Impact Factor
ISSN: 1837-9664
J Cancer 2021; 12(17):5249-5259. doi:10.7150/jca.52088 This issue Cite
Research Paper
Antiproliferative, Apoptotic Effects and Suppression of Oxidative Stress of Quercetin against Induced Toxicity in Lung Cancer Cells of Rats: In vitro and In vivo Study
1. Thoracic Surgery, Inner Mongolia People's Hospital, Hohhot City, Inner Mongolia Autonomous Region, 010020, China.
2. Forensic Medicine & Clinical Toxicology Department, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt.
3. Department of Pharmaceutical Chemistry, College of Pharmacy, Taif University, P.O. Box 11099,Taif 21944, Saudi Arabia.
4. Department of anatomy, Faculty of Medicine, Helwan University, Helwan, Egypt.
5. Department of anatomy, Faculty of Medicine, Al-Azhar University, Cairo, Egypt.
6. Department of zoology Faculty of science (Girls branch), Al-Azhar University, Cairo, Egypt.
7. Department of zoology Faculty of science, Al-Azhar University, Cairo, Egypt.
Received 2020-8-17; Accepted 2021-6-5; Published 2021-6-26
Abstract
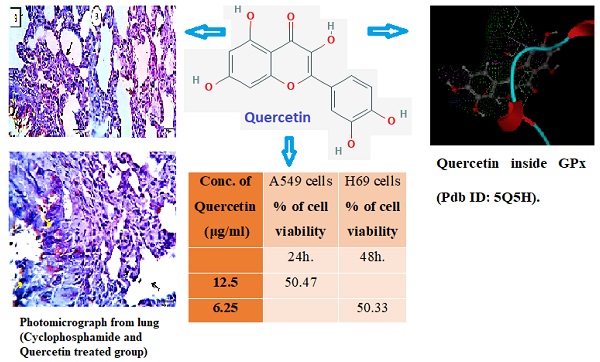
In the present study, quercetin was examined against lung human cancer cells using A549 and H69 cancer cell lines in addition to normal non cancer cells (W138). Two genes Bax and Bcl-2 that play an important role in apoptosis pathways were investigated. Also Immunohistochemical study for caspase-3 which is considered as indicator for apoptosis was performed. Quercetin showed good anti proliferative activity against tested lung cancer cell lines, IC50 values on A549 are 8.65, 7.96 and 5.14 µg/ml at 24, 48 and 72h respectively. Also significant effects of quercetin on Bax, Bcl-2 and caspase-3 were observed, that can prove its ability to induce apoptosis. On the other hand quercetin showed good therapeutic effects against cyclophosphamide induced lung toxicity that were observed in the histopathology study. In vitro studies were also performed such as cell cycle analysis through flowcytometry. The obtained results from all these performed analysis proved that quercetin can induce apoptosis in human lung cancer cells, additionally quercetin showed ability to reduce MDA and increase SOD and GSHP levels which indicates its ability in suppressing oxidative stress, Quercetin has played a therapeutic role in cyclophosphamide induced lung toxicity as it has improved restoring of the damaged lung tissue as discussed in this research work.
Keywords: lung cancer, quercetin, cyclophosphamide, cell cycle, molecular docking, antioxidant enzymes
Introduction
Lung cancer is a major cause of tumor-related deaths in humans worldwide; otherwise there is ability to prevent tumors by controlling weight, increase vegetarian eating, Physical activity, reducing alcohol and tobacco according to WHO reports [1]. Humans that have a 5-year survival rate are less than 15% [2]. As reported Non-small cell lung cancer (NSCLC) has largely formed heterogeneous group of tumors and accounts for about 85% of all lung cancers [3]. Recent studies have reported that surgical removal of the lung is not effective in tumor's treatment. Also, chemotherapy alone or in combination with radiation is good in treatment strategy for lung cancer [4, 5].
There are numbers of antitumor drugs which were approved for cancer treatment; they were derived from natural products [6]. Natural products have to be considered as anticancer active agents because their toxicity is very low. One of these natural products is quercetin (QEU), that have showed multiple pharmacological properties for example anti-inflammatory, antioxidant, antiatherosclerosis, anti-apoptotic and antiproliferation activities [7]. QEU has a good activity in some diseases with high potential effect against cancer, a lot of vegetables and fruits which human take in food daily contain a good quantity of QEU [8]. In vitro and in vivo studies illustrated that QEU has antitumor activity [9] and was reported to prevent cancer induced by chemotherapeutic agents in experimental studies [10]. QEU is reported to inhibit tumor growth in colon cancer [11]. Other studies suggest that quercetin inhibits the reactive oxygen species inside the cell and can cause programmed cell death for the cancer cells [12]. Former studies also postulated that QUE ameliorated cyclophosphamide-induced cardiotoxicity, urotoxicity, and genotoxicity in mice [13].
Cyclophosphamide (CYP) is a notable alkylating agent widely used alone or with other drugs in the treatment of chronic and acute leukemias, multiple myeloma, lymphomas. It is additionally used as an immunosuppressant for the treatment of rheumatoid arthritis, nephrotic syndrome, systemic lupus erythromatosis and bone marrow transplantation. In spite of its vital role in chemotherapy, cyclophosphamide produces serious effects including; hepatotoxicity, nephrotoxicity and lung toxicity [14]. As per the International Agency for Research on Cancer (IARC), CYP is widely used as reference mutagen and is classified as carcinogenic for both animals and humans [15].
Phosphoramide mustard and acrolein are the two active metabolites of CYP [16]. Phosphoramide mustard is responsible for the antineoplastic effects of CYP, while its toxic effects are linked to acrolein [17]. Acrolein, is responsible for chemically alkylating DNA as well as proteins, resulting in cross-links and thereby resulting in cytotoxicity [18].
Experimental evidence proposes that the occurrence of oxidative stress after CYP administration prompts decrease in the activities of antioxidant enzymes and increase in lipid peroxidation (LPO) level in liver and lung of mice [19, 20]. Alongside these, because of the absence of aldehyde oxidase and aldehyde dehydrogenase which are two key detoxifying enzymes, that catalyze the conversion of toxic aldehydes to less toxic carboxylic acids, in the lung tissues, CYP has been accounted to cause selective pulmonary injury [21]. CYP has a synergistic effect of matrix degrading proteases and adhesion proteins that enhances the process of pulmonary metastasis [22].
Free radicals cause peroxidation of lipid in cell components, leading to disruption of normal functions, results in stimulation of signaling pathways and subsequently increased apoptosis [23]. Alveolar epithelial type II cells are particularly more susceptible to the deleterious effects of oxidants [24]. Hence, lung damage is thought to be mediated by the generated reactive oxygen or nitrogen species and lipid peroxide formation in lung tissues. Moreover, elevated ROS production in this manner causes activation of cells of pulmonary defense system like neutrophils, monocytes, and macrophages resulting in pulmonary fibrosis [25].
Epidemiological studies have also reported CYP-induced pulmonary injury in humans. A comprehensive retrospective study has specified six patients over 20 years. In those patients, CYP was identified to be the sole factor that contributed to pulmonary injury. Clinically CYP-induced pulmonary toxicity was manifested by dyspnea, fever, cough, parenchymal infiltrates, gas exchange abnormalities, and pleural thickening [26]. Taking into account the drawbacks of chemotherapeutic agents, there is a critical need to develop new treatment strategies that can limit or antagonize the toxicity of CYP.
Reported results suggest that antioxidant supplementation can reduce the side effects of chemotherapeutic agents [27]. On the other hand cyclophosphamide causes oxidative stress and produces reactive oxygen species (ROS) [28]. Cyclophosphamide induces many pathological effects of lung injury; as it promotes lung toxicity that may lead to damage of lung cells [29]. It was reported that cyclophosphamide induced hepatotoxicity and caused DNA damage of hepatocytes [18, 30]. Cyclophosphamide also causes toxic effects on liver and lung because it inhibits lung and liver cells to defense against free radicals [31, 32]. Cyclophosphamide caused lung toxicity due to its ability to decrease detoxification enzymes [33]. The present study aimed to detect the antiproliferative and apoptotic effects of quercetin against lung cancer cells and to detect the in vivo therapeutic effect of quercetin in cyclophosphamide induced lung toxicity. Moreover, molecular docking studies will be performed to investigate quercetin binding into oxidative stress enzymes.
Materials and methods
In vitro studies
Cell culture
The human lung cancer cell lines were obtained from ATCC, then cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA); containing 1% penicillin/streptomycin (Hyclone; GE Healthcare Life Sciences, Logan, UT) and 10% fetal bovine serum (FBS; Gibco) in moist air with 5% CO2 at 37 °C. The study was performed as cells reached to 80% confluence.
Anti-proliferation assay
Anti-proliferation was examined using MTT assay, cells were seeded into 96-well plates at 2 × 104 cells per well and incubated for 24 h. Cells were treated by 100, 50, 25, 12.5, 6.25 µg from quercetin then after treatment the cells were incubated for 24, 48, 72 h. then 0.05 mL of MTT solution (final concentration of 1 mg/mL) was added to each well and incubated for 4 h. The MTT solution was then removed, and 0.05 mL of acidified isopropanol (with 0.4% HCl) was added to solubilize the formazan crystals. Three replicates were performed, and at 570 nm they were analysed using a microplate reader (BD Biosciences, Mountain View, CA, USA). The ratio of viability (%) was calculated as OD sample/OD control × 100%.
Gene expression detection (BAX and Bcl-2)
A549 cells were grown in RPMI 1640 containing 10% fetal bovine serum at 37 °C, to be tested for BAX and Bcl-2. Cells were treated with the IC50 value of the tested drug, and then cells were lysed with cell extraction buffer. It was diluted in standard buffer over the range of the assay and measured for activity of BAX and Bcl-2 contents (cells are plated in a density of 2 × 103 cells/well in a volume of 100 μl complete growth medium + 100 uL of the extracts per well in a 96-well plate for 48 h before enzyme assay using Invitrogen ELISA Kit (Cat. No. KHO1091) and DRG® Human Bax ELISA (EIA-4487) kit. Assays were repeated twice.
Cell cycle analysis
A549 cells at a density of 6 × 106 cells in T 75 company flasks were treated with IC50 value in µg/ml for 48h The cells were harvested by trypsinization, fxed with 70% (v/v) ethanol overnight at -20 °C and washed with PBS and then labelled with propidium iodide (50 μg/mL) and incubated at RT in the dark for 30 min. Samples were analysed with a FAC Scan flow cytometer (BD Biosciences, Mountain View, CA, USA), and the percentages of cells in the G1, S, and G2/M cell cycle phases were investigated using ModFIT software.
Annexin V/PI analysis
A549 cells were stained using an Annexin V-FITC detection kit. Cells in the exponential growth phase were seeded in 60-mm2 dishes, incubated at 37 °C for 24 h, pretreated with Z-VAD-FMK (20 μM) and SP600125 (10 μM) for 2 h, and then treated with the obtained IC50 values at 48h. The cells were then collected, washed twice with PBS, resuspended in 400 μL of Annexin binding buffer, stained with 5 μL of Annexin V-FITC and 10 μL of PI, and incubated for 15 min at RT in the dark. The stained cells were analyzed with a FAC Scan flow cytometer (BD Biosciences, Mountain View, CA, USA), and the percentages of apoptotic cells were investigated using Flow Jo software.
In vivo study
Male Swiss albino rats were obtained from animal house of faculty of science (220-250 g body weight), they were housed under 12:12 h light: dark cycles, at standard temperature (20 to 25 °C) and humidity (60 ± 10%) conditions, with food and water. A total number of 30 animals were randomly divided into three groups in the animal house. Group 1 was a control group, Group 2 received a single-dose intraperitoneal (i.p.) cyclophosphamide (200 mg/kg) on the 7th day of the study. Group 3 was given, single-dose intraperitoneal (ip) CYP (200 mg/kg) (Cyclophosphamide was purchased from Sigma company), then after one week quercetin was administered i.p. with doses of 100 mg/kg for 15 days. On the 16 day of the experiment, the rats in all groups were anesthetized, all animals were sacrificed. The lung tissue samples were collected for histopathological examination.
Histopathological assay
The formalin preserved experimental Albino rat specimens; lungs were processed in an automated tissue processor. The processing consisted of an initial 2 steps; fixation and dehydration. Fixation comprising tissue immersion in 10% buffered formalin for 48 hours, followed by removal of fixative in distilled water for 30 minutes. Dehydration was then carried out by running the tissues through a graded series of alcohol, initially exposed to 70% alcohol for 120 minutes followed by 90% alcohol for 90 minutes and then two cycles of absolute alcohol, each for one hour. Dehydration was then followed by clearing the samples in several changes of xylene. It consisted of tissue immersion for an hour in a mixture comprising 50% alcohol and 50% xylene, followed by pure xylene for one and a half hours. Samples were then impregnated with molten paraffin wax, then embedded and blocked out. Paraffin sections (4-5 μM) were stained with hematoxylin and eosin [34]. Stained sections were examined for inflammatory reactions, circulatory disturbances, degenerative, necrotic, apoptotic changes and any other pathological lesions in the examined tissues.
Immunohistochemical studies
The demonstration of antigen in tissues by immunostaining is a two-step process. The first step is binding of the primary antibody to the related antigen, followed by visualization of this reaction or by linking a secondary antibody to which different enzyme systems are attached, collectively known as the universal. The primary antibody determines the specificity of the reaction; whereas, the secondary antibody, with its linked enzyme, causes amplification of the reaction, hence, increase of the sensitivity of the test. The Biotin-Streptavidin (BSA) system was used to visualize the markers. Diaminobenzidine (DAB) was used as chromogen since it allows a permanent preparation. Hematoxylin counter stain was used.
Molecular docking studies
Pdb files were retrieved from protein data bank, refined, protonated and water molecules were removed, site of binding was detected by site finder in MOE software. Quercetin molecule was drawn by MOE builder and saved as mdb file. Swissdock was used for predicting the binding of quercetin with SOD (supplementary data) and MOE was used to predict binding of quercetin with glutathione peroxidase enzyme.
Oxidative parameters determination
Oxidative stress parameters (superoxide dismutase (SOD), malondialdehyde (MDA) and glutathione peroxidase (GSHP). were evaluated in tissues of lung using an ultraviolet spectrophotometer (UV-2410PC model, Shimadzu, Japan) using commercial diagnostic kits (Nanjing Jiancheng Biotechnology Co., Ltd, Jiangsu, China).
Results
Anti-proliferation assay
MTT assay was used to examine the effect of quercetin on A549 and H69 cancer cells, the results showed a great reduction of cell viability as shown in Table 1, after 24h, 48h and 72 hours, IC50 values of quercetin against A549 are 8.65, 7.96 and 5.14 µg/ml respectively while IC50 values of quercetin against H69 are 14.2, 10.57 and 9.18 respectively.
Gene expression (BAX and Bcl-2)
Table 2 is representing the effects of quercetin on Bax and Bcl-2 concentrations when compared to the control. Quercetin has increased the expression of Bax more than 7 times of its expression in the control cells (51.48). Also there is a high decrease in Bcl-2 concentration from 5.058 for the control to become 1.947 after treatment with quercetin.
Cell cycle analysis
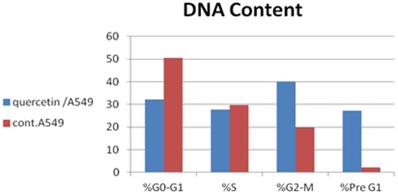
Data presented in the Table 3 and Figure 1 demonstrated that quercetin caused cell cycle arrest at G2/M phase, the percentage of A549 cells at that phase was 40.15% after treatment with quercetin, however the percentage was 19.87% in the untreated control cells. Also it is noticed that the percentage at pre G1 phase was increased after treating cells with quercetin to become 27.31%, when compared with 2.19% for the untreated cells, this indicates its high ability in promoting apoptosis. Figure 1 also illustrated the DNA content in the cells at different phases.
Annexin V/PI analysis
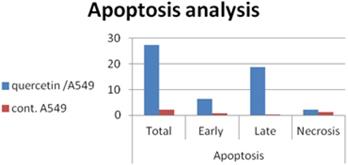
To examine if cell death that occurred in pre G1 phase is related to apoptosis or necrosis we have performed annexin V/PI analysis. The obtained results are represented in Table 4 and Fig. 2 quercetin induced apoptosis in both early and late stage.
DNA content in A549 cells after treatment with quercetin.
Early and late apoptosis in A549 cells after treatment with quercetin.
Percentage of cell viability of A549 and H69 cancer cells after treating them with various concentrations of quercetin at different times
| Concentration of quercetin (μg/ml) | Percentage (%) of cell viability ± SE | ||||||
|---|---|---|---|---|---|---|---|
| A549 | H69 | W138 (Normal cells) | |||||
| 24h | 48h | 72h | 24h | 48h | 72h | 48h | |
| 100 | 25.12 ± 0.16 | 21.47± 0.16 | 20.75± 0.14 | 30.19± 0.19 | 26.42± 0.12 | 22.49± 0.11 | 57.46± 0.29 |
| 50 | 32.82± 0.18 | 30.52± 0.15 | 30.14± 0.14 | 34.28± 0.16 | 31.64± 0.17 | 28.13± 0.13 | 64.19± 0.38 |
| 25 | 41.77± 0.23 | 38.56± 0.18 | 35.62± 0.17 | 44.62± 0.19 | 40.75± 0.17 | 37.25± 0.15 | 72.54± 0.45 |
| 12.5 | 45.63± 0.24 | 41.25± 0.22 | 40.19± 0.20 | 50.47± 0.24 | 47.22± 0.22 | 47.06± 0.21 | 85.17± 0.49 |
| 6.25 | 51.23± 0.26 | 50.33± 0.25 | 48.95± 0.23 | 58.37± 0.31 | 55.88± 0.28 | 52.81± 0.27 | 92.27± 0.57 |
| 0 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Each experiment was repeated three times for each concentration.
Effect of quercetin on apoptosis markers in A549 cancer cells
| Bax conc. pg/ml | Bcl2 Conc. pg/ml | |
|---|---|---|
| Quercetin | 375.22 | 1.947 |
| Cont. (A549) | 51.48 | 5.058 |
Each experiment was repeated twice.
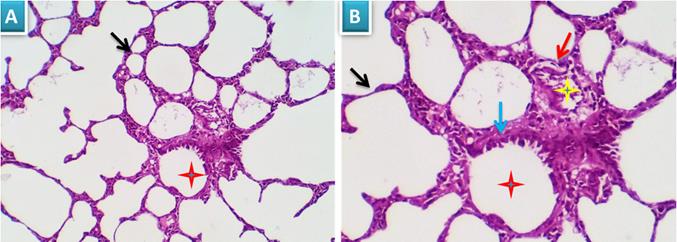
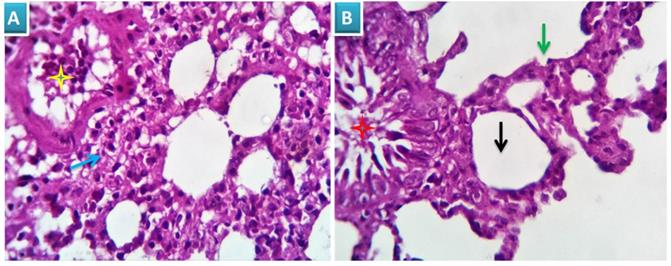
Photomicrograph from lung of group 1 showing normal histomorphological structures with preserved features of bronchial mucosal and submucoasal cellular features (red star and blue arrow). The peribronchial and interalveolar blood vessels are apparently normal (yellow star). The alveolar epithelial lining comprising the pneumocytes type I and II, the alveolar macrophages (pneumocyte type III) and perialveolar intersitium are of normal population and structure (black arrows). H&E X 100 (A), 200 (B).
Cell cycle analysis of A549 cells
| %G0-G1 | %S | %G2-M | %Pre G1 | |
|---|---|---|---|---|
| Quercetin/A549 | 32.14 | 27.71 | 40.15 | 27.31 |
| cont. A549 | 50.47 | 29.66 | 19.87 | 2.19 |
Each experiment was repeated twice.
Apoptosis analysis of A549/ quercetin cells
| Apoptosis | ||||
|---|---|---|---|---|
| Total | Early | Late | Necrosis | |
| quercetin/A549 | 27.31 | 6.37 | 18.7 | 2.24 |
| cont. A549 | 2.19 | 0.66 | 0.21 | 1.32 |
Each experiment was repeated twice.
In vivo study
Histopathological study
Group 1: Control
Examined sections revealed apparently normal bronchial and bronchiolar histomorphological structures with maintained features of mucosal and submucoasal cellular features. Mininmal peri-bronchiolar lympho-plasmacytic infiltration were seen. The peribronchial and interalveolar blood vessels were apparently normal. The alveolar epithelial lining comprising the pneumocytes type I and II, the alveolar macrophages (pneumocyte type III) and perialveolar intersitium were of normal population and structure (Fig. 3A & B).
Group 2: Cyclophosphamide group
Serial sections from lung of this group pointed out moderate perivascular edema with round cells infiltration, destruction of the vascular intima with thickening and hyalinization of the vascular walls (Fig. 4A) beside multifocal pneumonic areas with replacement of the alveoli by large number of round cells and macrophages. Compensatory focal alveolar emphysema was also seen (Fig. 4B).
Group 3: Cyclophosphamide and Quercetin
The pulmonary tissue underwent healing and remodeling process with presence of remnant of inflammatory cells in the interstitial tissue. The blood vessels washed out the toxic product and resumed apparently healthy intimal and medial structures. The bronchial and alveolar structures were apparently normal. Focal alveolar emphysema was still seen in some area of the pulmonary parenchyma (Fig. 5).
Immuno-histochemical results for caspase 3
Group1: control
Lung sections revealed about 4-6%/HPF (average 5.2%) of pneumocytes type 1 and 2 and alveolar macrophage cells positive for caspase 3 apoptotic marker signal, the remaining tissue cells were normal. Positive cells showed characteristic brownish cytoplasmic stainability. Bronchial and bronchiolar epithelial cells were negative for Caspase 3, however it seems that some bronchogenic cells with mucinous productive capacity (mucin producing cells) undergo programmed cell death following pouring of their mucin contents in the bronchial lumen with a resulting unrelated positive apoptotic reactions (Fig. 6).
Group 2: Cyclophosphamide group
Serial sections from lungs of this group pointed out a dramatic apoptotic changes in all of the pulmonary histologic structures as the bronchial epithelium, alveolar cells of pneumocytes type 1 and 2 beside the alveolar macrophages, all were affected with a mild to moderate proportional numbers 27-30% HPF (average 28.8%) of the morphometric analyzed cells, they were positively reactive to caspase -3, with the more pronounced effect in the alveolar macrophages and pneunocytes type 2, postulating an additional immune-depressive effect of the cyclophosphamide beside its real cytotoxic effect (Fig. 7).
Group 3: Cyclophosphamide and Quercetin
Serial sections from lungs of this group pointed out an optimizing protective effect of the used quercetin as the apoptotic index in most of the pulmonary tissue was very low. Nearly, 5-7% HPF (average 7%) of the morphometrically analysed cells were positively reacted, with the pneumocytes type 2 as the most affected cells, they were seen related to the alveolar wall and the interalveolar tissue. Other cells including bronchial, bronchiolar and alveolar cells (pneumocytes type 1) were normal or sparsely affected, especially the unrelated positively stained bronchial epithelial cells (Fig. 8).
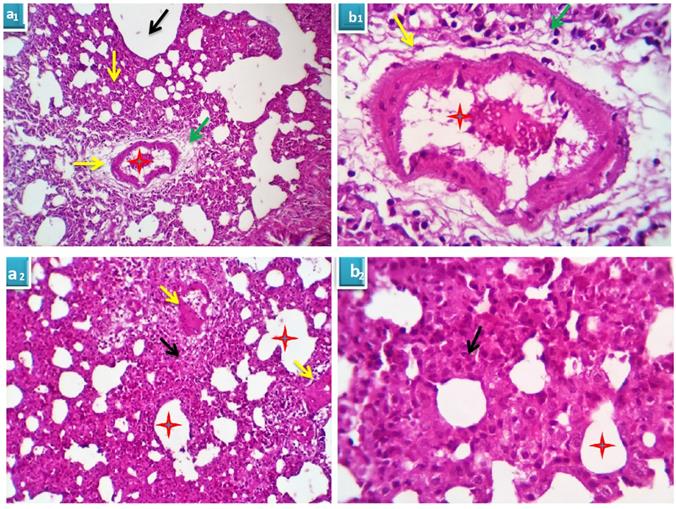
A. Photomicrograph from lung of group 2 showing perivascular edema (yellow arrows) with round cells infiltration (green arrows), destruction of the vascular intima with thickening and hyalinization of the vascular walls (red stars). H&E X 100 (a1), 400 (b1). B. Photomicrograph from lung of group 2 showing focal pneumonic areas with replacement of the alveoli by large number of round cells and macrophages (black arrows). Compensatory focal alveolar emphysema is seen (red stars). H&E X 100 (a2), 400 (b2).
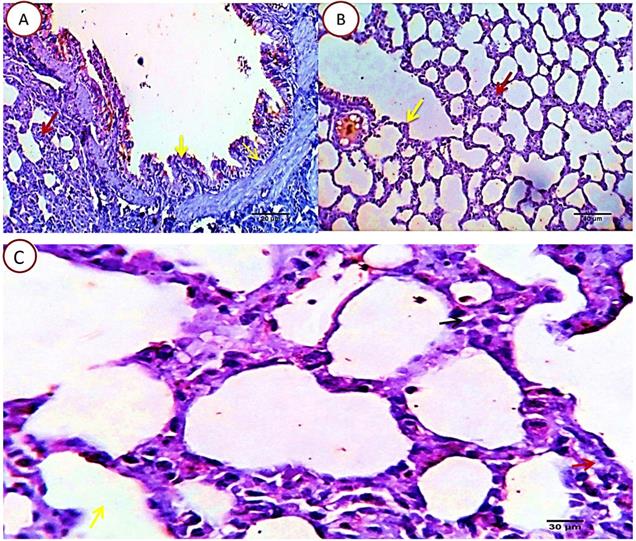
Photomicrograph from lung of group 3 showing healing and remodeling process with presence of remnant of inflammatory cells in the interstitial tissue (blue arrow). The bronchial and alveolar structures are normal (green arrow and red star). The blood vessels resume apparently healthy intimal and medial structures (yellow star). Focal alveolar emphysema was still seen (black arrow). H&E X 100 (A), 400 (B).
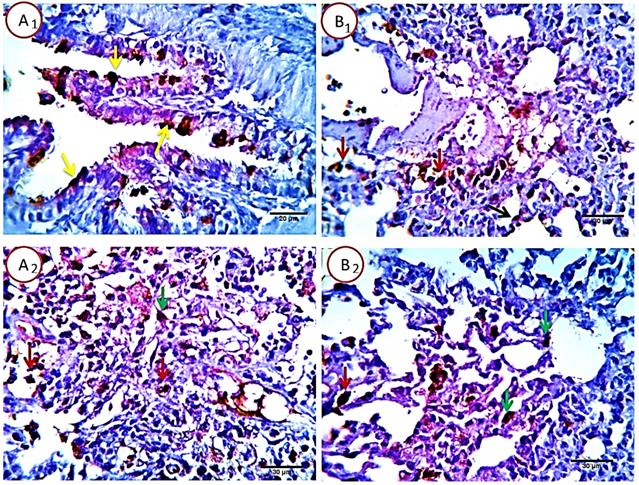
Photomicrograph from lung of normal (control rats), immune-stained with Caspase -3, showing very mild positive cytoplasmic dark brown staining reaction in the pneumocytes type 1 and 2 (2, yellow and red arrows) and in the alveolar macrophages (2, black arrow). Very few bronchogenic cells are uncreatively Caspase positive (1, yellow arrow). Scale bars 30, 40, 30 um.
Photomicrograph from lung of Cyclophosphamide treated group, immune-stained with Caspase-3, showing mild to moderate proportional numbers (27-30 %) of cells positively reactive to caspase-3, with the more pronounced effect in the alveolar macrophages (red arrows) and pneunocytes type 2 (green arrows). Pneumocytes type 1 (black arrow) and some bronchogenic cells (relative and un-relative) are also positively stained. Scale bars 20, 30 um.
Photomicrograph from lung of (Cyclophosphamide and Quercetin) immune-stained with Caspase-3, showing very low (5-7%) of the cells are positively stained. The pneumocytes type 2 are the most affected cells, they are seen related to the alveolar wall (red arrow) and the interalveolar tissue. Other cells including bronchial (yellow arrows), bronchiolar and alveolar cells (pneumocytes type 1) (black arrow) appears normal or sparsely affected, especially the unrelated positively stained bronchial epithelial cells (yellow arrows). Scale bars 20, 40 um.
Molecular docking studies of Quercetin with anti-oxidative stress enzymes (GSPH and SOD)
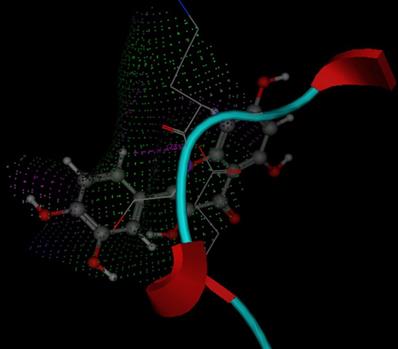
Molecular docking studies are performed in this research work to investigate if Quercetin has a good ability to bind with these enzymes, mode of binding, possible interactions and binding score. 1PM9 is the pdb file for SOD, it was retrieved from protein data bank (https://www.rcsb.org/structure/1PM9) as pdb file then uploaded to swissdock server (http://www.swissdock.ch/) with Quercetin to perform docking process, the obtained results revealed the ability of quercetin to bind to SOD with full fitness score equals -1876.34 kcal/mol and estimated ΔG = -8.2 kcal/mol which indicate promiscuity of quercetin to target and affect SOD enzyme (supplementary data). Additionally 5H5Q is the pdb file for glutathione peroxidase enzyme (https://www.rcsb.org/structure/5H5Q), it was also retrieved from protein data bank and used for docking with quercetin using MOE program, the obtained results revealed that quercetin has the ability to fit at the binding site of glutathione peroxidase enzyme with docking score energy equals -11.64 Kcal/mol (Fig. 9). These obtained in silico studies indicated the ability of quercetin to affect anti-oxidant enzymes and encouraged us to investigate the ant- oxidative stress activity of quercetin on SOD and GSPH to support molecular docking results.
3D mode for quercetin inside GPx (Pdb ID: 5Q5H).
Oxidative parameters determination
To determine the antioxidant effect of Quercetin, MDA and antioxidant enzymes (SOD and GSHP), they were evaluated in the lung of the control, CP-intoxicated lung of rats and after treatment of the CP-intoxicated lung with quercetin. The results are represented in Table 5, they showed decrease in GSHP and SOD and increase in MDA in intoxicated lung than the control. However after treating the intoxicated lung with quercetin the obtained results revealed increase in both GSHP and SOD and showed dramatic decrease in lung MDA to be nearly equal to the control as shown in the following table. From the obtained results we can conclude that Quercetin suppresses oxidative stress in induced cyclophosphamide lung intoxication of rats.
Oxidative parameters evaluation
| GSHP (ng/ml) | SOD (U/L) | MDA (nmol/ml) | |
|---|---|---|---|
| Control | 0.43± 0.05 | 0.29± 0.015 | 0.19± 0.02 |
| Cyclo. | 0.15± 0.01 | 0.13± 0.005 | 0.41± 0.021 |
| Cyclo. + qur. | 0.38±0.016 | 0.25±0.014 | 0.20±0.015 |
Each experiment was repeated three times.
Discussion
The aim of the present study was to detect the activity of quercetin against lung cancer cells. IC50 values revealed that quercetin has a good anticancer efficacy. Recent studies have reported that quercetin possess anticancer activity [35]. In the present study we focused on quercetin efficacy in treatment of human lung cancer cell lines and the results showed good inhibition of cell growth in MTT assay method. Therefore, we exposed A549 cells to further investigations to explore the mechanism of action of quercetin in cancer cell proliferation and the results that derived from these analysis showed that quercetin has induced apoptosis of cancer cells by increasing the level of expression of Bax gene and decrease the expression of Bcl-2. Caspases are known as a family of cysteine proteases that are required for cytokine maturation and apoptosis execution, they have been suggested to contribute to both apoptotic [36] and non-apoptotic cellular phenomena [37]. The effector caspases are activated through proteolytic cleavage more than that expected by the initiator caspases. Present data showed that caspase 3 are activated by quercetin in the apoptotic pathway. The obtained data have proved quercetin's ability to induce apoptotic pathway. Activation of caspase-3, will result in cell death [38]. In the present study, cell cycle analysis was performed to investigate the inhibition of the cancer cell cycle phases and apoptosis induction. In our study, cell cycle of A549 cells showed cell arrest at both S and G2-M phases. Wiśniewska et al. (2017) [38] have reported the effect of quercetin on the essential cytoskeletal elements, such as microfilaments, microtubules, cytoskeleton-driven processes and vimentin intermediate filaments in A549 non-small cell lung cancer cells. Their results pointed out that quercetin induced apoptosis via the regulation of BCL2/BAX, also necrosis and mitotic catastrophe, and suppressed the migration of those cells. The reduced migration of A549 cells detected with quercetin therapy might be due to the disassembling influence of quercetin on vimentin filaments, microtubules, and microfilaments associated with its suppression effect on N-cadherin and vimentin expression.
On the other hand the present study investigated the therapeutic effect of quercetin on cyclophosphamide induced lung damage and evaluated the parameters of immuno and histopathological examinations. Examination of lung tissues in the cyclophosphamide treated group suggested a dramatic apoptosis of bronchial epithelium, alveolar cells of pneumocytes type 1 and 2 beside the alveolar macrophages, all were affected with a mild to moderate proportional numbers 27-30% HPF (average 28.8%) of the morphometric analyzed cells were positively reactive to caspase-3 enzyme, with the more pronounced effect in the alveolar macrophages and pneumocytes type 2, postulating an additional immune-depressive effect of the cyclophosphamide beside its real cytotoxic effect. On the other hand, an optimizing protective effect was observed in quercetin treated rats as the apoptotic index in most of the pulmonary tissue was very low. The anti-inflammatory impact of QUE causes inhibition of inflammatory mediators and affects immunity and the inflammation response.
These findings were supported by a study that illustrated decreasing and changes in the epithelial cell components and alveolar capillary, lymphocytic interstitial pneumonia, extensive fibrous tissue proliferation, and thickened alveolar septa due to cyclophosphamide administration [39], while another study reported degenerated alveolar cells, morphological changes in the alveolar septa, cells of the alveolar lumen of cyclophosphamide group, whereas quercetin treatment diminished the degeneration and inflammatory changes in the CYP group [40]. So the obtained data of the present study demonstrated that using of quercetin in treatment has helped in decreasing CYP induced lung toxicity and maintained normal lung functions and lung structure. Molecular docking studies proved the ability of quercetin to bind to oxidative stress enzymes SOD and GSPH this studies were supported by the obtained results from evaluating oxidative stress parameters as shown in Table 5.
Conclusion
From the obtained results in this work, we can conclude that quercetin have good and remarkable anticancer activity against human lung cancer cells. Quercetin showed inhibitory effect on lung cancer cell proliferation, by inducing apoptosis through BAX, BCL-2 and caspase3. Quercetin caused G2-M phase arrest in lung cancer cells. Also quercetin treatment has shown therapeutic effect after cyclophosphamide induced lung toxicity in rats. The therapeutic role of quercetin could improve the lung tissue restoring. Therefore, our experimental results suggested that quercetin might potentially be a protective agent for cyclophosphamide induced lung toxicity. In addition, the promising activity of quercetin as antitumor against lung cancer cells. Prevention of lung cancer with natural extracts as quercetin is considered as a good therapeutic strategy for treatment of human lung cancer and hopeful strategy for replacing chemotherapy with natural sources. In addition quercetin showed potential effect in reducing oxidative stress.
Supplementary Material
Supplementary data.
Acknowledgements
Dr. Amany Belal is thankful to Taif University Researchers Supporting Project number (TURSP-2020/35), Taif University, Taif, Saudi Arabia.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Torre LA, Siegel RL, Jemal A, Lung Cancer Statistics. Adv Exp Med Biol. 2016; 893:1-19.
2. Oser MG, Niederst MJ, Sequist LV. et al. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol. 2015;16(4):65-72
3. Hirsch FR, Scagliotti GV, Mulshine JL. et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389(10066):299-311
4. Swanton C, Govindan R. Clinical Implications of Genomic Discoveries in Lung Cancer. N Engl J Med. 2016;374(19):1864-73
5. Gettinger S, Rizvi NA, Chow LQ. et al. Nivolumab Monotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2016;34(25):2980-7
6. Newman DJ, Cragg GM, Snader KM, Natural products as sources of new drugs over the period 1981-2002. J Nat Prod. 2003; 66(7):1022-37.
7. Harris Z, Donovan MG, Branco GM. et al. Quercetin as an Emerging Anti-Melanoma Agent: A Four-Focus Area Therapeutic Development Strategy. Front Nutr. 2016;3:48
8. Spagnuolo C, Russo M, Bilotto S. et al. Dietary polyphenols in cancer prevention: the example of the flavonoid quercetin in leukemia. Ann N Y Acad Sci. 2012;1259:95-103
9. Nam JS, Sharma AR, Nguyen LT. et al. Application of Bioactive Quercetin in Oncotherapy: From Nutrition to Nanomedicine. Molecules. 2016;21(1):E108
10. Jeong JH, An JY, Kwon YT. et al. Effects of low dose quercetin: cancer cell-specific inhibition of cell cycle progression. J Cell Biochem. 2009;106(1):73-82
11. Tanigawa S, Fujii M, Hou DX, Stabilization of p53 is involved in quercetin-induced cell cycle arrest, apoptosis in HepG2 cells. Biosci Biotechnol Biochem. 2008; 72(3):797-804.
12. Russo M, Nigro P, Rosiello R. et al. Quercetin enhances CD95- and TRAIL-induced apoptosis in leukemia cell lines. Leukemia. 2007;21(5):1130-3
13. Sekeroğlu V, Aydin B, Sekeroğlu ZA, Viscum album L extract, quercetin reduce cyclophosphamide-induced cardiotoxicity, urotoxicity, genotoxicity in mice. Asian Pac J Cancer Prev. 2011; 12(11):2925-31.
14. Pramita Chakraborty UHS, Murmu N, Das JK. et al. Modulation of cyclophosphamide-induced cellular toxicity by diphenylmethyl selenocyanate in vivo, an enzymatic study. J Journal of Cancer Molecules. 2009;4(6):183-189
15. Ludeman SM, The chemistry of the metabolites of cyclophosphamide. Curr Pharm Des. 1999; 5(8):627-43.
16. Weijl NI, Cleton FJ, Osanto S, Free radicals, antioxidants in chemotherapy-induced toxicity. Cancer Treat Rev. 1997; 23(4):209-40.
17. Kern JC, Kehrer JP, Acrolein-induced cell death. a caspase-influenced decision between apoptosis and oncosis/necrosis. Chem Biol Interact. 2002;139(1):79-95
18. Nafees S, Ahmad ST, Arjumand W. et al. Modulatory effects of gentisic acid against genotoxicity and hepatotoxicity induced by cyclophosphamide in Swiss albino mice. J Pharm Pharmacol. 2012;64(2):259-67
19. Basu A, Bhattacharjee A, Roy SS. et al. Vanadium as a chemoprotectant: effect of vanadium(III)-L-cysteine complex against cyclophosphamide-induced hepatotoxicity and genotoxicity in Swiss albino mice. J Biol Inorg Chem. 2014;19(6):981-96
20. Ghosh P, Bhattacharjee A, Basu A. et al. Attenuation of cyclophosphamide-induced pulmonary toxicity in Swiss albino mice by naphthalimide-based organoselenium compound 2-(5-selenocyanatopentyl)-benzo[de]isoquinoline 1,3-dione. Pharm Biol. 2015;53(4):524-32
21. Sulkowska M, Skrzydlewska E, Sobaniec-Lotowska M. et al. Effect of cyclophosphamide-induced generation reactive oxygen forms on ultrastructure of the liver and lung. BULLETIN-VETERINARY INSTITUTE IN PULAWY. 2002;46(2):239-246
22. Man S, Zhang Y, Gao W. et al. Cyclophosphamide promotes pulmonary metastasis on mouse lung adenocarcinoma. Clin Exp Metastasis. 2008;25(8):855-64
23. Bhagat R, Sporn TA, Long GD. et al. Amiodarone and cyclophosphamide: potential for enhanced lung toxicity. Bone Marrow Transplant. 2001;27(10):1109-11
24. Suntres ZE, Shek PN, Alleviation of paraquat-induced lung injury by pretreatment with bifunctional liposomes containing alpha-tocopherol, glutathione. Biochem Pharmacol. 1996; 52(10):1515-20.
25. Chaudhary NI, Schnapp A, Park JE, Pharmacologic differentiation of inflammation, fibrosis in the rat bleomycin model. Am J Respir Crit Care Med. 2006; 173(7):769-76.
26. Limper AH, Chemotherapy-induced lung disease. Clin Chest Med. 2004; 25(1):53-64.
27. Mythili Y, Sudharsan PT, Selvakumar E. et al. Protective effect of DL-alpha-lipoic acid on cyclophosphamide induced oxidative cardiac injury. Chem Biol Interact. 2004;151(1):13-9
28. Hubbard AK, Timblin CR, Shukla A. et al. Activation of NF-kappaB-dependent gene expression by silica in lungs of luciferase reporter mice. Am J Physiol Lung Cell Mol Physiol. 2002;282(5):L968-75
29. Bhattacharjee A, Basu A, Biswas J. et al. Nano-Se attenuates cyclophosphamide-induced pulmonary injury through modulation of oxidative stress and DNA damage in Swiss albino mice. Mol Cell Biochem. 2015;405(1-2):243-56
30. Zhu Y, Bazemore-Walker CR Mass spectrometry characterization of acrolein protein targets in the liver. Focus on site-specific analysis. Wiley Online Library. 2013
31. Kurutas EB, The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress. current state. Nutr J. 2016;15(1):71
32. Selvakumar E, Prahalathan C, Mythili Y. et al. Mitigation of oxidative stress in cyclophosphamide-challenged hepatic tissue by DL-alpha-lipoic acid. Mol Cell Biochem. 2005;272(1-2):179-85
33. Tang SM, Deng XT, Zhou J. et al. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed Pharmacother. 2020;121:109604
34. Suvarna S, Layton C, Bancroft J, Bancroft's Theory, Practice of Histological Techniques. 8th ed. 2018.
35. Boly R, Gras T, Lamkami T. et al. Quercetin inhibits a large panel of kinases implicated in cancer cell biology. Int J Oncol. 2011;38(3):833-42
36. Salvesen GS, Dixit VM, Caspases. intracellular signaling by proteolysis. Cell. 1997;91(4):443-6
37. Hashimoto T, Kikkawa U, Kamada S, Contribution of caspase(s) to the cell cycle regulation at mitotic phase. PLoS One. 2011; 6(3):e18449.
38. Klimaszewska-Wiśniewska A, Hałas-Wiśniewska M, Izdebska M. et al. Antiproliferative and antimetastatic action of quercetin on A549 non-small cell lung cancer cells through its effect on the cytoskeleton. Acta Histochem. 2017;119(2):99-112
39. Hassan M, Watari H, AbuAlmaaty A. et al. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int. 2014;2014:150845
40. Shokrzadeh M, Ahmadi A, Chabra A. et al. An ethanol extract of Origanum vulgare attenuates cyclophosphamide-induced pulmonary injury and oxidative lung damage in mice. Pharm Biol. 2014;52(10):1229-36
Author contact
![]() Corresponding author: Ahmed B. M. Mehany (E-mail: abelal_81com & abelal_81edu.eg).
Corresponding author: Ahmed B. M. Mehany (E-mail: abelal_81com & abelal_81edu.eg).

 Global reach, higher impact
Global reach, higher impact