3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2021; 12(18):5681-5686. doi:10.7150/jca.62853 This issue Cite
Research Paper
When to apply immune checkpoint inhibitor in patients with refractory advanced gastric cancer
Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea
Abstract
Background: Immune checkpoint inhibitors (ICIs) show clinical benefit in patients with refractory advanced gastric cancer (GC). The ICIs in routine clinical practice have been used in various treatment lines. Therefore, we investigated the timing for application of ICI in patients with refractory advanced GC.
Methods: We analyzed 187 patients with refractory advanced or recurrent GC who received ICIS as a 3rd- or 4th-line treatment between September 2015 and October 2020. Clinical outcomes of overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and disease control rate (DCR) were evaluated.
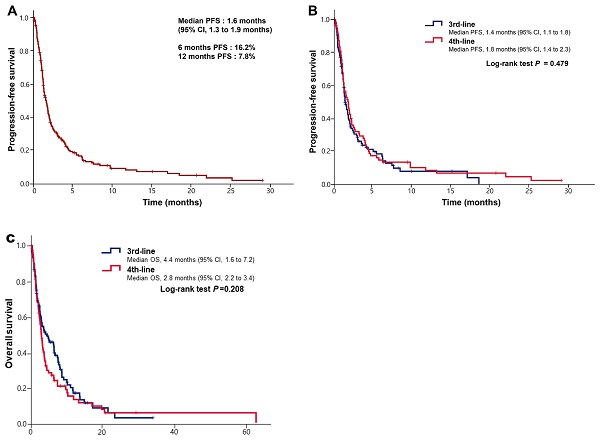
Results: Among 187 patients, 105 received ICIs as a 3rd-line treatment and 82 as a 4th line. The ORR for ICIs was 10.5% (11/105) in 3rd line and 8.5% (7/82) in 4th line. The DCR for ICIs was 36.2% (38/105) in 3rd-line treatment and 31.7% (26/82) in 4th line. There was no significant difference for ORR (P = 0.819) or DCR (P = 0.870). The median PFS and OS to ICIs was 1.4 months (95% CI, 1.1 to 1.8 months) and 4.4 months (95% CI, 1.6 to 7.2 months) in 3rd line and 1.8 months (95% CI, 1.4 to 2.3 months) and 2.8 months (95% CI, 2.2 to 3.4 months) in 4th line. The median PFS and OS to ICIs was not different between 3rd line and 4th line (P = 0.495 and P=0.208, respectively). There were also no significant difference for PFS and OS between PD-L1-positive tumors (CPS≥1) and PD-L1-negative tumors (P = 0.910 and P=0.931, respectively).
Conclusions: ICIs showed similar clinical benefits in the 3rd-line and 4th-line settings. ICIs might be a reasonable approach for patients with refractory GC in the setting of 3rd-line or 4th-line treatment options.
Keywords: Gastric cancer, Immune checkpoint inhibitor, Nivolumab, Pembrolizumab, Biomarker

 Global reach, higher impact
Global reach, higher impact