Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(14):2686-2693. doi:10.7150/jca.86635 This issue Cite
Research Paper
Colon Adenoma After Diagnosis of Immune Checkpoint Inhibitor-mediated Colitis
1. Department of Internal Medicine, The University of Texas Health Science Center, Houston, TX, USA.
2. Department of Gastroenterology, Hepatology, and Nutrition, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
3. Department of Medicine II, University Hospital, Ludwig-Maximilians-University Munich, Munich, Germany.
4. Inflammatory Bowel Disease Center, Cleveland Clinic, Cleveland, OH, USA.
5. Department of Gastrointestinal Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
6. Department of Hospital Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Received 2023-5-30; Accepted 2023-7-30; Published 2023-8-28
Abstract
Purpose: While the occurrence of colitis during immune checkpoint inhibitor (ICI) treatment is recognized as a sign of robust immune activation and correlates with better oncological outcomes, the long-term impact of ICI-mediated colitis on the colonic mucosa has not been studied. We thus aim to describe the colonoscopy and histology findings in patients at a follow-up time of ≥ 6 months post initial colitis event.
Methods: This retrospective analysis included adult cancer patients diagnosed with ICI colitis at a tertiary cancer center between October 2013 and June 2020. The study group included patients diagnosed with immune mediated colitis who had also undergone a follow up colonoscopy or flex sigmoidoscopy. The control group was patients exposed to ICI without immune mediated colitis. We reported patients' colitis clinical course, treatment, outcomes, and endoscopic and histologic features at diagnosis and at follow-up time of ≥ 6 months.
Results: Total 39 patients met the study criteria, with 82% being male, and 35.8% having melanoma. Most patients received a combination of CTLA-4 and PD-1/L1 inhibitors (82%). On initial endoscopic evaluation, inflammation without ulceration was reported in 76.9% of patients and active inflammation on histologic examination in 79.3% of patients. Most patients (79.4%) received corticosteroids, and 56.4% received add-on selective immunosuppressive therapy. Four patients received fecal microbiota transplantation. On follow-up, new incidence of colonic polyps was reported in 51.2% of patients, including adenomas in 33.3% among the colitis patients with median follow up duration of 12 months. The incidence of adenoma polyps 12 months after the colitis event was significantly higher compared to the control group without colitis based on the time-to-event analysis (p=0.041).
Conclusion: At a median follow up of 12 months after their initial colitis diagnosis, 51.2% of the patients had new incidence of colonic polyps, including a third with adenoma, at a significantly higher incidence than the control group without colitis. Studies with larger sample sizes are needed to further define the long-term impact of colitis and its treatments on colon health and to refine recommendations for surveillance of colonic adenomas and colorectal cancer.
Keywords: immune checkpoint inhibitor, colitis, adenoma, colonoscopy, surveillance
Introduction
Immune checkpoint inhibitors (ICIs) have been increasingly used as a treatment for various cancers. These agents act by releasing the immune system from specific inhibitory checkpoints to trigger a tumor-specific immune-mediated cytotoxic effect [1]. The best characterized targets of ICIs are cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1), and programmed cell death ligand 1 (PD-L1). Treatment with monoclonal antibodies targeting these proteins is associated with increased T-cell activation and effective anti-tumor immune responses. Yet, unleashing the immune system from negative regulation comes with well-known adverse reactions caused by loss of self-tolerance and excessive inflammatory activity, collectively known as immune-related adverse events [2].
Immune-mediated colitis, a common adverse reaction observed in patients undergoing treatment with ICIs, has been extensively studied over the past 5 years [3-6]. This condition requires exclusion of other alternative diagnosis as it frequently overlapped with features of inflammatory bowel disease, infectious colitis and graft-versus-host disease [7-8]. The initial treatment for moderate and severe colitis consists from dietary considerations to steroids, followed by selective immunosuppressive therapy (e.g., infliximab, vedolizumab) in refractory cases, and achieves response rates exceeding 80% [9-10]. Ustekinumab, tofacitinib, extracorporeal photopheresis and fecal microbiota transplantation are also reported to be effective [7, 11-14], and their use for steroid-refractory cases is being assessed.
It has been shown that prolonged course of colitis (>3 months) is associated with better long-term cancer outcomes and survival [6, 15]. However, there are indications that along with this beneficial effect on cancer-dependent prognosis, prolonged inflammation of the intestine following treatment with ICIs might also affect the long-term consequences on the health of colonic mucosa. This is exemplified by the fact that colitis can persist for years even after ICI cessation [16]. Therefore, we sought to elucidate the long-term outcome of immune-mediated colitis, in particular, the incidence of additional colon pathology.
Methods
Study design and population
This retrospective chart review is a descriptive, single-center study that included adult patients who were diagnosed with colitis after ICI treatment at a tertiary cancer center between October, 2013, and June, 2020. This study was approved by the institutional review board with a waiver of patients' informed consent. We identified patients 18 years or older who (1) were treated with ICIs for various types of cancer, (2) had a diagnosis of immune-mediated colitis, and (3) had colonoscopy/flex sigmoidoscopy at the time of colitis diagnosis and at follow-up at least 6 months later. Our control group included all patients that received ICI within the study window (1) that underwent serial colonoscopy or flexible sigmoidoscopy after initiating ICI, (2) had negative baseline scope, (3) without immune mediated colitis diagnosis. Patients with pre-existing inflammatory bowel disease (IBD), microscopic colitis, or other autoimmune gastrointestinal disorders were excluded.
Clinical data
Demographic and cancer-related information such as age, gender, primary cancer type, stage, cancer treatments received and doses, and Charlson Comorbidity Index score were collected. Also collected were data related to the onset of colitis, such as date, cycles of ICI before colitis, type of ICI, and peak Common Terminology Criteria for Adverse Events (CTCAE) grades for colitis and diarrhea. Diagnosis of colitis was based on the clinical presentation and endoscopic and histologic features after the exclusion of other etiologies. Information about the treatment for colitis such as steroids, infliximab, and vedolizumab, including doses and start and end dates, was obtained as well. Colonoscopy/sigmoidoscopy and pathology findings at the time of colitis diagnosis and at follow-up were collected based on previously established characteristics [17-20]; these included polyps, intervention (e.g., polypectomy, stricture dilation), and complications (e.g., bleeding, perforation). Polyp pathology was described as adenomatous or hyperplastic based on general terminology, given the lack of standardization on documentation for this population as what is well established in the IBD literature.
Statistical analysis
The statistical analyses performed were descriptive in nature. The distributions of continuous variables were summarized by medians and interquartile ranges. The distributions of categorical variables were summarized by frequencies and percentages. These were calculated using SPSS 26. To calculate the incidence of adenoma, we included all cases from both colitis and control groups with negative colonoscopy at baseline who had a follow-up colonoscopy/flex sigmoidoscopy done at least 6 months but not more than 1 year later. Time to adenoma detection was modeled for 6 months to 1 year after baseline colonoscopy using the Kaplan-Meier method. Survival curves were compared using the log-rank test.
Results
Patient population, characteristics, and oncologic history
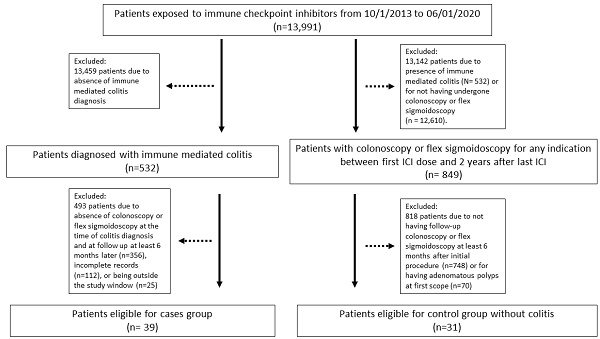
Thirty-nine patients met the inclusion criteria for the colitis group (patient selection flowchart shown in Figure 1). Their characteristics are summarized in Table 1. The most common cancer was melanoma, followed by genitourinary cancer, gastrointestinal cancer, and lung/head and neck malignancies. Thirty-two patients (82%) received a combination of PD-1/L1 and CTLA-4 inhibitors, 5 patients (12.8%) received a PD-1/L1 inhibitor as monotherapy, while only 2 patients (5.1%) received a CTLA-4 inhibitor as monotherapy. Clinical response of colitis symptom was achieved in 33 patients (84.6%). Six patients (15.4%) had screening colonoscopy before their colitis diagnosis within a median duration of 10 months (Table 3), and all had detected polyps that were removed. In addition, 31 patients met the inclusion criteria for the control group for comparison of incidence of colon adenoma polyps (Figure 1).
Endoscopic and histologic characteristics
At the time of colitis diagnosis, the most common endoscopic finding was non-ulcer inflammation in 30 patients (Table 2). In 7 patients, inflammation was evident only on histologic examination, with no obvious macroscopic changes of the colonic mucosa. Ulcers were found in only 2 patients. Histologic examination showed active inflammation in 31 patients. Polyps were found in 3 patients - 2 with hyperplastic polyps, 1 with adenoma, all of which were removed during the endoscopy.
Patient Characteristics, N=39
| Characteristic | No (%) |
|---|---|
| Age, years, median (IQR) | 64.2 (49-69.8) |
| Sex, male* | 32 (82) |
| Race, white† | 35 (89.7) |
| Cancer type | |
| Melanoma | 14 (35.8) |
| Genitourinary | 10 (25.6) |
| Lung/head and neck | 6 (15.3) |
| Gastrointestinal | 7 (17.9) |
| Hematological | 1 (2.5) |
| Renal | 1 (2.4) |
| Type of ICI | |
| Anti-CTLA-4 monotherapy | 2 (5.1) |
| Anti-PD-1/L1 monotherapy | 5 (12.8) |
| Combination anti-CTLA-4 and anti-PD-1/L1 | 32 (82) |
| Duration of follow-up from colitis diagnosis to last follow-up, months, median (IQR) | 14.2 (9.1-41.2) |
Abbreviations: CTLA-4, cytotoxic T lymphocyte antigen 4; ICI, immune checkpoint inhibitor; IQR, interquartile range; PD-1/PD-L1, programmed cell death 1/programmed death ligand 1.
*7 patients (18%) were females.
†3 patients (7.6%) were Hispanic and 1 patient (2.5%) African American.
Patient selection flowchart.
Endoscopy-related characteristics, N=39
| At the time of colitis diagnosis | No. (%) |
|---|---|
| Endoscopic findings | |
| Inflammation | |
| Ulcers | 2 (5.1) |
| Non-ulcer inflammation | 30 (76.9) |
| Normal | 7 (17.9) |
| Other findings | |
| Polyps ¶ | 3 (7.6) |
| Histologic findings | |
| Acute inflammation | 18 (46.1) |
| Chronic inflammation | 10 (25.6) |
| Microscopic colitis | 3 (7.6) |
| Normal | 8 (20.5) |
| Treatment of IMC | |
| Corticosteroids only | 16 (41.0) |
| Corticosteroids plus infliximab only | 8 (20.5) |
| Corticosteroids plus vedolizumab only | 7 (17.9) |
| Multiple treatment lines‡ | 8 (20.5) |
| Colitis maintenance treatment (> 3 doses of accumulative biologic agents: infliximab, vedolizumab, ustekinumab) | 8 (20.5) |
| At the time of follow-up endoscopy | |
| Duration from colitis diagnosis to first follow-up endoscopy, months, median (IQR) | 7.1 (2.9-21.7) |
| Duration from colitis diagnosis to last follow-up endoscopy, months, median (IQR) | 12.6 (8.2-18.8) |
| On active colitis treatment | 10 (25.6) |
| On active ICI treatment on follow-up endoscopy | 6 (15.3) |
| Endoscopic findings | |
| Inflammation | |
| Erythema, erosions, vascular congestion and inflammation | 16 (41) |
| Normal | 23 (58.9) |
| Others | |
| New polyps | 20 (51.2) |
| Stricture | 0 (0) |
| Perforation | 0 (0) |
| Histologic findings | |
| Inflammatory findings | |
| Inflammation† | 19 (48.7) |
| Normal histologic findings | 20 (51.2) |
| Others | |
| Adenoma polyps § | 13 (33.3) |
| Endoscopic intervention or surgery | |
| Polypectomy (out of 20 patients with polyps) | 20 (100%) |
| Stricture dilation | 0 (0) |
| Surgical resection of colon cancer | 0 (0) |
| All cause mortality | 7 (21.9) |
Abbreviations: CTCAE v5, Common Terminology Criteria for Adverse Events version 5; ICI, immune checkpoint inhibitor; IMC, immune-mediated colitis; IQR, interquartile range; TNF, tumor necrosis factor; FMT, fecal microbiota transplantation
*The 3 patients with cancer on histologic examination on follow-up colonoscopy had a previous diagnosis of colon cancer.
†Among patients with active histologic inflammation on follow-up: 4 patients (10.2%) had acute inflammation, 11 patients (28.2%) had chronic inflammation, 1 patient (2.5%) had acute and chronic inflammation, 3 patients (7.6%) had microscopic colitis.
¶ 2 patients had hyperplastic polyps and 1 patient had an adenoma. All of them were <5 mm in size and removed. These patients had non-ulcer inflammation at the time of colitis diagnosis.
§ 11 patients had tubular adenomas, 2 had tubulovillous adenomas. 1 patient had polyps <5 mm in size, and 11 had polyps >5 mm, and all were removed.
‡ 3 patients received both infliximab and vedolizumab, 1 patient received infliximab and ustekinumab, and 4 patients received FMT alongside other treatments (1 FMT + steroids, 1 FMT + infliximab + vedolizumab, 2 FMT + vedolizumab)
On follow-up endoscopic examination, signs of persistent inflammation (erythema, erosions, vascular congestion) were still present in 16 patients (Table 2). In 20 patients (51.2%), sessile polyps were reported and removed. Thirteen of these patients had detection of new adenomatous polyps: 11 had tubular adenomas, and 2 had tubulovillous adenomas. Two patients had polyps smaller than 5 mm, and 11 patients had polyps larger than 5 mm. The majority of the polyps were located in the ascending colon in 4 patients (30.6%) followed by transverse colon in 3 patients (23%). The median duration was 7.1 months from colitis diagnosis to the first follow-up endoscopic examination and 12.6 months to the last follow-up endoscopic examination. The endoscopic and histologic characteristics by cancer type are summarized in Supplemental Table 1.
Comparison of characteristics of patients with and without adenoma polyps
The median age of the 13 patients with adenoma polyps was 65 years (Supplemental Table 2), and the most common malignancy was melanoma. All the patients with adenoma received combination therapy with PD-1/L1 and CTLA-4 inhibitors. The endoscopic findings in most patients showed non-ulcer inflammation. In most patients, the distribution of inflammation and location of polyps was similar on follow-up endoscopy. Adenoma polyps were less likely to be found in patients who were receiving active colitis treatment at the time of follow-up endoscopy compared with patients who were not, despite similar ICI treatment status. Patients with adenoma polyps had a longer median duration between colitis onset and last follow-up endoscopy (14.8 vs 4.11 months, p=0.004). Otherwise, the characteristics were comparable between the patients with and without adenoma polyps.
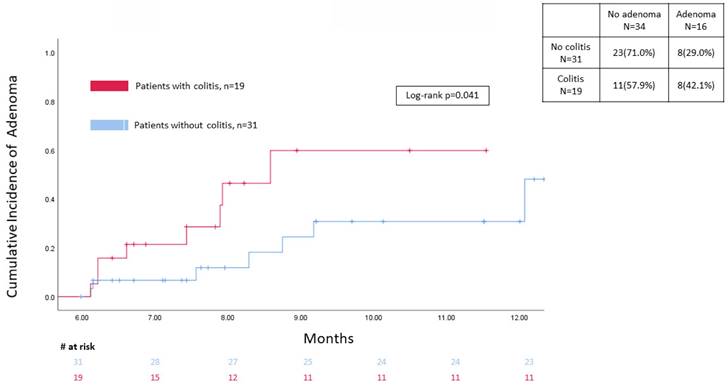
A total of 849 patients in the control group had a colonoscopy/flex sigmoidoscopy done at baseline after ICI treatment, with only 31 patients having a follow-up colonoscopy/sigmoidoscopy within 6 months to 1 year afterwards. The incidence of adenoma at different time points between the two groups on follow-up endoscopy exam in the study window was presented in Figure 2 showing that colitis patients had a significantly higher level on time-to-event analysis (p=0.041).
Univariate analysis (Table 3) identified longer duration from colitis onset to follow-up endoscopy as the only risk factor associated with adenoma. Age, cancer type, initial colonoscopy and histology findings, or colitis treatments were not associated with colon adenoma.
Kaplan-Meier for adenoma development between 6 months to 1 years of follow up.
Univariate analysis for colon adenoma, N=39
| Variables | OR (CI) | p |
|---|---|---|
| Age | 1.0 (0.9-1.1) | 0.191 |
| Cancer type (melanoma vs others) | 1.9 (0.5-7.6) | 0.348 |
| Initial colitis endoscopic findings (active inflammation vs normal) | 0.6 (0.1-3.2) | 0.557 |
| Initial colitis histologic findings (presence of inflammation vs normal) | 0.8 (0.2-4.0) | 0.779 |
| Immunosuppressant treatment for initial colitis (biologics vs no biologics) | 0.5 (0.1-2.1) | 0.364 |
| Active inflammation on follow-up endoscopy | 0.3 (0.1-1.3) | 0.120 |
| Active inflammation on follow-up histology | 0.4 (0.1-2.5) | 0.355 |
| Adequate or better preparation vs air or worse preparation for initial endoscopy | 0.8 (0.2-3.9) | 0.789 |
| Duration of last colonoscopy screening before colitis onset to the last follow-up colonoscopy | 1.1 (0.9-1.3) | 0.442 |
| Long term colitis maintenance with biologics vs no | 1.3 (0.3-6.4) | 0.779 |
| Adequate or better preparation vs fair or worse preparation for follow-up endoscopy | 0.4 (0.1-2.6) | 0.341 |
| Follow-up duration from colitis diagnosis to follow-up endoscopy | 1.05 (1.00-1.11) | 0.040 |
Discussion
The long-term outcome of ICI colitis after medical treatment has not been well studied. Our data found that colon adenomatous polyps were newly reported in 33.3% of patients on follow-up endoscopic evaluation around 12 months after colitis. Furthermore, 41% of our patients had persistent histologic inflammation. With the concerning rate of new polyp detection within the short 12 months window following a colitis event, it is critical to develop the optimal surveillance program for colon adenoma and thereby reduce the risk of colon dysplasia and/or colon cancer.
Colon cancer is the third most common malignancy and the second most deadly cancer in the US [21]. Certain chronic conditions, such as IBD, can increase the risk of colon cancer [22] secondary to persistent inflammation, long disease duration, extensive colitis distribution and higher severity, coexistent primary sclerosing cholangitis, and a family history of colon cancer [23] etc. In IBD patients, a histologic diagnosis of dysplasia (equivalent term for adenoma without IBD background) as well as serrated polyps carries a higher risk of developing more advanced neoplasia [24-25]. Different advanced techniques (such as chromoendoscopy) are recommended to improve the early and efficacious detection of dysplasia and cancer. Many international societies recommend a first surveillance colonoscopy 8-10 years after diagnosis with extensive colitis followed by subsequent surveillance on an interval determined by various risk factors [26-29]. Patients with primary sclerosing cholangitis specifically have a higher risk and require annual colonoscopy surveillance. Nonetheless common genetic and signaling pathways such as p53 should also be considered in the colitis associated cancer spectrum, inflammation can induce the production of pro inflammatory cytokines that can induce mutation in oncogenes such as p53, APC, K-ras and genomic instability by different mechanism promoting tumor proliferation and antiapoptotic properties or premalignant cells [30]. IBD and ICI colitis share multiple similitudes, such as the disruption in immune surveillance pathways and acute and/or chronic gastrointestinal inflammation. Especially concerning is the recently reported study showing the prolonged colitis disease course for years after ICI termination, with or without overt clinical symptoms [10]. Therefore, the elevated risk of colon cancer associated with IBD may also be anticipated and should be investigated in patients with ICI colitis. On the other hand, patients with mild ICI colitis on endoscopy evaluation, with shorter colitis course and minimal requirement of aggressive treatment, are usually considered a low-risk group. However, in our analysis, 7% of patients with normal endoscopy findings at colitis onset still experienced de novo colon adenomas later on, suggesting that even low-grade colonic inflammation may contribute to the pathogenesis of colon adenoma/cancer.
Available data have shown that early infliximab and vedolizumab treatment for moderate to severe immune-mediated colitis was associated with better colitis disease course and improved overall survival [10]. The decision to start infliximab and vedolizumab was frequently based on the CTCAE grade of diarrhea/colitis as well as high-risk endoscopic features that were previously described [6]. However, the safety of long-term use of potent immunosuppressants in patients with immune-mediated colitis remains unclear. Notably, a small increased risk of secondary malignancies such as lymphoma [31], skin cancers [32], and leukemia [33] was reported in IBD and rheumatological disease among patients on long term use of immunosuppressants who otherwise had no active malignancy. In particular, as the increasing volume of ICI-treated patients with chronic colitis with prolonged disease course, long-term maintenance with immunosuppressants has become more widely indicated and practiced. A recent study showed that the use of steroids and infliximab may impair overall survival among cancer patients [34]. This brought to providers' attention the potential negative impact of immunosuppressants on cancer-related outcomes, which has not been extensively studied previously. With all these risk factors, patients with immune-mediated colitis could be predisposed to colon adenoma/cancer, which can be monitored and prevented by implementing an optimal surveillance strategy via colonoscopy to remove dysplastic lesions early on.
The progression from small adenomatous polyps to sporadic colorectal cancers usually take years' duration [35]. Colonoscopy surveillance is associated with a substantially reduced incidence of colorectal cancer and its associated mortality, therefore, it has become the standard clinical practice for the general population [36]. Different characteristics and number of advanced adenomatous lesions may require more intense colonoscopy surveillance programs to maximize its benefit of cancer prevention, and thus screening intervals range from 3 to 10 years [37]. The surveillance for patients with existing colon cancer history is even more intensified [38]. In our cohort, only 6 patients (15.3%) had undergone screening colonoscopy before their colitis episode, a finding that could reiterate the need for up-to-date surveillance colonoscopy among cancer population. Despite the lack of high-quality data, multiple retrospective studies have observed a higher incidence of colon adenoma/cancer among cancer populations regardless of colon cancer history [39-42]. This association may further contribute to the outcome of advanced adenomatous lesions in the colon that we observed within a short time after the diagnosis of immune-mediated colitis in the setting of active malignancy and ICI treatment. Another confounding factor that should be considered is that the low rate of adenoma detection or reporting at the time of colitis diagnosis could be due to the masking effect from significant mucosal inflammation and higher threshold for endoscopists to perform polypectomy owing to concerns of bleeding, perforation, and poor healing [43]. This could also contribute to the rapid detection of adenoma just 6 months after colitis diagnosis following the initial scope evaluation. In addition, patients with colitis may have suboptimal bowel preparation for the endoscopy (up to 40% of cases in our data), which could impair the visualization of polyps.
Although this is the largest study on this topic to our knowledge, it has limitations. First, it is a single-center retrospective review. Second, the small sample size limits the power of subgroup analysis to compare different risk factors. Third, the primary cancer outcomes could be affected by multiple factors that limit the follow-up duration and the feasibility of repetitive colonoscopy, leading to a patient selection bias and different risk levels of second malignancy. Fourth, the lack of a standardized surveillance program after a colitis event can limit the risk assessment and stratification of subsequent management of colon health and colon cancer prevention.
In our study, more than 50% of patients diagnosed with immune-mediated colitis had de novo polyps reported on follow-up colonoscopy/flex sigmoidoscopy 12 months after the colitis diagnosis, and more than half the lesions were adenomatous, which is above the incidence of general patients without colitis history. This should raise concerns of rapid development and detection of colon adenoma after colitis diagnosis that is notably faster than in the general cancer population without colitis. To maximize the reduction of secondary colon cancer risk in this population, a prompt surveillance colonoscopy at 1 year after colitis diagnosis should be considered. Further assessment of this risk to determine the optimal interval for subsequent surveillance is warranted.
Supplementary Material
Supplementary tables.
Acknowledgements
Medical editing of this paper was provided by Bryan Tutt, Scientific Editor, Research Medical Library, The University of Texas MD Anderson Cancer Center.
Author contributions
Yinghong Wang, who was the senior author of this article, developed the concept, designed the study, interpreted the results, ensured the preservation of data accuracy and integrity at all stages, agreed to be accountable for all aspects of the study, was in charge of the overall direction and planning of the study, and contributed to the writing of the manuscript, with input from all authors. Antonio Pizuorno Machado collected the data for the study, conducted and interpreted the results of the manuscript. Antonio Pizuorno Machado drafted the manuscript. Malek Shatila conducted biostatistical analysis. All the authors critically revised the final version of the manuscript and approved the final manuscript.
Ethics approval and consent to participate
The ethics approval for this study was granted by the institutional review board at The University of Texas MD Anderson Cancer Center (PA18-0472). Patient consent was waived for this study.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50(12):1-11
2. Lozano AX, Chaudhuri AA, Nene A, Bacchiocchi A, Earland N, Vesely MD. et al. T cell characteristics associated with toxicity to immune checkpoint blockade in patients with melanoma. Nat Med. 2022;28(2):353-362
3. Schneider BJ, Naidoo J, Santomasso BD. et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2011;39(36):4073-4126
4. Thompson JA, Schneider BJ, Brahmer J et al. Management of Immunotherapy-Related Toxicities, Version 1.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20(4):387-405
5. Haanen J, Obeid M, Spain L. et al. on behalf of the ESMO Guidelines Committee*. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(12):1217-1238
6. Wang Y, Abu-Sbeih H, Mao E, Ali N, Ali FS, Qiao W. et al. Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: retrospective review at MD Anderson. J Immunother Cancer. 2018;6(1):37
7. Li H, Fu ZY, Arslan ME, Cho D, Lee H. Differential diagnosis and management of immune checkpoint inhibitor-induced colitis: A comprehensive review. World J Exp Med. 2021;11(6):79-92
8. Weingarden AR, Rubin SJS, Gubatan J. Immune checkpoint inhibitor-mediated colitis in gastrointestinal malignancies and inflammatory bowel disease. World J Gastrointest Oncol. 2021;13(8):772-798
9. Saha A, Dreyfuss I, Sarfraz H, Friedman M, Markowitz J. Dietary Considerations for Inflammatory Bowel Disease Are Useful for Treatment of Checkpoint Inhibitor-Induced Colitis. Cancers (Basel). 2022;15(1):84
10. Zou F, Faleck D, Thomas A, Harris J, Satish D, Wang X. et al. Efficacy and safety of vedolizumab and infliximab treatment for immune-mediated diarrhea and colitis in patients with cancer: a two-center observational study. J Immunother Cancer. 2021;9(11):e003277
11. Thomas AS, Ma W, Wang Y. Ustekinumab for Refractory Colitis Associated with Immune Checkpoint Inhibitors. N Engl J Med. 2021;384(6):581-583
12. Bishu S, Melia J, Sharfman W, Lao CD, Fecher LA, Higgins PDR. Efficacy and Outcome of Tofacitinib in Immune checkpoint Inhibitor Colitis. Gastroenterology. 2021;160(3):932-934.e933
13. Wang Y, Wiesnoski DH, Helmink BA, Gopalakrishnan V, Choi K, DuPont HL. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med. 2018;24(12):1804-1808
14. Ohwada S, Ishigami K, Akutsu N, Nakase H. Pharmacological Treatments Available for Immune-Checkpoint-Inhibitor-Induced Colitis. Biomedicines. 2022;10(6):1334
15. Weingarden AR, Gubatan J, Singh S. et al. Immune checkpoint inhibitor-mediated colitis is associated with cancer overall survival. World J Gastroenterol. 2022;28(39):5750-5763
16. Zou F, Abu-Sbeih H, Ma W, Peng Y, Qiao W. et al. Association of Chronic Immune-Mediated Diarrhea and Colitis With Favorable Cancer Response. J Natl Compr Canc Netw. 2020;19(6):700-708
17. Wang Y, Abu-Sbeih H, Mao E. et al. Endoscopic and Histologic Features of Immune Checkpoint Inhibitor-Related Colitis. Inflamm Bowel Dis. 2018;24(8):1695-1705
18. Abu-Sbeih H, Ali FS, Luo W, Qiao W, Raju GS, Wang Y. Importance of endoscopic and histological evaluation in the management of immune checkpoint inhibitor-induced colitis. J Immunother Cancer. 2018;6(1):95
19. Choi K, Abu-Sbeih H, Samdani R. et al. Can Immune Checkpoint Inhibitors Induce Microscopic Colitis or a Brand New Entity? Inflamm Bowel Dis. 2019;25(2):385-393
20. Shirwaikar Thomas A, Hanauer S, Wang Y. Immune Checkpoint Inhibitor Enterocolitis vs Idiopathic Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2023;21(4):878-890
21. Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14(10):101174
22. Kim ER, Chang DK. Colorectal cancer in inflammatory bowel disease: the risk, pathogenesis, prevention and diagnosis. World J Gastroenterol. 2014;20(29):9872-9881
23. Stidham RW, Higgins PDR. Colorectal Cancer in Inflammatory Bowel Disease. Clin Colon Rectal Surg. 2018;31(3):168-178
24. van Schaik FD, Mooiweer E, van der Have M, Belderbos TD, Ten Kate FJ, Offerhaus GJ. et al. Adenomas in patients with inflammatory bowel disease are associated with an increased risk of advanced neoplasia. Inflamm Bowel Dis. 2013;19(2):342-349
25. Jackson WE, Achkar JP, Macaron C, Lee L, Liu X, Pai RK. et al. The Significance of Sessile Serrated Polyps in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016;22(9):2213-2220
26. Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V. et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13(2):144-164
27. Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, et al.British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019; 68(Suppl 3): s1-s106.
28. Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD; ACG Clinical Guideline. Ulcerative Colitis in Adults. Am J Gastroenterol. 2019;114(3):384-413
29. Farraye FA, Odze RD, Eaden J, Itzkowitz SH, McCabe RP, Dassopoulos T. et al. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138(2):738-745
30. Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138(6):2101-2114
31. Dahmus J, Rosario M, Clarke K. Risk of Lymphoma Associated with Anti-TNF Therapy in Patients with Inflammatory Bowel Disease: Implications for Therapy. Clin Exp Gastroenterol. 2020;13:339-350
32. Long MD, Martin CF, Pipkin CA, Herfarth HH, Sandler RS, Kappelman MD. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012;143(2):390-399
33. Khan N, Patel D, Trivedi C. et al. Incidence of Acute Myeloid Leukemia and Myelodysplastic Syndrome in Patients With Inflammatory Bowel Disease and the Impact of Thiopurines on Their Risk. Am J Gastroenterol. 2021;116(4):741-747
34. Verheijden RJ, May AM, Blank CU, Aarts MJB, van den Berkmortel FWPJ, van den Eertwegh AJM. et al. Association of Anti-TNF with Decreased Survival in Steroid Refractory Ipilimumab and Anti-PD1-Treated Patients in the Dutch Melanoma Treatment Registry. Clin Cancer Res. 2020;26(9):2268-2274
35. Winawer SJ, Fletcher RH, Miller L, Godlee F, Stolar MH, Mulrow CD. et al. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology. 1997;112(2):594-642
36. Shaukat A, Kahi CJ, Burke CA, Rabeneck L, Sauer BG, Rex DK. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Am J Gastroenterol. 2021;116(3):458-479
37. Gupta S, Lieberman D, Anderson JC, Burke CA, Dominitz JA, Kaltenbach T. et al. Recommendations for Follow-Up After Colonoscopy and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2020;91(3):463-485
38. Kahi CJ, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, et al.Colonoscopy Surveillance after Colorectal Cancer Resection. Recommendations of the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2016;111(3):337-346
39. Abu-Sbeih H, Ali FS, Ge PS. et al. Patients with breast cancer may be at higher risk of colorectal neoplasia. Ann Gastroenterol. 2019;32(4):400-406
40. Abu-Sbeih H, Chen E, Ahmed O. et al. Patients with non-Hodgkin's lymphoma are at risk of adenomatous colon polyps, Dig Dis Sci. 2019; 64(10): 2965-2971.
41. Abu-Sbeih H, Ali FS, Qiao W, Lum P, Shafi MA, Bresalier RS. et al. Patients with non-colorectal cancers may be at elevated risk of colorectal neoplasia. J Cancer. 2020;11(11):3192-3198
42. Abu-Sbeih H, Ali FS, Tang T, Coronel E, Lee HJ, Pande M. et al. Rate of colorectal neoplasia in patients with Hodgkin lymphoma. Colorectal Dis. 2020;22(2):154-160
43. Neumann H, Vieth M, Langner C, Neurath MF, Mudter J. Cancer risk in IBD: how to diagnose and how to manage DALM and ALM. World J Gastroenterol. 2011;17(27):3184-3191
Author contact
![]() Corresponding author: Dr. Yinghong Wang, Department of Gastroenterology, Hepatology, and Nutrition, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Unit 1466, Houston, TX 77030. Tel.: (713) 794-5073. Fax (713) 794-5398. Email: ywang59org
Corresponding author: Dr. Yinghong Wang, Department of Gastroenterology, Hepatology, and Nutrition, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Unit 1466, Houston, TX 77030. Tel.: (713) 794-5073. Fax (713) 794-5398. Email: ywang59org

 Global reach, higher impact
Global reach, higher impact