Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(5):874-879. doi:10.7150/jca.67189 This issue Cite
Research Paper
Survival of NSCLC Patients Treated with Cimavax-EGF as Switch Maintenance in the Real-World Scenario
1. Department of Medical Oncology. National Institute of Oncology and Radiobiology. 29th Street, corner E, Vedado Plaza de la Revolución, Havana Cuba
2. Department of Radiotherapy. National Institute of Oncology and Radiobiology. 29th Street, corner E, Vedado Plaza de la Revolución, Havana Cuba
3. Department of Pneumology. National Institute of Oncology and Radiobiology. 29th Street, corner E, Vedado Plaza de la Revolución, Havana Cuba.
4. Department of Pathology, National Institute of Oncology and Radiobiology. 29th Street, corner E, Vedado Plaza de la Revolución, Havana Cuba.
5. Department of Imaging, National Institute of Oncology and Radiobiology. 29th Street, corner E, Vedado Plaza de la Revolución, Havana Cuba.
6. Department of Clinical Trials, National Institute of Oncology and Radiobiology. 29th Street, corner E, Vedado Plaza de la Revolución, Havana Cuba.
7. Clinical Direction. Center of Molecular Immunology, Ave 216, Esq 15. Atabey. Playa
Received 2021-9-1; Accepted 2021-12-5; Published 2023-4-1
Abstract
Introduction: In Cuba, lung cancer represents the first cause of mortality for both sexes. Non-small cell lung cancer (NSCLC) is the most prevalent histology. Overall, 75-85% of NSCLC overexpress EGFR and its ligands. EGFR overexpression has been implicated in the malignant transformation by promoting cell proliferation and survival. CIMAvax-EGF is a therapeutic vaccine composed of recombinant-human EGF conjugated to a carrier protein and Montanide as an adjuvant. CIMAvax-EGF is intended to induce antibodies against self-EGF that block the EGF-EGFR interaction.
Objectives: To characterize the efficacy and safety of CIMAvax-EGF as maintenance in NSCLC patients treated in the real-world setting.
Results: 106 patients diagnosed with advanced NSCLC at the National Institute of Oncology and Radiobiology, who had at least stable disease after first-line therapy, were enrolled in the study. The initial four CIMAvax-EGF doses were administered every 2 weeks and then, patients received monthly re-immunizations. Globally, 52.8% of the patients were 65 years or older, 77.4% had an ECOG 1 and 62.3% had an adenocarcinoma. The median survival time (MST) was 14.6 months. Patients younger than 65 years had a MST of 16.7 months and subjects with ECOG 0 survived for 29 months. The median progression-free survival was 8.16 months. Overall, 36.8% and 19.8% of patients maintained disease control at 6 and 12 months, respectively. The most frequent adverse events were pain (27.3%) or induration (7.3%) at the injection site and local erythema (10.9%).
Conclusion: CIMAvax-EGF, as an EGF depleting immunotherapy used as switch-maintenance was safe and effective in patients with NSCLC.
Keywords: CIMAvax-EGF, NSCLC, EGFR, vaccine, immunotherapy
Introduction
Lung cancer continues to be a global health problem. In 2020, 2,206,771 (11.4%) new patients with lung cancer were diagnosed. In addition, 1,796,144 (18.4%) deaths were reported from this pathology. Only 18% of patients survive more than five years after diagnosis [1].
In Cuba, lung cancer represents the first cause death for cancer in both sexes. According the 2019 statistical yearbook, there were 5026 deaths from this disease, which represents an adjusted crude rate to the world population (ACR) of 50 x 100 000 inhabitants [2].
Regarding incidence, lung cancer represents the third cause, preceded by skin cancer for both sexes and prostate cancer for men and breast cancer for women. In 2016, 3735 new male cases were diagnosed, representing an ACR of 68.5 x 100 000 inhabitants. In the case of females, 2176 new cases were reported, for an ACR of 20.7 x 100 000 inhabitants [2].
Using maintenance therapies after platinum-based chemotherapy as first line, increased the survival of those patients showing stable disease or better [3] [4]. Pemetrexed and bevacizumab, as well as tyrosine kinase inhibitors have shown clinical benefit in these patients [5-7].
Approximately 75-85% of non-small cell lung cancer (NSCLC) patients overexpress the epidermal growth factor receptor (EGFR) and its ligands. This overexpression is involved in the malignant transformation process, by promoting cell proliferation and survival. EGFR is a well-validated therapeutic target in patients with NSCLC [8, 9].
Immunotherapy has played an important role in the treatment of lung cancer. The main benefit of specific active immunotherapy is the ability to direct the immune response to the individual's own tumor. The induction of EGF deprivation by active immunotherapy is an emerging concept developed by Cuban researchers, which includes the manipulation of the individual's immune response to generate neutralizing antibodies (Abs) against self-EGF that reduce the size of the tumor or prevent its progression [8, 9].
CIMAvax-EGF consists of a chemical conjugate between EGF and P64, a recombinant protein from Neisseria meningitides and an adjuvant (Montanide ISA51, VG). The vaccine induces antibodies against EGF, capable of blocking the EGF-EGFR interaction [10, 11].
From 1995, our group is participating in the clinical trials to assess the efficacy and safety of the drug [12-14]. In 2008, the State Center for the Drug Quality Control and medical devices (CECMED), the Cuban regulatory agency, approved a conditional registration. In 2014, the definitive approval as maintenance treatment of NSCLC was granted by CECMED. In 2015, CIMAvax-EGF commercialization begins. The main objective of this study was to evaluate the efficacy and safety of the CIMAvax-EGF in patients with NSCLC in the real-world scenario.
Patients and methods
An observational, descriptive, longitudinal study was conducted in patients with NSCLC in stages IIIB and IV at the National Institute of Oncology and Radiobiology, from January 2015 to December 2017. Patients older than 18 years diagnosed with NSCLC, in clinical stage IIIB and IV, who have had an objective response or at least stabilization of the disease to first-line treatment were enrolled. Patients with brain metastases or progressive disease at the end of first-line treatment were excluded.
A hundred and six (106) patients were included. They were treated with CIMAvax-EGF after showing an objective response or disease stabilization to first line therapy including platinum doublets, chemotherapy and radiotherapy or single agent chemotherapy.
The study was approved by the scientific council, as well as by the ethics committee of the institution. All included patients signed the informed consent for the investigation. The study was conducted according to the Helsinki ethical principles for medical research involving human subjects. The study was funded by the Cuban Ministry of Health.
Patients received CIMAvax-EGF after the administration of cyclophosphamide at a dose of 200 mg/m2 on day 0. Three days after, they started CIMAvax-EGF administration every 14 days for 4 doses and subsequently, every 28 days until disease progression or unacceptable toxicity.
The response to treatment was evaluated according to the Response Evaluation Criteria in Solid Tumors (version 1.1) (RECIST v1.1), at 6 months and 1 year after treatment.
Statistical analysis
Categorical variables were displayed using descriptive summary statistics including number of patients and percentages while metric variables were presented using arithmetic mean ± standard deviation. Overall survival and progression-free survival were estimated by the Kaplan-Meier function and contrasted with the Log-Rank test. The mean and median survival times and confidence intervals (95%) were also determined.
Overall survival was defined as the time elapsed from the vaccine starting day until death or the date of the last news. In addition, overall survival from first line therapy was estimated.
Progression-free survival was defined as the time interval between CIMAvax-EGF first dose to the disease progression.
Results
Overall, 106 patients were included in the study. The median follow-up time was 14.2 months, 95% CI (11-15.8 months). Table 1 shows patients' characteristics as well as first line treatment, in addition to the response to the referred front line.
Characteristics of the patients treated with CIMAvax-EGF. Most patients had ECOG-1, adenocarcinoma histology and partial or stable disease after completing front line therapy.
| Patient Characteristics | No (106) | % | |
|---|---|---|---|
| Age | 65 years | 50 | 47.2 |
| 65 years and more | 56 | 52.8 | |
| Gender | Male | 62 | 58.5 |
| Female | 44 | 48.5 | |
| ECOG | 0 | 22 | 20.8 |
| 1 | 82 | 77.4 | |
| 2 | 2 | 1.9 | |
| Histology | Adenocarcinoma | 66 | 62.3 |
| Squamous cell | 28 | 26.4 | |
| Large cell carcinoma | 12 | 11.3 | |
| Stage | IIIB | 63 | 59.4 |
| IV | 43 | 40.6 | |
| First line therapy | Chemotherapy Radiotherapy | 63 | 59.4 |
| Chemotherapy | 43 | 40.6 | |
| Chemotherapy | Cisplatin/etoposide | 83 | 78.3 |
| Carboplatin/paclitaxel | 13 | 12.2 | |
| Carboplatin / gemcitabine | 10 | 9.4 | |
| Response to first-line treatment | Complete response | 8 | 7.5 |
| Partial response | 60 | 56.6 | |
| Stable disease | 38 | 35.8 | |
The predominant groups were patients with 65 years or older (52.8%), male-sex (58.5%), ECOG-1 (77.4%), adenocarcinoma histology (62.3%) and stage IIIB NSCLC (58.4 %). The combination of chemotherapy and radiotherapy was the most commonly used first line treatment (58.4 %). The most prevalent treatment modality was platinum plus etoposide (78.5%). Overall, 56.6% had a partial response to first-line treatment.
Median overall survival was estimated from the first line therapy and from the CIMAvax-EGF starting day. The median overall survival from the chemotherapy or chemo-radiotherapy initiation was 22.46 months, 95% CI (19.92-25.0) (graphic not shown) and the survival rates at 6, 12, and 24 months were 97.7 %, 82.7 % and 45.5 %, respectively.
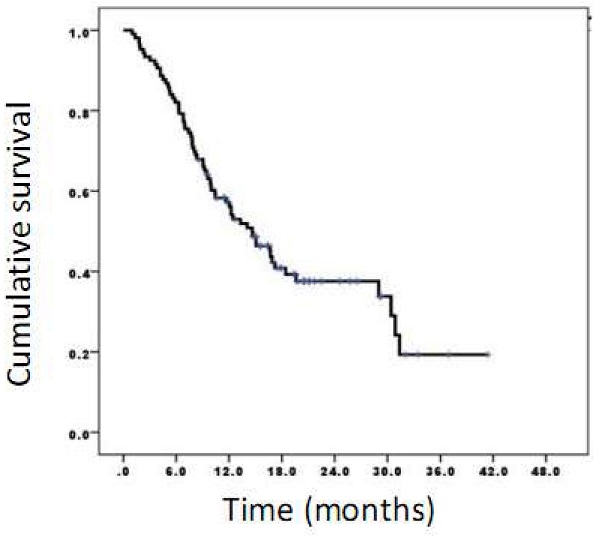
The time lag between chemotherapy completion and CIMAvax-EGF ranged from 8 to 12 weeks. In addition, survival time from the first dose of CIMAvax-EGF was estimated. The median overall survival was 14.6 months, 95% CI (10.6-18.8) (Figure 1).
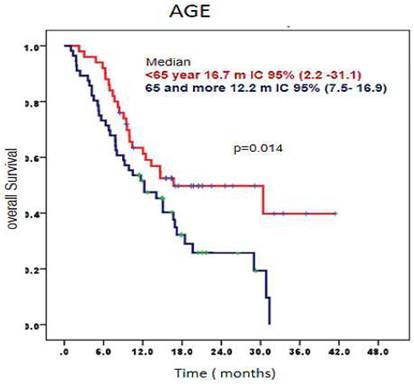
When analyzing overall survival according age, patients younger than 65 years had a median overall survival of 16.7 months, 95% CI (2.2-31.1) compared to those older than 65 years who had a median of overall survival 12.2 months, 95% CI (7.5-16.9) (p= 0.014) (Figure 2).
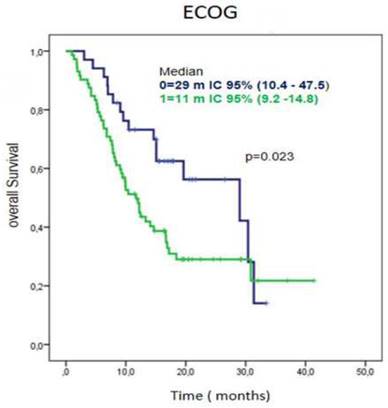
Regarding performance status, patients with ECOG 0 had a median overall survival of 29 months, 95% CI (10.4-47.5) while those with an ECOG-1 at diagnose, had a median overall survival of 11 months (95% CI 9.2-14.8) (Figure 3).
Survival time of patients vaccinated with CIMAvax-EGF. The median overall survival was 14.6 months while survival rates at 6, 12, and 24 months were 82.1%, 57.2%, and 37.6%, respectively.
Survival time according age of patients treated with CIMAvax-EGF. Patients younger than 65 had a significantly larger overall survival.
Survival time according ECOG in patients treated with CIMAvax-EGF. Patients with ECOG 0 had a significantly larger overall survival.
Progression-free survival of patients treated with CIMAvax-EGF. Progression-free survival rates at 6, 12, and 24 months were 55.4%, 36.4%, and 19.1%, respectively.
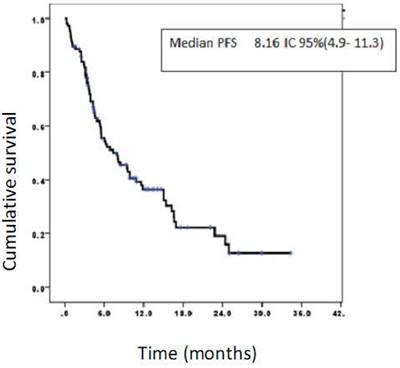
The median progression-free survival was 8.16 months, 95% CI (4.9-11.3) (Figure 4).
Globally, 36.8% and 19.8% of patients had objective response or disease stabilization at 6 and 12 months of treatment with CIMAvax-EGF, respectively (Table 2).
Evaluation of the response at 6 and 12 months in patients treated with CIMAvax-EGF. Overall, 36.5 % and 19.8 % of all patients, maintained disease control after 6 or 12 months of vaccination, respectively.
| 6 months | 12 months | |||
|---|---|---|---|---|
| Response | No | % | No | % |
| Complete response | 3 | 2.8 | 3 | 2.8 |
| Partial Response | 10 | 9.4 | 4 | 3.7 |
| Stable Disease | 26 | 24.5 | 14 | 13.2 |
| Progressive Disease | 67 | 63.2 | 85 | 80.1 |
| Global | 106 | 100 | 106 | 100 |
CIMAvax-EGF related adverse events. Most prevalent adverse events were injection site reactions including pain, erythema and induration.
| Adverse Event | Grade 1 | Grade 2 | ||
|---|---|---|---|---|
| No | % | No | % | |
| Pain in the site of injection | 33 | 27.3 | - | - |
| Local erythema | 12 | 10.9 | - | - |
| Nausea | 5 | 4.5 | - | - |
| Induration at the injection site | 8 | 7.3 | - | - |
| Dizziness | 5 | 4.5 | - | - |
| Vomiting | 1 | 0.9 | 2 | 1.8 |
| Fever | 2 | 1.8 | - | - |
| Others | 6 | 5.5 | - | - |
| All | 72 | 67.9 | 2 | 1.8 |
Treatment was safe as no serious adverse events were reported. The most frequent adverse events were grade 1 injection site pain (27.2 %) and local erythema (10.9 %). The most frequent grade 2 adverse event was vomiting (1.8 %) (Table 3).
Discussion
The objective of this study was to characterize the safety and efficacy of an EGF depleting immunotherapy (CIMAvax-EGF) as maintenance therapy in the real-world setting. When considering the most relevant demographic and tumor variables, patients were similar to the ones included in the phase III trial, except for the histology. In our scenario, the predominant histology was adenocarcinoma, while Rodríguez et al. recruited mostly patients with squamous cell carcinomas [14]. Other CIMAvax-EGF trials conducted by Ramos et al. [13] and Saavedra et al. [17] enrolled mostly adenocarcinomas, like our case.
Median overall survival (14.6 months) was larger than the one reported by Rodriguez and cols, in the multicentric phase III trial (10.8 months) [14]. Patients younger than 65 had better survival. It is well validated that older subjects had a decreased ability to respond to vaccination and to fight infections. These patient population also have a constitutive low-grade inflammation [15]. Elderly patients are more prone to an uncontrolled activation of innate immune response that leads to cytokine release syndrome and tissue damage [16].
In the phase II study, vaccinated patients with a good antibody response had a median overall survival of 11.7 months, significantly higher than the control group treated with best supportive care (5.33 months) [12]. In the referred study, the 6- and 12-months survival rates were 82.1% and 57.2% [12].
Other maintenance therapy studies including pemetrexed and bevacizumab reported similar median survival times. Median survival time (MST) after pemetrexed was 15 months [18-20] while after bevacizumab, MST was 14.4 months [20] [21]. In the KEYNOTE 024 clinical trial that evaluated the impact of using pembrolizumab in patients with PD-L1 expression larger than 50 %, the median overall survival was 30 months [22]. KEYNOTE 024 is not strictly comparable with our study, when considering the selection bias.
In our study, the median progression-free survival (PFS) was 8.16 months, higher than the PFS reported for pemetrexed (4-5 months), bevacizumab or gemcitabine [18-21], [6, 23]. For bevacizumab as maintenance therapy, the median PFS was 4 months [6]. Brodowicz et al. reported that gemcitabine used as continuation maintenance, had a PFS equivalent to 3.8 months [23]. However, in the KEYNOTE 024 study, patients with high PD-L1 expression treated with pembrolizumab had a PFS of 10.3 months [22]. In a different study including patients with PD-L1 of at least 1 % (KEYNOTE 189) the median PFS after using pembrolizumab as maintenance therapy was 8.8 months, similar to the patients treated with CIMAvax-EGF [5].
CIMAvax-EGF showed a disease control rate at 6 and 12 months of 36.8% and 19.8%. Ramalingam et al. found that the response rates after pemetrexed at the same time intervals were 18.5% and 12.5%, lower than the results seen in our study [20]. Recently, the Impower 150 study comparing chemotherapy plus atezolizumab/bevacizumab (ABCP) followed by maintenance with atezolizumab and bevacizumab (BCP) or atezolizumab (ACP) alone also achieved good results. In arm A where atezolizumab was used alone, the objective response rate was 40.6% while in arm B, which uses the combination atezolizumab and bevacizumab, the response rate was 56.4 % [24]. Improved progression-free survival with ABCP versus BCP or ACP was also observed. Median progression-free survival 8·4 months (ABCP) vs 6·8 months (BCP or ACP) [24]. CIMAvax-EGF's PFS data in our study compares favorably with the above referred.
Characterizing the safety profile of the vaccine in the real-world setting is very important. Across the previous studies, including phase I, II and III clinical trials, no serious adverse events were reported. [12-14]. In the phase II study, Neninger et al. found that the most common adverse events were grade 1-2 fever, headache, asthenia and injection site pain (13%). In this study, it was demonstrated that the vaccine was safe since no patients developed serious adverse events and the percentage of patients developing grade 1-2 adverse events was very small. Other maintenance trials with pemetrexed and bevacizumab, did find grade 3-4 adverse events [20]. In the Impower 150 study, 64 % of the patients treated with the atezolizumab/bevacizumab plus chemotherapy, followed by atezolizumab and bevacizumab, had grade 3-4 adverse events [24].
One of the limitations of the current study is that serum EGF levels, as well as anti EGF antibodies, were not evaluated. Previous studies have shown that EGF is a predictive marker of CIMAvax-EGF efficacy [17]. Better selection of the patient population would impact in a larger median PFS and overall survival.
Conclusions
In conclusion, in our standard practice, CIMAvax-EGF as an EGF depleting immunotherapy used after front line therapy was safe and effective in patients with NSCLC.
Acknowledgements
The authors are grateful to all patients that participated in this real-world study.
Author Contributions
Conceptualization: YIFV, DLPG, RRT and EGM; Data curation and formal analysis: YIFV, DLPG, RRT and EGM. Investigation: YIFV, DLPG, SAS, JRM, IBI, AEL, JEFR, JLG and DCO. Supervision: EGM, Roles/Writing - original draft: YIFV, DLPG, EGM and TCR. Writing, review & editing: All authors.
Competing Interests
One of the authors (TCR) currently work for the Center of Molecular Immunology, the institution that generated and originally patented CIMAvax-EGF. The remaining authors do not have any commercial or financial relationships that could be taken as a potential conflict of interest.
References
1. World Health Organization (WHO) Globocan IAfRoCI,. International Agency for Research on Cancer (IARC) 2020.. Available at:. https://gco.iarc.fr/
2. 2019 SYoH. Directorate of Medical Records and Health Statistics Ministry of Public Health of Cuba. Havana 2020; p 68 and 101. 2020; Available at. http://www.onei.gob.cu
3. Gridelli C, de Marinis F, Di Maio M, Ardizzoni A, Belani CP, Cappuzzo F. et al. Maintenance treatment of advanced non-small-cell lung cancer: results of an international expert panel meeting of the Italian association of thoracic oncology. Lung Cancer. 2012;76:269-79
4. Hashemi-Sadraei N, Pennell NA. Advanced non-small cell lung cancer (NSCLC): maintenance therapy for all? Curr Treat Options Oncol. 2012;13:478-90
5. Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F. et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378:2078-92
6. Patel JD, Hensing TA, Rademaker A, Hart EM, Blum MG, Milton DT. et al. Phase II study of pemetrexed and carboplatin plus bevacizumab with maintenance pemetrexed and bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer. J Clin Oncol. 2009;27:3284-9
7. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N. et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med. 2018;378:2288-301
8. Gonzalez G, Crombet T, Torres F, Catala M, Alfonso L, Osorio M. et al. Epidermal growth factor-based cancer vaccine for non-small-cell lung cancer therapy. Ann Oncol. 2003;14:461-6
9. Gonzalez G, Lage A. Cancer vaccines for hormone/growth factor immune deprivation: a feasible approach for cancer treatment. Curr Cancer Drug Targets. 2007;7:229-41
10. Lage A, Crombet T, Gonzalez G. Targeting epidermal growth factor receptor signaling: early results and future trends in oncology. Ann Med. 2003;35:327-36
11. Tagliamento M, Rijavec E, Barletta G, Biello F, Rossi G, Grossi F. et al. CIMAvax-EGF, a therapeutic non-small cell lung cancer vaccine. Expert Opin Biol Ther. 2018;18:829-35
12. Neninger Vinageras E, de la Torre A, Osorio Rodriguez M, Catala Ferrer M, Bravo I, Mendoza del Pino M. et al. Phase II randomized controlled trial of an epidermal growth factor vaccine in advanced non-small-cell lung cancer. J Clin Oncol. 2008;26:1452-8
13. Ramos TC, Vinageras EN, Ferrer MC, Verdecia BG, Rupale IL, Perez LM. et al. Treatment of NSCLC patients with an EGF-based cancer vaccine: report of a Phase I trial. Cancer Biol Ther. 2006;5:145-9
14. Rodriguez PC, Popa X, Martinez O, Mendoza S, Santiesteban E, Crespo T. et al. A Phase III Clinical Trial of the Epidermal Growth Factor Vaccine CIMAvax-EGF as Switch Maintenance Therapy in Advanced Non-Small Cell Lung Cancer Patients. Clin Cancer Res. 2016;22:3782-90
15. Sadighi Akha AA. Aging and the immune system: An overview. J Immunol Methods. 2018;463:21-6
16. Cunha LL, Perazzio SF, Azzi J, Cravedi P, Riella LV. Remodeling of the Immune Response With Aging: Immunosenescence and Its Potential Impact on COVID-19 Immune Response. Front Immunol. 2020;11:1748
17. Saavedra D, Neninger E, Rodriguez C, Viada C, Mazorra Z, Lage A. et al. CIMAvax-EGF: Toward long-term survival of advanced NSCLC. Semin Oncol. 2018;45:34-40
18. Paz-Ares L, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P. et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012;13:247-55
19. Paz-Ares LG, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P. et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31:2895-902
20. Ramalingam SS, Dahlberg SE, Belani CP, Saltzman JN, Pennell NA, Nambudiri GS. et al. Pemetrexed, Bevacizumab, or the Combination As Maintenance Therapy for Advanced Nonsquamous Non-Small-Cell Lung Cancer: ECOG-ACRIN 5508. J Clin Oncol. 2019;37:2360-7
21. Cohen MH, Cortazar P, Justice R, Pazdur R. Approval summary: pemetrexed maintenance therapy of advanced/metastatic nonsquamous, non-small cell lung cancer (NSCLC). Oncologist. 2010;15:1352-8
22. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A. et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol. 2019;37:537-46
23. Brodowicz T, Krzakowski M, Zwitter M, Tzekova V, Ramlau R, Ghilezan N. et al. Cisplatin and gemcitabine first-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non-small cell lung cancer: a phase III trial. Lung Cancer. 2006;52:155-63
24. Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F. et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019;7:387-401
Author contact
![]() Corresponding author: Yoanna Flores Vega, MD. National Institute of Oncology and Radiobiology. 29th Street, corner E, Vedado Plaza de la Revolución, Havana, Cuba. Phone number: 537 8369114. Email: yfloressld.cu or yoannafloresvegacom
Corresponding author: Yoanna Flores Vega, MD. National Institute of Oncology and Radiobiology. 29th Street, corner E, Vedado Plaza de la Revolución, Havana, Cuba. Phone number: 537 8369114. Email: yfloressld.cu or yoannafloresvegacom

 Global reach, higher impact
Global reach, higher impact