Impact Factor
ISSN: 1837-9664
J Cancer 2020; 11(5):1240-1249. doi:10.7150/jca.38286 This issue Cite
Research Paper
TRIM67 Promotes the Proliferation, Migration, and Invasion of Non-Small-Cell Lung Cancer by Positively Regulating the Notch Pathway
1. Department of Pathology, First Affiliated Hospital and College of Basic Medical Sciences, China Medical University, Shenyang, China.
2. Department of Thoracic Surgery, Shengjing Hospital, China Medical University, No. 36 Sanhao St., Heping District, Shenyang, China.
Received 2019-1-27; Accepted 2019-2-9; Published 2020-1-1
Abstract
Tripartite motif-containing 67 (TRIM67), an E3 ubiquitin ligase, belongs to the TRIM protein family. The relationship between TRIM67 and tumorigenesis is not fully clear. Here, we elucidated TRIM67 function in non-small cell lung cancer (NSCLC). TRIM67 immunostaining results were correlated with clinicopathological features. Moreover, the function of TRIM67 in cultured NSCLC cells was evaluated by MTT, colony formation, and Transwell assays. TRIM67 expression was associated with tumor size, lymph node metastasis, p-TNM stage, cancer cell differentiation, and poor prognosis. We altered TRIM67 expression in A549 and H1299 cell lines, and the results showed that TRIM67 promoted cell proliferation, migration, invasion and EMT by positively regulating the Notch pathway. Collectively, the results showed that TRIM67 promotes NSCLC progression through the Notch pathway and that TRIM67 expression is associated with clinicopathological features, indicating that TRIM67 may play an important role in promoting the development of NSCLC and could be applied as not only an important prognostic biomarker but also a therapeutic target in NSCLC.
Keywords: non-small-cell lung cancer, TRIM67, cell proliferation, cell migration, cell invasion, Notch pathway
Introduction
Lung cancer is one of the leading causes of death worldwide, is closely associated with lung disease, and its incidence continues to rise [1-5]. The 5-year survival rate remains at 15% and the prognosis of non-small cell lung cancer (NSCLC) patients is mainly related to tumor metastasis, which is related to transcriptional regulation of some key genes [5, 6-8]. Therefore, understanding the mechanism of NSCLC progression is very important for the treatment of NSCLC.
The Notch pathway is involved in development and cell proliferation, differentiation, and apoptosis. As a result of its multiple effects on tissue homeostasis and cancer, this signaling pathway has attracted increasing attention as a potential therapeutic target. Notch proteins belong to a highly conserved family of cell surface receptors. Ligand binding leads to the complex proteolysis of these receptors by the γ-secretase complex, followed by translocation of the active intracellular incision nuclei and transcriptional activation domains [9]. Notch signaling is activated by a ligand that binds to a receptor, initiating an intercellular communication system of five ligands (Delta-like 1, Delta-like 3, Delta-like 4, Jagged-1, and Jagged-2) and four receptors (Notch1-4). Ligand binding causes conformational changes of Notch, resulting in the exposure of the S2 site and sequence cleavage by the protease, disintegrase, a member of the metalloproteinase family and gamma secretase complex, to release the Notch intracellular domain (NICD). Finally, NICD is transferred to the nucleus, where it induces the target gene [10, 11] Yitong et al. 2018).
Tripartite motif-containing (TRIM) proteins are characterized by a common domain consisting of a RING finger, one or two B-box motifs and a coiled-coil motif, and are involved in many biological processes, including innate immunity, viral infection, cancer, and development [12-14]. TRIM67 belongs to the tripartite motif (TRIM) protein family [15]. Therefore, TRIM67 may be a specific E3 ubiquitin ligase, which is significant for target protein ubiquitin-dependent degradation or stabilization [16]. TRIM67 was reported to be associated with the nervous system [17-19], but its function in tumors has not been reported.
In this study, we demonstrated that TRIM67 promotes the proliferation, migration, invasion and EMT of NSCLC cells through the Notch pathway. Additionally, we detected the expression of TRIM67 in NSCLC tissues and cell lines by immunohistochemistry and western blotting and altered the expression of TRIM67 in NSCLC cells to study the variations of the cancer-related phenotype and determine its role in NSCLC.
Methods
Specimens and patient data
These assays were performed as described previously [5]. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards, and informed consent was obtained from all individual participants.
Immunohistochemistry
These assays were performed as described previously [5].
Cell culture
These assays were performed as described previously [5].
Plasmid construction, transfection, and administration of Notch pathway inhibitors
According to the manufacturer's instructions, Lipofectamine 3000 reagent was largely used for cell transfection (Invitrogen, Carlsbad, CA, USA). We used short hairpin RNA (shRNA) targeting TRIM67 to knockdown the expression of TRIM67, which was cloned into the SGU6 vector (Sangon Biotech, Shanghai), for 48 h. For upregulating the expression of TRIM67, we used TRIM67-expression plasmid and the corresponding empty pCMVNeo04 vector (LongqianBiotech, Shanghai, China).
To inhibit Notch signaling, we treated cells with 4 µM DAPT (MedChemExpress, Monmouth Junction, NJ, USA), a γ-secretase inhibitor blocking the Notch pathway, in dimethyl sulfoxide (DMSO), and an equal amount of DMSO was accordingly supplied to cells as a control 24 h after transfection.
Immunocytochemistry
Lung cancer cells cultured in 24-well plates for 24 h were immobilized in paraformaldehyde (4%) for 15 min and permeated with 0.1% Triton X-100 for 10 min. Next, the cells were washed with PBS and then blocked in bovine serum albumin (5%) for 1 h and incubated with anti-TRIM67 antibody (1:50) at 4 °C overnight. Thereafter, the cells were incubated with the secondary antibody, TRITC binding antibody, for 2 h; cell nuclei were re-stained with DAPI, and images were captured using the Olympus FV1000 laser scanning confocal microscope (Olympus, Tokyo, Japan).
Western blotting
Western blotting revealed several functional proteins that directly affect cell migration, cell invasion [28-30], and cell proliferation [31, 32]. Cellular proteins were extracted using lysis buffer (P0013; Beyotime Biosciences, Shanghai, China) containing protease-inhibitor cocktail (B14002; Biotool, Shanghai, China). Proteins were quantified using the Bradford method. Approximately 60 µg of each protein was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis on 10% gels, transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA), blocked in 5% skim milk (232100; Becton Dickenson, Franklin Lakes, NJ, USA) at 28 ºC, and incubated with antibodies (Table 1) overnight at 4 ºC. Membranes were then washed and incubated with horseradish peroxidase-conjugated anti-mouse/rabbit IgG (1:2000; ZSGB-BIO, Beijing, China) at 37 ºC for 2 h. Finally, immunoreactivity was detected using the BioImaging system (UVP, Inc., Upland, CA, USA).
Cell migration and invasion analyses
These assays were performed as described previously [5].
Cell proliferation and colony formation assays
These assays were performed as described previously [33].
Statistical analysis
Statistical analyses were mostly performed using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). Survival rates were analyzed by the Kaplan-Meier method and compared by the log rank test (Kaplan-Meier Plotter. Available from URL: http://www.kmplot.com/analysis/index.php?p=service&cancer=lung) [34]. Student's t-test was used to analyze statistically significant differences between the expression of TRIM67 and clinical features, and P < 0.05 was considered statistically significant.
Results
TRIM67 correlates with clinicopathological characteristics and poor prognosis
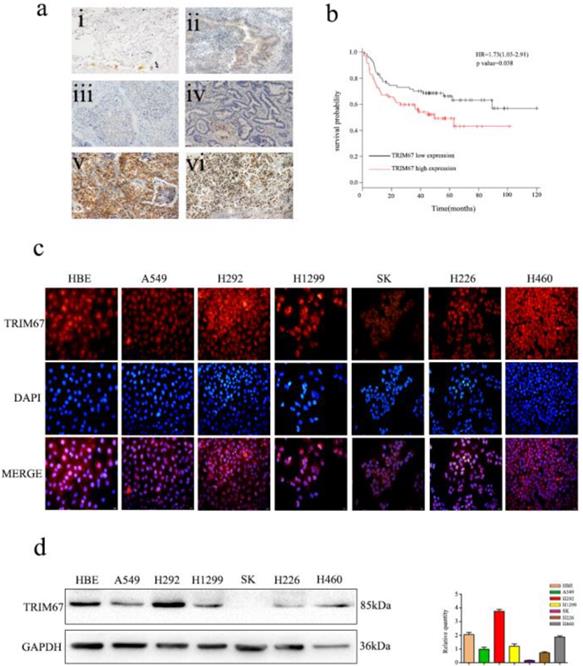
Immunofluorescence analysis of seven NSCLC cells showed that TRIM67 was localized in both the cytoplasm and nucleus (Fig. 1c). Moreover, immunohistochemical analysis showed that TRIM67 expression in low-differentiated NSCLC tissues was significantly higher than that in high-differentiated NSCLC tissues (Fig. 1a). Next, we investigated whether TRIM67 protein levels were associated with clinical features. The results showed that TRIM67 levels were positively correlated with differentiation (P < 0.001), tumor size (P = 0.018), lymph node metastasis (P < 0.001), and pathological TNM stage (P < 0.001), but not age (P = 0.424), sex (P = 0.328), and histological type (P = 0.514) as shown in Table 2. Moreover, high expression of TRIM67 was associated with lower survival in NSCLC patients according to the Kaplan-Meier database (Fig. 1b). Based on the above results, we suggest that TRIM67 might have an important effect on the biological functions of NSCLC.
Expression of TRIM67 in non-small cell lung cancer (NSCLC) is associated with poor clinical prognosis. a TRIM67 protein levels were analyzed by immunohistochemistry in (i) alveolar cells, (ii) normal bronchial epithelial cells, (iii) well-differentiated squamous cell carcinoma, (iv) well-differentiated adenocarcinoma, (v) poorly differentiated squamous cell carcinoma, (vi) poorly differentiated adenocarcinoma. b Survival of NSCLC patients with high and low TRIM67 expression based on Kaplan-Meier curves. Hazard ratio (HR) and P value are indicated. c Immunofluorescence assays were performed to detect TRIM67 localization in human normal bronchial epithelial and NSCLC cell lines. TRIM67 was localized to the cytoplasm and nucleus. d TRIM67 levels in HBE cells and six NSCLC cell lines according to western blot analysis. Relative quantification analysis was based on gray scale values.
List of antibodies used for western blotting in the study.
| Antibody name | Source | Catalog number | Host | Dilution |
|---|---|---|---|---|
| TRIM67 TRIM67 | Sigma-Aldrich Sigma-Aldrich | HPA034776 SAB2103188 | Rabbit Rabbit | 1:50 1:100 |
| GAPDH | Beyotime | AF0006 | Mouse | 1:1000 |
| RhoA RhoC | Cell Signaling Technology Inc Cell Signaling Technology Inc | 2117 3430 | Rabbit Rabbit | 1:500 1:500 |
| MMP9 | Cell Signaling Technology Inc | 13667 | Rabbit | 1:500 |
| P21 | Cell Signaling Technology Inc | 2947 | Rabbit | 1:1000 |
| c-myc | Cell Signaling Technology Inc | 13987 | Rabbit | 1:1000 |
| E-Cadherin | Proteintech | 20874 | Rabbit | 1:500 |
| N-Cadherin | Proteintech | 22018 | Rabbit | 1:500 |
| Vimentin Snail Slug Jagged1 Notch1 NICD | Proteintech Cell Signaling Technology Inc Cell Signaling Technology Inc Cell Signaling Technology Inc Cell Signaling Technology Inc Wanlei Bio | 10366 3879 9585 2620 3608 WL03097a | Rabbit Rabbit Rabbit Rabbit Rabbit Rabbit | 1:500 1:500 1:500 1:500 1:500 1:500 |
| HA | TransGen Biotech | HT301 | Mouse | 1:1000 |
Western blot analysis was also performed to determine the expression of TRIM67 in HBE normal bronchial epithelial cells and six NSCLC cell lines (Fig. 1d), and then we selected A549 and H1299 cell lines for subsequent studies because TRIM67 was moderately expressed in these two cell lines.
Association between TRIM67 expression and clinicopathological characteristics of non-small cell lung cancer patients.
| Clinicopathological characteristics | Total N | TRIM67-positive | TRIM67-negative | P value |
|---|---|---|---|---|
| Age (years) | ||||
| ≤ 60 | 148 | 81 | 67 | |
| > 60 | 106 | 70 | 36 | 0.424 |
| Sex | ||||
| Male | 143 | 78 | 65 | |
| Female | 111 | 44 | 67 | 0.328 |
| Histological type | ||||
| Squamous cell carcinoma | 106 | 60 | 46 | |
| Adenocarcinoma | 148 | 88 | 60 | 0.514 |
| Differentiation | ||||
| Well-moderate | 127 | 44 | 83 | |
| Poor | 127 | 74 | 53 | < 0.001 |
| Tumor size (cm) | ||||
| ≤ 5 | 224 | 98 | 126 | |
| > 5 | 30 | 20 | 10 | 0.018 |
| Lymph node metastasis | ||||
| Negative | 150 | 52 | 98 | |
| Positive | 104 | 66 | 38 | < 0.001 |
| TNM stage | ||||
| I | 148 | 50 | 98 | |
| II-III | 106 | 67 | 39 | < 0.001 |
TRIM67 expression promotes migration and invasion of NSCLC cells
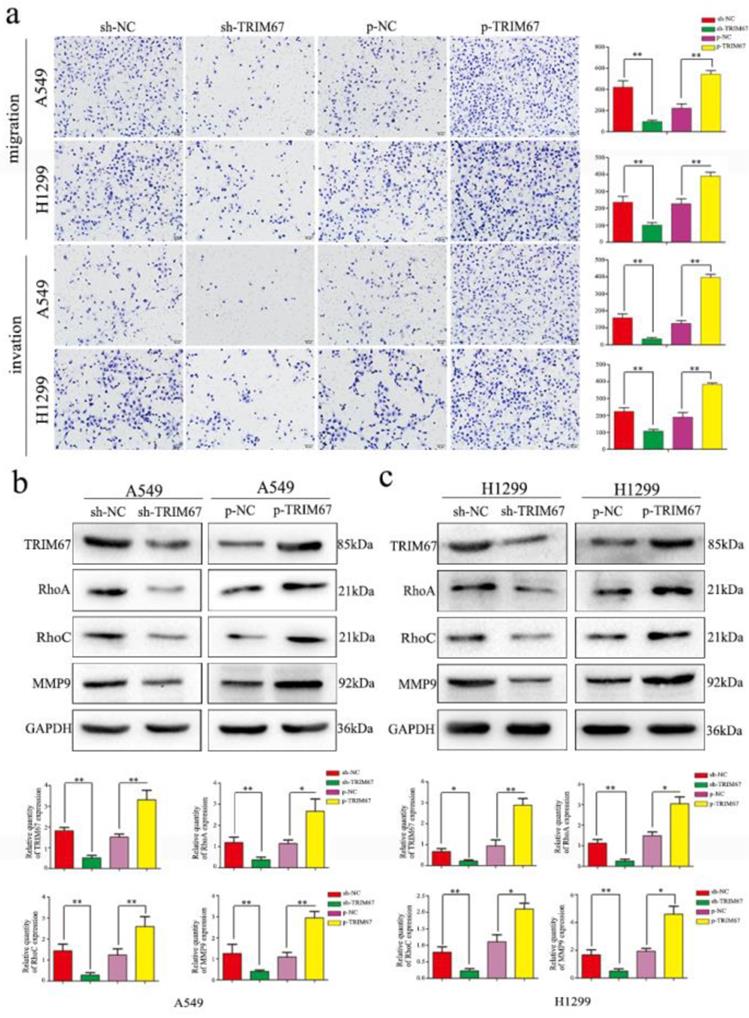
Based on the previously mentioned findings, we next studied the effect of TRIM67 protein in NSCLC. To this end, we altered TRIM67 expression by downregulation or upregulation in both A549 and H1299 cell lines. We found that TRIM67 knockdown inhibited the migration and invasion of A549 and H1299 cells as compared to that of control cells through Transwell assays, whereas migration and invasion were promoted by TRIM67 overexpression (P < 0.05; Fig. 2a). Further, alteration of TRIM67 protein expression had an effect on the expression of proteins related to cell migration and invasion, consistent with the aforementioned results. Transfection efficiency was also examined by western blotting 48 h post-transfection. Upon TRIM67 upregulation, the levels of RhoA, RhoC, and matrix metalloproteinase (MMP)-9 increased, whereas its downregulation exerted the opposite effect (Fig. 2b and c). Consistent with our immunohistochemical results, these results suggested that TRIM67 enhances the migration and invasion of NSCLC cells.
TRIM67 expression promotes the proliferation of NSCLC cells
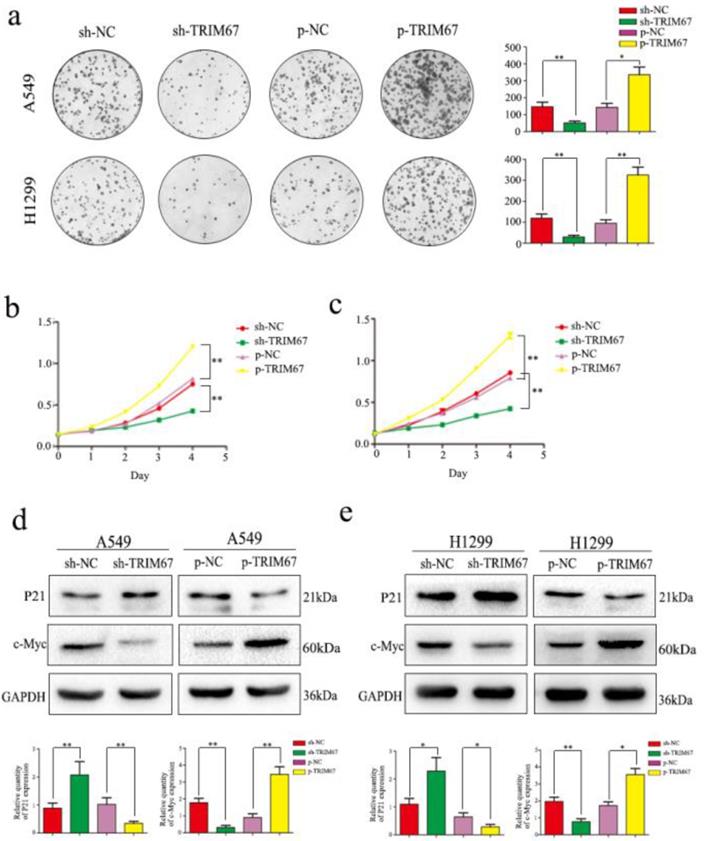
We next analyzed the effect of TRIM67 expression on cell proliferation. Colony formation analysis clearly demonstrated that TRIM67 expression and colony-formation ability of tumor cells were positively correlated in A549 and H1299 cells; specifically, downregulation inhibited clone formation, whereas upregulation had the opposite effect, when compared to that in respective control groups (Fig. 3a). Subsequent MTT assays revealed that upregulation of TRIM67 expression enhanced cell proliferation, whereas downregulation had an opposite effect in A549 cells (Fig. 3b), similar to the observation in H1299 cells (Fig. 3c). Consistent with these observations, TRIM67 overexpression promoted the proliferation of NSCLC cells, which also agreed with our immunohistochemical results showing that TRIM67 levels are associated with tumor size. We next analyzed the proliferation-related proteins, and the results showed that when TRIM67 was upregulated, the expression of c-Myc was also upregulated, whereas that of P21 was downregulated; knockdown of TRIM67 had the opposite effect on these proteins in both A549 and H1299 cells (Fig. 3d and e).
TRIM67 protein levels are positively correlated with non-small cell lung cancer (NSCLC) migration and invasion. a Transwell assays were performed to assess cell migration and invasion after TRIM67 overexpression and downregulation. TRIM67 overexpression in A549 and H1299 cells enhanced cell migration and invasion, whereas TRIM67 knockdown inhibited these processes. *P < 0.05; **P < 0.01. b, c Effects of TRIM67 levels on the expression of proteins associated with cell migration and invasion in A549 and H1299 cells. Relative quantification analysis was based on gray scale values. *P < 0.05; **P < 0.01.
TRIM67 positively regulates the expression of proteins related to epithelial-mesenchymal transition (EMT)
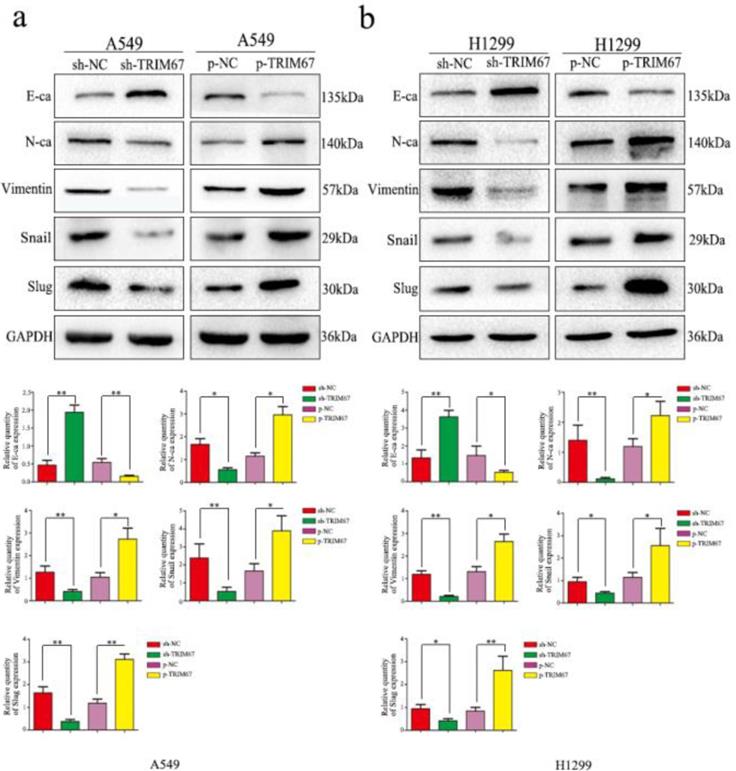
To elucidate the mechanism by which TRIM67 enhances the migration/invasion of lung cancer cells, we detected the expression of EMT-related proteins by western blotting. Results showed that increased TRIM67 expression enhanced N-cadherin, vimentin, Snail, and slug expression, but suppressed E-cadherin in A549 cells (Fig. 4a), similar to the observation in H1299 cells (Fig. 4b). These results revealed that TRIM67 can affect the migration and invasion of NSCLC cells by promoting epithelial-mesenchymal transition(EMT).
TRIM67 protein levels are positively correlated with non-small cell lung cancer (NSCLC) cell proliferation. a Colony formation assays were performed to assess cell proliferation after TRIM67 overexpression and downregulation. TRIM67 overexpression in A549 and H1299 cells enhanced cell proliferation, whereas TRIM67 knockdown inhibited cell proliferation. *P < 0.05; **P < 0.01. b, c MTT assays were performed to confirm that TRIM67 overexpression enhances cell proliferation and that TRIM67 knockdown inhibits this process. *P < 0.05; **P < 0.01. d, e The effects of TRIM67 upregulation or downregulation on the expression of proteins associated with cell proliferation in A549 and H1299 cells. Relative quantification analysis was based on gray scale values. *P < 0.05; **P < 0.01.
Effects of TRIM67 levels on the expression of epithelial-mesenchymal transition (EMT)-related proteins. a A549 and b H1299 cells were transfected with expression or shRNA vectors. Relative quantification analysis was based on gray scale values. *P < 0.05; **P < 0.01.
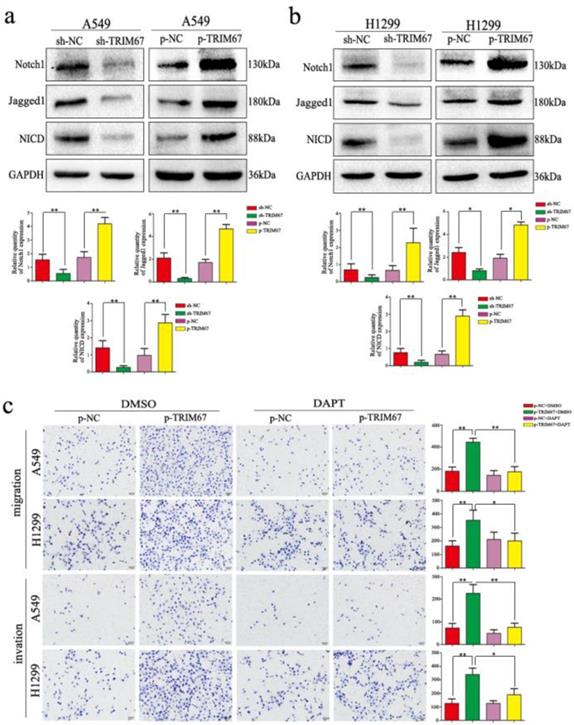
TRIM67 regulates migration, invasion, cell proliferation, and EMT of NSCLC cells through the Notch pathway
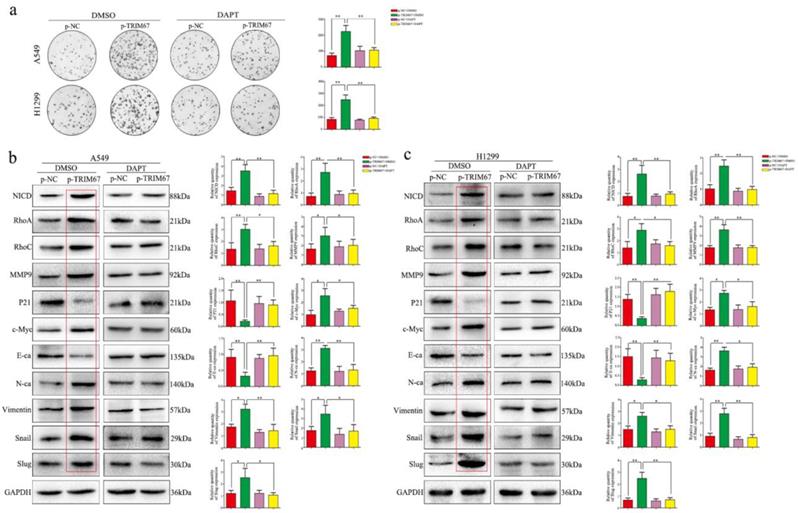
We clarified the role of TRIM67 in NSCLC cell migration, invasion, and proliferation and then investigated the biological mechanisms related to these effects. The Notch pathway is involved in the development of several cancers by influencing the expression of EMT-related proteins and other factors found to be altered by TRIM67 modulation; therefore, we examined whether TRIM67 is involved in the regulation of Notch signaling in NSCLC cells. Western blotting results showed that TRIM67 knockdown downregulated the expression of Notch1, Jagged1, and NICD in A549 and H1299 cell lines, whereas overexpression had the opposite effects (Fig. 5a and b). To investigate whether the function of TRIM67 was mediated through the Notch pathway, we used the inhibitor, DAPT, which inhibits NICD expression. DAPT inhibited cell migration, invasion, and proliferation in A549 and H1299 cells transfected with p-trim67 (Fig. 5c and 6a), and the effect of TRIM67 overexpression on protein expression of A549 and H1299 cells was reversed (Fig. 6b and c). These results indicated that activation of the Notch pathway contributes to TRIM67-mediated migration, invasion, and proliferation in NSCLC cells.
Discussion
TRIM67 is a protein that belongs to TRIM family and is localized to the cytoplasm and nucleus. Here, we demonstrated that TRIM67 is expressed in NSCLC and multiple lung cancer cell lines.
In this study, we showed that the expression of TRIM67 was associated with a range of clinicopathological features, such as tumor size, differentiation, lymph node metastasis, p-TNM staging, and prognosis. We also demonstrated that TRIM67 promotes the proliferation, migration, invasion and EMT of NSCLC cells through the Notch pathway and the expression of downstream proteins such as RhoA, RhoC, MMP-9, P21, c-myc, and EMT-related markers [17, 20, 21], thereby influencing the malignant behavior of tumor cells. However, the mechanisms involved in these functions are unclear, and further studies are needed.
The role of the Notch pathway in cancers has been determined according to the effect of Notch pathway on cell proliferation, migration, and invasion [22-26]. In the present study, we demonstrated that TRIM67 overexpression can upregulate Notch1, Jagged1, and NICD. NICD translocates to the nucleus, where it binds to several transcription factors and regulates the expression of target genes. The γ-secretase inhibitor DAPT blocks the Notch pathway by reducing the formation of NICD [27]. When we added DAPT, the effects of TRIM67 on NSCLC functions were reversed, which confirmed that TRIM67 functions through the regulation of Notch signaling.
TRIM67 modulates Notch signaling to regulate cell migration and invasion in non-small cell lung cancer. a, b Changes in the expression of Notch-related proteins in a A549 and b H1299 cells. Relative quantification analysis was based on gray scale values. *P < 0.05; **P < 0.01. c Changes in the migration and invasion capacity of A549 and H1299 cells in the presence or absence of treatment with the Notch inhibitor DAPT and transfection with p-TRIM67. *P < 0.05; **P < 0.01.
Notch signaling mediates the effects of TRIM67 on proliferation and epithelial-mesenchymal transition (EMT). a Changes in the proliferation activity of A549 and H1299 cells in the presence or absence of the Notch inhibitor DAPT after transfection with p-TRIM67. *P < 0.05; **P < 0.01. b, c Effect of TRIM67 in the presence or absence of Notch inhibition on protein expression in non-small cell lung cancer (NSCLC) cells. b A549 and c H1299 cells transfected with p-TRIM67 were treated with or without the Notch inhibitor DAPT and analyzed by western blotting for the expression of proteins involved in cell migration, invasion, proliferation, and EMT. Relative quantification analysis was based on gray scale values. *P< 0.05; **P< 0.01.
However, in this study, we did not confirm how TRIM67 acts on the Notch pathway. TRIM67 is an E3 ubiquitin ligase, so we speculate that this pathway is activated by ubiquitination of Notch pathway-inhibiting proteins. Even if this is true, it is still unclear which domain and binding site of TRIM67 are involved in this process, and thus further studies are needed.
In conclusion, our study demonstrated that TRIM67 plays a significant role in NSCLC via the Notch pathway, suggesting that TRIM67 might be a candidate prognostic biomarker and possible therapeutic target for NSCLC. Even though further studies are needed to determine the functions of TRIM67 in these biological processes, our results offer significant views and reflections about the functions and mechanisms of TRIM67 in tumorigenesis.
Abbreviations
EMT: epithelial-mesenchymal transition; FBS: fetal bovine serum; IP: immunoprecipitation; NICD: notch intracellular domain; NSCLC: Non-small cell lung cancer; TRIM: tripartite motif.
Acknowledgements
This work was supported by the Liaoning Province Colleges and Universities Innovation Team (LC2015029), Liaoning Provincial Education Department. We would like to thank Editage (www.editage.cn) for English-language editing.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Travis WD. Pathology of lung cancer. Clin Chest Med. 2002;23:65-81
2. Torre LA, Bray F, Siegel RL. et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108
3. Chen W, Zheng R, Baade PD. et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-32
4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30
5. Jiang J, Xu Y, Ren H. et al. MKRN2 inhibits migration and invasion of non-small-cell lung cancer by negatively regulating the PI3K/Akt pathway. J Exp Clin Cancer Res. 2018;37:189
6. Minna JD, Roth JA, Gazdar AF. Focus on lung cancer. Cancer Cell. 2002;1:49-52
7. Schiller JH, Harrington D, Belani CP. et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92-8
8. Jemal A, Siegel R, Ward E. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-49
9. Sandy AR, Maillard I. Notch signaling in the hematopoietic system. Expert Opin Biol Ther. 2009;9:1383-98
10. Andersson ER, Lendahl U. Therapeutic modulation of Notch signalling-are we there yet? Nat Rev Drug Discov. 2014;13:357-78
11. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674
12. Meroni G, Diez-Roux G. TRIM/RBCC, a novel class of 'single protein RING finger' E3 ubiquitin ligases. Bioessays. 2005;27:1147-57
13. Nisole S, Stoye JP, Saïb A. TRIM family proteins: retroviral restriction and antiviral defence. Nat Rev Microbiol. 2005;3:799-808
14. Yaguchi H, Okumura F, Takahashi H. et al. TRIM67 protein negatively regulates Ras activity through degradation of 80K-H and induces neuritogenesis. J Biol Chem. 2012;287:12050-59
15. Hatakeyama S. TRIM proteins and cancer. Nat Rev Cancer. 2011;1:792-804
16. Montell DJ. TRIMingneural connections with ubiquitin. Dev Cell. 2019;48:5-6
17. Hanna S, El-Sibai M. Signaling networks of Rho GTPases in cell motility. Cell Signal. 2013;25:1955-61
18. Boyer NP, Monkiewicz C, Menon S. et al. Mammalian TRIM67 functions in brain development and behavior. eNeuro. 2018:5
19. Do LD, Gupton SL, Tanji K. et al. TRIM9 and TRIM67 are new targets in paraneoplastic cerebellar degeneration. Cerebellum. 2018;2:245-54
20. Vandooren J, Van den Steen PE, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): the next decade. Crit Rev Biochem Mol Biol. 2013;48:222-72
21. Zheng H, Kang Y. Multilayer control of the EMT master regulators. Oncogene. 2014;33:1755-63
22. Belin de Chantemèle EJ, Retailleau K. et al. Notch3 is a major regulator of vascular tone in cerebral and tail resistance arteries. Arterioscler Thromb Vasc Biol. 2008;28:2216-24
23. Wang Z, Li Y, Kong D. et al. The role of Notch signaling pathway in epithelial-mesenchymal transition (EMT) during development and tumor aggressiveness. Curr Drug Targets. 2010;100:745-51
24. Cialfi S, Palermo R, Manca S. et al. Loss of Notch1-dependent p21 (Waf1/Cip1) expression influences the Notch1 outcome in tumorigenesis. Cell Cycle. 2014;13:2046-55
25. Ji Y, Chen S, Xiang B. et al. Jagged1/Notch3 signaling modulates hemangioma-derived pericyte proliferation and maturation. Cell Physiol Biochem. 2016;40:895-907
26. Lai XX, Li G, Lin B. et al. Interference of Notch 1 inhibits the proliferation and invasion of breast cancer cells: Involvement of the β catenin signaling pathway. Mol Med Rep. 2018;17:2472-8
27. Jiang J, Miao Y, Xiao S. et al. DAPT in the control of human hair follicle stem cell proliferation and differentiation. Postepy Dermatol Alergol. 2014;31:201-6
28. Spiering D, Hodgson L. Dynamics of the Rho-family small GTPases in actin regulation and motility. Cell Adh Migr. 2011;5:170-80
29. Bravo-Cordero JJ, Hodgson L, Condeelis J. Directed cell invasion and migration during metastasis. Curr Opin Cell Biol. 2012;24:277-83
30. Gong L, Wu D, Zou J. et al. Prognostic impact of serum and tissue MMP-9 in non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget. 2016;7:18458-68
31. Sherr CJ. D-type cyclins. Trends Biochem Sci. 1995;20:187-90
32. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-74
33. Dong QZ, Wang Y, Tang ZP. et al. Derlin-1 is overexpressed in non-small cell lung cancer and promotes cancer cell invasion via EGFR-ERK-mediated up-regulation of MMP-2 and MMP-9. Am J Pathol. 2013;182:954-64
34. Gyorffy B, Surowiak P, Budczies J. et al. Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS One. 2013;8:e8224
Author contact
![]() Corresponding author: Department of Pathology, First Affiliated Hospital and College of Basic Medical Sciences, China Medical University, No. 155 Nanjing North Street, Heping District, Liaoning, Shenyang 110001, China. Tel: +86-13386881964 E-mail: xsqiuedu.cn
Corresponding author: Department of Pathology, First Affiliated Hospital and College of Basic Medical Sciences, China Medical University, No. 155 Nanjing North Street, Heping District, Liaoning, Shenyang 110001, China. Tel: +86-13386881964 E-mail: xsqiuedu.cn

 Global reach, higher impact
Global reach, higher impact