3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(11):3189-3198. doi:10.7150/jca.74674 This issue Cite
Research Paper
The Effect of Intraoperative Fentanyl Consumption on Prognosis of Colorectal Liver Metastasis treated by Simultaneous Resection: A Propensity Score Matching Analysis
1. Department of Hepatobiliary Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, 100021, Beijing, China.
2. Department of Anesthesiology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, 100021, Beijing, China.
*Equal contribution
Received 2022-5-3; Accepted 2022-8-9; Published 2022-8-29
Abstract
Background: No previous studies have reported the effect of intraoperative opioid consumption in colorectal liver metastasis (CRLM).
Methods: Medical records of patients who received simultaneous resection of CRLM were retrospectively reviewed. Patients with epidural anesthesia, intraoperative morphine, or intraoperative oxycodone were excluded. Patients were separated into high- and low-dose groups by median intraoperative equianalgesic fentanyl dose. Short-term outcomes, progression-free surcical (PFS) and overall survival (OS) were compared between groups before and after 1:1 propensity score matching (PSM). Univariable and multivariable Cox regression analysis were performed to identify independent predictors of survival.
Results: The final study population included 343 patients. Patients were separated into the low dose group (n=172) and the high dose group (n=171) by median intraoperative equianalgesic fentanyl dose (8.33 μg/kg). After PSM, 55 patients in the low dose group were matched to 55 patients in the high dose group and the baseline characteristics of the two groups were balanced. The two groups had no statistically significance difference in severity and categories of postoperative complications before and after PSM. Before PSM, the two groups had similar PFS (median 10.2 vs. 12.4 months, P=0.54) and OS (median 59.0 vs. 58.3 months, P=0.76). Univariate and multivariate Cox regression analyses revealed no statistically significant association between intraoperative equianalgesic fentanyl and PFS (multivariate HR=0.852, 95% CI 0.655-1.11, P=0.235) and OS (multivariate HR=1, 95% CI 0.68-1.49, P = 0.981). After PSM, the two groups also had similar PFS (median 9.2 vs. 10.7 months, P=0.98) and OS (median 51.0 vs. 46.0 months, P=0.39). Univariate and multivariate Cox regression analyses revealed no statistically significant association between intraoperative equianalgesic fentanyl and PFS (multivariate HR=1.05, 95% CI 0.632-1.73, P=0.861) and OS (multivariate HR=1.74, 95% CI 0.892-3.38, P = 0.105).
Conclusion: Intraoperative opioids consumption was not correlated with outcomes of CRLM patients treated with simultaneous resection.
Keywords: Colorectal liver metastasis, fentanyl, opioids, prognosis, recurrence
Introduction
Opioids are the mainstay of analgesics in surgery and postoperative pain control of cancer. In recent years, clinicians were concerned that the use of opioids might promote tumorigenesis, cancer metastasis, or recurrence [1]. One possible mechanism is that opioids were long known to inhibit the activity of natural killer (NK) cells, the crucial component of antitumor immunity, in a dose-dependent manner [2, 3]. A large number of clinical studies have focused on the effect of perioperative opioid consumption on the prognosis of patients, and yielded conflicting results for various types of cancer [4-9]. Despite the great heterogeneity in the design and settings of these studies, the inconsistent results of these studies suggest that the prognostic effect of opioid consumption may depend on the type of malignancy. The expression of opioid receptors in certain types of cancer cells might present an alternative pathway for opioids to affect cancer progression and prognosis [10]. Thus, specific clinical studies were required to investigate the role of opioids in a particular type of cancer.
Previous clinical studies on colorectal cancer also yielded inconclusive results. Increased opioids consumption were correlated with worse prognosis in a study of metastatic or recurrent colorectal cancer [11], while other studies on colorectal cancer treated with surgeries found no correlation between perioperative opioids consumption on patient prognosis [4, 12]. These results indicated that the effect of opioids consumption on patients with colorectal cancer might depend on stage of the disease. In addition, preclinical study suggested that opioids could promote metastatic abilities of human colorectal cancer cells [13]. Therefore, colorectal cancer patients in metastatic stage disease may respond differently to opioids. More than 50% of colorectal cancer patients would develop colorectal liver metastasis (CRLM) in their lifetime, and approximately 20% of patients developed liver metastasis at diagnosis, termed synchronous CRLM [14]. Surgical resection is currently the treatment of choice for CRLM. With complete surgical resection, about one-sixth of CRLM patients can be cured [15]. The effect of perioperative opioids consumption on prognosis of patients with CRLM is still poorly understood, as previous studies on colorectal cancer excluded stage IV patients [4], enrolled inadequate number of patients with CRLM [12], or focused on unresectable patients [11]. No previous studies specifically investigated the effect of perioperative fentanyl consumption on prognosis of CRLM patients.
The aim of this study was to investigate the effect of intraoperative fentanyl consumption on the prognosis of CRLM patients treated with simultaneous resection.
Methods
Study population
This study focused on CRLM patients treated with simultaneous resection for: (1) simultaneous surgical resection of primary tumor and liver metastasis is the preferred surgical approach for synchronous CRLM at this institution; (2) patients treated with staged surgeries might experience opioid tolerance or opioid-induced hyperalgesia, which make it difficult to investigate the effect of intraoperative opioids on prognosis. We retrospectively reviewed medical records of patients who received simultaneous resection of CRLM at Cancer Hospital, Chinese Academy of Medical Sciences between December 2008 and May 2019. Patients receiving simultaneous surgery at this institution are required to be Child-Pugh A, while emergency surgery for symptomatic primary tumour is considered as a contraindication of simultaneous resection. Rectal resection/low rectal resection is not considered as a contraindication of simultaneous resection. Major hepatic resection (resection of more than 2 liver segments) is also not considered as a contraindication of simultaneous resection. Only adult patients who received surgery for a curative intent were included in this study. Patients with incomplete medical records were excluded from this study. We further excluded patients with epidural anesthesia, as a reliable approach to calculate the equianalgesic dose of epidural opioids was lacking. Finally, we excluded patients with intraoperative morphine or oxycodone from analysis, as dose conversion between opioids had been controversial [16], and these two opioids were only given to a small proportion of patients who received simultaneous resection of CRLM intraoperatively at this institution. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics approval of this study was obtained from the Institutional Review Board of the Cancer Hospital, Chinese Academy of Medical Sciences (ID:NCC2019C-016) and individual consent for this retrospective analysis was waived.
Anesthetic and analgesic methods
Patients who received simultaneous resection of CRLM generally underwent combined general anesthesia at this institution. The doses of strong opioids (fentanyl, sufentanil, remifentanil, morphine, oxycodone) were retrieved from surgical records of each patient, and the total dose during the operation was calculated. The doses of sufentanil and remifentanil were converted to equianalgesic fentanyl dose by the following manner: 0.1 μg sufentanil for 1 μg equianalgesic fentanyl, 1 μg remifentanil for 1 μg equianalgesic fentanyl. Intraoperative equianalgesic fentanyl dose per kilogram body weight is the exposure variable of this study.
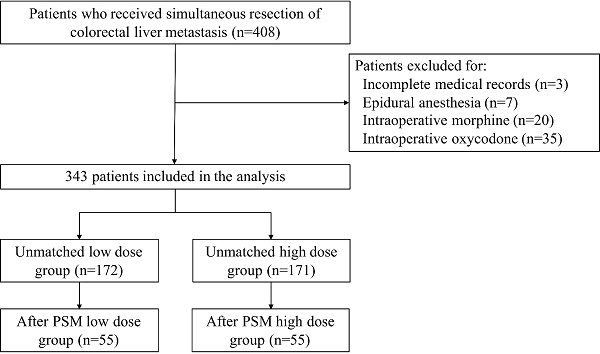
Flow diagram.
Follow-up and Outcomes
Postoperative complications were recorded until hospital discharge. The Clavian-Dindo classification system is applied to classify postoperative complications by severity [17]. Major complication is defined as Clavian-Dindo grade III or IV. For patients with multiple postoperative complications, complication with the highest grade was recorded. Postoperative complications were further classified into general complications and surgery-related complications. Anastomotic leak, gastrointestinal tract necrosis, intrathoracic or intraabdominal abscess, hemorrhage, and ileus were considered surgery-related complications while other complications were considered as general complications [18]. Hypertension and diabetes were recorded as comorbidities. As described previously, patients were followed up at regular intervals [19]. In brief, the initial follow-up was performed 1 month after surgery, then follow-ups were performed every 3 months thereafter. Progression-free survival (PFS) was defined from surgery to detection of tumor progression or the last follow-up. Overall survival (OS) was defined from surgery to death or the last follow-up.
Statistical analyses
Categorical variables were presented as percentages and compared by the Chi-square test. Continuous variables were present as median and interquartile ranges (IQR) and compared by the Mann-Whitney U test. The median intraoperative equianalgesic fentanyl dose was selected as the cut-off to separate patients into high- and low-dose groups accordingly. Survival was analyzed by the Kaplan-Meier method and compared by the log-rank test. Potential predictors of survival identified in the univariate analyses with P<0.10 and variable of interest (intraoperative equianalgesic fentanyl consumption) entered subsequent multivariate Cox regression analysis. To adjust for differences in baseline characteristics between groups, we performed 1:1 propensity score matching (PSM) by the 'nearest' method and without replacement. PSM was performed with the 'MatchIt' package of the R software. Two-sided P<0.05 indicates statistical significance. All statistical analyses were performed by the R software (Version 4.0.2).
Results
Study population
Medical records of 408 patients who received simultaneous resection of CRLM were retrospectively reviewed. Three patients were excluded for incomplete medical records, 7 patients were excluded for epidural anesthesia, and 55 patients were excluded for intraoperative morphine or oxycodone. Three hundred and forty-four patients remained in the final study population. The flow diagram of this study was shown in Figure 1. Each patient in the study population received at least one of fentanyl, sufentanil, or remifentanil intraoperatively. The median intraoperative equianalgesic fentanyl dose per kilogram body weight was 8.33 (6.53-14.0) μg/kg. The low-dose group consists of 172 patients with equianalgesic fentanyl dose≤8.33 μg/kg, while the high-dose group consists of 171 patients with equianalgesic fentanyl dose>8.33 μg/kg. Patient demographics, clinicopathological characteristics, and surgery and chemotherapy details were listed in Table 1. The low dose group had higher BMI (median 24.10 vs. 23.31, P=0.004), higher proportion of patients with comorbidities (51.7% vs. 36.8%, P=0.005), higher proportion of patients with ASA III (17.4% vs. 7.0%, P=0.013), and higher CEA (median 10.14 vs. 6.75 ng/μL, P=0.005).
Patient demographics, clinicopathological characteristics, and treatment details stratified by median intraoperative equianalgesic fentanyl dose
| Group | Before PSM | P | After PSM | P | |||
|---|---|---|---|---|---|---|---|
| Low dose (n=172) | High dose (n=171) | Low dose (n=55) | High dose (n=55) | ||||
| Demographics & clinicopathological characteristics | |||||||
| Age | 60.00 [52.75, 67.00] | 58.00 [52.00, 64.00] | 0.078 | 57.00 [50.00, 64.00] | 58.00 [52.00, 64.00] | 0.858 | |
| Gender | Male | 53 (30.8) | 65 (38.0) | 0.161 | 15 (27.3) | 17 (30.9) | 0.675 |
| Female | 119 (69.2) | 106 (62.0) | 40 (72.7) | 38 (69.1) | |||
| BMI | 24.10 [22.57, 26.49] | 23.31 [21.34, 25.66] | 0.004 | 23.66 [22.13, 25.11] | 23.71 [21.73, 25.63] | 0.914 | |
| comorbidity | Yes | 89 (51.7) | 63 (36.8) | 0.005 | 17 (30.9) | 17 (30.9) | 1 |
| No | 83 (48.3) | 108 (63.2) | 38 (69.1) | 38 (69.1) | |||
| ASA | I | 4 (2.3) | 4 (2.3) | 0.013 | 3 (5.5) | 2 (3.6) | 0.842 |
| II | 138 (80.2) | 155 (90.6) | 49 (89.1) | 49 (89.1) | |||
| III | 30 (17.4) | 12 (7.0) | 3 (5.5) | 4 (7.3) | |||
| Primary site | Rectum | 76 (44.2) | 78 (45.6) | 0.955 | 26 (47.3) | 22 (40.0) | 0.592 |
| Left colon | 62 (36.0) | 61 (35.7) | 16 (29.1) | 21 (38.2) | |||
| Right colon | 34 (19.8) | 32 (18.7) | 13 (23.6) | 12 (21.8) | |||
| Bilobular distribution of liver metastasis | Yes | 74 (43.0) | 64 (37.4) | 0.291 | 21 (38.2) | 17 (30.9) | 0.423 |
| No | 98 (57.0) | 107 (62.6) | 34 (61.8) | 38 (69.1) | |||
| Number of liver metastasis | 2.00 [1.00, 4.00] | 2.00 [1.00, 4.00] | 0.631 | 2.00 [1.00, 4.00] | 2.00 [1.00, 4.00] | 0.632 | |
| Maximum diameter of liver metastasis (cm) | 2.50 [1.50, 3.80] | 2.50 [1.50, 4.00] | 0.492 | 2.50 [1.55, 3.65] | 2.70 [1.50, 4.00] | 0.467 | |
| Poor differentiation | Yes | 59 (34.3) | 60 (35.1) | 0.879 | 19 (34.5) | 16 (29.1) | 0.539 |
| No | 113 (65.7) | 111 (64.9) | 36 (65.5) | 39 (70.9) | |||
| Primary tumor T stage | T1-T2 | 14 (8.1) | 16 (9.4) | 0.69 | 5 (9.1) | 6 (10.9) | 0.751 |
| T3-T4 | 158 (91.9) | 155 (90.6) | 50 (90.9) | 49 (89.1) | |||
| Primary lymph node metastasis | Yes | 128 (74.4) | 125 (73.1) | 0.781 | 42 (76.4) | 38 (69.1) | 0.392 |
| No | 44 (25.6) | 46 (26.9) | 13 (23.6) | 17 (30.9) | |||
| CEA (ng/μL) | 10.14 [5.32, 30.95] | 6.75 [3.07, 23.95] | 0.005 | 8.31 [4.40, 20.64] | 8.39 [2.96, 32.56] | 0.886 | |
| Extrahepatic metastasis | Yes | 12 (7.0) | 19 (11.1) | 0.182 | 5 (9.1) | 5 (9.1) | 1 |
| No | 160 (93.0) | 152 (88.9) | 50 (90.9) | 50 (90.9) | |||
| Chemotherapy | |||||||
| Neoadjuvant chemotherapy | Yes | 93 (54.1) | 100 (58.5) | 0.41 | 28 (50.9) | 33 (60.0) | 0.337 |
| No | 79 (45.9) | 71 (41.5) | 27 (49.1) | 22 (40.0) | |||
| Adjuvant chemotherapy | Yes | 116 (67.4) | 104 (60.8) | 0.201 | 39 (70.9) | 33 (60.0) | 0.229 |
| No | 56 (32.6) | 67 (39.2) | 16 (29.1) | 22 (40.0) | |||
| Surgical details | |||||||
| R0 resection | Yes | 128 (74.4) | 126 (73.7) | 0.877 | 36 (65.5) | 39 (70.9) | 0.539 |
| No | 44 (25.6) | 45 (26.3) | 19 (34.5) | 16 (29.1) | |||
| Intraoperative RFA | Yes | 19 (11.0) | 11 (6.4) | 0.13 | 4 (7.3) | 5 (9.1) | 0.728 |
| No | 153 (89.0) | 160 (93.6) | 51 (92.7) | 50 (90.9) | |||
| Surgical approach | Totally laparoscopic | 45 (26.2) | 40 (23.4) | 0.586 | 16 (29.1) | 13 (23.6) | 0.627 |
| Mixed | 87 (50.6) | 96 (56.1) | 23 (41.8) | 28 (50.9) | |||
| Totally open | 40 (23.3) | 35 (20.5) | 16 (29.1) | 14 (25.5) | |||
| Major hepatic resection | Yes | 80 (46.5) | 84 (49.1) | 0.628 | 24 (43.6) | 28 (50.9) | 0.445 |
| No | 92 (53.5) | 87 (50.9) | 31 (56.4) | 27 (49.1) | |||
| Intraoperative Pringle maneuver | Yes | 124 (72.1) | 131 (76.6) | 0.338 | 39 (70.9) | 42 (76.4) | 0.516 |
| No | 48 (27.9) | 40 (23.4) | 16 (29.1) | 13 (23.6) | |||
| Intraoperative blood loss (mL) | 200.00 [100.00, 400.00] | 200.00 [200.00, 400.00] | 0.279 | 200.00 [100.00, 500.00] | 200.00 [200.00, 300.00] | 0.47 | |
| Intraoperative blood transfusion | Yes | 39 (22.7) | 43 (25.1) | 0.592 | 16 (29.1) | 15 (27.3) | 0.832 |
| No | 133 (77.3) | 128 (74.9) | 39 (70.9) | 40 (72.7) | |||
| Operation time (min) | 324.50 [260.00, 410.25] | 348.00 [269.00, 420.00] | 0.451 | 310.00 [264.50, 380.50] | 350.00 [266.50, 400.00] | 0.453 | |
To adjust for differences in baseline characteristics between groups, we performed 1:1 PSM. After PSM, 55 patients in the low dose group were matched to 55 patients in the high dose group. The baseline characteristics were well-balanced between the two groups (Table 1).
Short-term outcomes
Details of short-term outcomes were listed in Table 2. Before PSM, the two groups were comparable in length of postoperative hospital stay (median 10 vs. 10 days, P=0.841). When classified into major complications and minor complications according to the Clavian-Dindo scoring system, a trend of the low dose group having lower proportion of no (48.3% vs. 54.4%) or minor (23.8% vs. 27.5%) complications and higher proportion of major complications (27.9% vs. 18.1%) was observed (P=0.099). The categories of postoperative complications were comparable (P=0.615). After PSM, the low dose group had numerically longer postoperative hospital stay (median 11 vs. 9 days, P=0.199). A trend of the low dose group having lower proportion of no (43.6% vs. 52.7%) or minor (23.6% vs. 32.7%) complications and higher proportion of major complications (32.7% vs. 14.5%) was observed (P=0.077). The categories of postoperative complications were comparable (P=0.272).
Progression-free survival and overall survival
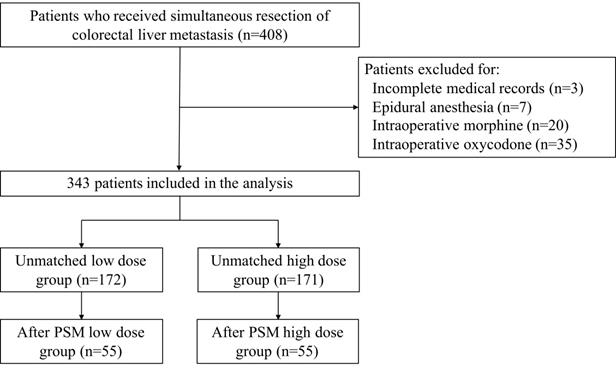
In the unmatched full cohort, the Kaplan-Meier survival plot and log-rank test revealed the two groups having comparable PFS (Figure 2a, median 10.2 vs. 12.4 months, P=0.54) and OS (Figure 2b, median 59.0 vs. 58.3 months, P=0.76). In univariate analysis, intraoperative equianalgesic fentanyl (high dose vs. low dose) was not associated with PFS (HR=0.925, 95% CI 0.720-1.19, P=0.541). In subsequent multivariate analysis, intraoperative equianalgesic fentanyl (high dose vs. low dose) was not associated with PFS (HR=0.852, 95% CI 0.655-1.11, P=0.235). Primary lymph node metastasis (HR=1.84, 95% CI 1.32-2.56, P<0.001), extrahepatic metastasis (HR=1.97, 95% CI 1.3-2.99, P=0.001), R0 resection (HR=0.686, 95% CI 0.509-0.924, P=0.013), and major hepatic resection (HR=1.49, 95% CI 1.04-2.12, P=0.029) were independent predictors of PFS.
Progression-free survival and overall survival in patients who received low- versus high-dose intraoperative equianalgesic fentanyl before propensity score matching: (a) Progression free survival; (b) overall survival.
Short-term outcomes of patients stratified by median intraoperative equianalgesic fentanyl dose
| Group | Before PSM | P | After PSM | P | |||
|---|---|---|---|---|---|---|---|
| Low dose (n=172) | High dose (n=171) | Low dose (n=55) | High dose (n=55) | ||||
| Postoperative Hospital stay | 10.00 [8.00, 13.25] | 10.00 [8.00, 13.50] | 0.841 | 11.00 [8.00, 15.00] | 9.00 [8.00, 13.00] | 0.199 | |
| Postoperative complication | No complication | 83 (48.3) | 93 (54.4) | 0.099 | 24 (43.6) | 29 (52.7) | 0.077 |
| Minor complication | 41 (23.8) | 47 (27.5) | 13 (23.6) | 18 (32.7) | |||
| Major complication | 48 (27.9) | 31 (18.1) | 18 (32.7) | 8 (14.5) | |||
| Postoperative complication category | No complication | 83 (48.3) | 93 (54.4) | 0.615 | 24 (43.6) | 29 (52.7) | 0.272 |
| Surgery-related | 31 (18.0) | 31 (18.1) | 14 (25.5) | 13 (23.6) | |||
| General complication | 30 (17.4) | 23 (13.5) | 4 (7.3) | 7 (12.7) | |||
| Surgery-related and general complication | 28 (16.3) | 24 (14.0) | 13 (23.6) | 6 (10.9) | |||
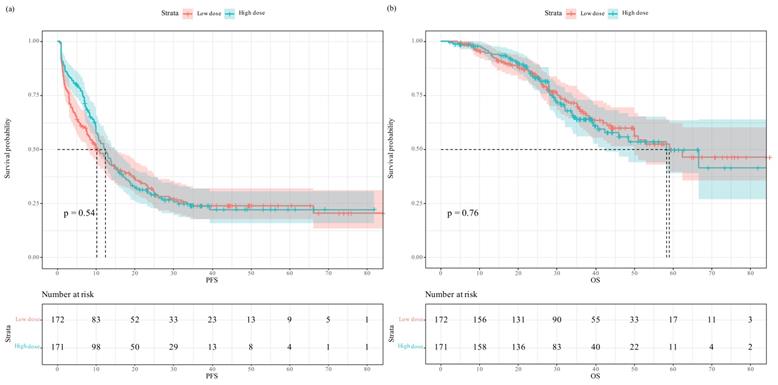
Progression-free survival and overall survival in patients who received low- versus high-dose intraoperative equianalgesic fentanyl after propensity score matching: (a) Progression free survival; (b) overall survival.
Univariate and Multivariate analysis of factors associated with progression-free survival and overall survival before PSM
| PFS | OS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate HR | P | Multivariate HR | P | Univariate HR | P | Multivariate HR | P | ||
| Intraoperative equianalgesic fentanyl | Low dose | Referent | Referent | Referent | Referent | ||||
| High dose | 0.925 (0.720-1.19) | 0.541 | 0.852 (0.655-1.11) | 0.235 | 1.06 (0.732-1.53) | 0.765 | 1 (0.68-1.49) | 0.981 | |
| Demographics & clinicopathological characteristics | |||||||||
| Age | 1.01 (0.991-1.02) | 0.495 | 1.03 (1.01-1.05) | 0.016 | 1.04 (1.02-1.06) | 0.002 | |||
| Gender | Male | Referent | Referent | Referent | |||||
| Female | 1.38 (1.05-1.8) | 0.020 | 1.1 (0.825-1.46) | 0.522 | 1.18 (0.797-1.74) | 0.413 | |||
| BMI | 1.02 (0.974-1.06) | 0.478 | 1.02 (0.958-1.09) | 0.507 | |||||
| Comorbidity | No | Referent | Referent | ||||||
| Yes | 1.09 (0.851-1.41) | 0.486 | 1.07 (0.755-1.55) | 0.725 | |||||
| ASA | I | Referent | Referent | ||||||
| II | 1.012 (0.476-2.15) | 0.975 | 1.22 (0.387-3.86) | 0.733 | |||||
| III | 1.002 (0.44-2.28) | 0.997 | 1.02 (0.288-3.62) | 0.975 | |||||
| Primary site | Rectum | Referent | Referent | Referent | |||||
| Left colon | 0.927 (0.703-1.22) | 0.588 | 1.002 (0.753-1.33) | 0.990 | 0.999 (0.664-1.5) | 0.995 | |||
| Right colon | 0.733 (0.513-1.05) | 0.089 | 0.711 (0.425-1.19) | 0.193 | 0.943 (0.567-1.57) | 0.822 | |||
| Bilobular distribution of liver metastasis | No | Referent | Referent | Referent | Referent | ||||
| Yes | 1.77 (1.38-2.28) | <0.001 | 0.91 (0.642-1.29) | 0.596 | 1.64 (1.14-2.37) | 0.009 | 0.666 (0.399-1.11) | 0.119 | |
| Number of liver metastasis | 1.15 (1.1-1.2) | <0.001 | 1.06 (0.989-1.13) | 0.100 | 1.18 (1.12-1.25) | <0.001 | 1.13 (1.02-1.24) | 0.015 | |
| Maximum diameter of liver metastasis (cm) | 1.11 (1.04-1.18) | 0.002 | 1.05 (0.971-1.13) | 0.229 | 1.15 (1.05-1.26) | 0.004 | 1.06 (0.957-1.18) | 0.257 | |
| Poor differentiation | No | Referent | Referent | Referent | Referent | ||||
| Yes | 1.28 (0.986-1.67) | 0.064 | 1.14 (0.87-1.5) | 0.342 | 1.41 (0.959-2.07) | 0.082 | 1.18 (0.787-1.77) | 0.425 | |
| Primary tumor T stage | T1-T2 | Referent | Referent | Referent | Referent | ||||
| T3-T4 | 1.65 (1.01-2.7) | 0.048 | 1.38 (0.832-2.3) | 0.211 | 4.2 (1.33-13.2) | 0.015 | 3.44 (1.06-11.1) | 0.040 | |
| Primary lymph node metastasis | No | Referent | Referent | Referent | Referent | ||||
| Yes | 2.14 (1.56-2.94) | <0.001 | 1.84 (1.32-2.56) | <0.001 | 3.4 (1.91-6.07) | <0.001 | 3.04 (1.65-5.58) | <0.001 | |
| CEA (ng/μL) | 1.001 (1-1.001) | 0.070 | 1 (0.999-1.001) | 0.989 | 1.001 (1-1.001) | 0.298 | |||
| Extrahepatic metastasis | No | Referent | Referent | Referent | |||||
| Yes | 2.06 (1.38-3.070 | <0.001 | 1.97 (1.3-2.99) | 0.002 | 1.36 (0.744-2.47) | 0.321 | |||
| Chemotherapy | |||||||||
| Neoadjuvant chemotherapy | No | Referent | Referent | Referent | |||||
| Yes | 1.02 (0.795-1.32) | 0.855 | 1.48 (1.01-2.17) | 0.046 | 1.19 (0.767-1.83) | 0.444 | |||
| Adjuvant chemotherapy | No | Referent | Referent | Referent | |||||
| Yes | 1.04 (0.797-1.35) | 0.794 | 0.616 (0.425-0.893) | 0.011 | 0.484 (0.324-0.725) | <0.001 | |||
| Surgical details | |||||||||
| R0 resection | No | Referent | Referent | Referent | Referent | ||||
| Yes | 0.553 (0.421-0.727) | <0.001 | 0.686 (0.509-0.924) | 0.013 | 0.556 (0.38-0.814) | 0.003 | 0.84 (0.552-1.28) | 0.416 | |
| Intraoperative RFA | No | Referent | Referent | Referent | Referent | ||||
| Yes | 1.71 (1.14-2.57) | 0.010 | 1.25 (0.772-2.01) | 0.369 | 2.16 (1.32-3.53) | 0.003 | 1.08 (0.584-2.01) | 0.802 | |
| Surgical approach | Totally laparoscopic | Referent | Referent | Referent | Referent | ||||
| Mixed | 1.52 (1.10-2.09) | 0.011 | 0.981 (0.685-1.41) | 0.918 | 1.58 (0.947-2.64) | 0.080 | 1.03 (0.565-1.86) | 0.934 | |
| Totally open | 1.24 (0.844-1.82) | 0.273 | 1.04 (0.634-1.72) | 0.867 | 1.62 (0.911-2.89) | 0.101 | 1.04 (0.543-1.98) | 0.913 | |
| Major hepatic resection | No | Referent | Referent | Referent | Referent | ||||
| Yes | 1.93 (1.5-2.49) | <0.001 | 1.49 (1.04-2.12) | 0.029 | 2.21 (1.51-3.22) | <0.001 | 1.84 (1.06-3.19) | 0.032 | |
| Intraoperative Pringle maneuver | No | Referent | Referent | Referent | |||||
| Yes | 1.18 (0.883-1.57) | 0.265 | 1.51 (0.974-2.32) | 0.066 | 0.646 (0.364-1.15) | 0.137 | |||
| Intraoperative blood loss (mL) | 1.001 (1-1.001) | <0.001 | 1 (1-1.001) | 0.772 | 1.001 (1-1.002) | 0.007 | 1 (0.999-1.001) | 0.893 | |
| Intraoperative blood transfusion | No | Referent | Referent | Referent | |||||
| Yes | 1.19 (0.891-1.58) | 0.243 | 1.63 (1.09-2.44) | 0.017 | 1.19 (0.679-2.1) | 0.539 | |||
| Operation time (min) | 1.002 (1.001-1.003) | <0.001 | 1.001 (0.999-1.002) | 0.330 | 1.003 (1.002-1.005) | <0.001 | 1.002 (1-1.004) | 0.056 | |
Univariate and Multivariate analysis of factors associated with progression-free survival and overall survival after PSM
| PFS | OS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate HR | P | Multivariate HR | P | Univariate HR | P | Multivariate HR | P | ||
| Intraoperative equianalgesic fentanyl | Low dose | Referent | Referent | Referent | Referent | ||||
| High dose | 0.996 (0.639-1.55) | 0.984 | 1.05 (0.632-1.73) | 0.861 | 1.31 (0.706-2.43) | 0.392 | 1.74 (0.892-3.38) | 0.105 | |
| Demographics & clinicopathological characteristics | |||||||||
| Age | 1.003 (0.982-1.03) | 0.764 | 1.04 (1.01-1.08) | 0.016 | 1.06 (1.02-1.11) | 0.003 | |||
| Gender | Male | Referent | Referent | Referent | |||||
| Female | 1.68 (1.02-2.79) | 0.047 | 1.23 (0.719-2.12) | 0.446 | 1.17 (0.587-2.34) | 0.653 | |||
| BMI | 1.03 (0.948-1.12) | 0.479 | 1.06 (0.939-1.19) | 0.366 | |||||
| Comorbidity | No | Referent | Referent | ||||||
| Yes | 1.25 (0.779-1.99) | 0.359 | 1.58 (0.822-3.04) | 0.170 | |||||
| ASA | I | Referent | Referent | ||||||
| II | 1.26 (0.46-3.46) | 0.652 | 3.15 (0.431-23) | 0.258 | |||||
| III | 0.609 (0.136-2.73) | 0.517 | 6.73 (0.692-65.4) | 0.101 | |||||
| Primary site | Rectum | Referent | Referent | Referent | |||||
| Left colon | 0.718 (0.434-1.19) | 0.197 | 0.808 (0.404-1.62) | 0.546 | 0.67 (0.322-1.39) | 0.284 | |||
| Right colon | 0.668 (0.37-1.21) | 0.181 | 0.482 (0.204-1.14) | 0.098 | 0.429 (0.171-1.07) | 0.070 | |||
| Bilobular distribution of liver metastasis | No | Referent | Referent | Referent | |||||
| Yes | 1.59 (1.02-2.49) | 0.043 | 1.06 (0.549-2.05) | 0.860 | 1.24 (0.663-2.33) | 0.497 | |||
| Number of liver metastasis | 1.12 (1.02-1.22) | 0.014 | 1.03 (0.918-1.15) | 0.638 | 1.09 (0.97-1.22) | 0.153 | |||
| Maximum diameter of liver metastasis (cm) | 1.09 (0.973-1.22) | 0.138 | 1.14 (0.973-1.34) | 0.105 | |||||
| Poor differentiation | No | Referent | Referent | ||||||
| Yes | 1.23 (0.768-1.98) | 0.386 | 1.13 (0.57-2.23) | 0.730 | |||||
| Primary tumor T stage | T1-T2 | Referent | Referent | ||||||
| T3-T4 | 1.36 (0.627-2.97) | 0.434 | 1.39 (0.427-4.5) | 0.588 | |||||
| Primary lymph node metastasis | No | Referent | Referent | Referent | Referent | ||||
| Yes | 2.33 (1.33-4.11) | 0.004 | 2.42 (1.34-4.36) | 0.004 | 2.45 (1.03-5.83) | 0.043 | 2.83 (1.14-7.02) | 0.025 | |
| CEA (ng/μL) | 1.002 (0.999-1.005) | 0.146 | 1 (0.997-1.01) | 0.613 | |||||
| Extrahepatic metastasis | No | Referent | Referent | Referent | |||||
| Yes | 2.3 (1.13-4.68) | 0.023 | 2.23 (1.07-4.67) | 0.033 | 1.21 (0.428-3.42) | 0.721 | |||
| Chemotherapy | |||||||||
| Neoadjuvant chemotherapy | No | Referent | Referent | Referent | |||||
| Yes | 0.676 (0.434-1.05) | 0.082 | 0.588 (0.364-0.951) | 0.031 | 1.26 (0.674-2.37) | 0.467 | |||
| Adjuvant chemotherapy | No | Referent | Referent | ||||||
| Yes | 1.06 (0.664-1.7) | 0.804 | 0.657 (0.352-1.23) | 0.187 | |||||
| Surgical details | |||||||||
| R0 resection | No | Referent | Referent | ||||||
| Yes | 0.815 (0.509-1.31) | 0.394 | 0.859 (0.454-1.62) | 0.639 | |||||
| Intraoperative RFA | No | Referent | Referent | ||||||
| Yes | 1.33 (0.609-2.89) | 0.477 | 1.48 (0.583-3.77) | 0.415 | |||||
| Surgical approach | Totally laparoscopic | Referent | Referent | ||||||
| Mixed | 1.01 (0.591-1.74) | 0.959 | 1.48 (0.653-3.33) | 0.349 | |||||
| Totally open | 0.792 (0.426-1.47) | 0.461 | 1.25 (0.508-3.05) | 0.632 | |||||
| Major hepatic resection | No | Referent | Referent | Referent | Referent | ||||
| Yes | 1.59 (1.02-2.48) | 0.042 | 1.28 (0.704-2.34) | 0.415 | 2.24 (1.2-4.2) | 0.012 | 1.49 (0.715-3.1) | 0.287 | |
| Intraoperative Pringle maneuver | No | Referent | Referent | Referent | |||||
| Yes | 1.27 (0.762-2.1) | 0.363 | 2.1 (0.968-4.56) | 0.061 | 1.09 (0.424-2.78) | 0.865 | |||
| Intraoperative blood loss (mL) | 1.001 (1-1.002) | 0.057 | 1.001 (1-1.002) | 0.105 | 1.001 (1-1.002) | 0.028 | 1.001 (1-1.003) | 0.035 | |
| Intraoperative blood transfusion | No | Referent | Referent | ||||||
| Yes | 1.14 (0.704-1.84) | 0.598 | 1.63 (0.861-3.09) | 0.133 | |||||
| Operation time (min) | 1.002 (1.001-1.004) | 0.007 | 1.001 (0.999-1.003) | 0.486 | 1.003 (1.001-1.005) | 0.004 | 1.002 (0.999-1.005) | 0.118 | |
In univariate analysis, intraoperative equianalgesic fentanyl (high dose vs. low dose) was not associated with OS (HR=1.06, 95% CI 0.732-1.53, P=0.765). In subsequent multivariate analysis, intraoperative equianalgesic fentanyl (high dose vs. low dose) was not associated with OS (HR=1, 95% CI 0.68-1.49, P=0.981). Age (HR=1.04, 95% CI 1.02-1.06, P=0.002), number of liver metastasis (HR=1.13, 95% CI 1.02-1.24, P=0.015), primary tumor T3 or T4 (HR=3.44, 95% CI 1.06-11.1, P=0.040), primary lymph node metastasis (HR=3.04, 95% CI 1.65-5.58, P<0.001), adjuvant chemotherapy (HR=0.484, 95% CI 0.324-0.725, P<0.001), and major hepatic resection (HR=1.84, 95% CI 1.06-3.19, P=0.032) were independent predictors of OS.
After propensity score matching, the Kaplan-Meier survival plot and log-rank test revealed the two groups having comparable PFS (Figure 3a, median 9.2 vs. 10.7 months, P=0.98) and OS (Figure 3b, median 51.0 vs. 46.0 months, P=0.39). In univariate analysis, intraoperative equianalgesic fentanyl (high dose vs. low dose) was not associated with PFS (HR=0.996, 95% CI 0.639-1.55, P=0.984). In subsequent multivariate analysis, intraoperative equianalgesic fentanyl (high dose vs. low dose) was not associated with PFS (HR=1.05, 95% CI 0.632-1.73, P=0.861). Primary lymph node metastasis (HR=2.42, 95% CI 1.34-4.36, P=0.004), extrahepatic metastasis (HR=2.23, 95% CI 1.07-4.67, P=0.033), and neoadjuvant chemotherapy (HR=0.588, 95% CI 0.364-0.951, P=0.031) were independent predictors of PFS.
In univariate analysis, intraoperative equianalgesic fentanyl (high dose vs. low dose) was not associated with OS (HR=1.31, 95% CI 0.706-2.43, P=0.392). In subsequent multivariate analysis, intraoperative equianalgesic fentanyl (high dose vs. low dose) was not associated with OS (HR=1.74, 95% CI 0.892-3.38, P=0.105). Age (HR=1.06, 95% CI 1.02-1.11, P=0.003), primary lymph node metastasis (HR=2.83, 95% CI 1.14-7.02, P=0.025), and intraoperative blood loss (mL) (HR=1.001, 95% CI 1-1.003, P=0.035) were independent predictors of OS.
Discussion
This is the first study to evaluate the effect of intraoperative opioid consumption on outcomes of CRLM patients. In this study, we demonstrated that for patients who received simultaneous resection of CRLM, intraoperative equianalgesic fentanyl dose was not associated with postoperative complications, PFS, or OS before and after PSM.
Previous clinical studies evaluating the prognostic effect of perioperative opioid consumption have associated increased opioid dose with a higher risk of developing disease recurrence in patients with non-small-cell lung cancer (NSCLC) [6], esophagus squamous cell carcinoma [20], and laryngeal squamous cell carcinoma [21]. Several plausible mechanisms were raised to explain the correlation between increased perioperative opioid consumption and a higher risk of disease recurrence in these types of cancer. First, opioids were known to have immunosuppressive effects, especially by inhibiting the activity of NK cells [3, 22]. As a critical component of tumor immunosurveillance, impaired cytotoxicity of NK cells may promote the growth and metastasis of tumors [23-25]. Second, opioids could directly interact with opioid receptors expressed by cancer cells, and stimulate tumor growth and metastasis [26]. Overexpression of mu-opioid receptor (MOR) has been observed in human colorectal cancer samples [27]. Activation of MOR by morphine promotes proliferation, invasion, and migration of human colorectal cancer cells, which is possibly due to transactivation of epidermal growth factor receptor (EGFR) and downstream signaling pathways [13].
In contrast, the association between decreased intraoperative opioid dose and worse recurrence-free survival (RFS) was observed in clinical studies of esophageal squamous cell carcinoma [5], and triple-negative breast cancer [28]. Different levels of MOR expression and polymorphisms of the OPRM1 gene may allow various cancer cells to respond differently to the stimulation of opioids [5]. While in the study of triple-negative breast cancer, data of bulk RNA-seq were available and showed almost no expression of the OPRM1 gene [28]. This mechanism is not likely to be favorable in the case of CRLM, as increased expression of the OPRM1 gene was observed in colorectal cancer tissue samples [27]. A low level of perioperative equianalgesic fentanyl dose might be associated with an increased risk of patients receiving inadequate pain control. Increased surgical stress response and activation of the sympathetic nervous system, possibly the consequence of insufficient pain control, is considered to be immunosuppressive or promote invasiveness of tumor cells [29]. Evaluation of the effectiveness of perioperative pain control, i.e. intraoperative hemodynamics and postoperative pain scoring, is lacking in this study, which limited further discussion.
In this study, we observed no statistically significant association between intraoperative equianalgesic fentanyl consumption and postoperative complications, PFS, or OS. These results were in line with a previous clinical study of intraoperative fentanyl consumption on prognosis of stage I-III colorectal cancer [4]. While in another study of metastatic or recurrent colorectal cancer treated with therapeutic chemotherapy, the use of opioids was associated with worse outcomes [11]. It is possible that long-term use of opioids for the management of cancer pain could involve higher cumulative dose of opioids compared to intraoperative opioids, and may lead to prolonged immunosuppression and worse prognosis of patients with colorectal cancer.
Several limitations are present in this study. First, results from analyses of the unmatched full cohort may be confounded by differences in baseline characteristics. The higher comorbidity burden and increased CEA of the low dose group could lead to worse outcomes of this group despite adjusted in multivariate analysis. While we tried to minimize confounding effect through PSM, the statistical power to tell the differences of outcomes between groups was reduced in the matched analysis. Second, data including NK cells activity, MOR expression, OPRM1 polymorphism, and evaluation of perioperative pain control are lacking in the study and limited further discussion of possible mechanisms contributing to the results. As the inhibitory effect of opioids on NK cells last for several days, the opioid consumption in a short time period after surgery may also have prognostic importance [3, 6]. Third, multiple anesthesiologists contributed to anesthesia and of patients in this study, and opioid doses prescribed by different anesthesiologists might be slightly biased. Finally, this is a retrospective cohort study with all patients treated at the same institution, and the results of this study require external validation.
Conclusion
Intraoperative opioids consumption was not correlated with outcomes of CRLM patients treated with simultaneous resection.
Abbreviations
NK: natural killer; CRLM: colorectal liver metastasis; PFS: progression-free survival; OS: overall survival; PSM: propensity score matching; IQR: interquartile ranges; BMI: body mass index; ASA: American Society of Anesthesiologists; RFA: radiofrequency ablation; NSCLC: non-small-cell lung cancer; MOR: mu-opioid receptor; EGFR: epidermal growth factor receptor; RFS: recurrence-free survival.
Acknowledgements
Funding
This study was supported by the National Natural Science Foundation of China (81672461, 81972311), CAMS Innovation Fund for Medical Sciences (CIFMS) (Grant no.2017-12M-4-002), the Non-profit Central Research Institution Fund of Chinese Academy of Medical Sciences (2019PT310026) and Sanming Project of Medicine in Shenzhen (No. SZSM202011010).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Buggy DJ, Borgeat A, Cata J, Doherty DG, Doornebal CW, Forget P. et al. Consensus statement from the BJA Workshop on Cancer and Anaesthesia. Br J Anaesth. 2015;114(1):2-3
2. Beilin B, Shavit Y, Hart J, Mordashov B, Cohn S, Notti I. et al. Effects of anesthesia based on large versus small doses of fentanyl on natural killer cell cytotoxicity in the perioperative period. Anesth Analg. 1996;82(3):492-497
3. Page GG. Immunologic effects of opioids in the presence or absence of pain. J Pain Symptom Manage. 2005;29(5 Suppl):S25-S31
4. Tai YH, Wu HL, Chang WK, Tsou MY, Chen HH, Chang KY. Intraoperative Fentanyl Consumption Does Not Impact Cancer Recurrence or Overall Survival after Curative Colorectal Cancer Resection. Sci Rep. 2017;7(1):10816
5. Du KN, Feng L, Newhouse A, Mehta J, Lasala J, Mena GE. et al. Effects of Intraoperative Opioid Use on Recurrence-Free and Overall Survival in Patients With Esophageal Adenocarcinoma and Squamous Cell Carcinoma. Anesth Analg. 2018;127(1):210-216
6. Maher DP, Wong W, White PF, McKenna R Jr, Rosner H, Shamloo B. et al. Association of increased postoperative opioid administration with non-small-cell lung cancer recurrence: a retrospective analysis. Br J Anaesth. 2014;113(Suppl 1):i88-i94
7. Oh TK, Jeon JH, Lee JM, Kim MS, Kim JH, Cho H. et al. Investigation of opioid use and long-term oncologic outcomes for non-small cell lung cancer patients treated with surgery. PLoS One. 2017;12(7):e0181672
8. Cata JP, Keerty V, Keerty D, Feng L, Norman PH, Gottumukkala V. et al. A retrospective analysis of the effect of intraoperative opioid dose on cancer recurrence after non-small cell lung cancer resection. Cancer Med. 2014;3(4):900-908
9. Oh TK, Kim K, Jheon SH, Do SH, Hwang JW, Jeon YT. et al. Long-Term Oncologic Outcomes, Opioid Use, and Complications after Esophageal Cancer Surgery. J Clin Med. 2018;7(2):33
10. Wigmore T, Farquhar-Smith P. Opioids and cancer: friend or foe? Curr Opin Support Palliat Care. 2016;10(2):109-118
11. Inoue Y, Iwata T, Okugawa Y, Kawamoto A, Hiro J, Toiyama Y. et al. Prognostic significance of opioid use in the active treatment of advanced colorectal cancer. Mol Clin Oncol. 2013;1(1):59-64
12. Wu HL, Tai YH, Chang WK, Chang KY, Tsou MY, Cherng YG. et al. Does postoperative morphine consumption for acute surgical pain impact oncologic outcomes after colorectal cancer resection?: A retrospective cohort study. Medicine (Baltimore). 2019;98(18):e15442
13. Lu H, Zhang H, Weng ML, Zhang J, Jiang N, Cata JP. et al. Morphine promotes tumorigenesis and cetuximab resistance via EGFR signaling activation in human colorectal cancer. J Cell Physiol. 2021;236(6):4445-4454
14. Leong SP, Cady B, Jablons DM, Garcia-Aguilar J, Reintgen D, Jakub J. et al. Clinical patterns of metastasis. Cancer Metastasis Rev. 2006;25(2):221-232
15. Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M. et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25(29):4575-4580
16. O'Bryant CL, Linnebur SA, Yamashita TE, Kutner JS. Inconsistencies in opioid equianalgesic ratios: clinical and research implications. J Pain Palliat Care Pharmacother. 2008;22(4):282-290
17. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213
18. Tabira Y, Okuma T, Kondo K, Yoshioka M, Mori T, Tanaka M. et al. Does neoadjuvant chemotherapy for carcinoma in the thoracic esophagus increase postoperative morbidity? Jpn J Thorac Cardiovasc Surg. 1999;47(8):361-367
19. Chen J, Chen Q, Yao J, Jiang Y, Zhou J, Zhao H. et al. Changes in serum lactate dehydrogenase levels as markers of pathological response and prognosis in colorectal liver metastases patients receiving neoadjuvant chemotherapy followed by resection. Ann Palliat Med. 2021;10(10):10276-10292
20. Oh TK, Jeon JH, Lee JM, Kim MS, Kim JH, Lim H. et al. Association of high-dose postoperative opioids with recurrence risk in esophageal squamous cell carcinoma: reinterpreting ERAS protocols for long-term oncologic surgery outcomes. Dis Esophagus. 2017;30(10):1-8
21. Cata JP, Zafereo M, Villarreal J, Unruh BD, Truong A, Truong DT. et al. Intraoperative opioids use for laryngeal squamous cell carcinoma surgery and recurrence: a retrospective study. J Clin Anesth. 2015;27(8):672-679
22. Liang X, Liu R, Chen C, Ji F, Li T. Opioid System Modulates the Immune Function: A Review. Transl Perioper Pain Med. 2016;1(1):5-13
23. Boland JW, Pockley AG. Influence of opioids on immune function in patients with cancer pain: from bench to bedside. Br J Pharmacol. 2018;175(14):2726-2736
24. Franchi S, Panerai AE, Sacerdote P. Buprenorphine ameliorates the effect of surgery on hypothalamus-pituitary-adrenal axis, natural killer cell activity and metastatic colonization in rats in comparison with morphine or fentanyl treatment. Brain Behav Immun. 2007;21(6):767-774
25. Gaspani L, Bianchi M, Limiroli E, Panerai AE, Sacerdote P. The analgesic drug tramadol prevents the effect of surgery on natural killer cell activity and metastatic colonization in rats. J Neuroimmunol. 2002;129(1-2):18-24
26. Lennon FE, Mirzapoiazova T, Mambetsariev B, Poroyko VA, Salgia R, Moss J. et al. The Mu opioid receptor promotes opioid and growth factor-induced proliferation, migration and Epithelial Mesenchymal Transition (EMT) in human lung cancer. PLoS One. 2014;9(3):e91577
27. Díaz-Cambronero O, Mazzinari G, Giner F, Belltall A, Ruiz-Boluda L, Marqués-Marí A. et al. Mu Opioid Receptor 1 (MOR-1) Expression in Colorectal Cancer and Oncological Long-Term Outcomes: A Five-Year Retrospective Longitudinal Cohort Study. Cancers (Basel). 2020;12(1):134
28. Montagna G, Gupta HV, Hannum M, Tan KS, Lee J, Scarpa JR. et al. Intraoperative opioids are associated with improved recurrence-free survival in triple-negative breast cancer. Br J Anaesth. 2021;126(2):367-376
29. Hiller JG, Perry NJ, Poulogiannis G, Riedel B, Sloan EK. Perioperative events influence cancer recurrence risk after surgery. Nat Rev Clin Oncol. 2018;15(4):205-218
Author contact
![]() Corresponding authors: Hong Zhao MD, E-mail: pumczhaohongcom; Department of Hepatobiliary Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. No. 17 Nanli, Panjiayuan, Chaoyang District, Beijing, China. Hongliang Wu MD, E-mail: wuhongliang2021com; Department of Anesthesiology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. No. 17 Nanli, Panjiayuan, Chaoyang District, Beijing, China.
Corresponding authors: Hong Zhao MD, E-mail: pumczhaohongcom; Department of Hepatobiliary Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. No. 17 Nanli, Panjiayuan, Chaoyang District, Beijing, China. Hongliang Wu MD, E-mail: wuhongliang2021com; Department of Anesthesiology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. No. 17 Nanli, Panjiayuan, Chaoyang District, Beijing, China.

 Global reach, higher impact
Global reach, higher impact