3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2021; 12(7):1945-1951. doi:10.7150/jca.50789 This issue Cite
Research Paper
Clinical importance of ADC in the prediction of 125I in the treatment for gliomas
1. Department of the Interventional Medical Center, the Affiliated Hospital of Qingdao University, Qingdao 266000, Shandong, China.
2. Department of Clinical Laboratory, the Affiliated Hospital of Qingdao University, Qingdao 266000, Shandong, China.
3. JinHua Municipal Central Hospital, JinHua, 321000, Zhejiang, China.
4. Jiangsu Key Laboratory of Molecular and Functional Imaging, Department of Radiology, Zhongda Hospital, Medical School of Southeast University, Nanjing, 210009, China.
Received 2020-7-27; Accepted 2020-12-26; Published 2021-1-30
Abstract
Objectives: To determine whether the minimum apparent diffusion coefficient (minADC) value can stratify survival in patients with glioma before 125I brachytherapy.
Methods: The study was approved by the Institutional Review Board, and the requirement for informed consent was waived. Twenty-three patients (16 male, 7 female; median age, 48 years) with high-grade glioma (HGG) (n=9) or recurrence after multimodal treatment (n=14) were included in this study. minADC values were obtained before 125I implantation. Overall survival (OS) and progression-free survival (PFS) were analyzed with Cox proportional hazards regression models and the Kaplan-Meier method with the log-rank test.
Results: For 125I-treated patients, the hazard ratio for OS in patients with ADC≥1.0*10^-3 mm2·sec-1 (high minADC) versus ADC<1.0*10^-3 mm2·sec-1 (low minADC) was 0.220 (95% confidence interval: 0.066, 0.735). The median OS was 12 months for patients with high minADC values and 6.0 months for those with low minADC values, and the differences were significant (p=0.032). The median PFS was 12 months for patients with high minADC values and 4 months for those with low minADC values. Significant differences were found in the long-rank test (p=0.013). The multivariate analysis results showed that minADC pre-125I implantation was an independent predictor of OS and PFS in patients receiving 125I brachytherapy.
Conclusions: Pre-125I implantation ADC analysis can stratify prognosis in 125I-treated patients with glioma, which may aid in choosing a suitable therapy for glioma patients.
Keywords: Gliomas, minimum ADC, 125I brachytherapy
Introduction
Malignant primary brain tumors, 80% of which are gliomas, account for a large number of deaths worldwide [1]. There are four different grades of gliomas (grade I-IV) according to the WHO criteria, and grade III and IV gliomas are also called high-grade or malignant gliomas [2]. The prognosis of high-grade gliomas (HGGs) is poor regardless of the variety of therapies. Gliomas easily recur, and even gross total resection has been performed following adjuvant radiotherapy and chemotherapy due to its characteristic of diffusely infiltrating spread from the origin site [3, 4].
125I brachytherapy has been suggested to improve the survival of patients with primary or recurrent glioma as an initial or a salvage therapy method in previous studies [5-8]. However, in the clinical setting, the prognosis of patients with glioma treated with 125I brachytherapy varies; some patients have a relatively favorable prognosis and some do not, even with the same histopathology and similar therapies. Diffusion-weighted imaging (DWI) shows additional information derived from the microscopic motion of water protons at the cellular or physiologic level in addition to what conventional MRI shows [9]. ADC values could be obtained from DWI. Several studies reported inverse correlations between the apparent diffusion coefficient (ADC) and tumor cellularity as well as glioma grade, and DWI has been used for the assignment of tumor grades or the differentiation of tumors [10-12]. A low ADC value reflects high cell density because most of the water molecules are restricted to move within cells rather than in the extracellular space [13]. Thus, ADC values have been applied for the assessment of therapy response in brain tumors and for the prediction of survival [14,15]. However, to the best of our knowledge, no studies have been reported on whether ADC could be used to assess the glioma response to 125I brachytherapy. Thus, the purpose of our study was to retrospectively evaluate whether pre-125I implantation ADC analysis has prognostic value in patients with glioma undergoing 125I brachytherapy.
Materials and Methods
Patient criteria
This retrospective study was approved by the institutional review board, and the requirement for informed patient consent was waived. The study spanned from March 4, 2014, to November 11, 2019. Patients who met the following inclusion criteria were included in our study: (a) patients with primary HGG or recurrent glioma; (b) patients who received 125I implantation; (c) patients with diffusion-weighted images obtained within 1 month before 125I implantation; and (d) patients with no previous or concurrent malignant diseases. Patients were excluded due to (a) the unavailability of digital pretreatment MRI data or (b) the presence of intratumoral hemorrhage.
Treatment
Two interventional radiologists who were both blinded to the research reviewed the patients' medical records to obtain the therapy information. Patients with recurrent glioma (n=14) underwent resection of the tumor at the time of the first diagnosis. Eleven of the patients underwent postoperative external-beam radiation therapy with radiation from 30 Gy to 64.2 Gy and underwent adjuvant chemotherapy with temozolomide (TMZ) using the Stupp protocol. Nine patients received 125I brachytherapy as their initial treatment due to extensive lesions, poor medical conditions, or the rejection of surgery and adjuvant radiochemotherapy. The 125I implantation was performed by one interventional radiologist with 17 years of experience. All patients underwent outpatient examinations monthly, and head MRI or CT was carried out almost every 2 months.
MR imaging and data processing
Pretreatment MRI was performed with a 3.0 Tesla scanner, including T1-weighted imaging (TSE sequence, field of view = 25 cm ×25 cm, slice thickness = 5 mm, interslice distance = 1.5 mm, matrix size=256 mm × 204 mm, TR = 1800 ms, TE =8.5 ms), T2-weighted imaging (TSE sequence, field of view = 25 cm × 25 cm, slice thickness = 5 mm, interslice distance = 1.5 mm, matrix size=320 mm × 296 mm, TR = 3800 ms, TE =92 ms), diffusion-weighted imaging (EPI sequence, field of view = 25 cm × 25 cm, slice thickness = 5 mm, interslice distance =1.5 mm, matrix size = 19.2 cm × 19.2 cm, TR = 6300 ms, TE=95 ms), and contrast material-enhanced T1-weighted imaging (gadopentetate dimeglumine, Magnevist; Berlex Laboratories, Wayne, NJ) (TSE sequence, field of view = 25 cm × 25 cm, slice thickness = 5 mm, interslice distance = 1.5 mm, TR = 1800 ms, TE=8.5 ms, matrix size = 256 × 204). ADCs were calculated with b=0 s/mm2 and b=1000 s/mm2. Postcontrast images were acquired immediately after contrast agent injection.
ADC maps were constructed via software installed on the MR unit. Two radiologists with 17 and 10 years of experience who were both blinded to the research identified the solid tumor components with or without enhancement on the MRI images in consensus. Five to ten regions of interest (ROIs) were manually placed within the solid tumor component on the ADC map by the above two radiologists. No cystic or necrotic areas or skull, which may influence the ADC values, were allowed to cover the ROI. minADC values were obtained for further analysis.
Study outcomes
The outcomes observed were the median overall survival (OS) and median progression-free survival (PFS). Overall survival was measured from the time of 125I implantation to the time of death or the last follow-up. Progression-free survival was measured from the time of 125I implantation to the time of tumor recurrence or progression or death.
Iodine-125 implantation
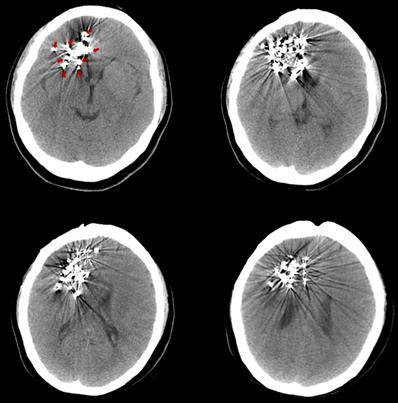
The pre-125I implantation plan was performed with the computerized treatment planning system (TPS, Beijing Astro Technology Ltd., Co., Beijing, China). With a negative pressure vacuum pad, the patients were fixed on the CT scan bed. After anesthetization with 2% lidocaine, an incision of approximately 2 mm was made on the scalp. Then, 2-4 mm diameter holes on the skull were made with an electric cranial drill. Flat needles were inserted into the tumor, and the angle and depth of the needle were dynamically adjusted under CT guidance. Iodine-125 seeds (diameter of 0.8 mm, length of 4.5 mm, half-life of 59.4 days; Model 7711, Beijing Atom and High Technique Industries, Inc., Beijing, China) were implanted, and dosimetric verification was performed with TPS during the operation. The scalp was sutured post-125I implantation to avoid leakage of the cerebrospinal fluid. After the implantation, vital signs were monitored, and the patients were required to be inactive on the bed for 24 hours. Routine dehydration medications were given for 7 to 14 days. Three days after the 125I implantation, a CT scan was carried out to exclude delayed damage to the brain (Fig. 1).
Three days after the 125I implantation, a CT scan was carried out to exclude delayed damage to the brain. The images show different slices of the brain with 125I seeds. (Arrows indicate the 125I seeds implanted in the brain).
Statistical analysis
The last follow-up date was April 22, 2020. The data were analyzed with SPSS (Version 18.0, IBM, NY, USA). A Cox proportional hazards regression model was used for the univariate analysis and all variables with P≤0.1 or variables that were clinically valuable were included in the multivariate analysis using a Cox proportional hazards regression model. Survival curves were calculated with the Kaplan-Meier method, and the differences were analyzed with the log-rank test. For all analyses, P <0.05 was considered to indicate a significant difference.
Results
Patient characteristics
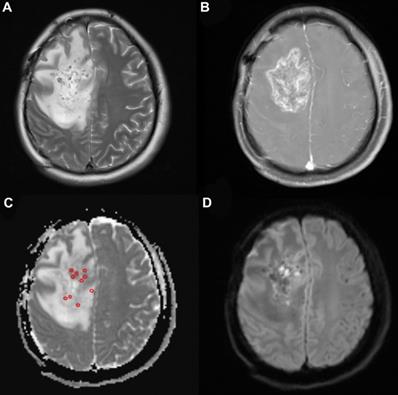
Table 1 shows the baseline characteristics of the patients with primary and recurrent glioma. Twenty-three patients (16 male and 7 female) were included in the present study. Four patients were over 65 years old. The median KPS (Karnofsky performance score) was 80 (range, 50-90). Of the patients with recurrent glioma, 12 underwent gross total resection and 2 underwent partial resection before. Eleven of the patients received radiotherapy with a total median radiation dose of 60 Gy (range: 30-64.2 Gy) and chemotherapy with temozolomide (TMZ) using the Stupp protocol. There were 3 patients who rejected radiochemotherapy after resection. Nine patients with primary glioma had MRI- and CT-proven high-grade glioma and received 125I brachytherapy as their initial treatment rather than conventional therapy with their informed consent due to extensive lesions, poor medical conditions or the rejection of surgery and adjuvant radiochemotherapy. Biopsy was performed during the 125I implantation to confirm the diagnosis. Minimum ADC values were obtained from the ADC map (Fig. 2). The mean minADC value was 0.96. The number of patients with tumors located in the left hemisphere, right hemisphere, both hemispheres and the cerebellum was 10, 9, 3, and 1, respectively. The median volume of the tumor was 37.33 cm3. Aggravating brain edema was found in seven of these patients, which was relieved after dehydration treatment approximately 15 days later. No severe postoperative complications were found in these patients.
Baseline characteristics of the patients
| Parameters | Values |
|---|---|
| Age (years) | |
| Median (range) | 48 (16-70) |
| ≥65 | 4 |
| <65 | 19 |
| Sex | |
| Male | 16 |
| Female | 7 |
| KPS | |
| Median (range) | 80 (50-90) |
| ≥80 | 12 |
| <80 | 11 |
| Resection | |
| Gross Total Resection | 12 |
| Partial Resection | 2 |
| Biopsy | 9 |
| EBRT | |
| Yes | 11 |
| No | 12 |
| Adjuvant Chemotherapy | |
| Yes | 11 |
| No | 12 |
| Minimum ADC (mm2.sec-1) | |
| Mean±SD | (0.96±0.32)×10^-3 |
| ≥1.0×10^-3 | 13 |
| <1.0×10^-3 | 10 |
| Tumor Location | |
| Left Hemisphere | 10 |
| Right Hemisphere | 9 |
| Both Hemispheres | 3 |
| Cerebellum | 1 |
| Tumor Type | |
| Recurrent | 14 |
| Primary (high-grade) | 9 |
| Tumor Volume (cm3) | |
| Median (range) | 37.44 (0.90-164.63) |
Note. KPS, Karnofsky performance score; EBRT, external beam radiotherapy; ADC, apparent diffusion coefficient.
A 33-year-old woman with high-grade glioma (WHO grade IV). (a) Pre-125I implantation T2WI, (b) contrast-enhanced T1WI, (c) ADC map (circles indicate the ADC values measured on the ADC map), and (d) DWI showing the glioma location in the brain. The minimum ADC value was detected on the ADC map.
Univariate analysis of OS and PFS in 125I-treated patients
| Variables | Odds Ratio | p value | 95% Confidence Interval |
|---|---|---|---|
| Overall Survival | |||
| Age | 9.028 | 0.004 | 1.990, 40.968 |
| Sex | 1.351 | 0.621 | 0.411, 4.444 |
| KPS | 0.299 | 0.038 | 0.096, 0.935 |
| Tumor Location | 1.292 | 0.479 | 0.636, 2.627 |
| Type | 0.892 | 0.838 | 0.297, 2.675 |
| Surgery | 1.121 | 0.838 | 0.374, 3.364 |
| EBRT | 1.676 | 0.356 | 0.560, 5.015 |
| Adjuvant Chemotherapy | 1.676 | 0.356 | 0.560, 5.015 |
| Minimum ADC | 3.256 | 0.042 | 1.041,10.186 |
| Progression-free Survival | |||
| Age | 10.878 | 0.002 | 2.347, 50.426 |
| Sex | 1.503 | 0.431 | 0.545, 4.148 |
| KPS | 0.148 | 0.020 | 0.029, 0.744 |
| Tumor Location | 1.469 | 0.181 | 0.837, 2.580 |
| Type | 1.302 | 0.601 | 0.484, 3.505 |
| Surgery | 0.768 | 0.601 | 0.285, 2.068 |
| EBRT | 1.876 | 0.230 | 0.671, 5.244 |
| Adjuvant Chemotherapy | 1.876 | 0.230 | 0.671, 5.244 |
| Minimum ADC | 3.861 | 0.022 | 1.214, 12.275 |
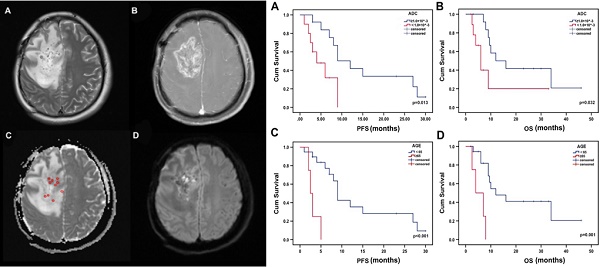
Survival analysis
The median PFS was 4 months for patients with low minADC values and 12 months for those with high minADC values (p=0.013, Fig. 3a). The median OS was 6 months for patients with low minADC values and 12 months for those with high minADC values. The differences between patients with low and high minADC values were statistically significant (p=0.032, Fig. 3b). The median PFS was 9 months in the younger group (age <65) and 2.5 months in the older group (age ≥65) (p<0.001, Fig. 3c). The median OS was 12 months in the younger group and 4 months in the older group, and the difference was significant (p=0.001, Fig. 3d). As shown in Table 2, in the univariate Cox model, age, minADC, and KPS were the most accurate predictors of OS (age: OR=9.028, p=0.004, 95% CI: 1.990, 40.968; minADC: OR=3.256, p=0.042, 95% CI: 1.041, 10.186; KPS: OR=0.299, p=0.038, 95% CI: 0.096, 0.935) and PFS (age: OR=10.878, p=0.002, 95% CI: 2.347, 50.426; minADC: OR=3.861, p=0.022, 95% CI: 1.214, 12.275; KPS: OR=0.148, p=0.020, 95% CI: 0.029, 0.744). When these factors were included in the multivariate Cox model, age and minADC were still the most accurate predictors of OS (age: HR=13.438, p=0.002, 95% CI: 2.655, 68.010; minADC: HR=0.220, p=0.014, 95% CI: 0.066, 0.735) and PFS (age: HR=42.533, p<0.001, 95% CI: 5.254, 344.303; minADC: HR=0.164, p=0.008, 95% CI: 0.043, 0.624) (Table 3).
Multivariate Analysis of OS and PFS in 125I-treated patients
| Variable | Hazard Ratio | p value | 95% Confidence Interval |
|---|---|---|---|
| Overall Survival | |||
| Age | 13.438 | 0.002 | 2.655,68.010 |
| Minimum ADC | 0.220 | 0.014 | 0.066,0.735 |
| Progression-free Survival | |||
| Age | 42.533 | 0.001 | 5.254,344.303 |
| Minimum ADC | 0.164 | 0.008 | 0.043,0.624 |
Discussion
Our results indicated that pre-125I implantation ADC analysis was valuable for assessing 125I treatment for HGG. HGG is a disease that mainly grows from the site of origin and can spread throughout the whole brain. Due to its diffusely infiltrating characteristics, gliomas can invade the adjacent brain for some distance, and they are invisible to clinical examination, preventing complete gross resection and promoting resistance to radiochemotherapy [16]. As a result, relapses of HGG are always a difficult subject for doctors, regardless of the multiple conventional therapies available [17, 18]. In addition, facing recurrent glioma, which therapy methods should be chosen remains a question.
In patients with glioma, 125I showed therapeutic efficacy, regardless of primary glioma or recurrent glioma [7, 8, 19]. However, not all patients respond well to 125I treatment. Therefore, research on biomarkers that predict the outcomes of 125I treatment is necessary for clinical treatment, which may aid in the selection of a suitable therapy for glioma patients. ADC reveals the microscopic structure of the tumor, which implies necrotic cell clusters and cell density at the cellular or physiological level that offer indirect information about tumor aggressiveness [20]. Previous studies confirmed the correlation of ADC with the prognosis of the tumor. Yamasaki et al.'s results showed an inverse relationship between ADC and astrocytic tumors [21]. Ryuji Murakami's research confirmed the value of ADC in the prediction of postoperative radiation therapy response in malignant gliomas. In another study, Wu CC et al indicated that lower ADC values correlated with poor survival in patients, regardless of WHO grade [22]. Although many studies have confirmed the predictive value of pretreatment ADC, iodine-125 seeds could play continuous roles within the tumor, with the continuous release of low-dose γ rays, which is different from EBRT. Thus, whether ADC analysis could be used for the prediction of 125I efficacy is unknown. In the present study, we hypothesized that pre-125I implantation ADC could be a prognostic predictor for 125I treatment for gliomas.
In our study, patients with primary or recurrent glioma were included in the analysis, and DWI was carried out within 1 month before the implantation of 125I. The cut-off value of 1.00×10^-3 mm2 sec-1 was chosen as previously reported [22]. As the univariate analysis results showed, age, KPS, and minADC were predictors of both OS and PFS. After the multivariate analysis, age and minADC were independent predictors of OS and PFS. However, KPS showed no effects on OS and PFS. These results indicated that younger patients with a minADC value ≥1.00×10^-3 mm2 sec-1 have a better prognosis with 125I brachytherapy. Previous studies suggested that KPS and age were independent predictors of OS in patients with glioma following resective surgery, which was partly in line with the results in our study [17]. Interestingly, the present study showed that the pre-125I implantation minADC was an important predictor of the prognosis of glioma patients receiving 125I brachytherapy, with p-values of 0.014 and 0.008 for OS and PFS, respectively, which has not been reported in previous studies to the best of our knowledge.
Kaplan-Meier estimates of OS and PFS from the time of 125I implantation. There were significant differences in PFS and OS according to minADC (cut-off value: 1.00×10^-3 mm2 sec-1) and age.
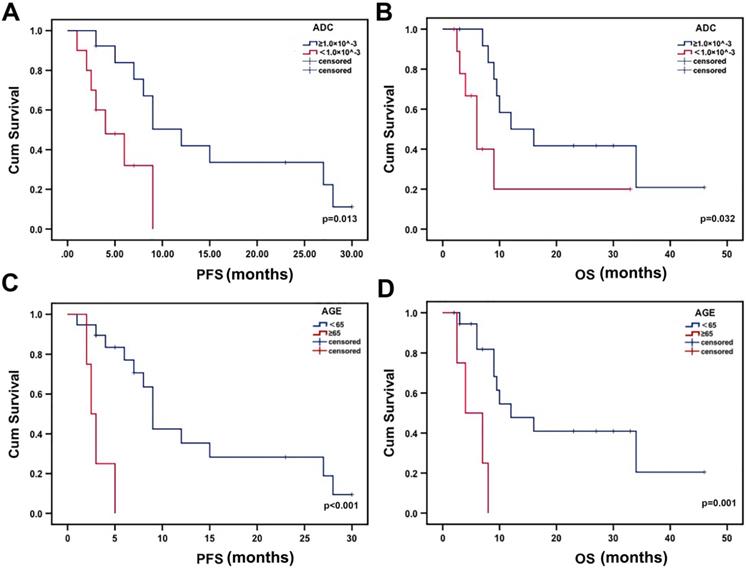
The results of the Kaplan-Meier analysis with the log-rank test showed significant differences in OS and PFS between patients with low and high minADC values. Additionally, differences were found between different age groups. The results indicated that low minADC values correlated with poor survival in patients receiving 125I treatment. Thus, the cut-off value of 1.00×10^-3 mm2 sec-1 could be used to evaluate the prognosis of patients before 125I implantation. The results confirmed the importance of pre-125I implantation ADC analysis, which could be used to evaluate the prognosis of patients receiving 125I implantation. In addition, our results also indicated that young patients receiving 125I brachytherapy for glioma had a better prognosis. ADC values were easy to obtain with the construction of the DWI image, allowing the evaluation of the different therapy responses to 125I implantation. Pre-125I implantation ADC analysis is an important and convenient method, helping patients with glioma select treatment options.
Nine patients received 125I seeds as their initial therapy method instead of the standard therapy due to extensive lesions, poor medical conditions, or the rejection of surgery and adjuvant radiochemotherapy. Patients with primary glioma underwent 125I brachytherapy as a salvage therapy method because they were not suitable for the conventional therapy methods, which may contribute to the results that no significant differences were found in OS and PFS between primary and recurrent gliomas. The greatest limitation of the study was that the sample size was not large enough; however, we still believe that the study offered valuable insights for clinical treatment with 125I brachytherapy. More patients with glioma who received 125I treatment will be included in future research.
We already carried out more than 600 cases of 125I implantation in the brain. Most operations were carried out under local anesthesia, which is tolerable. Although the implantation of 125I seeds was carried out with an 18G flat needle, which caused minor damage to the brain, the puncturing operation sometimes caused aggravating brain edema and bleeding of the tumor. The condition of patients with aggravating brain edema improved a few months later with dehydration treatment, and self-absorption of the cerebral hemorrhage was almost completed approximately 15 days later with the conservation treatment. Once heavy brain edema and brain bleeding happened after 125I implantation, which may cause cerebral hernia, a surgical operation will be carried out.
In conclusion, the present study showed that patients with high minADC values (<1.00×10^-3 mm2 sec-1) had better outcomes with 125I brachytherapy. Pretreatment minADC values supplied valuable information for the prognosis of 125I treatment, which had not been reported to the best of our knowledge and may supply an important and convenient method for therapy decision making.
Key points
Pre-125I implantation ADC analysis can stratify prognosis in 125I-treated patients with gliomas.
Patients with lower minADC values had a poor prognosis receiving 125I brachytherapy for gliomas.
Abbreviations
HGG: high-grade glioma; DWI: diffusion-weighted imaging; ADC: apparent diffusion coefficient; TMZ: Temozolomide; ROI: region of interest; KPS: Karnofsky performance score; TPS: treatment planning system; TSE: turbo spin-echo; EPI: Echo-planar imaging.
Acknowledgements
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Funding
This work was funded by the National Science Foundation for Youths of China (NO. 81901800).
Informed consent
This study has obtained IRB approval from the institutional review board of the Affiliated Hospital of Qingdao University and the need for informed consent was waived.
Authors' contributions
Congxiao Wang conducted all experiments, integrated data, edited figures, and wrote the manuscript; Zhijian Xu and Song Wang helped with the data analysis. Lijing Peng, Wei Zhang, Xueda Li, Lili Yang, Ying Luan, Tao Su, Zixiang Li provided essential assistance; X. Hu directed this study, designed the research, and gave key advice.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C. et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008-2012. Neuro-Oncology. 2015;17:iv1-iv62
2. Cancer Genome Atlas Research N. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061-8
3. Hu X, Qiu H, Zhang L, Zhang W, Ma Y, Qiao Z. et al. Recurrent gliomas. Cancer Biology & Therapy. 2014;13:840-7
4. Diamandis P, Aldape K. World Health Organization 2016 Classification of Central Nervous System Tumors. Neurol Clin. 2018;36:439-47
5. Ruge MI, Kickingereder P, Simon T, Treuer H, Sturm V. Stereotactic iodine-125 brachytherapy for treatment of inoperable focal brainstem gliomas of WHO grades I and II: feasibility and long-term outcome. Journal of Neuro-Oncology. 2012;109:273-83
6. Ruge MI, Kickingereder P, Grau S, Dorn F, Galldiks N, Treuer H. et al. Stereotactic iodine-125 brachytherapy for the treatment of WHO grades II and III gliomas located in the central sulcus region. Neuro Oncol. 2013;15:1721-31
7. Suchorska B, Hamisch C, Treuer H, Mahnkopf K, Lehrke RE, Kocher M. et al. Stereotactic brachytherapy using iodine 125 seeds for the treatment of primary and recurrent anaplastic glioma WHO° III. Journal of Neuro-Oncology. 2016;130:123-31
8. Larson DA, Suplica JM, Chang SM, Lamborn KR, McDermott MW, Sneed PK. et al. Permanent iodine 125 brachytherapy in patients with progressive or recurrent glioblastoma multiforme. Neuro-oncology. 2004;6:119-26
9. Higano S, Yun X, Kumabe T, Watanabe M, Mugikura S, Umetsu A. et al. Malignant Astrocytic Tumors: Clinical Importance of Apparent Diffusion Coefficient in Prediction of Grade and Prognosis. Radiology. 2006;241:839-46
10. Gupta RK, Sinha U, Cloughesy TF, Alger JR. Inverse correlation between choline magnetic resonance spectroscopy signal intensity and the apparent diffusion coefficient in human glioma. Magnetic Resonance in Medicine. 1999;41:2-7
11. Bulakbasi N, Kocaoglu M, Örs F, Tayfun C, Üçöz T. Combination of Single-Voxel Proton MR Spectroscopy and Apparent Diffusion Coefficient Calculation in the Evaluation of Common Brain Tumors. American Journal of Neuroradiology. 2003;24:225-33
12. Bulakbasi N, Guvenc I, Onguru O, Erdogan E, Tayfun C, Ucoz T. The Added Value of the Apparent Diffusion Coefficient Calculation to Magnetic Resonance Imaging in the Differentiation and Grading of Malignant Brain Tumors. Journal of Computer Assisted Tomography. 2004;28:735-46
13. Sugahara T, Korogi Y, Kochi M, Ikushima I, Shigematu Y, Hirai T. et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. Journal of Magnetic Resonance Imaging. 1999;9:53-60
14. Tomura N, Narita K, Izumi J-i, Suzuki A, Anbai A, Otani T. et al. Diffusion Changes in a Tumor and Peritumoral Tissue After Stereotactic Irradiation for Brain Tumors: Possible Prediction of Treatment Response. Journal of Computer Assisted Tomography. 2006;30:496-500
15. Babsky AM, Hekmatyar SK, Zhang H, Solomon JL, Bansal N. Predicting and monitoring response to chemotherapy by 1,3-bis(2-chloroethyl)-1-nitrosourea in subcutaneously implanted 9L glioma using the apparent diffusion coefficient of water and 23Na MRI. Journal of Magnetic Resonance Imaging. 2006;24:132-9
16. Nieder C, Andratschke N, Wiedenmann N, Busch R, Grosu AL, Molls M. Radiotherapy for high-grade gliomas. Does altered fractionation improve the outcome? Strahlenther Onkol. 2004;180:401-7
17. Eyüpoglu IY, Buchfelder M, Savaskan NE. Surgical resection of malignant gliomas - role in optimizing patient outcome. Nature Reviews Neurology. 2013;9:141-51
18. Kubben PL, Postma AA, Kessels AGH, van Overbeeke JJ, van Santbrink H. Intraobserver and Interobserver Agreement in Volumetric Assessment of Glioblastoma Multiforme Resection. Neurosurgery. 2010;67:1329-34
19. Ruge MI, Kickingereder P, Grau S, Dorn F, Galldiks N, Treuer H. et al. Stereotactic iodine-125 brachytherapy for the treatment of WHO grades II and III gliomas located in the central sulcus region. Neuro-oncology. 2013;15:1721-31
20. Murakami R, Sugahara T, Nakamura H, Hirai T, Kitajima M, Hayashida Y. et al. Malignant Supratentorial Astrocytoma Treated with Postoperative Radiation Therapy: Prognostic Value of Pretreatment Quantitative Diffusion-weighted MR Imaging. Radiology. 2007;243:493-9
21. Yamasaki F, Kurisu K, Satoh K, Arita K, Sugiyama K, Ohtaki M. et al. Apparent Diffusion Coefficient of Human Brain Tumors at MR Imaging. Radiology. 2005;235:985-91
22. Wu CC, Jain R, Radmanesh A, Poisson LM, Guo WY, Zagzag D. et al. Predicting Genotype and Survival in Glioma Using Standard Clinical MR Imaging Apparent Diffusion Coefficient Images: A Pilot Study from The Cancer Genome Atlas. American Journal of Neuroradiology. 2018;39:1814-20
Author contact
![]() Corresponding author: Xiaokun Hu, No. 1677 Wutaishan Road, Qingdao, 26600, Shandong, China. Tel: +86-0532-82919657; Fax: +86-0532-82919657; E-mail: huxiaokun770com.
Corresponding author: Xiaokun Hu, No. 1677 Wutaishan Road, Qingdao, 26600, Shandong, China. Tel: +86-0532-82919657; Fax: +86-0532-82919657; E-mail: huxiaokun770com.

 Global reach, higher impact
Global reach, higher impact