3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(10):3073-3083. doi:10.7150/jca.73385 This issue Cite
Research Paper
Association between the Co-administration of Histamine H2 Receptor Antagonists and the Effectiveness of Capecitabine in Patients with Colorectal Cancer: Propensity Score Analysis
1. Department of Pharmacy, Tochigi Cancer Center, 4-9-13 Yohnan, Utsunomiya, Tochigi 320-0834, Japan.
2. Department of Biomedical Statistics and Bioinformatics, Kyoto University Graduate School of Medicine, 54 Kawahara-cho, Shogoin, Sakyo-ku, Kyoto 606-8507, Japan.
3. Division of Pharmaceutical Care Sciences, Center for Social Pharmacy and Pharmaceutical Care Sciences, Keio University Faculty of Pharmacy, 1-5-30 Shibakoen, Minato-ku, Tokyo 105-8512, Japan.
4. Division of Pharmaceutical Care Sciences, Keio University Graduate School of Pharmaceutical Sciences, 1-5-30 Shibakoen, Minato-ku, Tokyo 105-8512, Japan.
5. Department of Pharmacy, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan.
6. Department of Pharmacy, Gifu University Hospital, 1-1 Yanagido, Gifu, Gifu 501-1194, Japan.
7. Department of Pharmacy, Osaka City University Hospital, 1-5-7 Asahi-machi, Abeno-ku, Osaka 545-8586, Japan.
8. Division of Pharmacy, Gunma Prefectural Cancer Center, 617-1 Takahayashi-nishi-cho, Ota, Gunma 373-0828, Japan.
9. Department of Pharmacy, Independent Administrative Institution Higashiosaka City Medical Center, 3-4-5 Nishiiwata, Higashiosaka, Osaka 578-8588, Japan.
10. Department of Frontier Science for Cancer and Chemotherapy, Osaka University Graduate School of Medicine, 2-2 Yamadaoka, Suita, Osaka 565-0871, Japan.
11. Department of Pharmacy, Nagoya City University West Medical Center, 1-1-1 Hirate-cho, Kita-ku, Nagoya, Aichi 462-8508, Japan.
12. Department of Pharmacy, Miyagi Cancer Center, 47-1 Nodayama, Medeshimashiote, Natori, Miyagi 981-1293, Japan.
13. Department of Pharmacy, Yokohama Minami Kyousai Hospital, 1-21-1 Mutsuurahigashi, Kanazawa-ku, Yokohama, Kanagawa 236-0037, Japan.
Received 2022-3-29; Accepted 2022-7-26; Published 2022-8-8
Abstract
Background: The association between the effectiveness of capecitabine and the concomitant administration of gastric acid suppressants remains controversial. We aimed to clarify whether the effectiveness of capecitabine is affected by the co-administration of histamine H2 receptor antagonists (H2RAs) in early-stage colorectal cancer (CRC) patients using real-world data.
Methods: This multicenter, retrospective, observational study included consecutive patients with stage II-III CRC who received either capecitabine monotherapy or the CapeOX regimen (capecitabine and oxaliplatin) as adjuvant therapy between January 2009 and December 2014 in Japan. Relapse-free survival (RFS) and overall survival were estimated using the Kaplan-Meier method. Additionally, multivariable Cox proportional hazards model, propensity score adjustment, and inverse probability of treatment weighting analyses were performed.
Results: In total, 552 patients were included in this study, of which 30 were co-administered H2RAs. RFS at five years was 76.7% (95% confidence interval [CI]: 57.2-88.1%) and 79.8% (95% CI: 76.0-83.0%) in the H2RA and non-H2RA groups, respectively. Multivariable Cox proportional hazards model and propensity score-adjusted analyses showed that the co-administration of H2RAs was associated with a poor RFS among those receiving capecitabine monotherapy (hazard ratio [HR], 2.01; 95% CI: 0.86-4.70 and HR, 1.81; 95% CI: 0.77-4.22, respectively). In contrast, these results were inconsistent with the group receiving the CapeOX regimen.
Conclusions: The study findings suggest that the co-administration of H2RAs may not reduce the effectiveness of capecitabine therapy in patients with early-stage CRC. To confirm this relationship, a prospective study with a pharmacokinetic approach is needed.
Keywords: capecitabine, CapeOX, histamine H2 receptor antagonist, drug-drug interaction, colorectal cancer
Introduction
According to a leading global cancer statistics source, colorectal cancer (CRC) was the third most commonly diagnosed cancer (10.0%) and the second leading cause of cancer death (9.4%) in both the sexes in the year 2020 [1]. Capecitabine is an oral prodrug designed for supplying high concentrations of 5-fluorouracil in tumor cells. It is commonly used for treating solid tumors, including CRC, gastric, as well as breast cancer, according to the package insert in Japan. Several recent studies have suggested that the concomitant use of proton pump inhibitors (PPIs) and capecitabine reduces the effectiveness of capecitabine in CRC [2-5]. Our previous study investigated the clinical consequences of the concomitant administration of PPIs and capecitabine monotherapy or the CapeOX regimen (capecitabine and oxaliplatin) in patients with early-stage CRC, where we found that the co-administration of PPIs led to poor survival outcomes [6]. PPIs are used to manage peptic ulcers and gastroesophageal reflux disease, and are among the most widely prescribed drugs among patients with cancer [7, 8]. Previous studies indicate that drug-drug interactions (DDIs), reduction of capecitabine solubility, or an increased CRC risk associated with PPIs may explain the association between PPI co-administration and the reduced effectiveness of capecitabine. However, the underlying mechanism remains unclear [9-12], which precludes the possibility of a therapeutic strategy that can effectively maintain the effectiveness of capecitabine.
Histamine H2 receptor antagonists (H2RAs) are acid-suppressive medications similar to PPIs [7]. In Japan, H2RAs are widely prescribed for gastrointestinal disorders. However, there are a few reports on combining capecitabine with H2RAs in CRC patients [4, 12]. Additionally, the clinical impact of the co-administration of H2RAs with capecitabine as postoperative adjuvant treatment in early-stage CRC patients has not been evaluated. Other studies have suggested that the carcinogenic risk of the long-term administration of H2RAs is nil or lower than that related to the administration of PPIs [13-15].
The purpose of this study was to clarify whether the co-administration of H2RAs affects the effectiveness of capecitabine monotherapy and CapeOX regimen in early-stage CRC patients using real-world data.
Methods
Patients
This was a multicenter, retrospective, observational study, and was conducted at nine institutions in Japan. Data were collected from the medical records of each institution, and compiled at the National Cancer Center Hospital; subsequently, data analyses were performed at the Keio University Faculty of Pharmacy and Kyoto University Graduate School of Medicine. The manuscript was prepared with reference to the STROBE checklist [16].
The inclusion criteria were as follows: 1) consecutive patients aged ≥ 20 years with pathologically diagnosed stage II-III CRC and who had undergone curative surgery; and 2) patients who had received at least one course of adjuvant capecitabine monotherapy (2,500 mg/m2, days 1-14, every 3 weeks) or the CapeOX regimen (capecitabine 2,000 mg/m2 on days 1-14, plus oxaliplatin 130 mg/m2 on day 1, every 3 weeks) between January 2009 and December 2014. The clinicopathological findings were used for reclassification according to the TNM Classification of Malignant Tumors 8th edition, published by the Union for International Cancer Control [17]. The treatment schedule and follow-up duration were modified at the clinician's discretion according to the toxicity profile of each patient.
The exclusion criteria were as follows: 1) refused use of medical records; 2) insufficient or missing information in the medical records; 3) history of the administration of capecitabine monotherapy or CapeOX regimen prior to the investigation period; 4) prior administration of any adjuvant chemotherapy, except for capecitabine monotherapy or the CapeOX regimen; 5) prior administration of any neoadjuvant chemotherapy; 6) concurrent radiotherapy during adjuvant chemotherapy; 7) inadequate bone marrow, liver, and renal function at baseline (neutrophil count < 1,500 cells/mm3 or white blood cell count < 3,000 cells/mm3; hemoglobin < 9.0 g/dL; platelet count < 75,000 cells/mm3; total bilirubin > 2.25 mg/dL; aspartate transaminase > 60 U/L, alanine transaminase > 84 U/L for men and > 46 U/L for women, and creatinine clearance ≤ 51 mL/min as calculated by the Cockcroft-Gault equation); 8) more than eight cycles of adjuvant chemotherapy; 9) development of other carcinomas after receiving adjuvant chemotherapy; and 10) co-administration of PPIs which was defined as a ≥ 20% overlap between PPI administration and adjuvant chemotherapy administration [3].
Data collection
Patient records were de-identified and analyzed anonymously. The following data were collected: age, sex, body surface area, cancer stage, TNM classification of malignant tumors, primary tumor site, chemotherapy regimen and dose, concomitant PPI or H2RA use, laboratory data before chemotherapy, and date of recurrence and/or death. The primary site included the right-sided colon (defined as the cecum, ascending colon, and transverse colon), left-sided colon (defined as the descending colon, sigmoid, and rectosigmoid junction), and rectum. The relative dose intensity (RDI) of the capecitabine or CapeOX regimens was defined as the percentage of actual dose intensity per scheduled dose intensity of eight courses. Concomitant use of H2RAs was defined as a ≥ 20% overlap between H2RA administration and capecitabine administration in accordance with a previous study [3]. The follow-up period ended on December 31, 2019.
Endpoints
Relapse-free survival (RFS) was defined as the period from the date of capecitabine administration to the date of radiographic recurrence or death from any cause. Overall survival (OS) was defined as the period from the date of capecitabine administration to the date of death from any cause. Patients who were still alive as well as those without documented radiographic recurrence were censored at the date of the last follow-up. The primary and secondary endpoints were RFS and OS, respectively.
Statistical analysis
RFS and OS in the H2RA and non-H2RA groups were estimated using the Kaplan-Meier method; confidence intervals (CIs) were calculated using the complementary log-log transformation and Greenwood's formula. The follow-up period was analyzed using reverse Kaplan-Meier estimates [18]. Subsequently, a multivariable Cox proportional hazards model was applied to compare the differences between the two groups. Hazard ratios (HRs) and 95% CIs were presented. Potential explanatory variables concerning patient background including chemotherapy regimen (CapeOX vs. capecitabine), concomitant use of H2RAs (yes vs. no), age (10-year intervals), sex (male vs. female), primary site (right-sided colon vs. others (left-sided colon and/or rectal)), cancer stage (III high-risk (T4, N2, or both cancers) vs. III low risk (T1, T2, or T3, and N1 cancers) vs. II), and RDI (10% intervals) were included as covariates in the multivariable model [19-21]. To account for indication bias due to the lack of randomization, propensity score-adjusted analyses were performed using the following: 1) a multivariable model including the propensity score as an additional covariate, and 2) an inverse probability of treatment weighting (IPTW) method [22, 23]. The propensity score of H2RA co-administration was estimated for each patient using a logistic regression model [24]. According to the recommendation of the American Statistical Association [25, 26], a P < 0.05 should be avoided when interpreting P-values; therefore, we interpreted the results on the basis of point estimates with their CIs. Furthermore, to supplement conventional CIs, we performed a post-hoc analysis using the Cox model re-expressed in a Bayesian statistical framework. We computed the Bayesian posterior probability [27] of HR < 1 based on a non-informative prior distribution as a reference to evaluate the hypotheses concerning the direction and magnitude of the unknown HR via the Cox model. We did not impute any missing data. All statistical analyses were performed using SAS version 9.4 and JMP version 16.2.0 (SAS Institute, Cary, NC, USA).
Ethics statement
Ethical approval was provided by the National Cancer Center Institutional Review Board (Approval No. 2019-294), ethics committee of the Tochigi Cancer Center (Approval No. 20-A001), medical review board of Gifu University Graduate School of Medicine (Approval No. 2020-069), ethical committee of Osaka City University Graduate School of Medicine (Approval No. 2020-042), ethics committee of the Gunma Prefectural Cancer Center (Approval No. 405-02012), ethical review board of Osaka University Hospital (Approval No. 20008), Nagoya City University East/West Medical Center Institutional Review Board (Approval No. 20-04-423-03), ethics review committee of the Miyagi Cancer Center (Approval No. 2020-003), and the ethics committee of the Yokohama Minami Kyosai Hospital (Approval No. 1-20-4-1) in Japan. This study was conducted in accordance with the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research involving Human Subjects promulgated by the Ministry of Education, Culture, Sports, Science, and Technology and the Ministry of Health, Labour, and Welfare of Japan. Acquiring written or oral informed consent from participants was waived considering the retrospective nature of the study. Therefore, we used an opt-out method through the official website of each participating institution.
Results
Patient characteristics
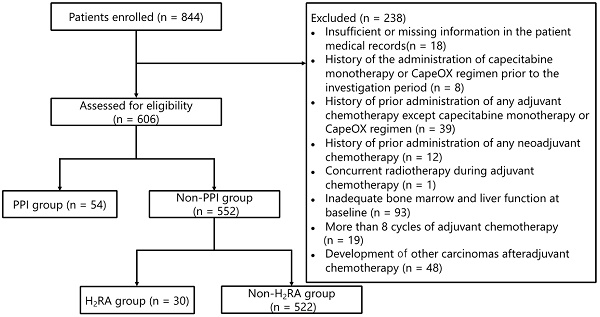
The patient flowchart illustrating the enrollment process is shown in Figure 1. Of the 844 patients who were initially screened, 238 were withdrawn from the analysis on the basis of the exclusion criteria as detailed in the Methods section. Subsequently, 54 patients who had received PPIs were further excluded from the analysis. Thus, data pertaining to 552 patients were evaluated in this study, of which 30 (5.4%) received H2RAs; of these 30 patients, 20 (66.7%) and 10 (33.3%) received capecitabine monotherapy and the CapeOX regimen, respectively.
The baseline patient characteristics are listed in Table 1. The median age of the patients was 63 years (interquartile range (IQR): 55-70 years), of which 305 (55.3%) were men, and 161 (29.2%) had right-sided colon cancer. In the H2RA group, the median duration of concomitant H2RA use was 100.0% (IQR: 87.5%-100%).
Endpoints
The median duration of follow-up was 6.1 years (95% CI: 5.9-6.3 years). Overall, there were 110 relapse events and 66 deaths. Among patients who received capecitabine monotherapy (2,500 mg/m2, days 1-14, every 3 weeks, 8 cycle), the median RDI of capecitabine was 81.1% (IQR: 64.2-87.8%) and 79.3% (IQR: 65.0-91.3%) in the H2RA and non-H2RA groups, respectively. Among patients who received the CapeOX regimen (capecitabine at 2,000 mg/m2 on days 1-14, plus oxaliplatin at 130 mg/m2 on day 1, every 3 weeks, 8 cycles), the median RDI of capecitabine was 84.9% (IQR: 79.1-91.1%) and 75.1% (IQR: 62.5-87.4%) in the H2RA and non-H2RA groups, respectively, and the median RDI of oxaliplatin was 72.4% (IQR: 59.6-81.8%) and 66.3% (IQR: 45.6-78.9%) in the H2RA and non-H2RA groups, respectively.
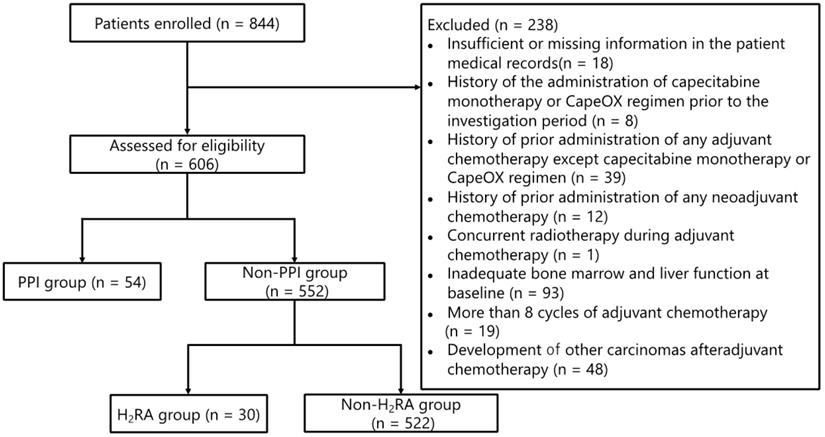
As shown in Figure 2, in the entire study population (capecitabine monotherapy and CapeOX regimen), the RFS at five years was 76.7% (95% CI: 57.2-88.1%) and 79.8% (95% CI: 76.0-83.0%) in the H2RA and non-H2RA groups, respectively. The OS at five years was 90.0% (95% CI: 72.1-96.7%) and 90.4% (95% CI: 87.5-92.7%) in the H2RA and non-H2RA groups, respectively. According to the univariable analysis, there were no significant differences in RFS and OS between the H2RA and non-H2RA groups (RFS: HR, 1.28; 95% CI: 0.59-2.74; P = 0.533 and OS: HR, 1.09; 95% CI: 0.40-3.00; P = 0.867, respectively).
As shown in Table 2, the multivariable Cox proportional hazards model and propensity score-adjusted analyses revealed that the co-administration of H2RAs was associated with shortened RFS to a small degree (HR, 1.12; 95% CI: 0.52-2.42, P = 0.772 and HR, 1.18; 95% CI: 0.55-2.53, P = 0.677, respectively). In contrast, OS was inconsistent as compared with RFS (Table 3).
Patient enrollment flowchart. Abbreviations: CapeOX: capecitabine and oxaliplatin; PPI: proton pump inhibitor; H2RA: Histamine H2 receptor antagonist
Baseline patient characteristics
| Characteristic | All (n = 552) | H2RAgroup (n = 30)a | Non-H2RA group (n = 522)a | |||
|---|---|---|---|---|---|---|
| Capecitabine monotherapy (n = 20) | CapeOX (n = 10) | Capecitabine monotherapy (n = 400) | CapeOX (n = 122) | |||
| Age, median (IQR), y | 63 (55-70) | 68 (60-74) | 59 (53-63) | 64 (57-71) | 60 (50-67) | |
| Sex | ||||||
| Male | 305 (55.3) | 12 (60.0) | 6 (60.0) | 214 (53.5) | 73 (59.8) | |
| Female | 247 (44.7) | 8 (40.0) | 4 (40.0) | 186 (46.5) | 49 (40.2) | |
| Primary sitea | ||||||
| Right-sided colon | 161 (29.2) | 2 (10.0) | 6 (60.0) | 121 (30.3) | 32 (26.2) | |
| Left-sided colon | 200 (36.2) | 12 (60.0) | 2 (20.0) | 149 (37.3) | 37 (30.3) | |
| Rectum | 191 (34.6) | 6 (30.0) | 2 (20.0) | 130 (32.5) | 53 (43.4) | |
| Stage | ||||||
| II | 66 (12.0) | 1 (5.0) | 1 (10.0) | 59 (14.8) | 5 (4.1) | |
| IIIA | 96 (17.4) | 4 (20.0) | 1 (10.0) | 77 (19.3) | 14 (11.5) | |
| IIIB | 315 (57.1) | 12 (60.0) | 6 (60.0) | 225 (56.3) | 72 (59.0) | |
| IIIC | 75 (13.6) | 3 (15.0) | 2 (20.0) | 39 (10.0) | 31 (25.4) | |
| Co-administered H2RA | ||||||
| Famotidine | 15 (75.0) | 7 (70.0) | ||||
| Ranitidine | 5 (25.0) | 0 ( 0.0) | ||||
| Lafutidine | 0 ( 0.0) | 3 (30.0) | ||||
Abbreviations: CapeOX: capecitabine and oxaliplatin; IQR: interquartile range; H2RA: histamine H2 receptor antagonist; y: years.
a Percentages may not add up to 100 because of rounding.
Multivariable Cox proportional hazards model, propensity score-adjustment, and IPTW analyses of the effect of the co-administration of H2RA on RFS with capecitabine monotherapy and the CapeOX regimen
| Multivariable analysis | Adjusted for propensity score | IPTW | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | No. | Event | Censored | HR (95% CI) | P | Posterior probability | HR (95% CI) | P | Posterior probability | HR (95% CI) | P | Posterior probability | |
| H2RA | Yes | 30 | 7 | 23 | 1.12 (0.52-2.42) | 0.772 | 0.435 | 1.18 (0.55-2.53) | 0.677 | 0.391 | 0.76 (0.32-1.80) | 0.527 | 0.776 |
| No | 522 | 103 | 419 | 1 | 1 | 1 | |||||||
| Age (10-year intervals) | - | - | - | 0.88 (0.74-1.04) | 0.130 | ||||||||
| Sex | Male | 305 | 69 | 236 | 1.43 (0.97-2.11) | 0.071 | |||||||
| Female | 247 | 41 | 206 | 1 | |||||||||
| Primary site | Right-sided colon | 161 | 34 | 127 | 1.05 (0.69-1.58) | 0.831 | |||||||
| Others | 391 | 76 | 315 | 1 | |||||||||
| Stage | III high-risk | 179 | 59 | 120 | 2.15 (1.15-4.00) | 0.016 | |||||||
| III low-risk | 307 | 39 | 268 | 0.70 (0.37-1.35) | 0.289 | ||||||||
| II | 66 | 12 | 54 | 1 | |||||||||
Abbreviations: H2RA: histamine H2 receptor antagonist; CapeOX: capecitabine and oxaliplatin; CI: confidence interval; HR: hazard ratio; IPTW: inverse probability of treatment weighting; RFS: relapse-free survival.
A comparison between the capecitabine monotherapy and the CapeOX regimen groups is shown in Tables 4 and 5. The multivariable Cox proportional hazards model and propensity score-adjusted analyses showed that the co-administration of H2RAs was relatively associated with a relatively poor RFS in the capecitabine monotherapy group (HR, 2.01; 95% CI: 0.86-4.70; P = 0.108 and HR, 1.81; 95% CI: 0.77-4.22; P = 0.172, respectively), although no significant difference was observed. In contrast, inconsistent results were obtained for the CapeOX regimen group with respect to RFS.
In the capecitabine monotherapy population, the Bayesian posterior probability showed that the HRs for the RFS of the H2RA group relative to that of the non-H2RA group would be < 1.00, ranging from 8.4% to 47.0% (Table 4). In the CapeOX regimen population, the Bayesian posterior probability showed that the HRs for the RFS of the H2RA group relative to that of the non-H2RA group would be < 1.00, ranging from 87.0% to 96.6% (Table 5).
Discussion
In the present study, we found that in real-world clinical practice, the effectiveness of capecitabine monotherapy and CapeOX regimen is unlikely to be affected by the combination of H2RAs in early-stage CRC patients. However, multivariable Cox proportional hazards model and propensity score-adjusted analyses showed that the co-administration of H2RAs was associated with a poor RFS among those receiving capecitabine monotherapy (HR, 2.01; 95% CI: 0.86-4.70 and HR, 1.81; 95% CI: 0.77-4.22, respectively). The HR of RFS was higher for capecitabine monotherapy and tended to fall in the overall population (capecitabine monotherapy and CapeOX). The difference in HR may have been attributed to the increased intensity of treatment with the addition of oxaliplatin and the different dosage of capecitabine in the two treatment regimens.
Kaplan-Meier survival curves estimate according to the absence or presence of H2RA co-administration. The solid and dashed lines are H2RA group and non-H2RA group, respectively. (A) Relapse-free survival. (B) Overall survival. Abbreviations: H2RA: histamine H2 receptor antagonist; RFS: relapse-free survival; OS: overall survival
Multivariable Cox proportional hazards model, propensity score-adjustment, and IPTW analyses of the effect of the co-administration of H2RA on OS with capecitabine monotherapy and the CapeOX regimen
| Multivariable analysis | Adjusted for propensity score | IPTW | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | No. | Event | Censored | HR (95% CI) | P | Posterior probability | HR (95% CI) | P | Posterior probability | HR (95% CI) | P | Posterior probability | |
| H2RA | Yes | 30 | 4 | 26 | 0.82 (0.30-2.27) | 0.704 | 0.699 | 0.90 (0.32-2.49) | 0.836 | 0.644 | 0.66 (0.22-1.96) | 0.458 | 0.816 |
| No | 522 | 62 | 460 | 1 | 1 | 1 | |||||||
| Age (10-year intervals) | - | - | - | 0.92 (0.73-1.15) | 0.450 | ||||||||
| Sex | Male | 305 | 44 | 261 | 1.74 (1.04-2.91) | 0.035 | |||||||
| Female | 247 | 22 | 225 | 1 | |||||||||
| Primary site | Right-sided colon | 161 | 24 | 137 | 1.33 (0.79-2.21) | 0.281 | |||||||
| Others | 391 | 42 | 349 | 1 | |||||||||
| Stage | III high-risk | 179 | 42 | 137 | 2.80 (1.19-6.61) | 0.018 | |||||||
| III low-risk | 307 | 18 | 289 | 0.65 (0.26-1.64) | 0.362 | ||||||||
| II | 66 | 6 | 60 | 1 | |||||||||
Abbreviations: H2RA: histamine H2 receptor antagonist; CapeOX: capecitabine and oxaliplatin; CI: confidence interval; HR: hazard ratio; IPTW: inverse probability of treatment weighting; OS: overall survival.
Multivariable Cox proportional hazards model, propensity score-adjustment, and IPTW analyses of the effect of the co-administration of H2RA on RFS with capecitabine monotherapy
| Multivariable analysis | Adjusted for propensity score | IPTW | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | No. | Event | Censored | HR (95% CI) | P | Posterior probability | HR (95% CI) | P | Posterior probability | HR (95% CI) | P | Posterior probability | |
| H2RA | Yes | 20 | 6 | 14 | 2.01 (0.86-4.70) | 0.108 | 0.084 | 1.81 (0.77-4.22) | 0.172 | 0.126 | 1.12 (0.31-4.04) | 0.864 | 0.470 |
| No | 400 | 71 | 329 | 1 | 1 | 1 | |||||||
| Age (10-year intervals) | - | - | - | 0.85 (0.69-1.04) | 0.115 | ||||||||
| Sex | Male | 226 | 47 | 179 | 1.45 (0.92-2.31) | 0.113 | |||||||
| Female | 194 | 30 | 164 | 1 | |||||||||
| Primary site | Right-sided colon | 123 | 24 | 99 | 1.13 (0.68-1.87) | 0.631 | |||||||
| Others | 297 | 53 | 244 | 1 | |||||||||
| Stage | III high-risk | 110 | 35 | 75 | 2.28 (1.12-4.63) | 0.023 | |||||||
| III low-risk | 250 | 32 | 218 | 0.77 (0.38-1.57) | 0.477 | ||||||||
| II | 60 | 10 | 50 | 1 | |||||||||
Abbreviations: H2RA: histamine H2 receptor antagonist; CI: confidence interval; HR: hazard ratio; IPTW: inverse probability of treatment weighting; RFS: relapse-free survival.
Multivariable Cox proportional hazards model, propensity score-adjustment, and IPTW analyses of the effect of the co-administration of H2RA on RFS with the CapeOX regimen
| Multivariable analysis | Adjusted for propensity score | IPTW | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | No. | Event | Censored | HR (95% CI) | P | Posterior probability | HR (95% CI) | P | Posterior probability | HR (95% CI) | P | Posterior probability | |
| H2RA | Yes | 10 | 1 | 9 | 0.27 (0.03-2.02) | 0.200 | 0.962 | 0.27 (0.04-2.05) | 0.206 | 0.966 | 0.52 (0.07-4.20) | 0.543 | 0.870 |
| No | 122 | 32 | 90 | 1 | 1 | 1 | |||||||
| Age (10-year intervals) | - | - | - | 0.95 (0.69-1.31) | 0.740 | - | |||||||
| Sex | Male | 79 | 22 | 57 | 1.33 (0.64-2.74) | 0.446 | |||||||
| Female | 53 | 11 | 42 | 1 | |||||||||
| Primary site | Right-sided colon | 38 | 10 | 28 | 1.14 (0.53-2.46) | 0.736 | |||||||
| Others | 94 | 23 | 71 | 1 | |||||||||
| Stage | III high-risk | 69 | 24 | 45 | 0.98 (0.22-4.35) | 0.979 | |||||||
| III low-risk | 57 | 7 | 50 | 0.29 (0.06-1.47) | 0.136 | ||||||||
| II | 6 | 2 | 4 | 1 | |||||||||
Abbreviations: H2RA: histamine H2 receptor antagonist; CI: confidence interval; CapeOX: capecitabine and oxaliplatin; HR: hazard ratio; IPTW: inverse probability of treatment weighting; RFS: relapse-free survival.
To date, few studies have examined the influence of H2RAs on the effectiveness of capecitabine therapy. Rhinehart et al. [4] observed that the co-administration of antacids (PPIs and H2RAs) affects the effectiveness of capecitabine, but the number of H2RAs users in that study was small in two cases. In a similar study, Kichenadasse et al. [12] reported no association between H2RA co-administration and worse OS and PFS (n = 362). The results were obtained from six randomized clinical trials, but patients with early-stage CRC were not included. In both the above-mentioned studies, the difference in RDI between the H2RA and non-H2RA groups was unclear, which may have affected the results. Notably, the present study evaluated the RDI of capecitabine; the difference in the RDI of capecitabine between the H2RA and non-H2RAr groups was only 1.8% in the capecitabine monotherapy-treated population (higher in the H2RA user group). Cancer patients may be prescribed H2RAs to reduce gastrointestinal symptoms; however, it was not clear whether this led to an increase in the RDI. The other known risk factors for recurrence after adjuvant chemotherapy in CRC include tumor invasiveness (T) and lymph node status (N) [28, 29]. To ascertain the impact of these factors, sensitivity analyses were performed with concomitant H2RA (yes vs. no), age (10-year interval), sex (male vs. female), primary site (right-sided colon vs. other), chemotherapy regimen (CapeOX vs. capecitabine), and cancer stage (III high-risk (T4, N2, or both cancers) vs. III low risk (T1, T2, or T3 and N1 cancers) vs. II) as covariates. The number of H2RA-treated patients in this study was relatively small (n = 30), but the overall study population was large (n = 552); the study included several cancer centers, university hospitals, and community hospitals. To the best of our knowledge, this is the first study to clarify whether the co-administration of H2RAs affects the effectiveness of capecitabine therapy in patients with early-stage CRC in a real-world setting, and therefore our results have considerable clinical implications.
Previous studies have reported that the co-administration of PPIs may have a negative impact on the effectiveness of capecitabine in patients with early-stage or advanced CRC and gastroesophageal cancer [2-6]. The results of this study suggest that although PPIs and H2RA are antacids, their impact on capecitabine therapy differs. The therapeutic effect of capecitabine therapy may be maintained by replacing PPIs with H2RAs. To determine whether this is possible, it is necessary to clarify the mechanism by which the PPIs combination reduces the effect of capecitabine. This evidence suggests that acid-reducing agents (ARAs) may reduce the effectiveness of capecitabine treatment. Several hypotheses have been proposed regarding the mechanism by which PPIs attenuate the effect of capecitabine. One hypothesis states that a DDI between the PPIs and capecitabine leads to reduced capecitabine efficacy. According to this hypothesis, capecitabine is sensitive to changes in pH, and a PPI-induced increase in gastric pH reduces the absorption of capecitabine. In previous studies, ARAs have been shown to affect the effectiveness of oral anticancer agents [30]. However, according to a systematic review of DDIs pertaining to ARAs [31], elevated gastric pH is a common characteristic of all three classes of ARAs (antacids, H2RAs, and PPIs). Therefore, clinically important gastric pH-mediated interactions should be observed with all ARAs, and if PPIs reduce the effect of capecitabine due to changes in pH, then H2RAs should have the same result. Several in vivo studies have examined the influence of ARAs on the pharmacokinetics of capecitabine. In vivo studies on DDIs between capecitabine and ARAs have reported no interaction between capecitabine and Maalox® (dried aluminum hydroxide gel, magnesium hydroxide) in 12 patients with solid tumors [32, 33], and no interaction between capecitabine and rabeprazole in patients with CRC [11]. Accordingly, PPIs may be less likely to show gastric pH-dependent interactions with capecitabine. Another hypothesis suggests that the use of PPIs itself may affect CRC. It has been reported that the suppression of gastric acidity by PPIs and H2RAs led to hypergastrinemia and induced the proliferation of colorectal epithelium and progression of colonic adenoma in in vivo models [34-36]. Additionally, while several case-control studies have concluded that PPI use was associated with an increased risk of CRC [13], one cohort study reported no increase in CRC risk [37]. The above studies agree that H2RA administration is not a risk factor for CRC. However, an increased risk of gastric cancer was reported with H2RA use [38]. Thus, risk evaluation pertaining to these drug classes must be performed and clarified by future studies. It is necessary to confirm that the concentrations of capecitabine and its metabolites are adequately high in patients receiving concomitant PPIs and H2RAs, and examine the in vivo pharmacokinetics of these drugs and evaluate differences among these and other drugs that are used to treat the same indication. The effect of the timing of the dose administration on DDIs should also be clarified. It is possible that multiple mechanisms are involved in the PPIs-induced reduction of capecitabine efficacy, and comorbidities may play a role as well. Therefore, prospective studies are needed to explore the mechanism underlying the reduction of capecitabine efficacy by PPIs. While the mechanism underlying this phenomenon could not be clarified in this study.
The present study has some limitations. First, it was a retrospective, observational study rather than a prospective study. The present study evaluated patients according to the guidelines of the Japanese Society for Cancer of Colon and Rectum (JSCCR); these guidelines are used during the treatment of CRC in actual clinical practice in Japan. Therefore, we excluded 93 patients whose major organ functions were not preserved at the time of therapy initiation. The JSCCR guidelines were published in 2009, 2010, and 2014 [39, 40]. The new additions in the JSCCR guidelines 2014 state that postoperative chemotherapy should be started 4-8 weeks after surgery, and that the CapeOX regimen has to be covered by insurance. As this is a retrospective study, information bias cannot be ruled out. Thus, multivariable analysis was performed to reduce the effect of potential confounding factors that were related to patient characteristics. Second, the sample sizes of the H2RA and non-H2RA groups were not equal. Notably, the number of H2RA-treated patients was relatively small, due to which it might not have been evaluated satisfactorily. We were unable to evaluate the OS in the population that received the CapeOX regimen due to the small number of events. Furthermore, there was a large variability regarding the Bayesian posterior probability shown in Tables 2 (range: 0.388-0.607) and 4 (range: 0.084-0.461), which might be an overestimation of IPTW owing to the small number of H2RA-treated patients. Third, the H2RA data were based on prescription information from the medical records; therefore, information on whether the patients purchased and used an over-the-counter drug was not available. Furthermore, it was unclear whether the patients took H2RAs during capecitabine treatment and whether medication adherence was adequate. Forth, the baseline laboratory data prior to chemotherapy represented the latest value in this study, and was therefore unable to identify the exact day within a given number of days. Fifth, we did not collect baseline CEA level or comorbidity data and therefore were not included as covariates in the multivariable model; the primary reasons for not collecting these data are as follows: First, information regarding comorbidity was not available in every institution because of the retrospective nature of the study. Second, patients with severe complications did not undergo surgery or postoperative adjuvant chemotherapy; hence, we focused on RFS, which included death due to any reason, rather than cancer-specific recurrence and death. Sixth, the frequency and timing of diagnostic imaging varies between facilities and may therefore affect the diagnosis of recurrence, which is a potentially confounding factor. Future prospective studies with larger sample sizes are necessary to confirm the study findings, evaluate the pharmacokinetic aspects of the drugs, and explore the mechanism underlying the effect of ARAs on the effectiveness of capecitabine therapy.
Conclusions
The findings of this study suggest that the concomitant use of H2RAs in patients with early-stage CRC receiving capecitabine therapy is unaffected by an increased risk of recurrence. Our data provide preliminary evidence for an association between the co-administration of H2RAs and capecitabine efficacy in Japanese patients with stage II-III CRC.
Abbreviations
ARAs: acid-reducing agents; CapeOX: capecitabine and oxaliplatin; CI: confidence interval; CRC: colorectal cancer; DDIs: drug-drug interactions; H2RAs: Histamine H2 receptor antagonists; HR: hazard ratio; ; IPTW: inverse probability of treatment weighting; IQR: interquartile range; JSCCR: Japanese Society for Cancer of Colon and Rectum; OS: overall survival; PPIs: proton pump inhibitors; RDI: relative dose intensity; RFS: relapse-free survival.
Acknowledgements
We are grateful to all the participants and medical staff of the nine participating institutions, including the National Cancer Center Hospital, Tochigi Cancer Center, Gifu University Hospital, Osaka City University Hospital, Gunma Prefectural Cancer Center, Osaka University Hospital, Nagoya City University West Medical Center, Miyagi Cancer Center, and Yokohama Minami Kyousai Hospital, who were involved in this study. We would like to thank Editage (www.editage.com) for English language editing.
Funding
This work was supported in part by the Keio Gijuku Fukuzawa Memorial Fund for the Advancement of Education and Research (2021) and the Policy-based Medical Services Foundation (2021) in Japan. The funders had no role in the study's design, collection, analysis, and interpretation of the data, writing of the manuscript, or the decision to submit the manuscript for publication.
Author Contributions
Conception/design: Yoshiko Kitazume, Hitoshi Kawazoe, and Hironobu Hashimoto.
Collection and/or assembly of data: Tomoko Yamazaki, Yoshiko Kitazume, Hirotoshi Iihara, Hironori Fujii, Masaya Takahashi, Takahiro Arai, Yasushi Murachi, Yumiko Sato, Takahiro Mikami, Koji Hashiguchi, Tomoe Yoshizawa, Katsuyuki Takahashi, Yukiyoshi Fujita, Yuki Hosokawa, Issei Morozumi, Masami Tsuchiya, Atsushi Yokoyama, and Hironobu Hashimoto.
Data analysis and interpretation: Ryuji Uozumi and Hitoshi Kawazoe.
Manuscript writing: Tomoko Yamazaki and Hitoshi Kawazoe.
Final approval of the manuscript: Tomoko Yamazaki, Ryuji Uozumi, Hitoshi Kawazoe, Yoshiko Kitazume, Hirotoshi Iihara, Hironori Fujii, Masaya Takahashi, Takahiro Arai, Yasushi Murachi, Yumiko Sato, Takahiro Mikami, Koji Hashiguchi, Tomoe Yoshizawa, Katsuyuki Takahashi, Yukiyoshi Fujita, Yuki Hosokawa, Issei Morozumi, Masami Tsuchiya, Atsushi Yokoyama, Hironobu Hashimoto, and Tetsuya Furukawa.
Competing Interests
Ryuji Uozumi: Eisai, Sawai Pharmaceutical, EP Croit (H). Hitoshi Kawazoe: Eli Lilly (RF).
The other authors have declared that no competing interest exists.
References
1. Sung H, Ferlay J, Siegel RL. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-49
2. Sun J, Ilich AI, Kim CA. et al. Concomitant administration of proton pump inhibitors and capecitabine is associated with increased recurrence risk in early stage colorectal cancer patients. Clin Colorectal Cancer. 2016;15:257-63
3. Chu MP, Hecht JR, Slamon D. et al. Association of proton pump inhibitors and capecitabine efficacy in advanced gastroesophageal cancer: secondary analysis of the TRIO-013/LOGiC randomized clinical trial. JAMA Oncol. 2017;3:767-73
4. Rhinehart HE, Phillips MA, Wade N. et al. Evaluation of the clinical impact of concomitant acid suppression therapy in colorectal cancer patients treated with capecitabine monotherapy. J Oncol Pharm Pract. 2019;25:1839-45
5. Kim SY, Lee JS, Kang J. et al. Proton pump inhibitor use and the efficacy of chemotherapy in metastatic colorectal cancer: a post hoc analysis of a randomized phase III trial (AXEPT). Oncologist. 2021;26:e954-62
6. Kitazume Y, Kawazoe H, Uozumi R. et al. Proton pump inhibitors effect on capecitabine efficacy in patients with stage II-III colorectal cancer: a multicenter retrospective study. Sci Rep. 2022;12:6561
7. Fox RK, Muniraj T. Pharmacologic therapies in gastrointestinal diseases. Med Clin North Am. 2016;100:827-50
8. Numico G, Fusco V, Franco P. et al. Proton pump inhibitors in cancer patients: how useful they are? A review of the most common indications for their use. Crit Rev Oncol Hematol. 2017;111:144-51
9. Wong GG, Ha V, Chu MP. et al. Effects of proton pump inhibitors on FOLFOX and CapeOx regimens in colorectal cancer. Clin Colorectal Cancer. 2019;18:72-9
10. Cheng V, Lemos M, Hunter N. et al. Concomitant use of capecitabine and proton pump inhibitors - Is it safe? J Oncol Pharm Pract. 2019;25:1705-11
11. Sekido M, Fujita KI, Kubota Y. et al. Rabeprazole intake does not affect systemic exposure to capecitabine and its metabolites, 5′-deoxy-5-fluorocytidine, 5′-deoxy-5-fluorouridine, and 5-fluorouracil. Cancer Chemother Pharmacol. 2019;83:1127-35
12. Kichenadasse G, Miners JO, Mangoni AA. et al. Proton pump inhibitors and survival in patients with colorectal cancer receiving fluoropyrimidine-based chemotherapy. J Natl Compr Canc Netw. 2021 19; 1037-44
13. Chubak J, Boudreau DM, Rulyak SJ. et al. Colorectal cancer risk in relation to use of acid suppressive medications. Pharmacoepidemiol Drug Saf. 2009;18:540-4
14. Cheung KS, Leung WK. Long-term use of proton-pump inhibitors and risk of gastric cancer: a review of the current evidence. Therap Adv Gastroenterol. 2019;12:1-11
15. Lai SW, Liao KF, Lai HC. et al. Use of proton pump inhibitors correlates with increased risk of colorectal cancer in Taiwan. Asia Pac J Clin Oncol. 2013;9:192-3
16. von Elm E, Altman DG, Egger M. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453-7
17. Brierley JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours, 8th ed. Hoboken, USA: Wiley-Blackwell. 2017
18. Korn EL. Censoring distributions as a measure of follow-up in survival analysis. Stat Med. 1986;5:255-60
19. Kim SE, Paik HY, Yoon H. et al. Sex- and gender-specific disparities in colorectal cancer risk. World J Gastroenterol. 2015;21:5167-75
20. Lee GH, Malietzis G, Askari A. et al. Is right-sided colon cancer different to left-sided colorectal cancer? - a systematic review. Eur J Surg Oncol. 2015;41:300-8
21. Hashiguchi Y, Muro K, Saito Y. et al. Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25:1-42
22. D'Agostino RBJr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265-81
23. Xu S, Ross C, Raebel MA. et al. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health. 2010;13:273-7
24. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41-55
25. Wasserstein RL, Lazar NA. The ASA statement on p-values: context, process, and purpose. Am Stat. 2016;70:129-33
26. Wasserstein RL, Schirm AL, Lazar NA. Moving to a world beyond “p < 0.05”. Am Stat. 2019;73:1-19
27. Spiegelhalter DJ, Abrams KR, Myles JP. Bayesian approaches to clinical trials and health-care evaluation. New York, USA: John Wiley & Sons. 2004
28. Grothey A, Sobrero AF, Shields AF. et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med. 2018;378:1177-88
29. Yoshino T, Yamanaka T, Oki E. et al. Efficacy and long-term peripheral sensory neuropathy of 3 vs. 6 months of oxaliplatin-based adjuvant chemotherapy for colon cancer: the ACHIEVE phase 3 randomized clinical trial. JAMA Oncol. 2019;5:1574-81
30. Indini A, Petrelli F, Tomasello G. et al. Impact of use of gastric-acid suppressants and oral anti-cancer agents on survival outcomes: a systematic review and meta-analysis. Cancers (Basel). 2020;12:998
31. Patel D, Bertz R, Ren S. et al. A systematic review of gastric acid-reducing agent-mediated drug-drug interactions with orally administered medications. Clin Pharmacokinet. 2020;59:447-62
32. Reigner B, Verweij J, Dirix L. et al. Effect of food on the pharmacokinetics of capecitabine and its metabolites following oral administration in cancer patients. Clin Cancer Res. 1998;4:941-8
33. Reigner B, Clive S, Cassidy J. et al. Influence of the antacid Maalox on the pharmacokinetics of capecitabine in cancer patients. Cancer Chemother Pharmacol. 1999;43:309-15
34. Koh TJ, Dockray GJ, Varro A. et al. Overexpression of glycine-extended gastrin in transgenic mice results in increased colonic proliferation. J Clin Invest. 1999;103:1119-26
35. Watson SA, Morris TM, McWilliams DF. et al. Potential role of endocrine gastrin in the colonic adenoma carcinoma sequence. Br J Cancer. 2002;87:567-73
36. Watson SA, Smith AM. Hypergastrinemia promotes adenoma progression in the APCMin-/+ mouse model of familial adenomatous polyposis. Cancer Res. 2001;61:625-31
37. Babic A, Zhang X, Morales-Oyarvide V. et al. Acid-suppressive medications and risk of colorectal cancer: results from three large prospective cohort studies. Br J Cancer. 2020;123:844-51
38. Ahn JS, Eom CS, Jeon CY. et al. Acid suppressive drugs and gastric cancer: a meta-analysis of observational studies. World J Gastroenterol. 2013;19:2560-8
39. Watanabe T, Itabashi M, Shimada Y. et al. Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol. 2012;17:1-29
40. Watanabe T, Itabashi M, Shimada Y. et al. Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol. 2015;20:207-39
Author contact
![]() Corresponding author: Hitoshi Kawazoe, Ph.D. Division of Pharmaceutical Care Sciences, Keio University Graduate School of Pharmaceutical Sciences, 1-5-30 Shibakoen, Minato-ku, Tokyo 105-8512, Japan. Tel: +81-3-5400-2639 Fax: +81-3-5400-2651 E-mail: kawazoe-htjp
Corresponding author: Hitoshi Kawazoe, Ph.D. Division of Pharmaceutical Care Sciences, Keio University Graduate School of Pharmaceutical Sciences, 1-5-30 Shibakoen, Minato-ku, Tokyo 105-8512, Japan. Tel: +81-3-5400-2639 Fax: +81-3-5400-2651 E-mail: kawazoe-htjp

 Global reach, higher impact
Global reach, higher impact