3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(15):3598-3605. doi:10.7150/jca.67569 This issue Cite
Research Paper
Bcl-6-dependent risk stratification by nuclear expression of Peli1 in diffuse large B-cell lymphoma
1. Department of Pathology, Seoul National University Bundang Hospital, Seongnam, South Korea
2. Department of Pathology, Seoul National University College of Medicine, Seoul, South Korea
3. Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, South Korea
4. Department of Internal Medicine, Seoul National University College of Medicine, Seoul, South Korea
Received 2021-9-29; Accepted 2022-11-1; Published 2022-11-14
Abstract
Background/Aim: Peli1 is an E3 ubiquitin ligase involving lymphomagenesis by lysine 63 ubiquitination-mediated stabilization of Bcl-6 with in diffuse large B-cell lymphoma (DLBCL).
Materials and Methods: We categorized nuclear expression of Peli1 according to Bcl-6 status by immunohistochemistry in DLBCL (n=100), and analyzed clinicopathologic association with prognosis.
Results: We established Bcl-6/Peli1 risk model composed of high risk (Bcl-6+/Peli1+ or Bcl-6-/Peli1-; n=64) and low risk (Bcl-6+/Peli1- or Bcl-6-/Peli1+; n=36). High risk group had more frequent non-GCB subtype (83% vs 64%; p=0.033) and Bcl-6-negativity (69% vs 28%; p<0.001) than low risk group. Univariate survival analysis for progression-free survival (PFS) and overall survival (OS) revealed Bcl-6/Peli1 risk group (p=0.026 and p=0.021) and other conventional variables including international prognostic index (IPI), stage, ECOG performance status, number of extranodal sites were significant prognostic factors, along with B symptoms for OS. In multivariate analysis for PFS, Bcl-6/Peli1 risk group (p=0.032; HR=3.29), IPI (p=0.013; HR=3.39) and ECOG PS (p=0.035; HR=3.08) were independent prognostic factors. In multivariate analysis for OS, Bcl-6/Peli1 risk group (p=0.048; HR=7.87) and IPI (p=0.001; HR=12.15) were associated with prognosis.
Conclusions: DLBCL had distinctive risk groups according to pairs of nuclear Peli1 and Bcl-6 expression. These results suggest the potential role of Peli1 and Bcl-6 in risk assessment in DLBCL.
Keywords: malignant lymphoma, diffuse large B-cell lymphoma, Bcl-6, Peli1
Introduction
Diffuse large B cell lymphoma (DLBCL) is a heterogeneous clinicopathologic entity composed of neoplastic large B cells with germinal center B cell-like (GCB) or non-GCB/activated B cell-like (ABC) molecular features [1]. While standard first-line immunochemotherapy with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) have good response in patients with DLBCL, about 1/3 or more patients remain refractory or experience relapse [2]. Therefore, it is necessary to develop risk assessment model to predict outcome of DLBCL patients. Notably, many molecular markers have been developed for this purpose on the basis of oncogenic mechanism and 'cell of origin' with various feasibilities [3].
Peli1 is a type of E3 ubiquitin ligase with RING (really interesting new gene) domain [4]. It has been known to play dual roles in biologic processes. It facilitates degradation of target protein via lysine 48 (K48) ubiquitination or stabilizes target protein via lysine 63 (K63) ubiquitination in a context-dependent manner [5]. In experimental models, transgenic mice overexpressing Peli1 developed B cell lymphoma and/or lupus-like autoimmune disease with high density of B cell aggregate [6, 7], suggesting its strong association in B cell lymphomagenesis and B cell-related autoimmunity. So far, clinical studies for use of Peli1 as a prognostic marker in various tumors have rarely been reported with various results according to target molecules [6, 8, 9]. In a few studies with tumor tissue samples of DLBCL with survival analysis, high level expression or nuclear-weighted expression of Peli1 showed poor prognosis, while its prognostic effects were limited in multivariate analysis in R-CHOP-treated cohort [6, 8]. Since Peli1 exerts its oncogenic role by K63 ubiquitination of Bcl-6, an important driver molecule of DLBCL [6], we hypothesized that Peli1 may have a distinct clinical significance according to Bcl-6 status on the basis of molecular oncogenic mechanism. We explored the clinicopathologic and prognostic implication of Peli1 expression by dissecting it with Bcl-6 status. In the present study, we constructed a new risk model with Peli1 and Bcl-6 for assessing DLBCL patients.
Materials and Methods
Patients and Samples
In this study, we retrospectively included the cases of diffuse large B cell lymphoma, not otherwise specified (DLBCL, NOS) by the criteria of the revised 4th edition of WHO classification of hematopoietic and lymphoid neoplasm (2016 WHO classification), as shown in supplementary figure 1 [10].
Briefly, we searched the database of pathology reports for the cases diagnosed at Seoul National University Bundang Hospital (SNUBH) as malignant lymphoma between May 2003 and January 2013. The cases were reviewed with pathological reports, histologic slides and molecular tests to specify the entity according to 2016 WHO classification, and the following cases were included: 1) the cases fitting the criteria of de novo DLBCL according to 2016 WHO classification, and 2) the cases with available paraffin blocks with sufficient residual tumor tissues. In addition, the following cases were excluded: 1) the cases with poor fixation or necrosis, 2) specific entities of large B cell lymphomas including 'EBV-positive DLBCL', 'primary mediastinal large B cell lymphoma', 'primary DLBCL of the central nervous system', 3) so-called 'gray-zone lymphomas', and 4) relapsed and/or transformed DLBCLs. In this way, a total of 100 cases of DLBCL were enrolled in this study.
The pathologic review process was performed by two pathologists (KRL and JHP) with histologic slides as well as immunohistochemical staining and molecular tests including EBV in situ hybridization and B cell clonality by using IgH gene rearrangement. All cases were positive for CD20 and 'cell of origin' classification including germinal center B cell-like (GCB) and non-GCB subtypes was performed by Hans algorithm [10, 11]. Medical records were reviewed and clinical variables including Ann Arbor stage, international prognostic index (IPI) were retrieved for clinicopathologic analysis. This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (B-1306-208-301) and informed consent was waived due to the retrospective design using archived material in this study.
Manufacturing tissue microarray, immunohistochemical staining, EBV in situ hybridization
Tumor tissues of round core with a diameter of 2 mm were sampled at well-fixed viable areas, and reconstructed into tissue microarray (TMA) blocks at Superbiochip (Seoul, South Korea) as previously described [12]. Peli1 antibody was purchased from Santa Cruz Biotechnology (F7; 1: 50; Santa Cruz Biotechnology, Dallas, TX, USA). Immunohistochemistry for Peli1 was performed by manual method (microwave 5 min x3). Benchmark XT and Benchmark ULTRA (Roche Diagnostics, Basel, Swiss) were used for immunohistochemical staining for other antibodies and EBV in situ hybridization (EBV-ISH). For immunophenotyping of DLBCL, immunostainings for Bcl-6 (std32, ready-to-use, Ventana Medical Systems, Oro Valley, AZ, USA), CD10 (std32, ready-to-use, Ventana), MUM1 (mild32, 1:150, Dako, Glostrup, Denmark), and Bcl-2 (std32, 1:50, Dako) were performed by using Ventana autostainers (Benchmark XT and Benchmark ULTRA). For interpretation of 'cell of origin' immunostaining markers including Bcl-6, CD10 and MUM1, cut-off criteria of 30% was used [11], while Bcl-2 was regarded as positive if 50% of tumor cells were stained. EBV-ISH was also performed by using Ventana autostainer, and EBV-positive cases were excluded from this study. Interpretation was performed by two pathologists (KRL and JHP). Considering the previous study of nuclear weighted interpretation method [6] and mechanistic assumption that Peli1 participating in Bcl-6 stabilization would be located in nucleus, we simplified criteria of Peli1 expression as nuclear expression of > 30% of tumor cells, regardless of cytoplasmic staining.
Statistical analysis
All statistical analysis was performed by using SPSS Statistics ver.19 (IBM, Chicago, IL). For clinicopathologic comparison, Chi square and Fisher's exact test were used. Univariate survival analysis was performed by using Kaplan-Meier analysis with log-rank test for statistical significance. Cox proportional hazard model was used for multivariate survival analysis with forward stepwise (conditional likelihood ratio) method incorporating all significant variables in univariate analysis. Overall survival (OS) was defined as the time interval from the date of diagnosis to the date of last follow-up or death. Progression-free survival (PFS) was defined as the time interval from the date of treatment to the date of progression with radiologic confirmation using computed tomography (CT) and/or positron emission tomography (PET)-CT after treatment or the date of death. All p values reported were two-sided. P values of less than 0.05 were regarded as statistically significant.
Results
Clinicopathologic characteristics of DLBCL
Clinicopathologic characteristics were summarized in Table 1. Briefly, male patients were slightly common (57%) with roughly equal distribution of age (>60, 52%). With similar distribution of stage (III-IV, 48%) and lactate dehydrogenase (elevated, 49%), cases with high IPI and extranodal sites (≥2) accounted for only 35% and 27%. Non-GCB subtype was predominant (76%).
Expression patterns of Peli1 according to Bcl-6 and building a risk model of Bcl-6/Peli1 based on clinicopathologic features
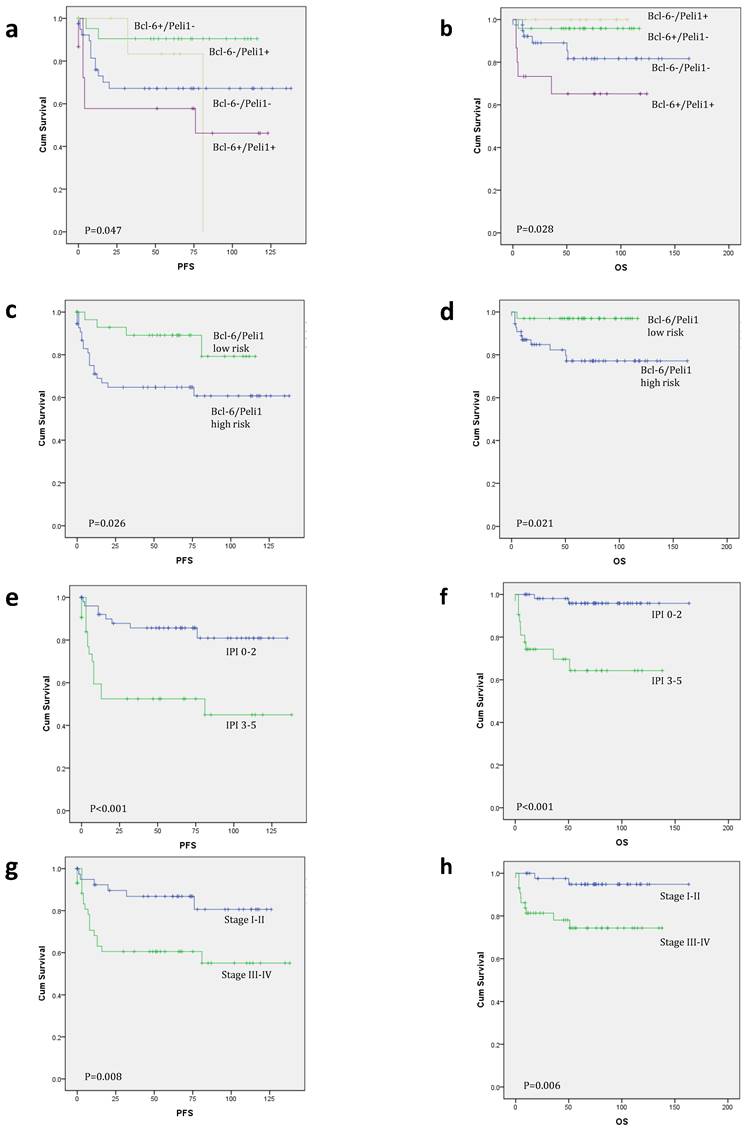
According to nuclear expression of Peli1 and Bcl-6, there were four subgroups of Bcl-6/Peli1 status, i.e., +/+, +/-, -/+ and -/-, as shown in Figure 1. In a mechanistic viewpoint, each subgroup may indicate the following status: 1) +/+, Bcl-6 stabilized by Peli1, 2) +/-, uncoupled (independent) Bcl-6 expression despite the absence of Peli1, 3) -/+, failure of Bcl-6 expression with intact Peli1, and 4) -/-, Bcl-6/Peli1-unrelated signaling mechanism. By the pilot analysis of PFS and OS for these 4 subgroups, two favorable subgroups, i.e., +/- and -/+, and two unfavorable subgroups, i.e., +/+ and -/-, were identified (Figure 2a-b). To develop practical risk classifier, we simplified these subgroups by making two-tier Bcl-6/Peli1 risk groups composed of high risk (+/+ and -/-) and low risk (+/- and -/+). By this process, high risk group accounted for 64% (64/100) and low risk group for 36% (36/100).
Associations between Bcl-6/Peli1 risk groups and clinicopathologic variables
Clinicopathologic associations between Bcl-6/Peli1 risk groups and clinicopathologic variables were analyzed (Table 2). Most of the conventional variables including international prognostic index (IPI) and Ann Arbor stage showed generally similar distribution. Of note, Bcl-6/Peli1 high risk group contained more frequent non-GCB subtype (83%; 53/64) by Hans classification than low risk group (64%; 23/36) (p=0.033). Among immunophenotypic markers, lack of Bcl-6 expression was also common in high risk group (69%; 44/64), compared to low risk group (28%; 10/36) (p <0.001), while other markers did not differ between the two groups.
Survival analysis
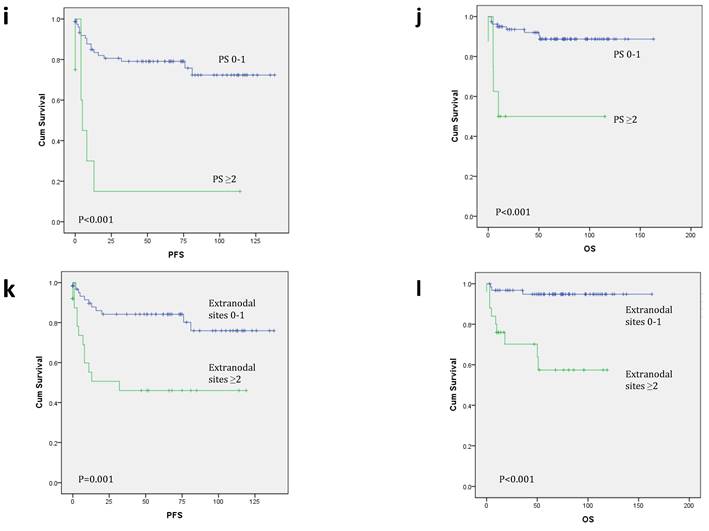
In univariate survival analysis for PFS and OS (Table 3 and Figure 2c-l), ECOG PS (p<0.001 for both), number of extranodal sites (p=0.001 and p<0.001), Ann Arbor stage (p=0.008 and p=0.006) and IPI (p<0.001 for both) were significant prognostic factors, as well as B symptoms for OS (p=0.030). Bcl-6/Peli1 risk group also predicted prognosis for PFS and OS (p=0.026 and p=0.021).
Clinicopathologic characteristics of diffuse large B cell lymphoma
| Clinicopathologic characteristics | No. of cases (%) | |
|---|---|---|
| Age | ≤60 | 48/100 (48%) |
| >60 | 52/100 (52%) | |
| Sex | Female | 43/100 (43%) |
| Male | 57/100 (57%) | |
| B symptoms | Absent | 79/100 (79%) |
| Present | 21/100 (21%) | |
| ECOG PS | <2 | 91/100 (91%) |
| ≥2 | 9/100 (9%) | |
| Serum LDH† | Normal | 49/97 (51%) |
| Elevated | 48/97 (49%) | |
| Ann Arbor stage | Ⅰ-Ⅱ | 52/100 (52%) |
| Ⅲ-Ⅳ | 48/100 (48%) | |
| Number of extranodal sites | <2 | 73/100 (73%) |
| ≥2 | 27/100 (27%) | |
| International prognostic index | 0-2 | 65/100 (65%) |
| 3-5 | 35/100 (35%) | |
| Bone marrow involvement† | Absent | 79/92 (86%) |
| Present | 13/92 (14%) | |
| Bulky disease (>10cm) | <10 | 91/100 (91%) |
| ≥10 | 9/100 (9%) | |
| Hans classification | GCB | 24/100 (24%) |
| Non-GCB | 76/100 (76%) | |
| BCL2 expression | Negative | 36/100 (36%) |
| Positive | 64/100 (64%) | |
| BCL6 expression | Negative | 54/100 (54%) |
| Positive | 46/100 (46%) | |
| CD10 expression | Negative | 85/100 (85%) |
| Positive | 15/100 (15%) | |
| MUM1 expression | Negative | 35/100 (35%) |
| Positive | 65/100 (65%) | |
| Peli1 nuclear expression | Negative | 70/100 (70%) |
| Positive | 30/100 (30%) | |
| Bcl-6/Peli1 combination | Negative/Negative | 44/100 (44%) |
| Positive /Negative | 26/100 (26%) | |
| Negative/Positive | 10/100 (10%) | |
| Positive/Positive | 20/100 (20%) | |
| Treatment | R-CHOP | 88/100 (88%) |
| R-others | 12/100 (12%) | |
| Total N (%) | 100 (100%) | |
ECOG PS, Eastern Cooperative Group Performance Status; LDH, lactate dehydrogenase; GCB, germinal center B cell-like; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone. †These variables excluded missing values.
Associations between Bcl-6/Peli1 risk groups and clinicopathologic variables in diffuse large B cell lymphoma.
| Clinicopathologic characteristics | Bcl-6/Peli1 risk group | |||
|---|---|---|---|---|
| High risk | Low risk | P value | ||
| Age | ≤60 | 32/64 (50%) | 16/36 (44%) | 0.594 |
| >60 | 32/64 (50%) | 20/36 (56%) | ||
| Sex | Female | 27/64 (42%) | 16/36 (44%) | 0.827 |
| Male | 37/64 (58%) | 20/36 (56%) | ||
| B symptoms | Absent | 51/64 (80%) | 28/36 (78%) | 0.822 |
| Present | 13/64 (20%) | 8/36 (22%) | ||
| ECOG PS | <2 | 57/64 (89%) | 34/36 (94%) | 0.482* |
| ≥2 | 7/64 (11%) | 2/36 (6%) | ||
| Serum LDH† | Normal | 30/62 (48%) | 19/35 (54%) | 0.577 |
| Elevated | 32/62 (52%) | 16/35 (46%) | ||
| Ann Arbor stage | Ⅰ-Ⅱ | 34/64 (53%) | 18/36 (50%) | 0.764 |
| Ⅲ-Ⅳ | 30/64 (47%) | 18/36 (50%) | ||
| Number of extranodal sites | <2 | 45/64 (70%) | 28/36 (78%) | 0.420 |
| ≥2 | 19/64 (30%) | 8/36 (22%) | ||
| International prognostic index | 0-2 | 40/64 (63%) | 25/36 (69%) | 0.485 |
| 3-5 | 24/64 (27%) | 11/36 (31%) | ||
| Bone marrow† involvement | Absent | 50/58 (86%) | 29/34 (85%) | 1.000* |
| Present | 8/58 (14%) | 5/34 (15%) | ||
| Bulky disease (>10cm) | <10 | 57/64 (89%) | 34/36 (94%) | 0.482* |
| ≥10 | 7/64 (11%) | 2/36 (6%) | ||
| Hans classification | GCB | 11/64 (17%) | 13/36 (36%) | 0.033 |
| Non-GCB | 53/64 (83%) | 23/36 (64%) | ||
| BCL2 expression | Negative | 21/64 (33%) | 15/36 (42%) | 0.376 |
| Positive | 43/64 (67%) | 21/36 (58%) | ||
| BCL6 expression | Negative | 44/64 (69%) | 10/36 (28%) | <0.001 |
| Positive | 20/64 (31%) | 26/36 (72%) | ||
| CD10 expression | Negative | 56/64 (88%) | 29/36 (81%) | 0.351 |
| Positive | 8/64 (12%) | 7/36 (19%) | ||
| MUM1 expression | Negative | 21/64 (33%) | 14/36 (39%) | 0.541 |
| Positive | 43/64 (67%) | 22/36 (61%) | ||
| Treatment | R-CHOP | 55/64 (86%) | 33/36 (92%) | 0.529* |
| R-others | 9/64 (14%) | 3/36 (8%) | ||
| Total N (%) | 64 (100%) | 36 (100%) | ||
ECOG PS, Eastern Cooperative Group Performance Status; LDH, lactate dehydrogenase; GCB, germinal center B cell-like; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone. †These variables excluded missing values. P values were calculated by Pearson's chi-square test (2-sided) or Fisher's exact test (2-sided).*
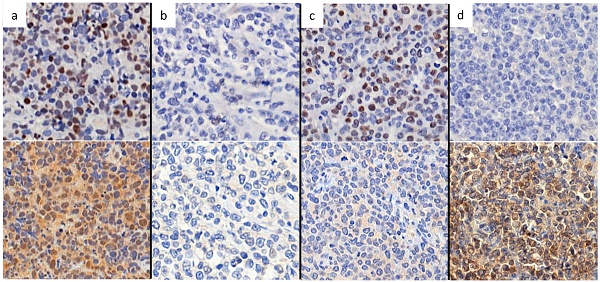
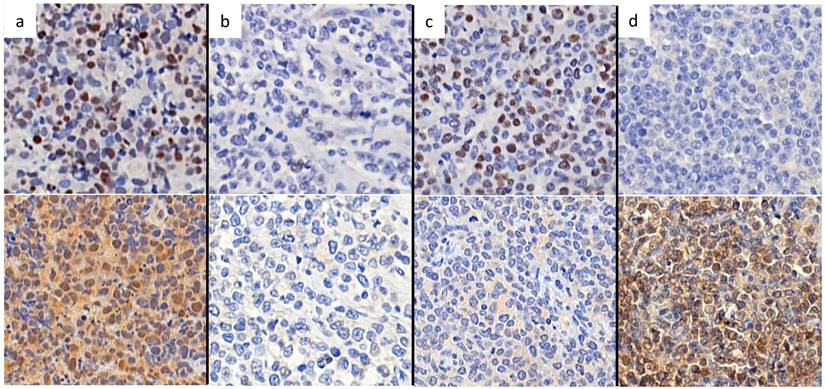
Representative figures of nuclear staining patterns of Bcl-6 and Peli1 by immunohistochemistry. When nuclei are stained as dark brown color contrasting with background, the case was interpreted as positive regardless of cytoplasmic stating. Figures on upper line show Bcl-6 status and figures on lower line show Peli1 status. (a) Bcl-6+, Peli1+, (b) Bcl-6-, Peli1- (c) Bcl-6+ Peli1- and (d) Bcl-6- Peli1+.
Kaplan-Meier survival curves for progression-free survival (PFS) and overall survival (OS). When analyzed by Bcl-6/Peli1 status, Bcl-6/Peli1 +/+ group showed worst PFS and OS followed by Bcl-6/Peli1 -/- group (a and b). With re-grouping as Bcl-6/Peli1 risk groups, high risk group showed worse prognosis (c and d). Other well-known prognostic factors such as high international prognostic index (e and f), high Ann Arbor stage (g and h), poor ECOG performance status (i and j) and extranodal sites (≥2) (k and l) showed worse PFS and OS.
Survival analysis with Bcl-6/Peli1 risk groups and clinicopathologic variables in diffuse large B cell lymphoma with R-CHOP treatment.
| Clinicopathologic variables | Progression-free survival | Overall survival | |||||
|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||
| P value | P value | HR [95% CI] | P value | P value | HR [95% CI] | ||
| Age | >60 | 0.068 | 0.064 | ||||
| Sex | Male | 0.940 | 0.870 | ||||
| ECOG PS | ≥ 2 | <0.001 | 0.035 | 3.08 [1.08-8.78] | <0.001 | 0.169 | |
| B symptoms | Present | 0.226 | 0.030 | 0.349 | |||
| Serum LDH | Elevated | 0.101 | 0.839 | ||||
| Number of extranodal sites | ≥ 2 | 0.001 | 0.595 | <0.001 | 0.127 | ||
| International prognostic index | 3-5 | <0.001 | 0.013 | 3.39 [1.30-8.86] | <0.001 | 0.001 | 12.15 [2.64-55.89] |
| Bone marrow involvement | Present | 0.529 | 0.551 | ||||
| Bulky disease | ≥ 10cm | 0.172 | 0.909 | ||||
| Ann Arbor stage | III-IV | 0.008 | 0.648 | 0.006 | 0.532 | ||
| Hans classification | Non-GCB | 0.642 | 0.912 | ||||
| Bcl-2 expression | Positive | 0.340 | 0.783 | ||||
| Bcl-6 expression | Positive | 0.605 | 0.708 | ||||
| CD10 expression | Positive | 0.931 | 0.926 | ||||
| MUM1 expression | Positive | 0.252 | 0.355 | ||||
| Bcl-6/Peli1 risk group | High risk | 0.026 | 0.032 | 3.29 [1.11-9.71] | 0.021 | 0.048 | 7.87 [1.01-62.50] |
R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone; ECOG PS: the Eastern Cooperative Group Performance Status; LDH: lactate dehydrogenase; GCB, germinal center B cell-like; HR, hazard ratio; 95% CI, 95% confidence interval.
In multivariate analysis for PFS with all significant variables observed in univariate analysis (Table 3), Bcl-6/Peli1 risk group (p=0.032, HR=3.29) was observed as an independent prognostic factor, along with IPI (p=0.013, HR=3.39) and ECOG PS (p=0.035, HR=3.08). In multivariate analysis for OS, Bcl-6/Peli1 risk group (p=0.048, HR=7.87) and IPI (p=0.001, HR=12.15) were significantly associated with prognosis.
Discussion
In the present study, we investigated the prognostic utility of Peli1 expression in association with Bcl-6 status, and observed Bcl-6/Peli1 risk group as an independent prognostic indicator for PFS and OS.
Since standard immunochemotherapy with R-CHOP regimen has been established in DLBCL, various methods for risk stratification have been developed due to its heterogeneous outcome [13], as shown in 'cell of origin' classification by using gene expression profiling or immunohistochemical algorithm [11]. In many studies, non-GCB or activated B cell-like (ABC) subtype predicts poor prognosis compared to GCB subtype [11], while proportion of non-GCB subtype and its prognostic value variably depends on geographic location, detection methods and various heterogeneous subset within each subtype [14-16].
BCL6 gene was known to function in germinal center (GC) formation during immune reaction. It exerts its role as a powerful transcriptional repressor targeting DNA damage sensing by TP53 and ARF and escape process from the GC reaction toward plasma cell differentiation by IRF4 and PRDM1, allowing sufficient time to get somatic hypermutation in immunoglobulin genes [17]. Deregulated Bcl-6 expression with disruption of many signals leads to lymphomagenesis in mice [18]. Among various deregulation mechanism leading to maintained expression of Bcl-6 [17, 19-21], ubiquitin-mediated stabilization is a feasible mechanism for sustained expression of nuclear factors [22, 23].
Prognostic effects of Bcl-6 protein are relatively complex in DLBCL. BCL6 itself is an oncogene of DLBCL. At the same time, Bcl-6 protein is a component of GCB marker, while it can also be variably expressed in non-GCB subtype and influences many other critical signaling pathways [24]. In many studies with clinical samples, Bcl-6 was observed as a good prognostic factor in DLBCL [25, 26]. From the previous mechanistic report [6], we focused on the functional relevance of Peli1 and Bcl-6, a known target of Peli1-induced lymphomagenesis. With nuclear co-localization status of both molecules, we divided two major prognostic groups, i.e., high risk and low risk groups.
In our clinicopathologic analysis, this risk modeling worked well as a significant prognostic indicator independently from IPI and other conventional variables. Considering that GCB and non-GCB subtypes contain heterogeneous prognostic subsets, our mechanism-based approach to dissect Bcl-6 expression according to E3 ligase Peli1 led to redefined clinical utility for risk stratification in addition to Bcl-6 or 'cell of origin' classification. It is of note that Bcl-6-/Peli1- subgroup was very aggressive nearly similar to Bcl-6+/Peli1+ subgroup, constituting high risk group in our risk model. Bcl-6-/Peli1- subset may largely belong to non-GCB subtype (41/44, 93%) and therefore, may be driven by Bcl-6-independent genetic alteration for non-GCB subtype. It remains to be clarified further to determine the detailed molecular mechanism of each subgroup, as well as clinical applicability via validation cohort.
A few previous studies addressed consistent results similar to our study [6, 8, 27], but with significant differences. Two previous studies focused on mechanism of Peli1-mediated cell survival or lymphomagenesis [6, 27]. In the studies for prognostic effects of Peli1 in DLBCL [6, 8], the interpretation criteria and prognostic significance were slightly different from our study. In the study by Park et al, DLBCL with ≥10% of cells showing nuclear expression of moderate to strong intensity were defined as Peli1hi, and otherwise Peli1lo. Peli1hi group had a worse prognosis than Peli1lo group for OS [6]. In their study, the prognostic significance of Peli1 for OS was independent of international index (IPI) in all DLBCL patients (p=0.010 for Peli1; p<0.001 for IPI), but not in R-CHOP-treated patients in multivariate analysis for OS (p=0.073 for Peli1; p=0.015 for IPI). In the setting of prognostic analysis, the meaning of Peli1 expression was not interpreted according to Bcl-6 expression status, while their expression levels showed a positive correlation in the Bcl-6-translocation-negative subset of DLBCL. In the study by Choe et al, they showed that Peli1>2 group was a significant prognostic indicator only for relapse-free survival (RFS) in univariate analysis (p=0.002), but not for OS or in multivariate analysis (p=0.591) [8]. In their study, the prognostic significance of Peli1 was not analyzed according to Bcl-6 expression status. In the present study, we focused on the close mechanistic relationship between Peli1 and Bcl-6, and the clinical effects of Peli1 expression according to expression status of Bcl-6 to make a clinically relevant indicator. Our survival analysis showed that Bcl-6/Peli1 risk group was significantly associated with prognosis for both PFS and OS in multivariate analysis, independently from IPI and conventional variables.
With the limitation of retrospective design of this analysis, our data suggest that combined interpretation of the two related molecular expression, Bcl-6 and Peli1, may harbor significant clinical implication. Validation study with larger independent cohort is needed to apply this model to clinical risk stratification.
In summary, we composed a new risk model of Bcl-6/Peli1 by using E3 ligase Peli1 and its known target, Bcl-6 in the basis of lymphomagenesis, and this model independently predicted prognosis in DLBCL patents for PFS and OS. With proper validation, it may contribute to risk assessment in patients with DLBCL.
Supplementary Material
Supplementary figure 1: enrollment criteria.
Acknowledgements
This study was supported by Basic Science Research Program through the National Research Foundation (NRF) of Korea funded by the Ministry of Science and ICT (NRF-2019R1F1A1061920) and research fund of SNUBH (No. 14-2015-019).
Author Contributions
KRL and JHP conceived and designed the experiments. KRL and JHP performed the experiments and analyzed pathologic data. JSL and JOL collected and analyzed clinical data. KRL and JHP performed clinicopathologic analysis and wrote the manuscript. All authors edited the final manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Menon MP, Pittaluga S, Jaffe ES. The histological and biological spectrum of diffuse large B-cell lymphoma in the World Health Organization classification. Cancer J. 2012;18:411-20
2. Coiffier B, Sarkozy C. Diffuse large B-cell lymphoma: R-CHOP failure-what to do? Hematology Am Soc Hematol Educ Program. 2016;2016:366-78
3. Harkins RA, Chang A, Patel SP, Lee MJ, Goldstein JS, Merdan S. et al. Remaining challenges in predicting patient outcomes for diffuse large B-cell lymphoma. Expert Rev Hematol. 2019;12:959-73
4. Sun Y. Targeting E3 ubiquitin ligases for cancer therapy. Cancer biology & therapy. 2003;2:623-9
5. Jin W, Chang M, Sun S-C. Peli: a family of signal-responsive E3 ubiquitin ligases mediating TLR signaling and T-cell tolerance. Cell Mol Immunol. 2012;9:113-22
6. Park HY, Go H, Song HR, Kim S, Ha GH, Jeon YK. et al. Pellino 1 promotes lymphomagenesis by deregulating BCL6 polyubiquitination. The Journal of clinical investigation. 2014;124:4976-88
7. Liu J, Huang X, Hao S, Wang Y, Liu M, Xu J. et al. Peli1 negatively regulates noncanonical NF-κB signaling to restrain systemic lupus erythematosus. Nature communications. 2018;9:1136
8. Choe JY, Park M, Yun JY, Na HY, Go H, Kim HJ. et al. PELI1 expression is correlated with MYC and BCL6 expression and associated with poor prognosis in diffuse large B-cell lymphoma. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2016;29:1313-23
9. Li D, Tavana O, Sun SC, Gu W. Peli1 Modulates the Subcellular Localization and Activity of Mdmx. Cancer research. 2018;78:2897-910
10. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R. et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-90
11. Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G. et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275-82
12. Kwon HJ, Yang JM, Lee JO, Lee JS, Paik JH. Clinicopathologic implication of PD-L1 and phosphorylated STAT3 expression in diffuse large B cell lymphoma. Journal of translational medicine. 2018;16:320
13. Susanibar-Adaniya S, Barta SK. 2021 Update on Diffuse large B cell lymphoma: A review of current data and potential applications on risk stratification and management. American journal of hematology. 2021;96:617-29
14. Shiozawa E, Yamochi-Onizuka T, Takimoto M, Ota H. The GCB subtype of diffuse large B-cell lymphoma is less frequent in Asian countries. Leukemia research. 2007;31:1579-83
15. Lee J, Hue SS, Ko SQ, Tan SY, Liu X, Girard LP. et al. Clinical impact of the cell-of-origin classification based on immunohistochemistry criteria and Lymph2Cx of diffuse large B-Cell lymphoma patients in a South-east Asian population: a single center experience and review of the literature. Expert Rev Hematol. 2019;12:1095-105
16. Li S, Young KH, Medeiros LJ. Diffuse large B-cell lymphoma. Pathology. 2018;50:74-87
17. Cardenas MG, Oswald E, Yu W, Xue F, MacKerell AD Jr, Melnick AM. The Expanding Role of the BCL6 Oncoprotein as a Cancer Therapeutic Target. Clinical cancer research: an official journal of the American Association for Cancer Research. 2017;23:885-93
18. Cattoretti G, Pasqualucci L, Ballon G, Tam W, Nandula SV, Shen Q. et al. Deregulated BCL6 expression recapitulates the pathogenesis of human diffuse large B cell lymphomas in mice. Cancer cell. 2005;7:445-55
19. Saito M, Gao J, Basso K, Kitagawa Y, Smith PM, Bhagat G. et al. A signaling pathway mediating downregulation of BCL6 in germinal center B cells is blocked by BCL6 gene alterations in B cell lymphoma. Cancer cell. 2007;12:280-92
20. Pasqualucci L, Migliazza A, Basso K, Houldsworth J, Chaganti RS, Dalla-Favera R. Mutations of the BCL6 proto-oncogene disrupt its negative autoregulation in diffuse large B-cell lymphoma. Blood. 2003;101:2914-23
21. Duan S, Cermak L, Pagan JK, Rossi M, Martinengo C, di Celle PF. et al. FBXO11 targets BCL6 for degradation and is inactivated in diffuse large B-cell lymphomas. Nature. 2012;481:90-3
22. Sun T, Liu Z, Yang Q. The role of ubiquitination and deubiquitination in cancer metabolism. Molecular cancer. 2020;19:146
23. Senft D, Qi J, Ronai ZA. Ubiquitin ligases in oncogenic transformation and cancer therapy. Nature reviews Cancer. 2018;18:69-88
24. Yang Y, Staudt LM. Protein ubiquitination in lymphoid malignancies. Immunological reviews. 2015;263:240-56
25. Maeshima AM, Taniguchi H, Fukuhara S, Morikawa N, Munakata W, Maruyama D. et al. Bcl-2, Bcl-6, and the International Prognostic Index are prognostic indicators in patients with diffuse large B-cell lymphoma treated with rituximab-containing chemotherapy. Cancer science. 2012;103:1898-904
26. Culpin RE, Sieniawski M, Angus B, Menon GK, Proctor SJ, Milne P. et al. Prognostic significance of immunohistochemistry-based markers and algorithms in immunochemotherapy-treated diffuse large B cell lymphoma patients. Histopathology. 2013;63:788-801
27. Wang H, Meng H, Li X, Zhu K, Dong K, Mookhtiar AK. et al. PELI1 functions as a dual modulator of necroptosis and apoptosis by regulating ubiquitination of RIPK1 and mRNA levels of c-FLIP. Proc Natl Acad Sci U S A. 2017;114:11944-9
Author contact
![]() Corresponding author: Jin Ho Paik, MD, PhD, Department of Pathology, Seoul National University Bundang Hospital, 82, Gumi-ro 173beon-gil, Bundang-gu, Seongnam-si, Gyeonggi-do 13620, South Korea. Tel.: +82-31-787-7717; Fax: +82-31-787-4012; E-mail: paikjhac.kr
Corresponding author: Jin Ho Paik, MD, PhD, Department of Pathology, Seoul National University Bundang Hospital, 82, Gumi-ro 173beon-gil, Bundang-gu, Seongnam-si, Gyeonggi-do 13620, South Korea. Tel.: +82-31-787-7717; Fax: +82-31-787-4012; E-mail: paikjhac.kr

 Global reach, higher impact
Global reach, higher impact