Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(11):3244-3250. doi:10.7150/jca.76108 This issue Cite
Research Paper
Evaluation of the necessity of Pulmonary Ligament Lymph Node Dissection for Upper Lobe Stage IB NSCLC: A Propensity Score-matched Study
1. Department of Minimally Invasive Surgery, Beijing Chest Hospital, Capital Medical University; Beijing Tuberculosis and Thoracic Tumor Research Institute, Beijing, China.
2. Department of Thoracic Surgery, National Cancer Center/National Clinical Research Center for Cancer, Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen, China.
3. State Key Laboratory of Oncology in South China, Department of Thoracic Surgery, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, China.
*These authors contributed equally to this work.
Received 2022-6-13; Accepted 2022-8-9; Published 2022-9-6
Abstract
Objective: The purpose of this study was to explore whether the resection of pulmonary ligament lymph nodes would affect the prognosis of patients with stage IB non-small cell lung cancer (NSCLC).
Methods: We retrospectively analyzed 341 patients with upper lobe stage IB NSCLC who underwent radical surgery for lung cancer at Sun Yat-Sen University Cancer Center from 1999 to 2009. The Cox proportional hazard regression model was used to analyze the prognostic factors. After propensity score matching (PSM), 204 cases were selected. The Kaplan-Meier method and log-rank test were applied to compare overall survival (OS) and recurrence-free survival (RFS).
Results: Among the 341 cases included in the study, 217 had no pulmonary ligament lymph nodes resected, and 124 had pulmonary ligament lymph nodes resected. They were divided into two groups according to whether the pulmonary ligament lymph nodes were resected; there were significant differences between the two groups in laterality, resected lymph node stations, and resected lymph node numbers (P<0.05). Univariate and multivariate analyses by the Cox proportional hazards model showed that age and family history of malignant tumors were prognostic factors for OS, and no variables were prognostic factors for RFS (P<0.05). Resection of the pulmonary ligament lymph node was not associated with OS or RFS. After propensity score matching (PSM), survival analysis was performed again using the Kaplan-Meier method and log-rank test; the results suggested that resection of the pulmonary ligament lymph node is not statistically associated with OS and RFS (P>0.05).
Conclusions: For stage IB NSCLC, resection of the pulmonary ligament lymph nodes was not statistically associated with OS or RFS. Pulmonary ligament lymph node resection is not necessary for early-stage NSCLC.
Keywords: non-small cell lung cancer (NSCLC), propensity score matching (PSM), pulmonary ligament lymph nodes, overall survival (OS), recurrence-free survival (RFS)
Introduction
Non-small cell lung cancer (NSCLC), accounting for approximately 85% of lung cancers, is the most common pathological type of lung cancer [1]. Surgery is currently the primary treatment option for NSCLC [2]. For many years, lobectomy with systematic lymph node dissection (SLND) has been the standard surgical treatment for lung cancer [3, 4]. However, for early-stage NSCLC, the method of lymph node dissection has been controversial. Many studies suggest that lobe-specific selective lymph node dissection (LSLND) and lymph node sampling (LNS) can be applied to early-stage NSCLC [5-11]. These studies indicate that for early-stage NSCLC, LSLND and LNS can reduce operative time and some perioperative complications and have no difference in survival compared with SLND [6-9, 12]. For tumors in the upper lobe, if SLND is performed, the pulmonary ligament lymph nodes need to be dissected. However, if LSLND or LNS is performed, pulmonary ligament lymph nodes do not need to be dissected. There are no studies on the necessity of dissection of pulmonary ligament lymph nodes (station 9 lymph nodes) in upper lobe NSCLC. The main purpose of this study was to explore the effect of pulmonary ligament lymph node dissection on survival in stage IB NSCLC.
Methods
Study cohort
We collected data from patients with NSCLC who underwent radical surgery at Sun Yat-Sen University Cancer Center from 1999 to 2009. The enrolled patients met the following requirements: 1) stage IB NSCLC located in the upper lobe; 2) treated with lobectomy; and 3) no history of neoadjuvant chemotherapy. Two experienced pathologists validated all pathological results. The exclusion criteria were as follows: 1) no detailed information on lymph nodes; 2) patients with autoimmune disease, preoperative infection, or severe cardiovascular disease; and 3) no detailed follow-up data. Ultimately, 341 cases were included in this study.
Follow-up process
Follow-up was performed every 3 months for the first 2 years, every 6 months for 3 to 5 years, and once a year after that. Blood tests, tumor markers, chest CT, and abdominal ultrasound were performed to assess the patient's status. Once a year, a brain MRI is performed. All cases were followed up until January 2013.
Statistical Analysis
To compare the variables between two groups, the chi-square test was used. Overall survival (OS) and recurrence-free survival (RFS) were calculated using the Kaplan-Meier method. The Cox proportional hazard regression model was performed for univariate and multivariate analyses. In multivariate analysis, variables with a P value less than 0.1 in univariate analysis were included. A P value of less than 0.05 was set as the statistical significance level.
R software (version 4.2.0, R Foundation, Austria) was used to perform the above statistical analyses. To conduct propensity score matching (PSM), the “matchit” package was used. Survival curves and forest plots were plotted using the “survival” and “survminer” packages. The optimal cutoff values for resected lymph node numbers were calculated using X-tile software (version 3.6.1, Yale University, United States).
Results
Patient characteristics
The clinicopathological characteristics of the patients in this study are shown in Table 1. A total of 341 patients were enrolled and divided into 2 groups according to whether the pulmonary ligament lymph nodes were resected. The chi-square test showed that there was a significant difference in laterality, resected lymph node stations, and resected lymph node numbers between the two groups (P < 0.05). We can see that male patients accounted for 72.7%. Patients older than 65 years accounted for approximately 31.4%. Patients with a history of smoking accounted for 58.7%. Most patients had no family history of malignant tumors (87.4%). Patients with grade I+II accounted for about 62.8%. The pathological type of the majority of patients was adenocarcinoma (67.7%). Squamous cell carcinoma accounted for approximately 29.9%, and the remaining included sarcomatoid carcinoma, mucoepidermoid carcinoma, and large cell carcinoma. Tumors located in the right lung accounted for 53.4%. Visceral pleural invasion and bronchial invasion accounted for 60.4% and 27.0%, respectively. A total of 28.4% of the patients had 6 or more lymph node dissection stations, and 59.5% had more than 12 lymph nodes dissected. Approximately 15.5% of patients received adjuvant chemotherapy after surgery.
Prognostic factors for OS and RFS before PSM
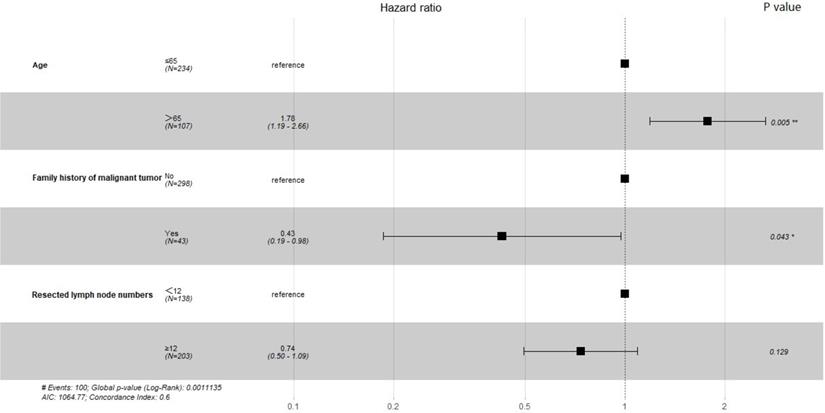
Prognostic factors for OS and RFS were assessed using Cox proportional hazards regression models before PSM. The results of univariate analysis for OS and RFS are shown in Table 2. For OS, age, family history of malignant tumors, and resected lymph node numbers were included in multivariate analyses (P<0.1). For RFS, in univariate analysis, no variable had a P value less than 0.1, so all variables were considered to be not statistically associated with RFS. The forest plots revealed the results of the multivariate analysis. Figure 1 shows that the P values for age and family history of malignant tumors were less than 0.05. These variables were found to be significant prognostic factors for OS. Pulmonary ligament lymph node resection is not a prognostic factor for OS and RFS.
Forest plot showing multivariate analysis for Overall Survival.
Comparison of clinicopathological characteristics between original and matched data set
| Variables | Original data set | Matched data set | ||||||
|---|---|---|---|---|---|---|---|---|
| Total | Pulmonary ligament lymph node resection | P value | Total | Pulmonary ligament lymph node resection | P value | |||
| N=341 | No (N=217) | Yes (N=124) | N=204 | No (N=102) | Yes (N=102) | |||
| Sex | 0.151 | 0.762 | ||||||
| Female | 93 (27.3%) | 53 (24.4%) | 40 (32.3%) | 63 (30.9%) | 30 (29.4%) | 33 (32.4%) | ||
| Male | 248 (72.7%) | 164 (75.6%) | 84 (67.7%) | 141 (69.1%) | 72 (70.6%) | 69 (67.6%) | ||
| Age | 0.559 | 0.641 | ||||||
| >65 | 107 (31.4%) | 71 (32.7%) | 36 (29.0%) | 58 (28.4%) | 27 (26.5%) | 31 (30.4%) | ||
| ≤65 | 234 (68.6%) | 146 (67.3%) | 88 (71.0%) | 146 (71.6%) | 75 (73.5%) | 71 (69.6%) | ||
| Smoking | 1.000 | 1.000 | ||||||
| No | 142 (41.6%) | 90 (41.5%) | 52 (41.9%) | 88 (43.1%) | 44 (43.1%) | 44 (43.1%) | ||
| Yes | 199 (58.4%) | 127 (58.5%) | 72 (58.1%) | 116 (56.9%) | 58 (56.9%) | 58 (56.9%) | ||
| Family history of malignant tumor | 0.770 | 1.000 | ||||||
| No | 298 (87.4%) | 191 (88.0%) | 107 (86.3%) | 178 (87.3%) | 89 (87.3%) | 89 (87.3%) | ||
| Yes | 43 (12.6%) | 26 (12.0%) | 17 (13.7%) | 26 (12.7%) | 13 (12.7%) | 13 (12.7%) | ||
| Grade | 0.695 | 0.662 | ||||||
| I+II | 214 (62.8%) | 134 (61.8%) | 80 (64.5%) | 130 (63.7%) | 63 (61.8%) | 67 (65.7%) | ||
| III+IV | 127 (37.2%) | 83 (38.2%) | 44 (35.5%) | 74 (36.3%) | 39 (38.2%) | 35 (34.3%) | ||
| Histology | 0.284 | 1.000 | ||||||
| Adenocarcinoma | 231 (67.7%) | 149 (68.7%) | 82 (66.1%) | 137 (67.2%) | 69 (67.6%) | 68 (66.7%) | ||
| Squamous cell carcinoma | 102 (29.9%) | 61 (28.1%) | 41 (33.1%) | 66 (32.4%) | 33 (32.4%) | 33 (32.4%) | ||
| Others | 8 (2.4%) | 7 (3.2%) | 1 (0.8%) | 1 (0.4%) | 0 (0.0%) | 1 (0.9%) | ||
| Laterality | <0.001 | 1.000 | ||||||
| Left | 159 (46.6%) | 82 (37.8%) | 77 (62.1%) | 110 (53.9%) | 55 (53.9%) | 55 (53.9%) | ||
| Right | 182 (53.4%) | 135 (62.2%) | 47 (37.9%) | 94 (46.1%) | 47 (46.1%) | 47 (46.1%) | ||
| Visceral pleura invasion | 0.746 | 0.771 | ||||||
| No | 135 (39.6%) | 84 (38.7%) | 51 (41.1%) | 75 (36.8%) | 36 (35.3%) | 39 (38.2%) | ||
| Yes | 206 (60.4%) | 133 (61.3%) | 73 (58.9%) | 129 (63.2%) | 66 (64.7%) | 63 (61.8%) | ||
| Bronchial invasion | 0.125 | 0.638 | ||||||
| No | 249 (73.0%) | 165 (76.0%) | 84 (67.7%) | 148 (72.5%) | 76 (74.5%) | 72 (70.6%) | ||
| Yes | 92 (27.0%) | 52 (24.0%) | 40 (32.3%) | 56 (27.5%) | 26 (25.5%) | 30 (29.4%) | ||
| Resected lymph node stations | <0.001 | 1.000 | ||||||
| <6 | 244 (71.6%) | 178 (82.0%) | 66 (53.2%) | 130 (63.7%) | 65 (63.7%) | 65 (63.7%) | ||
| ≥6 | 97 (28.4%) | 39 (18.0%) | 58 (46.8%) | 74 (36.3%) | 37 (36.3%) | 37 (36.3%) | ||
| Resected lymph node numbers | 0.002 | 0.883 | ||||||
| <12 | 138 (40.5%) | 102 (47.0%) | 36 (29.0%) | 70 (34.3%) | 34 (33.3%) | 36 (35.3%) | ||
| ≥12 | 203 (59.5%) | 115 (53.0%) | 88 (71.0%) | 134 (65.7%) | 68 (66.7%) | 66 (64.7%) | ||
| Chemotherapy | 0.316 | 1.000 | ||||||
| No | 288 (84.5%) | 187 (86.2%) | 101 (81.5%) | 166 (81.4%) | 83 (81.4%) | 83 (81.4%) | ||
| Yes | 53 (15.5%) | 30 (13.8%) | 23 (18.5%) | 38 (18.6%) | 19 (18.6%) | 19 (18.6%) | ||
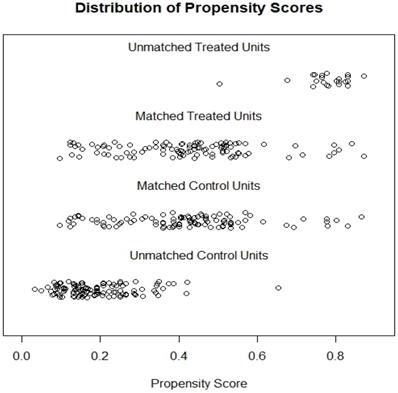
Distribution of propensity score.
Survival Analysis after PSM
To reduce potential bias in comparing the effects of pulmonary ligament lymph node resection on survival, a 1-to-1 PSM was performed. The distribution of propensity scores is shown in Figure 2; a perfect match is obtained between the 2 groups. A total of 204 cases were selected for survival analysis after PSM. As shown in Table 1, after PSM, there was no significant difference in any of the variables between the two groups (P>0.05).
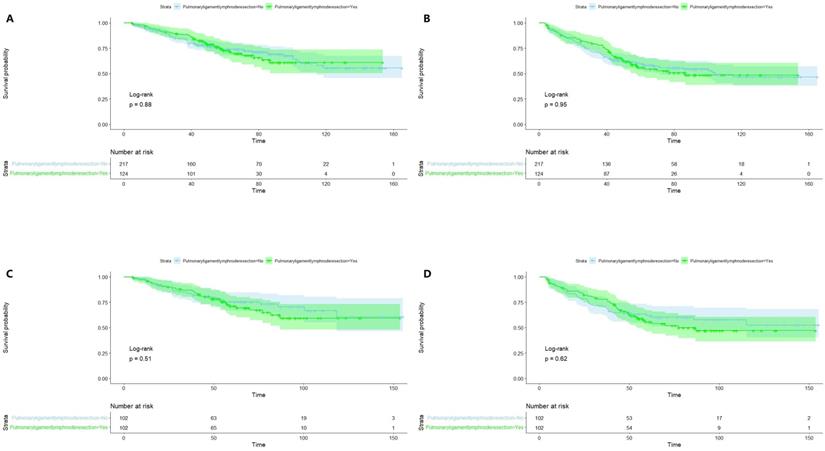
Figure 3 illustrates Kaplan-Meier curves of OS (A, C) and RFS (B, D) before and after PSM. All P values were greater than 0.05, suggesting that pulmonary ligament lymph node resection was not statistically associated with OS or RFS.
Discussion
Lymph node dissection is an integral part of lung cancer surgery, and the extent of lymph node dissection has always been a hot topic. SLND has been the standard surgical procedure for lung cancer surgery [13-15]. However, there are different views on the extent of lymph node dissection in lung cancer. Especially for early-stage non-small cell lung cancer, several studies have concluded that SLND is not necessary. Many recent studies have suggested that LSLND and LNS can achieve the same therapeutic effect as SLND for early-stage NSCLC [5, 6, 9, 12, 16, 17]. At the same time, some studies suggest that LSND and LNS can reduce the incidence of complications caused by lymph node dissection, such as lymphatic fistula, bleeding, and nerve damage [18]. However, this view has different opinions [19-21].
Kaplan-Meier curves of Overall Survival (A, C) and Recurrence-Free Survival (B, D) before and after propensity score matching.
Univariate analysis of overall survival and recurrence free survival before propensity score matching
| Variables | Total | Overall survival | Recurrence free survival | ||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Sex | 0.733 | 0.547 | |||
| Female | 93 (27.3%) | reference | reference | ||
| Male | 248 (72.7%) | 0.93 (0.59-1.44) | 0.90 (0.63-1.28) | ||
| Age | 0.004 | 0.109 | |||
| >65 | 107 (31.4%) | reference | reference | ||
| ≤65 | 234 (68.6%) | 0.55 (0.37-0.83) | 0.76 (0.54-1.06) | ||
| Smoking | 0.174 | 0.627 | |||
| No | 142 (41.6%) | reference | reference | ||
| Yes | 199 (58.4%) | 1.32 (0.88-1.99) | 1.08 (0.78-1.50) | ||
| Family history of malignant tumor | 0.023 | 0.273 | |||
| No | 298 (87.4%) | reference | reference | ||
| Yes | 43 (12.6%) | 0.40 (0.17-0.90) | 0.74 (0.44-1.27) | ||
| Grade | 0.181 | 0.254 | |||
| I+II | 214 (62.8%) | reference | reference | ||
| III+IV | 127 (37.2%) | 1.31 (0.88-1.95) | 1.21 (0.87-1.68) | ||
| Histology | 0.563 | 0.358 | |||
| Adenocarcinoma | 231 (67.7%) | reference | reference | ||
| Squamous cell carcinoma | 102 (29.9%) | 1.10 (0.72-1.68) | 0.81 (0.56-1.17) | ||
| Others | 8 (2.4%) | 1.82 (0.57-5.80) | 1.46 (0.54-3.97) | ||
| Laterality | 0.711 | 0.615 | |||
| Left | 159 (46.6%) | reference | reference | ||
| Right | 182 (53.4%) | 0.93 (0.63-1.37) | 1.09 (0.79-1.50) | ||
| Visceral pleura invasion | 0.216 | 0.516 | |||
| No | 135 (39.6%) | reference | reference | ||
| Yes | 206 (60.4%) | 0.78 (0.53-1.16) | 1.12 (0.80-1.56) | ||
| Bronchial invasion | 0.730 | 0.292 | |||
| No | 249 (73.0%) | reference | reference | ||
| Yes | 92 (27.0%) | 0.92 (0.58-1.47) | 0.81 (0.55-1.20) | ||
| Resected lymph node stations | 0.209 | 0.454 | |||
| <6 | 244 (71.6%) | reference | reference | ||
| ≥6 | 97 (28.4%) | 0.75 (0.47-1.18) | 0.87 (0.61-1.25) | ||
| Resected lymph node numbers | 0.096 | 0.123 | |||
| <12 | 138 (40.5%) | reference | reference | ||
| ≥12 | 203 (59.5%) | 0.72 (0.48-1.06) | 0.78 (0.56-1.07) | ||
| Pulmonary ligament lymph node resection | 0.881 | 0.947 | |||
| No | 217 (63.6%) | reference | reference | ||
| Yes | 124 (36.4%) | 1.03 (0.68-1.56) | 1.01 (0.72-1.41) | ||
| Chemotherapy | 0.173 | 0.630 | |||
| No | 288 (84.5%) | reference | reference | ||
| Yes | 53 (15.5%) | 0.65 (0.35-1.21) | 0.89 (0.57-1.41) | ||
Pulmonary ligament lymph nodes (station 9 lymph nodes) are located in the inferior pulmonary ligament, below the inferior pulmonary vein, and belong to the inferior mediastinal lymph nodes. In SLND, pulmonary ligament lymph nodes must be dissected. However, for LSLND and LNS, pulmonary ligament lymph nodes may not be dissected for upper lobe tumors [6, 8, 9, 17]. In addition, some studies have concluded that tumors in the upper lobe are more likely to metastasize to the upper mediastinal lymph nodes [12, 22, 23], and the pulmonary ligament lymph nodes are less likely to metastasize than other mediastinal lymph nodes [24]. Therefore, it is still controversial whether pulmonary ligament lymph nodes need to be dissected for early-stage upper lobe NSCLC. Since the dissection of the pulmonary ligament lymph nodes requires cutting the inferior pulmonary ligament, some studies have indicated that removing the inferior pulmonary ligament may lead to changes in the bronchial angle, resulting in bronchial obstruction and cough symptoms and shortness of breath [25, 26]. At the same time, other studies confirmed that pulmonary ligament resection is not necessary for upper lobectomy [26, 27]. This makes it more meaningful to study whether resection of pulmonary ligament lymph nodes is essential for early-stage upper lobe lung cancer.
This study retrospectively analyzed 341 patients with stage IB upper lobe NSCLC; prognostic analysis was performed using a Cox proportional hazard regression model before PSM. Univariate and multivariate analyses showed that pulmonary ligament lymph node resection was not a prognostic factor for OS or RFS. Subsequently, to reduce potential bias, all patients enrolled in the study were divided into two groups according to whether the pulmonary ligament lymph nodes were resected and matched with a 1:1 propensity score. Survival analysis was then performed by the Kaplan-Meier method, and the log-rank test indicated that the P values were all greater than 0.05 (Figure 3), suggesting that pulmonary ligament lymph node resection was not statistically associated with OS and RFS. The conclusions of this study are consistent with those of previous studies on early-stage NSCLC [8, 10, 28]. It should also be noted that for advanced NSCLC, SLND should be the standard surgical modality because of its survival benefit [29-31].
This study shows that the dissection of pulmonary ligament lymph nodes is not statistically associated with OS and RFS. For OS, only age and family history of malignant tumors were statistically significant prognostic factors. None of the variables included were prognostic factors for RFS. As shown in Figure 1, the hazard ratio of family history of malignant tumors was 0.43, with a P value less than 0.05. This means that patients with a family history of malignant tumors have a lower risk of death. This result may be related to the bias caused by the small sample size. We look forward to more studies with larger sample sizes to validate this conclusion.
Unavoidably, this study has several limitations. First, this is a single-center retrospective study, and prospective multicenter research is expected to confirm the conclusion. Second, 341 patient cases were enrolled in this study, and the small sample size may have led to bias. Finally, there are many surgical teams in our hospital, and the impact of the surgical procedures of the different surgical teams was not taken into account.
Conclusion
For stage IB NSCLC, resection of the pulmonary ligament lymph nodes was not statistically associated with OS or RFS. Pulmonary ligament lymph node resection is not necessary for early-stage NSCLC.
Abbreviations
NSCLC: non-small cell lung cancer; PSM: propensity score matching; SLND: systematic lymph nodes dissection; LSLND: lobe-specific selective lymph nodes dissection; LNS: lymph nodes sampling; OS: overall survival; RFS: recurrence-free survival.
Acknowledgements
Funding
Scientific Research Improvement Program of Beijing Chest Hospital; Capital Medical University (Kj2021cx009).
Ethical statement
The studies involving human participants were reviewed and approved by Ethics Committee of Sun Yat-Sen University Cancer Center and Beijing Chest Hospital Institutional Review Board. The patients/participants provided written informed consent to participate in this study.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
FW and XY drafted the manuscript. SL and LZ participated in the design and oversight of the study. YH and XY participated in the design of the study. FW, LZ and XY were involved in data collection and statistical analysis. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367-80
2. Masuda M, Kuwano H, Okumura M, Arai H, Endo S, Doki Y. et al. Thoracic and cardiovascular surgery in Japan during 2013: Annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg. 2015;63:670-701
3. De Leyn P, Lardinois D, Van Schil P, Rami-Porta R, Passlick B, Zielinski M. et al. European trends in preoperative and intraoperative nodal staging: ESTS guidelines. J Thorac Oncol. 2007;2:357-61
4. Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e278S-e313S
5. Adachi H, Maehara T, Nakayama H, Masuda M. Mediastinal lymph node dissection in surgical treatment for early stage non-small-cell lung cancer: lobe-specific or systematic? J Thorac Dis. 2017;9:2728-31
6. Adachi H, Sakamaki K, Nishii T, Yamamoto T, Nagashima T, Ishikawa Y. et al. Lobe-Specific Lymph Node Dissection as a Standard Procedure in Surgery for Non-Small Cell Lung Cancer: A Propensity Score Matching Study. J Thorac Oncol. 2017;12:85-93
7. Darling GE, Allen MS, Decker PA, Ballman K, Malthaner RA, Inculet RI. et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg. 2011;141:662-70
8. Hishida T, Miyaoka E, Yokoi K, Tsuboi M, Asamura H, Kiura K. et al. Lobe-Specific Nodal Dissection for Clinical Stage I and II NSCLC: Japanese Multi-Institutional Retrospective Study Using a Propensity Score Analysis. J Thorac Oncol. 2016;11:1529-37
9. Hughes MJ, Chowdhry MF, Woolley SM, Walker WS. In patients undergoing lung resection for non-small cell lung cancer, is lymph node dissection or sampling superior? Interact Cardiovasc Thorac Surg. 2011;13:311-5
10. Ishiguro F, Matsuo K, Fukui T, Mori S, Hatooka S, Mitsudomi T. Effect of selective lymph node dissection based on patterns of lobe-specific lymph node metastases on patient outcome in patients with resectable non-small cell lung cancer: a large-scale retrospective cohort study applying a propensity score. J Thorac Cardiovasc Surg. 2010;139:1001-6
11. Maniwa T, Okumura T, Isaka M, Nakagawa K, Ohde Y, Kondo H. Recurrence of mediastinal node cancer after lobe-specific systematic nodal dissection for non-small-cell lung cancer. Eur J Cardiothorac Surg. 2013;44:e59-64
12. Okada M, Sakamoto T, Yuki T, Mimura T, Miyoshi K, Tsubota N. Selective mediastinal lymphadenectomy for clinico-surgical stage I non-small cell lung cancer. Ann Thorac Surg. 2006;81:1028-32
13. Chansky K, Detterbeck FC, Nicholson AG, Rusch VW, Vallières E, Groome P. et al. The IASLC Lung Cancer Staging Project: External Validation of the Revision of the TNM Stage Groupings in the Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol. 2017;12:1109-21
14. Lardinois D, De Leyn P, Van Schil P, Porta RR, Waller D, Passlick B. et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg. 2006;30:787-92
15. Rice D, Chansky K, Nowak A, Pass H, Kindler H, Shemanski L. et al. The IASLC Mesothelioma Staging Project: Proposals for Revisions of the N Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Pleural Mesothelioma. J Thorac Oncol. 2016;11:2100-11
16. Huang X, Wang J, Chen Q, Jiang J. Mediastinal lymph node dissection versus mediastinal lymph node sampling for early stage non-small cell lung cancer: a systematic review and meta-analysis. PLoS ONE. 2014;9:e109979
17. Jett JR, Schild SE, Kesler KA, Kalemkerian GP. Treatment of small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e400S-e19S
18. Ma K, Chang D, He B, Gong M, Tian F, Hu X. et al. Radical systematic mediastinal lymphadenectomy versus mediastinal lymph node sampling in patients with clinical stage IA and pathological stage T1 non-small cell lung cancer. J Cancer Res Clin Oncol. 2008;134:1289-95
19. Allen MS, Darling GE, Pechet TT, Mitchell JD, Herndon JE 2nd, Landreneau RJ. et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg. 2006;81:1013-9 discussion 9-20
20. Bille A, Woo KM, Ahmad U, Rizk NP, Jones DR. Incidence of occult pN2 disease following resection and mediastinal lymph node dissection in clinical stage I lung cancer patients. Eur J Cardiothorac Surg. 2017;51:674-9
21. Doddoli C, Aragon A, Barlesi F, Chetaille B, Robitail S, Giudicelli R. et al. Does the extent of lymph node dissection influence outcome in patients with stage I non-small-cell lung cancer? Eur J Cardiothorac Surg. 2005;27:680-5
22. Asamura H, Nakayama H, Kondo H, Tsuchiya R, Naruke T. Lobe-specific extent of systematic lymph node dissection for non-small cell lung carcinomas according to a retrospective study of metastasis and prognosis. J Thorac Cardiovasc Surg. 1999;117:1102-11
23. Wang W, Mao F, Shen-Tu Y, Mei Y. [Research for mediastinal lymph node desection style of stage Ib upper lobe non-small cell lung cancer]. Zhongguo Fei Ai Za Zhi. 2013;16:584-90
24. Sun F, Zhan C, Shi M, Yang X, Wang L, Jiang W. et al. Is routine dissection of the station 9 lymph nodes really necessary for primary lung cancer? Int J Surg. 2016;34:53-7
25. Lv H, Zhou R, Zhan X, Di D, Qian Y, Zhang X. The choice of dissection or preservation of the inferior pulmonary ligament after an upper lobectomy: a systematic review and meta-analysis. World J Surg Oncol. 2020;18:5
26. Ueda K, Tanaka T, Hayashi M, Tanaka N, Li TS, Hamano K. Clinical ramifications of bronchial kink after upper lobectomy. Ann Thorac Surg. 2012;93:259-65
27. Khanbhai M, Dunning J, Yap KH, Rammohan KS. Dissection of the pulmonary ligament during upper lobectomy: is it necessary? Interact Cardiovasc Thorac Surg. 2013;17:403-6
28. Deng HY, Zhou J, Wang RL, Jiang R, Zhu DX, Tang XJ. et al. Lobe-Specific Lymph Node Dissection for Clinical Early-Stage (cIA) Peripheral Non-small Cell Lung Cancer Patients: What and How? Ann Surg Oncol. 2020;27:472-80
29. Keller SM, Adak S, Wagner H, Johnson DH. Mediastinal lymph node dissection improves survival in patients with stages II and IIIa non-small cell lung cancer. Eastern Cooperative Oncology Group. Ann Thorac Surg. 2000;70:358-65 discussion 65-6
30. Misthos P, Sepsas E, Kokotsakis J, Skottis I, Lioulias A. Prognosis of stage pIIIA non small cell lung cancer after mediastinal lymph node dissection or sampling. J buon. 2009;14:45-9
31. Wu Y, Huang ZF, Wang SY, Yang XN, Ou W. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer. 2002;36:1-6
Author contact
![]() Corresponding authors: Shuku Liu, Beijing Chest Hospital, Capital Medical University; Beijing Tuberculosis and Thoracic Tumor Research Institute, 9 Beiguan Street, Beijing 101149, China. E-mail: liushukucom; Lanjun Zhang, Department of Thoracic Surgery, Sun Yat-sen University Cancer Center, 651 Dong Feng east Road, 510060 guangzhou, guangdong, China. E-mail: zhangljorg.cn.
Corresponding authors: Shuku Liu, Beijing Chest Hospital, Capital Medical University; Beijing Tuberculosis and Thoracic Tumor Research Institute, 9 Beiguan Street, Beijing 101149, China. E-mail: liushukucom; Lanjun Zhang, Department of Thoracic Surgery, Sun Yat-sen University Cancer Center, 651 Dong Feng east Road, 510060 guangzhou, guangdong, China. E-mail: zhangljorg.cn.

 Global reach, higher impact
Global reach, higher impact