3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(1):174-182. doi:10.7150/jca.79747 This issue Cite
Review
Comparing the Diagnostic Performance of Contrast-Enhanced Mammography and Breast MRI: a Systematic Review and Meta-Analysis
1. GROW School for Oncology and Reproduction, Maastricht University, Universiteitssingel 40, 6229 ER Maastricht, the Netherlands.
2. Department of Radiology and Nuclear Medicine, Maastricht University Medical Center+, P. Debyelaan 25, 6229 HX Maastricht, the Netherlands.
3. Department of Radiology, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
4. Department of Medical Imaging, Laurentius hospital, Mgr. Driessenstrtaat 6, 6040AX Roermond, the Netherlands.
5. Department of Surgery, Maastricht University Medical Center+, P. Debyelaan 25, 6229 HX Maastricht, the Netherlands.
6. Department of Epidemiology, Maastricht University, P. Debyelaan 1, 6229 HA Maastricht, the Netherlands.
7. Department of Medical Imaging, Zuyderland Medical Center, Dr. H. van der Hoffplein 1, 6162 BG Sittard-Geleen, the Netherlands.
Received 2022-10-11; Accepted 2022-12-3; Published 2023-1-1
Abstract
Background: To provide a systematic review and meta-analysis that evaluates the diagnostic accuracy of contrast-enhanced mammography (CEM) compared to standard contrast-enhanced breast magnetic resonance imaging (breast MRI). Like breast MRI, CEM enables tumour visualization by contrast accumulation. CEM seems to be a viable substitute for breast MRI.
Methods: This systematic search assessed the diagnostic accuracy of these techniques in women with suspicious breast lesions on prior imaging or physical examination, who have undergone both breast MRI and CEM. CEM had to be performed on a commercially available system. The MRI sequence parameters had to be described sufficiently to ensure that standard breast MRI sequence protocols were used. Pooled values of sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio (DOR), were estimated using bivariate mixed-effects logistic regression modeling. Hierarchical summary receiver operating characteristic curves for CEM and breast MRI were also constructed.
Results: Six studies (607 patients with 775 lesions) met the predefined inclusion criteria. Pooled sensitivity was 96% for CEM and 97% for breast MRI. Pooled specificity was 77% for both modalities. DOR was 79.5 for CEM and 122.9 for breast MRI. Between-study heterogeneity expressed as the I2-index was substantial with values over 80%.
Conclusion: Pooled sensitivity was high for both CEM and breast MRI, with moderate specificity. The pooled DOR estimates, however, indicate higher overall diagnostic performance of breast MRI compared to CEM. Nonetheless, current scientific evidence is too limited to prematurely discard CEM as an alternative for breast MRI.
Keywords: contrast-enhanced mammography, breast MRI, diagnostic accuracy
Introduction
Breast cancer is currently the most common cancer in women, with 2.3 million newly diagnosed women worldwide in 2020 [1,2]. (Standard) contrast-enhanced breast magnetic resonance imaging (breast MRI) is considered the best imaging modality for breast cancer detection and evaluation of its extent [3]. However, it is associated with high costs, long acquisition and reading times, and limited availability in some regions [3,4]. It is contraindicated in patients with claustrophobia, some metal implants or foreign objects in their body, and known hypersensitivity reactions to Gadolinium-based contrast agents [5]. Therefore, alternative methods of evaluation must be considered. Hence, breast MRI is not a primary imaging modality in breast imaging, but is only indicated for very specific patient populations [6].
Since its introduction in 2011, the use of contrast-enhanced mammography (CEM) has been steadily increasing in both research and clinical settings [7-10]. A CEM examination, in which an iodinated contrast agent is administered followed by the acquisition of a dual-energy mammography, uses the same physiological contrast enhancement principle as breast MRI [8]. Reading CEM examinations utilizes the fifth edition of the American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) lexicon for mammography, including the supplemental chapter on CEM, combined with that for MRI without the contrast kinetics evaluation and with some descriptors specific for CEM [11,12]. Also, CEM seems to be easy to learn [13-16]. The costs of a CEM exam are significantly lower than that of breast MRI and patients tend to prefer CEM over breast MRI [4,17,18]. Consequently, CEM is becoming an attractive alternative for breast MRI [19].
Although several systematic reviews and meta-analyses assessing the diagnostic performance of CEM have been published before [20-24], most of them compared CEM with full-field digital mammography. Only two meta-analyses compared the diagnostic accuracy of CEM to that of breast MRI, showing conflicting results [23,24]. Both reviews used criteria for eligibility of studies, which may have introduced bias of the results. We therefore conducted a systematic review and meta-analysis, applying inclusion criteria that better correspond with everyday clinical practice, to compare the diagnostic accuracy of CEM and breast MRI.
Methods
This systematic review followed the checklist of Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy (PRISMA-DTA) [25].
Search strategy
A systematic literature search of the electronic databases PubMed (Central) and Embase was conducted to identify all published studies on CEM and breast MRI that reported on accuracy in the diagnostic setting. A combination of MeSH and EMTREE terms were used: “breast malignancy”, “contrast-enhanced mammography”, and “magnetic resonance imaging”. To define breast malignancy, the following keywords were used: “breast tumour”, “breast neoplasms”, “carcinoma”, “malignant neoplasms”, and “cancer”. In this primary search, studies published before May 2022 were identified and no language or publication restrictions were applied, such as publication language and conference abstracts or proceedings. Supplemental to the search, the reference lists of retrieved articles were reviewed for other potentially relevant studies. All the retrieved references were entered into a bibliography management software (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia) to facilitate the search for duplicate references and assist in our systematic approach.
Study eligibility
First, duplicate records were removed from the list of studies retrieved from the database search. All titles and abstracts of the remaining studies were independently screened by two researchers (LN and MR) to select studies that addressed the comparative value of CEM and breast MRI for diagnostic accuracy. Reviews, technical reports, letter to editors, comments to published studies, case reports, conference abstracts or proceedings, and publications in languages other than English were not considered eligible. After a first screening process, the full-text of the remaining studies was independently assessed for eligibility. Discrepancies between the two researchers were discussed and consensus was given by a third expert reviewer and radiologist (ML) when thought necessary.
To be eligible for inclusion in the review studies had to meet several inclusion criteria. Patients should have received both CEM and breast MRI for the evaluation of breast lesions. Absolute numbers of true positive (TP), true negative (TN), false positive (FP), and false negative (FN) lesions could be derived from the publication. CEM had to be performed on a commercially available system, not on a prototype system, and using a generally accepted image acquisition protocol. MRI sequence parameters had to be described sufficiently to ensure that standard contrast-enhanced breast MRI sequences were used. The study population had to consist of women with suspicious breast lesions on prior imaging (e.g., full-field digital mammography, ultrasound) or clinical examination. Studies that included only patients with histologically verified breast cancer were excluded, because such studies cannot provide information on the ability of CEM and breast MRI to distinguish between benign and malignant lesions. The estimates of sensitivity and specificity from studies, in which all patients had an already diagnosed index breast tumour, relate to the ability of both modalities to identify additional lesions, which corresponds with a different research question.
Data extraction
The two reviewers independently extracted the data from the eligible studies following a pre-defined extraction format. Variables that were extracted from all studies were: author and publication information, study population characteristics, CEM system characteristics and imaging protocol, MRI system characteristics and imaging sequences, and information on contrast administration for both CEM and breast MRI.
The QUADAS-2 score, the outcome of a tool for the quality assessment of diagnostic accuracy studies (QUADAS), was used to identify risk of bias and applicability concerns in the included studies [26].
Statistical analysis
For the comparison of pooled diagnostic parameters between CEM and breast MRI for diagnostic work-up, the bivariate model and hierarchical summary receiving operating characteristic (sROC) model were used [27]. Pairs of sensitivity and specificity are jointly analyzed, incorporating any correlation that might exist between these two measures using a random effects approach [28]. The bivariate model includes five parameters and for fitting of the model a minimum of 4 studies is required [27]. sROC curves with the prediction region, summary point and the confidence region were constructed to visualize the trade-off between sensitivity and specificity [27,29].
Statistical analyses were performed using STATA/SE 14.1 (StataCorp LLC, College Station, TX, USA). The metandi command in STATA was used, which provides summary estimates of sensitivity and specificity, positive and negative likelihood ratios and diagnostic odds ratios (DOR) [29]. The DOR is the odds of a positive test result on imaging in a case with breast cancer divided by the odds of a negative test result on imaging in a case without breast cancer. The midas command in STATA was used to construct Forest plots to give an overview of sensitivity and specificity with 95% confidence intervals of individual studies [30]. Between-study heterogeneity was expressed as the I2-index. This statistic quantifies the percentage of total variation across studies that is due to heterogeneity rather than chance [31].
Results
Eligible and extracted studies
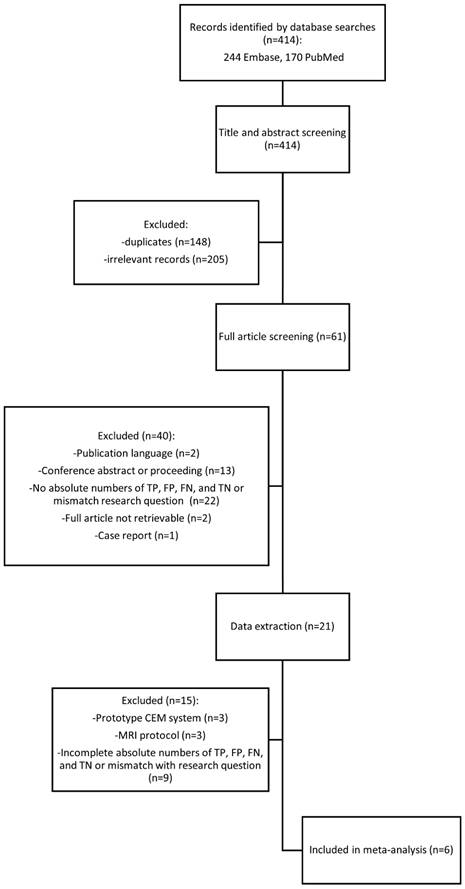
The literature search in Embase and PubMed (Central) databases resulted in the identification of 244 and 170 studies, respectively. After exclusion of 148 duplicate studies, screening of the title and abstract of the remaining 266 studies led to exclusion of another 205 studies. The full text of the 61 remaining studies was retrieved and read, and 21 studies remained for data extraction. During data extraction, three studies were excluded because of lack of sufficient details on MRI sequence parameters. Three other studies were excluded because CEM was performed on a prototype system. Nine studies were excluded because absolute numbers of of true positive (TP), true negative (TN), false positive (FP), and false negative (FN) lesions could not be derived or the study population included only women with histologically verified breast cancer. A list of the excluded publications is given in Supplementary materials 1. In the end, a total of six studies were included in the meta-analysis [32-37]. The flow chart in Figure 1 shows the selection of the studies included for review and reasons for exclusion.
Quality assessment of publications
The QUADAS-2 outcomes are listed in Table 1. Two studies showed low risks of bias and low concern on applicability for all domains [33,36]. The four other studies scored high for at least one domain in risk of bias and corresponding domain of concern on applicability [32,34,35,37]. A patient population with only BI-RADS 4 lesions resulted in a high score for risk of bias and applicability concerns in the patient selection domain [37]. High risk of bias and applicability concern in the index test and reference test domains was assigned in case of unknown reader experience or unknown blinding for the final diagnosis. One study specifically mentioned that the readers had no experience in assessing CEM exams [35]. Unknown or no experience in assessing CEM or breast MRI exams might affect the diagnostic accuracy. Not mentioning the time interval between the CEM exam and breast MRI led to a high score of risk of bias in the flow and timing domain [34]. With a larger time interval between the two imaging modalities, the tumour could have evolved, probably leading to better visibility, thus better sensitivity for the latter imaging exam.
Quality assessment of the publications using the QUADAS-2 format
| Study | Risk of Bias | Applicability concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient Selection | Index Test | Reference Standard | Flow and Timing | Patient Selection | Index Test | Reference Standard | |
| Kamal et al. [32] | Low | High | High | Low | Low | High | High |
| Luczynska et al. [33] | Low | Low | Low | Low | Low | Low | Low |
| Petrillo et al. [34] | Low | Low | Low | High | Low | Low | Low |
| Wang et al. [35] | Low | High | Low | Low | Low | High | Low |
| Xing et al. [36] | Low | Low | Low | Low | Low | Low | Low |
| Yasin & El Ghany [37] | High | High | Low | Low | High | High | Low |
Comparison of value of CEM and breast MRI for diagnostic work-up
The included six studies comprised 775 lesions in 607 patients with suspicious breast lesions on prior imaging or clinical examinations, of which 512 lesions were malignant [32-37]. One study was conducted on a Hologic system, the remaining studies on GE Healthcare systems. The CEM and breast MRI findings were matched with true disease state using cytological/histopathological results of all lesions, except in two studies. In these two studies lesions assessed as BI-RADS ≤2 on both CEM and breast MRI were considered true negative [35] or the lesions assessed as BI-RADS 2 were closely monitored for one year [32]. The same BI-RADS cut-off value was used in the six studies to define the absolute numbers of TP, FP, FN, TN. BI-RADS scores 0-3 were considered as negative cases on imaging and BI-RADS 4-5 as positive cases [32-37]. A comprehensive overview of the study characteristics is given in Table 2.
Flowchart of systematic search.
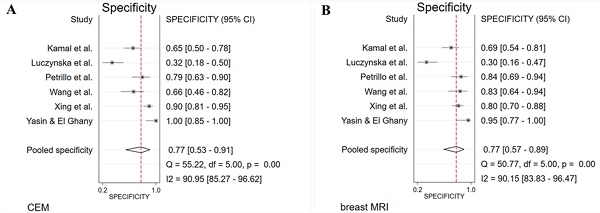
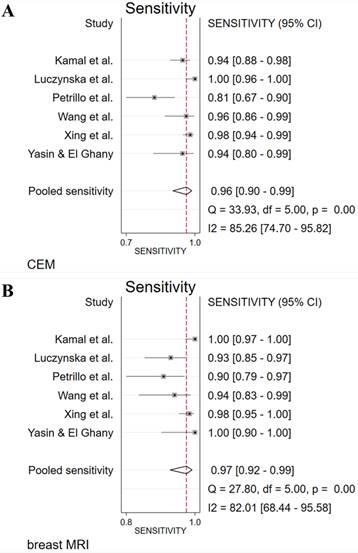
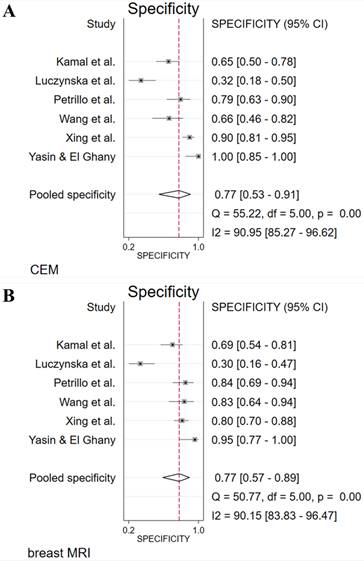
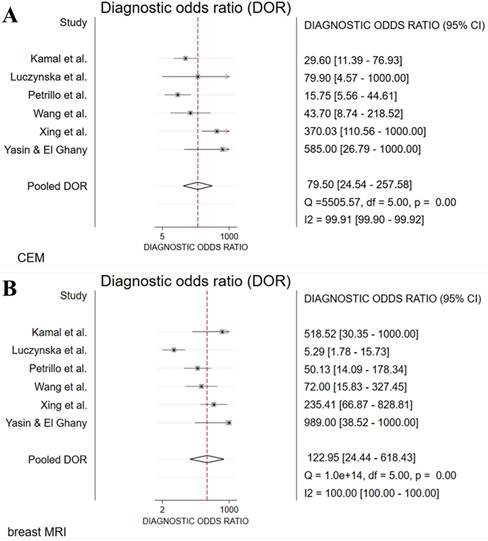
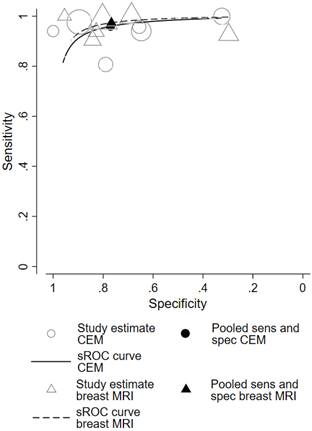
The absolute numbers of TP, FP, FN, TN, and the sensitivity, specificity, positive predictive value, and negative predictive value are given in Table S1. The pooled sensitivity of CEM and breast MRI was 96% (95% CI 90%-99%) and 97% (95% CI 92%-99%), respectively. Pooled specificity was, 77% (95% CI 53%-91%) for CEM and 77% (95% CI 57%-89%) for breast MRI. Figure 2 presents the forest plots for sensitivity and Figure 3 for specificity. Pooled positive likelihood ratio (LR) and pooled negative LR were 4.16 (95% CI 1.86-9.34) and 0.05 (95% CI 0.02-0.13) for CEM and 4.18 (95% CI 2.06-8.48) and 0.03 (95% CI 0.01-0.11) for breast MRI, respectively. The pooled DOR for breast MRI was 122.9 (95% CI 24.4-618.4) versus 79.5 (95% CI 24.5-257.6) for CEM (Figure 4). Between-study heterogeneity was considerable with I2 values exceeding 80%. The hierarchical sROC curves with summary points for CEM and breast MRI are shown in Figure 5. The hierarchical sROC curve is constructed by plotting the sensitivity (true positivity) and false positivity (1 - specificity) of each study.
Discussion
This systematic review and meta-analysis provide an evidence-based update on the comparative diagnostic performance of CEM and breast MRI in the diagnostic work-up in women with suspicious breast lesions. Pooled sensitivity of breast MRI was slightly higher than that of CEM (97% vs 96%) at similar pooled specificity (77%). The pooled DOR estimates indicate a higher overall diagnostic performance of breast MRI compared to CEM (122.9 vs 79.5). Strict eligibility criteria were applied to ensure that optimal imaging methods were used. Excluded were studies using prototype versions of CEM units and studies from which it could not be deduced with certainty that standard imaging protocols were used. Irrespective of the strict study eligibility criteria applied, the results show that there was still considerable between-study heterogeneity with I2 values higher than 80%. Although studies were performed on CEM systems by two vendors this probably did not contribute to the high I2 values, since recent research showed that diagnostic accuracy of CEM is likely to be vendor system independent [38]. One source of the variation between study results could be methodological issues, such as differences in patient populations and variation in reader experience.
Forest plots of sensitivity for CEM (A) and breast MRI (B) with pooled values, and the I2 values.
Only two meta-analyses have been published that directly compared CEM and breast MRI [23,24]. The meta-analysis published in 2019 by Xiang et al. resulted in a pooled sensitivity of 97% for both imaging modalities, but the pooled specificity for CEM (66%) was higher than that for breast MRI (52%) [23]. The authors concluded that the diagnostic performance of CEM appears to be more effective than that of breast MRI, because the pooled odds ratio for CEM was 60.15 versus 31.34 for breast MRI. The results of the present meta-analysis do not corroborate these results, because the pooled DOR for breast MRI was much higher (122.9 instead of 31.34) and exceeded the pooled DOR for CEM (79.5). Only two studies of the 13 studies included in the meta-analysis by Xiang et al. were also included in our meta-analysis [23,33,35]. The other eleven studies, listed in Supplementary materials 2, were excluded for this meta-analysis for several reasons. Results were untraceable, were published in a conference abstract or proceeding, or in a language other than English. In other studies, CEM was performed on a prototype system or the patient population consisted exclusively of women with an index tumour. The latter studies can answer the question whether CEM and breast MRI differ in the ability to detect additional lesions in the same breast or the contralateral breast [39-41], but cannot provide information on the ability of imaging modalities to discriminate between benign and malignant breast lesions.
Forest plots of specificity for CEM (A) and breast MRI (B) with pooled values, and the I2 values.
The most recent meta-analysis by Pötsch et al. resulted in a lower pooled sensitivity for CEM (91%) than for breast MRI (97%), whereas the pooled specificity for CEM (74%) was higher than that for breast MRI (69%) [24]. Overall diagnostic performance of breast MRI (pooled DOR: 73.0) was higher than that of CEM (pooled DOR: 30.4). The authors concluded that there is not yet a conclusive answer on the question as to whether breast MRI is superior to CEM for diagnostic work-up of women in a screening or recall setting. Four of the included studies by Pötsch et al. were also included in current meta-analysis [32,33,35,37]. The other three studies were excluded from the current meta-analysis because of the use of a prototype system for low dose CEM [42], a patient population in which all patients were already diagnosed with breast cancer [43], and incorrect absolute numbers of TP, FP, TN and FN [44]. In the latter study, Pötsch et al., wrongfully assumed that all 'non-papillomas' were malignant whereas only two non-papillomas were actually malignant. Based on the pooled estimates from the remaining four studies, the difference between sensitivity of breast MRI and CEM becomes much smaller: 98% versus 96% instead of 97% versus 91%, respectively.
Forest plots of diagnostic odds ratio (DOR) for CEM (A) and breast MRI (B) with pooled values, and I2 values.
Hierarchical sROC curves of CEM (black line) and breast MRI (black dotted line). The summary points of pooled sensitivity with pooled specificity for CEM and breast MRI are shown with the black circle and black triangle, respectively.
Diagnostic accuracy - study characteristics
| Author | Kamal et al. [32] | Luczynska et al. [33] | Petrillo et al. [34] | Wang et al. [35] | Xing et al. [36] | Yasin & El Ghany [37] |
|---|---|---|---|---|---|---|
| Year | 2020 | 2015 | 2020 | 2016 | 2019 | 2019 |
| Study design | P/F | P | P | P | P/F | P |
| Patients | 82 | 102 | 70 | 68 | 235 | 50 |
| Age mean ± SD (range) | 49.3 ± 10.8 (29-71) | NA | NA | 52.9 ± 10.7 (31-82) | 51 ± 10 (25-82) | 52 (33-83) |
| Lesions | 171 | 118 | 90 | 77 | 263 | 56 |
| Disease prevalence lesions | 70% [120/171] | 69% [81/118] | 58% [52/90] | 62% [48/77] | 67% [177/263] | 61% [34/56] |
| CEM system | GE Senographe Essential | GE SenoBright | Hologic Selenia | GE Senographe DS & GE Senographe Essential | GE Senographe Essential | GE Senographe Essential |
| CEM contrast | non-ionic contrast agent | Iopromide 370 | Visipaque 320 | Omnipaque 350 | Iohexol 300-350 | Visipaque 320 |
| MRI system | 1.5T Siemens | 1.5T Avanto Siemens | 1.5T Magnetom Symphony Siemens | 1.5T Signa HDx GE | 3.0T Signa HD XT GE | 1.5T Magnetom Aera Siemens |
| MRI contrast | Gd-DTPA (Magnevist) | Gadobutrol (Gadovist) | Gd-DOTA (Magnevist) | Gd-DTPA (Magnevist) | Gd-DTPA (Magnevist) | Gadolinium (not mentioned) |
Abbreviations: SD: standard deviation; CEM: contrast-enhanced mammography; MRI: magnetic resonance imaging; P: prospective; F: feasibility; NA: not available.
The large discrepancies between the results from the three meta-analyses, which are now available, raises concern. Use of different criteria for eligibility of studies resulted in the inclusion of different studies with only partial overlap. To be able to rely on the results of meta-analysis, robust results are needed to guide evidence-based practice. Therefore, we do not agree with the bold statements published earlier by experts Mann and Velthuis [45], which were based on the Pötsch review, stating that CEM would 'take us two steps back in breast imaging' compared to breast MRI. With such claims, we run the risk to prematurely exclude CEM from imaging, when in fact it is a modality which is increasingly being used with much potential [9]: more accessible to underserved populations, less expensive, shorter reading times and preferred by patients [4,17,18,46].
Indications for CEM are the same as for breast MRI: preoperative staging [33,36,47] (i.e., tumour size assessment and the detection of multifocal or contralateral breast cancer foci) and response monitoring of patients treated with neo-adjuvant systemic therapy [48-50]. We initially aimed to compare the diagnostic performance for these indications as well, but found that the available scientific literature did not provide the necessary data for a systematic review and meta-analysis. For comparison of accuracy of size measurement during pre-operative staging, mean values with standard deviation for size according to histopathology and imaging are needed to calculate summary estimates of mean differences, but these data were generally lacking [33,36,51,52]. We identified three studies that compared the performance of CEM and breast MRI in response monitoring, but the use of different definitions of pathological complete response (pCR) hinder comparison of study results [48-50]. More research comparing the diagnostic performance of CEM and MRI for these indications is required to draw more robust conclusions in the form of systematic reviews or meta-analyses.
Both this meta-analysis and the other two meta-analyses have limitations. The number of studies which directly compare the diagnostic accuracy of CEM and breast MRI for diagnostic work-up in a recall setting is very limited and the results from individual studies were very heterogeneous. The lack of robustness of the results from the three meta-analyses indicates that the definition of eligibility criteria for inclusion of studies is an important determining factor for the pooled measures of diagnostic accuracy. There is a need for more original diagnostic studies that meet the criteria for valid comparison of the diagnostic accuracy of optimal imaging with CEM and breast MRI using commercially available and generally accepted image acquisition protocols in a well-defined study population of women that are referred for diagnostic work-up.
In conclusion, we showed that sensitivity was high and specificity moderate for both CEM and breast MRI. The higher pooled DOR estimates for breast MRI indicate a higher overall diagnostic performance compared to CEM. Nevertheless, it seems premature to discard CEM as alternative for breast MRI due to the limited number of studies included in this review. Future studies that are directly comparing CEM and breast MRI for various indications are much needed.
Abbreviations
BI-RADS: breast imaging reporting and data system; CEM: contrast-enhanced mammography; breast MRI: contrast-enhanced breast magnetic resonance imaging; CI: confidence interval; DOR: diagnostic odds ratio; FFDM: full-field digital mammography; FN: false negative; FP: false positive; LR: likelihood ratio; NPV: negative predictive value; pCR: pathologic complete response; PPV: positive predictive value; PRISMA-DTA: preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy; QUADAS-2: quality assessment of diagnostic accuracy studies version 2; Sens: sensitivity; Spec: specificity; sROC: summary receiver operating characteristic; TN: true negative; TP: true positive.
Supplementary Material
Supplementary information, table.
Acknowledgements
As part of the RACER project, this study has received funding by the Netherlands Organization for Health Research and Development (ZonMw Efficiency Studies Grant Number 843001801) and GE Healthcare.
Competing Interests
MJ received a speaker's fee from GE Healthcare.
JW received institutional grants from AGFA, Bayer Healthcare, Bard Medical, GE Healthcare, Optimed, Philips Healthcare, Siemens Healthineers.
ML received a research grant and several speaker's fees from GE Healthcare, Tromp Medical, Bayer and Guerbet.
The other authors have declared that no competing interests exist.
References
1. Sung JS, Lebron L, Keating D. et al. Performance of dual-energy contrast-enhanced digital mammography for screening women at increased risk of breast cancer. Radiology. 2019;293:81-8
2. World Health Organization. Breast cancer 2021. https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed April 26, 2022).
3. Mann RM, Cho N, Moy L. Breast MRI: state of the art. Radiology. 2019;292:520-36
4. Patel BK, Gray RJ, Pockaj BA. Potential cost savings of contrast-enhanced digital mammography. Am J Roentgenol. 2017;208:W231-7
5. Richter V, Hatterman V, Preibsch H. et al. Contrast-enhanced spectral mammography in patients with MRI contraindications. Acta Radiol. 2018;59:798-805
6. Mann RM, Kuhl CK, Kinkel K. et al. Breast MRI: guidelines from the European Society of Breast Imaging. Eur Radiol. 2008;18:1307-18
7. GE Healthcare. GE Healthcare announces FDA 510(k) clearance of SenoBright Contrast Enhanced Spectral Mammography (CESM) for breast cancer diagnosis 2011. https://www.ge.com/news/press-releases/ge-healthcare-announces-fda-510k-clearance-senobright-contrast-enhanced-spectral (accessed April 26, 2021).
8. Jochelson MS, Lobbes MBI. Contrast-enhanced mammography: state of the art. Radiology. 2021;299:36-48
9. Zanardo M, Cozzi A, Trimboli RM. et al. Technique, protocols and adverse reactions for contrast-enhanced spectral mammography (CESM ): a systematic review. Insights Imaging. 2019;10:76
10. Neeter LMFH, Raat HPJ (Frank), Alcantara R. et al. Contrast-enhanced mammography: what the radiologist needs to know. BJR|Open. 2021;3:20210034
11. D'Orsi CJ, Sickles E, Mendelson E. et al. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA, USA. 2013
12. Lee CH, Phillips J, Sung JS. et al. Contrast enhanced mammography (CEM): a supplement to ACR BI-RADS® Mammography 2013. Reston, VA, USA. 2022
13. Lalji UC, Houben IPL, Prevos R. et al. Contrast-enhanced spectral mammography in recalls from the Dutch breast cancer screening program: validation of results in a large multireader, multicase study. Eur Radiol. 2016;26:4371-9
14. Van Nijnatten TJA, Smidt ML, Goorts B. et al. Can high school students help to improve breast radiologists in detecting missed breast cancer lesions on full-field digital mammography? J Cancer. 2019;10:765-71
15. Berg WA, Bandos AI, Zuley ML. et al. Training radiologists to interpret contrast-enhanced mammography: toward a standardized lexicon. J Breast Imaging. 2021;3:176-89
16. Cheung YC, Lin YC, Wan YL. et al. Diagnostic performance of dual-energy contrast-enhanced subtracted mammography in dense breasts compared to mammography alone: interobserver blind-reading analysis. Eur Radiol. 2014;24:2394-403
17. Hobbs MM, Taylor DB, Buzynski S. et al. Contrast-enhanced spectral mammography (CESM) and contrast enhanced MRI (CEMRI): patient preferences and tolerance. J Med Imaging Radiat Oncol. 2015;59:300-5
18. Phillips J, Miller MM, Mehta TS. et al. Contrast-enhanced spectral mammography (CESM) versus MRI in the high-risk screening setting: patient preferences and attitudes. Clin Imaging. 2017;42:193-7
19. Lewin J. Comparison of contrast-enhanced mammography and contrast-enhanced breast MR imaging. Magn Reson Imaging Clin N Am. 2018;26:259-63
20. Tagliafico AS, Bignotti B, Rossi F. et al. Diagnostic performance of contrast-enhanced spectral mammography: systematic review and meta-analysis. The Breast. 2016;28:13-9
21. Zhu X, Huang J ming, Zhang K. et al. Diagnostic value of contrast-enhanced spectral mammography for screening breast cancer: systematic review and meta-analysis. Clin Breast Cancer. 2018;18:e985-95
22. Suter MB, Pesapane F, Agazzi GM. et al. Diagnostic accuracy of contrast-enhanced spectral mammography for breast lesions : a systematic review and meta-analysis. The Breast. 2020;53:8-17
23. Xiang W, Rao H, Zhou L. A meta-analysis of contrast-enhanced spectral mammography versus MRI in the diagnosis of breast cancer. Thorac Cancer. 2020;11:1423-32
24. Pötsch N, Vatteroni G, Clauser P. et al. Contrast-enhanced mammography versus contrast- enhanced breast MRI : a systematic review and meta-analysis. Radiology. 2022;305:94-103
25. McInnes MDF, Moher D, Thombs BD. et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA - J Am Med Assoc. 2018;319:388-96
26. Whiting PF, Rutjes AWS, Westwood ME. et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-36
27. Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med. 2001;20:2865-84
28. Reitsma JB, Glas AS, Rutjes AWS. et al. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58:982-90
29. Harbord RM, Whiting P. metandi: Meta-analysis of diagnostic accuracy using hierarchical logistic regression. Stata J. 2009;9:211-29
30. Dwamena B. MIDAS: Stata module for meta-analytical integration of diagnostic test accuracy studies 2009
31. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-58
32. Kamal RM, Hanafy MM, Mansour SM. et al. Can contrast-enhanced mammography replace dynamic contrast-enhanced MRI in the assessment of sonomammographic indeterminate breast lesions? Egypt J Radiol Nucl Med. 2020;51:66
33. Luczynska E, Heinze-Paluchowska S, Hendrick E. et al. Comparison between breast MRI and contrast-enhanced spectral mammography. Med Sci Monit. 2015;21:1358-67
34. Petrillo A, Fusco R, Vallone P. et al. Digital breast tomosynthesis and contrast-enhanced dual-energy digital mammography alone and in combination compared to 2D digital synthetized mammography and MR imaging in breast cancer detection and classification. Breast J. 2020;26:860-72
35. Wang Q, Li K, Wang L. et al. Preclinical study of diagnostic performances of contrast-enhanced spectral mammography versus MRI for breast diseases in China. Springerplus. 2016;5:763
36. Xing D, Lv Y, Sun B. et al. Diagnostic value of contrast-enhanced spectral mammography in comparison to magnetic resonance imaging in breast lesions. J Comput Assist Tomogr. 2019;43:245-51
37. Yasin R, El Ghany EA. BIRADS 4 breast lesions: comparison of contrast-enhanced spectral mammography and contrast-enhanced MRI. Egypt J Radiol Nucl Med. 2019;50:34
38. Neeter LMFH, Raat HPJ, Meens-Koreman SD. et al. The diagnostic value of contrast-enhanced 2D mammography in everyday clinical use. Sci Rep. 2021;11:22224
39. Li L, Roth R, Germaine P. et al. Contrast-enhanced spectral mammography (CESM) versus breast magnetic resonance imaging (MRI): a retrospective comparison in 66 breast lesions. Diagn Interv Imaging. 2017;98:113-23
40. Lee-Felker SA, Tekchandani L, Thomas M. et al. Newly diagnosed breast cancer: comparison of contrast-enhanced spectral mammography and breast MR imaging in the evaluation of extent of disease. Radiology. 2017;285:389-400
41. Jochelson MS, Dershaw DD, Sung JS. et al. Bilateral contrast-enhanced dual-energy digital mammography: feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology. 2013;266:743-51
42. Clauser P, Baltzer PAT, Kapetas P. et al. Low-dose, contrast-enhanced mammography compared to contrast-enhanced breast MRI: a feasibility study. J Magn Reson Imaging. 2020;52:589-95
43. Fallenberg EM, Schmitzberger FF, Amer H. et al. Contrast-enhanced spectral mammography vs. mammography and MRI - clinical performance in a multi-reader evaluation. Eur Radiol. 2017;27:2752-64
44. Hegazy R, Adel L, Yasin R. The value of CESM in the evaluation of intraductal breast papilloma: a comparative study with DCE-MRI. Egypt J Radiol Nucl Med. 2020;51:1-13
45. Mann RM, Veldhuis WB. Contrast-enhanced mammography: moving ahead with perfusion imaging. Radiology. 2022;305:104-6
46. van Geel K, Kok EM, Krol JP. et al. Reversal of the hanging protocol of contrast enhanced mammography leads to similar diagnostic performance yet decreased reading times. Eur J Radiol. 2019;117:62-8
47. Lobbes MBI, Heuts EM, Moossdorff M. et al. Contrast enhanced mammography (CEM) versus magnetic resonance imaging (MRI) for staging of breast cancer: the pro CEM perspective. Eur J Radiol. 2021;142:109883
48. Barra FR, Sobrinho AB, Barra RR. et al. Contrast-enhanced mammography (CEM) for detecting residual disease after neoadjuvant chemotherapy: a comparison with breast magnetic resonance imaging (MRI). Biomed Res Int. 2018;2018:8531916
49. Iotti V, Ravaioli S, Vacondio R. et al. Contrast-enhanced spectral mammography in neoadjuvant chemotherapy monitoring: a comparison with breast magnetic resonance imaging. Breast Cancer Res. 2017;19:106
50. Patel BK, Hilal T, Covington M. et al. Contrast-enhanced spectral mammography is comparable to MRI in the assessment of residual breast cancer following neoadjuvant systemic sherapy. Ann Surg Oncol. 2018;25:1350-6
51. Youn I, Choi SH, Choi YJ. et al. Contrast enhanced digital mammography versus magnetic resonance imaging for accurate measurement of the size of breast cancer. Br J Radiol. 2019;92:20180929
52. Cheung Y-C, Juan Y-H, Lo Y-F. et al. Preoperative assessment of contrast-enhanced spectral mammography of diagnosed breast cancers after sonographic biopsy: correlation to contrast-enhanced magnetic resonance imaging and 5-year postoperative follow-up. Medicine (Baltimore). 2020;99:e19024
Author contact
![]() Corresponding author: E-mail: lidewij.neeternl.
Corresponding author: E-mail: lidewij.neeternl.

 Global reach, higher impact
Global reach, higher impact