tumor-infiltrating lymphocytes
Introduction
Esophageal cancer is one of the leading causes of cancer-related death worldwide [1]. The majority of cases in China is esophageal squamous cell carcinoma (ESCC) [2]. Although there has been progressive improvement in surgical resection and neoadjuvant chemoradiotherapy, the 5-year survival rate of patients with ESCC remains unsatisfactory [3]. Therefore, there is an urgent need to develop novel clinical strategies for treating patients with ESCC.
Recently, immunotherapies targeting immune checkpoints have been proven to be promising treatments across different cancers [4-6], including esophageal carcinoma [7]. Tumor responses to these therapies are mediated mainly by anti-tumor T cell immunity previously blocked by CTLA-4 (cytotoxic T lymphocyte-associated protein 4) or PD-1 (programmed cell death 1). Accordingly, clinical trials have observed increased T cell frequency in many patients who had received immunotherapy [8]. Despite the evaluation of T cell counts, only a minority of patients have objective tumor responses, indicating that the number of tumor-infiltrating lymphocytes (TILs), which comprise complex subpopulations with diverse activities, may be inadequate to reflect the efficacy of anti-tumor immunity. Therefore, identifying the T cell subpopulations involved in anti-tumor immunity and that influence disease progression should be helpful in clinical decisions on patient treatment.
CD103 is the αE integrin subunit of the heterodimeric αEβ7 complex whose ligand is the epithelial cell surface molecule E-cadherin [9]. CD103 expression has been found on different immune cells, such as dendritic cells (DC) [10], CD8+ cytotoxic T cells [11], and CD4+ T cells [12]. The adhesive interaction of CD103 with E-cadherin can mediate T cell recruitment and the localization of antigen-specific T cells in the lung and skin [13, 14]. Recent studies have found that increased CD103+ tissue-resident CD8+ T cells are associated with improved survival in melanoma [8], lung cancer [15], and cervical cancer [16], suggesting that CD103+ T cells are a potential predictive biomarker. Furthermore, it has been reported that TILs like CD8+ T cells correlated with improved survival in ESCC. However, some studies showed conflicting results [17, 18]. Therefore, there is an urgent need to identify an accurate marker that can determine the prognosis of TILs for enabling the development of novel clinical strategies for treating patients with ESCC.
In the present study, we analyzed the distribution, composition, and prognostic significance of CD103+ TILs in patients with ESCC. The majority of CD103-expressing cells were CD8+ T cells, and they could express CTLA-4 and granzyme B (GB). Moreover, high CD103+ cell numbers predicted favorable prognosis in patients with ESCC.
Materials and Methods
Patients and tissue specimens
Archived, formalin-fixed, paraffin-embedded tumor samples were obtained from patients with pathologically confirmed ESCC at the Sun Yat-sen University Cancer Center (SYSUCC) in 2002-2009 and at the Shantou Central Hospital (STCH) in 2010-2011. One hundred and seven patients from SYSUCC and ninety-one patients from STCH who had not received anti-tumor therapy preoperatively were enrolled randomly in the study. The patients' clinicopathological parameters were obtained from medical records and pathology reports. All tumor stages were determined according to American Joint Cancer Committee/Union International Center Cancer (AJCC/UICC) classification guidelines. ESCC specimen grading and histopathology subtyping was based on World Health Organization criteria. Patient consent was obtained prior to the use of the clinical materials for research purposes. The study was approved by the Institutional Review Boards of the participating hospitals, and it was implemented in strict accordance with the ethical guidelines of the Declaration of Helsinki.
Follow-up
Follow-up was performed with regular surveillance for recurrence using imageological examination at 2-4-month intervals. Disease-free survival (DFS) was defined by the time from diagnosis to death or relapse, whichever occurred first, or to the last follow-up date. Overall survival (OS) was defined as the interval between the time of surgery and either death or the last observation. Table 1 summarizes the patients' clinicopathological parameters.
Immunohistochemistry (IHC) and immunofluorescence staining
The formalin-fixed, paraffin-embedded samples were cut into 5-μm sections and subjected to IHC and immunofluorescence staining as described previously [19-21]. Briefly, tissue sections were incubated with primary antibodies against CD103 (rabbit anti-human, ab129202, Abcam, Cambridge, UK), CD8 (rabbit anti-human, MA5-14548, Thermo Fisher Scientific, Waltham, MA, USA), CD4 (mouse anti-human, ZM-0418, Zhongshan Bio-Tech, Guangdong, China), PD-1 (mouse anti-human, ZM-0381, Zhongshan Bio-Tech), CTLA-4 (mouse anti-human, 14-1529-80, eBioscience, San Diego, CA, USA), and CD11c (rabbit anti-human, ab52632, Abcam). Immunostaining was performed using horseradish peroxidase-conjugated anti-rabbit and anti-mouse antibodies from DAKO EnVision systems (Dako Cytomation, Glostrup, Denmark) and was developed with peroxidase and 3, 3′-diaminobenzidine tetrahydrochloride. All sections were counterstained with Mayer's hematoxylin and mounted in non-aqueous mounting medium.
For double immunofluorescence staining of CD3, CD11c, CD4, CD8, CTLA-4 or PD-1, and CD103, we used species-paired fluorescently labeled secondary antibodies [donkey anti-rat immunoglobulin G (IgG) (H+L) secondary antibody, Alexa Fluor 488, A-21206; donkey anti-mouse IgG (H+L) secondary antibody, Alexa Fluor 555, A-31570, Invitrogen, Waltham, MA, USA] and tyramide reagent (Alexa Fluor 488 Tyramide Reagent, B40953; Alexa Fluor 555 Tyramide Reagent, B40955, Invitrogen). Nuclei were counterstained using 4′, 6-diamidino-2-phenylindole (DAPI).
Image analysis
To evaluate CD103+ cell density, we used the Vectra-InForm image analysis system (Perkin-Elmer/Applied Biosystems, Foster City, CA, USA) as described previously [22, 23]. For IHC, the five most representative high-power fields were captured at ×200 magnification (0.284 mm2 per field) for each tumor region in all specimens. CD103+ cells in each field were counted and analyzed manually by two independent observers blinded to clinical outcome. Positively stained cells with morphological features characteristic of lymphocytes were counted based on localization in the intratumoral (IT) and adjacent non-tumor (ANT) regions. Data are reported as the mean (± SEM) number of cells per field.
Immunofluorescence images were captured using a confocal microscope (Olympus, Essex, UK) and analyzed using a FV10-ASW Viewer (Olympus). Two independent observers blinded to the outcome counted and analyzed single- or double-positive cells in each of five representative fields at ×400 magnification (0.07 mm2 per field). Data are reported as the mean (± SEM) number of cells per field.
Statistical analyses
All statistical analyses were performed using SPSS version 20.0 (SPSS Inc., Chicago, IL, USA). The significance of differences between groups was determined by the Wilcoxon signed rank test. Survival curves were calculated by the Kaplan-Meier method and analyzed by the log rank test. The Cox proportional hazards model was used to identify prognostic factors through univariate and multivariate analyses. The statistical significance of differences between groups was determined using the two-tailed Student t-test, where P < 0.05 was considered statistically significant.
Results
Study population
Table 1 summarizes the clinicopathological features of the patients. The average follow-up time was 38.0 months (range, 2.2-123.7 months) in SYSUCC cohort and 45.7 months (range, 3.0-84.8 months) in STCH cohort. During the follow-up, 121 patients (65.2%) died. Altogether, we enrolled 150 men and 48 women; their average survival time was 41.2 months and 41.7 months, respectively. Of all patients, 57.1% were > 55 years old. Based on the seventh edition of the AJCC staging manual, 22 cases (11.1%) were histologically graded as poorly differentiated, 162 cases (81.8%) as moderately or well-differentiated, and 14 cases (7.1%) as deficient. Among these patients, 19 (9.6%) had AJCC pathologic stage I disease and 179 (90.4%) had stage II-IV disease.
Clinicopathological characteristics of the patients
| Characteristics | Total | SYSUCC | STCH | P value* |
|---|---|---|---|---|
| Cases (%) | Cases (%) | Cases (%) | ||
| Gender | ||||
| Male | 150 (75.8) | 80 (74.8) | 70 (76.9) | 0.724 |
| Female | 48 (24.2) | 27 (25.2) | 21 (23.1) | |
| Age (year) | ||||
| > 55 | 113 (57.1) | 53 (49.5) | 60 (65.9) | 0.02 |
| ≤ 55 | 85 (42.9) | 54 (50.5) | 31 (34.1) | |
| T Classification | ||||
| T3 - T4 | 138 (69.7) | 63 (58.9) | 75 (82.4) | P < 0.001 |
| T1 - T2 | 60 (30.3) | 44 (41.1) | 16 (17.6) | |
| N Classification | ||||
| Yes | 78 (39.4) | 43 (40.2) | 35 (38.5) | 0.804 |
| No | 120 (60.6) | 64 (59.8) | 56 (61.5) | |
| Recurrence | ||||
| Yes | 77 (38.9) | 19 (17.8) | 58 (63.7) | P < 0.001 |
| No | 121 (61.1) | 88 (82.2) | 33 (36.3) | |
| TNM Staging | ||||
| Ⅱ-Ⅳ | 179 (90.4) | 100 (93.5) | 79 (86.8) | 0.114 |
| Ⅰ | 19 (9.6) | 7 (6.5) | 12 (13.2) | |
| Differentiation† | ||||
| Poor | 22 (12.0) | 13 (12.1) | 9 (11.0) | 0.736 |
| Well + Moderate | 162 (88.0) | 89 (83.2) | 73 (89.0) |
Abbreviations: TNM, tumor-node-metastasis. SYSUCC, Sun Yat-sen University Cancer Center. STCH, Shantou Central Hospital.
† Data was missing in these variables for some patients.*P values represent the correlation on clinicopathological features between SYSUCC cohort and STCH cohort.
Distribution of CD103+ cells
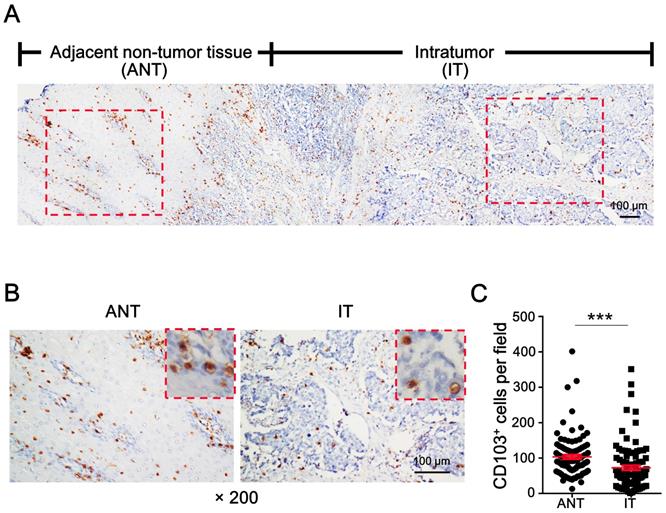
To evaluate the distribution of CD103+ cells in ESCC, tumor specimens containing the advancing edges of intratumoral (IT) and adjacent non-tumor (ANT) regions were used for IHC staining (Fig. 1A). Diffusely distributed CD103+ cells were detected in both the IT and ANT regions (Fig. 1A, 1B). CD103+ cell density was significantly higher in the ANT tissue (mean ± SEM: 103 ± 6 cells/field) than in the IT tissue (mean ± SEM: 73 ± 7 cells/field; P < 0.0001; Fig. 1C). These data suggest that CD103+ cell numbers might decrease during the transition from epithelial to tumor tissue.
Cellular source of CD103+ cells
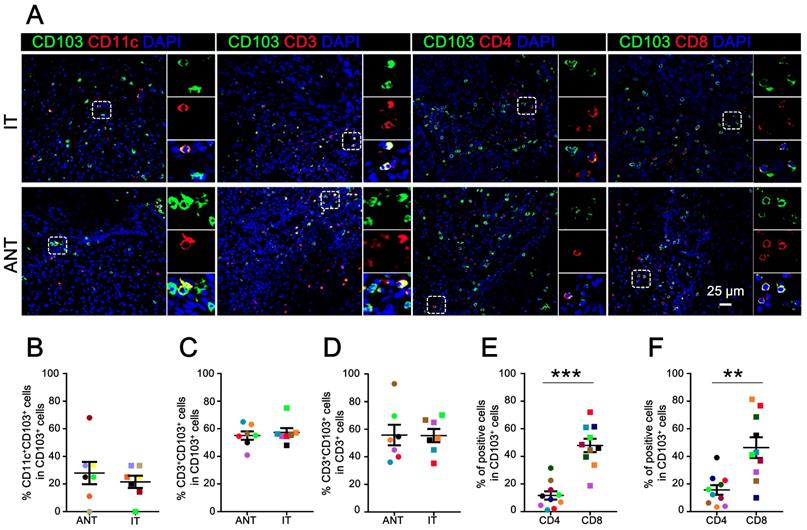
CD103+ cells are a heterogeneous population of cells [24]. Multicolor immunofluorescence staining was performed to characterize the cellular source of CD103+ cells in ESCC. As expected, CD103+ cells co-expressed CD11c in both the ANT (mean ± SEM: 27.97 ± 8.08%) and IT regions (mean ± SEM: 21.56 ± 4.42%) (Fig. 2A, 2B). Notably, we detected a high frequency of CD3+CD103+ cells in the total CD103+ cells from both the ANT (mean ± SEM: 55.04 ± 3.06%) and IT regions (mean ± SEM: 57.29 ± 3.16%) (Fig. 2A, 2C). Moreover, these CD3+CD103+ cells comprised about half of the CD3+ cells in the ANT (mean ± SEM: 55.75 ± 7.45%) and IT regions (mean ± SEM: 55.36 ± 4.79%) (Fig. 2D).
To identify the cellular source of CD103+ TILs, co-localization immunofluorescence was performed to calculate the proportion of CD4+CD103+ and CD8+CD103+ cells in the total CD103+ cells (Fig. 2A). In the IT tissue, the CD8+CD103+/CD103+ cell ratio (mean ± SEM: 46.35 ± 7.50%) was significantly higher than the CD4+CD103+/CD103+ cell ratio (mean ± SEM: 15.69 ± 3.50%; P < 0.01; Fig. 2F). The ANT region followed a similar trend (mean ± SEM: CD8+CD103+/CD103+ cell ratio, 47.93 ± 4.83%; CD4+CD103+/CD103+ cell ratio, 11.68 ± 3.00%; P < 0.001; Fig. 2E). Collectively, our findings indicate that the primary CD103-expressing cells in ESCC tissues are CD8+ T cells.
CD103+ cells are decreased in ESCC tumor tissue. (A) IHC staining shows CD103 in adjacent non-tumor tissue (ANT) and intratumor (IT) regions in ESCC tissue specimen. Scale bar = 100 μm. (B) Enlargements of the outlined areas in (A) showing outlined areas at higher magnification. Scale bar = 100 μm. (C) Quantification of CD103+ cell densities in the ANT and IT regions (n = 95). Results are the mean ± SEM (bars); ***P < 0.001.
Phenotype of CD103+ TILs
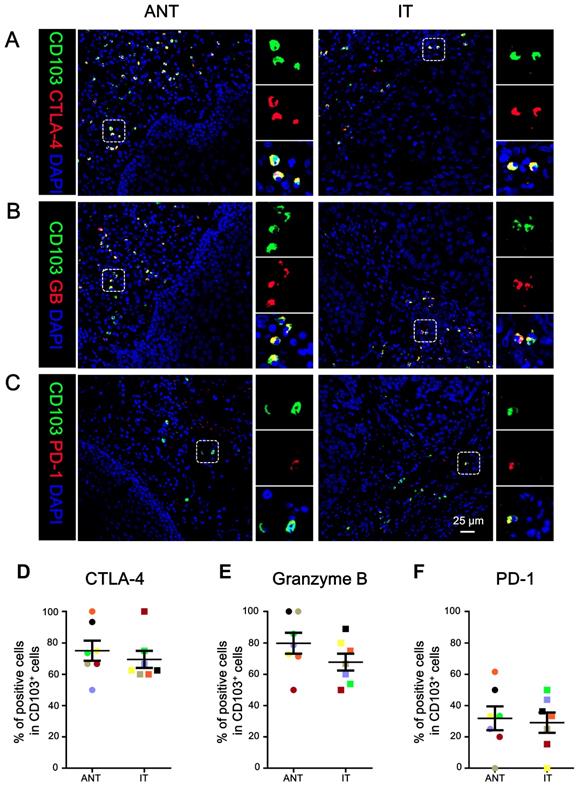
We determined the in-situ phenotype of the CD103+ TILs. Immunofluorescence staining showed that the majority of CD103+ cells were positive for CTLA-4 (mean ± SEM: ANT, 75.05 ± 6.41%; IT, 69.52 ± 5.45%; Fig. 3A, 3D) and granzyme B (mean ± SEM: ANT, 79.78 ± 6.66%; IT, 67.77 ± 5.39%; Fig. 3B, 3E). However, the CD103+ TILs expressed relatively lower levels of PD-1 (mean ± SEM: ANT, 31.89 ± 7.59%; IT, 29.12 ± 6.50%; Fig. 3C, 3F). Taken together, the intratumoral CD103+ cells had a CTLA-4hiGBhiPD-1low phenotype, suggesting that CD103+ TILs in ESCC exhibit an activated phenotype.
Prognostic value of CD103+ cells
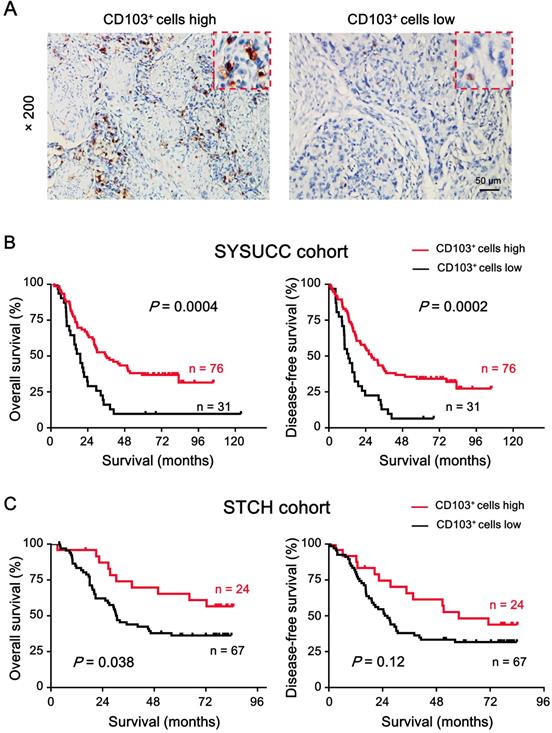
To investigate the relationship between CD103+ cell density in the ESCC tumor region and patient survival, we divided the patients into two groups based on the receiver operating characteristic. The CD103+ cell density cut-off value was 37.58 cells/field; approximately 71% of patients (76/107) had higher CD103+ cell density. Kaplan-Meier survival curves plotted to investigate the association with survival showed that patients with high CD103+ cell density had better OS (median: 38 months, P = 0.0004; Fig. 4A) and DFS (median: 32 months, P = 0.0002; Fig. 4B) than patients with low CD103+ cell density. The 5-year OS and DFS rates for patients with high CD103+ cell density were 34.21% and 34.21%, respectively, while that for patients with low CD103+ cell density were 9.67% and 12.9%, respectively. We also analyzed the correlation between CD103+ cell density and patient clinicopathological features. Table 2 shows that intratumoral CD103+ cell number was not significantly correlated with patient gender, age, T classification, lymph node metastasis, clinical staging, differentiation, or metastasis in both cohorts.
To further validate the prognostic role of CD103+ cell density, an independent external cohort of patients with pathologically confirmed ESCC from STCH was enrolled. The correlation of clinicopathological parameters between the two cohorts is showed in Table 1. The cut-off value derived from the internal cohort was applied to the external cohort. The results showed that patients with high CD103+ cell density had better OS (mean: 58.9 months, P = 0.038) and DFS (mean: 51.1 months, P = 0.12) as compared to patients with low CD103+ cell density (mean OS: 41.0 months; mean DFS: 36.0 months) in the external cohort (Fig. 4C).
Variables that were associated with survival by univariate analysis were adopted as covariates in the multivariate Cox model. Table 3 showed that high CD103+ cell density was associated with better OS (hazard ratio [HR] = 0.406, 95% confidence interval [CI] = 0.247-0.667, P = 0.0003) and DFS (HR = 0.385, 95% CI = 0.233-0.637, P = 0.0002) in the internal set, as well as in the external cohort (HR = 0.328, 95% CI = 0.141-0.763, P = 0.01; DFS: HR = 0.270, 95% CI = 0.113-0.644, P = 0.003). Therefore, CD103+ cell density could serve as an independent prognostic factor of OS and DFS in patients with ESCC.
Correlation between the density of CD103+ cells and clinicopathological parameters
| Characteristic | CD103+ Cells | ||||||
|---|---|---|---|---|---|---|---|
| SYSUCC | STCH | ||||||
| low | high | P value | low | high | P value | ||
| cases (%) | cases (%) | cases (%) | cases (%) | ||||
| Gender | Male | 23 (74.2) | 57 (75.0) | 0.931 | 51 (76.1) | 19 (79.2) | 0.761 |
| Female | 8 (25.8) | 19 (25.0) | 16 (23.9) | 5 (20.8) | |||
| Age (years) | > 55 | 13 (41.9) | 40 (52.6) | 0.315 | 43 (64.2) | 17 (70.8) | 0.555 |
| ≤ 55 | 18 (58.1) | 36 (47.4) | 24 (35.8) | 7 (29.2) | |||
| T classification | T3 - T4 | 21 (67.7) | 42 (55.3) | 0.234 | 55 (82.1) | 20 (83.3) | 0.582 |
| T1 - T2 | 10 (32.3) | 34 (44.7) | 12 (17.9) | 4 (16.7) | |||
| N classification | yes | 11 (35.5) | 32 (42.1) | 0.526 | 27 (40.3) | 7 (29.2) | 0.333 |
| no | 20 (64.5) | 44 (57.9) | 40 (59.7) | 17 (70.8) | |||
| Recurrence | yes | 4 (12.9) | 15 (19.7) | 0.578 | 45 (67.2) | 13 (54.2) | 0.256 |
| no | 27 (87.1) | 61 (80.3) | 22 (32.8) | 11 (45.8) | |||
| TNM staging | Ⅱ-Ⅳ | 30 (96.8) | 70 (92.1) | 0.671 | 58 (86.6) | 21 (87.5) | 0.608 |
| Ⅰ | 1 (3.2) | 6 (7.9) | 9 (13.4) | 3 (12.5) | |||
| Differentiation | poor | 6 (19.4) | 7 (9.9) | 0.186 | 7 (11.7) | 6 (27.3) | 0.086 |
| well + moderate | 25 (80.6) | 64 (90.1) | 53 (88.3) | 16 (72.7) | |||
P values were analyzed by χ2 test or Fisher's exact test, as appropriate.
Univariate and multivariate analyses of variables associated with overall survival and disease-free survival in the internal and external cohort
| variables | OS | DFS | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| univariate | multivariate | univariate | multivariate | ||||||||||||
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | ||||
| SYSUCC cohort | |||||||||||||||
| Gender (male/female) | 0.781 | 0.456-1.338 | 0.368 | 0.866 | 0.518-1.449 | 0.585 | |||||||||
| Age (>55/≤55) | 1.053 | 0.673-1.648 | 0.821 | 0.953 | 0.616-1.475 | 0.829 | |||||||||
| T classification (T3-T4/T1-T2) | 1.571 | 0.979-2.521 | 0.061 | 1.587 | 0.998-2.522 | 0.051 | |||||||||
| N classification (yes/no) | 2.050 | 1.307-3.215 | 0.002 | 2.265 | 1.416-3.623 | 0.001 | 1.879 | 1.209-2.919 | 0.005 | 1.657 | 1.030-2.664 | 0.037 | |||
| Recurrence (yes/no) | 1.646 | 0.976-2.773 | 0.061 | 2.143 | 1.265-3.630 | 0.005 | 2.146 | 1.190-3.869 | 0.011 | ||||||
| TNM staging (Ⅱ-Ⅳ/Ⅰ) | 4.041 | 0.991-16.478 | 0.051 | 4.246 | 1.042-17.306 | 0.044 | N.S. | ||||||||
| Differentiation (poor/well + moderate) | 2.138 | 1.141-4.007 | 0.018 | 1.952 | 1.023-3.726 | 0.043 | 2.038 | 1.094-3.798 | 0.025 | 2.318 | 1.206-4.455 | 0.012 | |||
| CD103+cells (low/high) | 0.432 | 0.269-0.694 | 0.001 | 0.406 | 0.247-0.667 | 0.0003 | 0.421 | 0.264-0.670 | 0.0003 | 0.385 | 0.233-0.637 | 0.0002 | |||
| ≥ 37.6 cells/field* | |||||||||||||||
| < 37.6 cells/field | |||||||||||||||
| STCH cohort | |||||||||||||||
| Gender (male/female) | 1.335 | 0.669-2.661 | 0.412 | 1.764 | 0.891-3.490 | 0.103 | |||||||||
| Age (>55/≤55) | 1.382 | 0.766-2.494 | 0.283 | 1.325 | 0.759-2.312 | 0.323 | |||||||||
| T classification (T3-T4/T1-T2) | 2.103 | 0.897-4.930 | 0.087 | 2.609 | 1.118-6.088 | 0.026 | N.S. | ||||||||
| N classification (yes/no) | 2.488 | 1.436-4.309 | 0.001 | N.S. | 2.745 | 1.624-4.642 | 0.0002 | N.S. | |||||||
| Recurrence (yes/no) | 63.692 | 8.743-463.987 | 0.0004 | 60.56 | 8.032-456.629 | 0.00006 | 153.010 | 17.363-1348.412 | 0.0001 | N.S. | |||||
| TNM staging (Ⅱ-Ⅳ/Ⅰ) | 6.019 | 1.462-24.780 | 0.013 | N.S. | 4.714 | 1.471-15.106 | 0.009 | N.S. | |||||||
| Differentiation (poor/well + moderate) | 0.763 | 0.323-1.803 | 0.538 | 1.089 | 0.511-2.323 | 0.825 | |||||||||
| CD103+cells (low/high) | 0.492 | 0.246-0.982 | 0.044 | 0.328 | 0.141-0.763 | 0.01 | 0.615 | 0.331-1.142 | 0.123 | 0.270 | 0.113-0.644 | 0.003 | |||
| ≥ 37.6 cells/field* | |||||||||||||||
| < 37.6 cells/field | |||||||||||||||
*Each number represents mean value/field of all cases analyzed.
Univariate analysis, Cox proportional hazards regression model.
Multivariate analysis, Cox proportional hazards regression model. Variables were adopted by univariate analysis.
Abbreviations: OS, overall survival; DFS, disease-free survival; HR, hazard ratio; CI, confidence interval. N.S., not significant.
Cellular source of CD103+ cells in ESCC tissues. (A) Double immunofluorescence staining shows CD103 (green), CD3 (red), CD11c (red), CD4 (red), and CD8 (red) expression and co-localization of double-positive cells (yellow) in ESCC tissue. (B) The percentages of CD11c+CD103+ cells identified as CD11c and CD103 double-positive cells and calculated in total numbers of CD103+ cells in the ANT and IT regions. Scale bar = 25 μm. (C, D) The percentages of CD3+CD103+ cells identified as double-positive cells and calculated in total numbers of CD103+ or CD3+ cells in the ANT and IT regions (n = 7). (E, F) The percentages of CD4+CD103+ and CD8+CD103+ cells identified as double-positive cells and compared to the total numbers of CD103+ cells in the ANT (E) and IT regions (F), respectively (n = 10). Results are the means ± SEM (bars); **P < 0.01; ***P < 0.001.
Discussion
CD103+ cells are an important component of inflammatory infiltrating lymphocytes in tissue, where they exhibit diverse subpopulations and a distinct phenotype [24-26]. In the present study, we delineated the distribution and prognostic value of CD103+ cells in ESCC tissue. Intratumoral tissues had decreased CD103+ cell density compared with non-tumor tissues. CD8+ T cells accounted for most CD103+ cells in the ESCC tissue and exhibited a CTLA-4hiGBhiPD-1low activated phenotype. Furthermore, we demonstrated that intratumoral CD103+ cell density was associated with favorable prognosis and could be served as an independent risk factor in patients with ESCC.
The heterogeneity of T cells is responsible for their diverse functions in tumor progression [27-29]. Previous studies have found that clinical significance of T cell subsets remains controversial [30-34]. Therefore, there is an urgent need to identify accurate prognostic factors to enable the development of novel clinical strategies for treating patients with ESCC. CD103 is a convenient cell surface marker for tissue-resident memory T cells [26, 35]. We observed that most CD103+ cells were CD3+ T cells in ESCC tissues and the intratumoral tissues had decreased CD103+ cell density compared to the non-tumor tissues. Moreover, high CD103+ TILs density was associated with better OS and DFS. Multivariate analyses revealed that CD103+ TILs number was an independent and significant prognostic factor in ESCC. In accordance with our results previous studies showed that CD103+ TIL density has been associated with improved prognosis in patients with non-small cell lung cancer [15], endometrial adenocarcinoma [36], ovarian cancer [37], cervical cancer [16], and bladder cancer [11]. Recent study has found that five features (MYC, ANO1, SLC52A3, Age and N-stage, MASAN) provided a precise prediction of ESCC survival outcome [38]. The combination of CD103 (ITGAE) with MASAN may has the potential to be further studied as the optimal predictor for ESCC prognosis. Moreover, the applicability of these cells across heterogeneous populations of ESCC patients should be further validated.
TILs subsets span the innate-adaptive continuum and include innate lymphoid cells, unconventional T cells (e.g., natural killer T cells, γδ T cells), and tissue-resident memory T cells, which have diverse phenotypes and functions in homeostasis, infection, and tumors [39]. Many TILs express CD103, which binds E-cadherin, mediating cellular localization in tumor tissue [9]. The CD103+CD39+ CD8 TILs identified tumor-reactive CD8 T cells in many human solid tumor [40]. Upon infection, in contrast to CD103-CD8+ T cells, most CD103+CD8+ cells can quickly upregulate cytotoxic mediators when exposed to their specific antigen, which might provide a rapid and efficient response to influenza infection without causing cytotoxic damage to the epithelial barrier [41]. Moreover, the CD11b-CD103+PD-L1high DCs in mesenteric lymph nodes (MLNs) could take up intestinal luminal antigens, and highly induce Foxp3+ regulatory T cells through TGF-β activation [42]. Accordingly, we observed that most CD103+ TILs were CD8+ T cells, which accounted for about 46% of such cells in the ESCC tissues; CD11c+ DC made up fewer of the numbers, suggesting that the CD8+CD103+ subset in CD103+ TILs play a primary role in the anti-tumor immune response. Therefore, the underlying mechanisms that regulate CD103+ TILs subset migration, location, and development, including their diverse functions, which local environmental signals might influence, warrant further investigation.
CD103+ cells co-express CTLA-4, granzyme B (GB), and PD-1 in ESCC. (A-C) Double immunofluorescence staining shows CD103, CTLA-4 (A), granzyme B (GB) (B), and PD-1 (C) expression and co-localization of double-positive cells in ESCC tissue. DAPI (blue) was used as a counterstain. Scale bar = 25 μm. The percentages were identified as double-positive cells compared to the total numbers of CD103+ cells in the ANT and T regions, respectively (n = 7). Results are the means ± SEM (bars).
High intratumoral CD103+ cell density is a predictor of favorable prognosis in patients with ESCC. (A) Representative IHC images demonstrate high (left) and low (right) number of CD103+ cells. Brown areas indicate positively stained cells. Scale bar = 50 μm. (B) Kaplan-Meier survival curves compare OS and DFS rates in patients (n = 107) with low or high intratumoral CD103+ cell density in SYSUCC cohort. (C) Kaplan-Meier survival curves compare OS and DFS rates in patients (n = 91) with low or high intratumoral CD103+ cell density in STCH cohort. P < 0.05 was considered statistically significant (log-rank test).
In a normal physiological environment, immune checkpoints are crucial for maintaining self-tolerance [43]. CTLA-4 is expressed mainly on T cells, where it primarily regulates the amplitude of the early stages of T cell activation [44]. On the other hand, when exposed to chronic antigens, such as viral infection and tumor, T cells are educated to a state of exhaustion or dysfunction, with increased PD-1 expression, which limits their lytic activity [45]. In the present study, CD103+ TILs displayed a CTLA-4hiGBhiPD-1low activated phenotype in the tumor. In cervical cancer, ITGAE gene expression has been strongly correlated with cytotoxic T cell markers (e.g., CD8/granzyme B), and in a preclinical mouse model, human papillomavirus (HPV) E6/E7 targeted therapeutic vaccination combined with radiotherapy increased the number of intratumoral CD103+CD8+ T cells, indicating that CD103 is a promising response biomarker of HPV E6/E7 targeted immunotherapy [16]. We found that most CD103+ TILs co-located with CTLA-4+ cells, but confocal microscopic analysis showed that fewer co-expressed PD-1 in situ. Moreover, the majority of CD103+ TILs secreted granzyme B, which could kill the target cells. Taken together, our results suggest that CD103+ TILs have anti-tumor potential and are deserved in-depth mechanism research on their anti-tumor activity, which might have functional roles in cancer immunotherapy.
Conclusions
In ESCC, CD103+ cells are mainly activated, and not exhausted T cells that exhibit a CTLA-4hiGBhiPD-1low phenotype. The density of CD103+ TILs predicts favorable prognostic value during tumor progression. These results can be used as a promising prognostic biomarker for patients with ESCC.
Acknowledgements
This work was supported by the project grants from the National Key R&D Program of China (2018ZX10302205) and Fundamental Research Funds for the Central Universities under Grant 171gjc32. We appreciate for the support from Sun Yat-sen University Cancer Center and Shantou Central Hospital.
Author Contributions
Data curation: Yifan Chu, Jing Liao.
Funding acquisition: Limin Zheng, Jing Xu, Lian Li.
Investigation: Yifan Chu, Jing Liao, Xingjuan Yu, Jinqing Li, Yongchun Wang, Junfeng Wang, Xiue Xu, Liyan Xu.
Writing-original: Yifan Chu.
Writing-editing: Limin Zheng, Jing Xu, Lian Li.
All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34
2. Chen W, Zheng R, Baade PD. et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-32
3. Shapiro J, van Lanschot JJB, Hulshof M. et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090-8
4. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-64
5. Su Q, Zhang X, Shen X. et al. Risk of immune-related colitis with PD-1/PD-L1 inhibitors vs chemotherapy in solid tumors: systems assessment. J Cancer. 2018;9:1614-22
6. Guo L, Zhang H, Chen B. Nivolumab as Programmed Death-1 (PD-1) Inhibitor for Targeted Immunotherapy in Tumor. J Cancer. 2017;8:410-6
7. Chen X, Wang L, Li P. et al. Dual TGF-beta and PD-1 blockade synergistically enhances MAGE-A3-specific CD8(+) T cell response in esophageal squamous cell carcinoma. Int J Cancer. 2018;143:2561-74
8. Edwards J, Wilmott JS, Madore J. et al. CD103(+) Tumor-Resident CD8(+) T Cells Are Associated with Improved Survival in Immunotherapy-Naive Melanoma Patients and Expand Significantly During Anti-PD-1 Treatment. Clin Cancer Res. 2018;24:3036-45
9. Hardenberg JB, Braun A, Schon MP. A Yin and Yang in Epithelial Immunology: The Roles of the alphaE(CD103)beta7 Integrin in T Cells. J Invest Dermatol. 2018;138:23-31
10. de Mingo Pulido A, Gardner A, Hiebler S. et al. TIM-3 Regulates CD103(+) Dendritic Cell Function and Response to Chemotherapy in Breast Cancer. Cancer Cell. 2018;33:60-74 e6
11. Wang B, Wu S, Zeng H. et al. CD103+ Tumor Infiltrating Lymphocytes Predict a Favorable Prognosis in Urothelial Cell Carcinoma of the Bladder. J Urol. 2015;194:556-62
12. Braun A, Dewert N, Brunnert F. et al. Integrin alphaE(CD103) Is Involved in Regulatory T-Cell Function in Allergic Contact Hypersensitivity. J Invest Dermatol. 2015;135:2982-91
13. Zikos TA, Donnenberg AD, Landreneau RJ. et al. Lung T-cell subset composition at the time of surgical resection is a prognostic indicator in non-small cell lung cancer. Cancer Immunol Immunother. 2011;60:819-27
14. Jenkinson SE, Whawell SA, Swales BM. et al. The alphaE(CD103)beta7 integrin interacts with oral and skin keratinocytes in an E-cadherin-independent manner*. Immunology. 2011;132:188-96
15. Djenidi F, Adam J, Goubar A. et al. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol. 2015;194:3475-86
16. Komdeur FL, Prins TM, van de Wall S. et al. CD103+ tumor-infiltrating lymphocytes are tumor-reactive intraepithelial CD8+ T cells associated with prognostic benefit and therapy response in cervical cancer. Oncoimmunology. 2017;6:e1338230-43
17. Donnem T, Hald SM, Paulsen EE. et al. Stromal CD8+ T-cell Density-A Promising Supplement to TNM Staging in Non-Small Cell Lung Cancer. Clin Cancer Res. 2015;21:2635-43
18. Wakabayashi O, Yamazaki K, Oizumi S. et al. CD4+ T cells in cancer stroma, not CD8+ T cells in cancer cell nests, are associated with favorable prognosis in human non-small cell lung cancers. Cancer Sci. 2003;94:1003-9
19. Li L, Xu L, Yan J. et al. CXCR2-CXCL1 axis is correlated with neutrophil infiltration and predicts a poor prognosis in hepatocellular carcinoma. J Exp Clin Cancer Res. 2015;34:129-38
20. Xu J, Ding T, He Q. et al. An in situ molecular signature to predict early recurrence in hepatitis B virus-related hepatocellular carcinoma. J Hepatol. 2012;57:313-21
21. Ding T, Xu J, Zhang Y. et al. Endothelium-coated tumor clusters are associated with poor prognosis and micrometastasis of hepatocellular carcinoma after resection. Cancer. 2011;117:4878-89
22. Zhang Y, Li JQ, Jiang ZZ. et al. CD169 identifies an anti-tumour macrophage subpopulation in human hepatocellular carcinoma. J Pathol. 2016;239:231-41
23. Wu C, Ning H, Liu M. et al. Spleen mediates a distinct hematopoietic progenitor response supporting tumor-promoting myelopoiesis. J Clin Invest. 2018;128:3425-38
24. Fan X, Rudensky AY. Hallmarks of Tissue-Resident Lymphocytes. Cell. 2016;164:1198-211
25. Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity. 2014;41:886-97
26. Gebhardt T, Palendira U, Tscharke DC. et al. Tissue-resident memory T cells in tissue homeostasis, persistent infection, and cancer surveillance. Immunol Rev. 2018;283:54-76
27. Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269-81
28. Kuang DM, Xiao X, Zhao Q. et al. B7-H1-expressing antigen-presenting cells mediate polarization of protumorigenic Th22 subsets. J Clin Invest. 2014;124:4657-67
29. Zhang W, Bai JF, Zuo MX. et al. PD-1 expression on the surface of peripheral blood CD4(+) T cell and its association with the prognosis of patients with diffuse large B-cell lymphoma. Cancer Med. 2016;5:3077-84
30. Zhu Y, Li M, Mu D. et al. CD8+/FOXP3+ ratio and PD-L1 expression associated with survival in pT3N0M0 stage esophageal squamous cell cancer. Oncotarget. 2016;7:71455-65
31. Nabeki B, Ishigami S, Uchikado Y. et al. Interleukin-32 expression and Treg infiltration in esophageal squamous cell carcinoma. Anticancer Res. 2015;35:2941-7
32. Lv L, Pan K, Li XD. et al. The accumulation and prognosis value of tumor infiltrating IL-17 producing cells in esophageal squamous cell carcinoma. PLoS One. 2011;6:e18219-25
33. Xue L, Lu HQ, He J. et al. Expression of FOXP3 in esophageal squamous cell carcinoma relating to the clinical data. Dis Esophagus. 2010;23:340-6
34. Cho Y, Miyamoto M, Kato K. et al. CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res. 2003;63:1555-9
35. Sathaliyawala T, Kubota M, Yudanin N. et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38:187-97
36. Workel HH, Komdeur FL, Wouters MC. et al. CD103 defines intraepithelial CD8+ PD1+ tumour-infiltrating lymphocytes of prognostic significance in endometrial adenocarcinoma. Eur J Cancer. 2016;60:1-11
37. Webb JR, Milne K, Nelson BH. PD-1 and CD103 Are Widely Coexpressed on Prognostically Favorable Intraepithelial CD8 T Cells in Human Ovarian Cancer. Cancer Immunol Res. 2015;3:926-35
38. Liu W, He JZ, Wang SH. et al. MASAN: a novel staging system for prognosis of patients with oesophageal squamous cell carcinoma. Br J Cancer. 2018;118:1476-84
39. Savage PA, Leventhal DS, Malchow S. Shaping the repertoire of tumor-infiltrating effector and regulatory T cells. Immunol Rev. 2014;259:245-58
40. Duhen T, Duhen R, Montler R. et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat Commun. 2018;9:2724-36
41. Piet B, de Bree GJ, Smids-Dierdorp BS. et al. CD8(+) T cells with an intraepithelial phenotype upregulate cytotoxic function upon influenza infection in human lung. J Clin Invest. 2011;121:2254-63
42. Shiokawa A, Kotaki R, Takano T. et al. Mesenteric lymph node CD11b(-) CD103(+) PD-L1(High) dendritic cells highly induce regulatory T cells. Immunology. 2017;152:52-64
43. Baumeister SH, Freeman GJ, Dranoff G. et al. Coinhibitory Pathways in Immunotherapy for Cancer. Annu Rev Immunol. 2016;34:539-73
44. Rowshanravan B, Halliday N, Sansom DM. CTLA-4: a moving target in immunotherapy. Blood. 2018;131:58-67
45. Kamphorst AO, Wieland A, Nasti T. et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science. 2017;355:1423-7
Author contact
![]() Corresponding authors: Lian Li, School of Life Sciences, Sun Yat-sen University, No. 135, Xingang Xi Road, Guangzhou 510275, P. R. China, Tel: 86-20-84115531, E-mail: lilian-75com; Jing Xu, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, No. 651, Dongfeng East Road, Guangzhou 510060, P. R. China, E-mail: xujingorg.cn.
Corresponding authors: Lian Li, School of Life Sciences, Sun Yat-sen University, No. 135, Xingang Xi Road, Guangzhou 510275, P. R. China, Tel: 86-20-84115531, E-mail: lilian-75com; Jing Xu, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, No. 651, Dongfeng East Road, Guangzhou 510060, P. R. China, E-mail: xujingorg.cn.

 Global reach, higher impact
Global reach, higher impact