Impact Factor
ISSN: 1837-9664
J Cancer 2020; 11(5):1094-1103. doi:10.7150/jca.30682 This issue Cite
Research Paper
Androgen Receptor (AR)-TLR4 Crosstalk Mediates Gender Disparities in Hepatocellular Carcinoma Incidence and Progression
Institute of Immunopharmaceutical Sciences, School of Pharmaceutical Sciences, Shandong University, Jinan 250012, Shandong, China
*These authors contributed equally.
Received 2018-10-15; Accepted 2019-8-2; Published 2020-1-1
Abstract
Background: Androgen receptor (AR) has a role in regulating malignancies and gender disparities in hepatocellular carcinoma (HCC). Recently, TLR4 activation is demonstrated to be required for HCC progression; however, whether and how TLR4 interacts with AR is largely unknown.
Methods: The tumorigenesis was detected in female and male mice induced by DEN/CCL4, then TLR4 and AR signals were detected in liver tissues by qPCR and FACS. The proliferation, colony formation and migration of HCC cell treated with TLR4 agonist LPS, or/and androgen DHT were evaluated in vitro. Furthermore, the expression of TLR4 and AR was detected by IHC in tissue microarray of HCC, and correlation of AR and TLR4 was defined.
Results: Male mice are more susceptible to develop HCC than female mice. Meanwhile, we found baseline TLR4 levels were higher in male mice than in female mice. AR expression in male mice was increased by treatment with DEN/CCL4. And, AR was constitutively expressed in human HCC cell lines. Dihydrotestosterone (DHT) stimulated TLR4 expression in both HepG2 and HepG2 2.15 cells, which could be blocked by silencing AR. On the other hand, treatment with LPS stimulated AR expression, but it was blocked by treatment with TLR4 antagonist and in cells deficient for TLR4. DHT treatment exacerbated TLR4-induced cellular proliferation, colony formation, migration, and invasion of HepG2 cells. The positive relationship between AR and TLR4 was confirmed in human HCC samples.
Conclusions: DHT-AR-TLR4 signaling enhances the development of HCC cells and facilitates their migration and invasion, demonstrating a mechanism underlying gender disparity in HCC.
Keywords: AR, Gender bias, TLR4, Hepatocellular Carcinoma
Introduction
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related death, and is notably more prevalent in men than in women, with a male-to-female ratio ranging from 2:1 to 8:1 [1-3]. Many risk factors contribute to gender disparity in HCC incidence, including gender-specific lifestyle differences, alcohol consumption, smoking habits, and infection with hepatitis B virus (HBV) or hepatitis C virus (HCV) [4, 5].
The androgen receptor (AR) is a member of the nuclear steroid receptor superfamily [6, 7], and is a transcription factor activated by ligand-dependent and ligand-independent mechanisms [8]. In response to stimulation by androgens, AR translocates into the nucleus, where it binds to androgen response elements (AREs), and regulates expression of genes related to cell growth and survival; it is in this role that AR serves an important regulator of pathogenesis in hepatocarcinogenesis [4]. AR has also been shown to promote expression of HBV viral RNAs [9]. Furthermore, both higher serum androgen concentrations and the presence of AR gene variants containing shorter CAG repeats (leading to higher AR activity) have been linked to increased risk of hepatocarcinogenesis, especially of HBV-mediated HCC [7, 10]. In addition, androgen/AR signals promote cancer cell stemness through direct activation of Nanog homeobox (NANOG) transcription in HCC [10]. These data suggest that androgen-AR signals may play key roles in promoting the development of HCC. However, clinical studies using anti-androgens (e.g. flutamide and cyproterone acetate) yielded disappointing results, and demonstrated no apparent clinical benefits for patients [11, 12]. The association between AR and gender disparity in HCC initiation and progression has been well documented, but the detailed mechanisms underlying how AR regulates HCC remain to be completely elucidated [2, 13].
The role of the gut-liver axis in the development of liver diseases, including non-alcoholic steatohepatitis and HCC, has recently attracted a great deal of attention [14, 15]. Pattern recognition receptors (PRR), including Toll-like receptors (TLRs), recognize gut-derived bacterial components, and stimulate the innate immune system in response. TLR4 has been demonstrated to play an important role in the promotion of HCC promotion, but not in the initiation of HCC, and it mediates increased cellular proliferation, expression of the hepatomitogen epiregulin, and prevention of apoptosis [14, 16, 17]. TLR4 signaling promotes the development of HCC development in HCC induced by N′-N′-diethylnitrosamine (DEN) by increasing production of proinflammatory and cancer-related biomolecules, including cytokines, NANOG, Caspase-1, Ephrin-A1, NO, and B-cell CLL/lymphoma 6 (BCL6) [18, 19]. Furthermore, TLR4 signaling plays a role in the migration and invasion of HCC cells. We reported that TLR4 signaling contributes to a COX2/PGE2/STAT3 positive feedback loop in HCC cells [20]. In addition, the TLR4 rs1927914 polymorphism is associated with increased risk of HCC recurrence [21]. Taken together, these data suggest a critical role for TLR4 in the promotion of HCC.
Based on the importance of TLR4 and AR in HCC development, we wanted to know whether and how TLR4 crosstalk occurs with sex hormone receptors, especially AR, during the development of HCC. Here, we report that the baseline expression of TLR4 was higher in male mice than in female mice. This gender disparity was further increased by treatment with DEN/CCL4. TLR4 showed a positive relationship with AR expression in human HCC tissues. Further investigation demonstrated that DHT-AR is critical for TLR4 signal-mediated promotion of HCC, suggesting that TLR4 is essential for gender disparities observed in HCC. These findings provide new insight for improving the efficacy of HCC treatment in the clinic.
Material and Methods
Mouse model of DEN-induced HCC
A mouse model of DEN-induced HCC was generated (described in Supplementary Methods). 6-week-old C57BL/6 mice were obtained from HFK Bioscience Co, Ltd (Beijing, China). Beginning at fourteen days of age, female and male mice (5 mice per group) received weekly intraperitoneal (i.p.) injections of 20 mg/kg DEN for three weeks (Sigma, USA), followed by weekly i.p. injections of 100 μl CCL4 dissolved in olive oil for six weeks. The procedure was divided into three stages; early (7-21 weeks), middle (22-42 weeks), and late (43 week-sacrificial endpoint), as previously described [16]. Evaluation of tumor number and size was determined as described by counting the number of visible tumors and measuring the size of the largest tumor with calipers, the rate of tumor incidence was is recorded [16]. All animals were kept in standard laboratory conditions and provided with food and water ad libitum. All animal experiments were approved by the Ethics Committee of Shandong University.
Cell lines and reagents
The HCC cell lines HepG2, H7402, Hepa1-6, and HepG2.2.15 were cultured and maintained in our laboratory. All cell lines were grown in DMEM (Gibco, USA), containing 1% penicillin-streptomycin and supplemented with 10% fetal bovine serum (FBS). Cell cultures were incubated at 37°C in 5% CO2. LPS isolated from Escherichia coli (0111:B4), natural AR ligand dihydrotestosterone (DHT; T1500), and estrogen (E2) (E2758) were purchased from Sigma-Aldrich (St. Louis, MO). The TLR4 signaling inhibitor, TAK-242, was obtained from Invivogen (San Diego, CA, USA).
Quantitative real-time PCR analysis
1.5×105 HepG2 or Hep1-6 cells/well were plated into 12-well plates, and were treated with DHT or LPS. Total RNA from cells and liver tissues was extracted using the Trizol reagent (Invitrogen, Carlsbad, CA, USA), and was used to generated cDNA using Moloney Murine Leukemia Virus Reverse Transcriptase (M-MLV; Invitrogen) according to the manufacturer's protocol. cDNA amplification was performed using real-time PCR with FastStart Universal SYBR Green Master (Roche, Switzerland) on an iCycleriQ real-time PCR system (Bio-Rad, Hercules, CA, USA). GAPDH and β-actin genes were used to normalize gene expression. The primers used in this study are described in Table 1.
Immunofluorescence microscopy
Immunofluorescence was performed using primary antibodies to AR (Abcam Cambridge, UK), and DyLightTM 549-conjugated goat anti-rabbit secondary antibody (Abbkine, Wuhan, China). Quantification was performed using Image J and Photoshop software, analyzing at least 10 images per mouse for AR staining.
Cell proliferation assay
The MTT cell proliferation reagent (Roche Molecular Biochemical, Mannheim, Germany) was used to evaluate cell growth, according to the manufacturer's instructions. Briefly, 5×103 cells/well were plated into 96-well plates. After sample preparation, 20 µL of MTT reagent was added, and cells were incubated at 37 °C for another 3h. Absorbance at 570 nm was recorded by a scanning multi-well spectrophotometer (Bio-Rad, Hercules, CA).
The primers used in this study
| Gene name | Sequence 5' → 3' | Size (bp) |
|---|---|---|
| Human GAPDH | F:GAAGGTGAAGGTCGGAGT | 155 |
| R:CATGGGTGGAATCATATTGGAA | ||
| Human AR | F: GACGACCAGATGGCTGTCATT | 106 |
| R: GGGCGAAGTAGAGCATCCT | ||
| Human ERα | F: GGGAAGTATGGCTATGGAATCTG | 220 |
| R: TGGCTGGACACATATAGTCGTT | ||
| Human ERβ | F: AGCACGGCTCCATATACATACC | 199 |
| Mouse β-actin | F:AGAGGGAAATCGTGCGTGAC | 128 |
| R:CAATAGTGATGACCTGGCCGT | ||
| R: CACCTCCATTGTCCCTGTTTTAT | ||
| Mouse AFP | F: CTTCCCTCATCCTCCTGCTAC | 145 |
| R: ACAAACTGGGTAAAGGTGATGG | ||
| Mouse PCNA | F: TTTGAGGCACGCCTGATCC | 135 |
| R: GGAGACGTGAGACGAGTCCAT | ||
| Mouse Ki67 | F: ACCGTGGAGTAGTTTATCTGGG | 126 |
| R: TGTTTCCAGTCCGCTTACTTCT | ||
| Mouse Acta2 | F: GTCCCAGACATCAGGGAGTAA | 102 |
| R: TCGGATACTTCAGCGTCAGGA | ||
| Mouse AR | F: CTGGGAAGGGTCTACCCAC | 128 |
| R: GGTGCTATGTTAGCGGCCTC |
Western blot analysis
Cells or liver tissue was homogenized using lysis buffer with containing a protease inhibitor cocktail (Beyotime Biotechnology, Shanghai, China). Total protein (30 µg) from each sample was separated on a 10% SDS-polyacrylamide gel, and then transferred onto PVDF membranes. Membranes were incubated for 1 h in blocking buffer containing 5% milk, then incubated with anti-β-actin (Santa Cruz Biotechnology, CA), anti-AR (Abcam, CA), or rabbit anti-TLR4 (PL Laboratories, British Columbia), diluted 1:2000 overnight at 4 °C. After overnight incubation, membranes were incubated with peroxidase conjugated goat anti-rabbit IgG (Santa Cruz Biotechnology, CA) for 1 h at room temperature. Proteins were visualized using Immobilon Western Chemiluminescent HRP Substrate (Millipore, Billerica, MA) and detected with Alpha Ease FC software (Bio-Rad, Hercules, CA) [22].
Immunohistochemical Staining (IHC)
A human hepatoma tissue microarray (OD-CT-DgLiv02 and HLiv-HCC060CD-01) was obtained from Outdo Biotech (Shanghai, China). Paraffin-embedded and formalin-fixed mouse liver samples were cut into 5 μm sections, which were then processed for immunohistochemistry as described [23]. After incubation with an antibody against TLR4 (Santa Cruz Biotechnology) or AR (Abcam), samples were kept overnight at 4 °C. IHC staining was evaluated using Image-Pro Plus v6.2 software. The relative expression level of each protein was quantified according to integrated optical density.
Analysis of apoptosis by FACS
1.5×105 cells/well were plated into 12-well plates. Cells were treated with DHT or LPS. Twenty-four hours later, cells were washed, harvested, and re-centrifuged in 200 μl 1× annexin-binding buffer. Cells were incubated with 5 μl Alexa Fluor® 488 Annexin V and 2.5 μl of propidium iodide (PI) (Bestbio, Shanghai, China) at room temperature for 15 min, then analyzed by flow cytometry (FACSCalibur, BD).
Transwell migration assays
The migration assay was performed using 24-well transwell inserts with a pore size of 8 μm (Corning, USA). Initially, HepG2 cells treated with DHT or LPS were seeded into the upper chamber in serum-free medium. DMEM with 10% FBS was added to the lower chamber, as a chemoattractant. After 6 h incubation at 37 °C, the cells that traversed the membrane were fixed in methanol and stained with 0.1% crystal violet. For quantification, the number of migrated cells was calculated by counting at least five random separate fields.
Colony formation assay
For the clonogenic colony formation assay, 500 cells/well were seeded in 6-well plates. After 14 days, visible colonies were observed using the naked eye, fixed with 4% formaldehyde, and stained with 0.1% crystal violet. Colonies with a diameter greater than 1 mm were counted.
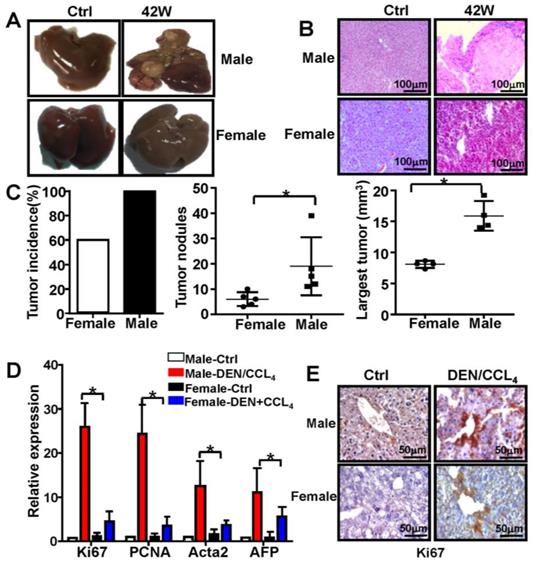
Male mice are more susceptible to develop HCC than female mice. C57BL/6 mice were injected three times with DEN (100 mg/kg i.p.) starting at the age of 2 weeks, followed by six injections of CCl4 (0.5 ml/kg i.p.); mice were sacrificed different time points after DEN treatment (×200). B&C. Male and female mice were sacrificed 42 weeks after DEN injection. The appearance of liver tissue (A) and H&E staining (B) from DEN-induced mice were shown. (C) Tumor incidence, tumor number, and largest tumor size were assessed 42-weeks after DEN injection in female and male mice. (D) The proliferation marker Ki67, PCNA, and clinical indicators of HCC, AFP and Acta2, were analyzed by q-PCR. Data are represented as mean ± SEM (n=5). (E) Proliferation of hepatocytes indicated by Ki67 protein expression was analyzed by IHC (×400). * P < 0.05: Male-DEN/CCL4 versus Female-DEN/CCL4.
Statistical analysis
Statistical analysis was performed using paired Student's t-test. Statistical significance was determined as ** P < 0.01 and * P < 0.05 compared with control.
Results
Male mice exhibit increased susceptibility to HCC development
The incidence and mortality of liver cancer incidence is significantly higher in male mice than in female mice [1, 24]. In the present study, male and female mice were subjected to the combination of treatment with DEN and CCl4 (Figure S1). The tumors induced by this treatment demonstrated typical features of HCC in male mice (Figure 1A & 1B), and the spleens of male and female mice did not show any significant differences (data not shown). Moreover, compared to female mice, there was a profound increase in tumor number and size in male mice 44 weeks after the initial DEN/CCl4 treatment (n=5) (Figure 1C). qPCR analysis revealed that expression of Ki67, proliferating cell nuclear antigen (PCNA), Acta2, and alpha-fetoprotein (AFP) was markedly increased in male mice treated with DEN/CCl4, and displayed expression profiles characteristic of HCC (n=5) (Figure 1D). By IHC analysis, Ki67 protein expression was higher in the liver tissue from male mice treated with DEN/CCl4 than in female mice (Figure 1E). These data confirm that there is a gender disparity associated with the development of HCC.
Both TLR4 and AR expression is increased in this model of male HCC
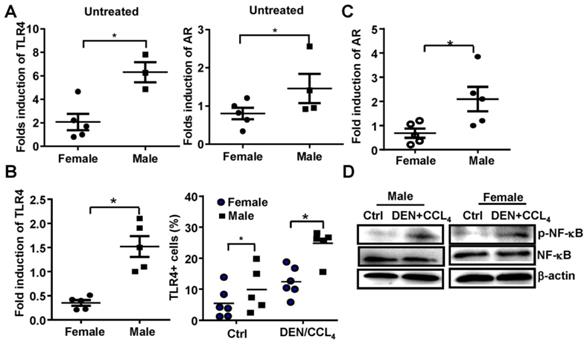
Previous reports demonstrate that AR promotes gender disparities in cancer [10], and TLR4 plays an important role in promoting liver cancer [16]. Therefore, we evaluated whether AR was associated with TLR4 signaling at the initiation and promotion stages of HCC in this model. We initially found that the baseline mRNA levels of TLR4 and AR were significantly higher in male mice than that in female mice (Figure 2A). Following DEN/CCL4 injection, we observed TLR4 positive hepatocytes were increased in liver tissue of male mice (n=5) (Figure 2B); this was accompanied by an elevation of AR mRNA (Figure 2C). Furthermore, NF-κB activation in liver tissue from male mice were confirmed by western blot assays (Figure 2D). These findings indicate there may be a relationship between TLR4 and AR in HCC development.
The expression of both TLR4 and AR is increased in a male HCC model. (A) TLR4 and AR expression were evaluated by qPCR in untreated-female and male mice. (B) The mRNA of TLR4 and TLR4-positive hepatocytes from female and male mice treated with DEN were evaluated by qPCR and FACS. (C) AR levels were measured in DEN treated female and male mice by qRT-PCR. (D) NF-κB activation in hepatocytes from female and male mice was detected by Western Blot. Data are represented as mean ± SEM, (n=5). Bar graph shows quantification results.
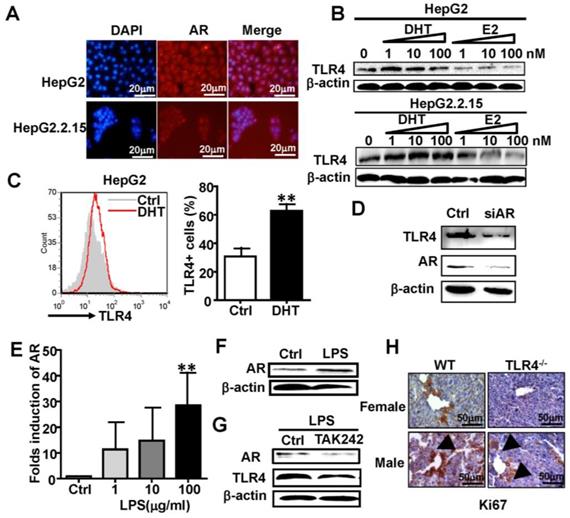
TLR4 and AR up-regulate each other. (A) AR expression was analyzed by immunofluorescent staining in HepG2 and HepG2.2.15 cells; nuclear stain indicated by DAPI stain. The MERGE is the merge of DyLight 549 and DAPI (×400). (B) Western blot analysis showing the protein expression of TLR4 in HepG2 and HepG2.2.15 cells treated by different concentrations of DHT or E2 for 48 h. (C) TLR4 assay by FACS for HepG2 cells was performed following treatment with control or 100 nM DHT for 24h. Bar graph shows quantification results; data are represented as mean ± SEM. (D) Western blot analysis showing the protein expression of TLR4 and AR in HepG2 cells transfected with siRNA targeting AR (siAR), or scramble siRNA (Ctrl), for 48h. AR levels were measured in LPS treated HepG2 cells by qRT-PCR (E) and western blot (F). (G) TLR4 and AR protein expression levels were assayed by western blot in HepG2 cells treated with the TLR4 inhibitor TAK242. (H) Ki67 protein expression was analyzed by IHC at 23 weeks after DEN treatment in wild type (WT) and TLR4-/- male and female mice (×400).
AR and TLR4 positively regulate each other
To clarify the relationship between AR and TLR4 signaling in HCC, we studied the human HCC cell lines HepG2 and HepG2.2.15, which constitutively express AR at a relatively high level (Figure S2). Immunofluorescence staining confirmed the expression of AR in HepG2 and HepG2.2.15 cells (Figure 3A). HepG2 and HepG2.2.15 cells were treated with androgen DHT or estrogen (E2) for 48 h. Treatment with DHT elevated TLR4 the expression of TLR4, while E2 treatment down-regulated TLR4 in HepG2 cells (Figure 3B, top). A similar trend in TLR4 expression changes was observed in HepG2.2.15 cells (Figure 3B, bottom). Meanwhile, FACS assay revealed the percentage of TLR4 positive HepG2 cells was increased by DHT treatment (Figure 3C). However, silencing of AR by siRNA abolished the DHT-induced up-regulation of TLR4 in HepG2 cells (Figure 3D). We stimulated HepG2 cells with the TLR4 agonist, LPS, for 24 h; the expression of AR at both the mRNA and protein levels was increased (Figure 3E-F); this increase in AR was abrogated by the TLR4 inhibitor TAK242 (Figure 3G). In addition, the level of Ki67 in WT male mice treated with DEN/CCL4 was higher than that in WT female mice; this was profoundly decreased in TLR4-/- mice (Figure 3H). These results suggest that the positive regulation between AR and TLR4 signaling is critical for HCC initiation and development.
AR enhances TLR4-induced tumor characteristics in HCC cells
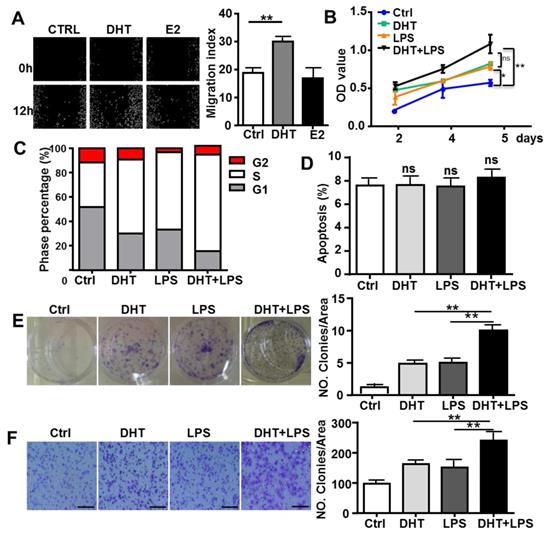
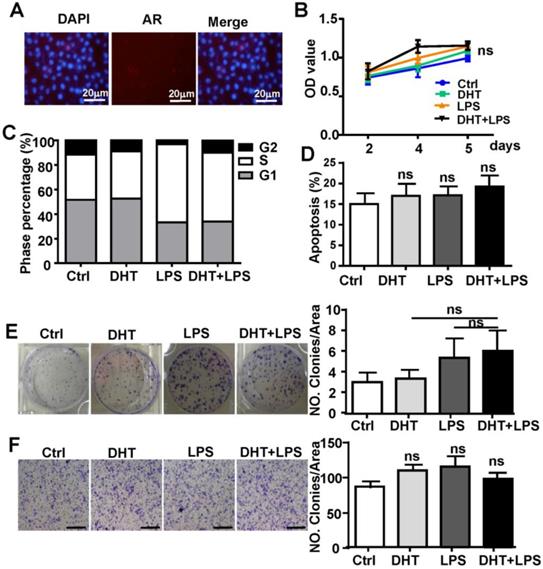
To determine if AR-associated signaling facilitates TLR4-induced HCC development, HepG2 cells were treated with 100 nM DHT or 100 nM E2. After stimulation for 6 h, we observed that DHT promoted the wound healing capacity of HCC cells, while E2 did not show significant effects (Figure 4A). Compared to control, DHT- and LPS-treated cells, the proliferation of HCC cells was augmented by treatment with a combination of LPS and DHT (LPS+DHT) (Figure 4B); simultaneously, the proportion of HepG2 cells in S-phase was increased (Figure 4C), but the apoptosis of HepG2 cells was not affected by DHT+LPS (Figure 4D). Furthermore, colony formation assays revealed that both DHT and LPS stimulated colony growth (Figure 4F) and enhanced the migratory ability (Figure 4G) of HepG2 cells, which was further enhanced by treatment with a combination of LPS and DHT. However, these phenomena were not observed in Hepa1-6 cells, which exhibited low levels of AR (Figure 5). Therefore, these data suggested that AR signaling exacerbates TLR4-induced proliferation, colony formation, and migration of HCC cells.
AR enhances TLR4-induced HCC proliferation, colony formation, and migration. (A) HepG2 cells were treated with DHT (100 nM) or E2 (100 nM) for 12 h. The migration rate was detected by Image-Pro Plus. Bar graph shows quantification results. Data are represented as mean ± SEM. (B) AR positive-HepG2 cells were treated with LPS (10 µg/ml), DHT (100 nM), or with a combination for 2, 4, and 5 days, and the proliferation rate was assessed by the MTT assay. (C) HepG2 cells were incubated with LPS (10 µg/ml) and DHT (100 nM) for 48 h, and cell cycle distribution of was analyzed by FACS. (D) HepG2 cells were treated with DHT, LPS, or with the combination indicated for 24 h, then Annexin V/PI staining was employed to detect the apoptosis of cells. (E) HepG2 cells were treated with LPS (10 µg/ml), DHT (100 nM), or with a combination for 6 h. After 14 days, colony formation was detected by Crystal Violet stain. (F) HepG2 cells were treated LPS (10 µg/ml) and DHT (100 nM) for 6 h. The migration rate was evaluated by Crystal Violet staining (the scale bar is 100nm). Bar graph shows quantification results. Data are represented as mean ±SEM.
Positive relationship between TLR4 and AR expression in human HCC tissues
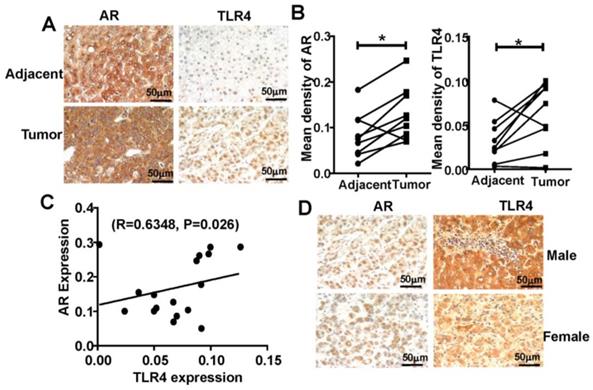
To extend our findings in mice to human HCC, we analyzed TLR4 and AR expression using tissue microarray, containing matched pairs of tumor and peritumoral liver tissue from 22 male and 8 female patients with primary HCC. We found that TLR4 and AR were expressed more highly in HCC tumor tissue than in adjacent liver tissue (Figure 6A-B), and we found that the expression of TLR4 was positively correlated with AR in HCC tumors (R=0.6348, P=0.026; Figure 6C). Furthermore, we found that the expression of AR in male is higher than that in female HCC. And the similar trend was observed for detection of TLR4 (Figure 6D).These results support the positive relationship between TLR4 and AR in HCC development.
Discussion
Here, we show that TLR4 expression was increased in male mice treated with DEN/CCL4. We discovered that a positive feedback loop involving DHT-AR and TLR4 signaling is critical for the initiation and development of HCC. We conclude that DHT-AR-TLR4 signaling mediates promotion of HCC, which is a critical aspect of the gender disparity in HCC.
TLR4 promotes HCC via several mechanisms, including increasing expression of NANOG, Caspase-1, Ephrin-A1, NO, and BCL6 and increasing the number of T regulatory lymphocytes [17, 18, 25]. TLR4 also facilitates the proliferation of HCC cells, mediated by a COX-2/PGE2/STAT3 positive feedback loop, and induces the production of pro-inflammatory and malignancy related molecules [20]. We propose that TLR4 signaling also contributes to the gender disparity observed in HCC. Our data demonstrate that TLR4 levels are increased in DEN/CCL4-treated male mice, that and DHT-AR and TLR4 signaling are involved in a positive feedback loop (Figure 2 and Figure 3). In addition, in line with our findings, estrogen restricts TLR4 and TNF-α, but not TLR3 and NLRP3 responses, contributing to a proinflammatory hepatic microenvironment in males, which imparts increased risk to men with chronic viral hepatitis to progress to cirrhosis or HCC [26].
DHT has no effect on the growth of Hepa1-6 cells. (A) AR expression was analyzed by immunofluorescent staining in Hepa1-6 cells; nuclear staining is indicated by DAPI stain. The MERGE is the merge of DyLight 549 and DAPI (×400). (B) Hepa1-6 cells were treated with LPS (10 µg/ml), DHT (100 nM), or with a combination for 2, 4, and 5 days, and the proliferation rate was assessed by the MTT assay. (C) Hepa1-6 cells were incubated with LPS (10 µg/ml) and DHT (100 nM) for 48 h, then the cell cycle distribution was analyzed by FACS. (D) Hepa1-6 cells treated with DHT, LPS, or with the combination indicated for 24 h, then Annexin V/PI staining was employed to detect apoptosis of the cells. (E) Hepa1-6 cells were treated with LPS (10 µg/ml), DHT (100 nM), or with a combination for 6 h. After 14 days, colony formation was assessed by Crystal Violet stain (×200). (F) Hepa1-6 cells were treated with LPS (10 µg/ml) and DHT (100 nM) for 6 h. The migration rate was evaluated by Crystal Violet staining (the scale bar is 100nm). Bar graph shows quantification results. Data are represented as mean ± SEM.
Positive correlation of AR and TLR4 in HCC patients. (A) IHC analysis of TLR4 and AR expression in 30 paired HCC tissues and their corresponding adjacent normal tissue (×400). (B) IHC staining was evaluated using Image-Pro Plus v6.2 software, and the relative expression level of each protein was quantified based on integrated optical density. Statistical significance of TLR4 and AR between HCC and adjacent tissues was indicated at P < 0.05. (C) Correlation between the relative protein levels of AR and TLR4 in 30 HCC tissue specimens. (D) IHC analysis of TLR4 and AR expression in male and female HCC tissue microarray (×400).
Previous studies found that AR activation could convene more than 200 kinds of regulatory molecules, including transcriptional factors, kinases, molecular chaperones, cytoskeleton proteins, and molecules related to histone modification. Importantly, PGE and STAT3 are among them, and we preciously found that TLR4 signaling could promote the proliferation and induce multidrug resistance of HCC cells to chemotherapeutics, in which COX-2/PGE2/STAT3 positive feedback loop [20, 27, 28]. In this study, although we found AR and TLR4 positively regulate each other (Figure 3), the exact molecular mechanism has not been enucleated, we still need further study to explore it.
Our study demonstrates that AR can promote HCC tumorigenicity; this is supported by evidence that mice lacking hepatic AR had less hepatocarcinogenesis after exposure to a higher dose of DEN [4, 29]. Treatment with the chemotherapeutic agent cisplatin suppresses AR expression by two distinct mechanisms: by increasing miR-34a-5p to suppress AR expression and by altering AR ubiquitination to accelerate AR protein degradation; suppressed AR expression may result in up-regulation of ULBP2, a natural-killer group 2 member D (NKG2D) ligand, to enhance the cytotoxicity of NK cells against HCC [30]. Another study reported that LPS-induced apoptosis in HepG2 cells stably expressing shAR, but not in control HepG2 cells [31], suggesting that AR may be involved in the apoptotic response of hepatocytes. Our data is not consistent with this observation, as we observed no effect of DHT treatment on HCC cell apoptosis, while AR signaling exacerbated TLR4-induced HCC proliferation, colony formation, and migration (Figure 4).
Similar to the gender disparities seen in human HCC, gender differences are also observed in mouse models of HCC, including both genetically or chemically induced HCC. Sex hormones and inflammatory responses have been implicated in the gender disparity of HCC; such studies have often used a mouse model that develops HCC upon exposure to DEN. This model incorporates chronic injury, inflammation, fibrogenesis, and CCl4-mediated increases in endotoxin levels [16, 32]. Thus, male mice are usually used to model HCC.
In addition, various immune cells in the tumor microenvironment influence the progression of HCC [3, 19]. AR alters NK cells cytotoxicity by suppressing Interleukin (IL)-12A expression in HCC cells, revealing a relationship between the cytotoxicity of NK cells and AR in HCC [13, 33]. Thus, the potential connection between AR and immune surveillance during HCC progression merits further evaluation. Although we found expression of TLR4 was positively correlated with TLR4 in tumors (Figure 6), the relationship of these molecules with survival time needs further clarification. Targeting AR with ASC-J9® or other small molecules may have potential to be developed to suppress HCC progression more effectively in near future [34].
Conclusions
Our data suggest that DHT-AR-TLR4 signaling enhances the development of HCC cells and facilitates their migration and invasion. These findings suggest that AR and TLR4 are potential therapeutic targets in the treatment of HCC, although further mechanistic studies are warranted.
Abbreviations
AR: androgen receptor; HCC: Hepatocellular carcinoma; DEN: N′-N′-diethylnitrosamine; AFP: alpha-fetoprotein; PCNA: proliferating cell nuclear antigen; IL: Interleukin; TLR4: Toll like receptor 4; E2: Estradiol; DHT: Dihydrotestosterone; NANOG: Nanog homeobox; IHC: Immunohistochemical; BCL6: B-cell CLL/lymphoma 6; PRR: pattern recognition receptor; COX2: cyclooxygenase 2.
Supplementary Material
Supplementary figures.
Acknowledgements
Ethics approval and consent to participate
The animal research was approved by the Animal Research Ethics Committee of Shandong University, and complied with the Guidelines for Animal Experiments of Laboratory Animals.
Availability of Data and Materials
Please contact author for data requests.
Authors' contributions
Study concept and design: Z J; acquisition of data: QJH, DY; analysis and interpretation of data: YD; drafting of the manuscript: QJH, YCL; critical revision of the manuscript for important intellectual content: QJH and DY; statistical analysis: QJH and DY; obtained funding and study supervision: ZJ and QJH. All authors read and approved the final manuscript.
Funding
This work was supported by National Natural Science Foundation of China (81972686, 81972694), Shandong Provincial Key Research and Development Program [grant number 2017GSF18159] and Shandong Provincial Natural Science Foundation, China [grant number ZR2017BH029] and the Fundamental Research Fund of Shandong University (2017JC004).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Li Y, Li H, Spitsbergen JM, Gong Z. Males develop faster and more severe hepatocellular carcinoma than females in krasV12 transgenic zebrafish. Sci Rep. 2017;7:41280
2. Shi L, Feng Y, Lin H, Ma R, Cai X. Role of estrogen in hepatocellular carcinoma: is inflammation the key? J Transl Med. 2014;12:93
3. Zhang J, Zhang Q, Lou Y, Fu Q, Chen Q, Wei T. et al. Hypoxia-inducible factor-1alpha/interleukin-1beta signaling enhances hepatoma epithelial-mesenchymal transition through macrophages in a hypoxic-inflammatory microenvironment. Hepatology. 2018;67:1872-89
4. Yeh SH, Chen PJ. Gender disparity of hepatocellular carcinoma: the roles of sex hormones. Oncology. 2010;78(Suppl 1):172-9
5. Bertoletti A, Kennedy PTF, Durantel D. HBV infection and HCC: the 'dangerous liaisons'. Gut. 2018;67:787-8
6. Tian YE, Xie XU, Lin Y, Tan G, Zhong WU. Androgen receptor in hepatocarcinogenesis: Recent developments and perspectives. Oncol Lett. 2015;9:1983-8
7. Zhang H, Li XX, Yang Y, Zhang Y, Wang HY, Zheng XFS. Significance and mechanism of androgen receptor overexpression and androgen receptor/mechanistic target of rapamycin cross-talk in hepatocellular carcinoma. Hepatology. 2018;67:2271-86
8. Wang SH, Chen PJ, Yeh SH. Gender disparity in chronic hepatitis B: Mechanisms of sex hormones. J Gastroenterol Hepatol. 2015;30:1237-45
9. Wang SH, Yeh SH, Lin WH, Wang HY, Chen DS, Chen PJ. Identification of androgen response elements in the enhancer I of hepatitis B virus: a mechanism for sex disparity in chronic hepatitis B. Hepatology. 2009;50:1392-402
10. Jiang L, Shan J, Shen J, Wang Y, Yan P, Liu L. et al. Androgen/androgen receptor axis maintains and promotes cancer cell stemness through direct activation of Nanog transcription in hepatocellular carcinoma. Oncotarget. 2016;7:36814-28
11. Groupe d'Etude et de Traitement du Carcinome H. Randomized trial of leuprorelin and flutamide in male patients with hepatocellular carcinoma treated with tamoxifen. Hepatology. 2004;40:1361-9
12. Di Maio M, Daniele B, Pignata S, Gallo C, De Maio E, Morabito A. et al. Is human hepatocellular carcinoma a hormone-responsive tumor? World J Gastroenterol. 2008;14:1682-9
13. Shi L, Lin H, Li G, Jin RA, Xu J, Sun Y. et al. Targeting Androgen Receptor (AR)->IL12A Signal Enhances Efficacy of Sorafenib plus NK Cells Immunotherapy to Better Suppress HCC Progression. Mol Cancer Ther. 2016;15:731-42
14. Miura K, Ishioka M, Minami S, Horie Y, Ohshima S, Goto T. et al. Toll-like Receptor 4 on Macrophage Promotes the Development of Steatohepatitis-related Hepatocellular Carcinoma in Mice. J Biol Chem. 2016;291:11504-17
15. Miura K, Ohnishi H. Role of gut microbiota and Toll-like receptors in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:7381-91
16. Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I. et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504-16
17. Li W, Xiao J, Zhou X, Xu M, Hu C, Xu X. et al. STK4 regulates TLR pathways and protects against chronic inflammation-related hepatocellular carcinoma. J Clin Invest. 2015;125:4239-54
18. Uthaya Kumar DB, Chen CL, Liu JC, Feldman DE, Sher LS, French S. et al. TLR4 Signaling via NANOG Cooperates With STAT3 to Activate Twist1 and Promote Formation of Tumor-Initiating Stem-Like Cells in Livers of Mice. Gastroenterology. 2016;150:707-19
19. Sepehri Z, Kiani Z, Kohan F, Alavian SM, Ghavami S. Toll like receptor 4 and hepatocellular carcinoma; A systematic review. Life Sci. 2017;179:80-7
20. Lin A, Wang G, Zhao H, Zhang Y, Han Q, Zhang C. et al. TLR4 signaling promotes a COX-2/PGE2/STAT3 positive feedback loop in hepatocellular carcinoma (HCC) cells. Oncoimmunology. 2016;5:e1074376
21. Shi G, Wang C, Zhang P, Ji L, Xu S, Tan X. et al. Donor Polymorphisms of Toll-like Receptor 4 rs1927914 Associated with the Risk of Hepatocellular Carcinoma Recurrence Following Liver Transplantation. Arch Med Res. 2017;48:553-60
22. Xu D, Han Q, Hou Z, Zhang C, Zhang J. miR-146a negatively regulates NK cell functions via STAT1 signaling. Cell Mol Immunol. 2017;14:712-20
23. Lan P, Zhang C, Han Q, Zhang J, Tian Z. Therapeutic recovery of hepatitis B virus (HBV)-induced hepatocyte-intrinsic immune defect reverses systemic adaptive immune tolerance. Hepatology. 2013;58:73-85
24. Shalini S, Nikolic A, Wilson CH, Puccini J, Sladojevic N, Finnie J. et al. Caspase-2 deficiency accelerates chemically induced liver cancer in mice. Cell Death Differ. 2016;23:1727-36
25. Wang Y, Cai J, Zeng X, Chen Y, Yan W, Ouyang Y. et al. Downregulation of toll-like receptor 4 induces suppressive effects on hepatitis B virus-related hepatocellular carcinoma via ERK1/2 signaling. BMC Cancer. 2015;15:821
26. Hartwell HJ, Petrosky KY, Fox JG, Horseman ND, Rogers AB. Prolactin prevents hepatocellular carcinoma by restricting innate immune activation of c-Myc in mice. Proc Natl Acad Sci U S A. 2014;111:11455-60
27. van de Wijngaart DJ, Dubbink HJ, van Royen ME, Trapman J, Jenster G. Androgen receptor coregulators: recruitment via the coactivator binding groove. Mol Cell Endocrinol. 2012;352:57-69
28. Heemers HV, Tindall DJ. Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev. 2007;28:778-808
29. Shi L, Yan P, Liang Y, Sun Y, Shen J, Zhou S. et al. Circular RNA expression is suppressed by androgen receptor (AR)-regulated adenosine deaminase that acts on RNA (ADAR1) in human hepatocellular carcinoma. Cell Death Dis. 2017;8:e3171
30. Shi L, Lin H, Li G, Sun Y, Shen J, Xu J. et al. Cisplatin enhances NK cells immunotherapy efficacy to suppress HCC progression via altering the androgen receptor (AR)-ULBP2 signals. Cancer Lett. 2016;373:45-56
31. Jiang X, Kanda T, Nakamoto S, Miyamura T, Wu S, Yokosuka O. Involvement of androgen receptor and glucose-regulated protein 78 kDa in human hepatocarcinogenesis. Exp Cell Res. 2014;323:326-36
32. Ngo HKC, Kim DH, Cha YN, Na HK, Surh YJ. Nrf2 Mutagenic Activation Drives Hepatocarcinogenesis. Cancer Res. 2017;77:4797-808
33. Lin SJ, Chou FJ, Li L, Lin CY, Yeh S, Chang C. Natural killer cells suppress enzalutamide resistance and cell invasion in the castration resistant prostate cancer via targeting the androgen receptor splicing variant 7 (ARv7). Cancer Lett. 2017;398:62-9
34. Cheng MA, Chou FJ, Wang K, Yang R, Ding J, Zhang Q. et al. Androgen receptor (AR) degradation enhancer ASC-J9((R)) in an FDA-approved formulated solution suppresses castration resistant prostate cancer cell growth. Cancer Lett. 2018;417:182-91
Author contact
![]() Corresponding author: Jian Zhang, M.D, Ph.D, Institute of Immunopharmaceutical Sciences, School of Pharmaceutical Sciences, Shandong University, Jinan 250012, Shandong, China, E-mail: zhangj65edu.cn, Tel:86-531-88383781, Fax: 86-531-88383782.
Corresponding author: Jian Zhang, M.D, Ph.D, Institute of Immunopharmaceutical Sciences, School of Pharmaceutical Sciences, Shandong University, Jinan 250012, Shandong, China, E-mail: zhangj65edu.cn, Tel:86-531-88383781, Fax: 86-531-88383782.

 Global reach, higher impact
Global reach, higher impact