3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(5):759-769. doi:10.7150/jca.82410 This issue Cite
Research Paper
Nanoformulation of Paclitaxel: Exploring the Cyclodextrin / PLGA Nano Delivery Carrier to Slow Down Paclitaxel Release, Enhance Accumulation in Vivo
1. Guangdong Pharmaceutical University, Guangzhou, People's Republic of China
2. The Sixth Affiliated Hospital, School of Medicine, South China University of Technology, Guangzhou, People's Republic of China
3. The Innovation Team for Integrating Pharmacy with Entrepreneurship, Guangdong Pharmaceutical University, Guangzhou, People's Republic of China
4. Guangdong Provincial Key Laboratory of Advanced Drug Delivery Systems, Guangdong Pharmaceutical University, Guangzhou, People's Republic of China
5. Guangdong Provincial Engineering Center of Topical Precision Drug Delivery System, Center for Drug Research and Development, Guangdong Pharmaceutical University, Guangzhou, People's Republic of China
Abstract
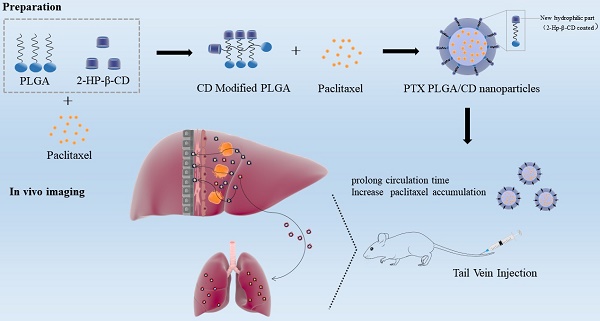
Background: Improving the aggregation and penetration in tumor sites increases the anti-tumor efficacy of nanomedicine. In the current study, we designed cyclodextrin modified PLGA nanoparticles loaded with paclitaxel to elevate the accumulation and prolong circulation of chemotherapy drugs in vivo.
Methods: The PLGA nanoparticles loaded with paclitaxel (PTX PLGA NPs) and cyclodextrin (CD) modified PLGA nanoparticles loaded with paclitaxel (PTX PLGA/CD NPs) were prepared using the emulsification solvent evaporation method. The nanoparticles were characterized by particle size, zeta potential, encapsulation efficiency, infrared spectroscopy analysis and X-Ray diffraction (XRD). Then, drug release of the nanoparticles was evaluated via reverse dialysis method in vitro. Finally, the in vivo distribution fate and pharmacokinetic characteristics of the nanoparticles were assessed in mice and rats.
Results: The average particle size, zeta potential, and encapsulation efficiency of PTX PLGA NPs were (163.57±2.07) nm, - (20.53±2.79) mV and (60.44±6.80)%. The average particle size, zeta potential, and encapsulation efficiency of PTX PLGA/CD NPs were (148.57±1.66) nm, - (11.42±0.84) mV and (85.70±2.06)%. In vitro release studies showed that PTX PLGA/CD NPs were released more slowly compared to PTX PLGA NPs under normal blood pH conditions, while PTX PLGA/CD NPs were released more completely under tumor site pH conditions. The modified PLGA nanocarrier (PLGA/CD NPs) increased drug residence time and accumulation than the plain PLGA nanocarrier (PLGA NPs) in vivo distribution. In addition, the elimination half-life, area under the drug-time curve, and maximum blood concentration of the nanoparticle group were higher than those of Taxol®, especially the PTX PLGA/CD NPs group, which was significantly different from Taxol® and plain nanoparticle groups (p<0.001).
Conclusions: The 2-HP-β-CD modified PLGA nanoparticles prolonged circulation time and accumulation of the chemotherapy drug paclitaxel in vivo.
Keywords: PLGA nanoparticles, 2-HP-β-CD, in vivo imaging, pharmacokinetics.

 Global reach, higher impact
Global reach, higher impact