Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(14):2644-2654. doi:10.7150/jca.87169 This issue Cite
Research Paper
Doublet or Triplet Antiemetic Prophylaxis for Nausea and Vomiting Induced by Trastuzumab Deruxtecan: an Open-Label, Randomized, and Multicenter Exploratory Phase 2 Study
1. Department of Pharmacy, Gifu University Hospital, 1-1 Yanagido, Gifu, Gifu 501-1194, Japan.
2. Patient Safety Division, Gifu University Hospital, 1-1 Yanagido, Gifu, Gifu 501-1194, Japan.
3. Laboratory of Pharmacy Practice and Social Science, Gifu Pharmaceutical University, 1-25-4 Daigakunishi, Gifu, Gifu 501-1196, Japan.
4. Department of Biostatistics, Yamaguchi University Graduate School of Medicine, 1-1-1 Minamikogushi, Ube, Yamaguchi 755-8505, Japan.
5. Cancer Biostatistics Laboratory, Clinical Research Institute, National Hospital Organization Kyushu Cancer Center, 3-1-1 Notame, Minami-ku, Fukuoka 811-1395, Japan.
6. Institute of Medicine, Breast and Endocrine Surgery, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki 305-8575, Japan.
7. Department of Breast Surgery, Gifu University Hospital, 1-1 Yanagido, Gifu, Gifu 501-1194, Japan.
8. Department of Breast Surgery, Yokkaichi Municipal Hospital, 2-2-37 Shibata, Yokkaichi, Mie 510-8567, Japan.
9. Department of Breast Surgery, Asahi University Hospital, 3-23 Hashimoto-cho, Gifu, Gifu 500-8523, Japan.
10. Department of Breast Center, Ehime University Hospital, 454 Shitsukawa, Toon, Ehime 791-0295, Japan.
11. Department of Breast Surgery, Miyagi Cancer Center, 47-1 Aza-nodayama, Medeshima-shiote, Natori, Miyagi 981-1239, Japan.
12. Department of First Surgery, Yamagata University Graduate School of Medicine, 2-2-2 Iida-Nishi, Yamagata, Yamagata, 990-9585, Japan (present address).
13. Department of Surgery, Iwate Medical University, 2-1-1 Idaidori, Yahaba, Shiwa, Iwate 028-3695, Japan.
14. Department of Breast Surgery, Central Japan International Medical Hospital, 1-1 Kenkonomachi, Minokamo, Gifu 505-8510, Japan.
15. Department of Surgery, Gihoku Kosei Hospital, 1187-3 Takatomi, Yamagata, Gifu 501-2105, Japan.
16. Department of Pharmacy, Fukushima Medical University Hospital, 1 Hikariga-oka, Fukushima 960-1247, Japan.
17. Department of Breast Surgery, Gifu Municipal hospital, 7-1 Kashimacho, Gifu, Gifu 500-8513, Japan.
18. Department of Breast Surgery, Gifu Prefectural General Medical Center, 4-6-1 Noishiki, Gifu, Gifu 500-8717, Japan.
19. Department of Breast and Endocrine Surgery, St. Marianna University School of Medicine, 2-16-1 Sugao, Miyamae, Kawasaki, Kanagawa 216-8511, Japan.
20. Department of Pharmacy, Tohoku Rosai Hospital, 4-3-21 Dainohara, Miyagino, Sendai, Miyagi 981-8563, Japan.
21. Department of breast, thyroid, endocrine surgery, University of Tsukuba Hospital, 2-1-1 Amakubo, Tsukuba, Ibaraki 305-8576, Japan.
22. Department of Pharmacy, Yokkaichi Municipal Hospital, 2-2-37 Shibata, Yokkaichi, Mie 510-8567, Japan.
23. Department of Pharmacy, Asahi University Hospital, 3-23 Hashimoto-cho, Gifu, Gifu 500-8523, Japan.
24. Department of Pharmacy, Miyagi Cancer Center, 47-1 Nodayama, Medeshimashiote, Natori, Miyagi 981-1293, Japan.
25. Laboratory of Advanced Medical Pharmacy, Gifu Pharmaceutical University, 1-25-4 Daigakunishi, Gifu, Gifu, 501-1196, Japan.
Received 2023-6-14; Accepted 2023-8-17; Published 2023-8-28
Abstract
Background: Trastuzumab deruxtecan is classified as an anticancer agent that poses a moderate emetic risk in the international guidelines for antiemetic therapy. The guidelines recommend emesis prophylaxis using a two-drug combination therapy comprising a 5-hydroxytryptamine-3 receptor antagonist (5-HT3RA) and dexamethasone (DEX). However, the high incidence of nausea and vomiting associated with trastuzumab deruxtecan is problematic. The National Comprehensive Cancer Network guideline version 1.2023 classified trastuzumab deruxtecan as having a high risk of emesis and changed its recommendation to a triplet regimen including a neurokinin-1 receptor antagonist (NK1RA). However, the emetogenic potential of trastuzumab-deruxtecan and the optimal antiemetic prophylaxis are controversial. Hence, this exploratory phase 2 study aimed to assess the efficacy and safety of treatment comprising 5-HT3RA and DEX with or without a NK1RA in preventing trastuzumab deruxtecan-induced nausea and vomiting.
Methods: We conducted an open-label and randomized exploratory phase 2 study at 14 centers in Japan. Patients with breast cancer who were scheduled to receive trastuzumab deruxtecan were enrolled in this study. The patients were randomly assigned to receive granisetron and DEX (arm GD) or granisetron, DEX, and aprepitant (fosaprepitant; arm GDA). The primary endpoint was complete response (CR; no emesis or no rescue therapy) during the overall phase (120 h after the start of trastuzumab deruxtecan).
Results: Between September 2020 and March 2023, 40 patients were randomly assigned to the GD (n = 19) or GDA (n = 21) arm. In the GDA arm, one patient who did not complete the use of the rescue medication listed in the diary was excluded from the efficacy analysis, which included the use of rescue medication. The CR rates during the overall phase were 36.8% and 70.0% in the GD and GDA arms, respectively (odds ratio 0.1334; 95% confidence interval [CI]: 0.0232-0.7672; P = 0.0190), with a difference of 33.2%. No grade 3 or 4 toxicity related to antiemetic therapy was observed.
Conclusions: Patients receiving trastuzumab deruxtecan require triple therapy, including mandatory NK1RA administration.
Keywords: breast cancer, antiemetic regimen, trastuzumab deruxtecan, nausea, vomiting
Introduction
Trastuzumab deruxtecan is a conjugate of the humanized anti-human epidermal growth factor receptor 2 (HER2) antibody with topoisomerase I inhibitor payload using an enzymatically cleavable peptide-based linker [1]. The cytotoxic payload is deruxtecan, which is a camptothecin analog. Trastuzumab deruxtecan has been developed for the treatment of HER2-expressing solid tumors [2,3]. In the DESTINY-Breast01 trial, trastuzumab deruxtecan showed an extremely high level of clinical activity with an overall response rate of 60.9% and progression-free survival of 16.4 months in a previously treated patient population with HER2-positive metastatic breast cancer [4]. On December 20, 2019, the drug received an accelerated approval from the United States Food and Drug Administration for treating patients with unresectable or metastatic HER2-positive breast cancer. It has since been approved for use in Japan and Europe. Trastuzumab deruxtecan is highly effective against HER2-positive gastric cancer, HER2-mutated non-small-cell lung cancer, and HER2-expressing metastatic colorectal cancer [5-7]. However, it was associated with an elevated rate of nausea and vomiting in the corresponding trials. The respective incidence rates of nausea and vomiting in patients with breast, gastric, non-small cell lung, and colorectal cancers were as follows: 77.7% and 45.7%, 63% and 26%, 73% and 40%, and 62.3% and 29.5% [5-7]. In response, the American Society of Clinical Oncology guideline for antiemetics and the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology (Antiemesis version 1.2020), classified trastuzumab deruxtecan as an anticancer agent with a moderate emetic risk [8,9]. However, the DESTINY trials did not explore antiemetic therapy or the antiemetic effects against trastuzumab deruxtecan action. Moreover, there is no information available in terms of antiemetic therapy. DESTINY-Breast04 study, the protocol recommended prophylactic antiemetic therapy with a 5-hydroxytryptamine receptor antagonist (5-HT3RA) or neurokinin-1 receptor antagonist (NK1RA) and/or a steroid, such as dexamethasone (DEX) since 2020. However, it is unclear which combination regimen was most often administered. [10].
Recent guidelines recommend emesis prophylaxis using a two-drug combination therapy comprising a 5-HT3RA and DEX for chemotherapy with a moderate emetic risk [8,9,11,12]. However, these guidelines suggest a three-drug combination therapy comprising a 5-HT3RA, DEX, and NK1RA prophylactic regimen for patients with additional risk factors or those in whom previous treatment with a 5HT3RA and DEX failed [9]. There are also anticancer agents, such as carboplatin, that are considered highly emetogenic with a moderate emetic risk (classified as high emetic risk by the NCCN) but are recommended as a special exception for triplet therapy comprising 5-HT3RA, DEX, and NK1RA. In January 2023, the NCCN guidelines (version 1.2023) reclassified trastuzumab deruxtecan as having a high emetic risk [9].
It is unclear whether two- or three-drug combinations should be recommended for trastuzumab deruxtecan-treated patients with a high incidence of nausea and vomiting. Complete response (CR) is a standard method of evaluating antiemetic therapy. This study aimed to investigate the CR (no emesis or rescue therapy) rate of trastuzumab deruxtecan-treated patients with breast cancer using a two-drug combination of granisetron and DEX or a three-drug combination of granisetron, DEX, and aprepitant (fosaprepitant) as antiemetic treatments.
Methods
Study design
This open-label, multicenter, and randomized exploratory phase 2 study was conducted in 14 Japanese hospitals (cancer centers, private hospitals, public hospitals, and university hospitals) in accordance with the Declaration of Helsinki and the Ethical Guidelines for Clinical Studies. The study was approved by the institutional review board of each participating hospital and independently monitored by the Alliance Data Center and Safety Monitoring Board. Data collection and analysis were conducted using alliance statistics and data centers. Data quality was ensured by a review of the data performed by the Alliance Statistics and Data Center and by the principal investigator according to Alliance policies. The study was registered with the University Hospital Medical Information Network (UMIN000041004; principal investigators: HI and MF; primary statistician: MS).
Randomization
Eligible patients were randomized (1:1) to receive either granisetron and DEX (GD arm) or granisetron, DEX, and aprepitant/fosaprepitant (GDA arm). Registration and randomization were performed using a web entry system requiring a personal account and password. The participants were stratified and randomly allocated to either the GD or GDA arm by using permuted blocks. The stratification was based on two factors, age (≥ 55 years vs < 55 years) and previous experience with chemotherapy-induced nausea and vomiting (CINV; yes vs no). The block sizes were set to 2 and 4 to guarantee balanced allocation.
Patient selection
Patients with breast cancer scheduled to receive trastuzumab deruxtecan for the first time were enrolled in this study. Other eligibility criteria were as follows: age of ≥ 20 years; absence of current use of any drug with antiemetic activity or drugs with a risk of nausea, for example, 5-HT3RA, NK1RA, corticosteroids, antidopamine agonists, phenothiazine tranquilizers, serotonin dopamine antagonists, multi-acting receptor-targeted antipsychotics, benzodiazepine agents, selective serotonin reuptake inhibitors, and serotonin noradrenaline reuptake inhibitors; aspartate aminotransferase and alanine aminotransferase levels of ≤ 100U/L; total bilirubin level of ≤ 2.0 mg/dL; and provision of written informed consent.
The exclusion criteria were as follows: patients with a history of hypersensitivity or allergy due to the study drugs or similar compounds; patients who needed antiemetics at enrollment; patients who started taking opioids within 48 h before enrollment; patients with unstable angina, ischemic heart disease, cerebral hemorrhage or apoplexy, or an active gastric or duodenal ulcer within 6 months before enrollment; patients with convulsive disorders requiring anticonvulsant therapy; patients with ascites effusion requiring paracentesis; patients with gastrointestinal obstruction; breastfeeding or expecting women or those who did not wish to use contraception; patients with psychosis or psychiatric symptoms that interfere with daily life; and patients who were judged to be unsuitable for the study by the investigators.
Treatment regimen
Antiemetic therapy was administered according to the ASCO 2020 recommendations for carboplatin as follows [8]: patients in both treatment arms were administered granisetron (1 mg intravenous infusion 30 min before trastuzumab deruxtecan on day 1). Those in the GD arm were administered DEX (6.6 mg intravenous infusion 30 min before trastuzumab deruxtecan administration on day 1 and 8 mg oral administration on days 2-3). The patients in the GDA arm were administered DEX (9.9 mg intravenous infusion 30 min before trastuzumab deruxtecan on day 1) and NK1RA (125 mg of aprepitant orally administered 60 min before chemotherapy on day 1 and 80 mg oral administration on days 2 and 3, or 150 mg fosaprepitant intravenous infusion 60 min before trastuzumab deruxtecan on day 1). Patients were allowed to take rescue medication for CINV, if necessary, throughout the study period. All the patients were prescribed rescue medications. The choice of the recommended rescue medication was determined from among metoclopramide, domperidone, and prochlorperazine specified in the protocol by each investigator.
Assessment procedures
All pertinent demographic characteristics and medical data were recorded during the pre-study period. Patients' physical examinations and blood tests were scheduled before treatment initiation. Data were collected from the patient diaries. Patients filled out the diary every 24 h from the start of trastuzumab deruxtecan treatment until 168 h, in which they reported daily the presence of nausea and decreased appetite by using a four-item scale on which the symptom severity was rated as none, mild, moderate, and severe. The frequency of vomiting was reported using a five-item scale as follows: 0 times, 1-2 times, 3-5 times, 6 times or more, and almost always. The use of rescue medication was reported using a four-item scale as follows: 0 times, 1 time, 2 times, and 3 times or more. In addition, the following items of the Patient Reported Outcome (PRO) Common Terminology Criteria for Adverse Events (CTCAE) version 1.0. were reported after the extended-overall phase (0-168 h): nausea, vomiting, decreased appetite, taste changes, hiccups, constipation, and diarrhea. To obtain baseline data, an assessment was performed before the start of trastuzumab deruxtecan therapy. Patient satisfaction with antiemetic therapy was measured using a seven-grade scale (very satisfied, satisfied, somewhat satisfied, rather satisfied, rather dissatisfied, dissatisfied, and very dissatisfied) after the extended-overall phase. The CINV assessment was performed on the basis of the patient's diary.
Outcomes
The primary endpoint was the CR rate during the overall phase (0-120 h) after the initiation of trastuzumab deruxtecan therapy. The secondary endpoints were the CR rates during the extended-overall phase (0-168 h), acute phase (0-24 h), delayed phase (24-120 h), and extended-delayed phase (24-168 h) and the complete control (CC) rate, defined as no emetic episode; no rescue medication use; and no significant nausea during the acute, delayed, extended-delayed, overall phase, and extended-overall phases. Significant nausea was defined as any rating greater than “mild nausea” on the four-point scale. The total control (TC) rate was defined as no emetic episodes, no rescue medication use, and no nausea during the acute, delayed, extended-delayed, overall phase, and extended-overall phases. The rates of nausea and vomiting were assessed during the acute, delayed, extended-delayed, overall, and extended-overall phases. Time to treatment failure (TTF) was defined as the time from the initiation of trastuzumab deruxtecan therapy to the first episode of vomiting or to rescue medication use, whichever happened first; and patient satisfaction with antiemetic therapy. Adverse events were graded according to PRO-CTCAE version 1.0 and CTCAE version 5.0.
Statistical analysis
This exploratory phase 2 study aimed to determine the optimal antiemetic therapy for trastuzumab deruxtecan-treated patients with breast cancer. To date, there is no knowledge about the CR rate during the 5-day phase (within 120 h of the start of trastuzumab deruxtecan administration), which is the primary endpoint. Therefore, because the number of cases could not be based on a statistical hypothesis, the number of cases that could be evaluated clinically with minimal accuracy against the obtained results was set (GD arm: 20 cases; GDA arm: 20 cases) by considering the number of cases that could be accumulated within the enrollment phase.
The primary endpoint was estimated using the CR rate and 95% confidence intervals (CIs) according to the Pearson-Clopper method, and the Cochran-Mantel-Haenszel test was used to compare exploratory between the two treatment arms with strata of age and previous experience with CINV. Logistic regression analyses with the backward elimination method, while keeping established prognostic factors age and previous experience of CINV fixed, were performed to determine the risk factors associated with non-achievement CR, non-achievement CC, and non-achievement TC in the overall phase and extended-overall phase. All potential explanatory variables reported in previous studies were included as independent variables. These were patient-related risk factors such as age, Eastern Cooperative Oncology Group performance status, motion sickness, habitual alcohol consumption, morning sickness, previous experience with CINV, and NK1RA use [13-16]. TTF was estimated using the Kaplan-Meier method, and the curves were compared with the log-rank test and a Cox proportional hazards model. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). All P-values were two-sided, and P < 0.05 was considered statistically significant.
Results
Study patients
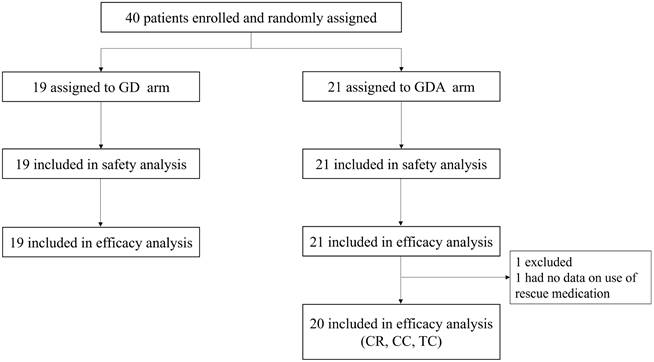
A total of 40 patients were enrolled in this study between September 2020 and March 2023. Forty patients were randomly assigned to the GD (n = 19) or GDA (n = 21) arm. In the GDA arm, one patient who did not complete the use of rescue medications, as recorded in their diary, was excluded from efficacy analysis (CR, CC, and TC rates; shown in Figure 1). Demographic data and patient characteristics are summarized in Table 1.
Efficacy
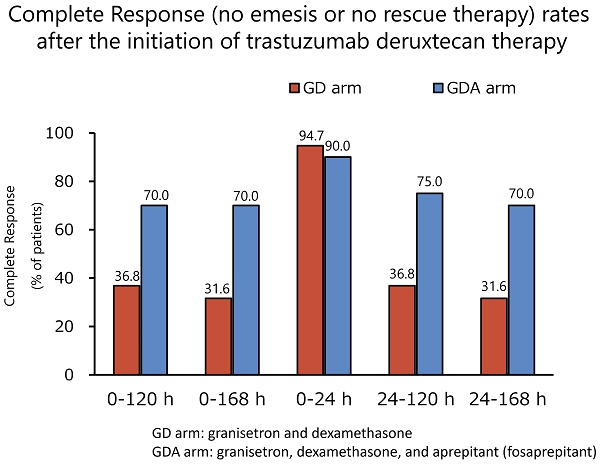
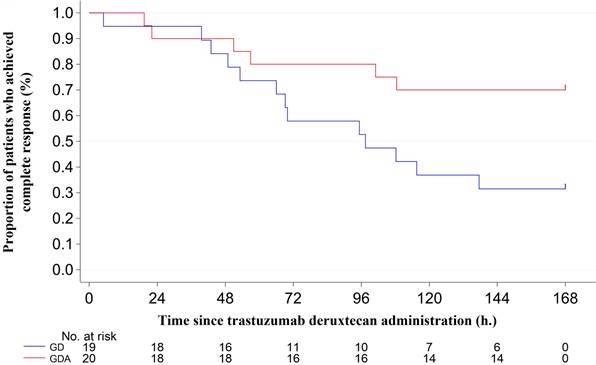
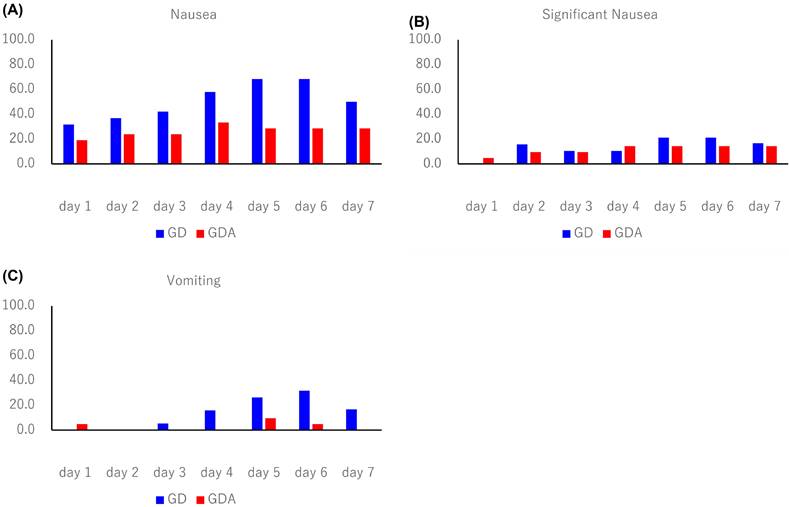
The antiemetic effects are summarized in Table 2. The CR rates during the overall phase were 36.8% in the GD arm and 70.0% in the GDA arm (odds ratio [OR]: 0.1334; 95% confidence interval [CI]: 0.0232-0.7672; P = 0.0190), with a difference of 33.2%. The CR rates during the extended-overall phase were 31.6% in the GD arm and 70.0% in the GDA arm (OR: 0.1073; 95% CI: 0.0185-0.6239; P = 0.0087), with a difference of 38.4%. In one patient in the GDA arm who was excluded from the evaluation of CR, CC, and TC because the rescue medication diary was not completed, nausea or vomiting did not occur during the 7-day period. The incidence of nausea, significant nausea, and vomiting after trastuzumab deruxtecan administration (shown in Figure 2) tended to increase after day 4 in both groups. The Kaplan-Meier analysis of the TTF is shown in Figure 3. TTF was significantly longer in the GDA arm than in the GD arm (hazard ratio: 0.346; 95% CI: 0.13-0.914; log-rank test, P = 0.0251).
Trial profile. GD: patients on two-drug combination of granisetron and dexamethasone (DEX); GDA: patients on three-drug combination of granisetron, DEX, and aprepitant (fosaprepitant) CR: complete response; CC: complete control; TC: total control.
Patients' characteristics
| Characteristic | GD (N = 19) n (%) | GDA (N = 21) n (%) | |
|---|---|---|---|
| Age | Mean ± SD | 59.1 ± 11.3 | 60.1 ± 10.2 |
| Min - Max | 41.0 - 77.0 | 41.0 - 75.0 | |
| Median | 58 | 60 | |
| < 55 | 7 ( 36.8) | 7 ( 33.3) | |
| >= 55 | 12 ( 63.2) | 14 ( 66.7) | |
| Trastuzumab deruxtecan dose (mg/kg) | Mean ± SD | 5.3 ± 0.2 | 5.4 ± 0.0 |
| Min - Max | 4.5 - 5.4 | 5.3 - 5.4 | |
| Median | 5.4 | 5.4 | |
| ECOG performance status | 0 | 16 ( 84.2) | 14 ( 66.7) |
| 1 | 3 ( 15.8) | 7 ( 33.3) | |
| Previous lines of therapy for metastatic disease | 2 | 2 ( 10.5) | 3 ( 14.3) |
| 3 | 8 ( 42.1) | 8 ( 38.1) | |
| 4 | 3 ( 15.8) | 3 ( 14.3) | |
| 6 | 3 ( 15.8) | 3 ( 14.3) | |
| 7 | 0 ( 0.0) | 1 ( 4.8) | |
| 8 | 1 ( 5.3) | 1 ( 4.8) | |
| 9 | 1 ( 5.3) | 1 ( 4.8) | |
| 10 | 1 ( 5.3) | 0 ( 0.0) | |
| 11 or more | 0 ( 0.0) | 1 ( 4.8) | |
| Motion sickness | No | 14 ( 73.7) | 19 ( 90.5) |
| Yes | 5 ( 26.3) | 2 ( 9.5) | |
| Habitual alcohol consumption | No | 18 ( 94.7) | 15 ( 71.4) |
| Yes | 1 ( 5.3) | 6 ( 28.6) | |
| Morning sickness | No | 5 ( 26.3) | 8 ( 38.1) |
| Yes | 11 ( 57.9) | 9 ( 42.9) | |
| no experience | 3 ( 15.8) | 4 ( 19.0) | |
| Previous experience of nausea and vomiting with chemotherapy | No | 9 ( 47.4) | 11 ( 52.4) |
| Yes | 10 ( 52.6) | 10 ( 47.6) |
Control of CINV
| Endpoints | GD (N = 19) | GDA (N = 20) | ||
|---|---|---|---|---|
| No. | (%) | No. | (%) | |
| Complete response | ||||
| Overall phase | 7 | 36.8 | 14 | 70.0 |
| Extended-overall phase | 6 | 31.6 | 14 | 70.0 |
| Acute phase | 18 | 94.7 | 18 | 90.0 |
| Delayed phase | 7 | 36.8 | 15 | 75.0 |
| Extended-delayed phase | 6 | 31.6 | 14 | 70.0 |
| Complete control | ||||
| Overall phase | 5 | 26.3 | 12 | 60.0 |
| Extended-overall phase | 4 | 21.1 | 12 | 60.0 |
| Acute phase | 18 | 97.4 | 17 | 85.0 |
| Delayed phase | 5 | 26.3 | 13 | 65.0 |
| Extended-delayed phase | 4 | 21.1 | 13 | 65.0 |
| Total control | ||||
| Overall phase | 2 | 10.5 | 9 | 45.0 |
| Extended-overall phase | 2 | 10.5 | 8 | 40.0 |
| Acute phase | 13 | 68.4 | 14 | 70.0 |
| Delayed phase | 2 | 10.5 | 11 | 55.0 |
| Extended-delayed phase | 2 | 10.5 | 9 | 45.0 |
| Endpoints | GD (N = 19) | GDA (N = 21) | ||
| No. | (%) | No. | (%) | |
| No nausea | ||||
| Overall phase | 3 | 15.8 | 12 | 57.1 |
| Extended-overall phase | 2 | 10.5 | 11 | 52.4 |
| Acute phase | 13 | 68.4 | 17 | 81.0 |
| Delayed phase | 4 | 21.1 | 13 | 61.9 |
| Extended-delayed phase | 3 | 15.8 | 12 | 57.1 |
| No significant nausea | ||||
| Overall phase | 14 | 73.7 | 18 | 85.7 |
| Extended-overall phase | 13 | 68.4 | 18 | 85.7 |
| Acute phase | 19 | 100.0 | 20 | 95.2 |
| Delayed phase | 14 | 73.7 | 18 | 85.7 |
| Extended-delayed phase | 13 | 68.4 | 18 | 85.7 |
| No vomiting | ||||
| Overall phase | 13 | 68.4 | 18 | 85.7 |
| Extended-overall phase | 10 | 52.6 | 17 | 81.0 |
| Acute phase | 19 | 100.0 | 20 | 95.2 |
| Delayed phase | 13 | 68.4 | 19 | 90.5 |
| Extended-delayed phase | 10 | 52.6 | 18 | 85.7 |
Incidence of nausea, significant nausea, and vomiting over 7 days starting on day 1 of trastuzumab deruxtecan therapy. (A) nausea, (B) significant nausea, and (C) vomiting. GD: patients on two-drug combination of granisetron and dexamethasone (DEX); GDA: patients on three-drug combination of granisetron, DEX, and aprepitant (fosaprepitant)
Adverse events
Major adverse events are summarized in Table 3. According to the investigator's evaluation, there was no grade 3 or 4 toxicity related to antiemetic therapy.
Risks analysis
Logistic regression analysis revealed that NK1RA use was associated with a significant decrease in the non-achievement of CR, CC, and TC in overall phase and extended-overall phases (Table 4).
Kaplan-Meier plot showing the time to treatment failure. GD: patients on two-drug combination of granisetron and dexamethasone (DEX); GDA: patients on three-drug combination of granisetron, DEX, and aprepitant (fosaprepitant)
Adverse events
| Symptom Term | GD (N=19) n (%) | GDA (N=21) n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade1 | Grade2 | Grade3 | Grade4 | Unknown | Grade1 | Grade2 | Grade3 | Grade4 | Unknown | |
| Nausea | 9 ( 47.4) | 6 ( 31.6) | 0 ( 0.0) | 0 ( 0.0) | 1 ( 5.3) | 8 ( 38.1) | 2 ( 9.5) | 0 ( 0.0) | 0 ( 0.0) | 0 ( 0.0) |
| Vomiting | 4 ( 21.1) | 0 ( 0.0) | 0 ( 0.0) | 0 ( 0.0) | 1 ( 5.3) | 1 ( 4.8) | 0 ( 0.0) | 0 ( 0.0) | 0 ( 0.0) | 0 ( 0.0) |
| Anorexia | 9 ( 47.4) | 6 ( 31.6) | 1 ( 5.3) | 0 ( 0.0) | 1 ( 5.3) | 10 ( 47.6) | 3 ( 14.3) | 0 ( 0.0) | 0 ( 0.0) | 0 ( 0.0) |
| Dysgeusia | 6 ( 31.6) | 1 ( 5.3) | 0 ( 0.0) | 0 ( 0.0) | 1 ( 5.3) | 2 ( 9.5) | 1 ( 4.8) | 0 ( 0.0) | 0 ( 0.0) | 0 ( 0.0) |
| Hiccups | 2 ( 10.5) | 0 ( 0.0) | 0 ( 0.0) | 0 ( 0.0) | 1 ( 5.3) | 1 ( 4.8) | 0 ( 0.0) | 0 ( 0.0) | 0 ( 0.0) | 0 ( 0.0) |
| Constipation | 7 ( 36.8) | 2 ( 10.5) | 0 ( 0.0) | 0 ( 0.0) | 1 ( 5.3) | 9 ( 42.9) | 2 ( 9.5) | 0 ( 0.0) | 0 ( 0.0) | 0 ( 0.0) |
| Diarrhea | 3 ( 15.8) | 0 ( 0.0) | 0 ( 0.0) | 0 ( 0.0) | 1 ( 5.3) | 2 ( 9.5) | 0 ( 0.0) | 0 ( 0.0) | 0 ( 0.0) | 0 ( 0.0) |
| Symptom Term (PRO-CTCAE/CTCAE) | GD (N=19) n (%) | GDA (N=21) n (%) | ||||||||
| Mild | Moderate | Severe | Very severe | Unknown | Mild | Moderate | Severe | Very severe | Unknown | |
| Nausea (severity) | 6 ( 31.6) | 6 ( 31.6) | 1 ( 5.3) | 0 ( 0.0) | 1 ( 5.3) | 7 ( 33.3) | 1 ( 4.8) | 2 ( 9.5) | 0 ( 0.0) | 0 ( 0.0) |
| Nausea (interference) | 5 ( 26.3) | 9 ( 47.4) | 2 ( 10.5) | 0 ( 0.0) | 1 ( 5.3) | 3 ( 14.3) | 4 ( 19.0) | 2 ( 9.5) | 2 ( 9.5) | 0 ( 0.0) |
| Vomiting (severity) | 1 ( 5.3) | 2 ( 10.5) | 1 ( 5.3) | 0 ( 0.0) | 1 ( 5.3) | 2 ( 9.5) | 0 ( 0.0) | 0 ( 0.0) | 1 ( 4.8) | 0 ( 0.0) |
| Vomiting (interference) | 0 ( 0.0) | 4 ( 21.1) | 0 ( 0.0) | 0 ( 0.0) | 1 ( 5.3) | 1 ( 4.8) | 0 ( 0.0) | 0 ( 0.0) | 0 ( 0.0) | 0 ( 0.0) |
| Anorexia (severity) | 3 ( 15.8) | 9 ( 47.4) | 4 ( 21.1) | 0 ( 0.0) | 1 ( 5.3) | 6 ( 28.6) | 5 ( 23.8) | 1 ( 4.8) | 1 ( 4.8) | 1 ( 4.8) |
| Anorexia (interference) | 7 ( 36.8) | 5 ( 26.3) | 6 ( 31.6) | 0 ( 0.0) | 1 ( 5.3) | 8 ( 38.1) | 4 ( 19.0) | 2 ( 9.5) | 2 ( 9.5) | 0 ( 0.0) |
| Taste changes/Dysgeusia | 6 ( 31.6) | 3 ( 15.8) | 1 ( 5.3) | 1 ( 5.3) | 1 ( 5.3) | 1 ( 4.8) | 4 ( 19.0) | 1 ( 4.8) | 0 ( 0.0) | 0 ( 0.0) |
| Hiccups (severity) | 2 ( 10.5) | 0 ( 0.0) | 0 ( 0.0) | 0 ( 0.0) | 1 ( 5.3) | 3 ( 14.3) | 0 ( 0.0) | 0 ( 0.0) | 0 ( 0.0) | 0 ( 0.0) |
| Hiccups (interference) | 0 ( 0.0) | 2 ( 10.5) | 0 ( 0.0) | 0 ( 0.0) | 1 ( 5.3) | 4 ( 19.0) | 1 ( 4.8) | 0 ( 0.0) | 0 ( 0.0) | 0 ( 0.0) |
| Constipation | 2 ( 10.5) | 9 ( 47.4) | 4 ( 21.1) | 0 ( 0.0) | 1 ( 5.3) | 5 ( 23.8) | 7 ( 33.3) | 2 ( 9.5) | 0 ( 0.0) | 0 ( 0.0) |
| Diarrhea | 3 ( 15.8) | 2 ( 10.5) | 0 ( 0.0) | 0 ( 0.0) | 1 ( 5.3) | 2 ( 9.5) | 3 ( 14.3) | 1 ( 4.8) | 0 ( 0.0) | 1 ( 4.8) |
Risk analysis for CINV in overall phase and extended-overall phase
| Non-achievement of CR (overall phase) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age (< vs. >=55 years) | 1.273 (0.343 - 4.726) | 0.7186 | 0.996 (0.227 - 4.371) | 0.9957 |
| PS (1 vs. 0) | 1.714 (0.329 - 8.943) | 0.5225 | ||
| Motion sickness (Yes vs. No) | 0.203 (0.037 - 1.127) | 0.0683 | ||
| Habitual alcohol consumption (Yes vs. No) | 1.714 (0.329 - 8.943) | 0.5225 | ||
| Morning sickness (Yes vs. No) | 0.714 (0.169 - 3.027) | 0.6479 | ||
| Morning sickness (No experience vs. No) | 0.952 (0.144 - 6.281) | 0.9596 | ||
| Previous experience of nausea and vomiting with chemotherapy (Yes vs. No) | 2.095 (0.581 - 7.555) | 0.2583 | 2.215 (0.529 - 9.284) | 0.2767 |
| With APR (GDA vs. GD) | 0.250 (0.066 - 0.950) | 0.0419 | 0.242 (0.062 - 0.950) | 0.0420 |
| Non-achievement CC (overall phase) | Univariate analysis | Multivariate analysis | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age (< vs. >=55 years) | 2.708 (0.668 - 10.98) | 0.1631 | 2.565 (0.540 - 12.19) | 0.2363 |
| PS (1 vs. 0) | 1.037 (0.199 - 5.410) | 0.9656 | ||
| Motion sickness (Yes vs. No) | 0.226 (0.048 - 1.067) | 0.0604 | ||
| Habitual alcohol consumption (Yes vs. No) | 2.206 (0.372 - 13.09) | 0.3839 | ||
| Morning sickness (Yes vs. No) | 1.500 (0.355 - 6.347) | 0.5817 | ||
| Morning sickness (No experience vs. No) | 1.333 (0.204 - 8.708) | 0.7638 | ||
| Previous experience of nausea and vomiting with chemotherapy (Yes vs. No) | 2.063 (0.570 - 7.470) | 0.2698 | 1.709 (0.402 - 7.275) | 0.4682 |
| With APR (GDA vs. GD) | 0.238 (0.061 - 0.925) | 0.0383 | 0.220 (0.053 - 0.913) | 0.0370 |
| Non-achievement TC (overall phase) | Univariate analysis | Multivariate analysis | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age (< vs. >=55 years) | 1.725 (0.374 - 7.953) | 0.4844 | 1.397 (0.245 - 7.952) | 0.7066 |
| PS (1 vs. 0) | 2.727 (0.289 - 25.75) | 0.3811 | ||
| Motion sickness (Yes vs. No) | 0.261 (0.056 - 1.206) | 0.0854 | ||
| Habitual alcohol consumption (Yes vs. No) | 0.978 (0.160 - 5.988) | 0.9810 | ||
| Morning sickness (Yes vs. No) | 0.467 (0.078 - 2.807) | 0.4051 | ||
| Morning sickness (No experience vs. No) | 0.267 (0.032 - 2.249) | 0.2243 | ||
| Previous experience of nausea and vomiting with chemotherapy (Yes vs. No) | 2.333 (0.554 - 9.834) | 0.2483 | 2.340 (0.456 - 12.00) | 0.3080 |
| With APR (GDA vs. GD) | 0.144 (0.026 - 0.795) | 0.0262 | 0.136 (0.024 - 0.784) | 0.0256 |
| Non-achievement CR (extended-overall phase) | Univariate analysis | Multivariate analysis | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age (< vs. >=55 years) | 1.083 (0.293 - 4.011) | 0.9046 | 0.877 (0.196 - 3.935) | 0.8641 |
| PS (1 vs. 0) | 1.511 (0.290 - 7.869) | 0.6239 | ||
| Motion sickness (Yes vs. No) | 0.176 (0.032 - 0.982) | 0.0477 | ||
| Habitual alcohol consumption (Yes vs. No) | 1.511 (0.290 - 7.869) | 0.6239 | ||
| Morning sickness (Yes vs. No) | 1.711 (0.403 - 7.271) | 0.4668 | ||
| Morning sickness (No experience vs. No) | 1.050 (0.159 - 6.924) | 0.9596 | ||
| Previous experience of nausea and vomiting with chemotherapy (Yes vs. No) | 1.681 (0.473 - 5.967) | 0.4220 | 1.823 (0.429 - 7.748) | 0.4160 |
| With APR (GDA vs. GD) | 0.198 (0.051 - 0.771) | 0.0195 | 0.194 (0.049 - 0.770) | 0.0197 |
| Non-achievement CC (extended-overall phase) | Univariate analysis | Multivariate analysis | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age (< vs. >=55 years) | 2.308 (0.569 - 9.359) | 0.2417 | 2.348 (0.476 - 11.57) | 0.2944 |
| PS (1 vs. 0) | 0.912 (0.174 - 4.773) | 0.9134 | ||
| Motion sickness (Yes vs. No) | 0.193 (0.040 - 0.922) | 0.0393 | ||
| Habitual alcohol consumption (Yes vs. No) | 1.944 (0.327 - 11.55) | 0.4648 | ||
| Morning sickness (Yes vs. No) | 1.857 (0.432 - 7.978) | 0.4052 | ||
| Morning sickness (No experience vs. No) | 1.333 (0.204 - 8.708) | 0.7638 | ||
| Previous experience of nausea and vomiting with chemotherapy (Yes vs. No) | 1.671 (0.462 - 6.051) | 0.4339 | 1.389 (0.315 - 6.122) | 0.6642 |
| With APR (GDA vs. GD) | 0.178 (0.043 - 0.736) | 0.0171 | 0.167 (0.039 - 0.724) | 0.0168 |
| Non-achievement TC (extended-overall phase) | Univariate analysis | Multivariate analysis | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age (< vs. >=55 years) | 1.426 (0.304 - 6.695) | 0.6530 | 1.191 (0.212 - 6.701) | 0.8424 |
| PS (1 vs. 0) | 2.348 (0.247 - 22.34) | 0.4578 | ||
| Motion sickness (Yes vs. No) | 0.208 (0.043 - 1.001) | 0.0502 | ||
| Habitual alcohol consumption (Yes vs. No) | 0.833 (0.134 - 5.167) | 0.8447 | ||
| Morning sickness (Yes vs. No) | 0.212 (0.022 - 2.032) | 0.1786 | ||
| Morning sickness (No experience vs. No) | 0.121 (0.010 - 1.531) | 0.1029 | ||
| Previous experience of nausea and vomiting with chemotherapy (Yes vs. No) | 1.846 (0.428 - 7.961) | 0.4111 | 1.814 (0.356 - 9.245) | 0.4733 |
| With APR (GDA vs. GD) | 0.177 (0.032 - 0.982) | 0.0477 | 0.174 (0.031 - 0.985) | 0.0480 |
Abbreviations, CR: complete response; CC: complete control; TC: total control
Patient satisfaction
Patient satisfaction is summarized in Table 5. Only patients in the GDA arm chose to report being “very satisfied” with the treatment.
Discussion
To our knowledge, this exploratory phase 2 study is the first to evaluate the antiemetic effects of a two-drug combination of granisetron and DEX and a three-drug combination of granisetron, DEX, and aprepitant (fosaprepitant) in trastuzumab deruxtecan-treated patients with breast cancer. Notably, the CR rates for the overall phase were 36.8% in the GD arm and 70.0% in the GDA arm, with a difference of 33.2% attributable to the addition of NK1RA. In this study, the use of the combination of 5-HT3RA and DEX with NK1RA for controlling CINV in trastuzumab deruxtecan-treated breast cancer patients resulted in a clinically meaningful difference. Additionally, multivariate analysis revealed that the addition of NK1RA significantly reduced the risk of non-achievement of CR. The Multinational Association for Supportive Care in Cancer/European Society for Medical Oncology 2016 guidelines call for a >10% improvement in CR rates on adding drugs for the prevention of CINV [11]. Although the comparison between the GD and GDA arms in this study was secondary, the results met this criterion of a >10% difference.
Patient satisfaction
| GD (N = 19) n (%) | GDA (N = 21) n (%) | |
|---|---|---|
| Very satisfied | 0 ( 0.0) | 7 ( 33.3) |
| Satisfied | 8 ( 42.1) | 5 ( 23.8) |
| Somewhat satisfied | 4 ( 21.1) | 2 ( 9.5) |
| Rather satisfied | 2 ( 10.5) | 2 ( 9.5) |
| Rather dissatisfied | 2 ( 10.5) | 1 ( 4.8) |
| Dissatisfied | 1 ( 5.3) | 1 ( 4.8) |
| Very dissatisfied | 1 ( 5.3) | 1 ( 4.8) |
| Blank | 1 ( 5.3) | 2 ( 9.5) |
Our study suggests that a combination of a 5-HT3RA, DEX, and an NK1RA may be more effective than that of a 5-HT3RA and DEX for the prevention of nausea and vomiting associated with trastuzumab deruxtecan. This finding is consistent with those of previous research indicating that adding an NK1RA to standard antiemetic therapy is effective and safe in preventing CINV in patients treated with highly emetogenic and carboplatin-based moderately emetogenic chemotherapy. This is because carboplatin is considered highly emetogenic even among moderately emetogenic agents [17,18].
The mechanisms underlying the high incidence of nausea and vomiting associated with trastuzumab deruxtecan treatment are not fully understood. It is possible that prolonged exposure to trastuzumab deruxtecan, which has a half-life of over six days, may contribute to these side effects [3]. Extended exposure to trastuzumab deruxtecan may result in the activation of the emetic pathway, leading to nausea and vomiting. The long half-life of trastuzumab deruxtecan may be related to the high incidence of CINV observed at the 168-h time point in this study. This suggests that the prolonged presence of the drug in the body could contribute to persistent CINV symptoms. The NCCN Clinical Practice Guidelines in Oncology for Antiemetics, version 1.2023, have updated the emetic potential of trastuzumab deruxtecan from moderate to high risk. Our results support this change [9].
The present study had several limitations. First, this was an exploratory phase 2 study with a small number of patients, and an open label design, which cannot rule out the possibility of a placebo effect, particularly in patients receiving aprepitant in the GDA arm. Second, this study focused only on Japanese patients, although the results may be applicable to patients of other ethnicities. Third, our 0-168 h observation period is longer than the standard 0-124 h period for evaluating antiemetic therapy. However, trastuzumab deruxtecan, which has a half-life of more than 6 days, will require a longer observation period than 168 h in the future [19]. Finally, at the start of the study, trastuzumab deruxtecan was only indicated for breast cancer in Japan despite its use in the management of a wide range of diseases; hence, the study only targeted patients with breast cancer.
Although trastuzumab deruxtecan has shown promise in the treatment of certain types of cancer, its use is associated with considerable nausea and vomiting. Unfortunately, current antiemetic regimens, including 5-HT3RAs, DEX, and NK1RAs, appear to be insufficient for controlling CINV. Therefore, it is imperative that future studies be conducted to better understand the underlying mechanisms of trastuzumab deruxtecan-induced nausea and vomiting and to develop more effective treatment strategies to manage these adverse effects in patients receiving this therapy.
Conclusion
Breast cancer patients receiving trastuzumab deruxtecan require triple therapy, including mandatory NK1RA administration, for antiemetic prophylaxis. Future investigations are warranted to explore the development of a four-drug combination therapy including olanzapine.
Abbreviations
CC: complete control; CI: confidence interval; CINV: chemotherapy-induced nausea and vomiting; CR: complete response; CTCAE: Common Terminology Criteria for Adverse Events; DEX: dexamethasone; 5-HT3RA: 5-hydroxytryptamine-3 receptor antagonist; GD: granisetron and dexamethasone; GDA: granisetron, dexamethasone, and aprepitant (fosaprepitant); HER2: human epidermal growth factor receptor 2; NCCN: National Comprehensive Cancer Network; NK1RA: neurokinin-1 receptor antagonist; OR: odds ratio; PRO: patient-reported outcome; TC: total control; TTF: time to treatment failure.
Acknowledgements
We thank all the patients and their families who participated in this study. We thank the Independent Alliance Data Centre and Ms. Mami Matsumaru (Gifu University Hospital). We express our sincere gratitude to our advisors Prof. Shigehira Saji (Fukushima Medical University), Prof. Tohru Ohtake (Fukushima Medical University), Prof. Koichiro Tsugawa (St. Marianna University School of Medicine), Dr. Koji Ohnuki (Miyagi Cancer Center), Dr. Hiroshi Honda (Tohoku Rosai Hospital), Prof. Nobuhisa Matsuhashi (Gifu University), and Pres. Kazuhiro Yoshida (Gifu University) for their invaluable guidance and support throughout this research project. Moreover, we thank Editage (www.editage.com) for English language editing.
Funding
This research received no specific grant from any funding agency in the public, commercial, or nonprofit sectors.
Author Contributions
HI, MF, and MS contributed significantly to the conception and design of the study protocol. MS provided statistical expertise. The protocol was written by HI, MF, MS, MK, and AS. HI, HB, YN, YM, YK, KM, AM, MK, KI, MT, KI, TI, TN, AO, YK, FK, AS, RM, KH, TF, YK, MT, AT, SY, MK, MO, and MF collected and/or assembled the data. HI, MF, and MS drafted the manuscript. All the authors have read and approved the final version of the manuscript.
Competing Interests
Dr. Iihara received personal fees from Taiho, Chugai, Yakult, Astellas, Eli Lilly, Daiichi Sankyo, AstraZeneca, Nippon Kayaku, Ono, and Nippon Boehringer Ingelheim as well as consulting fees for their institution from Taiho and Eisai outside the submitted work. Dr. Bando received personal fees from Daiichi Sankyo outside the submitted work. Dr. Mizuno received personal fees from Daiichi Sankyo, Chugai, Eisai, and AstraZeneca outside the submitted work. Dr. Kawaguchi received personal fees from Daiichi Sankyo, Chugai, and AstraZeneca outside the submitted work. Dr. Kawai received personal fees from AstraZeneca, Eisai, Kyowa-Kirin, Novartis, Chugai, Eli Lilly, MSD, and Pfizer outside the submitted work. Dr. Ishihara received personal fees from Nippon Kayaku, Kyowa-Kirin, Daiichi Sankyo, Eisai, and Chugai outside the submitted work. Dr. Nakada received personal fees from Daiichi Sankyo, Novartis Pharma, Pfizer, Chugai, and Eli Lilly outside the submitted work. Dr. Kojima received personal fees from Chugai, Novartis, Daiichi Sankyo, Eli Lilly, Kyowa-Kirin Eisai, Taiho, Pfizer, AstraZeneca, and MSD outside the submitted work. Dr. Higuchi and Ms. Furuta received personal fees from Daiichi Sankyo outside the submitted work. Dr. Suzuki received personal fees from Toa Eiyo, Asahi Kasei, Daiichi Sankyo, Pfizer, Eisai, Nippon Shinyaku, Celltrion Healthcare Japan, Otsuka, Sandoz, Tsumura, Nipro, Taiho, Kyowa-Kirin, Nippon Chemiphar, Japan Blood Products Organization, Takeda, and Nippon Boehringer Ingelheim, as well as grants for their institution from Nippon Kayaku, Asahi Kasei, Chugai, Taiho, Daiichi Sankyo, Japan Blood Products Organization, Mochida, and Sun Pharma outside the submitted work. Dr. Futamura received personal fees from Daiichi Sankyo, Taiho, Chugai, Eisai, Eli Lilly, and Nihon-Kayaku outside the submitted work.
References
1. Nakada T, Sugihara K, Jikoh T. et al. The latest research and development into the antibody-drug conjugate, [fam-] trastuzumab deruxtecan (DS-8201a), for HER2 cancer therapy. Chem Pharm Bull (Tokyo). 2019;67:173-85 doi: 10.1248/cpb.c18-00744
2. Tamura K, Tsurutani J, Takahashi S. et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol. 2019;20:816-26 doi: 10.1016/S1470-2045(19)30097-X
3. Doi T, Shitara K, Naito Y. et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol. 2017;18:1512-22 doi: 10.1016/S1470-2045(17)30604-6
4. Modi S, Saura C, Yamashita T. et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382:610-21 doi: 10.1056/NEJMoa1914510
5. Shitara K, Bang YJ, Iwasa S. et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382:2419-30 doi: 10.1056/NEJMoa2004413
6. Li BT, Smit EF, Goto Y. et al. Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N Engl J Med. 2022;386:241-51 doi: 10.1056/NEJMoa2112431
7. Siena S, Di Bartolomeo M, Raghav K. et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2021;22:779-89 doi: 10.1016/S1470-2045(21)00086-3
8. Hesketh PJ, Kris MG, Basch E. et al. Antiemetics: ASCO guideline update. J Clin Oncol. 2020;38:2782-97 doi: 10.1200/JCO.20.01296
9. NCCN Clinical Practice Guidelines in Oncology. Antiemesis. Version 1. 2020 https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf
10. Modi S, Jacot W, Yamashita T. et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387:9-20 doi: 10.1056/nejmoa2203690
11. Roila F, Molassiotis A, Herrstedt J. et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. 2016;27(suppl 5):v119-33 doi: 10.1093/annonc/mdw270
12. Aogi K, Takeuchi H, Saeki T. et al. Optimizing antiemetic treatment for chemotherapy-induced nausea and vomiting in Japan: update summary of the 2015 Japan Society of Clinical Oncology Clinical Practice Guidelines for antiemesis. Int J Clin Oncol. 2021;26:1-17 doi: 10.1007/s10147-020-01818-3
13. Hesketh PJ, Aapro M, Street JC. et al. Evaluation of risk factors predictive of nausea and vomiting with current standard-of-care antiemetic treatment: analysis of two phase III trials of aprepitant in patients receiving cisplatin-based chemotherapy. Support Care Cancer. 2010;18:1171-7 doi: 10.1007/s00520-009-0737-9
14. Warr DG, Street JC, Carides AD. Evaluation of risk factors predictive of nausea and vomiting with current standard-of-care antiemetic treatment: analysis of phase 3 trial of aprepitant in patients receiving adriamycin-cyclophosphamide-based chemotherapy. Support Care Cancer. 2011;19:807-13 doi: 10.1007/s00520-010-0899-5
15. Hilarius DL, Kloeg PH, van der Wall E. et al. Chemotherapy-induced nausea and vomiting in daily clinical practice: A community hospital-based study. Support Care Cancer. 2012;20:107-17 doi: 10.1007/s00520-010-1073-9
16. Sekine I, Segawa Y, Kubota K. et al. Risk factors of chemotherapy-induced nausea and vomiting: index for personalized antiemetic prophylaxis. Cancer Sci. 2013;104:711-7 doi: 10.1111/cas.12146
17. Yokoe T, Hayashida T, Nagayama A. et al. Effectiveness of antiemetic regimens for highly emetogenic chemotherapy-induced nausea and vomiting: A systematic review and network meta-analysis. Oncologist. 2019;24:e347-57 doi: 10.1634/theoncologist.2018-0140
18. Jordan K, Blättermann L, Hinke A. et al. Is the addition of a neurokinin-1 receptor antagonist beneficial in moderately emetogenic chemotherapy? -a systematic review and meta-analysis. Support Care Cancer. 2018;26:21-32 doi: 10.1007/s00520-017-3857-7
19. Navari RM, Qin R, Ruddy KJ. et al. Olanzapine for the Prevention of Chemotherapy-Induced Nausea and Vomiting. N Engl J Med. 2016;375:134-42 doi: 10.1056/nejmoa1515725
Author contact
![]() Corresponding authors: Hirotoshi Iihara, Ph.D. Phone: +81-58-230-7088; E-mail: iihara.hirotoshi.p7gifu-u.ac.jp and Manabu Futamura, M.D., Ph.D. Phone: +81-58-230-6000; E-mail address: futamura.manabu.m3gifu-u.ac.jp.
Corresponding authors: Hirotoshi Iihara, Ph.D. Phone: +81-58-230-7088; E-mail: iihara.hirotoshi.p7gifu-u.ac.jp and Manabu Futamura, M.D., Ph.D. Phone: +81-58-230-6000; E-mail address: futamura.manabu.m3gifu-u.ac.jp.

 Global reach, higher impact
Global reach, higher impact