Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(5):676-688. doi:10.7150/jca.80517 This issue Cite
Research Paper
Prognostic Model of Baseline Medications plus Neutrophil-to-lymphocyte Ratio in Patients with Advanced Non-small-cell Lung Cancer Receiving Immune Checkpoint Inhibitor plus Platinum Doublet: A Multicenter Retrospective Study
1. Division of Pharmaceutical Care Sciences, Keio University Graduate School of Pharmaceutical Sciences, 1-5-30 Shibakoen, Minato-ku, Tokyo 105-8512, Japan
2. Department of Pharmacy, Toranomon Hospital, 2-2-2 Toranomon, Minato-ku, Tokyo 105-8470, Japan
3. Department of Pharmacy, Nagoya City University Hospital, 1-Kawasumi, Mizuho-cho, Miskuho-ku, Nagoya, Aichi 467-8602, Japan
4. Department of Industrial Engineering and Economics, Tokyo Institute of Technology, 2-12-1 Ookayama, Meguro-ku, Tokyo 152-8552, Japan
5. Department of Pharmacy, National Hospital Organization Hokkaido Cancer Center, 4-2-3-54, Kikusui, Shiroishi-ku, Sapporo 003-0804, Japan
6. Department of Hospital Pharmaceutics, School of Pharmacy, Showa University, 1-5-8 Hatanodai, Shinagawa-ku, Tokyo 142-8666, Japan
7. Department of Pharmacy, Gifu University Hospital, 1-1 Yanagido, Gifu, Gifu 501-1194, Japan
8. Department of Pharmacy, Keio University Hospital, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan
9. Department of Respiratory Medicine, Allergy and Clinical Immunology, Nagoya City University Graduate School of Medical Sciences, 1-Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya, Aichi 467-8602, Japan
10. Department of Respiratory Medicine, National Hospital Organization Hokkaido Cancer Center, 4-2-3-54, Kikusui, Shiroishi-ku, Sapporo 003-0804, Japan
11. Respiratory Medicine and Allergology, Showa University School of Medicine, 1-5-8 Hatanodai, Shinagawa-ku, Tokyo 142-8666, Japan
12. Department of Cardiology and Respiratory Medicine, Gifu University Graduate School of Medicine, 1-1 Yanagido, Gifu, Gifu 501-1194, Japan
13. Division of Pulmonary Medicine, Department of Medicine, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan
14. Department of Respiratory Medicine, Toranomon Hospital, 2-2-2 Toranomon, Minato-ku, Tokyo 105-8470, Japan
15. Division of Drug Development and Regulatory Science, Keio University Faculty of Pharmacy, 1-5-30 Shibakoen, Minato-ku, Tokyo 105-8512, Japan
Received 2022-11-4; Accepted 2023-2-19; Published 2023-3-11
Abstract
Background: Association between baseline medications plus neutrophil-to-lymphocyte ratio (NLR) and the effectiveness of immune checkpoint inhibitor (ICI) plus platinum doublet remains unknown, despite several reported prognostic models. We used real-world data to investigate whether baseline medications plus NLR predict survival outcomes in patients with advanced non-small-cell lung cancer (NSCLC) receiving ICI plus platinum doublet.
Methods: This multicenter, retrospective, observational study conducted in Japan between December 2018 and March 2021 used real-world data of consecutive patients with advanced NSCLC who received ICI (pembrolizumab or atezolizumab) plus platinum doublet as first-line treatment. Progression-free survival (PFS) and overall survival (OS) were estimated using the Kaplan-Meier method. The prognostic score for baseline medications plus NLR was weighted by regression β coefficients and used to categorize patients into good, intermediate, and poor prognoses groups. In addition, time-dependent receiver operating characteristic curve analyses and univariable and multivariable Cox proportional hazards models were constructed.
Results: Overall, 241 patients were included. Poor prognosis was significantly associated with worse PFS (hazard ratio [HR]: 1.78; 95% confidence interval [CI]: 1.08-2.94; P = 0.025) and OS (HR: 3.59; 95% CI: 2.05-6.28; P < 0.001) than good prognosis. Harrell's C-index for this prognostic model was 0.648.
Conclusions: Baseline medication plus NLR could predict progressively worse survival outcomes in patients with advanced NSCLC receiving ICI plus platinum doublet and could be used as a prognostic index for poor outcomes.
Keywords: pembrolizumab, atezolizumab, immune checkpoint inhibitors, baseline medications, prognostic model, non-small-cell lung cancer
Introduction
Lung cancer is one of the most commonly diagnosed cancers and the leading cause of cancer-related deaths for both sexes [1]. Approximately 19.3 million new cancer cases and 10 million cancer-related deaths occurred in 2020. Lung cancer accounts for an estimated 1.8 million deaths (18%) among men and women combined. Furthermore, most patients initially diagnosed with non-small-cell lung cancer (NSCLC) are already at an advanced stage. Combination therapy comprising an immune checkpoint inhibitor (ICI) plus platinum doublet such as cisplatin and carboplatin has become the first-line treatment for advanced NSCLC [2-4]. The median overall survival (OS) is approximately 5 months longer with an ICI plus platinum doublet than with placebo combination therapy [2-4], which has remarkably changed the survival outcomes of these patients.
The effect of baseline medications on the effectiveness of ICI monotherapy is considered controversial. Baseline concomitant medications include corticosteroids, antibiotics, non-steroidal anti-inflammatory drugs (NSAIDs), and proton pump inhibitors (PPIs) [5-7]. However, the gut microbiome can affect ICI effectiveness. Responders and non-responders to ICI monotherapy for melanoma showed significant differences in the diversity and composition of the gut microbiome [8]. Patients with a high abundance of Faecalibacterium in their gut microbiome had a higher density of immune cells and markers of antigen processing and presentation than those with a high abundance of Bacteroidales, thereby suggesting a possible mechanism through which the gut microbiome modulates anti-tumor immune responses. Another study [9] showed that the gut microbiome might modulate responses to anti-programmed cell death 1 immunotherapy. Patients with a good gut microbiome, including those with a high diversity and abundance of Ruminococcaceae and Faecalibacterium, have enhanced systemic and anti-tumor immune responses mediated by increased antigen presentation and improved effector T cell function in the tumor periphery and microenvironment. Antibiotics, NSAIDs, and PPIs can alter the gut microbiota and reduce its diversity. Additionally, the use of antibiotics and PPIs is associated with decreased OS and progression-free survival (PFS) after ICI monotherapy [10].
Systemic inflammation is associated with the prognosis of solid tumors. The neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR) are biomarkers of the general immune response to various stress stimuli [11,12]. A high NLR is associated with poor OS after ICI monotherapy [11,12]. Moreover, some studies reported a direct correlation between NLR and intra-tumoral levels of granulocyte myeloid-derived suppressor cells, which are closely related to neutrophils [11,12].
Importantly, these studies evaluated baseline medications and NLR or PLR separately. Buti et al. [13] and Ogiwara et al. [14] studied NLR combined with baseline medications in patients receiving ICI monotherapy. Recently, Ogura et al. [15] reported an association between immunological and nutritional markers and the outcomes of a first-line ICI plus platinum doublet treatment; however, they did not consider baseline medication usage. To the best of our knowledge, no study has investigated the association between baseline medications and NLR, PLR, or LMR and survival outcomes associated with ICI plus platinum doublet as first-line treatment. Therefore, we hypothesized that baseline medications plus NLR, PLR, or LMR could predict survival outcomes after ICI plus platinum doublet treatment in clinical practice.
This study is novel because we focused on ICI plus platinum doublet as the first-line therapy for patients with advanced NSCLC. Furthermore, several prognostic models have been reported for ICI monotherapy [13] or combination therapy [16,17]. In this study, all enrolled patients had NSCLC and received ICI plus platinum doublet as first-line treatment. Moreover, we collected data of over 200 patients from six facilities across the country. Furthermore, we constructed a prognostic model that combined NLR and baseline medications in this study. The individual usefulness of NLR and baseline medications has been evaluated by Joshi et al. [17], however, there are no prognostic models that consider both NLR and baseline medications to date.
In this study, we used real-world data to investigate the prognostic model according to weighted scores in patients with advanced NSCLC treated with ICI plus platinum doublet as first-line treatment.
Materials and Methods
Study design
This study utilized a multicenter, retrospective, observational design. Patients' data were collected from the electronic medical records of six participating medical institutions: Toranomon Hospital, Nagoya City University Hospital, National Hospital Organization Hokkaido Cancer Center, Showa University Hospital, Gifu University Hospital, and Keio University Hospital in Japan. Data integration and analyses were performed at the Keio University Faculty of Pharmacy. This study adhered to the STROBE statement [18] and followed the methods used in previous studies [14,19,20].
The inclusion criteria were as follows: 1) consecutive patients aged ≥20 years with postoperative relapse or stage IV NSCLC and 2) patients who had received at least one course of combination therapy of ICI (pembrolizumab or atezolizumab) plus platinum doublet as first-line treatment between December 2018 and March 2021. The treatment schedule and follow-up were modified at the discretion of the clinician, according to the efficacy and/or toxicity profile of each patient.
The exclusion criteria were as follows: 1) incomplete medical records or lack of baseline laboratory data, 2) having active cancers other than advanced NSCLC, 3) comorbid autoimmune disease, 4) history of tuberculosis, 5) history of interstitial pneumonia, and 6) use of unapproved medicine in clinical trials. In addition, routine clinical follow-up of the enrolled patients was performed daily.
Data collection
Patient data were deidentified and analyzed anonymously. Data on patients' age; sex; chemotherapy regimen; Eastern Cooperative Oncology Group performance status (ECOG PS); counts of absolute neutrophils, lymphocytes, monocytes, and platelets at baseline; date of progression and/or death at the time of initiation of the immune-platinum doublet; and baseline medications (corticosteroids [dose ≥10 mg prednisolone equivalent per day and cancer-related use], antibiotics, fibrates, statins, metformin, PPIs, and NSAIDs) were collected. Baseline medication was defined as use within 30 days of oral or intravenous administration before initiating the immune-platinum regimen [21]. We calculated NLR, PLR, and LMR at baseline using routinely available blood cell counts. NLR was calculated as the absolute neutrophil count divided by the absolute lymphocyte count, PLR as the absolute platelet count divided by the absolute lymphocyte count, and LMR as the absolute lymphocyte count divided by the absolute monocyte count. The follow-up period ended on September 30, 2021.
Endpoints
PFS was defined as the period from the date of initiating the ICI plus platinum doublet treatment to the date of progression disease (PD), whereas OS was the period from the date of initiating the ICI plus platinum doublet treatment to the date of death from any cause. Patients without documented PD or who were still alive were defined as censored to PFS and OS, respectively, on the date of the last follow-up. Computed tomography (CT) evaluation is routinely assessed and is commonly conducted after about two months from the treatment initiation. At the maintenance period, CT evaluation is assessed every around three months.
Statistical analyses
The study endpoints were OS and PFS, which were estimated using the Kaplan-Meier method. The log-rank test was performed to compare the survival curves. Time-dependent receiver operating characteristic (ROC) curve analyses [22] and Youden's index were used to determine the optimal cutoff values for NLR associated with OS. In the ROC curve analyses, a higher area under the curve (AUC) indicated better predictive ability. We calculated prognostic scores using regression β coefficients via a univariable analysis, as reported in previous studies [7,13,14]. Univariable and multivariable Cox proportional hazards models were used to examine the associations between the groups based on prognostic scores and survival outcomes. In sensitivity analysis, potential explanatory variables regarding patients' background, including age (10-year intervals) and ECOG PS (2 vs. 0-1), were included in the multivariable model as covariates. The explanatory variables were included in multivariable analysis by the enter method. These explanatory variables were determined based on our clinical judgment. Additionally, we calculated another prognostic scores using regression β coefficients via a multivariable analysis.
Discrimination was assessed by concordance probability estimates using Harrell's C-index [23]. All statistical analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC, USA) and SPSS Statistics (version 25; IBM, Armonk, NY, USA). All P-values were two-sided, and statistical significance was set at a P-value of <0.05.
Ethics statement
The study protocol was approved by the Ethics Committee of Toranomon Hospital (approval number: 2225), Nagoya City University Hospital (approval number: 60-21-0074), National Hospital Organization Hokkaido Cancer Center (approval number: 03-15), Showa University Hospital (approval number: 3503), Gifu University Hospital (approval number: 2021-0188), Keio University Hospital (approval number: 20210049), and Keio University Faculty of Pharmacy (approval number: 220518-4). This study was conducted in accordance with the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research involving Human Subjects by the Ministry of Education, Culture, Sports, Science, and Technology, and the Ministry of Health, Labour and Welfare of Japan. Written informed consent for participation in this study was waived in accordance with the national legislation and institutional requirements.
Results
Patient characteristics
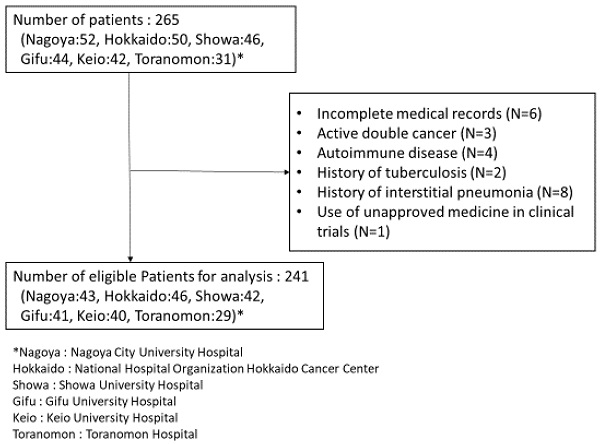
The patient enrollment flowchart is shown in Figure 1. Of the 265 patients initially screened, 24 were excluded from the analysis. Data of the remaining 241 patients were finally evaluated in this study. Patient characteristics are listed in Table 1. The median patient age was 68 years (interquartile range [IQR]: 61-72 years). Overall, 211 (87.6%) patients were in good condition, with an ECOG PS score of 0-1. In total, 195 (80.9%) and 46 (19.1%) patients received pembrolizumab and atezolizumab, respectively. The most frequently used ICI plus platinum doublet treatment was pembrolizumab + carboplatin + pemetrexed in 119 patients (49.4%), and the most frequently used baseline medications were PPIs and NSAIDs in 87 (36.1%) and 83 (34.4%) patients, respectively.
Endpoints
The median PFS and OS were 0.73 and 2.04 years, respectively. In the univariable analysis, the concomitant use of PPIs and NSAIDs resulted in significantly decreased OS (Table 2). In contrast, no significant association was observed between other concomitant medications and OS.
Flowchart of the patient enrollment process
Baseline patient characteristics
| Characteristic | Total (n = 241) |
|---|---|
| Age, years | |
| Median (IQR) | 68 (61-72) |
| Sex, n (%) | |
| Male | 177 (73.4) |
| Female | 64 (26.6) |
| ECOG PS, n (%) | |
| 0 | 98 (40.7) |
| 1 | 113 (46.9) |
| 2 | 20 (8.3) |
| Unknown | 10 (4.1) |
| ICI plus platinum doublet, n (%) | |
| Pembrolizumab + CBDCA + PEM | 119 (49.4) |
| Pembrolizumab + CBDCA + nab-PTX | 52 (21.6) |
| Pembrolizumab + CDDP + PEM | 18 (7.5) |
| Pembrolizumab + CBDCA + PTX ± bevacizumab | 6 (2.5) |
| Atezolizumab + CBDCA + PTX ± bevacizumab | 25 (10.4) |
| Atezolizumab + CBDCA + nab-PTX | 19 (7.9) |
| Atezolizumab + CBDCA + PEM | 1 (0.4) |
| Atezolizumab + CDDP + PEM | 1 (0.4) |
| Baseline concomitant medications, n (%) | |
| Corticosteroidsa | 10 (4.1) |
| Antibiotics | 59 (24.5) |
| PPIs | 87 (36.1) |
| NSAIDs | 83 (34.4) |
| Metformin | 14 (5.8) |
| Fibrates | 8 (3.3) |
| Statins | 46 (19.1) |
| Baseline peripheral blood counts (cells/mm3), median (IQR) | |
| Absolute neutrophil count | 4846 (3694-6699) |
| Absolute lymphocyte count | 1303 (889-1687) |
| Platelet count | 275000 (225500-351000) |
| Absolute monocyte count | 483 (338-656) |
| NLR, median (IQR) | 3.89 (2.70-5.87) |
| PLR, median (IQR) | 230.8 (158.4-329.2) |
| LMR, median (IQR) | 2.52 (1.78-3.78) |
a Dose ≥10 mg prednisolone equivalent per day and cancer-related use
Abbreviations: IQR: interquartile range; ECOG PS: Eastern Cooperative Oncology Group performance status; ICI: immune checkpoint inhibitor; CBDCA: carboplatin; PEM: pemetrexed; nab-PTX: albumin-binding paclitaxel; CDDP: cisplatin; PTX: paclitaxel; PPIs: proton pump inhibitors; NSAIDs: non-steroidal anti-inflammatory drugs; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; LMR: lymphocyte-to-monocyte ratio
Regression β coefficients from univariable and multivariable analyses for overall survival
| Univariable analysis | Multivariable analysis | ||||||
|---|---|---|---|---|---|---|---|
| Variables | Regression β coefficient | Crude HR (95% CI) | P-value | Regression β coefficient | Adjusted HR (95% CI) | P-value | |
| NLR ≥4.2 | 0.939 | 2.56 (1.68-3.90) | <0.001 | 0.865 | 2.38 (1.50-3.77) | <0.001 | |
| PPIs | 0.597 | 1.82 (1.21-2.73) | 0.004 | 0.318 | 1.38 (0.86-2.19) | 0.181 | |
| NSAIDs | 0.452 | 1.57 (1.04-2.37) | 0.032 | 0.125 | 1.13 (0.69-1.86) | 0.622 | |
| Corticosteroids | 0.211 | 1.23 (0.45-3.37) | 0.681 | 0.110 | 1.12 (0.34-3.68) | 0.856 | |
| Age (10-year interval) | 0.120 | 1.13 (0.90-1.41) | 0.293 | 0.069 | 1.01 (0.98-1.38) | 0.586 | |
| ECOG PS ≥2 | 1.926 | 6.86 (3.92-12.01) | <0.001 | 1.683 | 5.38 (2.95-9.82) | <0.001 | |
Ten patients with unknown ECOG PS were excluded from this analysis.
Abbreviations: HR: hazard ratio; CI: confidence interval; NLR: neutrophil-to-lymphocyte ratio; PPIs: proton pump inhibitors; NSAIDs: non-steroidal anti-inflammatory drugs; ECOG PS: Eastern Cooperative Oncology Group performance status.
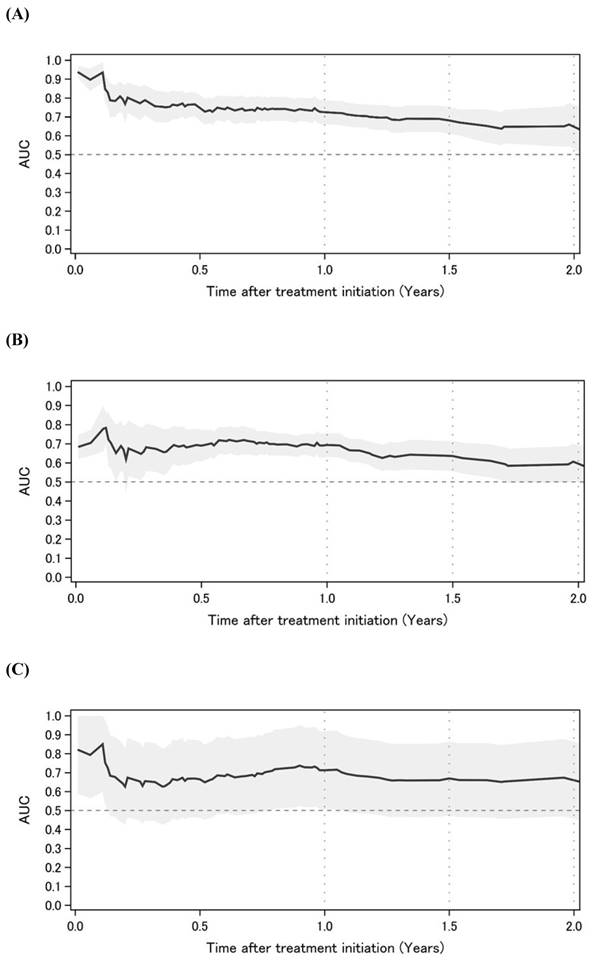
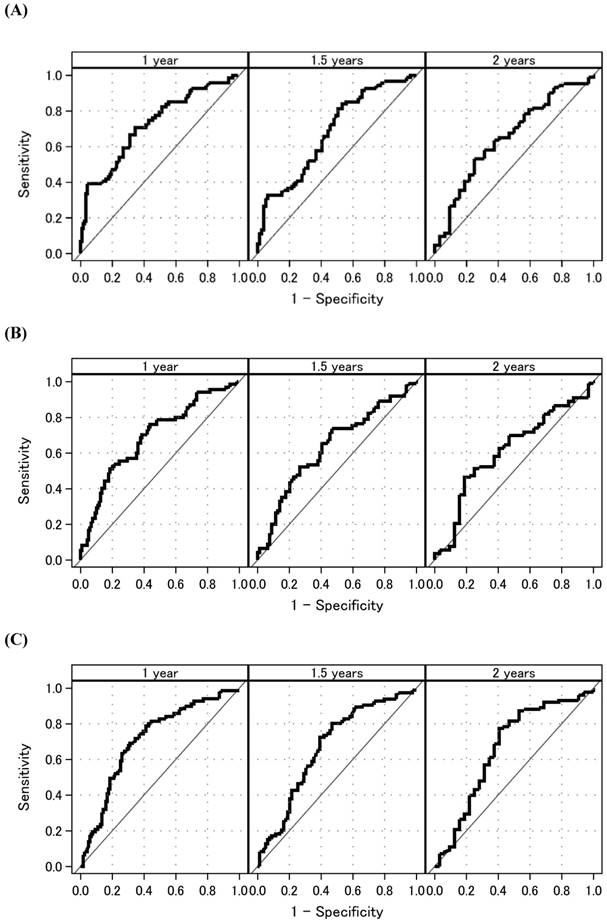
Figure 2 shows the time-dependent AUCs of NLR, PLR, and LMR. The time-dependent AUC of NLR was slightly higher than that of PLR and LMR. However, the time-dependent AUC of NLR remained higher than 0.6 over time. Subsequently, time-dependent ROC curve analyses to determine the optimal cutoff values for NLR, PLR, and LMR to predict OS are shown in Figure 3. At 1, 1.5, and 2 years after initial treatment, the optimal NLR cutoff values were 4.2, 3.2, and 4.9 (Figure 3A); the optimal PLR cutoff values were 296, 209, and 298 (Figure 3B); and the optimal LMR cutoff values were 2.4, 3.1, and 3.2 (Figure 3C), respectively. In particular, the AUC (95% confidence interval [CI]) for NLR was 0.725 (0.661-0.788), 0.681 (0.609-0.772), and 0.653 (0.539-0.767) at 1, 1.5, and 2 years after initial treatment, respectively (Figure 3A). However, the AUCs for PLR and LMR were lower than that for NLR (Figures 3B and 3C). Therefore, we selected NLR for subsequent analysis. Given the clinical importance and statistical analysis, we chose the NLR cutoff value of 4.2 at 1 year after initial treatment.
Time-dependent AUC of NLR, PLR, and LMR (A) NLR. (B) PLR. (C) LMR. Abbreviations: AUC: area under the curve; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; LMR: lymphocyte-to-monocyte ratio.
Time-dependent ROC curve analyses to determine the optimal cutoff values for NLR, PLR, and LMR to predict overall survival (A) NLR. (B) PLR. (C) LMR. Time-dependent ROC curves at 1, 1.5, and 2 years. Abbreviations: ROC: receiver operating characteristic; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; LMR: lymphocyte-to-monocyte ratio.
Prognostic factors and scoring
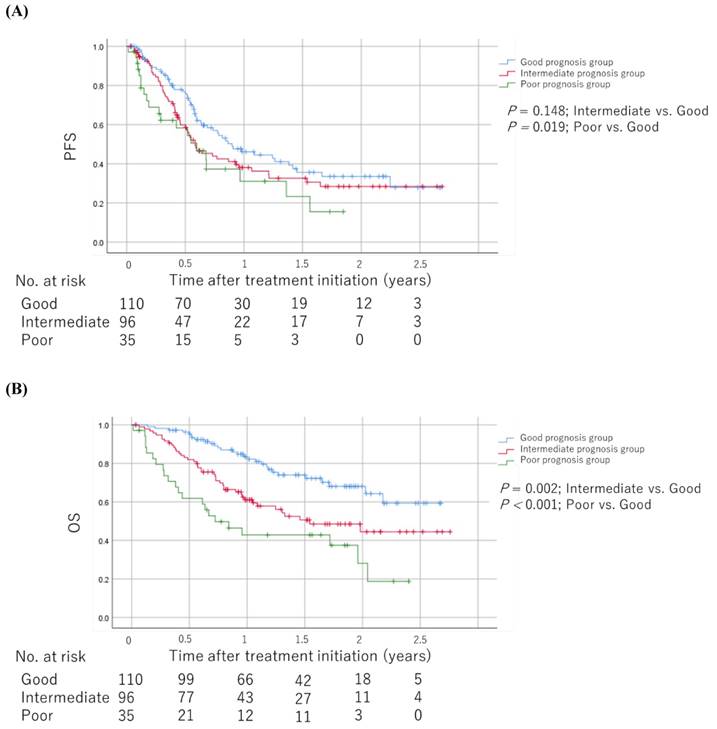
As shown in Table 2, we developed prognostic scores with baseline medications plus NLR based on regression β coefficients via a univariable analysis as follows: patients with an NLR ≥4.2 were assigned 2 points, whereas 1 point each was allotted for PPIs, NSAIDs, and corticosteroid use. We allotted 0 points each for an NLR <4.2 and no PPIs, NSAIDs, and corticosteroid use. Therefore, each patient was assigned a score ranging from 0 to 5. We categorized patients into the good (score 0-1), intermediate (score 2-3), and poor (score 4-5) prognosis groups based on their scores. The Kaplan-Meier survival curves for PFS and OS among the groups are shown in Figure 4. The poor prognosis group had a significantly worse PFS than the good prognosis group (log-rank test; P = 0.019). In addition, the poor and intermediate prognosis groups had significantly worse OS than the good prognosis group (log-rank test; P < 0.001 and P = 0.002, respectively). The univariable Cox proportional hazards model showed that the poor prognosis group had significantly worse PFS and OS than the good and intermediate prognosis groups (hazard ratio [HR]: 1.78; 95% CI: 1.08-2.94; P = 0.025 and HR: 3.59; 95% CI: 2.05-6.28; P < 0.001, respectively). Sensitivity analysis using the multivariable Cox proportional hazards model for OS revealed consistent results (Table 3). The HRs for OS were 3.03 (95% CI: 1.66-5.52, P < 0.001) and 2.30 (95% CI: 1.39-3.79, P = 0.001) in the poor and intermediate prognosis groups, respectively. Applying the computed score to this population, Harrell's C-index for OS was 0.643.
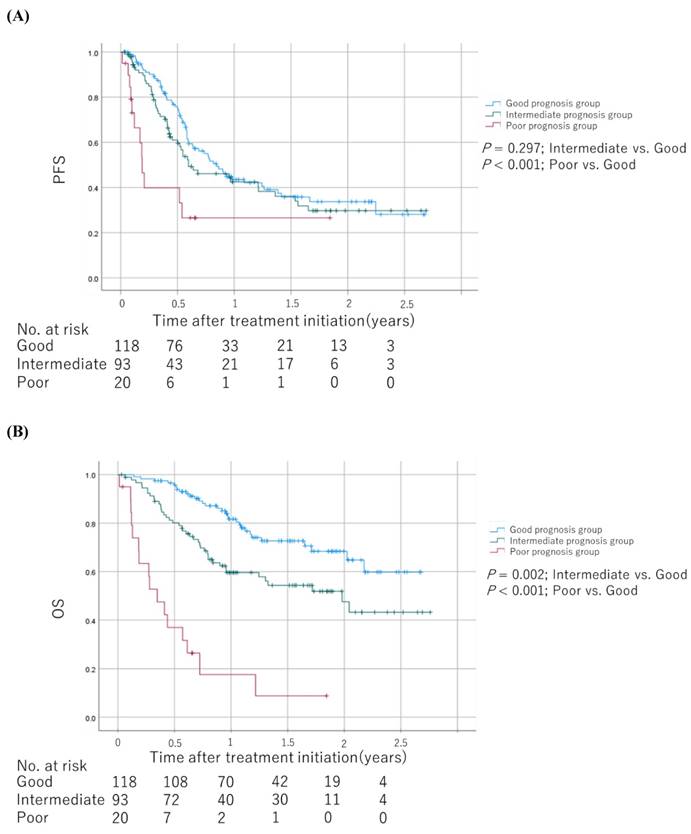
We calculated another prognostic scores based on regression β coefficients via a multivariable analysis as follows: patients with ECOG PS ≥2 and NLR ≥4.2 were assigned 2 points and 1 point, respectively (Table 2). Therefore, each patient was assigned a score ranging from 0 to 3. We categorized patients into the good (score 0), intermediate (score 1), and poor (score 2-3) prognosis groups based on their scores. The Kaplan-Meier survival curves for PFS and OS among the groups are shown in Figure 5. The poor and intermediate prognosis groups had a significantly worse OS than the good prognosis group (log-rank test; P < 0.001 and P = 0.002, respectively).
Kaplan-Meier survival curves for PFS and OS among the groups scored based on regression β coefficients via a univariable analysis (A) PFS. (B) OS. The log-rank test was used to compare survival curves. Abbreviations: PFS: progression-free survival; OS: overall survival.
Kaplan-Meier survival curves for PFS and OS among the groups scored based on regression β coefficients via a multivariable analysis (A) PFS. (B) OS. The log-rank test was used to compare survival curves. Abbreviations: PFS: progression-free survival; OS: overall survival.
Multivariable Cox proportional hazards model for overall survival
| Variables | No. | Event | Censored | Adjusted HR (95% CI) | P-value | |
|---|---|---|---|---|---|---|
| Group | Poor | 33 | 21 | 12 | 3.03 (1.66-5.52) | <0.001 |
| Intermediate | 92 | 40 | 52 | 2.30 (1.39-3.79) | 0.001 | |
| Good | 106 | 26 | 80 | 1 | ||
| Age (10-year interval) | - | - | - | 1.09 (0.85-1.39) | 0.489 | |
| ECOG PS | 2 | 20 | 16 | 4 | 6.01 (3.25-11.10) | <0.001 |
| 0-1 | 211 | 71 | 140 | 1 | ||
Ten patients with unknown ECOG PS were excluded from this analysis.
Abbreviations: HR: hazard ratio; CI: confidence interval; ECOG PS: Eastern Cooperative Oncology Group performance status.
Discussion
Our study findings suggest that baseline medications plus NLR can progressively worsen survival outcomes in patients with advanced NSCLC receiving ICI plus platinum doublet as first-line treatment in clinical practice. The poor and intermediate prognosis groups had lower OS than the good prognosis group. To the best of our knowledge, this is the first study to investigate baseline medications plus NLR as a prognostic factor in patients treated with the ICI plus platinum doublet for advanced NSCLC using real-world data. Additionally, weighted scoring combined with baseline medications and NLR can predict prognosis. Several prognostic models have also been reported for ICI monotherapy. The mechanism underlying the association between a higher NLR and survival outcomes remains unclear. However, ICIs interrupt immune suppression and activate CD8+ T-lymphocytes in the tumor microenvironment. Similarly, the mechanism underlying the association between baseline medications and survival outcomes remains unclear, except for corticosteroids. The diversity of the gut microbiota might influence the effectiveness of ICIs because of their association with immune status [24]. The present study showed an association between a higher NLR and survival outcomes and between baseline medications and poor survival outcomes. Integrating the time-dependent AUC of NLR showed a good prognostic factor that maintained a high predictive ability over time. The lymphocyte count is immutable and impossible to modify before ICI treatment. In contrast, baseline medications can be stopped or changed to a different medication. New agents with different mechanisms of action from ICIs or those that do not increase lymphocyte counts are needed for patients with poor prognosis. Furthermore, some agents may enhance the effects of ICIs by altering the gut microbiota [25]. Therefore, the prognosis can be improved by concomitant oral administration of this prebiotics with ICIs.
This study had a gap of over 1 year between OS and PFS. A previous study reported that ICIs have a carry-over effect [26], and there is sometimes a pseudo-progression before ICIs prove to be effective [27]. This may be because patients diagnosed with the progressive disease based on the pseudo-progression who stop ICIs still present a continuous effect of ICIs, leading to longer OS.
Regarding baseline medications, Buti et al. [14] reported significant differences in survival between the use of corticosteroids and antibiotics. Compared to NLR, the contribution of NSAIDs and PPIs are less; thus, we assigned 1 point for NSAIDs and PPIs and 2 points for NLR ≥4.2. In the cohort training study by Buti et al. [7], corticosteroids are strongly associated with the worsened outcome ICI treatment. In our study, 10 among 241 cases used corticosteroids, whereas Buti et al. reported 50 of 217 such cases. It is obvious clinically that corticosteroids are the first class of medication identified to be significantly related to worse clinical outcomes among patients treated with ICIs. We considered the detection power in our study to be low because of a small number of cases of corticosteroid use. However, whether corticosteroids should be used for palliative or non-palliative purposes for cancer-related conditions remains controversial [28-30]. While corticosteroids could reduce the efficacy of ICIs, a reduction in efficacy has not been seen [6]. In the present study, 10 (4.1%) patients were treated with concomitant corticosteroids, whereas in other studies, 93 (14.3%) of 650 patients received more than 10 mg of prednisone equivalents in both palliative and non-palliative settings. However, this study was conducted only in Japan, and antibiotic use might differ from that in other countries and in the extent of change in microbiota.
In this study, the settings of patients' backgrounds were different from those in previous studies because ICI plus platinum doublet for advanced NSCLC is a new regimen as first-line treatment. Buti et al. [13] reported that in 950 patients with advanced NSCLC who received pembrolizumab monotherapy as first-line treatment, a drug-based prognostic score of baseline medications showed a predictive ability for survival outcomes. Each concomitant drug was assigned a different score, and patients were categorized into three groups using regression β coefficients. Ogiwara et al. [14] reported that in 259 patients with advanced NSCLC who received nivolumab and pembrolizumab monotherapy as first- and later-line treatments, respectively; the prognostic score of baseline medications plus NLR had a higher predictive value than that reported by Buti et al. In the present study, Harrell's C-index was comparable to that reported by Buti et al. and Ogiwara et al. [13,14].
Buti et al. constructed a prognostic model based on regression β coefficients via a univariable analysis [13]. We also developed a prognostic model based on regression β coefficients via a univariable analysis and clinical decision. Additionally, we developed another prognostic model consisting of ECOG PS ≥2 and NLR ≥4.2 based on regression β coefficients via a multivariable analysis. The poor and intermediate prognosis groups had lower OS than the good prognosis group, as in the previous prognostic model.
The present study has several strengths. First, it was a multicenter study of six participating institutions in Japan, including cancer centers, university hospitals, and community hospitals. Therefore, our data may be generalizable to similar populations in the clinical setting. Second, we focused on the ICI plus platinum doublet treatment for patients with advanced NSCLC because of administering a new regimen as first-line treatment. Third, we performed time-dependent ROC curve and sensitivity analyses using the multivariable Cox proportional hazards model, which resulted in consistent results. These analyses increased the robustness of our results and are the novel features of our study.
This study has several limitations. First, this was a retrospective observational study. Therefore, information bias could not be excluded. There is also the possibility of missing clinical and drug history data. While we did not determine the tumor proportion score (TPS) using programmed death-ligand 1 expression data, it can be assumed that this did not affect the impact of baseline medications plus NLR on survival outcomes, and there was no need to determine the TPS when using ICI plus platinum doublet for advanced NSCLC. Additionally, oncogenic driver mutation data were not collected. When oncogenic driver mutations are present, tyrosine kinase inhibitors should be used as first-line treatment. We assumed that the histological type, such as squamous cell carcinoma and adenocarcinoma, is not a prognostic factor in patients receiving ICIs; therefore, data on the histologic type were not collected. However, besides the unmeasured confounders mentioned above, other baseline medications and second-line treatment were not adjusted in the multivariable analyses, which is a major limitation of our study because controlling for these could have affected the results. Second, the sample size was relatively smaller than that in previous studies that developed a drug-based prognostic score for the first time [7,14]. The present study only included a training cohort. Further validation cohorts are warranted to confirm the clinical application of this prognostic model. Third, we did not collect data on immune-related adverse events (irAEs), although the development of irAEs was positively correlated with survival outcomes in previous reports [31-33]. In contrast, Miura et al. [6] reported that baseline medications were not significantly associated with the onset of irAEs in 300 Japanese patients with advanced NSCLC treated with nivolumab or pembrolizumab monotherapy. In addition, the longer the patients survive, the higher the incidence of irAEs. Fourth, we did not consider the patients with no confirmed PD were treated as censors on the date of their last CT evaluation due to a clinical practice setting. There may be an overestimation of PFS. However, we believe that OS analyses were robust. Finally, this study mainly included a Japanese population. If the microbiota is associated with the effectiveness of ICI plus platinum doublet treatment, the outcome might change based on the country, the trend of medication use, food culture, and race, which are associated with microbiota diversity. Overall, the findings should be validated in a large-scale multicenter study with a large sample of patients treated with various baseline medications that affect the microbiota.
Conclusions
Baseline medications plus NLR at baseline could predict shorter survival in patients treated with ICI plus platinum doublet as first-line treatment for advanced NSCLC. In addition, the findings from this multicenter nationwide study conducted in Japan can be translated to a Japanese population.
Abbreviations
CBDCA: carboplatin; CDDP: cisplatin; CI: confidence interval; ECOG PS: Eastern Cooperative Oncology Group performance status; ICI: immune checkpoint inhibitor; irAE: immune-related adverse event; IQR: interquartile range; LMR: lymphocyte-to-monocyte ratio; nab-PTX: albumin-binding paclitaxel; NLR: neutrophil-to-lymphocyte ratio; NSAIDs: non-steroidal anti-inflammatory drugs; NSCLC: non-small-cell lung cancer; OS: overall survival; PD: progression disease; PEM: pemetrexed; PFS: progression-free survival; PLR: platelet-to-lymphocyte ratio; PPIs: proton pump inhibitors; PTX: paclitaxel; ROC: receiver operating characteristic.
Acknowledgements
We are grateful to all patients and medical staff involved in this study. We thank Prof. Kazunori Kimura, Dr. Takahiro Okada, and the Effectiveness of Platinum chemotherapy and Immune checkpoint inhibitors by Concomitant use (EPIC) investigators for their participation. We would like to thank Editage (www.editage.jp) for English language editing.
Funding
This research was partly supported by the JSPS KAKENHI (grant numbers 20H04147 to RU and 22K06776 to HK). The funders had no role in the study's design, collection, analysis, and interpretation of the data, writing of the manuscript, or the decision to submit the manuscript for publication.
Author Contributions
Conception/design: Hitoshi Kawazoe.
Collection and/or assembly of data: Izumi Nasu, Masahiro Kondo, Shinya Takada, Shuichi Nawata, Hirotoshi Iihara, Yohei Okumura, Masashi Takemoto, Kozo Mino, Tadanori Sasaki, Chiemi Hirose, Tohru Aomori, and Rena Shimano.
Data analysis and interpretation: Izumi Nasu, Ryuji Uozumi, and Azusa Hara.
Manuscript writing: Izumi Nasu and Hitoshi Kawazoe.
Final approval of the manuscript: Izumi Nasu, Masahiro Kondo, Ryuji Uozumi, Shinya Takada, Shuichi Nawata, Hirotoshi Iihara, Yohei Okumura, Masashi Takemoto, Kozo Mino, Tadanori Sasaki, Chiemi Hirose, Tohru Aomori, Rena Shimano, Ken Maeno, Satoshi Oizumi, Sojiro Kusumoto, Yasushi Ohno, Shinnosuke Ikemura, Daiya Takai, Azusa Hara, Hitoshi Kawazoe, and Tomonori Nakamura.
Competing Interests
Ryuji Uozumi received consulting fees from Eisai, Sawai Pharmaceutical, and EPS. Tadanori Sasaki received research grants from Nippon Kayaku, Daiichi Sankyo, Ono Pharmaceutical, and Mochida Pharmaceutical. Ken Maeno received research grants from Boehringer Ingelheim and Eli Lilly and payment for lectures from AstraZeneca, Chugai Pharmaceutical, and Ono Pharmaceutical. Satoshi Oizumi received payment for lectures from AstraZeneca, Bristol-Myers Squibb, Chugai Pharmaceutical, Ono Pharmaceutical, Takeda, Novartis, Eli Lilly, Merck, Merck Sharp & Dohme, Pfizer, Taiho Pharmaceutical, and Nippon Kayaku. Sojiro Kusumoto received payment for lectures from AstraZeneca, Taiho Pharmaceutical, Kracie Pharmaceutical, Chugai Pharmaceutical, and Novartis Pharma KK. Daiya Takai received payment for lectures from Bristol-Myers Squibb, MSD, and Ono Pharmaceutical. Hitoshi Kawazoe received research funding from Eli Lilly. Tomonori Nakamura received research funding from Astellas Pharma, Chugai, Daiichi Sankyo, Kyowa Kirin, Otsuka Pharmaceutical, Sanofi, Sato Pharmaceutical, and Shionogi. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Sung H, Ferlay J, Siegel RL. et al. Global cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-49 doi:10.3322/caac.21660
2. Gandhi L, Rodríguez-Abreu D, Gadgeel S. et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078-92 doi:10.1056/NEJMoa1801005
3. Socinski MA, Jotte RM, Cappuzzo F. et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288-301 doi:10.1056/NEJMoa1716948
4. Paz-Ares L, Luft A, Vicente D. et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040-51 doi:10.1056/NEJMoa1810865
5. Chalabi M, Cardona A, Nagarkar DR. et al. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: pooled post hoc analyses of the OAK and Poplar trials. Ann Oncol. 2020;31:525-31 doi:10.1016/j.annonc.2020.01.006
6. Miura K, Sano Y, Niho S. et al. Impact of concomitant medication on clinical outcomes in patients with advanced non-small cell lung cancer treated with immune checkpoint inhibitors: a retrospective study. Thorac Cancer. 2021;12:1983-94 doi:10.1111/1759-7714.14001
7. Buti S, Bersanelli M, Perrone F. et al. Effect of concomitant medications with immune-modulatory properties on the outcomes of patients with advanced cancer treated with immune checkpoint inhibitors: development and validation of a novel prognostic index. Eur J Cancer. 2021;142:18-28 doi:10.1016/j.ejca.2020.09.033
8. Gopalakrishnan V, Spencer CN, Nezi L. et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:397-403 doi:10.1126/science.aan4236
9. Sivan A, Corrales L, Hubert N. et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084-9 doi:10.1126/science.aac4255
10. Giordan Q, Salleron J, Vallance C. et al. Impact of antibiotics and proton pump inhibitors on efficacy and tolerance of anti-PD-1 immune checkpoint inhibitors. Front Immunol. 2021;12:716317. doi:10.3389/fimmu.2021.716317
11. Zhang N, Jiang J, Tang S. et al. Predictive value of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in non-small cell lung cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Int Immunopharmacol. 2020;85:106677. doi:10.1016/j.intimp.2020.106677
12. Sacdalan DB, Lucero JA, Sacdalan DL. Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: a review and meta-analysis. Onco Targets Ther. 2018;11:955-65 doi:10.2147/OTT.S153290
13. Buti S, Bersanelli M, Perrone F. et al. Predictive ability of a drug-based score in patients with advanced non-small-cell lung cancer receiving first-line immunotherapy. Eur J Cancer. 2021;150:224-31 doi:10.1016/j.ejca.2021.03.041
14. Ogiwara T, Kawazoe H, Egami S. et al. Prognostic value of baseline medications plus neutrophil-to-lymphocyte ratio in the effectiveness of nivolumab and pembrolizumab in patients with advanced non-small-cell lung cancer: a retrospective study. Front Oncol. 2021;11:770268. doi:10.3389/fonc.2021.770268
15. Ogura Y, Kataoka N, Kunimatsu Y. et al. Predictors of survival among Japanese patients receiving first-line chemoimmunotherapy for advanced non-small cell lung cancer. Thorac Cancer. 2021;12:97-105 doi:10.1111/1759-7714.13720
16. Chen S, Li R, Zhang Z. et al. Prognostic value of baseline and change in neutrophil-to-lymphocyte ratio for survival in advanced non-small cell lung cancer patients with poor performance status receiving PD-1 inhibitors. Lung Cancer Res. 2021;10:1397-407 doi:10.21037/tlcr-21-43
17. Joshi I, Peravali M, Geng X. et al. Impact of baseline clinical biomarkers on treatment outcomes in patients with advanced NSCLC receiving first-line pembrolizumab-based therapy. Clin Lung Cancer. 2022;23:438-45 doi:10.1016/j.cllc.2022.03.010
18. von Elm E, Altman DG, Egger M. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495-9 doi:10.1016/j.ijsu.2014.07.013
19. Egami S, Kawazoe H, Hashimoto H. et al. Absolute lymphocyte count predicts immune-related adverse events in patients with non-small-cell lung cancer treated with nivolumab monotherapy: a multicenter retrospective study. Front Oncol. 2021;11:618570. doi:10.3389/fonc.2021.618570
20. Egami S, Kawazoe H, Hashimoto H. et al. Peripheral blood biomarkers predict immune-related adverse events in non-small cell lung cancer patients treated with pembrolizumab: a multicenter retrospective study. J Cancer. 2021;12:2105-12 doi:10.7150/jca.53242
21. Cortellini A, Di Maio M, Nigro O. et al. Differential influence of antibiotic therapy and other medications on oncological outcomes of patients with non-small cell lung cancer treated with first-line pembrolizumab versus cytotoxic chemotherapy. J Immunother Cancer. 2021;9:e002421. doi:10.1136/jitc-2021-002421
22. Uno H, Cai T, Tian L. et al. Evaluating prediction rules for t-year survivors with censored regression models. J Am Stat Assoc. 2007;102:527-37 doi:10.1198/016214507000000149
23. Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361-87 doi:10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4
24. Routy B, Le Chatelier E, Derosa L. et al. Gut microbiome influences efficacy of PD-1-Based immunotherapy against epithelial tumors. Science. 2018;359:91-7 doi:10.1126/science.aan3706
25. Tanoue T, Morita S, Plichta DR. et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature. 2019;565:600-5 doi:10.1038/s41586-019-0878-z
26. Osa A, Uenami T, Koyama S, Fujimoto K. et al. Clinical implications of monitoring nivolumab immunokinetics in non-small cell lung cancer patients. JCI Insight. 2018;3:e59125. doi:10.1172/jci.insight.59125
27. Park HJ, Kim KW, Pyo J. et al. Incidence of pseudoprogression during immune checkpoint inhibitor therapy for solid tumors: a systematic review and meta-analysis. Radiology. 2020;297:87-96 doi:10.1148/radiol.2020200443
28. De Giglio A, Mezquita L, Auclin E. et al. Impact of intercurrent introduction of steroids on clinical outcomes in advanced non-small-cell lung cancer (NSCLC) patients under immune-checkpoint inhibitors (ICI). Cancers (Basel). 2020;12:2827. doi:10.3390/cancers12102827
29. Ricciuti B, Dahlberg SE, Adeni A. et al. Immune checkpoint inhibitor outcomes for patients with non-small-cell lung cancer receiving baseline corticosteroids for palliative versus nonpalliative indications. J Clin Oncol. 2019;37:1927-34 doi:10.1200/JCO.19.00189
30. Arbour KC, Mezquita L, Long N. et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018;36:2872-8 doi:10.1200/JCO.2018.79.0006
31. Haratani K, Hayashi H, Chiba Y. et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4:374-8 doi:10.1001/jamaoncol.2017.2925
32. Naqash AR, Ricciuti B, Owen DH. et al. Outcomes associated with immune-related adverse events in metastatic non-small cell lung cancer treated with nivolumab: a pooled exploratory analysis from a global cohort. Cancer Immunol Immunother. 2020;69:1177-87 doi:10.1007/s00262-020-02536-5
33. Maillet D, Corbaux P, Stelmes JJ. et al. Association between immune-related adverse events and long-term survival outcomes in patients treated with immune checkpoint inhibitors. Eur J Cancer. 2020;132:61-70 doi:10.1016/j.ejca.2020.03.017
Author contact
![]() Corresponding author: Hitoshi Kawazoe, Ph.D. Division of Pharmaceutical Care Sciences, Keio University Graduate School of Pharmaceutical Sciences, 1-5-30 Shibakoen, Minato-ku, Tokyo 105-8512, Japan Tel: +81-3-5400-2639 Fax: +81-3-5400-2651 E-mail: kawazoe-htjp
Corresponding author: Hitoshi Kawazoe, Ph.D. Division of Pharmaceutical Care Sciences, Keio University Graduate School of Pharmaceutical Sciences, 1-5-30 Shibakoen, Minato-ku, Tokyo 105-8512, Japan Tel: +81-3-5400-2639 Fax: +81-3-5400-2651 E-mail: kawazoe-htjp

 Global reach, higher impact
Global reach, higher impact