Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(8):2673-2682. doi:10.7150/jca.71514 This issue Cite
Research Paper
Fasting serum glucose and lymph node metastasis in non-diabetic PTC patients: a 10-Year multicenter retrospective study
1. Department of Thyroid Surgery, Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China.
2. The second clinical medicine college, Medical Department, Nanchang University, Nanchang, Jiangxi, China.
3. Department of General Surgery, The First Affiliated Hospital of Jiangxi Medical College, Shangrao, Jiangxi, China.
4. Department of Otolaryngology, Yichun People's Hospital, Yichun, Jiangxi, China.
5. Department of Otolaryngology, Xinfeng County People's Hospital, Ganzhou, Jiangxi, China.
*Yushu Liu, Jiantao Gong and Yanyi Huang contributed equally to this work.
Received 2022-1-28; Accepted 2022-5-8; Published 2022-5-20
Abstract
Background: Mostly current studies are limited to the impact of lymph node metastasis(LNM) on the prognosis of papillary thyroid cancer(PTC) or the impact of glucose metabolism on the occurrence of PTC, but no one has paid attention to the connection between fasting serum glucose(FSG) and LNM. The purpose of our study was to explore the relationship between FSG and LNM in non-diabetic PTC patients.
Methods: In this study, we performed a multicenter, retrospective study on 6034 non-diabetic patients with PTC. The associations of FSG with three types of LNM including central lymph node metastasis (CLNM), lateral cervical lymph node metastasis (LLNM) and both were estimated.
Results: Compared with PTC patients without LNM, those with LNM had higher FSG. We also found that FSG was associated with tumor extension, maximum tumor diameter and TSH. In order to further explore the association between FSG and different types of LNM, we analyzed three groups of data separately. Our study reveals that by comparing FSG between patients without LNM and patients with three LNM types, it was statistically different in the PTC patients with CLNM and the PTC patients with CLNM combined with LLNM.
Conclusion: Our study provides evidence for the association of FSG and LNM in non-diabetic PTC patients, with a gradual increase in FSG over the course of the PTC from no lymph node metastasis to CLNM combined with LLNM. Meanwhile, higher FSG is a risk factor for CLNM and CLNM combined with LLNM. In the future, FSG might be used as an indicator for lymph node dissection in PTC patients. However, larger relative studies are needed.
Keywords: Papillary thyroid carcinoma, Fasting serum glucose, Lymph node metastasis
Introduction
According to the American Cancer Society in 2019, the incidence of thyroid cancer has become the fifth leading cause of cancer in women [1], and the incidence of thyroid cancer has increased faster than any other types of cancer in the world [2, 3]. Papillary thyroid cancer (PTC) is the most common type, accounting for 84% of all thyroid cancers [4]. Lymph node metastasis (LNM) is one of the accepted prognostic indicators of PTC [5], and compared with lateral cervical lymph node metastasis (LLNM), central lymph node metastasis (CLNM) is more common [6]. This is a manifestation of cancer's ability to invade and metastasize. Therefore, the indicators affecting LNM in PTC patients deserve more attention.
The most important three metabolisms in human body are glucose metabolism, lipid metabolism and amino acid metabolism. Any kind of metabolic disorder will affect other metabolisms, which is particularly important in the course of PTC [7]. The main forms of sugar in the human body are glucose and glycogen. Glucose is the main form of sugar in blood and it is an important source of energy. The concentration of blood glucose is an important index to reflect the state of glucose metabolism. The oxidation process of glucose can provide energy for cells and promote the growth, development, invasion and metastasis of cells [8]. Many researchers have studied the association between diabetes and cancer [9], but according to the data we collected, the role of glucose in the occurrence and development of cancer in PTC patients without diabetes has not been reported. Mostly current studies are limited to the impact of LNM on the prognosis of PTC [10] or the impact of glucose metabolism on the occurrence of PTC [11], but no one has paid attention to the connection between the glucose metabolism and LNM in PTC patients. Therefore, the purpose of our study was to explore the relationship between fasting serum glucose (FSG) and LNM in PTC patients without diabetes.
In this study, we collected the clinicopathological data of 5440 PTC patients from the Second Affiliated Hospital of Nanchang University and 594 PTC patients from The People's Hospital of Yichun City and Xinfeng County People's Hospital for analyzing the relationship between FSG and three types of LNM (CLNM, LLNM, CLNM + LLNM). According to the information we collected, this study is the first to reveal an association between LNM and FSG. In addition, we found that with the course of the PTC from no lymph node metastasis to CLNM combined with LLNM, FSG gradually increased. Higher FSG is a risk factor for CLNM and CLNM combined with LLNM.
Materials and Methods
Data sources
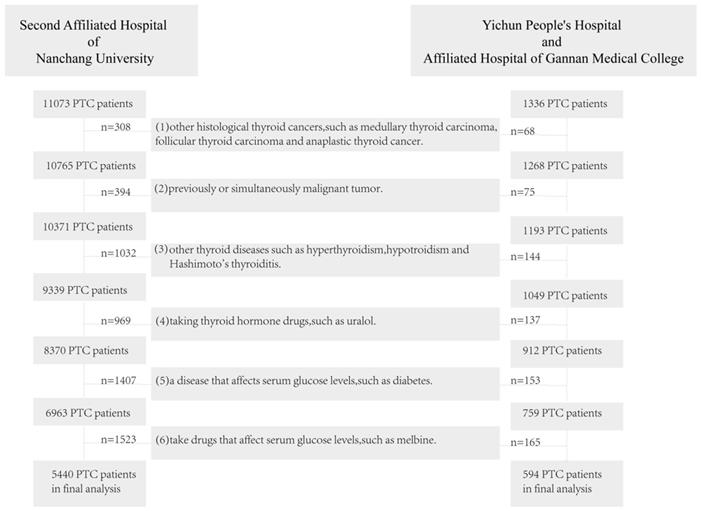
In this study, we performed a multicenter, retrospective study on 11073 patients with PTC treated at the Second Affiliated Hospital of Nanchang University from May 2011 to July 2021 and 1336 patients with PTC treated at the Yichun People's Hospital and Xinfeng County People's Hospital from August 2015 to May 2021. The inclusion criteria were histology-confirmed PTC, and complete baseline data (including age and gender), tumor biopsy data (including CLNM, LLNM, number of lesions (hereinafter referred to as 'lesions'), the condition of tumor extension (hereinafter referred to as 'extension'), maximum tumor diameter) and preoperative laboratory data (including preoperative serum TSH, free triiodothyronine (fT3), free thyroxine (fT4) and FSG). The exclusion criteria are as follows: (1) other histological thyroid cancers, such as medullary thyroid carcinoma, follicular thyroid carcinoma and anaplastic thyroid cancer; (2) previously or simultaneously malignant tumor; (3) other thyroid diseases such as hyperthyroidism, hypothyroidism and Hashimoto's thyroiditis; (4) taking thyroid hormone drugs, such as Euthyrox; (5) a disease that affects FSG levels, such as diabetes; (6) taking drugs that affect FSG levels, such as melbine. All patients signed informed consent and were approved by the ethics committee. Screening through exclusion criteria, the remaining 5440 patients from the Second Affiliated Hospital of Nanchang University and 594 patients from the Yichun People's Hospital and Xinfeng County People's Hospital were included in our study.
Data collection
Baseline data were obtained from outpatient data. Tumor biopsy data were obtained from patient pathology and color Doppler ultrasound reports. All laboratory data (blood chemistry analysis) were acquired in the next morning after the patient's admission to hospital (between 6:00 and 8:00 AM).Patients were required to fast for at least 8 hours before sample collection. The serum was immediately separated.
Treatment
According to the National Comprehensive Cancer Network guidelines, the standard treatment for our study was thyroidectomy. Laboratory data included preoperative serum TSH, fT3, fT4 and FSG. They were tested through blood samples which were collected from each patient 8 to 10 hours before surgery. An automatic chemiluminescence detection system (Cobas E411) was commonly used machine for the testing.
Statistical analysis
All statistical analyses were performed using R software (3.6.1). Categorical variables are represented by number and percentage, and continuous variables are represented by mean ± standard deviation. Mann-Whitney U test was used to analyze the difference between continuous variables and Chi-square test was used to analyze the difference between categorical variables. Kolmogorov-Smirnov test was used to analyze the difference among the groups. The Receiver operating characteristic (ROC) curves were used to determine the optimal cut-off value of the variables and the area under the curve (AUC) was used to reflect theirs predictive power. In our study, p-value < 0.05 was statically significant.
Results
Clinical baseline characteristics
According to the inclusion criteria and exclusion criteria, we finally selected 5440 PTC patients from the Second Affiliated Hospital of Nanchang University and 594 PTC patients from the Yichun People's Hospital and Xinfeng County People's Hospital for data analysis (Figure 1). Our data analysis process is shown in Figure 2.For analyzing the factors which affected LNM in PTC patients, we used the sample function in R to randomly divide the 5440 patients from the Second Affiliated Hospital of Nanchang University into the development cohort (n=2720) and internal validation cohort (n=2720) according to the ratio of 1:1. The patients from the Yichun People's Hospital and Xinfeng County People's Hospital were used as external validation cohort. The development cohort was used to construct the model predicting the status of LNM. The internal and external validation cohorts were used to verify the predictive effect of the model. By using Kolmogorov-Smirnov test [12], we could confirm that there was no statistical difference among three groups. More details were shown in Table 1.
Baseline characteristics of PTC patients
| Charateristics | Development cohort | Internal validation cohort | External validation cohort | P |
|---|---|---|---|---|
| N/Mean±SD | ||||
| Number of patients | n = 2720 | n = 2720 | n = 594 | - |
| Age | 42.72±11.74 | 43.11±11.95 | 43.56±12.93 | 0.253 |
| Gender | ||||
| Male | 676(24.85%) | 646(23.75%) | 135(22.73%) | 0.443 |
| Female | 2044(75.15%) | 2074(76.25%) | 459(77.27%) | |
| LNM | ||||
| Yes | 918(33.75%) | 921(33.86%) | 208(35.02%) | 0.836 |
| No | 1802(66.25%) | 1799(66.14%) | 386(64.98%) | |
| Clnm | ||||
| Yes | 852(31.32%) | 838(30.81%) | 182(30.64%) | 0.899 |
| No | 1868(68.68%) | 1882(69.19%) | 412(69.36%) | |
| LLNM | ||||
| Yes | 312(11.47%) | 295(10.85%) | 67(11.28%) | 0.762 |
| No | 2408(88.53%) | 2425(89.15%) | 527(88.72%) | |
| Lesions | ||||
| Unifocal | 1934(71.10%) | 1882(69.19%) | 405(68.18%) | 0.187 |
| Multifocal | 786(28.90%) | 838(30.81%) | 189(31.82%) | |
| Extension | ||||
| Yes | 646(23.75%) | 683(25.11%) | 141(23.74%) | 0.471 |
| No | 2074(76.25%) | 2037(74.89%) | 453(76.26%) | |
| Maximum tumor diameter (cm) | 1.16±0.96 | 1.12±0.93 | 1.22±1.03 | 0.115 |
| FSG | 5.81±1.32 | 5.84±1.33 | 5.82±1.17 | 0.239 |
| TSH | 2.22±2.48 | 2.17±2.29 | 2.18±2.14 | 0.275 |
| fT3 | 3.23±0.52 | 3.25±0.56 | 3.13±1.01 | 0.170 |
| fT4 | 1.28±0.31 | 1.29±0.37 | 1.28±0.38 | 0.516 |
| fT3/fT4 | 2.59±0.50 | 2.61±0.55 | 2.63±1.16 | 0.148 |
Abbreviations: PTC, papillary thyroid cancer; LNM, lymph node metastasis; CLNM, central lymph node metastasis; LLNM, lateral cervical lymph node metastasis; TSH,t hyrotrophin; fT3, free triiodothyronine; fT4, free thyroxine; extension, extension of tumor
Explore the association FSG and LNM
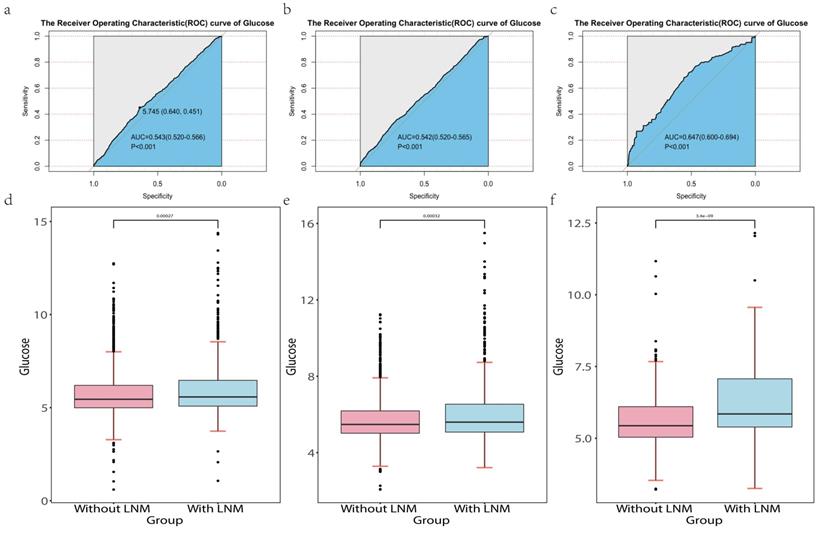
We used the "pROC" package [13]to explore the association between FSG and lymph node metastasis. In our study, P<0.05 was statically significant. The result was shown in Figure 3 a-c. In the development cohort, the AUC and 95%CI of FSG were 0.543 (0.520-0.566, P<0.001). The optimal cut-off values for FSG was 5.745(specificity 64.0%, sensitivity 45.1%).In the internal and external validation cohort, the AUC and 95%CI of FSG were 0.542 (0.520-0.565,P<0.001) and 0.647 (0.600-0.694,P<0.001). It indicates that blood glucose concentration has certain diagnostic value in judging whether lymph node metastasis occurs in PTC patients. In additional, "ggplot2" package [14] was used which visually showed the differential level of FSG in the group with LNM and without LNM in three cohorts (Figure 3d-f). The results revealed that the higher FSG is associated with the greater possibility of LNM. Therefore, higher FSG is an independent risk factor for LNM.
The correlation between FSG and other indicators
According to the optimal cut-off value in the development cohort, patients were divided into the high and the low FSG groups. In the development cohort, there were statistically significant differences in age (P<0.001), gender (P<0.001), LNM (P<0.001), extension (P<0.001), maximum tumor diameter (P<0.001) and TSH (P<0.001) between the high and the low FSG groups (Table 2).We performed the same analysis in the internal validation cohort and the external validation cohort. In the internal validation cohort, there were statistically significant differences in age (P<0.001), gender (P<0.001), LNM (P=0.010), lesions (P=0.012), extension (P=0.005), maximum tumor diameter (P=0.028) and TSH (P<0.001) between the high and the low FSG groups. In the external validation cohort, there were statistically significant differences in LNM (P<0.001), lesions (P<0.001), extension (P=0.012), maximum tumor diameter (P=0.020) and TSH (P=0.003) between the high and the low FSG groups. In the analysis of the three cohorts, except for lesions, the groups showed statistical differences in LNM, extension, maximum tumor diameter and TSH. It can be seen from the three cohorts that compared with the low FSG group, the incidence of LNM and tumor extension in the high FSG group increased, and the mean TSH also increased. Therefore, it is reasonable to believe that there is some correlation among FSG, TSH, extension, maximum tumor diameter and LNM.
PTC patients exclusion flowchart. Abbreviation: PTC, papillary thyroid cancer
Correlation between FSG and other indicators of PTC patients in the development cohort,internal and external validation cohort
| Characteristics | Development cohort | Internal validation cohort | External validation cohort | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FSG<5.745 (n=1658) | FSG≥5.745 (n=1062) | P | FSG<5.745 (n=1624) | FSG≥5.745 (n=1096) | P | FSG<5.745 (n=359) | FSG≥5.745 (n=235) | P | ||
| Age (years)# | 40.97(11.5) | 45.46(11.6) | <0.001* | 41.58(11.9) | 45.39(11.6) | <0.001* | 42.81(12.9) | 44.71(12.9) | 0.073 | |
| Gender | ||||||||||
| Male | 369(22.3%) | 307(28.9%) | <0.001* | 348(21.4%) | 298(27.2%) | <0.001* | 72(20.1%) | 63(26.8%) | 0.069 | |
| Female | 1289(77.7%) | 755(71.1%) | 1276(78.6%) | 798(72.8%) | 287(79.9%) | 172(73.2%) | ||||
| LNM | ||||||||||
| Yes | 504(30.4%) | 414(39.0%) | <0.001* | 518(31.9%) | 403(36.8%) | 0.010* | 101(28.1%) | 107(45.5%) | <0.001* | |
| No | 1154(69.6%) | 648(61.0%) | 1106(68.1%) | 693(63.2%) | 258(71.9%) | 128(54.5%) | ||||
| Lesions | ||||||||||
| Unifocal | 1198(72.3%) | 736(69.3%) | 0.107 | 1154(71.1%) | 728(66.4%) | 0.012* | 300(83.6%) | 153(65.1%) | <0.001* | |
| Multifocal | 460(27.7%) | 326(30.7%) | 470(28.9%) | 368(33.6%) | 59(16.4%) | 82(34.9%) | ||||
| Extension | ||||||||||
| Yes | 353(21.3%) | 293(27.6%) | <0.001* | 376(23.2%) | 307(28.0%) | 0.005* | 131(36.5%) | 111(47.2%) | 0.012* | |
| No | 1305(78.7%) | 769(72.4%) | 1248(76.8%) | 789(72.0%) | 228(63.5%) | 124(52.8%) | ||||
| Maximum tumor diameter(cm)# | 1.07(0.8) | 1.3(1.1) | <0.001* | 1.08(0.9) | 1.19(1.0) | 0.028* | 1.10(0.9) | 1.39(1.2) | 0.020* | |
| TSH# | 2.04(2.7) | 2.49(2.1) | <0.001* | 2.02(2.4) | 2.39(2.1) | <0.001* | 2.13(2.3) | 2.29(1.8) | 0.003* | |
| fT3# | 3.24(0.5) | 3.23(0.5) | 0.622 | 3.26(0.5) | 3.23(0.6) | 0.076 | 3.08(1.1) | 3.21(0.9) | 0.093 | |
| fT4# | 1.28(0.3) | 1.29(0.3) | 0.961 | 1.28(0.3) | 1.29(0.4) | 0.967 | 1.27(0.3) | 1.30(0.5) | 0.636 | |
| fT3/fT4# | 2.60(0.5) | 2.57(0.4) | 0.270 | 2.62(0.6) | 2.60(0.5) | 0.286 | 2.58(1.7) | 2.69(1.1) | 0.327 | |
Abbreviations: FSG, fasting serum glucose; PTC, papillary thyroid cancer; TSH, thyroid stimulating hormone; extension, extension of tumor; fT3, free triiodothyronine; fT4, free thyroxine
#Mean (standard deviation)
*P < 0.05 considered as statistically significant.
Effects of FSG on three types of LNM
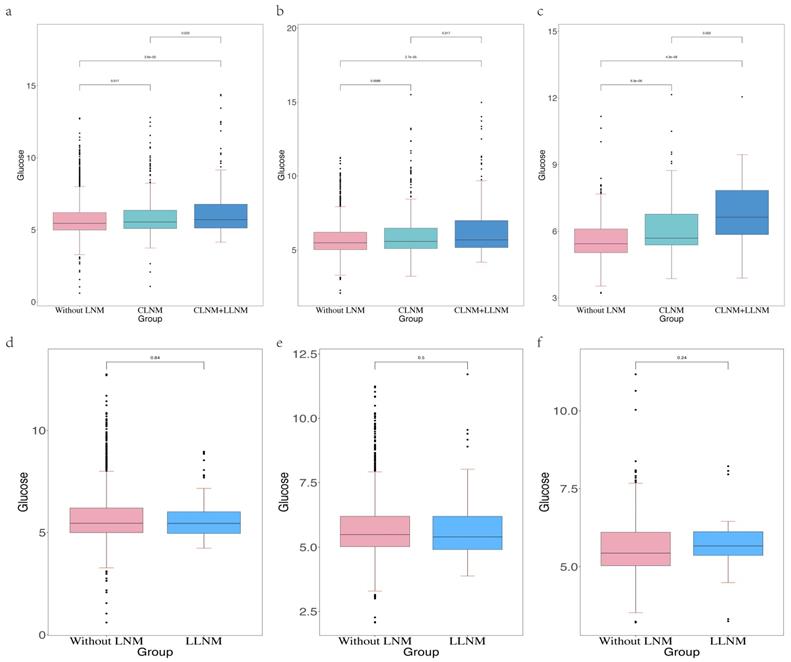
Cervical lymph node metastasis (CLNM) in PTC patients is usually considered to first metastasized to the central region of the neck, followed by the lateral neck region [15]. However, there is a special condition of LLNM without CLNM. This condition is called skip metastases. The incidence of skip metastases ranged from 6.8% to 27.8% [16, 17]. It is consistent with our data (Table 3). Our study has shown that higher FSG is an independent risk factor for LNM, but the predictive power of FSG in the three types of LNM was still not clear. Therefore, in order to further determine whether FSG also has strong predictive power among the three LNM types and in which types of LNM it has the best predictive power, we compared the distribution of FSG between patients without LNM and patients with three LNM types (Figure 4). We found that higher FSG could be a risk factor for CLNM and CLNM combined with LLNM. The occurrence and location of LNM can be seen as one of the diagnostic criteria for the course of cancer. In the three groups of patients without LNM, only with CLNM, and with CLNM combined with LLNM, the FSG value of patients without LNM was the lowest, and the FSG value of patients with CLNM combined with LLNM was the highest. And there was a statistically significant difference in FSG. Therefore, we believed that the FSG gradually increased with the course of PTC. Clinically, when patients have high blood glucose manifestations, we are supposed to be alert to the occurrence of CLNM and CLNM combined with LLNM. Central lymph node dissection can be performed if necessary. However, there was no statistical difference in FSG between patients without LNM and patients with skip metastasis, this may be because skip metastasis is mainly affected by the location and size of the primary tumor. According to Dou Y et al. [18], PTC patients are more prone to skip metastasis when their tumors are located at the upper pole of the thyroid gland. In addition, skip metastasis is more likely to occur when the diameter of primary tumor is less than 0.5cm [19]. It can be inferred that skip metastasis is more likely to occur early in the course of PTC.
LNM in the development cohort,internal and external validation cohort
| LNM | Development cohort (n=918) | Internal validation cohort (n=921) | External validation cohort (n=208) |
|---|---|---|---|
| CLNM | 606(66.01%) | 626(67.97%) | 141(67.79%) |
| CLNM+LLNM | 246(26.80%) | 212(23.02%) | 41(19.71%) |
| LLNM | 66(7.19%) | 83(9.01%) | 26(12.5%) |
Abbreviations: PTC, papillary thyroid cancer; LNM, lymph node metastasis; CLNM, central lymph node metastasis; LLNM, lateral cervical lymph node metastasis.
The data analysis process of the article. Abbreviations: PTC, Papillary thyroid cancer; FSG, fasting serum glucose; LNM, lymph node metastasis; LLNM, lateral cervical lymph node metastasis; CLNM, central lymph node metastasis
Abbreviation: LNM, lymph node metastasis. The Receiver Operating Characteristics (ROC) curve of glucose in the development cohort (a), internal validation cohort (b) and external validation cohort (c).The differential level of glucose in the patients with LNM and without LNM in the development cohort (d), internal validation cohort (e) and external validation cohort (f).
Discussion
LNM is a significant risk factor affecting the prognosis of cancer patients [5], so it is very useful to explore the related factors of LNM. Glucose is an important factor reflecting human metabolic status [20], but no one has analyzed the correlation between FSG and LNM yet. For the first time, we analyzed the association between FSG and LNM in non-diabetic PTC patients. In this study, we analyzed it from 2,720 PTC patients spanning 10 years and validated our result in both an internal validation cohort of 2,720 PTC patients and an external validation cohort of 594 PTC patients. We drew a conclusion that FSG and LNM are indeed correlated to a certain extent. FSG gradually increased throughout the progression of PTC from no lymph metastasis to LLNM (excluding skip metastasis). Meanwhile, higher FSG is a risk factor for CLNM and CLNM combined with LLNM. The long time span and large number of patients enhance the reliability of the results of our study.
Others have studied the effects of many factors on LNM [21-23]. Compared with previous studies, the current work is a significant advance. Prior studies were limited to data from a single institution and were not validated by external data. More importantly, no studies have been conducted on the relationship between FSG and LNM in PTC patients without diabetes. Our study not only included multiple cohorts from three institutions for training and validation, but also for the first time analyzed the association between FSG and LNM in PTC patients without diabetes and strictly screened eligible patients. Furthermore, we analyzed PTC patients without lymph node metastasis, only with CLNM, CLNM combined with LLNM and skip metastasis, and found that FSG increased over the course of the PTC from no lymph node metastasis to CLNM combined with LLNM.
Our data confirm previous studies. Xiang Y et al. [24] showed that elevated TSH is a risk factor for LNM, which may be related to the ability of LncPVT1 to regulate the proliferation of thyroid cancer cell by recruiting EZH2 and regulating thyroid stimulating hormone receptors [25]. Lower fT3 concentration[26] is associated with increased thyroid cancer, and its bioavailability is regulated by three pyiodimidine deiodinase (D) [type I(D1), type II(D2), and type III(D3)] [27]. Maximum tumor diameter [28], lesions [29] and extension [6] are visual representations of tumor growth, which are also reported to be closely related to LNM. Existing studies [18, 30] have claimed that tumor site, maximum tumor diameter, gender, extension and CLNM are risk factors for LLNM, but the association between FSG and it has not been reported.
Our study was the first to find an association between FSG and LNM, with a gradual increase in FSG over the course of the PTC from no lymph node metastasis to CLNM combined with LLNM. Meanwhile, higher FSG is a risk factor for CLNM and CLNM combined with LLNM.
TSH is considered to be a major stimulant of thyroid function [31] and cell proliferation [32]. TRH stimulates the pituitary and promotes the synthesis and secretion of TSH. TSH acts on the thyroid gland and stimulates thyroid hormone biosynthesis and secretion. T3 and T4 regulate the secretion of TRH and TSH through negative feedback [33]. TSH induces thyroid cell growth directly by binding to its own receptors, and indirectly by stimulating the production of autocrine or parocrine growth factors such as vascular endothelial growth factor (VEGF) [34] and amyloid precursors [35]. VEGF-C induces lymphangiectasia (lymphangiogenesis) in primary tumors and drained sentinel lymph nodes to promote the growth of tumor-associated lymphatic vessels and enhance LNM [36]. But other pathways such as mitogen-activated protein kinase (MAPK) [37], phosphoinositide 3 kinase (PI3-K) [38], mammalian target of rapamycin (mTOR) [39] and insulin growth factor (IGF) system [40] play an equally important role in the proliferation and growth of thyroid cells and their precursors/stem cells.
The differential level of glucose in the patients without LNM, with CLNM and with CLNM combined with LLNM in the development cohort (a), internal validation cohort (b) and external validation cohort (c).The differential level of glucose in the patients without LNM, with CLNM and with LLNM in the development cohort (d), internal validation cohort (e) and external validation cohort (f).
Insulin /IGF axis is an important pathway for the proliferation of normal thyroid cells and tumor cells. In addition to its own effects, the stimulation of TSH on thyroid growth is partially dependent on other growth hormones, including insulin and IGF [41]. They coordinate with cAMP to regulate the expression of thyroid transcription factor 2(TTF-2) [42]. Studies have shown that crosstalk between insulin/IGF axis and TSH also plays a role in abnormal thyroid cell proliferation, and insulin and IGF can greatly enhance the tumor-promoting effect of TSH [43]. At the same time, stimulation of TSH on FRTL-5 thyroid cells enhances tyrosine phosphorylation of insulin receptor substrate (IRS)-2 triggered by IGF-1, leading to enhanced IGF-1-dependent proliferation [44].
Overexpression of IGF-1, IGF-1R, IGF-2 and insulin receptor (IR) can be seen in the early stage of thyroid cancer [45]. Insulin-like growth factor-1 receptor (IGF-1R) plays an important role in diabetic insulin resistance and hyperinsulinemia, and it has been reported that the expression of IGF-1R is significantly increased in lung cancer combined with type 2 diabetes [46]. Yan Y et al. [47] showed that in PTC tissues, the expression of IGF-1R in diabetic patients was significantly higher than that in non-diabetic patients. Hyperglycemia can promote the production of advanced glycation end products (AGEs), and then tumor cell proliferation, and directly or indirectly promote IGF-1R phosphorylation (activation) [48, 49]. Elevated circulating insulin levels are associated with an increased risk of cancer and aggressive and metastatic cancer phenotypes [50]. Our study showed that in PTC patients without diabetes, higher FSG was positively associated with cancer progression, and hyperglycemia was strongly associated with an increased maximum tumor diameter and extension, which may also be associated with IGF-1-associated insulin resistance and hyperinsulinemia. However, more data is needed to prove our conjecture. Whether IGF-1 is activated in PTC patients without diabetes and the specific mechanism and pathway of activation also need more research and demonstration.
The advantage of our study is that for the first time, we found a potential association between FSG and LNM. Based on a large amount of clinical data and a long time span, FSG increased over the course of the PTC from no lymph node metastasis to CLNM combined with LLNM. Higher FSG is a risk factor for CLNM and CLNM combined with LLNM. However, our study still has some shortcomings. First, the retrospective nature of the study may cause bias. Meanwhile, the patients included in our study were limited to Jiangxi Province, and patients from other regions of China or other countries were not studied. Regional environmental differences and ethnic differences may also cause certain bias. Our data span 10 years, which can enhance the generalizability of the analysis results, but given the update of testing technology during the decade, different testing instruments may bias the analysis results. In addition, there are technical limitations in detecting CLNM, so the diagnosis of skip metastases may not have high sensitivity and specificity. The lack of prospective studies to validate the prediction model is a major limitation of this study. In spite of this, the LNM prediction model established by us still has satisfactory predictive power, and the increase of FSG with the course of the PTC from no lymph node metastasis to CLNM combined with LLNM is credible.
Conclusion
Our results suggest that FSG have a good ability to predict LNM in PTC patients, with a gradual increase in FSG over the course of the PTC. Meanwhile, higher FSG is a risk factor for CLNM and CLNM combined with LLNM. However, prospective studies and more data are needed to confirm our conclusion.
Abbreviations
PTC: Papillary thyroid carcinoma; LNM: lymph node metastasis; CLNM: central lymph node metastasis; LLNM: lateral cervical lymph node metastasis; TSH: thyrotrophin; fT3: free triiodothyronine; fT4: free thyroxine; AUC: the area under the curve; CI: confidence interval; ROC: receiver operating characteristic curves.
Acknowledgements
This work received a grant from the National Natural Science Foundation of China (nfsc: 81660294) and the Young and Middle-aged Doctor Research Project of the Beijing Bethune Public Welfare Foundation and the General Project of Jiangxi Provincial Health Commission (no.202210576).
Author contributions
YSL, JTG, YYH, ZYW and YXL jointly designed this study. YSL worked with JTG and YYH to collect clinical data from PTC patients. SSX, LC and TY further collated and preliminarily analyzed the data. YSL conducted statistical analysis and drew the figures and tables of the whole article. JTG wrote the results section of the manuscript, while YYH wrote the rest of the manuscript. LC, TY and ZYW reviewed and revised the manuscript. All authors read and approved the finally manuscript.
Ethics approval and consent to participate
The experimental protocol was established, according to the ethical guidelines of the Helsinki Declaration and was approved by the Human Ethics Committee of The Second Affiliated Hospital of Nanchang University Written informed consent was obtained from individual or guardian participants.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30
3. Wiltshire JJ, Drake TM, Uttley L. et al. Systematic Review of Trends in the Incidence Rates of Thyroid Cancer. Thyroid. 2016;26:1541-52
4. Brito JP, Gionfriddo MR, Al Nofal A. et al. The accuracy of thyroid nodule ultrasound to predict thyroid cancer: systematic review and meta-analysis. J Clin Endocrinol Metab. 2014;99:1253-63
5. Faro FN, Bezerra  MLB, Scalissi NM. et al. Intermediate-risk thyroid carcinoma: indicators of a poor prognosis. Arch Endocrinol Metab. 2021;64:764-71
6. Feng JW, Yang XH, Wu BQ. et al. Predictive factors for central lymph node and lateral cervical lymph node metastases in papillary thyroid carcinoma. Clin Transl Oncol. 2019;21:1482-91
7. Tang Z, Xu Z, Zhu X. et al. New insights into molecules and pathways of cancer metabolism and therapeutic implications. Cancer Commun (Lond). 2021;41:16-36
8. Hantzidiamantis PJ, Lappin SL. Physiology, Glucose. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC. 2022
9. Malaguarnera R, Vella V, Nicolosi ML. et al. Insulin Resistance: Any Role in the Changing Epidemiology of Thyroid Cancer? Front Endocrinol (Lausanne). 2017;8:314
10. Genpeng L, Jianyong L, Jiaying Y. et al. Independent predictors and lymph node metastasis characteristics of multifocal papillary thyroid cancer. Medicine (Baltimore). 2018;97:e9619
11. Suh HY, Choi H, Paeng JC. et al. Comprehensive gene expression analysis for exploring the association between glucose metabolism and differentiation of thyroid cancer. BMC Cancer. 2019;19:1260
12. Antoneli F, Passos FM, Lopes LR. et al. A Kolmogorov-Smirnov test for the molecular clock based on Bayesian ensembles of phylogenies. PLoS One. 2018;13:e0190826
13. Robin X, Turck N, Hainard A. et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77
14. Maag JLV. gganatogram: An R package for modular visualisation of anatograms and tissues based on ggplot2. F1000Res. 2018;7:1576
15. Song M, Huang Z, Wang S. et al. Predictive factors of lateral lymph node metastasis in conventional papillary thyroid carcinoma. Gland Surg. 2020;9:1000-7
16. Lee YS, Shin SC, Lim YS. et al. Tumor location-dependent skip lateral cervical lymph node metastasis in papillary thyroid cancer. Head Neck. 2014;36:887-91
17. Park JH, Lee YS, Kim BW. et al. Skip lateral neck node metastases in papillary thyroid carcinoma. World J Surg. 2012;36:743-7
18. Dou Y, Hu D, Chen Y. et al. PTC located in the upper pole is more prone to lateral lymph node metastasis and skip metastasis. World J Surg Oncol. 2020;18:188
19. Nie X, Tan Z, Ge M. Skip metastasis in papillary thyroid carcinoma is difficult to predict in clinical practice. BMC Cancer. 2017;17:702
20. Naifeh J, Dimri M, Varacallo M. Biochemistry, Aerobic Glycolysis. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC. 2022
21. Ruiz EML, Niu T, Zerfaoui M. et al. A novel gene panel for prediction of lymph-node metastasis and recurrence in patients with thyroid cancer. Surgery. 2020;167:73-9
22. Thompson AM, Turner RM, Hayen A. et al. A preoperative nomogram for the prediction of ipsilateral central compartment lymph node metastases in papillary thyroid cancer. Thyroid. 2014;24:675-82
23. Wu Y, Rao K, Liu J. et al. Machine Learning Algorithms for the Prediction of Central Lymph Node Metastasis in Patients With Papillary Thyroid Cancer. Front Endocrinol (Lausanne). 2020;11:577537
24. Xiang Y, Xu Y, Bhandari A. et al. Serum TSH levels are associated with postoperative recurrence and lymph node metastasis of papillary thyroid carcinoma. Am J Transl Res. 2021;13:6108-16
25. Zhou Q, Chen J, Feng J. et al. Long noncoding RNA PVT1 modulates thyroid cancer cell proliferation by recruiting EZH2 and regulating thyroid-stimulating hormone receptor (TSHR). Tumour Biol. 2016;37:3105-13
26. Gul K, Ozdemir D, Dirikoc A. et al. Are endogenously lower serum thyroid hormones new predictors for thyroid malignancy in addition to higher serum thyrotropin? Endocrine. 2010;37:253-60
27. Bianco AC, Salvatore D, Gereben B. et al. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23:38-89
28. Wang W, Yang Z, Ouyang Q. A nomogram to predict skip metastasis in papillary thyroid cancer. World J Surg Oncol. 2020;18:167
29. Al Afif A, Williams BA, Rigby MH. et al. Multifocal Papillary Thyroid Cancer Increases the Risk of Central Lymph Node Metastasis. Thyroid. 2015;25:1008-12
30. Liu C, Xiao C, Chen J. et al. Risk factor analysis for predicting cervical lymph node metastasis in papillary thyroid carcinoma: a study of 966 patients. BMC Cancer. 2019;19:622
31. Schwarz C, Leichtle AB, Arampatzis S. et al. Thyroid function and serum electrolytes: does an association really exist? Swiss Med Wkly. 2012;142:w13669
32. Kimura T, Van Keymeulen A, Golstein J. et al. Regulation of thyroid cell proliferation by TSH and other factors: a critical evaluation of in vitro models. Endocr Rev. 2001;22:631-56
33. Ortiga-Carvalho TM, Chiamolera MI, Pazos-Moura CC. et al. Hypothalamus-Pituitary-Thyroid Axis. Compr Physiol. 2016;6:1387-428
34. Hoffmann S, Hofbauer LC, Scharrenbach V. et al. Thyrotropin (TSH)-induced production of vascular endothelial growth factor in thyroid cancer cells in vitro: evaluation of TSH signal transduction and of angiogenesis-stimulating growth factors. J Clin Endocrinol Metab. 2004;89:6139-45
35. Pietrzik CU, Hoffmann J, Stöber K. et al. From differentiation to proliferation: the secretory amyloid precursor protein as a local mediator of growth in thyroid epithelial cells. Proc Natl Acad Sci U S A. 1998;95:1770-5
36. Karaman S, Detmar M. Mechanisms of lymphatic metastasis. J Clin Invest. 2014;124:922-8
37. Su X, Shen Z, Yang Q. et al. Vitamin C kills thyroid cancer cells through ROS-dependent inhibition of MAPK/ERK and PI3K/AKT pathways via distinct mechanisms. Theranostics. 2019;9:4461-73
38. Richards JS. New signaling pathways for hormones and cyclic adenosine 3',5'-monophosphate action in endocrine cells. Mol Endocrinol. 2001;15:209-18
39. Saji M, Ringel MD. The PI3K-Akt-mTOR pathway in initiation and progression of thyroid tumors. Mol Cell Endocrinol. 2010;321:20-8
40. Manzella L, Massimino M, Stella S. et al. Activation of the IGF Axis in Thyroid Cancer: Implications for Tumorigenesis and Treatment. Int J Mol Sci. 2019 20
41. Dumont JE, Lamy F, Roger P. et al. Physiological and pathological regulation of thyroid cell proliferation and differentiation by thyrotropin and other factors. Physiol Rev. 1992;72:667-97
42. Santisteban P, Acebrón A, Polycarpou-Schwarz M. et al. Insulin and insulin-like growth factor I regulate a thyroid-specific nuclear protein that binds to the thyroglobulin promoter. Mol Endocrinol. 1992;6:1310-7
43. van der Laan BF, Freeman JL, Asa SL. Expression of growth factors and growth factor receptors in normal and tumorous human thyroid tissues. Thyroid. 1995;5:67-73
44. Fukushima T, Okajima H, Yamanaka D. et al. HSP90 interacting with IRS-2 is involved in cAMP-dependent potentiation of IGF-I signals in FRTL-5 cells. Mol Cell Endocrinol. 2011;344:81-9
45. Vella V, Sciacca L, Pandini G. et al. The IGF system in thyroid cancer: new concepts. Mol Pathol. 2001;54:121-4
46. Ding J, Tang J, Chen X. et al. Expression characteristics of proteins of the insulin-like growth factor axis in non-small cell lung cancer patients with preexisting type 2 diabetes mellitus. Asian Pac J Cancer Prev. 2013;14:5675-80
47. Yan Y, Hu F, Wu W. et al. Expression characteristics of proteins of IGF-1R, p-Akt, and survivin in papillary thyroid carcinoma patients with type 2 diabetes mellitus. Medicine (Baltimore). 2017;96:e6393
48. Yang SJ, Chen CY, Chang GD. et al. Activation of Akt by advanced glycation end products (AGEs): involvement of IGF-1 receptor and caveolin-1. PLoS One. 2013;8:e58100
49. Lopez R, Arumugam A, Joseph R. et al. Hyperglycemia enhances the proliferation of non-tumorigenic and malignant mammary epithelial cells through increased leptin/IGF1R signaling and activation of AKT/mTOR. PLoS One. 2013;8:e79708
50. De Marco P, Cirillo F, Vivacqua A. et al. Novel Aspects Concerning the Functional Cross-Talk between the Insulin/IGF-I System and Estrogen Signaling in Cancer Cells. Front Endocrinol (Lausanne). 2015;6:30
Author contact
![]() Corresponding authors: Zhiyong Wang: Department of Otolaryngology, Xinfeng County People's Hospital, Ganzhou, Jiangxi, China. E-mail address:zhiyongwcom. Yunxia Lv: Department of Thyroid Surgery, Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China. E-mail address:ndefy12389edu.cn
Corresponding authors: Zhiyong Wang: Department of Otolaryngology, Xinfeng County People's Hospital, Ganzhou, Jiangxi, China. E-mail address:zhiyongwcom. Yunxia Lv: Department of Thyroid Surgery, Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China. E-mail address:ndefy12389edu.cn

 Global reach, higher impact
Global reach, higher impact