Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(8):2528-2539. doi:10.7150/jca.67977 This issue Cite
Review
FSCN1 acts as a promising therapeutic target in the blockade of tumor cell motility: a review of its function, mechanism, and clinical significance
1. Shanxi Key Laboratory of Otorhinolaryngology Head and Neck Cancer, First Hospital of Shanxi Medical University, Taiyuan 030001, Shanxi, China
2. Shanxi Province Clinical Medical Research Center for Precision Medicine of Head and Neck Cancer, Department of Otolaryngology Head & Neck Surgery, First Hospital of Shanxi Medical University, Taiyuan 030001, Shanxi, China
3. Clinical Medical Academy & General Hospital, Shenzhen University, Shenzhen 518055, Guangdong, China
4. Department of Otolaryngology Head & Neck Surgery, Shanxi Bethune Hospital, Taiyuan 030032, Shanxi, China
5. Translational Research Institute, People's Hospital of Zhengzhou University, Academy of Medical Science, Henan International Joint Laboratory of Non-coding RNA and Metabolism in Cancer, Zhengzhou University, Zhengzhou 450003, Henan, China
# These authors contributed equally to this work.
Received 2021-10-11; Accepted 2022-4-28; Published 2022-5-9
Abstract
Fascin actin-bundling protein 1 (FSCN1) is an actin-bundling protein that is capable of inducing membrane protrusions and plays critical roles in cell migration, motility, adhesion, and other cellular interactions. FSCN1 also plays a role in forming and stabilizing filopodia or microspikes, which assist during cell migration. Furthermore, FSCN1 is a downstream target of several microRNAs and participates in various biological processes, such as epithelial-to-mesenchymal transition and autophagy, which regulate the invasion and migration ability of cells in various cancers. Increased FSCN1 levels have been associated with enhanced migration and invasion of multiple cancers as well as poor patient prognosis. Promising results from in vitro experimental studies using docosahexaenoic acid (DHA) in breast cancer and recombinant porcine NK-lysin A in hepatocellular carcinoma have revealed that anticancer drugs targeting FSCN1 have significant potential clinical applications. This review discusses FSCN1 in terms of five aspects: structure and function, biological processes, regulatory mechanisms, clinical applications, and future prospects.
Keywords: FSCN1, Actin-binding protein, Epithelial-mesenchymal transition, Metastasis and invasion, Therapeutic target of cancer
Introduction
Fascin actin-bundling protein 1 (FSCN1), also called fascin or fascin-1, is a globular, filamentous, actin-binding protein, that belongs to the actin cytoskeletal protein family [1]. Through stabilization of actin bundles, FSCN1 supports various cell structures, including microspikes, filopodia, lamellar pseudopods, and other actin-based protrusions under the plasma membrane, which are important for processes including cell migration and cell matrix adhesion [2, 3]. Recently, FSCN1 has attracted much attention, because multiple studies have indicated that it may be a candidate biomarker or therapeutic target for the treatment of several different aggressive, metastatic carcinomas [2, 4, 5]. In this review, we discuss, at length, the structure and biological function of FSCN1, and describe the expression patterns, biological processes, and regulatory mechanisms of FSCN1 in various cancers. Furthermore, we discuss the clinical significance of FSCN1 in various cancers.
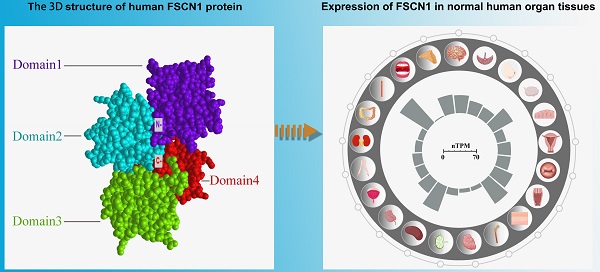
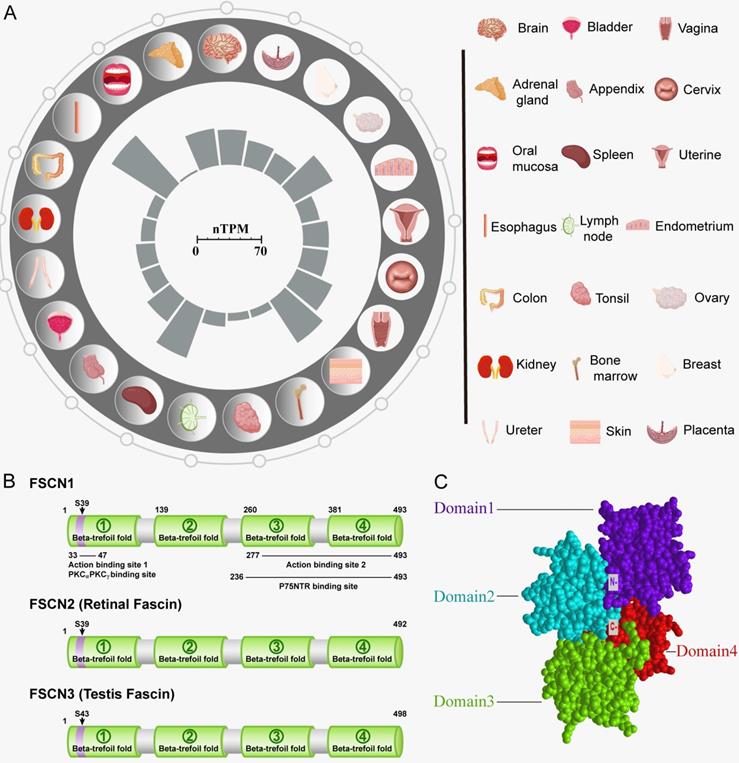
Structure and biological functions of FSCN1. A: Expression of FSCN1 in normal human organ tissues. The inner barchart on the left panel shows the expression profile of FSCN1 in normal human organs. The normalized expression (nTPM) values were obtained from The Human Protein Atlas; The right panel shows the name of the corresponding organ in the left panel. B: Schematic diagram of model proteins in three vertebrates; C: The three-dimensional structure of human FSCN1 protein; “N-” represents the N-terminal of FSCN1; “C-” represents the C-terminal of FSCN1.
FSCN1 gene and protein structures
In vertebrates, the expansion of the FSCN family has proceeded through a unique sequence of gene duplications. Three forms of FSCN exist in vertebrates, namely FSCN1, FSCN2, and FSCN3. FSCN1 is widely expressed in the nervous system as well as mesenchymal tissues (Figure 1A). FSCN2 is expressed in retinal photoreceptor cells, whereas FSCN3 is only expressed in the testis [2, 6]. In humans, FSCN1 is located on chromosome 7p22 [7], and FSCN2 is located on chromosome 17q25. In addition, each gene is adjacent to a member of the actin gene family [8, 9]. FSCN1 and FSCN2 share a 56% sequence identity, whereas FSCN3 exhibits 27% sequence identityhomology to FSCN1 and 28% to FSCN2 (Figure 1B) [4].
The FSCN1 protein comprises four β-trefoil domains (Domain 1: residues 1-139, Domain 2: residues 140-259, Domain 3: residues 260-382, and Domain 4: residues 383-493) (Figure 1C) [1]. Evidence suggests that Ser39 and Ser274 are related to phosphorylation-dependent regulation of actin-binding in FSCN1 [10, 11]. In addition, phosphorylation of FSCN1 at tyrosine 23 and Ser 38 is important for cell migration and filopodia formation in esophageal squamous cancer cells [12]. Monoubiquitination is a type of post-translational modification that regulates the actin-bundling activity and dynamics of FSCN1. FSCN1 is monoubiquitinated at two lysine residues (Lys247 and Lys250) in Domain 2 [13]. FSCN1 plays a role in tissues through two types of actin-based structures, namely, dynamic cortical cell protrusions and cytoplasmic microfilament bundles. The cortical structures include filopodia, spikes, lamellipodial ribs, oocyte microvilli, and the dendrites on dendritic cells, which play roles in cell-matrix adhesion, cell interactions, and cell migration. In contrast, the cytoplasmic actin bundles appear to be involved in cell architecture. The main function of FSCN1 is crosslinking of actin microfilaments into tight, rigid, and parallel bundles [14].
FSCN1 expression patterns and distribution in various cells and tissues
FSCN1 expression patterns exhibit spatiotemporal specificity. In Drosophila, FSCN1 is expressed in numerous cell types throughout the life cycle. In mouse embryos, Fscn1 is expressed in the nervous system (brain, spinal cord, and eye), developing somites, the condensing mesenchyme of limb buds, the skeletal and smooth muscle of various organs, and in heart ventricles at low levels [15]. The expression pattern of FSCN1 in human embryos and tissues is similar to that observed in mouse embryos [16]. Knockout of FSCN1 in an inbred strain of mice resulted in about 48% neonatal lethality, and the bodyweight of the surviving mice in the strain was observed to be reduced [17]. FSCN1 also plays a role in the formation and stability of filopodia or microspikes, which assist in cell migration [17-19]. The contractile phenotype of vascular smooth muscle cells (VSMCs) is converted to a migratory phenotype upon blood vessel injury, and FSCN1 is an important component of the dynamic podosomes that mediate VSMC migration in response to PDGF receptor signaling through c-Src kinase [20]. FSCN1 also plays a critical role in the formation of specialized podosomes, known as invadopodia, which mediate the invasion of melanoma-derived cells into a three-dimensional extracellular matrix [21]. This process is closely related to the invasion and migration capabilities and processes of cancer cells.
Biological processes involving FSCN1
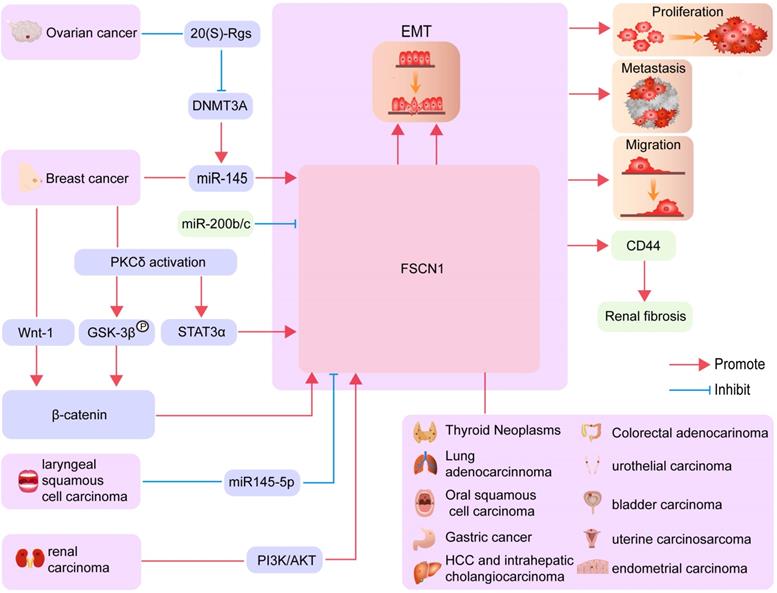
Elevated FSCN1 levels promote epithelial-to-mesenchymal transition (EMT)
EMT is a dedifferentiation process that converts adherent epithelial cells into singular migrating cells, which are essential for embryonic development, oncogenic progression, and metastasis [22]. EMT promotes stemness in normal breast tissues and breast cancer cells [22]. In addition, embryonic stem cell genes, including Oct4 and Nanog, positively regulate tumor metastasis through the enhancement of EMT in lung adenocarcinoma cells [23]. FSCN1 is a downstream effector of SNAI2 in the promotion of EMT, and FSCN1 mRNA levels are significantly elevated after the induction of transforming growth factor β (TGF-β) expression [24]. FSCN1 regulates EMT in various cancers, including ovarian cancer [25], squamous cell carcinoma, and lung cancer [26, 27]. Moreover, data obtained from 80 samples of gastric cancer patients indicated that FSCN1 promotes EMT in gastric cancer [28]. EMT is also a primary mechanism that contributes to resistance to chemotherapy in hepatocellular carcinoma [29].
The microRNA (MiR)-200b/c family is known to regulate the EMT process through directly targeting the FSCN1/CD44 axis and thereby inhibiting renal fibrosis [30]. A recent study reported that the suppression of miR‑145 expression in breast cancer cells affects cell migration by targeting FSCN1 and inhibiting EMT [31]. Inhibition of FSCN1 significantly suppresses vimentin expression and increases E-cadherin expression. Therefore, increased FSCN1 expression levels may promote the resistance of HCC against DOX by inducing EMT [32]. Research in the field of ovarian cancer has revealed that 20-(S)-Rg3 prevents EMT by targeting the DNMT3A/miR-145/FSCN1 pathway (Figure 2) [25].
Increased FSCN1 expression inhibits autophagy
Autophagy is a mechanism involving self-digestion in eukaryotic cells that removes abnormal proteins and damaged organelles through lysosomal degradation [33]. The role of autophagy in tumor biology is relatively complex [34]. During the early stages of tumor development, inhibition of autophagic activity induces continuous precancerous cell growth [35]. However, autophagy also enables the survival of advanced tumor cells in conditions of nutrient-limitation and low oxygen [36, 37]. In the endometrium, elevated FSCN1 levels reverse the inhibitory effect of autophagy on cell invasion. Additionally, the activation of autophagy inhibits the formation of filopodia by FSCN1 (Figure 2) [38, 39].
Biological functions and molecular mechanisms of FSCN1 in cancer Cancers of the nervous system
FSCN1 is expressed in cells of the central nervous system, such as microglia, astrocytes, and neurons, and is present in glioblastomas of all grades [40], wherein its function appears to be associated with cell motility, invasion, and immune response [41, 42]. FSCN1-mediated formation of filopodia is essential for mouse development [17]. In rat spinal cords, FSCN1 contributes to neuropathic pain through the promotion of inflammation [43]. FSCN1 also regulates the migration of subventricular zone-derived neuroblasts in the postnatal brain [44]. Furthermore, there is a correlation between increased FSCN1 expression and increasing grades of astrocytomas [45]. FSCN1 is also one of four proteins that are downregulated in the thalamus of stargazer mutant mice after γ-butyrolactone-induced seizures [46]. Additionally, increased FSCN1 expression has been observed in the hippocampus of Down's syndrome poly transgenic mice exhibiting impaired learning and memory [47].
Malignancy of the lymphatic system
Multiple cell types within the cardiovascular system express FSCN1, including dendritic cells, B lymphocytes, T lymphocytes, macrophages, neutrophils, platelets, vessel wall endothelial cells, smooth muscle cells, and fibroblasts [48]. In normal human peripheral blood, FSCN1 expression is restricted to dendritic cells, which play a primary role in the initiation of acquired immune responses (Figure 2) [49-51].
In a previous study, 187 patients with Hodgkin's disease, including 132 patients with nodular sclerosis, 34 with mixed cellularity, 14 with lymphocyte predominance (nodular), 2 with lymphocyte depleted, and 5 with unclassified types were evaluated. In all patients, except those with nodular lymphocyte predominance type, Reed-Sternberg cells and variants were found to be uniformly reactive for FSCN1. In most cases, almost all Reed-Sternberg cells and variants exhibited strong diffuse cytoplasmic staining. In some cases, the intensity of staining was varied. In the nodular sclerosis type, reactive cells frequently appeared in the form of aggregates, sheets, or syncytial masses mixed with recognizable interdigitating reticulum cells. These results indicated that FSCN1 represents a highly effective marker for the detection of specific dendritic cells in normal and neoplastic tissues and is a highly consistent marker for Reed-Sternberg cells and variants of the Hodgkin's disease [50].
The biological processes involving FSCN1 in different cancers. FSCN1 mediated EMT, proliferation, metastasis, and migration in various cancers are shown.
Head and neck cancers
Laryngeal squamous cell carcinoma (LSCC) is a common form of head and neck cancer and is generally associated with a poor prognosis. Quantitative RT-PCR and western blot analyses revealed that FSCN1 expression is significantly upregulated in LSCC tissues compared with that in adjacent normal mucosa tissues [52]. Loss-of-function studies showed that FSCN1 knockdown inhibited LSCC migration, invasion, and growth through the suppression of EMT [53]. Furthermore, Gao et al. identified that FSCN1 binds with AIMP1 and LTA4H in LSCC, and their results suggested that AIMP1 and LTA4H were possible effectors involved in FSCN1-mediated malignant progression of LSCC [54]. In addition, expression of FSCN1 is higher in tongue squamous cell carcinoma (TSCC) tissues and cells than in adjacent non-carcinoma tissues and normal control cells. Knockdown of FSCN1 inhibited TSCC cell viability and trans-migration in vitro and impaired tumor growth in vivo [55].
Cancers of the respiratory system
In the lungs, FSCN1 is directly recruited to mitochondria under metabolic stress conditions to stabilize mitochondrial actin filaments, thereby promoting mitochondrial oxidative phosphorylation by increasing the biogenesis of respiratory complex I. Similarly, FSCN1 promotes the metastatic colonization of lung cancer cells by enhancing metabolic stress resistance and mitochondrial oxidative phosphorylation [56]. Furthermore, FSCN1 is differentially expressed in non-small cell lung cancer (NSCLC) tissues and normal para-carcinoma tissues; moreover, FSCN1 expression in cancer tissues is associated with poor prognosis in patients with NSCLC (Figure 2) [57].
Cancers of the digestive system
Oral squamous cell carcinoma exhibits aggressive progression with a high incidence of nodal metastasis, even in the early stage [58]. In 2007, it was first demonstrated that FSCN1 overexpression was significantly associated with lymph node metastasis and tumor recurrence but not tumor stage or differentiation in oral squamous cell carcinoma (Figure 2) [59].
FSCN1 expression levels in esophageal squamous cell carcinoma are often increased compared with those in the normal epithelium, and FSCN1 overexpression is significantly associated with poor prognoses [60, 61]. Furthermore, non-phosphorylation mutations at tyrosine 23, serine 38, and serine 39 in β-trefoil domain 1 and at serine 274 in β-trefoil domain 3 promote cell motility and filopodia formation, while phosphorylation mutations at these sites inhibit cell functions and filopodia formation [12].
Normal gastric epithelial cells are negative for FSCN1 expression, whereas endothelial cells, lymphocytes, and stromal cells in the underlying lamina propria are positive. FSCN1 is mainly located in the cytoplasm of gastric adenocarcinoma cells (Figure 2) [62]. Enhanced immunostaining intensity of FSCN1 is correlated with higher histological grades, AJCC staging, and worse prognoses in Chinese patients with gastric adenocarcinoma [63].
In HCC, FSCN1-positive tumors are larger and less differentiated than are FSCN1-negative tumors, and they are more prone to portal venous invasion, bile duct invasion, and intrahepatic metastasis. In intrahepatic cholangiocarcinoma, FSCN1 exhibits similar expression characteristics (Figure 2) [64]. Additionally, FSCN1 expression is significantly correlated with high alpha-fetoprotein levels in HCC. Patients with FSCN1-positive HCC exhibit significantly poorer outcomes than patients with FSCN1-negative HCC, and FSCN1 is an independent prognostic factor for disease-free survival of HCC patients [65]. Through global gene expression analysis, FSCN1 levels were found to be increased during the transition from carcinoma in situ to invasive adenocarcinoma [66].
FSCN1 expression is downregulated in normal colonic epithelial cells. However, FSCN1 is expressed in subsets of adenomas and colorectal adenocarcinomas. Specifically, FSCN1 is widely expressed in 16% of adenomas and between 17% and 26% of adenocarcinomas. In 47% of colorectal tumors, FSCN1 expression is increased in the surrounding stroma regardless of the level of FSCN1 in the tumor. In adenomas, FSCN1 and Ki67 expressions are often inversely correlated at the cellular level, but this trend is less evident in adenocarcinomas. In advanced tumors, strong FSCN1 staining is significantly associated with poor prognosis. FSCN1 regulates cell morphology and migration and may represent a potential, novel marker or therapeutic target for the identification and treatment of patients with aggressive forms of colorectal adenocarcinoma (Figure 2) [67].
Cancers of the urinary system
FSCN1 expression is increased in actively growing renal carcinoma cell lines [7] compared with that in normal kidney cells [68]. Differences in the extent and intensity of FSCN1 immunohistochemical staining are useful to predict patient prognosis [69]. Similarly, increased FSCN1 levels are associated with aggressiveness of renal cell carcinoma in patients [70]. Additionally, increasing evidence suggests that FSCN1 is an effective predictive factor of tumor clinicopathological parameters in renal cell carcinoma [71]. In renal carcinoma cell lines, treatment with a PI3K or AKT inhibitor reduces FSCN1 protein and mRNA expression, indicating that FSCN1 may be regulated through the PI3K/AKT axis (Figure 2) [72].
In urothelial carcinoma tissues, increased FSCN1 expression is positively correlated with histological grade and pT stage. FSCN1 is also positively correlated with increased migration and invasion of cancer cells [73]. Furthermore, FSCN1 overexpression is associated with increased invasiveness of carcinomas in the urinary bladder (Figure 2) [74].
Gynecologic Cancer
FSCN1 directly affects the constitutive expression of the downstream targets of β-catenin and enhances the self-renewal ability. It is also crucial for the expression and function of the downstream targets of β-catenin induced by glycogen synthase kinase 3b inhibition. Moreover, the constitutive and inducible expression of the downstream targets of β-catenin mediated by FSCN1 depends, at least in part, on focal adhesion kinase (FAK). Among breast cancer patients, those with co-expression of FSCN1high and FAKhigh or high β-catenin downstream targets exhibit the least favorable survival outcomes, whereas in the FSCN1low group, the co-expression of FAKhigh or high β-catenin downstream targets has a less significant effect on patient survival [75].
In uterine carcinosarcoma, FSCN1 is abnormally expressed and is associated with aggressive metastatic behavior and poor prognoses [76]. Moreover, increased FSCN1 levels contribute to the highly invasive properties of endometrial carcinoma and can predict an epithelial-mesenchymal transition-like process. FSCN1 overexpression in intravascular tumor cells indicated increased metastatic risk, suggesting that FSCN1 may be an independent prognostic indicator for the different steps of extracellular matrix invasion [77-79].
The mechanisms underlying aberrant FSCN1 expression in cancer
Hypoxia-inducible factor 1 promotes FSCN1 expression
Hypoxia is one of the most common types of microenvironmental stress conditions observed in solid tumors. It is the result of excessive tumor growth and insufficient blood supply, which play a central role in tumor metastasis [80-82]. Hypoxia‑inducible factor‑1 (HIF‑1) is an important heterodimeric transcription factor comprising a highly regulated α subunit and a constitutively expressed β subunit. HIF‑1α is a key mediator of cellular responses to hypoxia and plays a critical role in regulating HIF‑1 transcriptional activity [83]. Additionally, it regulates the expression of target genes related to tumor invasion and metastasis [84]. HIF‑1α plays an important role in the tumor invasion and metastasis of head and neck squamous cell carcinoma [85]. FSCN1 is a direct target of HIF‑1α, and HIF‑1α may promote invasion and metastasis by upregulating the expression of FSCN1 in hypopharyngeal squamous cell carcinoma and pancreatic ductal adenocarcinoma (Figure 3) [86].
Long non-coding RNAs regulate FSCN1 expression
Long non-coding RNAs (lncRNAs) are a class of regulatory transcripts, longer than 200 nucleotides, with no protein-coding potential. lncRNAs act as molecular sponges for microRNAs, thereby reducing their influence on target mRNAs. The lncRNA ZEB1 anti-sense RNA 1 (ZEB1-AS1) functions as an oncogenic lncRNA in many types of cancers [87]. In recent studies, ZEB1-AS1 was identified as a downstream target of TGF-β1 and reported to contribute to the TGF-β1-mediated regulation of cell migration and invasion by upregulating the expression of the FSCN1 axis in bladder cancer cells (Figure 3) [88].
As the main effector of the Hippo pathway, Yes-associated protein 1 (YAP1) plays a key role in the regulation of a variety of biological functions, including intercellular contact inhibition, proliferation, and differentiation [89, 90]. YAP1 is a transcription coactivator that is highly expressed in colorectal cancer (CRC) [91-93]. Recently, a novel YAP1 regulatory model was proposed, which has attracted widespread attention. In this model, YAP1 transcriptionally regulates noncoding RNAs (ncRNAs) in CRC, including microRNAs such as miR-130a [93], miR-29 [94] and lncRNAs such as RMRP [95], BCAR4 [96], MALAT1 [97], and lncARSR [98]. Among these lncRNAs, LINC00152 is expressed at high levels in human CRC tissues. The suppression of LINC00152 expression results in the downregulation of 159 genes, thereby resulting in the inhibition of the malignant proliferation, invasion, and metastasis of CRC cells. LINC00152, as a novel YAP1 target, promotes the biological characteristics of CRC cells by sponging miR-185-3p and miR-632 to upregulate FSCN1 expression. These results suggest that the YAP1/LINC00152/FSCN1 axis promotes the malignant proliferation, migration, and metastasis of CRC (Figure 3) [99].
Regulatory mechanism of FSCN1 in cancers. Regulatory factors or signaling pathways that affect FSCN1 expression in various cancers are shown.
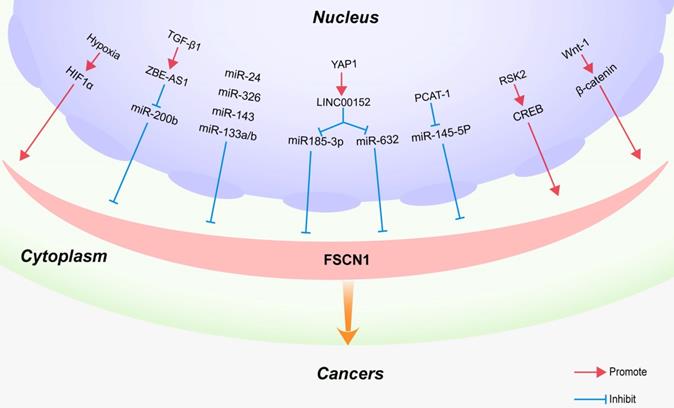
Bioinformatics analyses revealed that the seed regions of PCAT-1 and miR-145-5p exhibit some complementary pairing [100]. MiR-145-5p has been reported to exhibit an anti-oncogenic function in various cancers, including laryngeal cancer, gastric cancer, breast cancer, and renal cell carcinoma [101-103]. Moreover, miR-145 inhibits cell proliferation, migration, and invasion by targeting FSCN1 in prostate cancer [104]. Recent studies indicate that PCAT-1 acts as a competing endogenous RNA of miR-145-5p to upregulate FSCN1 expression, thereby promoting the development of prostate cancer (Figure 3) [100].
MicroRNAs regulate FSCN1 expression
MicroRNAs (miRNAs) are non-coding RNAs that have a length of 18-25 nucleotides. miRNAs suppress gene expression at the post-transcriptional level by binding to the 3'‑untranslated region of mRNAs [105]. MiR-133a negatively regulates various types of human malignant cancer cells, including NSCLC [106], gastric cancer [101], osteosarcoma [107], esophageal squamous cell carcinoma [108], ovarian cancer [109], and colorectal cancer [110]. By inhibiting the protein expression of FSCN1, miR‑133a partially inhibits the invasion of colorectal cancer cells (Figure 3) [111]. Wu et al. revealed that miR-488 inhibits the proliferation and motility of breast cancer cells by downregulating FSCN1 expression [112]. Furthermore, the FSCN1 gene has been identified as a direct target of several miRs, such as miR-145-5p in laryngeal cancer [53], miR-143 in chondrosarcoma and esophageal carcinoma [113], miR-24 in nasopharyngeal and prostate cancers [114, 115], and miR-326 in lung and gastric cancers (Figure 3) [116].
CREB signaling upregulates FSCN1 expression
cAMP response element-binding protein (CREB) and CREB-binding protein (CBP)/p300 play critical roles in epithelial tumorigenesis [117]. CREB is a transcription factor, whose signaling is implicated in the promotion of tumor progression, growth stimulation, apoptotic resistance, and the support of angiogenesis [118, 119]. Li et al. reported that activation of the CREB signaling pathway upregulates FSCN1 expression in breast adenocarcinoma, head and neck squamous cell carcinoma, and lung adenocarcinoma cells, leading to enhanced cancer cell invasion in vitro and tumor metastasis in vivo [120]. In neuronal precursor cells, knockdown of CBP downregulates mRNA and protein expression levels of FSCN1, indicating that FSCN1 is a downstream target of CBP [121].
Activation of the Wnt/βcatenin signaling pathway upregulates FSCN1 expression
The canonical Wnt/β-catenin signaling pathway has multiple functions in epithelial cancers, and increased levels of β-catenin accumulate in the nucleus during the late stages of tumor progression. β-catenin accumulation in the nucleus leads to its interaction with TCF/LEF factors, driving the transcription of target genes [122]. It has been demonstrated that the FSCN1 gene is a direct downstream target of the Wnt/β-catenin signaling pathway in colorectal cancer cells. Immunohistochemical staining revealed that FSCN1 was specifically localized at the invasive front of colon carcinomas that display nuclear β-catenin [123]. Consistent with these findings, Kim et al. demonstrated that inhibition of the Wnt/β-catenin pathway through the silencing of galectin-3 reduced FSCN1 expression in gastric cancer cells [124]. Furthermore, Hölsken et al. reported that knockdown of β-catenin through RNA interference significantly downregulated FSCN1 mRNA expression levels in adamantinomatous craniopharyngioma cells (Figure 2) [125].
Clinical significance of FSCN1 in cancer treatment
Owing to the extensive investigations into the functions of FSCN1, which have led to a greater understanding of its biological functions and mechanisms, FSCN1 is increasingly being used as a therapeutic target in cancer treatment. As a biological marker for EMT, FSCN1 is more sensitive and specific than the current standards that are used to diagnose antibody-mediated rejection, and it also exhibits good prognostic value for predicting future graft dysfunction [126]. Knockdown of the lncRNA CCAT1 enhances paclitaxel sensitivity in prostate cancer by regulating the expression of miR-24-3p and FSCN1 [114]. Additionally, FSCN1 knockdown inhibits cell migration and invasion in NSCLC through the regulation of the MAPK signaling pathway [127]. Co-targeting the epidermal growth factor receptor and FSCN1 has been proposed as a novel treatment strategy for the treatment of triple-negative breast cancer [128]. Furthermore, FSCN1-targeting anti-tumor drugs have been used in the treatment of various cancers. Temozolomide induces the expression of big potassium ion channels and inhibits FSCN1 expression in glioma, indicating that FSCN1 is a possible target for the treatment of glioma [129]. Docosahexaenoic acid inhibits breast cancer cell migration through the inhibition of FSCN1 [130]. Recombinant porcine NK-lysin inhibits the invasion of HCC in vitro, suggesting that this compound may be a potential therapeutic candidate that can be used in HCC treatment [131].
FSCN1 is a key downstream component of several crucial pathways associated with tumor progression, including EMT, the Wnt/β-catenin signaling pathway, and the expression of microRNAs. FSCN1 expression can help predict poor prognosis in patients with nasopharyngeal carcinoma [132]. Remarkably, several recent studies have highlighted the potential of FSCN1 as a novel prognostic biomarker and therapeutic target for the treatment of a variety of cancers [Table 1].
The inhibition of FSCN1 can lead to the reduction of invasion and migration capabilities of cells in a variety of cancers. The success of in vitro experimental studies regarding the role of DHA in breast cancer and that of recombinant porcine NK-lysin A in HCC have demonstrated that anticancer drugs targeting FSCN1 have significant potential for clinical applications [130, 131]. Co-targeting the epidermal growth factor receptor and FSCN1 has proved to be a promising novel therapeutic strategy for the treatment of triple-negative breast cancer [128]. With the advent of molecular targeted drugs, FSCN1 may have the potential to become a key target in cancer treatment. Improving our understanding of the mechanisms by which FSCN1 promotes cancer cell migration and invasion is therefore an essential initial step toward the development of FSCN1-specific drugs for cancer therapy in the future.
Role of FSCN1 in the diagnosis and treatment of different cancers
| Tumor Type | Related Drugs | Diagnosis | Treatment | References |
|---|---|---|---|---|
| Adrenocortical Carcinoma | None | poor prognostic markers | a potential therapeutic target | [135, 136] |
| Advanced Breast Cancer | None | poor prognostic markers | / | [137] |
| Bladder Urothelial Carcinoma | None | poor prognostic markers | a potential therapeutic target | [74, 138, 139] |
| Borderline Ovarian Tumor | None | poor prognostic markers | / | [140] |
| Breast Cancer | None | None | a potential therapeutic target | [128, 141-144] |
| Cholangiocarcinoma | None | poor prognostic markers | a potential therapeutic target | [145, 146] |
| Colorectal Cancer | None | poor prognostic markers | a potential therapeutic target | [147-149] |
| Esophageal Carcinoma | None | poor prognostic markers | a potential therapeutic target | [150-154] |
| Gastric Cancer | None | None | a potential therapeutic target | [155] |
| Hepatocellular Carcinoma | Doxycycline, Recombinant Porcine NK-lysin | poor prognostic markers | a potential therapeutic target | [32, 65, 131, 156, 157] |
| Laryngeal Squamous Cell Carcinoma | None | poor prognostic markers | a potential therapeutic target | [52-54, 134] |
| Lung Cancer | None | poor prognostic markers | a potential therapeutic target | [57, 158-160] |
| Nasopharyngeal Carcinoma | Doxorubicin | None | a potential therapeutic target | [161] |
| Oral Squamous Cell Carcinoma. | None | poor prognostic markers | a potential therapeutic target | [162, 163] |
| Ovarian Cancer | None | None | a potential therapeutic target | [164-167] |
| Pancreatic Cancer | None | poor prognostic markers | a potential therapeutic target | [66, 86, 168-171] |
| Renal Cell Carcinoma | None | poor prognostic markers | / | [70, 172] |
| Thyroid Neoplasms | None | poor prognostic markers | / | [173] |
Abbreviations
FSCN1: Fascin actin-bundling protein 1; LSCC: laryngeal squamous cell carcinoma; CBP: CREB-binding protein; CRC: colorectal cancer; CREB: cAMP response element-binding protein; DLD: dihydrolipoamide dehydrogenase; EMT: epithelial-to-mesenchymal transition; ENO2: enolase 2; HCC: hepatocellular carcinoma; HIF‑1: hypoxia‑inducible factor‑1; lncRNA: long non-coding RNA; miR: microRNA; miRs: microRNAs; mRNAs: messenger RNAs; NSCLC: non-small cell lung cancer; PCAT-1: prostate cancer-related lncRNA transcript 1; TGF: transforming growth factor; VSMC: vascular smooth muscle cells; ZEB1: zinc finger E-box binding homeobox 1; ZEB1-AS1: ZEB antisense RNA 1.
Acknowledgements
Funding
This work was supported by the National Natural Science Foundation of China (No. 82073101, and 82022054), Shenzhen Science and Technology Program (RCJC20210706091950028), Shenzhen Key Laboratory Foundation (ZDSYS20200811143757022), the Research Funds for China Central Government-guided Development of Local Science and Technology (No. 2020-165-19). Research Project Supported by Shanxi Scholarship Council of China (No. 2020165), Fund for the Scientific Activities of Selected Return Overseas Professionals in Shanxi Province (No. 20200034), Anhui Science Fund for Distinguished Young Scholars (No. 2008085J36).
Author Contributions
Conceptualization: WG, YYW, WLH. Compilation of literature: ZXL, JS, NNZ, and XWZ. Article writing and editing: ZXL, JS, NNZ, XWZ, and YKJ. Figure organization: ZXL, JS, NNZ, YKJ, and SXW. Supervision: WG, YYW, WLH. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ponting CP, Russell RB. Identification of distant homologues of fibroblast growth factors suggests a common ancestor for all beta-trefoil proteins. J Mol Biol. 2000;302:1041-7
2. Hashimoto Y, Skacel M, Adams JC. Roles of fascin in human carcinoma motility and signaling: prospects for a novel biomarker. Int J Biochem Cell Biol. 2005;37:1787-804
3. Pelosi G, Pastorino U, Pasini F, Maissoneuve P, Fraggetta F, Iannucci A. et al. Independent prognostic value of fascin immunoreactivity in stage I nonsmall cell lung cancer. Br J Cancer. 2003;88:537-47
4. Hashimoto Y, Kim DJ, Adams JC. The roles of fascins in health and disease. J Pathol. 2011;224:289-300
5. Kulasingam V, Diamandis EP. Fascin-1 is a novel biomarker of aggressiveness in some carcinomas. BMC Med. 2013;11:53
6. Adams JC. Roles of fascin in cell adhesion and motility. Curr Opin Cell Biol. 2004;16:590-6
7. Duh FM, Latif F, Weng Y, Geil L, Modi W, Stackhouse T. et al. cDNA cloning and expression of the human homolog of the sea urchin fascin and Drosophila singed genes which encodes an actin-bundling protein. DNA Cell Biol. 1994;13:821-7
8. Saishin Y, Ishikawa R, Ugawa S, Guo W, Ueda T, Morimura H. et al. Retinal fascin: functional nature, subcellular distribution, and chromosomal localization. Invest Ophthalmol Vis Sci. 2000;41:2087-95
9. Tubb BE, Bardien-Kruger S, Kashork CD, Shaffer LG, Ramagli LS, Xu J. et al. Characterization of human retinal fascin gene (FSCN2) at 17q25: close physical linkage of fascin and cytoplasmic actin genes. Genomics. 2000;65:146-56
10. Jansen S, Collins A, Yang C, Rebowski G, Svitkina T, Dominguez R. Mechanism of actin filament bundling by fascin. J Biol Chem. 2011;286:30087-96
11. Zanet J, Jayo A, Plaza S, Millard T, Parsons M, Stramer B. Fascin promotes filopodia formation independent of its role in actin bundling. J Cell Biol. 2012;197:477-86
12. Zeng FM, Wang XN, Shi HS, Xie JJ, Du ZP, Liao LD. et al. Fascin phosphorylation sites combine to regulate esophageal squamous cancer cell behavior. Amino Acids. 2017;49:943-55
13. Lin S, Lu S, Mulaj M, Fang B, Keeley T, Wan L. et al. Monoubiquitination Inhibits the Actin Bundling Activity of Fascin. J Biol Chem. 2016;291:27323-33
14. Kureishy N, Sapountzi V, Prag S, Anilkumar N, Adams JC. Fascins, and their roles in cell structure and function. Bioessays. 2002;24:350-61
15. De Arcangelis A, Georges-Labouesse E, Adams JC. Expression of fascin-1, the gene encoding the actin-bundling protein fascin-1, during mouse embryogenesis. Gene Expr Patterns. 2004;4:637-43
16. Zhang FR, Tao LH, Shen ZY, Lv Z, Xu LY, Li EM. Fascin expression in human embryonic, fetal, and normal adult tissue. J Histochem Cytochem. 2008;56:193-9
17. Yamakita Y, Matsumura F, Yamashiro S. Fascin1 is dispensable for mouse development but is favorable for neonatal survival. Cell Motil Cytoskeleton. 2009;66:524-34
18. Cohan CS, Welnhofer EA, Zhao L, Matsumura F, Yamashiro S. Role of the actin bundling protein fascin in growth cone morphogenesis: localization in filopodia and lamellipodia. Cell Motil Cytoskeleton. 2001;48:109-20
19. Adams JC, Schwartz MA. Stimulation of fascin spikes by thrombospondin-1 is mediated by the GTPases Rac and Cdc42. J Cell Biol. 2000;150:807-22
20. Quintavalle M, Elia L, Condorelli G, Courtneidge SA. MicroRNA control of podosome formation in vascular smooth muscle cells in vivo and in vitro. J Cell Biol. 2010;189:13-22
21. Li A, Dawson JC, Forero-Vargas M, Spence HJ, Yu X, König I. et al. The actin-bundling protein fascin stabilizes actin in invadopodia and potentiates protrusive invasion. Curr Biol. 2010;20:339-45
22. Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442-54
23. Chiou SH, Wang ML, Chou YT, Chen CJ, Hong CF, Hsieh WJ. et al. Coexpression of Oct4 and Nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell-like properties and epithelial-mesenchymal transdifferentiation. Cancer Res. 2010;70:10433-44
24. Keshamouni VG, Jagtap P, Michailidis G, Strahler JR, Kuick R, Reka AK. et al. Temporal quantitative proteomics by iTRAQ 2D-LC-MS/MS and corresponding mRNA expression analysis identify post-transcriptional modulation of actin-cytoskeleton regulators during TGF-beta-Induced epithelial-mesenchymal transition. J Proteome Res. 2009;8:35-47
25. Li J, Lu J, Ye Z, Han X, Zheng X, Hou H. et al. 20(S)-Rg3 blocked epithelial-mesenchymal transition through DNMT3A/miR-145/FSCN1 in ovarian cancer. Oncotarget. 2017;8:53375-86
26. Wang L, Jia Y, Jiang Z, Gao W, Wang B. FSCN1 is upregulated by SNAI2 and promotes epithelial to mesenchymal transition in head and neck squamous cell carcinoma. Cell Biol Int. 2017;41:833-41
27. Pan Y, Chen J, Tao L, Zhang K, Wang R, Chu X. et al. Long noncoding RNA ROR regulates chemoresistance in docetaxel-resistant lung adenocarcinoma cells via epithelial mesenchymal transition pathway. Oncotarget. 2017;8:33144-58
28. Wang G, Gu Y, Lu W, Liu X, Fu H. Fascin1 promotes gastric cancer progression by facilitatingcell migrationand epithelial-mesenchymal transition. Pathol Res Pract. 2018;214:1362-9
29. Piska K, Koczurkiewicz P, Bucki A, Wójcik-Pszczoła K, Kołaczkowski M, Pękala E. Metabolic carbonyl reduction of anthracyclines - role in cardiotoxicity and cancer resistance. Reducing enzymes as putative targets for novel cardioprotective and chemosensitizing agents. Invest New Drugs. 2017;35:375-85
30. Fu H, Gu YH, Yang YN, Liao S, Wang GH. MiR-200b/c family inhibits renal fibrosis through modulating epithelial-to-mesenchymal transition via targeting fascin-1/CD44 axis. Life Sci. 2020;252:117589
31. Zhao H, Kang X, Xia X, Wo L, Gu X, Hu Y. et al. miR-145 suppresses breast cancer cell migration by targeting FSCN-1 and inhibiting epithelial-mesenchymal transition. Am J Transl Res. 2016;8:3106-14
32. Zhang Y, Lu Y, Zhang C, Huang D, Wu W, Zhang Y. et al. FSCN-1 increases doxorubicin resistance in hepatocellular carcinoma through promotion of epithelial-mesenchymal transition. Int J Oncol. 2018;52:1455-64
33. Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069-75
34. Levy J, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17:528-42
35. Finkel T, Serrano M, Blasco MA. The common biology of cancer and ageing. Nature. 2007;448:767-74
36. Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891-906
37. Alva AS, Gultekin SH, Baehrecke EH. Autophagy in human tumors: cell survival or death. Cell Death Differ. 2004;11:1046-8
38. Luo X, Cheng W, Wang S, Chen Z, Tan J. Autophagy Suppresses Invasiveness of Endometrial Cells through Reduction of Fascin-1. Biomed Res Int. 2018;2018:8615435
39. Luo X, Cheng W, Wang S, Chen Z. [Involvement of fascin-1-mediated autophagy in the biological behavioral of endometrial cells]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018;43:957-63
40. Roma AA, Prayson RA. Fascin expression in 90 patients with glioblastoma multiforme. Ann Diagn Pathol. 2005;9:307-11
41. Hwang JH, Smith CA, Salhia B, Rutka JT. The role of fascin in the migration and invasiveness of malignant glioma cells. Neoplasia. 2008;10:149-59
42. Al-Alwan MM, Rowden G, Lee TD, West KA. Fascin is involved in the antigen presentation activity of mature dendritic cells. J Immunol. 2001;166:338-45
43. Wang B, Fan B, Dai Q, Xu X, Jiang P, Zhu L. et al. Fascin-1 Contributes to Neuropathic Pain by Promoting Inflammation in Rat Spinal Cord. Neurochem Res. 2018;43:287-96
44. Sonego M, Gajendra S, Parsons M, Ma Y, Hobbs C, Zentar MP. et al. Fascin regulates the migration of subventricular zone-derived neuroblasts in the postnatal brain. J Neurosci. 2013;33:12171-85
45. Peraud A, Mondal S, Hawkins C, Mastronardi M, Bailey K, Rutka JT. Expression of fascin, an actin-bundling protein, in astrocytomas of varying grades. Brain Tumor Pathol. 2003;20:53-8
46. Ryu MJ, Lee C, Kim J, Shin HS, Yu MH. Proteomic analysis of stargazer mutant mouse neuronal proteins involved in absence seizure. J Neurochem. 2008;104:1260-70
47. Shin JH, Guedj F, Delabar JM, Lubec G. Dysregulation of growth factor receptor-bound protein 2 and fascin in hippocampus of mice polytransgenic for chromosome 21 structures. Hippocampus. 2007;17:1180-92
48. Adams JC. Fascin protrusions in cell interactions. Trends Cardiovasc Med. 2004;14:221-6
49. Mosialos G, Birkenbach M, Ayehunie S, Matsumura F, Pinkus GS, Kieff E. et al. Circulating human dendritic cells differentially express high levels of a 55-kd actin-bundling protein. Am J Pathol. 1996;148:593-600
50. Pinkus GS, Pinkus JL, Langhoff E, Matsumura F, Yamashiro S, Mosialos G. et al. Fascin, a sensitive new marker for Reed-Sternberg cells of hodgkin's disease. Evidence for a dendritic or B cell derivation. Am J Pathol. 1997;150:543-62
51. Hart DN. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245-87
52. Gao W, Zhang C, Feng Y, Chen G, Wen S, Huangfu H. et al. Fascin-1, ezrin and paxillin contribute to the malignant progression and are predictors of clinical prognosis in laryngeal squamous cell carcinoma. PLoS One. 2012;7:e50710
53. Gao W, Zhang C, Li W, Li H, Sang J, Zhao Q. et al. Promoter Methylation-Regulated miR-145-5p Inhibits Laryngeal Squamous Cell Carcinoma Progression by Targeting FSCN1. Mol Ther. 2019;27:365-79
54. Gao W, An C, Xue X, Zheng X, Niu M, Zhang Y. et al. Mass Spectrometric Analysis Identifies AIMP1 and LTA4H as FSCN1-Binding Proteins in Laryngeal Squamous Cell Carcinoma. Proteomics. 2019;19:e1900059
55. Chen Y, Tian T, Li ZY, Wang CY, Deng R, Deng WY. et al. FSCN1 is an effective marker of poor prognosis and a potential therapeutic target in human tongue squamous cell carcinoma. Cell Death Dis. 2019;10:356
56. Lin S, Huang C, Gunda V, Sun J, Chellappan SP, Li Z. et al. Fascin Controls Metastatic Colonization and Mitochondrial Oxidative Phosphorylation by Remodeling Mitochondrial Actin Filaments. Cell Rep. 2019;28:2824-36
57. Zhang Y, Liang B, Dong H. Expression of fascin_1 protein in cancer tissues of patients with nonsmall cell lung cancer and its relevance to patients' clinicopathologic features and prognosis. J Cancer Res Ther. 2018;14:856-9
58. Yuen AP, Wei WI, Wong YM, Tang KC. Elective neck dissection versus observation in the treatment of early oral tongue carcinoma. Head Neck. 1997;19:583-8
59. Lee TK, Poon RT, Man K, Guan XY, Ma S, Liu XB. et al. Fascin over-expression is associated with aggressiveness of oral squamous cell carcinoma. Cancer Lett. 2007;254:308-15
60. Hashimoto Y, Ito T, Inoue H, Okumura T, Tanaka E, Tsunoda S. et al. Prognostic significance of fascin overexpression in human esophageal squamous cell carcinoma. Clin Cancer Res. 2005;11:2597-605
61. Zhang H, Xu L, Xiao D, Xie J, Zeng H, Cai W. et al. Fascin is a potential biomarker for early-stage oesophageal squamous cell carcinoma. J Clin Pathol. 2006;59:958-64
62. Hashimoto Y, Shimada Y, Kawamura J, Yamasaki S, Imamura M. The prognostic relevance of fascin expression in human gastric carcinoma. Oncology. 2004;67:262-70
63. Tsai WC, Jin JS, Chang WK, Chan DC, Yeh MK, Cherng SC. et al. Association of cortactin and fascin-1 expression in gastric adenocarcinoma: correlation with clinicopathological parameters. J Histochem Cytochem. 2007;55:955-62
64. Iguchi T, Aishima S, Taketomi A, Nishihara Y, Fujita N, Sanefuji K. et al. Fascin overexpression is involved in carcinogenesis and prognosis of human intrahepatic cholangiocarcinoma: immunohistochemical and molecular analysis. Hum Pathol. 2009;40:174-80
65. Iguchi T, Aishima S, Umeda K, Sanefuji K, Fujita N, Sugimachi K. et al. Fascin expression in progression and prognosis of hepatocellular carcinoma. J Surg Oncol. 2009;100:575-9
66. Swierczynski SL, Maitra A, Abraham SC, Iacobuzio-Donahue CA, Ashfaq R, Cameron JL. et al. Analysis of novel tumor markers in pancreatic and biliary carcinomas using tissue microarrays. Hum Pathol. 2004;35:357-66
67. Hashimoto Y, Skacel M, Lavery IC, Mukherjee AL, Casey G, Adams JC. Prognostic significance of fascin expression in advanced colorectal cancer: an immunohistochemical study of colorectal adenomas and adenocarcinomas. BMC Cancer. 2006;6:241
68. Sonderbye L, Meehan S, Palsson R, Ahsan N, Ladefoged J, Langhoff E. Immunohistochemical study of actin binding protein (p55) in the human kidney. Transplantation. 1998;65:1004-8
69. Craven RA, Stanley AJ, Hanrahan S, Dods J, Unwin R, Totty N. et al. Proteomic analysis of primary cell lines identifies protein changes present in renal cell carcinoma. Proteomics. 2006;6:2853-64
70. Jin JS, Yu CP, Sun GH, Lin YF, Chiang H, Chao TK. et al. Increasing expression of fascin in renal cell carcinoma associated with clinicopathological parameters of aggressiveness. Histol Histopathol. 2006;21:1287-93
71. Tsai WC, Sheu LF, Nieh S, Yu CP, Sun GH, Lin YF. et al. Association of EMMPRIN and fascin expression in renal cell carcinoma: correlation with clinicopathological parameters. World J Urol. 2007;25:73-80
72. Zhang M, Zhao Z, Duan X, Chen P, Peng Z, Qiu H. FSCN1 predicts survival and is regulated by a PI3K-dependent mechanism in renal cell carcinoma. J Cell Physiol. 2018;233:4748-58
73. Bi JB, Zhu Y, Chen XL, Yu M, Zhang YX, Li BX. et al. The role of fascin in migration and invasion of urothelial carcinoma of the bladder. Urol Int. 2013;91:227-35
74. Tong GX, Yee H, Chiriboga L, Hernandez O, Waisman J. Fascin-1 expression in papillary and invasive urothelial carcinomas of the urinary bladder. Hum Pathol. 2005;36:741-6
75. Barnawi R, Al-Khaldi S, Bakheet T, Fallatah M, Alaiya A, Ghebeh H. et al. Fascin Activates β-Catenin Signaling and Promotes Breast Cancer Stem Cell Function Mainly Through Focal Adhesion Kinase (FAK): Relation With Disease Progression. Front Oncol. 2020;10:440
76. Richmond AM, Blake EA, Torkko K, Smith EE, Spillman MA, Post MD. Fascin Is Associated with Aggressive Behavior and Poor Outcome in Uterine Carcinosarcoma. Int J Gynecol Cancer. 2017;27:1895-903
77. Stewart CJ, Crook ML. Fascin expression in undifferentiated and dedifferentiated endometrial carcinoma. Hum Pathol. 2015;46:1514-20
78. Gun BD, Bahadir B, Bektas S, Barut F, Yurdakan G, Kandemir NO. et al. Clinicopathological significance of fascin and CD44v6 expression in endometrioid carcinoma. Diagn Pathol. 2012;7:80
79. Xue LY, Zou SM, Zheng S, Xie YQ, Wen P, Liu XY. et al. [Expression of fascin and CK14 in different histological types of cancer and its differential diagnostic significance]. Zhonghua Zhong Liu Za Zhi. 2010;32:838-44
80. Shay JE, Celeste Simon M. Hypoxia-inducible factors: crosstalk between inflammation and metabolism. Semin Cell Dev Biol. 2012;23:389-94
81. Subarsky P, Hill RP. The hypoxic tumour microenvironment and metastatic progression. Clin Exp Metastasis. 2003;20:237-50
82. Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer. 2014;14:430-9
83. Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda). 2004;19:176-82
84. Brahimi-Horn MC, Bellot G, Pouysségur J. Hypoxia and energetic tumour metabolism. Curr Opin Genet Dev. 2011;21:67-72
85. Zhu G, Peng F, Gong W, She L, Wei M, Tan H. et al. Hypoxia promotes migration/invasion and glycolysis in head and neck squamous cell carcinoma via an HIF-1α-MTDH loop. Oncol Rep. 2017;38:2893-900
86. Zhao X, Gao S, Ren H, Sun W, Zhang H, Sun J. et al. Hypoxia-inducible factor-1 promotes pancreatic ductal adenocarcinoma invasion and metastasis by activating transcription of the actin-bundling protein fascin. Cancer Res. 2014;74:2455-64
87. Li J, Li Z, Leng K, Xu Y, Ji D, Huang L. et al. ZEB1-AS1: A crucial cancer-related long non-coding RNA. Cell Prolif. 2018 51
88. Gao R, Zhang N, Yang J, Zhu Y, Zhang Z, Wang J. et al. Long non-coding RNA ZEB1-AS1 regulates miR-200b/FSCN1 signaling and enhances migration and invasion induced by TGF-β1 in bladder cancer cells. J Exp Clin Cancer Res. 2019;38:111
89. Yu FX, Zhao B, Guan KL. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell. 2015;163:811-28
90. Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246-57
91. Ou C, Sun Z, Li X, Li X, Ren W, Qin Z. et al. MiR-590-5p, a density-sensitive microRNA, inhibits tumorigenesis by targeting YAP1 in colorectal cancer. Cancer Lett. 2017;399:53-63
92. Zhao B, Li L, Lei Q, Guan KL. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 2010;24:862-74
93. Shen S, Guo X, Yan H, Lu Y, Ji X, Li L. et al. A miR-130a-YAP positive feedback loop promotes organ size and tumorigenesis. Cell Res. 2015;25:997-1012
94. Tumaneng K, Schlegelmilch K, Russell RC, Yimlamai D, Basnet H, Mahadevan N. et al. YAP mediates crosstalk between the Hippo and PI(3)K-TOR pathways by suppressing PTEN via miR-29. Nat Cell Biol. 2012;14:1322-9
95. Park J, Jeong S. Wnt activated β-catenin and YAP proteins enhance the expression of non-coding RNA component of RNase MRP in colon cancer cells. Oncotarget. 2015;6:34658-68
96. Zheng X, Han H, Liu GP, Ma YX, Pan RL, Sang LJ. et al. LncRNA wires up Hippo and Hedgehog signaling to reprogramme glucose metabolism. EMBO J. 2017;36:3325-35
97. Sun Z, Ou C, Liu J, Chen C, Zhou Q, Yang S. et al. YAP1-induced MALAT1 promotes epithelial-mesenchymal transition and angiogenesis by sponging miR-126-5p in colorectal cancer. Oncogene. 2019;38:2627-44
98. Qu L, Wu Z, Li Y, Xu Z, Liu B, Liu F. et al. A feed-forward loop between lncARSR and YAP activity promotes expansion of renal tumour-initiating cells. Nat Commun. 2016;7:12692
99. Ou C, Sun Z, He X, Li X, Fan S, Zheng X. et al. Targeting YAP1/LINC00152/FSCN1 Signaling Axis Prevents the Progression of Colorectal Cancer. Adv Sci (Weinh). 2020;7:1901380
100. Xu W, Chang J, Du X, Hou J. Long non-coding RNA PCAT-1 contributes to tumorigenesis by regulating FSCN1 via miR-145-5p in prostate cancer. Biomed Pharmacother. 2017;95:1112-8
101. Qiu T, Zhou X, Wang J, Du Y, Xu J, Huang Z. et al. MiR-145, miR-133a and miR-133b inhibit proliferation, migration, invasion and cell cycle progression via targeting transcription factor Sp1 in gastric cancer. FEBS Lett. 2014;588:1168-77
102. Yan X, Chen X, Liang H, Deng T, Chen W, Zhang S. et al. miR-143 and miR-145 synergistically regulate ERBB3 to suppress cell proliferation and invasion in breast cancer. Mol Cancer. 2014;13:220
103. Lu R, Ji Z, Li X, Zhai Q, Zhao C, Jiang Z. et al. miR-145 functions as tumor suppressor and targets two oncogenes, ANGPT2 and NEDD9, in renal cell carcinoma. J Cancer Res Clin Oncol. 2014;140:387-97
104. Fuse M, Nohata N, Kojima S, Sakamoto S, Chiyomaru T, Kawakami K. et al. Restoration of miR-145 expression suppresses cell proliferation, migration and invasion in prostate cancer by targeting FSCN1. Int J Oncol. 2011;38:1093-101
105. Ambros V. The functions of animal microRNAs. Nature. 2004;431:350-5
106. Wang LK, Hsiao TH, Hong TM, Chen HY, Kao SH, Wang WL. et al. MicroRNA-133a suppresses multiple oncogenic membrane receptors and cell invasion in non-small cell lung carcinoma. PLoS One. 2014;9:e96765
107. Fujiwara T, Katsuda T, Hagiwara K, Kosaka N, Yoshioka Y, Takahashi RU. et al. Clinical relevance and therapeutic significance of microRNA-133a expression profiles and functions in malignant osteosarcoma-initiating cells. Stem Cells. 2014;32:959-73
108. Akanuma N, Hoshino I, Akutsu Y, Murakami K, Isozaki Y, Maruyama T. et al. MicroRNA-133a regulates the mRNAs of two invadopodia-related proteins, FSCN1 and MMP14, in esophageal cancer. Br J Cancer. 2014;110:189-98
109. Guo J, Xia B, Meng F, Lou G. miR-133a suppresses ovarian cancer cell proliferation by directly targeting insulin-like growth factor 1 receptor. Tumour Biol. 2014;35:1557-64
110. Wang H, An H, Wang B, Liao Q, Li W, Jin X. et al. miR-133a represses tumour growth and metastasis in colorectal cancer by targeting LIM and SH3 protein 1 and inhibiting the MAPK pathway. Eur J Cancer. 2013;49:3924-35
111. Zheng K, Liu W, Liu Y, Jiang C, Qian Q. MicroRNA-133a suppresses colorectal cancer cell invasion by targeting Fascin1. Oncol Lett. 2015;9:869-74
112. Wu Y, Yuan MH, Wu HT, Chen WJ, Zhang ML, Ye QQ. et al. MicroRNA-488 inhibits proliferation and motility of tumor cells via downregulating FSCN1, modulated by Notch3 in breast carcinomas. Cell Death Dis. 2020;11:912
113. Urdinez J, Boro A, Mazumdar A, Arlt MJ, Muff R, Botter SM. et al. The miR-143/145 Cluster, a Novel Diagnostic Biomarker in Chondrosarcoma, Acts as a Tumor Suppressor and Directly Inhibits Fascin-1. J Bone Miner Res. 2020;35:1077-91
114. Li X, Han X, Wei P, Yang J, Sun J. Knockdown of lncRNA CCAT1 enhances sensitivity of paclitaxel in prostate cancer via regulating miR-24-3p and FSCN1. Cancer Biol Ther. 2020;21:452-62
115. Li YQ, Lu JH, Bao XM, Wang XF, Wu JH, Hong WQ. MiR-24 functions as a tumor suppressor in nasopharyngeal carcinoma through targeting FSCN1. J Exp Clin Cancer Res. 2015;34:130
116. Zhang N, Nan A, Chen L, Li X, Jia Y, Qiu M. et al. Circular RNA circSATB2 promotes progression of non-small cell lung cancer cells. Mol Cancer. 2020;19:101
117. Karamouzis MV, Konstantinopoulos PA, Papavassiliou AG. Roles of CREB-binding protein (CBP)/p300 in respiratory epithelium tumorigenesis. Cell Res. 2007;17:324-32
118. Abramovitch R, Tavor E, Jacob-Hirsch J, Zeira E, Amariglio N, Pappo O. et al. A pivotal role of cyclic AMP-responsive element binding protein in tumor progression. Cancer Res. 2004;64:1338-46
119. Jiang H, Chen SS, Yang J, Chen J, He B, Zhu LH. et al. CREB-binding protein silencing inhibits thrombin-induced endothelial progenitor cells angiogenesis. Mol Biol Rep. 2012;39:2773-9
120. Li D, Jin L, Alesi GN, Kim YM, Fan J, Seo JH. et al. The prometastatic ribosomal S6 kinase 2-cAMP response element-binding protein (RSK2-CREB) signaling pathway up-regulates the actin-binding protein fascin-1 to promote tumor metastasis. J Biol Chem. 2013;288:32528-38
121. Megiorni F, Indovina P, Mora B, Mazzilli MC. Minor expression of fascin-1 gene (FSCN1) in NTera2 cells depleted of CREB-binding protein. Neurosci Lett. 2005;381:169-74
122. Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192-205
123. Vignjevic D, Schoumacher M, Gavert N, Janssen KP, Jih G, Laé M. et al. Fascin, a novel target of beta-catenin-TCF signaling, is expressed at the invasive front of human colon cancer. Cancer Res. 2007;67:6844-53
124. Kim SJ, Choi IJ, Cheong TC, Lee SJ, Lotan R, Park SH. et al. Galectin-3 increases gastric cancer cell motility by up-regulating fascin-1 expression. Gastroenterology. 2010;138:1035-45 e1-2
125. Hölsken A, Buchfelder M, Fahlbusch R, Blümcke I, Buslei R. Tumour cell migration in adamantinomatous craniopharyngiomas is promoted by activated Wnt-signalling. Acta Neuropathol. 2010;119:631-9
126. Xu-Dubois YC, Peltier J, Brocheriou I, Suberbielle-Boissel C, Djamali A, Reese S. et al. Markers of Endothelial-to-Mesenchymal Transition: Evidence for Antibody-Endothelium Interaction during Antibody-Mediated Rejection in Kidney Recipients. J Am Soc Nephrol. 2016;27:324-32
127. Zhao D, Zhang T, Hou XM, Ling XL. Knockdown of fascin-1 expression suppresses cell migration and invasion of non-small cell lung cancer by regulating the MAPK pathway. Biochem Biophys Res Commun. 2018;497:694-9
128. Wang CQ, Li Y, Huang BF, Zhao YM, Yuan H, Guo D. et al. EGFR conjunct FSCN1 as a Novel Therapeutic Strategy in Triple-Negative Breast Cancer. Sci Rep. 2017;7:15654
129. Hoa NT, Ge L, Martini F, Chau V, Ahluwalia A, Kruse CA. et al. Temozolomide induces the expression of the glioma Big Potassium (gBK) ion channel, while inhibiting fascin-1 expression: possible targets for glioma therapy. Expert Opin Ther Targets. 2016;20:1155-67
130. Lii CK, Chang JW, Chen JJ, Chen HW, Liu KL, Yeh SL. et al. Docosahexaenoic acid inhibits 12-O-tetradecanoylphorbol-13- acetate-induced fascin-1-dependent breast cancer cell migration by suppressing the PKCδ- and Wnt-1/β-catenin-mediated pathways. Oncotarget. 2016;7:25162-79
131. Khan A, Fan K, Sun N, Yin W, Sun Y, Sun P. et al. Recombinant porcine NK-lysin inhibits the invasion of hepatocellular carcinoma cells in vitro. Int J Biol Macromol. 2019;140:1249-59
132. Wu D, Chen L, Liao W, Ding Y, Zhang Q, Li Z. et al. Fascin1 expression predicts poor prognosis in patients with nasopharyngeal carcinoma and correlates with tumor invasion. Ann Oncol. 2010;21:589-96
133. Mahdiannasser M, Haghpanah V, Damavandi E, Kabuli M, Tavangar SM, Larijani B. et al. Investigation of promoter methylation of FSCN1 gene and FSCN1 protein expression in differentiated thyroid carcinomas. Mol Biol Rep. 2020;47:2161-9
134. Liu H, Cui J, Zhang Y, Niu M, Xue X, Yin H. et al. Mass spectrometry-based proteomic analysis of FSCN1-interacting proteins in laryngeal squamous cell carcinoma cells. IUBMB Life. 2019;71:1771-84
135. Poli G, Ceni E, Armignacco R, Ercolino T, Canu L, Baroni G. et al. 2D-DIGE proteomic analysis identifies new potential therapeutic targets for adrenocortical carcinoma. Oncotarget. 2015;6:5695-706
136. Poli G, Ruggiero C, Cantini G, Canu L, Baroni G, Armignacco R. et al. Fascin-1 Is a Novel Prognostic Biomarker Associated With Tumor Invasiveness in Adrenocortical Carcinoma. J Clin Endocrinol Metab. 2019;104:1712-24
137. Min KW, Chae SW, Kim DH, DO SI, Kim K, Lee HJ. et al. Fascin expression predicts an aggressive clinical course in patients with advanced breast cancer. Oncol Lett. 2015;10:121-30
138. Gomaa W, Al-Maghrabi H, Al-Attas M, Al-Ghamdi F, Al-Maghrabi J. Fascin expression in urinary bladder urothelial carcinoma correlates with unfavourable prognosis. Int J Clin Exp Pathol. 2019;12:3901-7
139. Bi J, Chen X, Zhang Y, Li B, Sun J, Shen H. et al. Fascin is a predictor for invasiveness and recurrence of urothelial carcinoma of bladder. Urol Oncol. 2012;30:688-94
140. El-Balat A, Arsenic R, Sänger N, Karn T, Becker S, Holtrich U. et al. Fascin-1 expression as stratification marker in borderline epithelial tumours of the ovary. J Clin Pathol. 2016;69:142-8
141. Götte M, Mohr C, Koo CY, Stock C, Vaske AK, Viola M. et al. miR-145-dependent targeting of junctional adhesion molecule A and modulation of fascin expression are associated with reduced breast cancer cell motility and invasiveness. Oncogene. 2010;29:6569-80
142. Wang C, Huang B, Wu Z, Sun X, Zeng Y, Wang Y. [Expression of Fascin-1 protein in breast cancer and its clinicopathologic correlation]. Zhonghua Bing Li Xue Za Zhi. 2014;43:451-4
143. Osanai M, Lee GH. The retinoic acid-metabolizing enzyme CYP26A1 upregulates fascin and promotes the malignant behavior of breast carcinoma cells. Oncol Rep. 2015;34:850-8
144. Esnakula AK, Ricks-Santi L, Kwagyan J, Kanaan YM, DeWitty RL, Wilson LL. et al. Strong association of fascin expression with triple negative breast cancer and basal-like phenotype in African-American women. J Clin Pathol. 2014;67:153-60
145. Ruys AT, Groot Koerkamp B, Wiggers JK, Klümpen HJ, ten Kate FJ, van Gulik TM. Prognostic biomarkers in patients with resected cholangiocarcinoma: a systematic review and meta-analysis. Ann Surg Oncol. 2014;21:487-500
146. Zhao H, Yang F, Zhao W, Zhang C, Liu J. Fascin Overexpression Promotes Cholangiocarcinoma RBE Cell Proliferation, Migration, and Invasion. Technol Cancer Res Treat. 2016;15:322-33
147. Montoro-García S, Alburquerque-González B, Bernabé-García Á, Bernabé-García M, Rodrigues PC, den-Haan H. et al. Novel anti-invasive properties of a Fascin1 inhibitor on colorectal cancer cells. J Mol Med (Berl). 2020;98:383-94
148. Oh SY, Kim YB, Suh KW, Paek OJ, Moon HY. Prognostic impact of fascin-1 expression is more significant in advanced colorectal cancer. J Surg Res. 2012;172:102-8
149. Adams JC. Fascin-1 as a biomarker and prospective therapeutic target in colorectal cancer. Expert Rev Mol Diagn. 2015;15:41-8
150. Wang C, Wang J, Chen Z, Gao Y, He J. Immunohistochemical prognostic markers of esophageal squamous cell carcinoma: a systematic review. Chin J Cancer. 2017;36:65
151. Cao HH, Zheng CP, Wang SH, Wu JY, Shen JH, Xu XE. et al. A molecular prognostic model predicts esophageal squamous cell carcinoma prognosis. PLoS One. 2014;9:e106007
152. Xue LY, Hu N, Song YM, Zou SM, Shou JZ, Qian LX. et al. Tissue microarray analysis reveals a tight correlation between protein expression pattern and progression of esophageal squamous cell carcinoma. BMC Cancer. 2006;6:296
153. Hsu KF, Lin CK, Yu CP, Tzao C, Lee SC, Lee YY. et al. Cortactin, fascin, and survivin expression associated with clinicopathological parameters in esophageal squamous cell carcinoma. Dis Esophagus. 2009;22:402-8
154. Shen SN, Li K, Liu Y, Yang CL, He CY, Wang HR. Silencing lncRNAs PVT1 Upregulates miR-145 and Confers Inhibitory Effects on Viability, Invasion, and Migration in EC. Mol Ther Nucleic Acids. 2020;19:668-82
155. Lai C, Chen Z, Li R. MicroRNA-133a inhibits proliferation and invasion, and induces apoptosis in gastric carcinoma cells via targeting fascin actin-bundling protein 1. Mol Med Rep. 2015;12:1473-8
156. Elewa MA, Al-Gayyar MM, Schaalan MF, Abd El Galil KH, Ebrahim MA, El-Shishtawy MM. Hepatoprotective and anti-tumor effects of targeting MMP-9 in hepatocellular carcinoma and its relation to vascular invasion markers. Clin Exp Metastasis. 2015;32:479-93
157. Gkretsi V, Bogdanos DP. Experimental evidence of Migfilin as a new therapeutic target of hepatocellular carcinoma metastasis. Exp Cell Res. 2015;334:219-27
158. Zhao W, Gao J, Wu J, Liu QH, Wang ZG, Li HL. et al. Expression of Fascin-1 on human lung cancer and paracarcinoma tissue and its relation to clinicopathological characteristics in patients with lung cancer. Onco Targets Ther. 2015;8:2571-6
159. Zhang Y, Yang X, Wu H, Zhou W, Liu Z. MicroRNA-145 inhibits migration and invasion via inhibition of fascin 1 protein expression in non-small-cell lung cancer cells. Mol Med Rep. 2015;12:6193-8
160. Choi PJ, Yang DK, Son CH, Lee KE, Lee JI, Roh MS. Fascin immunoreactivity for preoperatively predicting lymph node metastases in peripheral adenocarcinoma of the lung 3 cm or less in diameter. Eur J Cardiothorac Surg. 2006;30:538-42
161. Alaeddini M, Fouladdel S, Etemad-Moghadam S, Azizi E. Expression of fascin protein and mRNA in the KB carcinoma cell line following treatment with doxorubicin. J Cancer Res Ther. 2011;7:427-32
162. Alam H, Bhate AV, Gangadaran P, Sawant SS, Salot S, Sehgal L. et al. Fascin overexpression promotes neoplastic progression in oral squamous cell carcinoma. BMC Cancer. 2012;12:32
163. Rodrigues PC, Sawazaki-Calone I, Ervolino de Oliveira C, Soares Macedo CC, Dourado MR, Cervigne NK. et al. Fascin promotes migration and invasion and is a prognostic marker for oral squamous cell carcinoma. Oncotarget. 2017;8:74736-54
164. McGuire S, Kara B, Hart PC, Montag A, Wroblewski K, Fazal S. et al. Inhibition of fascin in cancer and stromal cells blocks ovarian cancer metastasis. Gynecol Oncol. 2019;153:405-15
165. Zhang S, Lin QD, DI W. Suppression of human ovarian carcinoma metastasis by the metastasis-suppressor gene, BRMS1. Int J Gynecol Cancer. 2006;16:522-31
166. Lin CK, Su HY, Tsai WC, Sheu LF, Jin JS. Association of cortactin, fascin-1 and epidermal growth factor receptor (EGFR) expression in ovarian carcinomas: correlation with clinicopathological parameters. Dis Markers. 2008;25:17-26
167. Kabukcuoglu S, Oner U, Ozalp SS, Bildirici K, Yalcin OT, Colak E. The role of actin bundling protein fascin in the progression of ovarian neoplasms. Eur J Gynaecol Oncol. 2006;27:171-6
168. Lu Z, Hu L, Evers S, Chen J, Shen Y. Differential expression profiling of human pancreatic adenocarcinoma and healthy pancreatic tissue. Proteomics. 2004;4:3975-88
169. Tsai WC, Lin CK, Lee HS, Gao HW, Nieh S, Chan DC. et al. The correlation of cortactin and fascin-1 expression with clinicopathological parameters in pancreatic and ampulla of Vater adenocarcinoma. APMIS. 2013;121:171-81
170. Yoshida K, Kuramitsu Y, Murakami K, Ryozawa S, Taba K, Kaino S. et al. Proteomic differential display analysis for TS-1-resistant and -sensitive pancreatic cancer cells using two-dimensional gel electrophoresis and mass spectrometry. Anticancer Res. 2011;31:2103-8
171. Schenk M, Aykut B, Teske C, Giese NA, Weitz J, Welsch T. Salinomycin inhibits growth of pancreatic cancer and cancer cell migration by disruption of actin stress fiber integrity. Cancer Lett. 2015;358:161-9
172. Zigeuner R, Droschl N, Tauber V, Rehak P, Langner C. Biologic significance of fascin expression in clear cell renal cell carcinoma: systematic analysis of primary and metastatic tumor tissues using a tissue microarray technique. Urology. 2006;68:518-22
173. Chen G, Zhang FR, Ren J, Tao LH, Shen ZY, Lv Z. et al. Expression of fascin in thyroid neoplasms: a novel diagnostic marker. J Cancer Res Clin Oncol. 2008;134:947-51
Author contact
![]() Corresponding authors: Professor Wei Gao, M.D., Email: wei.gaoedu.cn, ORCID: 0000-0001-7836-2851; Professor Yongyan Wu, Ph.D., Email: wuyongyanedu.cn, ORCID: 0000-0003-1669-3860; Professor Wanglai Hu, Ph.D., Email: wanglaihuedu.cn, ORCID: 0000-0003-0194-4006
Corresponding authors: Professor Wei Gao, M.D., Email: wei.gaoedu.cn, ORCID: 0000-0001-7836-2851; Professor Yongyan Wu, Ph.D., Email: wuyongyanedu.cn, ORCID: 0000-0003-1669-3860; Professor Wanglai Hu, Ph.D., Email: wanglaihuedu.cn, ORCID: 0000-0003-0194-4006

 Global reach, higher impact
Global reach, higher impact