Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(12):2315-2328. doi:10.7150/jca.85966 This issue Cite
Review
Combined Use of Immune Checkpoint Inhibitors and Phytochemicals as a Novel Therapeutic Strategy against Cancer
1. Genomics Research Center (Key Laboratory of Gut Microbiota and Pharmacogenomics of Heilongjiang Province, State-Province Key Laboratory of Biomedicine-Pharmaceutics of China), College of Pharmacy, Harbin Medical University, Harbin, 150081, China.
2. National Key Laboratory of Frigid Zone Cardiovascular Diseases (NKLFZCD) College of Pharmacy, Harbin Medical University, Harbin, 150081, China.
3. Harbin Medical University-University of Calgary Cumming School of Medicine Centre for Infection and Genomics, Harbin Medical University, Harbin, 150081, China.
4. Department of Biochemistry and Molecular Biology, University of Calgary, Calgary, T2N 4N1, Canada.
5. Department of Microbiology, Immunology and Infectious Diseases, University of Calgary, Calgary, T2N 4N1, Canada.
6. Division of Anti-Tumor Pharmacology, State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201203, China.
Received 2023-5-8; Accepted 2023-7-3; Published 2023-7-24
Abstract
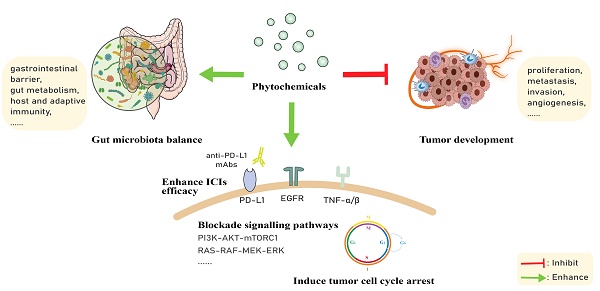
Immune checkpoint inhibitor (ICI) therapy has dramatically changed cancer treatment, opening novel opportunities to cure malignant diseases. To date, most prevalently targeted immune checkpoints are programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), with many others being under extensive investigations. However, according to available data, only a fraction of patients may respond to ICI therapy. Additionally, this therapy may cause severe adverse immune-related side effects, such as diarrhea, headache, muscle weakness, rash, hepatitis and leucopenia, although most of them are not fatal, they can affect the patient's treatment outcome and quality of life. On the other hand, growing evidence has shown that phytochemicals with anticancer effects may combine ICI therapy to augment the safety and effectiveness of the treatment against cancer while reducing the adverse side effects. In this review, we summarize the state of art in the various experiments and clinical application of ICIs plus phytochemicals, with a focus on their combined use as a novel therapeutic strategy to cure cancer.
Keywords: Immune checkpoint inhibitor, Phytochemicals, Combination therapy, Immune-related adverse events, Predictive biomarker, Gut microbiota
Introduction
The past decade has witnessed enormous advances on understanding the immune checkpoints regarding their roles to help cancer cells elude immune surveillance via decreasing immune cell activity and suppressing immune responses. In the tumor microenvironment (TME), cancer cells escape from immune functions through the expression of immune checkpoints such as programmed cell death ligand 1 (PD-L1), cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), T-cell immunoglobulin and mucin domain-containing molecule-3 (Tim-3), lymphocyte-activation gene 3 (LAG-3) and T cell immunoglobulin and ITIM domain (TIGIT) [1-5]. Tumor cells can adopt normal physiologic immune checkpoints and hence disrupt the balance between tumor cell proliferation and immune-surveillance, ultimately escaping from the host elimination. To reactivate the suppressed immune system and restore the suppressed ability of immune cells to recognize and kill cancer cells, scientists have developed monoclonal antibodies (mAbs) as immune checkpoint inhibitors to block the immune checkpoint interaction between cancer cells and immune cells [6]. To date, seven inhibitors of PD-L1/PD-1 or CTLA-4 have received FDA (U.S. Food and Drug Administration) approval for treating malignant tumors ([7-20]; see Table 1). However, to date only a subset of patients may respond to this novel therapy, whereas the vast majority of patients do not benefit or even experience multiple immune-related adverse events (irAEs), such as diarrhea, endocrine disorders, thyroid dysfunction or diabetes [21]. The side effects can be rather devastating to the immune system or other physiological functions and may lead to exacerbation of the disease or even death of the patient, calling urgently for a solution for the ICI therapy to achieve greater efficacy and in the meantime minimize or even completely remove such unfavorable reactions.
Plants harbor a great diversity of chemicals, many of which may have biological properties to augment ICI efficacy or ameliorate ICI adverse effects. For example, phytochemicals derived from several plants show multiple activities in the human body such as anti-oxidation, immune-protection and anti-inflammation [22-24], which may improve the outcomes of cancer patients and may possibly be used in conjunction with immune checkpoint inhibitors. In fact, a large number of phytochemicals, such as curcumin, resveratrol and epigallocatechin-3-gallate (EGCG), show positive results in combination therapies with ICIs for cancer treatment ([25-36]; see Table 2). At the same time, phytochemicals also influence the diversity and abundance of intestinal microbiota that exist in a symbiotic relationship with the host. Previous studies have exhibited that some microbial metabolites are immunoprotective while others contribute to chronic inflammation and cancer promotion. Increased level of toxic microbial metabolites plays a role in the formation of tumor microenvironment (TME) because of imbalance of intestinal microbiota, so maybe we can regard the balance of gut microbes as indicators of the efficacy of cancer treatment [37]. Additionally, predictive biomarkers such as immune cells, PD-L1 overexpression, neoantigens, genetic and epigenetic signatures may also help screen patients for those who may have high responses to ICI therapy. Investigations on biomarkers for patient selection to achieve maximum efficacy and in the meantime alleviate side effects are rapidly progressing [38].
Immune checkpoint inhibitors (ICIs) approved by FDA.
| Agent | Target | Target cancer type | Ref |
|---|---|---|---|
| Ipilimumab | CTLA-4 | Melanoma | [7] |
| Nivolumab | PD-1 | Melanoma, NSCLC, SCLC, SCC, Urothelial carcinoma, Classic Hodgkin's lymphoma, RCC, HCC | [8, 10] |
| Pembrolizumab | PD-1 | Melanoma, NSCLC, Gastric or gastroesophageal junction, Merkel cell carcinoma, RCC, HCC, head and neck SCC, Endometrial carcinoma | [11-13] |
| Cemiplimab | PD-1 | Advanced SCC | [14] |
| Atezolizumab | PD-L1 | Urothelial carcinoma, NSCLC, PD-L1 triple-negative breast cancer, SCLC, Melanoma, HCC | [15, 16] |
| Durvalumab | PD-L1 | Urothelial carcinoma, NSCLC, SCLC | [17] |
| Avelumab | PD-L1 | Merkel cell carcinoma, RCC, Urothelial carcinoma | [18, 19] |
List of phytochemicals and their effects on PD-L1 regulation in several anti-cancer studies.
| Classification | Plant Origin | Compound | Effect | Molecule mechanism | Cancer type (model) | Ref |
|---|---|---|---|---|---|---|
| Flavonoids | Matricaria chamomilla | Apigenin | PD-L1 ↓ | Inhibits PD-L1 expression via IFN-γ-induced STAT1 activation | Breast cancer (cell lines) | [27] |
| Scutellaria baicalensis | Baicalein | PD-L1 ↓ | Downregulates PD-L1 expression via inhition of STAT3 | Hepatocellular carcinoma (mouse model) | [28] | |
| Baicalin | ||||||
| Arbutus unedo | Cyanidin-3-O- glucoside | PD-1 and PD-L1 ↓ | Inhibits VEGF and PD-L1 expression | Colon cancer (cell lines) | [31] | |
| Dictamnus dasycarpus | EGCG (Epigallocate-chin gallate) | PD-L1 ↓ | Reduces IFN-γ-induced PD-L1 expression via inhibition of JAK2/STAT1 signaling and decreases EGF-induced PD-L1 expression through inhibition of EGFR/Akt signaling | Lung cancer, mouse melanoma (mouse model) | [26] | |
| Citrus species | Hesperidin | PD-L1 ↓ | Suppresses Akt and NF-κB signaling and induces PD-L1 downregulation | Breast cancer, oral cancer (cell lines) | [33, 129] | |
| Epimedium koreanum | Icaritin | Synergistic effect with anti-PD-1 Ab ↓ PD-L1 ↓ | Blocks IKK complex formation and NF-κB translocation which promote PD-L1 expression | Liver cancer (clinical trial) | [34] | |
| Matricaria chamomilla | Luteolin | PD-L1 ↓ | Inhibits the IFN-γ dependent PD-L1 upregulation | Breast cancer (cell lines) | [27] | |
| Non-flavonoids | Curcuma longa | Curcumin | PD-L1 ↓ | Inhibits STAT3 pathway and reduces Treg (CD4+CD25+FOXP3+) and MDSC | Tongue squamous cell carcinoma (cell lines and mouse model) external cancerous lesions (clinical trial) | [29, 130] |
| Camellia sinensis | Gallic acid | PD-L1 ↓ | Suppresses EGF binding on EGFR resulting PI3K/AKT pathway inhibition, upregulates p53 and miR-34a and downregulates PD-L1 expression | Lung cancer (cell lines) | [32] | |
| Polygonum cuspidatum | Polydatin | PD-L1 ↓ | Enhances miR-382 and inhibits miR-382-induced PD-L1 expression | Colon cancer (cell lines) | [35] | |
| Vitis vinifera | Resveratrol | PD-L1 ↓ | Inhibits thyroxine-induced PD-L1 expression | Oral cancer (cell lines) | [25] | |
| Inhibits PD-L1 Glycosylation and Dimerization | Breast cancer (cell lines) | [119] | ||||
| Terpenes | Epimedium koreanum | β-Elemene | PD-L1 ↓ | Regulates AKT signaling, thereby controlling the expression of PD-L1 | Esophageal cancer (cell lines and mouse model) | [131] |
| Panax ginseng | Ginsenoside Rg3 | PD-L1 ↓, CD8+T↑ | Suppresses Akt and NF-κB signaling and induces PD-L1 downregulation, as well as promote CD8+T cells | Lung cancer (cell lines) | [132] | |
| Ginsenoside Rh2 | PD-L1 ↓ | Suppresses PI3K-Akt and EGFR signalings and induces PD-L1 downregulation | Lung cancer (cell lines) | [133] | ||
| Ginsenoside Rk1 | PD-L1 ↓ | Suppresses NF-κB signaling and induces PD-L1 downregulation | Lung adenocarcinoma normal lung epithelial cell (mouse model) | [134] | ||
| Platycodon grandiflorus | Platycodin D | PD-L1 ↓ | Reduces the level of PD-L1 in lung cancer cells via triggering its release into the cell culture medium | Lung cancer (cell lines) | [135] | |
| Alkaloids | Berberis plants | Berberine | PD-L1 ↓ | Enhances the sensitivity of tumor cells to co-cultured T-cells by decreasing the level of PD-L1 in cancer cells | Non-small-cell lung cancer (NSCLC) (mouse model) | [136] |
| Ecteinascidia turbinate | Trabectedin | blocks the PD-1/PD-L1 axis | Modulates transcription and translation of IL6, CCL2, and IFNα in myeloid cells and FOXP3 in regulatory T cells, also blocks the PD-1/PD-L1 axis by targeting PD-L1+ CLL cells, PD-L1+ monocytes/macrophages, and PD-1+ T cells | Chronic lymphocytic leukemia (CLL) (mouse model) | [137] | |
| Others | Chaenomeles speciosa | Ethanol extract (EEC) | PD-L1, Foxp3, TGF-β ↓ | Inhibits the expression of PD-L1, Foxp3 and TGF-β to suppress tumor growth | Hepatoma (cell line) | [138] |
| Sarcodon imbricatus | Aqueous extract | IL-6↑ PD-L1↓ | Increases serum concentrations of IL-2, IL-6 and tumor necrosis factor-α, natural killer cell activity and the viability of splenocytes and reduces the expression of PD-L1 | Breast cancer (mouse model) | [139] |
In this review, we first summarize the currently known immune checkpoints and relevant FDA-approved blockades, briefly discuss ICI therapy-induced side effects, and then introduce representative phytochemicals along with their advantages and shortcomings in cancer treatment. Finally, we comment on combination therapies of immune checkpoint inhibitors with phytochemicals, with a focus on the future development of this novel anti-cancer therapy.
Immune checkpoints
Numerous immune checkpoint proteins, such as PD-1 (programmed cell death protein 1)/ PD-L1 (programmed cell death ligand 1) and CTLA-4 (cytotoxic T lymphocyte-associated antigen-4), are involved in tumor proliferation, angiogenesis, metastasis and chemoresistance via suppressing cytotoxic T cell activation, resulting in cancer immune escape and immune tolerance [39, 40]. Relevant laboratory or clinical studies and public interest in ICI therapy keep increasing; here we introduce five well-studied immune checkpoints and their influence in cancer development.
PD-1
PD-1 is a member of the CD28 superfamily expressed on CD4 and CD8 T cells, B cells, monocytes, natural killer (NK) cells, and dendritic cells (DCs) [41, 42]. PD-1 interacts with its two ligands, PD-L1 or PD-L2, to deliver negative signals and exert multiple immunoregulatory roles in T cell activation and tolerance [43]. PD-L1 is expressed on many cancer [44], and, on the other hand, PD-L2 is mostly restricted to activated DCs and macrophages [45]. Interactions of PD-1 with PD-L1/PD-L2 have been reported in a wide variety of solid tumors and hematologic malignancies, with patients having tumors positive for PD-L1 or PD-L2 showing dramatically lower survival rates than those having tumors negative for both of these ligands (46% vs. 83% for 5-year survival) [46].
Currently, the modulatory effects of PD-1 on tumor growth and on immunity have been extensively studied, leading to the development of targeting antibodies such as pembrolizumab, nivolumab, cemiplimab, etc. [47, 48]. However, a large proportion of patients do not respond to such treatments, which, additionally, may cause various immune-related side effects [49], calling for further studies to elucidate the involved mechanisms and relevant biomarkers for patient selection.
CTLA-4
CTLA-4 is a homolog of CD28 expressed on activated T cells and Treg cells. It binds to B7 molecules with high affinity and negatively regulates immune responses [50]. Although CD28 also binds to B7 molecules and co-stimulates T cells together with the T cell receptor (TCR), CTLA-4 has higher affinity for B7-1 (CD80) and B7-2 (CD86) and hence can outcompete CD28, leading to reduced release of pro-effector cytokines such as IL-12 and cytotoxic enzymes [51]. As one of the most extensively studied immune checkpoints, CTLA-4 has been the target of inhibitor development, with ipilimumab being the first to be approved by FDA in 2011, for attacking melanoma cells [7]. Currently, anti-CTLA-4 monoclonal antibodies (mAbs) are widely used in the treatment of multiple solid cancers, including melanoma, renal cell carcinoma, and colorectal cancer. Due to adverse side effects and limited observation, other CTLA-4 blockades that have shown good anti-cancer effects in many trials, such as tremelimumab and nivolumab, have not received FDA approval. Additionally, the combined use of anti-CTLA-4 mAbs and other anti-cancer molecules has shown positive outcomes, although further work is needed regarding potential adverse effects of such applications [52].
LAG-3
LAG-3 (Lymphocyte activation gene 3) is one of the immune inhibitory receptors (IRs), expressed on activated T cells, Treg cells and NK cells, and is upregulated by IL-2, IL-7, IL-10 and IL-12. Major histocompatibility complex class II (MHC-II), LSECtin and Galectin-3 are representative ligands of LAG-3 [53]. The expression of LAG-3 and MHC-II may be coordinated in regulating T-cell-mediated immune responses. While the binding of these two activation antigens negatively regulates activated T cells, LAG-3 alone may work as a negative regulator to prevent the exacerbation of a variety of autoimmunity diseases, and has expected to be a promising therapeutic target in autoimmune diseases [54, 55]. Recent studies support the view that blockade of LAG-3 significantly enhanced antitumor immunity, and its combination with other immune checkpoint inhibitors remarkably improves the efficacy of antitumor therapy [56].
Tim-3
Tim-3, a member of the novel T cell immunoglobulin and mucin domain (Tim) family originally identified as a specific marker for Th1 and Tc1 cells, is also expressed on Treg cells, NK cells, monocytes, macrophages, and DCs [5]. Upon interaction with a ligand, Tim-3 suppresses the immune responses, facilitating the development of diseases. As a negative immune regulator, Tim-3 is implicated for a role in autoimmune diseases. Galectin-9 has been confirmed as a classical ligand for Tim-3 and their binding may cause Th1 cell death and induce immune tolerance in tumor microenvironment [57]. Additionally, several clinical trials have demonstrated that the expression of Tim-3 is associated with severe dysfunction of T cells in different types of cancers such as non-small-cell lung carcinoma (NSCLC), renal cell carcinoma (RCC), colon cancer and gastric cancer [58, 59]. Indeed, on exhausted CD8+ T cells isolated from patients infected with virus, Tim-3 expression is often upregulated, which is probably responsible for inefficient antiviral activity [60]. However, growing evidence indicates that, upon binding with Galectin-9, Tim-3 helps reduce tissue inflammation and hence suppress autoimmunity [61].
TIGIT
TIGIT (T cell immunoglobulin and ITIM domain) is an inhibitory receptor expressed on the surface of NK cells, Treg cells, follicular T helper cells and subsets of regulatory and memory CD4+ and CD8+ T cells [62]. TIGIT possesses a single extracellular immunoglobulin variable (IgV) domain that is responsible for binding nectin-2 (CD112), nectin-3 (CD113) and necl-5 (CD155). TIGIT interacts with CD155 expressed on antigen-presenting cells or tumor cells to downregulate T cell and NK cell functions, resulting in T cell suppression and the limitation of NK cell activation [63]. TIGIT up-regulation has been observed in various malignancies, including melanoma, breast cancer, non-small-cell lung carcinoma (NSCLC); indeed TIGIT expression on peripheral blood CD8+ T cells of various cancer patients has been associated with metastases and poor survival [64]. Solid tumors are currently targeted with anti-TIGIT mAbs in various stages of clinical trials, and blockades of TIGIT, such as tiragolumab, BGB-A1217 and AB154, are ongoing. Of note, compared to either monotherapy, dual TIGIT blockades, especially when combined with other ICIs, are likely to be more effective and promising [65, 66].
Immune checkpoint inhibitor therapy
Many compromised measures have been used for cancer treatment, such as surgery (much normal tissues have to be removed) and chemo- and radio-therapies (severe side effects including damage to immune and hemopoietic systems), but cure or even alleviation is often hardly achievable. This situation calls for novel strategies for cancer treatment. Among the newly developed anti-cancer strategies ICI therapy are promising research direction. Many of immune checkpoint blockades are under assessment in clinical trials or have been approved by the FDA. Below, we summarize some representative inhibitors of immune checkpoints - their effectiveness and application, along with their adverse effects (Table 3).
Common immune-related adverse effects.
| Affected area | Symptoms |
|---|---|
| Gastrointestinal system | Diarrhea, abdominal pain, nausea, bowel perforations |
| Skin | Rash, pruritus |
| Liver | Right upper quadrant abdominal pain, nausea, vomiting |
| Nervous system | Muscle weakness, sensory neuropathies |
| Endocrine system | Headache, visual-field defects, fatigue, weakness, asthenia, nausea and vomiting, fever, hypotension, behavioral changes |
PD-1/PD-L1 inhibition
In the past few years, inhibitors of PD-1 and its ligand PD-L1 are increasingly used for the treatment of selected cancer patients and are highly effective in many cases. Nivolumab (MDX-1106 or BMS-936558), an antibody targeting PD-1, has been approved by FDA for the treatment of a broad panel of malignancies, including advanced NSCLC, melanoma, classical Hodgkin lymphoma, squamous cell carcinoma of the head and neck (HNSCC), renal cell carcinoma (RCC), urothelial carcinoma (UC), hepatocellular carcinoma (HCC), and colorectal cancer (CRC) [8, 67, 68]. In a Phase I trial of nivolumab, patients with melanoma, renal cell carcinoma and NSCLC had response rates of 28%, 27% and 17%, respectively [69].
Pidilizumab (CT-011) is the first PD-1-targeting humanized mAb to be tested in clinical trials for melanoma, NSCLC, renal cell carcinoma, head and neck cancers, lymphoma and several other cancers [70]. Other PD-1 inhibitors encompass pembrolizumab (MK-3475, lambrolizumab) and cemiplimab, which respectively exhibit acceptable tolerance and remarkable efficacy in several tumors such as advanced NSCLC and advanced squamous cell carcinoma (SCC) [14].
Atezolizumab (MPDL3280A) inhibits the PD-L1/PD-1 pathway by binding to PD-L1 and has been approved for the treatment of advanced NSCLC, triple-negative breast cancer and urothelial carcinoma [15, 71]. Another humanized PD-L1 monoclonal antibody durvalumab (MEDI4736) with T cell dependent anti-tumor activity shows efficacy in the treatment of patients with urothelial carcinoma [72].
CTLA-4 inhibition
Ipilimumab (MDX-010, Yervoy; Bristol-Myers Squibb) is a fully human monoclonal antibody against CTLA-4 (cytotoxic T-lymphocyte antigen-4) for the treatment of melanoma patients and was approved by FDA in 2011 [7]. Over the past decade, ipilimumab has demonstrated efficacy in the treatment of renal cell cancer (RCC), lung cancer, metastatic melanoma and prostate cancer in addition to melanoma [73, 74]. In a series of trials on combined applications of immune checkpoint inhibitors in cancer treatment, cohort A (nivolumab plus ipilimumab) demonstrated better treatment outcomes than cohort B (nivolumab alone), as judged by reduced tumor metastases and effective extension of progression-free survival. Unfortunately, however, these improvements were not of significance and in many cases this combined therapy resulted in more serious side effects [75].
Melanoma patients with ipilimumab monotherapy showed excellent overall response rates, with survival time of many patients being significantly prolonged [76]. In a phase 2 dose-ranging study, patients with pretreated advanced melanoma were randomly assigned a fixed dose of ipilimumab of either 10 mg/kg (n=73), 3 mg/kg (n=72), or 0.3 mg/kg (n=72) every 3 weeks for four cycles, followed by maintenance therapy every 3 months. The best overall response rate was 11.1% (95% CI 4.9-20.7), 4.2% (0.9-11.7), and 0% (0.0-4.9) respectively. Of note, in the trial, some patients showed immune-related adverse events (irAEs) [77]. Nevertheless, considering the favorable clinical outcomes, National Comprehensive Cancer Network (NCCN) has added ipilimumab as a category 1 recommendation in the guidelines of systemic therapy options for advanced or metastatic melanoma. The irAEs, such as enterocolitis, rash, hepatitis, hypophysitis, uveitis, pancreatitis and leucopenia, could be rather serious, so it is critical to carefully select the patients and use ipilimumab at appropriate stages for optimal treatment.
Tremelimumab (CP-675206) is also a humanized monoclonal antibody specific for treatment of cancers such as malignant mesothelioma [78], mostly as an adjuvant agent with other mAbs [79].
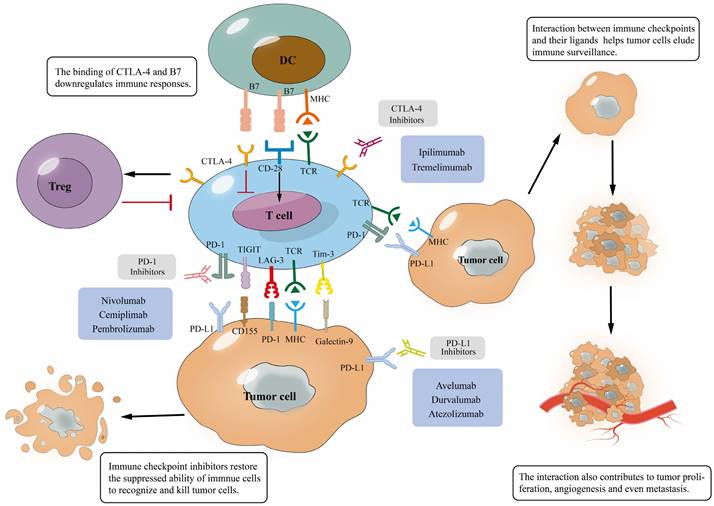
Interaction of immune checkpoints with their ligands and mechanism of immune checkpoint blockades. (A), The binding of immune checkpoints with their ligands can inhibit T cells and help tumor cells evade immune surveillance, which will promote tumor development; (B), The binding of the antibody to immune checkpoints prevents them from binding to ligands, inducing T cell activation and tumor cell apoptosis, which eventually improves anti-tumor immunity.
Measures against ICI related adverse effects - combined use of phytochemicals
Immune-related adverse events most commonly involve the gastrointestinal tract, endocrine glands, skin, liver, and the nervous and endocrine systems [80]. Common symptoms include diarrhea, rash, muscle weakness, headache, fatigue and other minor discomfort, which are not fatal but affect patients' quality of life [81] (Table 3). The precise pathophysiology underlying the immune-related adverse events is under scrutiny, and different blockades of immune checkpoints may cause different adverse effects on patients with different or even histologically indistinguishable cancers, often presenting a dilemma for the physician to decide whether to use ICI. However, the prestigious efficacy of ICI calls for measures to make full use of this special anticancer strategy and in the meantime mitigate its side effects. One way is to combine ICI therapies with phytochemicals.
Owing to their ubiquitous availability and special biological activities, often of great medical significance, plant-derived natural compounds have had tremendous impacts on drug discovery and continuously receive FDA approval [82]. Phytochemicals can be divided into multiple groups, such as polyphenols, terpenes, alkaloids and others, and polyphenols can be classified into flavonoids (e.g., apigenin and EGCG) and no-flavonoids (e.g., resveratrol and curcumin). We previously have demonstrated the efficacy of plant materials against diseases including malignancies and the beneficial effects of these natural products could be attributed to the phytochemicals such as lignans [23, 24, 83-85]. According to previous studies, the combination of phytochemicals with standard chemotherapeutic drugs could significantly improve cancer patient survival [86-88]. Furthermore, phytochemicals contribute to the balance of intestinal microbiota, increasing beneficial microbial metabolites, thus exerting anti-tumor effects and helping improve the efficacy of immunotherapy [89, 90] (Figure 4).
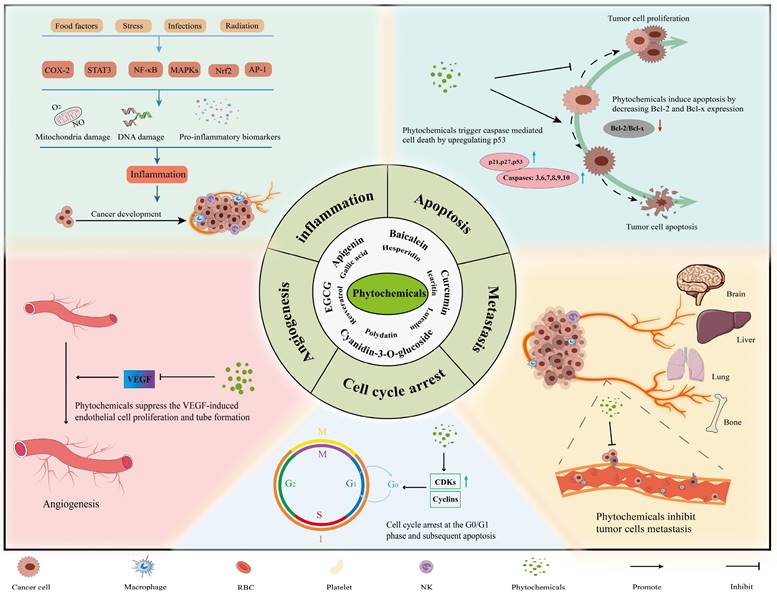
Phytochemicals fight against tumors mainly through inducing apoptosis of cancer cells, facilitating cell cycle arrest, disabling immunosuppressive Tregs and inhibiting neoangiogenesis (Figure 2). In experiments with the combined application of phytochemicals and immune checkpoint inhibitors, we found that phytochemicals promote the efficacy of ICIs by down-regulating the expression of immune checkpoints or/and their ligands (e.g., PD-1/PD-L1), as well as by blocking the pathways associated with cancer progression (e.g., PI3K/AKT, EGFR) (Figure 3). In addition, they can also affect drug metabolism by regulating intestinal microbiota [84]. Below are representative phytochemicals that have significantly enhanced cancer treatments alone or combined with ICI therapies.
Resveratrol
Resveratrol, richly present in a variety of plants such as grapevine, peanut and pine, exhibits potent anti-inflammatory, antioxidant, anti-viral activities and has demonstrated therapeutic properties against several cancers including breast, gastric, lung, prostate and thyroid cancers [91, 92]. Resveratrol inhibits the growth and development of tumors through nuclear factor-κB (NF-κB) signaling. Activation of nuclear factor-κB (NF-κB) often occurs in tumor cells and contributes to aggressive tumor growth and resistance to chemotherapy and radiotherapy, whereas resveratrol may downregulate NF-κB and thus increase the therapeutic efficacy by lowering the threshold for tumor cell apoptosis [93]. Of significant importance, resveratrol may enhance the effects of ICI therapy by modulating the expression of immune checkpoint and its ligand, PD-1, PD-L1 and CTLA-4, and hence mitigate immune-related adverse effects caused by ICIs. Indeed, the combined use of resveratrol and PD-1 antibody could greatly inhibit tumor growth in ovarian carcinoma, while anti-CD8 antibody co-treatment would restore the tumor growth [94]. Verdura et al. reported that resveratrol can inhibit glyco-PD-L1-processing enzymes (α-glucosidase/α-mannosidase) that modulate N-linked glycan decoration of PD-L1, thereby promoting the endoplasmic reticulum retention of a mannose-rich, abnormally glycosylated form of PD-L1, ultimately impeding its targeting to the cancer cell membrane and promoting immunity [95]. These well-conducted experiments and the unforeseen immunomodulating mechanisms by resveratrol might illuminate new approaches to restore T-cell function by targeting the interaction of PD-1 and PD-L1. It is worth mentioning that resveratrol has a boosting effect on gut microbiota, the microbiota can modulate multiple cellular events such as cellular metabolism and host immune function to regulate internal homeostasis and regress the development of virous cancers [96].
The main mechanisms and effects of phytochemicals against tumors. Most phytochemicals participate in tumor suppression by inhibiting tumor metastasis, angiogenesis and inducing tumor cell apoptosis and cell cycle arrest.
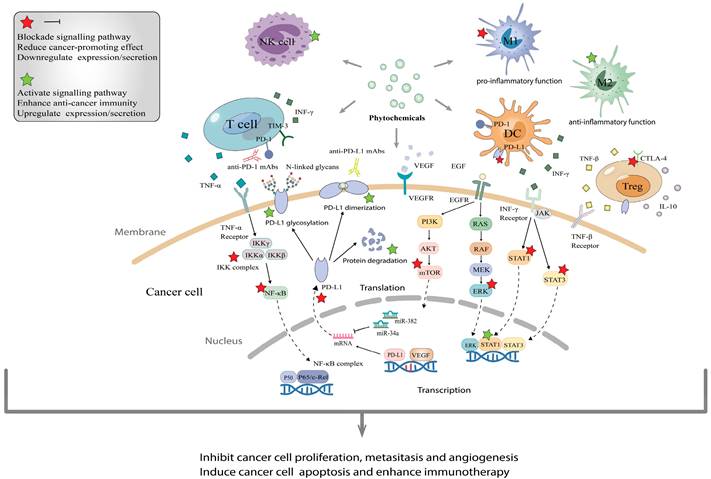
The molecular mechanisms of phytochemicals in combined application with immune checkpoint inhibitors. (A), Phytochemicals usually enhance immune checkpoint inhibitor therapy via activating immune cells that recognize and kill cancer cells such as T cells, NK cells and DC, as well as suppressing pro-inflammatory and carcinogenetic function of immunosuppressive cells. (B), Phytochemicals block the interaction between PD-1 and PD-L1 by decreasing the expression and promoting dimerization/glycosylation of PD-L1, even facilitating their degradation. (C), Through blocking various signaling pathways such as PI3K/AKT/mTOR, JAK/STAT3 and VEGF, phytochemicals help ICIs to inhibit tumor proliferation, metastasis and angiogenesis, then induce apoptosis.
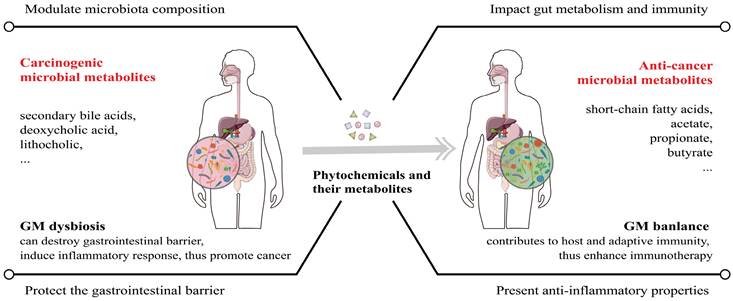
The regulation function of phytochemicals in GM (gut microbiota) balance and potential anticancer effects. GM dysbiosis is a major cause of cancer, both within and outside the gastrointestinal tract. Phytochemicals and their active metabolites can regulate GM balance to repair the gastrointestinal barrier and strengthen the host immunity, thus exerting cancer suppressive effects and enhancing ICI therapy efficacy.
Curcumin
Curcumin is an active component of the dietary spice turmeric that has been widely used for treatment of illnesses in Asian countries, such as China and India, and some European regions [97]. Over several centuries, curcumin has been a key ingredient in many traditional medical recipes for treatment of a large variety of diseases, including digestive disorders, common infections, dermatologic diseases and depressive states [98].
As a kind of phytochemicals, curcumin has many well documented bioactive effects, including anti-inflammatory, anti-oxidative, immune-protective, metabolic stability and anti-tumor functions and, therefore, may potentially be used in the treatment of diseases such as cancer therapy [97]. Many lines of evidence demonstrate that curcumin inhibits tumor progression by modulating multiple pathways (e.g., NF-κB, MAPK, STAT3; [99]), coordinating tumor suppressor factors (e.g., P53, cytokines IL-1, -2, -6, -8, or -12, IFN-γ) to promote cancer cell apoptosis and clearance [100, 101], and inhibiting angiogenesis [102]. Curcumin, used alone or combined with other treatments, has shown robust effects in suppressing the progression and metastasis of cancers, such as tongue squamous cell carcinoma, prostate cancer, pancreatic cancer, breast cancer and colorectal cancer, and, especially, in enhancing the response of immune therapy [103]. When combined with immune checkpoint inhibitors such as anti-PD-1/PD-L1 and anti-CTLA-4 mAbs, curcumin presented excellent antitumor effects by downregulating cytokine secretion, inhibiting NF-κB pathway and promoting the infiltration of anti-tumor T cells [101]. Analogs of curcumin, e.g., bisdemethoxycurcumin, in combination with an anti-PD-L1 antibody, may significantly boost immune responses and prolong survival in animal experiments [104]. Hayakawa et al. combined curcumin with PD-1/PD-L1 Abs in MC38 murine tumor models and achieved synergistic anti-tumor effects and induced tumor antigen-specific T cells, significantly augmenting the efficacy of treatment [105]. In addition to showing excellent results in the cell lines and animal models, the combined application of curcumin and ICIs is also beginning to be studied in some clinical trials, accelerating their clinical use in cancer patients [106]. In this trial, patients obtained good therapeutic results in cervical and uterine cancer by combining PD-1 blockades Pembrolizumab with curcumin as a dietary supplement. Additionally, researchers think the alterations in gut microbiota could contribute to identify mechanisms of therapy, resulting in predictive biomarkers for efficacy and improved patient selection in future clinical applications.
EGCG (epigallocatechin-3-gallate)
Catechins are bioactive constituents of tea with a variety of beneficial effects on human health and have long been the targets of active research for cancer prevention and therapy [107]. EGCG (epigallocatechin-3-gallate) is among the most potent anti-cancer elements in tea and is widely used in the treatment of cancer and many other diseases, alone or with traditional or ICI cancer therapies to reduce side effects [108, 109]. Its antitumor mechanisms encompass inducing cell cycle arrest, apoptosis and autophagic cell death, and it can reduce cisplatin-induced ototoxicity, DOX-induced cardiotoxicity, and cisplatin-induced neurotoxicity via inhibiting the STAT1 (signal-transducer-and-activator-of-transcription-1) and NF-κB signaling pathways [110]. EGCG could suppress lung tumor growth by inhibiting PD-L1 expression through numerous pathways, such as JAK2/STAT1 signaling and EGFR/Akt signaling, and therefore significantly restore T cell function [26]. While there are limited examples of the combination of EGCG and ICIs, a previous study has reported that EGCG may inhibit PD-1/PD-L1 interactions by dimerizing PD-L1. Through utilizing molecular modeling and computer analysis, researchers illustrate how EGCG induce and stabilize PD-L1 dimerization, highlighting its potential as a small anti-cancer molecule or complementary therapeutic drug in cancer immunotherapy [111]. However, further experiments verification is demanded to provide more comprehensive understandings on the inhibitory mechanism of EGCG in anti-PD-1/PD-L1 treatment, especially regarding its combination with immune checkpoint inhibitors.
Remaining questions and promising solutions about phytochemicals
The greatest advantage of phytochemicals is their potent anti-tumor properties with low toxicity, ideal for effective and safer cancer treatment. They suppress cancer cells mostly by modulating the expression of immune checkpoints or their ligands, activating T cells, inducing cell cycle arrest and apoptosis, and cooperating with general defense mechanisms of the human body such as gut microbiota and the innate immune functions [24, 112, 113] (Figure 2-4), rather than simply and directly killing cancer cells, resistance by the targeted cancer cells can maximally be reduced or avoided. Additionally, the majority of phytochemicals used in cancer treatment possess anti-oxidative, anti-inflammatory, or immune-protective properties, thus they can protect normal tissues from chemotherapy- and radiotherapy-induced toxicity and therefore mitigate related side effects. Collectively, phytochemicals have a great potential to be a very effective adjuvant to help improve the effectiveness of other anti-cancer treatments including traditional therapy and novel immunotherapy.
Unfortunately, phytochemicals, though ubiquitous and richly present in dietary plants, usually do not have the required amounts sufficient for cancer treatment or prevention. Furthermore, they may not stay long enough in the body to exert the desired anti-cancer functions. Many of them may have low water solubility and poor absorption (when phytochemicals reach the intestinal tract, they are rapidly metabolized), leading to low bioavailability to the cancer cells [114, 115]. Aim to increase the dispersibility, stability and bioactivity of phytochemicals, several delivery systems including nanoparticles, liposomes, and micelles have been investigated. With the continuous development of nanotechnology, new nano-materials and nanotechnology are increasingly used in medicine and pharmacy [116]. After the phytochemical is nano-sized, the physical properties (e.g., saturation solubility and hydrophilicity) and biological properties (e.g., molecular affinity) of it, are changed. For curcumin, low bioavailability is a common problem with oral administration of it [117], curcumin oral nanoformulations show higher solubility in various trials, encouraging us to develop formulations used in clinic with higher bioavailability [118]. On the other hand, not all phytochemicals are non-toxic, especially high dose of them should be used to meet effective dose, probably causing serious adverse effects such as allergic reactions, or liver or kidney toxicity, which is a topic awaiting systematic investigations.
As mentioned above, we found that many phytochemicals inhibit tumor proliferation by down-regulating PD-L1 expression, but some studies have shown that different phytochemicals have opposite effects on PD-L1 levels in different types of tumors, some even increase PD-L1 expression which may decrease the efficacy of the treatment [28, 119]. At the same time, many observations, including our previously published article, have disclosed the relevance of phytochemicals in the abundance of beneficial bacteria with contributions to the health of human beings [84, 120]. Furthermore, mounting evidence support that microbiome exhibit anti-cancer effects as a factor rather than a biomarker in assessing the efficacy of ICI therapy [121]. Therefore, there is a great need to rationally conduct more experiments such as cellular lines and animal models to elucidate the anti-tumor mechanisms of the combined application of phytochemicals plus ICIs, which will provide sufficient rationale to facilitate precision medicine. Considering the pivotal role of phytochemicals on human gut microbiome, establishing corresponding antitumor models to analyze the connection between differential phytochemicals and the abundance of beneficial bacteria is feasible and promising, which will also broaden application prospects of phytochemicals.
Combination therapy: conclusions and perspectives
In the current clinical setting, the main strategies to treat cancer still, like the last decades, consist of conventional methods including surgery, chemo- and radio-therapies, which do not provide adequate efficacy and often face recurrence, although novel therapies such as ICI therapy are becoming a choice. However, the low response of the disease to ICI and the high instance of adverse effects of ICI have seriously hampered this kind of treatment. Many patients receiving this therapy do not respond positively or experience immune-related adverse events (irAEs), such as gastrointestinal toxicity, endocrine toxicity, and dermatologic toxicity.
To overcome such problems, combination therapy comes to clinical application and provides great potential. Compared to monotherapies, combination therapy offers three major advantages, including considerably improved anti-cancer efficacy, expanded drug usage, and delayed drug resistance. The key approach to successful application of combination therapy is the selection of drugs based on the personalized medical information of the patient that act on different pathways or different mechanisms without cross-resistance or overlapping adverse effects.
In order to achieve good treatment results, reduce patient suffering and ease the economic burden, the continuous discovery and improvement of novel economical phytochemicals is a core issue. For example, lignans, such as enterolactone, enterodiol, secoisolariciresinol diglucoside or other compounds [24, 122, 123], have excellent suppressive effects on cancers alone, and we are in the process of evaluating these phytochemicals for combination therapy with ICI. To date, numerous experiments (e.g., in vitro, in vivo, clinical trials) has shown enormous potential of ICI plus phytochemicals combination in virous tumors, so it's hopeful to see their application in clinic in the near future [106, 124, 125]. With the development of high-throughput sequencing technology and popularization of personalized therapy, precision medicine based on genomic analysis (e.g., Next Generation Sequencing) has been popular and approved for clinical application of various diseases including cancer [126, 127]. In the meanwhile, gut microbiota genomic sequencing (e.g., 16S rRNA, whole genome sequencing) is also gradually applied in the field of cancer treatment, having a great prospect for choosing the appropriate treatment plan for patients and evaluating the treatment efficacy [128]. Additionally, toxicity assessment is also critical to the development of phytochemicals to aid less side effects and better treatment efficacy, whether evaluating single agent or agents used in combination. And the studies in predictive biomarkers for differentiating responders and nonresponders will greatly help in patient selection and alleviate immune-related adverse events.
The application of ICI plus phytochemicals combination has a promising perspective, not only should we be optimistic about this therapy, but also face their shortcomings and obstacle in clinical use. As we sated above, elucidating the activity and potential toxicity of various phytochemicals in different tumors as well as designing the mode of administration to promote their biological activity are of vital interest to boost the combination with ICI therapy. Furthermore, in order to facilitate the development of personalized therapies and the progress of immunotherapy, we should pay more attention to the discovery of relevant indicators to measure the effectiveness of the combination therapy.
Abbreviations
CDK: cyclin dependent kinase; CRC: colorectal cancer; CTLA-4: cytotoxic T-lymphocyte-associated antigen 4; DC: dendritic cells; EGCG: epigallocatechin-3-gallate; ELAM-1: endothelial leukocyte adhesion molecule-1; FDA: U.S. Food and Drug Administration; HCC: hepatocellular carcinoma; HNSCC: head and neck squamous cell carcinoma; ICAM-1: intracellular adhesion molecule-1; ICI: immune checkpoint inhibitor; IFN-g: interferon-g; IL-1: interleukin-1; IL-2: interleukin-2; IL-6: interleukin-6; IL-7: interleukin-7; IL-8: interleukin-8; Il-10: interleukin-10; IL-12: interleukin-12; irAEs: immune-related adverse events; IRs: inhibitory receptors; JAK2: Janus kinase 2; LAG-3: Lymphocyte activation gene 3; lgV: immunoglobulin variable; mAb: monoclonal antibody; MAPK: mitogen-activated protein kinase; MHC-II: Major histocompatibility complex class II; NCCN: National Comprehensive Cancer Network; NF-κB: nuclear factor-κB; NK cell: natural killer cell; NSCLC: non-small-cell lung carcinoma; PD-1: programmed cell death protein 1; PD-L1: programmed cell death-Ligand 1; PD-L2: programmed cell death-Ligand 2; RCC: renal cell carcinoma; STAT-1: signal-transducer-and-activator-of-transcription-1; STAT3: signal-transducer-and-activator-of-transcription-3; TIGIT: T cell immunoglobulin and ITIM domain; Tim-3: T cell immunoglobulin and mucin domain-containing protein 3; Tregs: regulatory T cells; TSCC: tongue squamous cell carcinoma; UC: urothelial carcinoma; VCAM-1: vascular cell adhesion molecule-1.
Acknowledgements
The authors thank all the colleagues of Genomics Research Center for comments on earlier versions of this manuscript.
Funding
The authors would like to express their gratitude to all the colleagues of Genomics Research Center for comments on the manuscript. This work was supported by the National Natural Science Foundation of China (82020108022, 82104217), the College Students' Innovation and Entrepreneurship Project in Heilongjiang Province (S202010226035), and the National College Students' Innovation and Entrepreneurship Project (201910226005). H.D.L. is financed by a grant from The Young Innovative Talents of Heilongjiang Province Universities (UNPYSCT-2018064), the China Postdoctoral Science Foundation (2018M630380), Heilongjiang Postdoctoral Financial Assistance (LBH-Z18198, LBH-Q21139), Merit-based Funding for Returned Oversea Students in Heilongjiang Province (2019QD0026), College of Pharmacy, Harbin Medical University, Excellent Young Talents Funding (2019-YQ-08), Harbin Medical University College of Pharmacy COVID-19 Funding for Medicine Research (CoV-202007) and HMU Marshal Initiative Funding (HMUMIF-21026).
Author contributions
LJL, a major contributor in writing the manuscript, conceived and drafted the manuscript. CJL, JLW, DLC and YRL contributed to provision of study thought, design and production of pictures, form making and data analysis. HDL, PFW, YFZ, YWS, ZXG, WXW, XK, SW, YYZ and MZX are involved in the revision of this article. LJL and CJL finalized the manuscript. HDL and SLL supervised LJL and provided the final approval of the version to be published. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Quatrini L, Mariotti FR, Munari E, Tumino N, Vacca P, Moretta L. The Immune Checkpoint PD-1 in Natural Killer Cells: Expression, Function and Targeting in Tumour Immunotherapy. Cancers. 2020 12
2. Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM. et al. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972-5
3. Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B. et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nature immunology. 2009;10:48-57
4. Hemon P, Jean-Louis F, Ramgolam K, Brignone C, Viguier M, Bachelez H. et al. MHC class II engagement by its ligand LAG-3 (CD223) contributes to melanoma resistance to apoptosis. Journal of immunology. 2011;186:5173-83
5. Anderson AC. Tim-3: an emerging target in the cancer immunotherapy landscape. Cancer immunology research. 2014;2:393-8
6. Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. The Journal of experimental medicine. 2010;207:2187-94
7. Trinh VA, Hwu WJ. Ipilimumab in the treatment of melanoma. Expert opinion on biological therapy. 2012;12:773-82
8. Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M. et al. Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2017;35:3924-33
9. Tomita Y, Fukasawa S, Shinohara N, Kitamura H, Oya M, Eto M. et al. Nivolumab versus everolimus in advanced renal cell carcinoma: Japanese subgroup 3-year follow-up analysis from the Phase III CheckMate 025 study. Japanese journal of clinical oncology. 2019;49:506-14
10. Tomita Y, Fukasawa S, Shinohara N, Kitamura H, Oya M, Eto M. et al. Nivolumab versus everolimus in advanced renal cell carcinoma: Japanese subgroup analysis from the CheckMate 025 study. Japanese journal of clinical oncology. 2017;47:639-46
11. Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn MJ. et al. Five-Year Overall Survival for Patients With Advanced NonSmall-Cell Lung Cancer Treated With Pembrolizumab: Results From the Phase I KEYNOTE-001 Study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2019;37:2518-27
12. Joshi SS, Maron SB, Catenacci DV. Pembrolizumab for treatment of advanced gastric and gastroesophageal junction adenocarcinoma. Future oncology. 2018;14:417-30
13. de Sousa LG, Ferrarotto R. Pembrolizumab in the first-line treatment of advanced head and neck cancer. Expert review of anticancer therapy. 2021;21:1321-31
14. Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD. et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. The New England journal of medicine. 2018;379:341-51
15. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J. et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837-46
16. Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J. et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389:67-76
17. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R. et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. The New England journal of medicine. 2018;379:2342-50
18. Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H. et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. The New England journal of medicine. 2020;383:1218-30
19. Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D'Angelo SP. et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. The Lancet Oncology. 2016;17:1374-85
20. Vaddepally RK, Kharel P, Pandey R, Garje R, Chandra AB. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers. 2020 12
21. Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chavez A, Keegan N, Khamashta MA. et al. Immune-related adverse events of checkpoint inhibitors. Nature reviews Disease primers. 2020;6:38
22. Wang CZ, Ma XQ, Yang DH, Guo ZR, Liu GR, Zhao GX. et al. Production of enterodiol from defatted flaxseeds through biotransformation by human intestinal bacteria. BMC Microbiol. 2010;10:115
23. Wang YF, Xu ZK, Yang DH, Yao HY, Ku BS, Ma XQ. et al. The antidepressant effect of secoisolariciresinol, a lignan-type phytoestrogen constituent of flaxseed, on ovariectomized mice. Journal of natural medicines. 2013;67:222-7
24. Wu H, Wang Y, Chen JT, Wang LP, Liu GR, Liu SL. Anticancer effects of dietary administration of secoisolariciresinol diglucoside in a patient of gastrointestinal stromal tumor: a case report. International Journal of Surgery Oncology. 2021 5
25. Lin CC, Chin YT, Shih YJ, Chen YR, Chung YY, Lin CY. et al. Resveratrol antagonizes thyroid hormone-induced expression of checkpoint and proliferative genes in oral cancer cells. Journal of dental sciences. 2019;14:255-62
26. Rawangkan A, Wongsirisin P, Namiki K, Iida K, Kobayashi Y, Shimizu Y. et al. Green Tea Catechin Is an Alternative Immune Checkpoint Inhibitor that Inhibits PD-L1 Expression and Lung Tumor Growth. Molecules. 2018 23
27. Coombs MRP, Harrison ME, Hoskin DW. Apigenin inhibits the inducible expression of programmed death ligand 1 by human and mouse mammary carcinoma cells. Cancer letters. 2016;380:424-33
28. Ke M, Zhang Z, Xu B, Zhao S, Ding Y, Wu X. et al. Baicalein and baicalin promote antitumor immunity by suppressing PD-L1 expression in hepatocellular carcinoma cells. International immunopharmacology. 2019;75:105824
29. Liao F, Liu L, Luo E, Hu J. Curcumin enhances anti-tumor immune response in tongue squamous cell carcinoma. Archives of oral biology. 2018;92:32-7
30. Lim SO, Li CW, Xia W, Cha JH, Chan LC, Wu Y. et al. Deubiquitination and Stabilization of PD-L1 by CSN5. Cancer cell. 2016;30:925-39
31. Mazewski C, Kim MS, Gonzalez de Mejia E. Anthocyanins, delphinidin-3-O-glucoside and cyanidin-3-O-glucoside, inhibit immune checkpoints in human colorectal cancer cells in vitro and in silico. Scientific reports. 2019;9:11560
32. Kang DY, Sp N, Jo ES, Rugamba A, Hong DY, Lee HG. et al. The Inhibitory Mechanisms of Tumor PD-L1 Expression by Natural Bioactive Gallic Acid in Non-Small-Cell Lung Cancer (NSCLC) Cells. Cancers. 2020 12
33. Kongtawelert P, Wudtiwai B, Shwe TH, Pothacharoen P, Phitak T. Inhibitory Effect of Hesperidin on the Expression of Programmed Death Ligand (PD-L1) in Breast Cancer. Molecules. 2020 25
34. Bailly C. Molecular and cellular basis of the anticancer activity of the prenylated flavonoid icaritin in hepatocellular carcinoma. Chemico-biological interactions. 2020;325:109124
35. Jin Y, Zhan X, Zhang B, Chen Y, Liu C, Yu L. Polydatin Exerts an Antitumor Effect Through Regulating the miR-382/PD-L1 Axis in Colorectal Cancer. Cancer biotherapy & radiopharmaceuticals. 2020;35:83-91
36. Lucas J, Hsieh TC, Halicka HD, Darzynkiewicz Z, Wu JM. Upregulation of PDL1 expression by resveratrol and piceatannol in breast and colorectal cancer cells occurs via HDAC3/p300mediated NFkappaB signaling. International journal of oncology. 2018;53:1469-80
37. Peredo-Lovillo A, Romero-Luna HE, Jiménez-Fernández M. Health promoting microbial metabolites produced by gut microbiota after prebiotics metabolism. Food research international (Ottawa, Ont). 2020;136:109473
38. Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Experimental & molecular medicine. 2018;50:1-11
39. Hamidi H, Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nature reviews Cancer. 2018;18:533-48
40. Farhood B, Najafi M, Mortezaee K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: A review. Journal of cellular physiology. 2019;234:8509-21
41. Dermani FK, Samadi P, Rahmani G, Kohlan AK, Najafi R. PD-1/PD-L1 immune checkpoint: Potential target for cancer therapy. Journal of cellular physiology. 2019;234:1313-25
42. Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. The EMBO journal. 1992;11:3887-95
43. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annual review of immunology. 2008;26:677-704
44. Kluger HM, Zito CR, Turcu G, Baine MK, Zhang H, Adeniran A. et al. PD-L1 Studies Across Tumor Types, Its Differential Expression and Predictive Value in Patients Treated with Immune Checkpoint Inhibitors. Clinical cancer research: an official journal of the American Association for Cancer Research. 2017;23:4270-9
45. Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I. et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nature immunology. 2001;2:261-8
46. Abiko K, Matsumura N, Hamanishi J, Horikawa N, Murakami R, Yamaguchi K. et al. IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. British journal of cancer. 2015;112:1501-9
47. Sul J, Blumenthal GM, Jiang X, He K, Keegan P, Pazdur R. FDA Approval Summary: Pembrolizumab for the Treatment of Patients With Metastatic Non-Small Cell Lung Cancer Whose Tumors Express Programmed Death-Ligand 1. The oncologist. 2016;21:643-50
48. Mahoney KM, Freeman GJ, McDermott DF. The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma. Clinical therapeutics. 2015;37:764-82
49. Jin KT, Wang SB, Ying XJ, Lan HR, Lv JQ, Zhang LH. et al. Immune-mediated adverse effects of immune-checkpoint inhibitors and their management in cancer. Immunology letters. 2020;221:61-71
50. Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM. et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405-13
51. Greenwald RJ, Boussiotis VA, Lorsbach RB, Abbas AK, Sharpe AH. CTLA-4 regulates induction of anergy in vivo. Immunity. 2001;14:145-55
52. Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. Journal of experimental & clinical cancer research: CR. 2019;38:255
53. Huard B, Mastrangeli R, Prigent P, Bruniquel D, Donini S, El-Tayar N. et al. Characterization of the major histocompatibility complex class II binding site on LAG-3 protein. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:5744-9
54. Bettini M, Szymczak-Workman AL, Forbes K, Castellaw AH, Selby M, Pan X. et al. Cutting edge: accelerated autoimmune diabetes in the absence of LAG-3. Journal of immunology. 2011;187:3493-8
55. Kim D, Le HT, Nguyen QT, Kim S, Lee J, Min B. Cutting Edge: IL-27 Attenuates Autoimmune Neuroinflammation via Regulatory T Cell/Lag3-Dependent but IL-10-Independent Mechanisms In Vivo. Journal of immunology. 2019;202:1680-5
56. Maruhashi T, Sugiura D, Okazaki IM, Okazaki T. LAG-3: from molecular functions to clinical applications. Journal for immunotherapy of cancer. 2020 8
57. Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ. et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nature immunology. 2005;6:1245-52
58. Granier C, Dariane C, Combe P, Verkarre V, Urien S, Badoual C. et al. Tim-3 Expression on Tumor-Infiltrating PD-1(+)CD8(+) T Cells Correlates with Poor Clinical Outcome in Renal Cell Carcinoma. Cancer research. 2017;77:1075-82
59. Kang CW, Dutta A, Chang LY, Mahalingam J, Lin YC, Chiang JM. et al. Apoptosis of tumor infiltrating effector TIM-3+CD8+ T cells in colon cancer. Scientific reports. 2015;5:15659
60. Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ. et al. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. Journal of virology. 2009;83:9122-30
61. Joller N, Peters A, Anderson AC, Kuchroo VK. Immune checkpoints in central nervous system autoimmunity. Immunological reviews. 2012;248:122-39
62. Deuss FA, Gully BS, Rossjohn J, Berry R. Recognition of nectin-2 by the natural killer cell receptor T cell immunoglobulin and ITIM domain (TIGIT). The Journal of biological chemistry. 2017;292:11413-22
63. Bottino C, Castriconi R, Pende D, Rivera P, Nanni M, Carnemolla B. et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. The Journal of experimental medicine. 2003;198:557-67
64. Kong Y, Zhu L, Schell TD, Zhang J, Claxton DF, Ehmann WC. et al. T-Cell Immunoglobulin and ITIM Domain (TIGIT) Associates with CD8+ T-Cell Exhaustion and Poor Clinical Outcome in AML Patients. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016;22:3057-66
65. Zhang Q, Bi J, Zheng X, Chen Y, Wang H, Wu W. et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nature immunology. 2018;19:723-32
66. Dixon KO, Schorer M, Nevin J, Etminan Y, Amoozgar Z, Kondo T. et al. Functional Anti-TIGIT Antibodies Regulate Development of Autoimmunity and Antitumor Immunity. Journal of immunology. 2018;200:3000-7
67. Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH. et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32:1020-30
68. Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA. et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33:2004-12
69. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366:2443-54
70. Pal SK, Hu A, Chang M, Figlin RA. Programmed death-1 inhibition in renal cell carcinoma: clinical insights and future directions. Clinical advances in hematology & oncology: H&O. 2014;12:90-9
71. Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H. et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. The New England journal of medicine. 2018;379:2108-21
72. Powles T, van der Heijden MS, Castellano D, Galsky MD, Loriot Y, Petrylak DP. et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. The Lancet Oncology. 2020;21:1574-88
73. Blansfield JA, Beck KE, Tran K, Yang JC, Hughes MS, Kammula US. et al. Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. Journal of immunotherapy. 2005;28:593-8
74. Rijavec E, Genova C, Barletta G, Burrafato G, Biello F, Dal Bello MG. et al. Ipilimumab in non-small cell lung cancer and small-cell lung cancer: new knowledge on a new therapeutic strategy. Expert opinion on biological therapy. 2014;14:1007-17
75. Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP. et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. The Lancet Oncology. 2018;19:672-81
76. Lipson EJ, Drake CG. Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17:6958-62
77. Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L. et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. The Lancet Oncology. 2010;11:155-64
78. Guazzelli A, Hussain M, Krstic-Demonacos M, Mutti L. Tremelimumab for the treatment of malignant mesothelioma. Expert opinion on biological therapy. 2015;15:1819-29
79. He M, Chai Y, Qi J, Zhang CWH, Tong Z, Shi Y. et al. Remarkably similar CTLA-4 binding properties of therapeutic ipilimumab and tremelimumab antibodies. Oncotarget. 2017;8:67129-39
80. Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J. et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients With Advanced Melanoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2017;35:785-92
81. Abu-Sbeih H, Ali FS, Wang Y. Immune-checkpoint inhibitors induced diarrhea and colitis: a review of incidence, pathogenesis and management. Current opinion in gastroenterology. 2020;36:25-32
82. Patridge E, Gareiss P, Kinch MS, Hoyer D. An analysis of FDA-approved drugs: natural products and their derivatives. Drug discovery today. 2016;21:204-7
83. Li T, Ferns K, Yan ZQ, Yin SY, Kou JJ, Li D. et al. Acanthopanax senticosus: Photochemistry and Anticancer Potential. The American journal of Chinese medicine. 2016;44:1543-58
84. Lin C, Zeng Z, Lin Y, Wang P, Cao D, Xie K. et al. Naringenin suppresses epithelial ovarian cancer by inhibiting proliferation and modulating gut microbiota. Phytomedicine: international journal of phytotherapy and phytopharmacology. 2022;106:154401
85. Liu H, Liu SL. Pharmacological Effects of Natural Components Against Ovarian Cancer and Mechanisms. Advances in experimental medicine and biology. 2021;1330:55-73
86. Ranjan A, Ramachandran S, Gupta N, Kaushik I, Wright S, Srivastava S. et al. Role of Phytochemicals in Cancer Prevention. International journal of molecular sciences. 2019 20
87. Zhu F, Du B, Xu B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Critical reviews in food science and nutrition. 2018;58:1260-70
88. Ho JW, Cheung MW. Combination of phytochemicals as adjuvants for cancer therapy. Recent patents on anti-cancer drug discovery. 2014;9:297-302
89. Lu Y, Yuan X, Wang M, He Z, Li H, Wang J. et al. Gut microbiota influence immunotherapy responses: mechanisms and therapeutic strategies. Journal of hematology & oncology. 2022;15:47
90. Li X, Zhang S, Guo G, Han J, Yu J. Gut microbiome in modulating immune checkpoint inhibitors. EBioMedicine. 2022;82:104163
91. Rauf A, Imran M, Butt MS, Nadeem M, Peters DG, Mubarak MS. Resveratrol as an anti-cancer agent: A review. Critical reviews in food science and nutrition. 2018;58:1428-47
92. Bishayee A. Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. Cancer prevention research. 2009;2:409-18
93. Malaguarnera L. Influence of Resveratrol on the Immune Response. Nutrients. 2019 11
94. Zhang Y, Yang S, Yang Y, Liu T. Resveratrol induces immunogenic cell death of human and murine ovarian carcinoma cells. Infectious agents and cancer. 2019;14:27
95. Verdura S, Cuyas E, Cortada E, Brunet J, Lopez-Bonet E, Martin-Castillo B. et al. Resveratrol targets PD-L1 glycosylation and dimerization to enhance antitumor T-cell immunity. Aging. 2020;12:8-34
96. Chaplin A, Carpéné C, Mercader J. Resveratrol, Metabolic Syndrome, and Gut Microbiota. Nutrients. 2018 10
97. Kocaadam B, Sanlier N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Critical reviews in food science and nutrition. 2017;57:2889-95
98. Tilak JC, Banerjee M, Mohan H, Devasagayam TP. Antioxidant availability of turmeric in relation to its medicinal and culinary uses. Phytotherapy research: PTR. 2004;18:798-804
99. Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. Journal of immunology. 2003;171:3863-71
100. Shanmugam MK, Rane G, Kanchi MM, Arfuso F, Chinnathambi A, Zayed ME. et al. The multifaceted role of curcumin in cancer prevention and treatment. Molecules. 2015;20:2728-69
101. Xiao Z, Su Z, Han S, Huang J, Lin L, Shuai X. Dual pH-sensitive nanodrug blocks PD-1 immune checkpoint and uses T cells to deliver NF-κB inhibitor for antitumor immunotherapy. Science advances. 2020;6:eaay7785
102. Bhandarkar SS, Arbiser JL. Curcumin as an inhibitor of angiogenesis. Advances in experimental medicine and biology. 2007;595:185-95
103. Giordano A, Tommonaro G. Curcumin and Cancer. Nutrients. 2019 11
104. Shao Y, Zhu W, Da J, Xu M, Wang Y, Zhou J. et al. Bisdemethoxycurcumin in combination with α-PD-L1 antibody boosts immune response against bladder cancer. OncoTargets and therapy. 2017;10:2675-83
105. Hayakawa T, Yaguchi T, Kawakami Y. Enhanced anti-tumor effects of the PD-1 blockade combined with a highly absorptive form of curcumin targeting STAT3. Cancer science. 2020;111:4326-35
106. Tuyaerts S, Van Nuffel AMT, Naert E, Van Dam PA, Vuylsteke P, De Caluwé A. et al. PRIMMO study protocol: a phase II study combining PD-1 blockade, radiation and immunomodulation to tackle cervical and uterine cancer. BMC cancer. 2019;19:506
107. Zubair H, Azim S, Ahmad A, Khan MA, Patel GK, Singh S. et al. Cancer Chemoprevention by Phytochemicals: Nature's Healing Touch. Molecules. 2017 22
108. Pons-Fuster López E, Gómez García F, López Jornet P. Combination of 5-Florouracil and polyphenol EGCG exerts suppressive effects on oral cancer cells exposed to radiation. Archives of oral biology. 2019;101:8-12
109. Chen D, Wan SB, Yang H, Yuan J, Chan TH, Dou QP. EGCG, green tea polyphenols and their synthetic analogs and prodrugs for human cancer prevention and treatment. Advances in clinical chemistry. 2011;53:155-77
110. Arafa MH, Atteia HH. Protective Role of Epigallocatechin Gallate in a Rat Model of Cisplatin-Induced Cerebral Inflammation and Oxidative Damage: Impact of Modulating NF-κB and Nrf2. Neurotoxicity research. 2020;37:380-96
111. Guo Y, Liang J, Liu B, Jin Y. Molecular Mechanism of Food-Derived Polyphenols on PD-L1 Dimerization: A Molecular Dynamics Simulation Study. International journal of molecular sciences. 2021 22
112. Zhuang H, Cheng L, Wang Y, Zhang YK, Zhao MF, Liang GD. et al. Dysbiosis of the Gut Microbiome in Lung Cancer. Frontiers in cellular and infection microbiology. 2019;9:112
113. Ferreira de Oliveira JMP, Santos C, Fernandes E. Therapeutic potential of hesperidin and its aglycone hesperetin: Cell cycle regulation and apoptosis induction in cancer models. Phytomedicine: international journal of phytotherapy and phytopharmacology. 2020;73:152887
114. Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. The American journal of clinical nutrition. 2005;81:230s-42s
115. Vareed SK, Kakarala M, Ruffin MT, Crowell JA, Normolle DP, Djuric Z. et al. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17:1411-7
116. Angelova A, Garamus VM, Angelov B, Tian Z, Li Y, Zou A. Advances in structural design of lipid-based nanoparticle carriers for delivery of macromolecular drugs, phytochemicals and anti-tumor agents. Advances in colloid and interface science. 2017;249:331-45
117. Hu Q, Luo Y. Chitosan-based nanocarriers for encapsulation and delivery of curcumin: A review. International journal of biological macromolecules. 2021;179:125-35
118. Ma Z, Wang N, He H, Tang X. Pharmaceutical strategies of improving oral systemic bioavailability of curcumin for clinical application. Journal of controlled release: official journal of the Controlled Release Society. 2019;316:359-80
119. Lucas J, Hsieh TC, Halicka HD, Darzynkiewicz Z, Wu JM. Upregulation of PD-L1 expression by resveratrol and piceatannol in breast and colorectal cancer cells occurs via HDAC3/p300-mediated NF-κB signaling. International journal of oncology. 2018;53:1469-80
120. Wu HJ, Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut microbes. 2012;3:4-14
121. Chung MW, Kim MJ, Won EJ, Lee YJ, Yun YW, Cho SB. et al. Gut microbiome composition can predict the response to nivolumab in advanced hepatocellular carcinoma patients. World journal of gastroenterology. 2021;27:7340-9
122. Liu H, Liu J, Wang S, Zeng Z, Li T, Liu Y. et al. Enterolactone has stronger effects than enterodiol on ovarian cancer. Journal of ovarian research. 2017;10:49
123. Haque A, Brazeau D, Amin AR. Perspectives on natural compounds in chemoprevention and treatment of cancer: an update with new promising compounds. European journal of cancer (Oxford, England: 1990). 2021;149:165-83
124. Emens LA, Molinero L, Loi S, Rugo HS, Schneeweiss A, Diéras V. et al. Atezolizumab and nab-Paclitaxel in Advanced Triple-Negative Breast Cancer: Biomarker Evaluation of the IMpassion130 Study. Journal of the National Cancer Institute. 2021;113:1005-16
125. Ge YX, Zhang TW, Zhou L, Ding W, Liang HF, Hu ZC. et al. Enhancement of anti-PD-1/PD-L1 immunotherapy for osteosarcoma using an intelligent autophagy-controlling metal organic framework. Biomaterials. 2022;282:121407
126. Morganti S, Tarantino P, Ferraro E, D'Amico P, Duso BA, Curigliano G. Next Generation Sequencing (NGS): A Revolutionary Technology in Pharmacogenomics and Personalized Medicine in Cancer. Advances in experimental medicine and biology. 2019;1168:9-30
127. Jackson SE, Chester JD. Personalised cancer medicine. International journal of cancer. 2015;137:262-6
128. Cammarota G, Ianiro G, Ahern A, Carbone C, Temko A, Claesson MJ. et al. Gut microbiome, big data and machine learning to promote precision medicine for cancer. Nature reviews Gastroenterology & hepatology. 2020;17:635-48
129. Wudtiwai B, Makeudom A, Krisanaprakornkit S, Pothacharoen P, Kongtawelert P. Anticancer Activities of Hesperidin via Suppression of Up-Regulated Programmed Death-Ligand 1 Expression in Oral Cancer Cells. Molecules. 2021 26
130. Kuttan R, Sudheeran PC, Josph CD. Turmeric and curcumin as topical agents in cancer therapy. Tumori. 1987;73:29-31
131. Liang Y, Li S, Zheng G, Zhang L. β-elemene suppresses the malignant behavior of esophageal cancer cells by regulating the phosphorylation of AKT. Acta histochemica. 2020;122:151538
132. Jiang Z, Yang Y, Yang Y, Zhang Y, Yue Z, Pan Z. et al. Ginsenoside Rg3 attenuates cisplatin resistance in lung cancer by downregulating PD-L1 and resuming immune. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2017;96:378-83
133. Chen Y, Zhang Y, Song W, Zhang Y, Dong X, Tan M. Ginsenoside Rh2 Improves the Cisplatin Anti-tumor Effect in Lung Adenocarcinoma A549 Cells via Superoxide and PD-L1. Anti-cancer agents in medicinal chemistry. 2020;20:495-503
134. Hu M, Yang J, Qu L, Deng X, Duan Z, Fu R. et al. Ginsenoside Rk1 induces apoptosis and downregulates the expression of PD-L1 by targeting the NF-κB pathway in lung adenocarcinoma. Food & function. 2020;11:456-71
135. Huang MY, Jiang XM, Xu YL, Yuan LW, Chen YC, Cui G. et al. Platycodin D triggers the extracellular release of programed death Ligand-1 in lung cancer cells. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2019;131:110537
136. Liu Y, Liu X, Zhang N, Yin M, Dong J, Zeng Q. et al. Berberine diminishes cancer cell PD-L1 expression and facilitates antitumor immunity via inhibiting the deubiquitination activity of CSN5. Acta pharmaceutica Sinica B. 2020;10:2299-312
137. Banerjee P, Zhang R, Ivan C, Galletti G, Clise-Dwyer K, Barbaglio F. et al. Trabectedin Reveals a Strategy of Immunomodulation in Chronic Lymphocytic Leukemia. Cancer immunology research. 2019;7:2036-51
138. Yao G, Liu C, Huo H, Liu A, Lv B, Zhang C. et al. Ethanol extract of Chaenomeles speciosa Nakai induces apoptosis in cancer cells and suppresses tumor growth in mice. Oncology letters. 2013;6:256-60
139. Tan X, Chen W, Jiao C, Liang H, Yun H, He C. et al. Anti-tumor and immunomodulatory activity of the aqueous extract of Sarcodon imbricatus in vitro and in vivo. Food & function. 2020;11:1110-21
Author contact
![]() Corresponding authors: Huidi Liu (hdliuedu.cn) and Shu-Lin Liu (slliuedu.cn), Genomics Research Center (Key Laboratory of Gut Microbiota and Pharmacogenomics of Heilongjiang Province, State-Province Key Laboratory of Biomedicine-Pharmaceutics of China) College of Pharmacy, Harbin Medical University. 157 Baojian Road, Harbin, 150081, China; Tel.: +86 151 0469 5693; Fax: +86 451 86692236.
Corresponding authors: Huidi Liu (hdliuedu.cn) and Shu-Lin Liu (slliuedu.cn), Genomics Research Center (Key Laboratory of Gut Microbiota and Pharmacogenomics of Heilongjiang Province, State-Province Key Laboratory of Biomedicine-Pharmaceutics of China) College of Pharmacy, Harbin Medical University. 157 Baojian Road, Harbin, 150081, China; Tel.: +86 151 0469 5693; Fax: +86 451 86692236.

 Global reach, higher impact
Global reach, higher impact