3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2020; 11(18):5511-5517. doi:10.7150/jca.46414 This issue Cite
Research Paper
Value of combining PET/CT and clinicopathological features in predicting EGFR mutation in Lung Adenocarcinoma with Bone Metastasis
1. Department of Internal Oncology, Shanghai Jiao Tong University Affiliated Sixth People's Hospital, Shanghai, 200030, China.
2. Department of Radiology, Shanghai Jiao Tong University Affiliated Sixth People's Hospital, Shanghai, 200030, China.
3. Department of Nuclear Medicine, Shanghai Jiao Tong University Affiliated Sixth People's Hospital, Shanghai, 200030, China.
*These authors contributed equally to this work.
Received 2020-3-27; Accepted 2020-6-29; Published 2020-7-11
Abstract
Purpose: Epidermal growth factor receptor (EGFR) mutation is the most common target for precision treatment in metastatic lung adenocarcinoma. We investigated the predictive role of 18F-FDG PET/CT and clinicopathological features for EGFR mutations in lung adenocarcinoma with bone metastasis.
Methods: Seventy-five lung adenocarcinoma patients with histologically confirmed bone metastasis were included. They all received EGFR status test and PET/CT before systemic treatment. The differences of maximum standardized uptake value (SUVmax) in primary tumor (pSUVmax), regional lymph node (nSUVmax) and bone metastasis (bmSUVmax) between different EGFR status groups were compared, alongside with common clinicopathological features. Multivariate logistic regression analysis was performed to evaluate predictors of EGFR mutations.
Results: EGFR mutations were found in 37 patients (49.3%). EGFR mutations were more common in females, non-smokers, expression of Thyroid Transcription Factor-1 (TTF-1) and NaspinA. Low bmSUVmax was significantly associated with EGFR mutations, while no significant difference was observed in pSUVmax and nSUVmax. Multivariate analysis showed that bmSUVmax ≤7, non-smoking, expression of TTF-1 were predictors of EGFR mutations. The area under the curve (AUC) of receiver operating characteristic (ROC) curve was 0.84 for the combination of the three factors.
Conclusion: Low bmSUVmax is more frequently in EGFR mutations, and bmSUVmax is an independent predictor of EGFR mutations. Combining bmSUVmax with other clinicopathological features could forecast the EGFR status in lung adenocarcinoma with unavailable EGFR gene testing.
Keywords: Adenocarcinoma, Bone metastasis, EGFR mutations, PET/CT, SUVmax
Introduction
Precision medicine has become a prevailing approach in the treatment of malignant tumors [1]. A typical representative is the EGFR mutations [2], which is the most common druggable target in non-small cell lung cancer (NSCLC) [3]. In metastatic NSCLC with EGFR mutations, tyrosine kinase inhibitors (TKI) have been established in front-line treatment status [4]. Now the molecular profiling of EGFR status in advanced NSCLC is a recommended standard [5, 6]. However, in clinical practice, a successful gene analysis depends on sufficient tumor samples of good quality, which can be sometimes difficult to obtain [7, 8]. Even in some clinical trials, effective detection rate of EGFR was no more than 40% [4, 9]. Furthermore, in a real-world study, the proportions of patients receiving TKI therapy among those with unknown EGFR mutation status could be as high as 45.8% [10]. Seeking some other predictors of EGFR status may offer assistance.
18F-FDG PET/CT is a noninvasive diagnostic and staging tool for NSCLC [11]. FDG uptake was associated with tumor invasiveness [12], meanwhile EGFR signaling regulated cell proliferation and glucose metabolic pathway in NSCLC with EGFR mutations [13]. Using FDG tumor uptake as a predictor of EGFR status could be worth exploring. Several studies have investigated the association between SUVmax and EGFR mutations [8, 14-25]. However, the results were controversial and conflicting. Previous studies included NSCLC patients with I-IV stages and various histological subtypes, which increased diversity. Furthermore, most studies focused on the SUVmax of primary lung tumor, only few of them [8, 14] discussed the relationship between metastatic FDG uptake and EGFR status. EGFR status in NSCLC may vary according to the tumor staging or histological subtypes, and heterogeneity could exist between primary and metastatic site, making results from above researches inconclusive. Therefore, in this retrospective study, we aimed to evaluate whether 18F-FDG PET/CT and common clinicopathological features could predict EGFR status in advanced lung adenocarcinoma with histologically confirmed bone metastasis.
Materials and Methods
Patients
This retrospective study included lung adenocarcinoma patients with histologically confirmed bone metastasis from November 2016 to November 2019. The study was approved by the ethics committee of our hospital and formal consent was waived. An initial 80 patients were included according to inclusion criteria: PET/CT scan indicated suspicious bone metastasis; bone biopsy confirmed metastasis from lung adenocarcinoma; available results for EGFR mutation status. Then 5 patients were excluded due to exclusion criteria: treatment before PET/CT within 6 months (2 patients); interval between PET/CT and bone biopsy exceeded 1 month (3 patients). Finally, 75 patients were included.
18F-FDG PET/CT
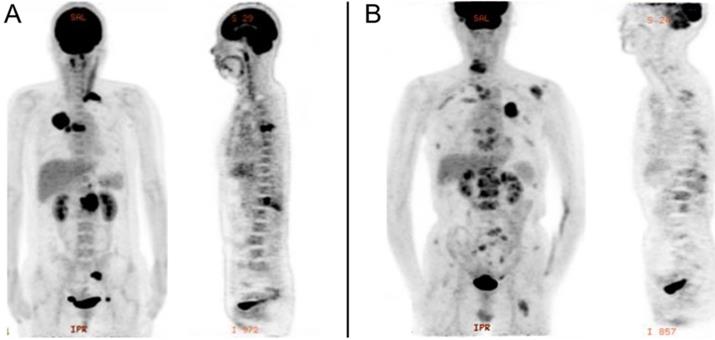
PET/CT was performed using an integrated PET/CT system (Discovery VCT; GE Medical Systems). All patients were required to fast for at least 6h and undergo a peripheral blood sugar test to avoid hyperglycemia. Approximately 1 h after the intravenous injection of 18F-FDG (3.7MBq/kg), CT was done from head to lower limbs with the following setting: 120 V and 80 mA, 64 slices, with a slice thickness of 3.75 mm. PET scans were performed with 2.5 min per bed position. Finally, the CT and PET images were reconstructed iteratively using ordered subset expectation maximization. Attenuation correction was done by unenhanced CT. A senior nuclear medicine doctor then evaluated all of the combined 18F-FDG PET/CT scans whilst blinded to the EGFR status. The region of interest (ROI) over the primary lung tumor, regional nodal metastasis and bone metastasis were drawn on PET/CT images on each transaxial slice. SUVmax was defined at the peak value on one pixel with the highest counts within the ROI. Representative image is shown in Figure 1.
PET image of stage IV lung adenocarcinoma with bone metastasis. A, EGFR-wild: primary tumor in the right upper lobe (SUVmax, 20.3), with regional node (SUVmax, 11.4), and bone metastasis (SUVmax, 16.0). B, EGFR-mutant: primary tumor in the left upper lobe (SUVmax, 9.6), with intrapulmonary (SUVmax, 2.6), distant node (SUVmax, 2.4), adrenal gland (SUVmax, 6.1), and bone metastasis (SUVmax, 6.0).
Pathological evaluation and EGFR mutation
Bone biopsy was performed in each patient. The specimen was then handled with modified Ethylene Diamine Tetraacetic Acid (EDTA) decalcification (which is good at preserving antigenicity and DNA quality), instead of traditional acid decalcification. If bone metastasis form NSCLC was confirmed by the morphology and immunohistochemistry (IHC) results of specimen, EGFR mutations were analyzed under of the amplification refractory mutation system (ARMS) using the EGFR 29 Mutations Detection Kit (Amoy Diagnostics, Xiamen, PRC).
Statistical analysis
The characteristics of included patients were compared, using Fisher's exact test for binary data and the Wilcoxon rank-sum test for continuous data. All tests were two-sided and P values less than 0.05 were considered statistically significant. ROC curve was constructed to obtain the cutoff value of bmSUVmax in predicting EGFR mutation status. Logistic regression analysis was performed to identify independent predictors of the EGFR status. Variables with p < 0.05 in the multivariate analysis were independent predictors for EGFR mutations. The ROC curves were constructed for individual predictor and combined factors in predicting EGFR mutations. Statistical analyses were performed using STATA/SE version 15.1 (StataCorp LLC USA).
Results
Patient and clinical characteristics
Among the final 75 patients, all of them received EGFR status test from bone biopsy. 66 patients received PET/CT as an initial diagnosis. 9 patients had resection of primary site before PET/CT, and then PET/CT was performed as an overall assessment (The interval between resection and PET/CT was not exceed 1.5 months). EGFR mutations were identified in 37 (49.3%). The mutations types were Exon 18 (2, 5.5%), Exon 19 (22, 59.5%), Exon 21 (13, 35%). Further clinical characteristics were summarized in Table 1.
SUVmax of tumors
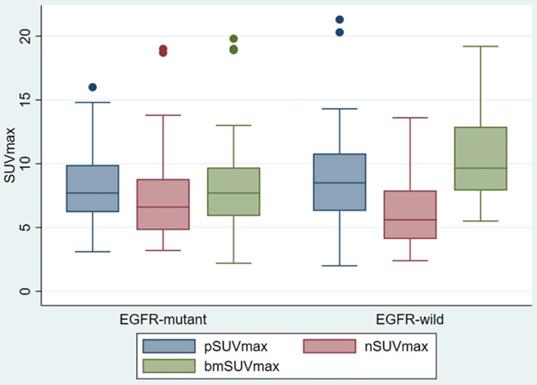
pSUVmax, nSUVmax and bmSUVmax were compared according to the EGFR status (Figure 2). There was no difference in the pSUVmax (median 7.7 vs 8.5, p < 0.454) and nSUVmax (6.6 vs 5.6, p < 0.208) between the EGFR mutant and EGFR wild groups. The EGFR mutant bone metastasis had lower bmSUVmax (median 7.7 vs 9.7, p < 0.015). The ROC curve showed the cutoff value of bmSUVmax was 7.0, with sensitivity 45.9%, specificity 84.2% and AUC 0.65 (p < 0.01).
Clinicopathological features and PET/CT parameters in study
| Characteristics | EGFR Mutant | EGFR Wild | Total | p value |
|---|---|---|---|---|
| Age (years, median) | 63 | 64.5 | 64 (37-85) | 0.767 |
| Gender | ||||
| Male/Female | 16/21 | 26/12 | 42/33 | 0.037 |
| Non-smoking | 26 (66.67%) | 13 (33.33%) | 39 (52%) | 0.003 |
| Primary tumor size (cm, median) | 3.8 | 3.2 | 3.5 | 0.979 |
| Regional node involvement | 25 (53.19%) | 22 (46.81%) | 47 (63%) | 0.476 |
| Bone metastasis | ||||
| Oligo (<5) | 12 (46.15%) | 14 (53.85%) | 26 (35%) | 0.809 |
| Multiple (≥5) | 25 (51.02%) | 24 (48.98%) | 49 (65%) | |
| Other distant organ metastasis | 8 (42.11%) | 11 (57.89%) | 19 (25.33%) | 0.597 |
| Interval between bone biopsy and PET/CT (day, median) | 6 | 4 | 5 | 0.175 |
| pSUVmax (median) | 7.7 | 8.5 | 8.3 | 0.454 |
| nSUVmax (median) | 6.6 | 5.6 | 6.4 | 0.208 |
| bmSUVmax (median) | 7.7 | 9.7 | 8.9 | 0.015 |
| CK7 | 75 (100%) | |||
| Positive | 37 (50.68%) | 36 (49.32%) | 0.493 | |
| Negative | 0 | 2 (100%) | ||
| Vilin | 72 (96%) | |||
| Positive | 11 (42.31%) | 15 (57.69%) | 0.469 | |
| Negative | 24 (52.17%) | 22 (47.83%) | ||
| TTF-1 | 75 (100%) | |||
| Positive | 35 (60.34%) | 23 (39.26%) | 0.001 | |
| Negative | 2 (11.76%) | 15 (88.24%) | ||
| NaspinA | 75 (100%) | |||
| Positive | 34 (59.65%) | 23 (40.35%) | 0.002 | |
| Negative | 3 (16.67%) | 15 (83.33%) | ||
| Ki67 | 55 (73%) | |||
| >20% | 12 (50%) | 12 (50%) | 0.789 | |
| ≤20% | 14 (45.16%) | 17 (54.84%) |
Univariate and multivariate analysis of predictors in EGFR status
| Characteristics | Univariate Analysis OR (95% CI) | p value | Multivariate Analysis OR (95% CI) | p value |
|---|---|---|---|---|
| Gender | ||||
| Female | 2.84 (1.11-7.31) | 0.030 | 0.97 (0.17-5.53) | 0.971 |
| Male | Reference | Reference | ||
| Smoking status | ||||
| Non-smoking | 4.55 (1.72-12.02) | 0.002 | 6.42 (1.17-35.28) | 0.032 |
| Smoking | Reference | Reference | ||
| bmSUVmax | ||||
| ≤7 | 4.53 (1.53-13.42) | 0.006 | 4.23 (1.02-17.49) | 0.047 |
| >7 | Reference | Reference | ||
| TTF-1 | ||||
| Positive | 11.41 (2.38-54.66) | 0.002 | 11.65 (1.69-80.21) | 0.013 |
| Negative | Reference | Reference | ||
| NaspinA | ||||
| Positive | 7.39 (1.92-28.45) | 0.004 | 2.45 (0.43-13.88) | 0.311 |
| Negative | Reference | Reference |
Prediction of the EGFR mutation status
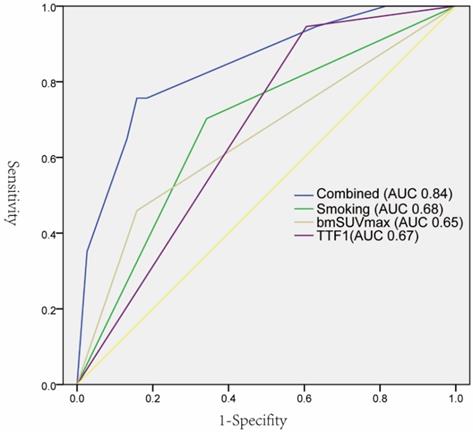
As summarized in Table 1, EGFR mutations were found more frequently in female patients (21 vs 12, p 0.037), non-smoking (66.67% vs 33.33%, p 0.003), lower bmSUVmax (7.7 vs 9.7, p 0.015), positive expression of TTF-1(60.34% vs 39.26%, p 0.001), and NaspinA (59.65% vs 40.35%, p 0.002). The univariate logistic regression analysis showed that five factors were significantly correlated with EGFR mutations. The subsequent multivariate regression analysis demonstrated that non-smoking, lower bmSUVmax, and positive expression of TTF-1 were independent predictors of EGFR mutations (Table 2). The ROC curves analysis revealed that each factor could predict EGFR mutation with AUC ranging from 0.65 to 0.68. When the three factors were combined, the AUC could reach 0.84 (Figure 3).
Discussion
EGFR mutations had a decisive role in systematic therapy of NSCLC. In this study, we found that bone metastasis from EGFR mutant lung adenocarcinoma had lower SUVmax than EGFR wild types. However, SUVmax of primary tumors and regional lymph nodes didn't seem significantly different between two EGFR status lung adenocarcinomas. Further analyses demonstrated that lower bmSUVmax was an independent predictor for EGFR mutations. When combining with other accessible factors, an AUC of predicting EGFR mutations could reach 0.84.
Box Plot of SUVmax of primary tumor, regional lymph node, and bone metastasis, according to different EGFR status.
ROC curves of individual predictors and combined factors (bmSUVmax, smoking status, and TTF-1) in predicting EGFR mutation status.
Similar studies of relationship between SUVmax and EGFR mutation status in NSCLC
| Author | N | Histology | Stage | EGFR mutation | Lesions Measured | Favor factor in mutation |
|---|---|---|---|---|---|---|
| Lee et al. [11] | 71 | ADC | IV | 48 (68%) | pSUVmax; nSUVmax; SUVmax | Low nSUVmax; mSUVmax |
| Huang et al. [12] | 77 | ADC | III-IV | 49 (64%) | pSUVmax | High pSUVmax |
| Qiang et al. [13] | 97 | ADC | I-III | 44 (45%) | pSUVmax | Low pSUVmax |
| Li et al. [14] | 115 | ADC, | I-IV | 64 (56%) | pSUVmax | Low pSUVmax |
| Minamimoto et al. [15] | 131 | ADC | I-IV | 32 (24%) | pSUVmax | Low pSUVmax |
| Ko et al. [16] | 132 | ADC | I-IV | 69 (52%) | pSUVmax | High pSUVmax |
| Zhu et al. [17] | 139 | ADC | I-IV | 74 (53%) | pSUVmax | Low pSUVmax |
| Yang et al. [18] | 200 | ADC | I-IV | 115 (58%) | pSUVmax | Low pSUVmax |
| Gu et al. [19] | 210 | ADC, non-ADC | I-IV | 70 (33%) | pSUVmax | Low pSUVmax |
| Guan et al. [20] | 316 | ADC, non-ADC | I-IV | 126 (40%) | pSUVmax | Low pSUVmax |
| Yip et al. [21] | 348 | ADC, non-ADC | I-IV | 44 (13%) | pSUVmax | Low pSUVmax |
| Kazuya et al. [22] | 734 | ADC | I-IV | 334 (46%) | pSUVmax | Low pSUVmax |
| Lv et al. [8] | 849 | ADC, non-ADC | I-IV | 371 (46%) | pSUVmax; nSUVmax; mSUVmax | Low pSUVmax; nSUVmax; mSUVmax |
ADC: adenocarcinoma; pSUVmax: SUVmax in primary tumor; nSUVmax: SUVmax in regional lymph node; mSUVmax: SUVmax in distant metastasis.
Several studies [8, 12-23] had evaluated the value of FDG uptake for predicting EGFR status in NSCLC. As described in Table 3, the results were not very consistent. Almost all previous studies solely focused on the pSUVmax. Ten of them [8, 14-16, 18-23] revealed that lower pSUVmax was associated with EGFR mutations, and three studies [8, 22, 23] found lower pSUVmax was an independent predictor for EGFR mutations by multivariate analysis. Meanwhile, two studies [15, 19] demonstrated that high pSUVmax was a significant predictor of EGFR mutations, and one [14] didn't show statistical difference in pSUVmax between different EGFR statuses. Possible explanation for these controversies may the clinicopathological features. These studies included NSCLC patients of all stages and various histological types. EGFR status could vary according to the different clinical stage [8], while NSCLC FDG uptake across histologic subtypes may be discrepant [26]. As a result, conflicts existed in the previous related studies.
Our included patients contained only stage IV lung adenocarcinoma with bone metastasis. Final results demonstrated lower bmSUVmax was significant predictor of EGFR mutations, while pSUVmax and nSUVmax weren't statistically different. Few studies identified predictive values of FDG uptake in primary tumor, lymph node, and metastasis simultaneously. Lee et al. [14] reported 71 stage IV lung adenocarcinoma patients, finding SUVmax of metastasis (both nodal and distant) was a significant independent predictor of EGFR mutations, meanwhile SUVmax of primary tumor wasn't significant. Another study by Lv et al. [8] included 849 NSCLC patients with different stages and histologic subtypes. In their analyses, low SUVmax of primary tumor, lymph node, and distant metastasis were associated with EGFR mutations. Furthermore, in a subgroup analysis of stage IV adenocarcinoma, the pSUVmax wasn't meaningful between different EGFR status.
Our data were consistent with above studies to a great extent, whereby low SUVmax of metastasis could predict EGFR mutations of NSCLC, while SUVmax of primary tumor was less useful. EGFR was responsible for the tumor invasiveness, which is an interesting finding that EGFR mutations were associated with lower SUVmax. FDG uptake in malignant tumors was mediated by Glucose transportase-1 (GLUT-1) [27], Higashi et al. [28] proposed the correlations between GLUT-1 expression and FDG uptake in NSCLC. EGFR, on the other hand, has a strong interaction with sodium/glucose cotransporter 1 (SGLT1) in various tumors [29-31]. As SGLT1 opposed to GLUT-1 [27], this mechanism may partly explaine less FDG avid in EGFR mutations. However, further mechanism studies should be sponsored for investigating the relationship between tumor metabolic activity and EGFR mutations.
In patients with stage IV NSCLC, SUVmax of primary tumor seemed to have no predictive value, a reasonable explanation being heterogeneity in tumor metastasis. Lymph node FDG uptake was also significant in the studies of Lv et al. [8] and Lee et al. [14], nevertheless, our data didn't find significance in different EGFR status. Only 47 patients in the study had regional node involvement, which may affect the statistical analysis. We also considered the confounding factor of inflammatory lymph nodes which is difficult to identify from metastatic nodes.
One novel aspect of our research was the histologic tissue origin. All of the included patients received bone biopsy; subsequent pathological evaluation and EGFR analysis were performed by bone metastasis tissue. Hardness was the distinguishing feature of bone tissue; decalcification should be performed first for histological analyses [32]. Traditional acid decalcification inevitably affected IHC [32] and molecular pathology [33]. In our study, EDTA decalcification was applied, which was more suitable for preserving the bone structure, antigenicity, and DNA quality [32-34]. EGFR mutations were identified in 37 (49.3%), which was consistent with the mutation rate in Asian populations (36.8-76.2%) [35]. To our knowledge, our study was the first using bone metastasis tissue as reference, comparing to the similar others. The molecular analysis from same tissue source could avoid metastatic tumor heterogeneity to a great extent.
In the diagnosis of lung cancer, lung biopsy was the more traditional selection for pathological examination and gene test in the past. However, pneumothorax and air embolism arising from lung biopsy could be potentially life-threatening [36, 37]. By contrast, bone biopsy was proven to be a safe method without serious biopsy-related complications [38-40]. In our clinical practice, biopsy was usually performed by outpatient. Patients left the hospital directly after the biopsy. Consequently, quite a proportion of patients preferred to a safer biopsy method after consideration. As a result, in this study, there existed patients with bone biopsy, rather than lung biopsy.
In the multivariate analysis, we demonstrated another two predictors of EGFR mutations: non-smoking, and positive expression of TTF-1. The predictive value of non-smoking has been proven by previous studies [8, 41]. TTF-1, a routine IHC index of lung cancer, may be a biomarker to predict the unknown EGFR mutation status. In a meta-analysis containing 9764 patients [42], TTF-1 expression significantly correlated with EGFR mutations in patients with NSCLC. When specimens weren't good enough for gene testing, those clinicopathological features may assist to guide targeted therapy.
There were several limitations in our study. First, it was a retrospective study that may introduce selection bias. We included lung adenocarcinoma patients with histologically confirmed bone metastasis, further validation of our results would be performed by other stage IV lung cancer. Moreover, not every patient received ALK and ROS1 rearrangement tests; we couldn't know whether other mutations have an influence on FDG uptake. As clinically impractical, not all bone metastasis lesions were biopsied, we chose the bone lesion with highest bmSUVmax to analyze.
In conclusion, the bone metastasis of EGFR-mutant lung carcinoma has lower FDG uptake compared with EGFR wild-type, and SUVmax could be a valuable noninvasive predictor for EGFR mutations. Combining SUVmax with other clinicopathological features could forecast the EGFR status in lung adenocarcinoma with unavailable EGFR gene testing.
Acknowledgements
This research was funded by the Natural Science Foundation of China (grant No.81672852), Interdisciplinary program of Shanghai Jiao Tong University, a three-year action plan to promote clinical skills and clinical innovation in municipal hospitals (16CR2007A). Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant Support (20172024). We appreciate the data analyst Ms. Yiru Shen from PerkinElmer for the preliminary data grabbing during the study.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Reck M, Rabe KF. Precision Diagnosis and Treatment for Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377:849-61
2. Grant S, Qiao L, Dent P. Roles of ERBB family receptor tyrosine kinases, and downstream signaling pathways, in the control of cell growth and survival. Front Biosci. 2002;7:d376-89
3. Reck M, Heigener DF, Mok T, Soria JC, Rabe KF. Management of non-small-cell lung cancer: recent developments. Lancet. 2013;382:709-19
4. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N. et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947-57
5. Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G. et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Mol Diagn. 2013;15:415-53
6. Lindeman NI, Cagle PT, Aisner DL, Arcila ME, Beasley MB, Bernicker EH. et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Mol Diagn. 2018;20:129-59
7. Dacic S. EGFR assays in lung cancer. Adv Anat Pathol. 2008;15:241-7
8. Lv Z, Fan J, Xu J, Wu F, Huang Q, Guo M. et al. Value of (18)F-FDG PET/CT for predicting EGFR mutations and positive ALK expression in patients with non-small cell lung cancer: a retrospective analysis of 849 Chinese patients. Eur J Nucl Med Mol Imaging. 2018;45:735-50
9. Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R. et al. Prognostic factors in patients with advanced non-small cell lung cancer: data from the phase III FLEX study. Lung Cancer. 2012;77:376-82
10. Sun JM, Rampal S, Lee G, Lee J, Choi YL, Parasuraman B. et al. Real world impact of epidermal growth factor receptor mutation status on treatment patterns in patients with non-small cell lung cancer. Lung Cancer. 2013;80:191-6
11. Lee JW, Lee SM. Radiomics in Oncological PET/CT: Clinical Applications. Nucl Med Mol Imaging. 2018;52:170-89
12. Vesselle H, Schmidt RA, Pugsley JM, Li M, Kohlmyer SG, Vallires E. et al. Lung cancer proliferation correlates with [F-18]fluorodeoxyglucose uptake by positron emission tomography. Clin Cancer Res. 2000;6:3837-44
13. Siegelin MD, Borczuk AC. Epidermal growth factor receptor mutations in lung adenocarcinoma. Lab Invest. 2014;94:129-37
14. Lee EY, Khong PL, Lee VH, Qian W, Yu X, Wong MP. Metabolic phenotype of stage IV lung adenocarcinoma: relationship with epidermal growth factor receptor mutation. Clin Nucl Med. 2015;40:e190-5
15. Huang CT, Yen RF, Cheng MF, Hsu YC, Wei PF, Tsai YJ. et al. Correlation of F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value and EGFR mutations in advanced lung adenocarcinoma. Med Oncol. 2010;27:9-15
16. Qiang G, Huang WEI, Liang C, Xu RUI, Yan JUE, Xu Y. et al. Association between histopathological subtype, 18F-fluorodeoxyglucose uptake and epidermal growth factor receptor mutations in lung adenocarcinoma. Oncology Letters. 2016;11:1769-77
17. Li X, Yin G, Zhang Y, Dai D, Liu J, Chen P. et al. Predictive Power of a Radiomic Signature Based on 18F-FDG PET/CT Images for EGFR Mutational Status in NSCLC. Frontiers in Oncology. 2019 9
18. Minamimoto R, Jamali M, Gevaert O, Echegaray S, Khuong A, Hoang CD. et al. Prediction of EGFR and KRAS mutation in non-small cell lung cancer using quantitative (18)F FDG-PET/CT metrics. Oncotarget. 2017;8:52792-801
19. Ko KH, Hsu HH, Huang TW, Gao HW, Shen DH, Chang WC. et al. Value of (1)(8)F-FDG uptake on PET/CT and CEA level to predict epidermal growth factor receptor mutations in pulmonary adenocarcinoma. Eur J Nucl Med Mol Imaging. 2014;41:1889-97
20. Zhu L, Yin G, Chen W, Li X, Yu X, Zhu X. et al. Correlation between EGFR mutation status and F(18) -fluorodeoxyglucose positron emission tomography-computed tomography image features in lung adenocarcinoma. Thorac Cancer. 2019;10:659-64
21. Yang B, Wang QG, Lu M, Ge Y, Zheng YJ, Zhu H. et al. Correlations Study Between (18)F-FDG PET/CT Metabolic Parameters Predicting Epidermal Growth Factor Receptor Mutation Status and Prognosis in Lung Adenocarcinoma. Front Oncol. 2019;9:589
22. Gu J, Xu S, Huang L, Li S, Wu J, Xu J. et al. Value of combining serum carcinoembryonic antigen and PET/CT in predicting EGFR mutation in non-small cell lung cancer. J Thorac Dis. 2018;10:723-31
23. Guan J, Xiao NJ, Chen M, Zhou WL, Zhang YW, Wang S. et al. 18F-FDG uptake for prediction EGFR mutation status in non-small cell lung cancer. Medicine (Baltimore). 2016;95:e4421
24. Yip SS, Kim J, Coroller TP, Parmar C, Velazquez ER, Huynh E. et al. Associations Between Somatic Mutations and Metabolic Imaging Phenotypes in Non-Small Cell Lung Cancer. J Nucl Med. 2017;58:569-76
25. Takamochi K, Mogushi K, Kawaji H, Imashimizu K, Fukui M, Oh S. et al. Correlation of EGFR or KRAS mutation status with 18F-FDG uptake on PET-CT scan in lung adenocarcinoma. PLoS One. 2017;12:e0175622
26. Vesselle H, Salskov A, Turcotte E, Wiens L, Schmidt R, Jordan CD. et al. Relationship between non-small cell lung cancer FDG uptake at PET, tumor histology, and Ki-67 proliferation index. J Thorac Oncol. 2008;3:971-8
27. Gerbaudo VH, Kim CK. PET Imaging-Based Phenotyping as a Predictive Biomarker of Response to Tyrosine Kinase Inhibitor Therapy in Non-small Cell Lung Cancer: Are We There Yet? Nucl Med Mol Imaging. 2017;51:3-10
28. Higashi K, Ueda Y, Sakurai A, Mingwang X, Xu L, Murakami M. et al. Correlation of Glut-1 glucose transporter expression with [(18)F]FDG uptake in non-small cell lung cancer. Eur J Nucl Med. 2000;27:1778-85
29. Weihua Z, Tsan R, Huang WC, Wu Q, Chiu CH, Fidler IJ. et al. Survival of cancer cells is maintained by EGFR independent of its kinase activity. Cancer Cell. 2008;13:385-93
30. Cossu-Rocca P, Muroni MR, Sanges F, Sotgiu G, Asunis A, Tanca L. et al. EGFR kinase-dependent and kinase-independent roles in clear cell renal cell carcinoma. Am J Cancer Res. 2016;6:71-83
31. Liu H, Ertay A, Peng P, Li J, Liu D, Xiong H. et al. SGLT1 is required for the survival of triple-negative breast cancer cells via potentiation of EGFR activity. Mol Oncol. 2019;13:1874-86
32. Savi FM, Brierly GI, Baldwin J, Theodoropoulos C, Woodruff MA. Comparison of Different Decalcification Methods Using Rat Mandibles as a Model. J Histochem Cytochem. 2017;65:705-22
33. Schrijver WA, van der Groep P, Hoefnagel LD, Ter Hoeve ND, Peeters T, Moelans CB. et al. Influence of decalcification procedures on immunohistochemistry and molecular pathology in breast cancer. Mod Pathol. 2016;29:1460-70
34. Liu H, Zhu R, Liu C, Ma R, Wang L, Chen B. et al. Evaluation of Decalcification Techniques for Rat Femurs Using HE and Immunohistochemical Staining. Biomed Res Int. 2017;2017:9050754
35. Jiang L, Mino-Kenudson M, Roden AC, Rosell R, Molina MA, Flores RM. et al. Association between the novel classification of lung adenocarcinoma subtypes and EGFR/KRAS mutation status: A systematic literature review and pooled-data analysis. Eur J Surg Oncol. 2019;45:870-6
36. Hwang EJ, Hong JH, Lee KH, Kim JI, Nam JG, Kim DS. et al. Deep learning algorithm for surveillance of pneumothorax after lung biopsy: a multicenter diagnostic cohort study. Eur Radiol. 2020
37. Liu SH, Fu Q, Yu HL, Yang Q, Hu YB, Zhang ZX. et al. A retrospective analysis of the risk factors associated with systemic air embolism following percutaneous lung biopsy. Exp Ther Med. 2020;19:347-52
38. Cox M, Pukenas B, Poplawski M, Bress A, Deely D, Flanders A. CT-guided Cervical Bone Biopsy in 43 Patients: Diagnostic Yield and Safety at Two Large Tertiary Care Hospitals. Acad Radiol. 2016;23:1372-5
39. Guo W, Hao B, Chen HJ, Zhao L, Luo ZM, Wu H. et al. PET/CT-guided percutaneous biopsy of FDG-avid metastatic bone lesions in patients with advanced lung cancer: a safe and effective technique. Eur J Nucl Med Mol Imaging. 2017;44:25-32
40. Wu MH, Xiao LF, Liu HW, Yang ZQ, Liang XX, Chen Y. et al. PET/CT-guided versus CT-guided percutaneous core biopsies in the diagnosis of bone tumors and tumor-like lesions: which is the better choice? Cancer Imaging. 2019;19:69
41. Shi Z, Zheng X, Shi R, Song C, Yang R, Zhang Q. et al. Radiological and Clinical Features associated with Epidermal Growth Factor Receptor Mutation Status of Exon 19 and 21 in Lung Adenocarcinoma. Sci Rep. 2017;7:364
42. Kim HS, Kim JH, Han B, Choi DR. Correlation of Thyroid Transcription Factor-1 Expression with EGFR Mutations in Non-Small-Cell Lung Cancer: A Meta-Analysis. Medicina (Kaunas). 2019 55
Author contact
![]() Corresponding authors: Hui Zhao, E-mail: zhao-huiedu.cn, and Quanyong Luo, E-mail: lqynnet.
Corresponding authors: Hui Zhao, E-mail: zhao-huiedu.cn, and Quanyong Luo, E-mail: lqynnet.

 Global reach, higher impact
Global reach, higher impact