Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(14):2707-2719. doi:10.7150/jca.86996 This issue Cite
Review
The Dual Effects of Exosomes on Glioma: A Comprehensive Review
1. Department of Neurosurgery, the Affiliated Hospital of Southwest Medical University, Luzhou 646000, China.
2. Department of Neurosurgery, the Affiliated Hospital of PanZhiHua University, PanZhiHua 617000, China.
3. Southwest Medical University, Luzhou 646000, China.
4. Sichuan Clinical Research Center for Neurosurgery, Luzhou 646000, China.
5. Academician (Expert) Workstation of Sichuan Province, Luzhou 646000, China.
*Maowen Luo and Xingzhao Luan contributed equally to this work.
Received 2023-6-10; Accepted 2023-8-21; Published 2023-9-4
Abstract
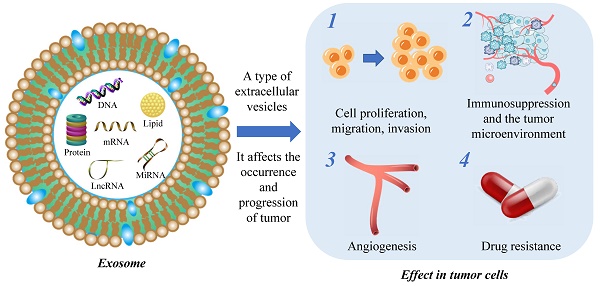
Glioma is a frequently occurring type of cancer that affects the central nervous system. Despite the availability of standardized treatment options including surgical resection, concurrent radiotherapy, and adjuvant temozolomide (TMZ) therapy, the prognosis for glioma patients is often unfavorable. Exosomes act as vehicles for intercellular communication, contributing to tissue repair, immune modulation, and the transfer of metabolic cargo to recipient cells. However, the transmission of abnormal substances can also contribute to pathologic states such as cancer, metabolic diseases, and neurodegenerative disorders. The field of exosome research in oncology has seen significant advancements, with exosomes identified as dynamic modulators of tumor cell proliferation, migration, and invasion, as well as angiogenesis and drug resistance. Exosomes have negligible cytotoxicity, low immunogenicity, and small size, rendering them an ideal therapeutic candidate for glioma. This comprehensive review discusses the dual effects of exosomes in glioma, with an emphasis on their role in facilitating drug resistance. Furthermore, the clinical applications and current limitations of exosomes in glioma therapy are also discussed in detail.
Keywords: Glioma, exosome, diagnosis, therapy, drug resistance.
Introduction
Gliomas are tumors arising from glial and neuronal cells in the nervous system, representing 78.2% of all malignant central nervous system tumors in individuals aged over 65 years[1]. The high morbidity and mortality rates are concerning, highlighting the urgent need for effective treatment options. In 2021, the World Health Organization updated the classification and grading of gliomas based on molecular markers, providing significant opportunities for immunotherapy and targeted therapy[2]. However, owing to technical limitations and unclear molecular mechanisms, the standard treatment remains surgical resection, synchronous radiotherapy, and adjuvant TMZ therapy[3]. The generation of TMZ resistance and limited drug delivery through the blood-brain barrier (BBB) significantly impairs the efficacy of treatment, leading to a poor median survival of only 14.6 months in glioma patients[4]. Therefore, the development of novel therapeutic strategies is imperative for mitigating the clinical challenge posed by gliomas.
Exosomes are extracellular vesicles (EVs) with a diameter of 30-150nm and a density of 1.13-1.19g/mL, containing a complex repertoire of metabolites such as lipids, proteins, and nucleic acids[5]. Current research suggests that exosomes are generated through the fusion of multivesicular bodies (MVBs) with the plasma membrane, a process that can be modulated by diverse regulatory mechanisms, resulting in the heterogeneous composition of exosomes[6, 7]. MVBs can undergo either lysosomal digestion or exosomal release, depending on their degree of maturation. Exosomes can play both beneficial and detrimental roles in intercellular communication, facilitating the transfer of functional proteins, metabolites, and nucleic acids from donor cells to recipient cells in both physiological and pathological contexts[8, 9]. However, accumulating evidence suggests that exosomes are also implicated in the pathogenesis and progression of tumors such as glioma[10], breast cancer[11], lung cancer[12], and colorectal cancer[13], as well as drug resistance. In the case of glioma, exosomes are crucial in promoting tumor cell proliferation, invasion, migration, angiogenesis, immune invasion, and mediating drug resistance[14-16]. Targeting exosomes represents a promising strategy for diagnosing and treating glioma, holding potential to yield innovative breakthroughs.
Exosomes exhibit advantageous characteristics such as broad applicability, ability to readily penetrate the BBB, and low immunogenicity, rendering them an attractive therapeutic strategy for glioma. The rapid advancements in separation and purification technologies, as well as nanotechnology, have presented new possibilities for the clinical diagnosis and treatment of glioma with exosomes. This review provides an overview of the role and mechanism of exosomes in glioma, with a particular emphasis on their contribution to the development of drug resistance. Additionally, the clinical prospects of exosomes in glioma therapy are discussed.
Exosome biogenesis
Initially believed to function as cellular waste disposal mechanisms, exosomes were first discovered in 1983[17] and officially named in 1987[18]. Exosomes are a subgroup of extracellular vesicles that can be distinguished from microvesicles (MVs) and apoptotic bodies. Exosomes originate from MVBs which fuse with the plasma membrane[19] for secretion. The biogenesis of exosomes involves multiple key steps, including substrate sorting, MVB formation, MVB trafficking, the fusion of MVBs with the plasma membrane to generate intraluminal vesicles (ILVs), and the release of exosomes containing the ILVs. This exquisitely orchestrated process is governed by various mechanisms that contribute to the heterogeneous composition and diverse biological functions of exosomes.
According to data from the latest exosome database, exosomes contain diverse types of RNA, proteins, and lipids[20]. Exosomal RNA encompasses mRNA, micro-RNA (miRNA), long non-coding RNA (lncRNA), circular RNA (circRNA), among other species, with crucial implications for the diagnosis and treatment of glioma. Exosomal proteins consist of membrane transport and fusion-related proteins, tetraspanin family proteins, and MVB-associated proteins, characterized by ubiquitination and vitally contributing to the formation of ILVs[21].
Current studies on exosomes, as a subtype of extracellular vesicles, have predominantly focused on their function in mediating intercellular communication and transferring materials, thereby participating in a wide array of physiological processes. Exosomes are involved in tissue maintenance and repair[22], intercellular communication[23], immune regulation[24], anti-inflammatory and anti-infective responses[25-27], and facilitating tissue regeneration after injury[28-30]. Unlike normal cells, tumor cells secrete exosomes under the control of genetic factors, which maintain cellular homeostasis, evade immune detection, and promote drug resistance[31] (Figure 1).
The biological functions and properties of exosomes from different sources.
| Exosome source | Present site | Biological function | Clinical significance |
|---|---|---|---|
| Normal cells[102] | Every part | Intercellular communication | / |
| Tumor cells[13, 103] | Cerebrospinal fluid, serum | It plays an important role in tumor growth, metastasis, and formation of tumor immunosuppressive microenvironment | It can be used as the target of tumor therapy and drug carrier |
| Mesenchymal stem cell (MSC)[104, 105] | Bone marrow, umbilical cord blood, placental tissue, adipose tissue | Substance transport, immune regulation, maintenance of homeostasis, tissue repair | It can be used as the main source of exosomes |
| Macrophage[106] | Bone marrow and serum | Immunoregulation | It plays an important role in tumor invasion and drug resistance formation |
| Dendritic cell (DC)[107, 108] | T cell dependent region of peripheral immune organs | Immunoregulation | It plays an important role in tumor invasion and drug resistance formation |
| Virus infected cell[109, 110] | From the site of the virus gradually spread to all parts | Transfer of bioactive ingredients associated with viral infection | An important therapeutic target for controlling certain virus-infected diseases |
Origin and mechanism of exosomes and their effects on glioma progression.
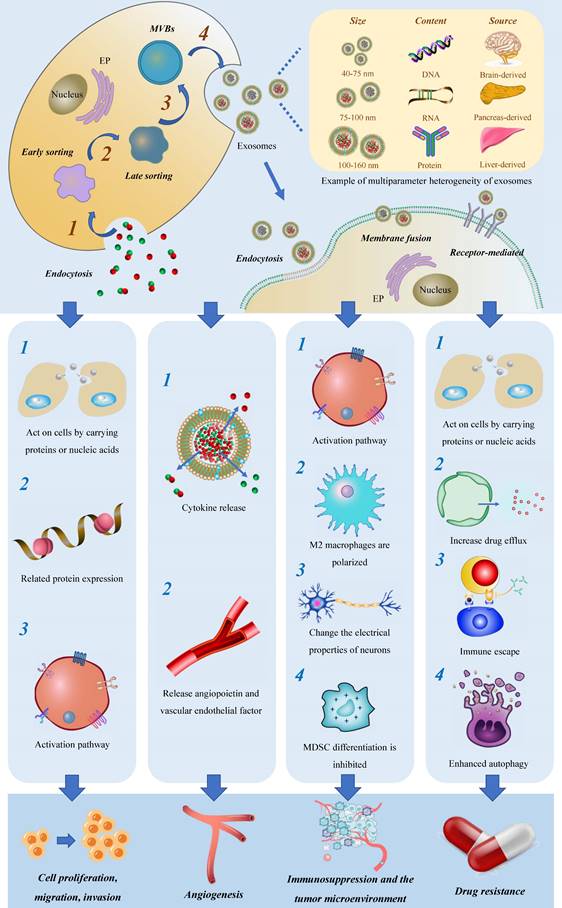
Exosomes are derived from diverse human tissues and cells and can be found in various extracellular fluids, including plasma, cerebrospinal fluid, interstitial fluid, lymph fluid, and others[32]. Furthermore, they have the capability to interact with different target cells, facilitating the transmission of biological information[33]. Here we briefly summarize the biological functions and properties of exosomes from different sources (Table 1).
Advances in molecular biology technologies, coupled with advancements in exosome isolation and preparation, have facilitated highly detailed studies of these tiny vesicles. Differential ultracentrifugation (UC) has been widely recognized as a trusted method for isolating exosomes from biological fluids, albeit considered cumbersome in recent times[34]. Sung Jin Back et al. designed CaTiO3:Eu3@Fe3O4 multifunctional nanocomposites for the direct capture and separation of exosomes from complex biological systems, opening up new avenues for portable exosome detection[35].
Although research on exosomes has made significant progress in recent years, there are still limitations in the separation methods, including their cumbersome nature, low speed, yield, and purity. These limitations hinder the advancement of both basic research and clinical applications of exosomes. As a result, researchers have been actively seeking new, convenient, and efficient separation methods. In 2021, a highly efficient exosome detection method, known as the ultrafast separation system (EXODUS), was introduced. This method utilizes negative pressure oscillations and double-coupled harmonic oscillators to induce membrane vibration, enabling the ultra-efficient purification of exosomes. Excitingly, the EXODUS system allows for automatic labeling-free purification of exosomes from various biological fluids. The development of this method has created vast opportunities for the separation and extraction of exosomes. However, despite its promising potential, the widespread application of this method still requires further popularization in both the research community and clinical settings[36].
In terms of exosome preparation, biogenic exosomes are typically processed and modified through genetic or chemical engineering[37]. With the development of nanotechnology, attempts to design exosome-mimetic nanoscale structures that replicate the unique features of exosomes are becoming feasible[38-40].
Given the strides made in exosome isolation and preparation technologies, a growing body of research has begun exploring the use of exosomes in tumor treatment. In comparison to artificial nanoplatforms, exosomes offer several advantages, including low cytotoxicity, minimal immunogenicity, high biocompatibility, and readily manipulatable for synthetic modifications. Exosomes have emerged as promising therapeutic agents and gene carriers for potential clinical applications.
The role and mechanism of exosomes in glioma progression
Exosomal effects on glioma cell proliferation, invasion, and migration
As carriers of materials, exosomes facilitate the transmission of normal and aberrant substances between cells and the tumor microenvironment (TME), providing an ideal mechanism for the migration and invasion of tumor cells. Furthermore, exosomes act as information carriers, mediating the transmission of abnormal signals through membrane fusion events, leading to altered gene expression and aberrant tumor cell proliferation activity.
The process of cell proliferation involves the modulation of cycle-related proteins, which encompasses various steps such as gene expression, transcription, and translation. In this review, we focus on three main aspects of previous research. Firstly, certain components within exosomes act as essential regulatory factors, directly influencing the proliferation of tumor cells. For example, in sero-derived exosomes, the overexpression of lnc-LINC00470 has been identified as a key regulator of glioma autophagy and proliferation. This molecule binds to miR-580-3p in glioma cells, resulting in the regulation of WEE1 expression and activation of the PI3K/AKT/mTOR pathway, ultimately inhibiting autophagy[14]. There is a strong correlation between this mechanism and the degree of malignant progression and survival time in glioma patients. Secondly, exosomes indirectly contribute to tumor progression through a sorting mechanism. Glioma cells selectively eliminate tumor-suppressive miRNAs via exosomes, which are then transferred to immune cells within the TME. This, in turn, induces a transformation of immune cells into cancer-promoting phenotypes. Conversely, miRNAs that enhance tumor proliferation are selectively retained within tumor cells, continuously promoting cell proliferation. These findings highlight the intricate role of exosomes in tumor proliferation, shedding light on both direct and indirect regulatory mechanisms[41]. What's more, in terms of migration and invasion, several investigations have shown that circNEIL3[42], miR-3184-3p[43], miR-3591-3p[44], miR-148a[45], miR-15a, and miR-92a[46] can all be packaged into exosomes via specific pathways and are critical to the migration and invasion of glioma cells. Nonetheless, most of the research remains in the stage of bioinformation analysis and experimental verification, and its mechanism has yet to be effectively proven.
Exosomes and their impact on glioma angiogenesis
Glioma is often characterized by angiogenesis and hypoxia, which have been linked to unfavorable clinical outcomes[47]. Exosomes have been shown to play a significant role in mediating angiogenesis through various pathways. Firstly, exosomes release cytokines such as IL-8 and VEGF into the TME, which act on endothelial cells to rapidly form peripheral blood vessels. Secondly, exosomes are complex and heterogeneous, especially those derived from tumors, and exhibit significant changes in protein components, often containing angiopoietin and vascular endothelial factors[48-50].
Among these pathways, vasculogenic mimicry (VM) is a unique phenomenon observed in glioma, where independent channels composed of basement membranes without endothelial cells and fibroblasts are formed[51]. The presence of VM can increase the feeding blood flow to hypoxic glioma cells, leading to enhanced glioma cell proliferation[52]. Recent studies by J. Jiang have revealed a downregulation of miR-376b-3p in serum exosomes of malignant glioma patients. Intriguingly, the upregulation of miR-376b-3p in serum-derived exosomes has been shown to enhance the expression of HOXD10, leading to a reduction in glioma cell proliferation and invasion. Additionally, this upregulation impedes the formation of angiogenic mimics. These findings indirectly suggest that serum-derived exosomes have the ability to promote glioma angiogenesis [53]. In addition, the delivery of miRNA-29a-3p through exosomes secreted by human MSCs has been found to inhibit VM production and thus impede the progression of glioma[54].
What's more, hypoxia profoundly affects the repertoire and composition of proteins present in exosomes derived from glioma cells. Notably, selective upregulation of protein-lysine 6-oxidase (LOX-6), VEGF, thrombospondin-1 (TSP1) and other proteins has been observed, which converge in the tumor neovascularization region and may serve as key players in tumor angiogenesis[55].
Exosomes induce the formation of glioma immunosuppressive microenvironment
Immune cells, particularly macrophages and monocytes, comprise the majority of immune cells within the TME[56]. Increasingly, studies have revealed that exosomes are key mediators of the critical link between glioma cells and the surrounding microenvironment[57]. Simultaneously, exosomes are closely associated with immune tolerance in glioma. They not only aid tumor cells in evading immune surveillance and creating an environment conducive to tumor cell generation through direct inhibition of immune cells, but also play adverse roles in immune regulation within the body. They achieve this by transmitting inhibitory signals that promote the establishment of immune tolerance in glioma cells.
Exosomes play a pivotal role in inducing the formation of an immunosuppressive microenvironment through three major mechanisms. Firstly, they stimulate the polarization of M2 macrophages, contributing to the maintenance of chronic inflammation and increased tumor aggressiveness. This polarization can be achieved through the delivery of tumor suppressor miR-3591-3p[44] and miR-1246[58, 59] by tumor-derived exosomes. Secondly, exosomes facilitate the generation of myeloid suppressor cells (MDSC), which play a critical role in establishing immunosuppressive microenvironments and assisting tumors in evading host immune responses[60]. MiR-29a and miR-92a promote the proliferation of MDSC by targeting high-mobility group box transcription factor 1 (Hbp1) and protein kinase cAMP-dependent type I regulatory subunit alpha (Prkar1a), respectively[61]. Furthermore, the presence of stem-like brain tumor initiation cells (BTIC) with enhanced resistance to radiation and chemotherapy contributes to the poor prognosis of glioblastoma. Exosomes released by BTIC carry tenascin-C (TNC) and have been shown to inhibit T cell activity[62].
In several clinical studies, mounting evidence suggests that the TME has a crucial role in epileptic seizure onset in glioma patients. Recent findings suggest that exosomes can be released by tumor cells through synapses and carry out abnormal information transfer between neurons. As a result, specific rearrangements occur in neuronal connections, exerting a far-reaching influence on neuronal network activity and synchronization, leading to cognitive decline in glioma patients, and severe cognitive impairment in more severe cases[63]. These studies provide valuable insights into the debilitating complications yielded by glioma, and hold considerable significance in terms of enhancing the quality of life of affected patients.
The association between exosomes and drug resistance in glioma
Surgical treatment combined with radiotherapy and TMZ is one of the most frequently employed methods for glioma treatment. TMZ is an oral alkylating agent that directly damages tumor cell DNA by attacking the N-7 and O6 sites of guanine, as well as, the N-3 sites of adenine. The formation of O6-methylguanine (O6-meG) is a crucial product that plays a role in inducing apoptosis[64]. Various factors, such as DNA damage repair, immune stress response, abnormal expression of proto-oncogenes and tumor suppressor genes, contribute to TMZ resistance. At present, O6-methylguanine-DNA methyltransferase (MGMT) is the most common marker of TMZ resistance, with its presence leading to resistance to TMZ by removing TMZ-induced alkylation[65]. Exosomes have a dual influence on glioma drug resistance. On one hand, they can promote drug resistance, even inducing the transition of TMZ-sensitive cells into drug-resistant ones. On the other hand, certain exosomes possess the ability to reverse TMZ-resistant glioma cells, rendering them sensitive to treatment. In this discussion, we will explore the former scenario first.
Exosomes can carry a diverse range of substances, with the direct or indirect targeting mediated by RNA being one significant contributor to drug resistance, according to current research[66]. Numerous studies have highlighted the importance of exosome-associated ncRNA in regulating the chemo- and radiation resistance of glioma cells through various pathways, demonstrating their strong connection to the development of glioma resistance (Figure 2, Table 2).
Occurrence and development of exosomal RNA and glioma resistance.
| Source | Exosomal-RNA | Mode of action | Medicine | Drug susceptibility | Clinical significance |
|---|---|---|---|---|---|
| Hypoxic glioma cells | MiR-106a-5p[69] | By regulating PTEN/Akt signaling | TMZ | Reduce | Provide new ideas for targeted therapy |
| MSC | MiR-199a[95] | Down-regulating AGAP2 inhibits glioma progression | TMZ | Improve | Provide new molecular targets |
| TMZ resistant glioma cells | MiR-1238[71] | From non-sensitive cells to sensitive cells | TMZ | Reduce | Promising molecular targets |
| TMZ resistant glioma cells | MiR-25-3p[70] | Target the regulation of FBXW7 | TMZ | Reduce | Prognostic marker |
| TMZ resistant glioma cells | MiR-151a[96] | Exogenous miRNA is transferred through exosomes | TMZ | Improve | Used for TMZ combined therapy |
| TMZ resistant glioma cells | LncSBF2-AS1[75] | Remodel the TME | TMZ | Reduce | As a diagnostic marker of refractory glioma |
| TMZ resistant glioma cells | Circ_0043949[111] | Up-regulated ITGA1 axis of oncogene | TMZ | Reduce | Providing potential molecular targets |
| TMZ resistant glioma cells | Circ_0072083[77] | Regulate the expression of NANOG and ALKBH5 | TMZ | Reduce | Important targeting marker |
| TMZ resistant glioma cells | CircWDR62[78] | Adjust the miR-370-3p/MGMT axis | TMZ | Reduce | Targeting markers and prognostic markers |
| Glioma cells | Circ_0042003[80] | Mediated by heparinase | TMZ | Reduce | To provide ideas for the development of new treatment strategies |
| TMZ resistant glioma cells | Circ-HIPK3[79] | Regulate the miR-421 / ZIC5 axis | TMZ | Reduce | Improve the therapeutic effect of TMZ |
Exosomes are associated with glioma drug resistance.
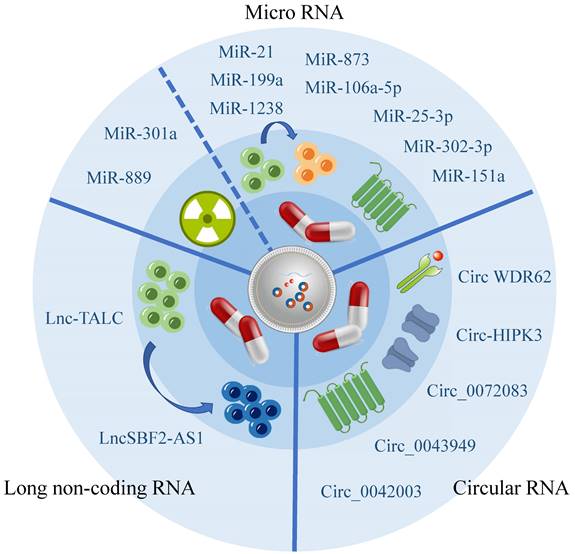
Exosomal miRNA influence glioma drug resistance
MiRNA is a class of regulatory molecules that are around 22 nucleotides long, and processed by RNA precursors, playing multiple functions such as down-regulating gene expression[67], and transmitting information[68].
Recent studies have been summarized, revealing that miRNA has been shown to decrease the vulnerability of glioma cells towards TMZ. Additionally, certain miRNAs have the ability to penetrate exosomes released by TMZ-resistant cells and be absorbed by TMZ-sensitive cells, resulting in the transmission of TMZ resistance. Exosomes originating from hypoxic glioma cells have demonstrated the capability to diminish PTEN expression through the transportation of miR-106a-5p, consequently reducing the sensitivity of glioma cells to TMZ[69]. Furthermore, bioactive miR-1238 and miR-25-3p have been observed to be incorporated into exosomes released by TMZ-resistant cells, thus having the potential to be absorbed by TMZ-sensitive cells and propagate TMZ resistance[70, 71].
In addition to the increased chemotherapeutic resistance associated with TMZ, Xiao Yue's research has also identified that exosome miRNA is associated with a decrease in the effectiveness of radiotherapy. Specifically, exosome miR-301a has been found to activate the Wnt/ beta-catenin signaling pathway by targeting TCEAL7 under hypoxic conditions, which reduces radiation sensitivity and ultimately minimizes the radiotherapy effects of glioma[72].
Exosomal lncRNA and its influence on glioma drug resistance
LncRNA are a class of ncRNA that are over 200 nucleotides in length. They are characterized by low stability, poor conservation, and are closely associated with the occurrence and progression of several cancers[73]. Some studies have demonstrated that exosomes can shield lncRNA from degradation[74]. In glioma, exosome lncSBF2-AS1 is secreted from glioma cells into the TME to promote the development of drug resistance in tumors[75]. This mode of material transfer significantly complicates the treatment of glioma.
Exosomal circRNA Influence Glioma Drug Resistance
CircRNA hold a unique annular structure, which distinguishes them from miRNA and lncRNA. With the rapid advancements in gene sequencing and high-throughput bioinformatics, their crucial role in diverse physiological and pathological processes, including cell proliferation, growth, differentiation, and senescence, has been established[76].
CircRNA has been increasingly recognized as having a significant impact on glioma drug resistance. However, its effect on drug resistance is primarily indirect, as it affects miRNA expression. This suggests that exosomal miRNA plays a crucial role in the development of glioma drug resistance. The Warburg effect plays a significant role in promoting the release of exosomal circ_0072083 from TMZ-resistant glioma cells, which elevates glioma resistance to TMZ by selectively targeting miR-5-1252p[77]. Likewise, exosomal circWDR62 from TMZ-resistant glioma cells participates in transmitting resistance between TMZ-sensitive and non-sensitive cells through the miR-370-3p/MGMT axis[78]. Additionally, exosomal circ-HIPK3 from TMZ-resistant glioma cells regulates glioma via the miR-421/ZIC5 axis, thereby enhancing its drug resistance[79].
Besides its indirect impact on glioma drug resistance through miRNA, circRNA may also have an influence on other aspects. However, further research is required to explore these potential roles. Recent research has identified heparinase as a key regulator of exosomal secretion that plays a crucial role in the development of drug resistance. This mechanism may be attributed to the delivery of hsa_circ_0042003 through exosomes[80].
Other Implications of Exosomes in Glioma Resistance
Apart from the well-established role of exosomal RNA in drug resistance, various other factors continually influence the development of glioma resistance. In recent years, it has been observed that exosomes are capable of transferring therapeutic drugs from within tumor cells to the extracellular environment, thereby rendering them ineffective. Moreover, exosomes facilitate the formation of an effective fibrous barrier by inducing fibroblast response, which prevents therapeutic drugs from reaching specific sites.
As mentioned earlier, MGMT is an essential biomarker in glioma drug resistance prediction. Barbara Oldrini et al. have demonstrated the significance of MGMT rearrangement in inducing drug resistance, which effectively addresses the limitations of grouping MGMT methylation. Furthermore, they have successfully detected the fusion gene after MGMT rearrangement in tumor-derived exosomes, thereby offering a promising avenue for future medication guidance through exosome detection[81]. Rajshekhar A Kore et al. have reported a smaller volume of tumor-derived exosomes in hypoxic environments, which may be attributed to exosomal metabolism. This finding may help elucidate the intricate relationship between drug resistance and hypoxia, but further research is required to uncover the underlying mechanism[55].
In this review, we have primarily focused on the impact of non-coding RNA in exosomes on glioma drug resistance. However, we are also intrigued by the potential involvement of proteins and lipids in exosomes. Despite our extensive literature review, we have yet to establish a definitive link between these components and the development of glioma resistance. This area warrants further investigation in future studies due to the complex and diverse nature of proteins and other metabolites in exosomes.
Clinical application of exosomes in glioma
Exosomes for diagnosis and prognosis of glioma
Brain tumors present distinct diagnostic challenges due to the BBB. Hence, conventional circulating biomarkers, including circulating tumor cells, are often not of diagnostic value in glioma. Exosomes, a type of extracellular vesicle, carry rich genetic information and are widely found in various bodily fluids, presenting as potential markers for tumor diagnosis and prognosis[82], and as an auxiliary modality for existing imaging techniques to improve the early diagnosis of glioma[83].
From a diagnostic perspective, exosomes play a significant role in determining the pathological grade of glioma. Notably, exosomes derived from serum samples of glioma patients exhibit abnormal overexpression of miR-210, and this expression level increases with the progression of glioma grade[84]. Additionally, the elevation of miR-301a levels is associated with higher pathological grades and lower Karnofsky physical status (KPS) scores. In serum samples, miR-301a is predominantly found in exosomes[85]. Furthermore, glioma cells release exosomes enriched with the cancer-associated lncRNA, lncSBF2-AS1, which reshapes the TME and contributes to the development of chemotherapy resistance. Therefore, the presence of lncSBF2-AS1 in serum may serve as an indicator of refractory glioma[75].
In terms of prognosis, tumor-associated macrophages (TAMs) are the predominant cell population within the glioma microenvironment and play a crucial role in glioma initiation and malignant progression[86, 87]. Recent studies have demonstrated that circNEIL3, facilitated by hnRNPA1B2, can be packaged into exosomes and delivered to infiltrating TAMs. This process leads to the acquisition of immunosuppressive properties by TAMs, as circNEIL3 stabilizes IGF3BP3, thereby promoting glioma progression. The detection of exosomal circNEIL3 levels may serve as a prognostic indicator for glioma[42]. Additionally, exosome-derived miR-1246 has been shown to impact the polarization of M2 macrophages. Notably, this microRNA is enriched in the cerebrospinal fluid of glioma patients and its levels decrease after tumor resection, indicating that it may also be one of the prognostic indicators of glioma[58].
It is noteworthy to consider the relevance of exosome detection in predicting the efficacy of radiotherapy. Zihuang Li conducted a study examining 34 genes that exhibited differential expression, with 11 of them being associated with glioma. Notably, after radiotherapy, a significant reduction in miR-574-3p was observed. This finding suggests that miR-574-3p may serve as a promising biomarker candidate for monitoring the effectiveness of radiotherapy[82].
Exosomes serve as high-quality, nanoscale information transmitters that are ubiquitous in cerebrospinal fluid and blood[88]. While we acknowledge the significant potential of exosomes as biomarkers, it is important to highlight that current studies only demonstrate that the elevation of exosome levels is a consequence of tumorigenesis, rather than a causative factor. The evidence regarding exosomes as a cause of tumorigenesis necessitates further examination and discussion.
Exosomes as targeted therapy vectors for glioma
Glioma, a central nervous system tumor, presents a poor prognosis, primarily due to several factors. Firstly, the presence of the BBB limits the efficacy of drugs due to their inability to directly reach the tumor area. Secondly, glioma exhibits resistance to lipophilic drugs such as TMZ, further hindering appropriate treatment[89]. Lastly, the therapeutic effect of a single drug may be insufficient, leading to incomplete treatment and disease recurrence. Consequently, the exploration of more effective and accurate treatment methodologies that improve glioma's therapeutic effect and prognosis is critical.
Nano-drugs have shown promise in improving chemotherapy drugs' targeted accumulation, biological distribution, and penetration efficiency, providing significant clinical prospects for diagnosing and treating next-generation tumors[90]. Exosomes, nanoscale structures with bilayer membranes, carry low immunogenicity, high protective ability, and strong penetration capacities across the BBB[91]. Current research has reported the use of exosomes as tumor drug hosts, demonstrating clinically relevant achievements. Exosomes loaded with therapeutic molecules serve as promising biomolecules that can enhance targeted accumulation, improve drug efficacy, and reduce side effects. Hence, exosomes may hold great potential in revolutionizing current tumor therapy approaches (table 3).
Despite exosomes' potential as loading materials, they present several limitations, such as time-consuming purification procedures, low yield, and difficulties related to large-scale production. Recent comparative studies have demonstrated that biomimetic nanovesicles (BNVs) exhibit similar targeting properties, BBB penetration, and load capacity as exosomes[92]. To some extent, BNVs may serve as substitutes for exosomes in industrial production and large-scale applications, creating new opportunities for future exosome research.
Exosomes in reversing therapeutic resistance in glioma
TMZ is an FDA-approved first-line chemotherapy drug for glioma and a DNA alkylating agent[3]. However, some glioma patients experience drug resistance after TMZ chemotherapy, hindering treatment efficacy. DNA repair, transporter expression, and MGMT overexpression commonly contribute to drug resistance development[93]. As previously mentioned, exosomes play a dual role in glioma drug resistance. In this section, we will specifically explore the capacity of exosomes to convert drug-resistant glioma cells into drug-sensitive ones.
To overcome chemotherapy resistance in glioma, exosomes can be utilized as drug carriers. Examples of this include the design of a double-receptor-specific exosome loaded with TMZ and BG, which aims to repair O-alkylation damage caused by TMZ[94]. Another approach involves the direct delivery of miR-199a to glioma cells through exosomes, which are derived from MSCs. This delivery inhibits glioma cell proliferation, invasion, and migration while enhancing chemotherapy sensitivity[95]. Similarly, the loading of exogenous miR-151a into exosomes emerges as a potential strategy to reverse glioma resistance[96]. Recognizing the clinical significance of exosomes, Fawad Ur Rehman investigated the utilization of exosomes (BMSCEXO) derived from allogeneic bone marrow MSCs for TMZ-resistant glioblastoma. These exosomes were employed as carriers for heme oxygenase-1 (HMOX1) specific short peptides (HSSP) and siRNA[97].
Collectively, these exosome-related molecular biological findings hold promise as adjuvant therapy with TMZ chemotherapy to enhance the therapeutic efficacy of refractory gliomas.
Other clinical applications of exosomes in glioma
Nucleic acids and proteins are fundamental regulatory molecules that affect almost every aspect of the tumor process. Exosomes serve as carriers for these major molecules, providing a viable approach for their transportation[98]. Current studies aim to block abnormal exosome transmission to treat tumors, including glioma, by inhibiting exosome production, release, and reuptake[41, 58, 99].
Tumor-derived exosomes carry antigens that induce immune responses[100]. Ning Bu et al. discovered that DC-derived exosomes produce cytotoxic effects against autogenous tumors, while exosomes obtained from ascites and pleural effusion of ovarian cancer patients demonstrate anti-tumor therapy effectiveness[101]. These findings suggest that exosomes also relate to current research on tumor immunotherapy, which is an area in need of further exploration.
Application of exosomes in drug or gene loading.
| Load material | Carrier | Clinical significance |
|---|---|---|
| Superparamagnetic iron oxide nanoparticles (SPIONs) and curcumin (Cur) | RGE-Exo-SPION / Cur[112] | As a targeting ligand of NRP-1, it is used for targeted imaging and therapy of glioma cells |
| DOX | Bioinspired neutrophil-exosomes (NEs-Exos) system[113] | It has strong neutrophil chemotaxis and BBB penetration |
| TMZ and O6-benzylguanine (BG) | Dual-receptor-specific exosomes[94] | The activity of O6-alkylguanine-DNA alkyl transferase (AGT) was inhibited by transferring alkyl to Cys145, which played a role in enhancing TMZ |
| Panobinostat and PPM1D-siRNA | A biomimetic nano drug delivery system (cEM@DEP‐siRNA)[114] | For targeted therapy of diffuse intrinsic pontine glioma (DIPG) caused by PPM1D mutation |
| Selumetinib | Exosomes derived from U87MG cells[115] | Targeting gliomas without cytotoxicity |
| Magnetic nanoparticles (MNPs) | engineered exosomes[40] | It provides a new idea for enhancing iron death in collaborative GBM therapy. |
| DOX and PTX | Exosomes were loaded through a microfluidic device (Exo-Load)[116] | Further optimized potential drug loading devices for exosomes were designed |
| DOX | Endothelium-derived exosomes[117] | Immunogenic chemotherapy for glioma |
| TMZ and Dihydrotanshinone (DHT) | Reassembly-exosomes (R-EXO)[118] | It is used to reverse TMZ resistance |
Advantages of exosome as the therapeutic nano-carrier.
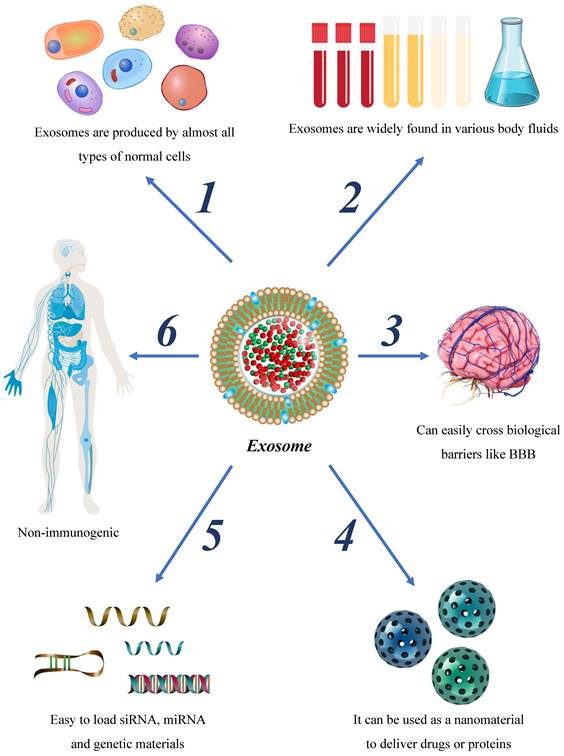
Future perspectives
Glioma is a prevalent central nervous system neoplasm with a high mortality rate. Although combined therapies, including surgical excision, radiotherapy, and chemotherapy have been employed in recent years, refinements are required to optimize their therapeutic efficacy. Exosomes, a type of extracellular vesicle characterized by their bilayer membrane structure, have unique properties that make them important in the regulation of glioma. While the impacts of exosomes on glioma are multifaceted, exhibiting effects on glioma migration, invasion, immune regulation, drug resistance, and other aspects, many of their underlying mechanisms remain elusive. Considering the molecular size, molecular origin, and immune properties of exosomes, these vesicles possess unique advantages over other approaches for the diagnosis and treatment of glioma (Figure 3). In this study, we comprehensively review the origin and mechanisms of exosomes, their influence on glioma progression, and their role in the treatment of glioma.
The utilization of exosomes for clinical diagnosis and therapy remains a challenging task based on current research. The foremost challenge is the difficulty in achieving high levels of exosome purity through currently available methods and techniques. Furthermore, as exosomes are natural extracellular vesicles, their production is currently limited, and strategies to significantly increase their production require further investigation. Additionally, the transport mechanism of miRNA within exosomes has yet to be fully elucidated, and the cytotoxicity and side effects of miRNA-loaded exosomes have yet to be thoroughly studied. Ultimately, the clinical application of exosomes lacks standardization, and inappropriate usage may result in irreversible adverse effects on patient outcomes. These issues significantly impede the clinical application of exosomes and hamper their potential efficacy in the treatment of glioma.
Continuous research and exploration on the loading mechanism, cytotoxicity, and standardized use of exosomes remain necessary for future developments. Innovative approaches, such as simulating the natural structure and biological behavior of exosomes and developing bio-cell derived nanocarriers yielding higher quantities, could potentially overcome current limitations of low exosome production and accessibility. Further investigating the effects of exosomes on glioma angiogenesis, TME, and immunosuppression is necessary to meet the varying needs of patients and achieve targeted treatment.
Overall, exosomes provide a promising avenue for targeted and personalized therapy in the diagnosis and treatment of glioma. In conclusion, while exosomes have both benefits and drawbacks in relation to gliomas, we must strive to leverage their advantages to advance glioma treatment and mitigate their adverse influence on malignant progression.
Abbreviations
MiRNA: micro-RNA; LncRNA: long non-coding RNA; CircRNA: circular RNA; TMZ: temozolomide; BBB: blood-brain barrier; EV: extracellular vesicle; MVB: multivesicular body; MV: micro vesicle; ILV: intraluminal vesicles; LOX-6: protein-lysine 6-oxidase; VEGF: vascular derived endothelial factor; TSP1: thrombospondin-1; GAM: glioblastoma-associated microglia; hBMEC: human brain micro vessel endothelial cell; MDSC: Myeloid-derived suppressor cell; BTIC: Brain tumor initiating cell; TNC: tenascin-C; MGMT: O6-methylguanine-DNA methyltransferase; ncRNA: non-coding RNA; DOX: doxorubicin; Nes-Exo: Bioinspired neutrophil-exosome; DHT: Dihydrotanshinone; Cur: curcumin; SPION: Superparamagnetic iron oxide nanoparticle; AGT: O6-alkylguanine-DNA alkyl transferase; BG: O6-benzylguanine; DIPG: diffuse intrinsic pontine glioma; MNP: magnetic nanoparticle; PTX: paclitaxel; BNV: biomimetic nanovesicle; HMOX1: Heme Oxygenase-1; MSC: mesenchymal stem cell; DC: dendritic cell; siRNA: small interfering RNA; TME: tumor microenvironment.
Acknowledgements
Funding
This research was supported by Joint project of Hejiang County and Southwest Medical University (Grant No. 2020-HJXNYD-6), and Sichuan Science and Technology Program (Grant No. 2022YFS0630).
Author contributions
ML, XL, and GJ collected the related papers and drafted the manuscript.KY, SL, WX and JZ participated in the design of the review and drafted the manuscript. GJ and LY revised the manuscript. All authors reviewed the manuscript and all approved of the final version.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Low JT, Ostrom QT, Cioffi G, Neff C, Waite KA, Kruchko C. et al. Primary brain and other central nervous system tumors in the United States (2014-2018): A summary of the CBTRUS statistical report for clinicians. Neuro-oncology practice. 2022;9:165-82
2. Gritsch S, Batchelor TT, Gonzalez Castro LN. Diagnostic, therapeutic, and prognostic implications of the 2021 World Health Organization classification of tumors of the central nervous system. Cancer. 2022;128:47-58
3. Fisher JP, Adamson DC. Current FDA-Approved Therapies for High-Grade Malignant Gliomas. Biomedicines. 2021;9:324
4. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine. 2005;352:987-96
5. Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Current protocols in cell biology. 2006 Chapter 3: Unit 3.22
6. Kwon SH, Oh S, Nacke M, Mostov KE, Lipschutz JH. Adaptor Protein CD2AP and L-type Lectin LMAN2 Regulate Exosome Cargo Protein Trafficking through the Golgi Complex. The Journal of biological chemistry. 2016;291:25462-75
7. Rabas N, Palmer S, Mitchell L, Ismail S, Gohlke A, Riley JS. et al. PINK1 drives production of mtDNA-containing extracellular vesicles to promote invasiveness. The Journal of cell biology. 2021;220:e202006049
8. Sung BH, Parent CA, Weaver AM. Extracellular vesicles: Critical players during cell migration. Developmental cell. 2021;56:1861-74
9. Noonin C, Thongboonkerd V. Exosome-inflammasome crosstalk and their roles in inflammatory responses. Theranostics. 2021;11:4436-51
10. Karami Fath M, Azami J, Masoudi A, Mosaddeghi Heris R, Rahmani E, Alavi F. et al. Exosome-based strategies for diagnosis and therapy of glioma cancer. Cancer cell international. 2022;22:262
11. Dong X, Bai X, Ni J, Zhang H, Duan W, Graham P. et al. Exosomes and breast cancer drug resistance. Cell Death Dis. 2020;11:987
12. Li MY, Liu LZ, Dong M. Progress on pivotal role and application of exosome in lung cancer carcinogenesis, diagnosis, therapy and prognosis. Molecular cancer. 2021;20:22
13. Zhao S, Mi Y, Guan B, Zheng B, Wei P, Gu Y. et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J Hematol Oncol. 2020;13:156
14. Ma W, Zhou Y, Liu M, Qin Q, Cui Y. Long non-coding RNA LINC00470 in serum derived exosome: a critical regulator for proliferation and autophagy in glioma cells. Cancer cell international. 2021;21:149
15. Wang ZF, Liao F, Wu H, Dai J. Glioma stem cells-derived exosomal miR-26a promotes angiogenesis of microvessel endothelial cells in glioma. Journal of experimental & clinical cancer research: CR. 2019;38:201
16. Yin K, Liu X. CircMMP1 promotes the progression of glioma through miR-433/HMGB3 axis in vitro and in vivo. IUBMB life. 2020;72:2508-24
17. Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967-78
18. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). The Journal of biological chemistry. 1987;262:9412-20
19. Crescitelli R, Lässer C, Lötvall J. Isolation and characterization of extracellular vesicle subpopulations from tissues. Nature protocols. 2021;16:1548-80
20. Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic acids research. 2012;40:D1241-4
21. Moreno-Gonzalo O, Fernandez-Delgado I, Sanchez-Madrid F. Post-translational add-ons mark the path in exosomal protein sorting. Cellular and molecular life sciences: CMLS. 2018;75:1-19
22. Lai RC, Chen TS, Lim SK. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regenerative medicine. 2011;6:481-92
23. Lakkaraju A, Rodriguez-Boulan E. Itinerant exosomes: emerging roles in cell and tissue polarity. Trends in cell biology. 2008;18:199-209
24. Chaput N, Théry C. Exosomes: immune properties and potential clinical implementations. Seminars in immunopathology. 2011;33:419-40
25. Kim SH, Bianco NR, Shufesky WJ, Morelli AE, Robbins PD. Effective treatment of inflammatory disease models with exosomes derived from dendritic cells genetically modified to express IL-4. J Immunol. 2007;179:2242-9
26. Miksa M, Wu R, Dong W, Das P, Yang D, Wang P. Dendritic cell-derived exosomes containing milk fat globule epidermal growth factor-factor VIII attenuate proinflammatory responses in sepsis. Shock (Augusta, Ga). 2006;25:586-93
27. Tran TH, Mattheolabakis G, Aldawsari H, Amiji M. Exosomes as nanocarriers for immunotherapy of cancer and inflammatory diseases. Clinical immunology (Orlando, Fla). 2015;160:46-58
28. Camussi G, Deregibus MC, Bruno S, Grange C, Fonsato V, Tetta C. Exosome/microvesicle-mediated epigenetic reprogramming of cells. American journal of cancer research. 2011;1:98-110
29. Jang YY, Collector MI, Baylin SB, Diehl AM, Sharkis SJ. Hematopoietic stem cells convert into liver cells within days without fusion. Nature cell biology. 2004;6:532-9
30. Ratajczak MZ, Kucia M, Jadczyk T, Greco NJ, Wojakowski W, Tendera M. et al. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia. 2012;26:1166-73
31. Takahashi A, Okada R, Nagao K, Kawamata Y, Hanyu A, Yoshimoto S. et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nature communications. 2017;8:15287
32. Tkach M, Théry C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell. 2016;164:1226-32
33. Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A. et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. The Journal of biological chemistry. 2009;284:34211-22
34. Jeppesen DK, Hvam ML, Primdahl-Bengtson B, Boysen AT, Whitehead B, Dyrskjøt L. et al. Comparative analysis of discrete exosome fractions obtained by differential centrifugation. Journal of extracellular vesicles. 2014;3:25011
35. Back SJ, Kim W, Kim DY, Kim SJ, Hwang SR, Jung GB. Rapid and simple isolation and detection of exosomes using CaTiO(3):Eu(3+)@Fe(3)O(4) multifunctional nanocomposites. Analytical biochemistry. 2023;673:115161
36. Chen Y, Zhu Q, Cheng L, Wang Y, Li M, Yang Q. et al. Exosome detection via the ultrafast-isolation system: EXODUS. Nature methods. 2021;18:212-8
37. Rashidi M, Bijari S, Khazaei AH, Shojaei-Ghahrizjani F, Rezakhani L. The role of milk-derived exosomes in the treatment of diseases. Frontiers in genetics. 2022;13:1009338
38. Mondal J, Pillarisetti S, Junnuthula V, Saha M, Hwang SR, Park IK. et al. Hybrid exosomes, exosome-like nanovesicles and engineered exosomes for therapeutic applications. Journal of controlled release: official journal of the Controlled Release Society. 2023;353:1127-49
39. Zhang Z, Yu Y, Zhu G, Zeng L, Xu S, Cheng H. et al. The Emerging Role of Plant-Derived Exosomes-Like Nanoparticles in Immune Regulation and Periodontitis Treatment. Frontiers in immunology. 2022;13:896745
40. Li B, Chen X, Qiu W, Zhao R, Duan J, Zhang S. et al. Synchronous Disintegration of Ferroptosis Defense Axis via Engineered Exosome-Conjugated Magnetic Nanoparticles for Glioblastoma Therapy. Advanced science (Weinheim, Baden-Wurttemberg, Germany). 2022;9:e2105451
41. Qi Y, Jin C, Qiu W, Zhao R, Wang S, Li B. et al. The dual role of glioma exosomal microRNAs: glioma eliminates tumor suppressor miR-1298-5p via exosomes to promote immunosuppressive effects of MDSCs. Cell Death Dis. 2022;13:426
42. Pan Z, Zhao R, Li B, Qi Y, Qiu W, Guo Q. et al. EWSR1-induced circNEIL3 promotes glioma progression and exosome-mediated macrophage immunosuppressive polarization via stabilizing IGF2BP3. Molecular cancer. 2022;21:16
43. Xu H, Li M, Pan Z, Zhang Z, Gao Z, Zhao R. et al. miR-3184-3p enriched in cerebrospinal fluid exosomes contributes to progression of glioma and promotes M2-like macrophage polarization. Cancer science. 2022;113:2668-80
44. Li M, Xu H, Qi Y, Pan Z, Li B, Gao Z. et al. Tumor-derived exosomes deliver the tumor suppressor miR-3591-3p to induce M2 macrophage polarization and promote glioma progression. Oncogene. 2022;41:4618-32
45. Cai Q, Zhu A, Gong L. Exosomes of glioma cells deliver miR-148a to promote proliferation and metastasis of glioblastoma via targeting CADM1. Bulletin du cancer. 2018;105:643-51
46. Yao J, Wang Z, Cheng Y, Ma C, Zhong Y, Xiao Y. et al. M2 macrophage-derived exosomal microRNAs inhibit cell migration and invasion in gliomas through PI3K/AKT/mTOR signaling pathway. Journal of translational medicine. 2021;19:99
47. Plate KH, Scholz A, Dumont DJ. Tumor angiogenesis and anti-angiogenic therapy in malignant gliomas revisited. Acta Neuropathol. 2012;124:763-75
48. Guo Q, Fan Y, Wang Q, Li B, Qiu W, Qi Y. et al. Glioblastoma upregulates SUMOylation of hnRNP A2/B1 to eliminate the tumor suppressor miR-204-3p, accelerating angiogenesis under hypoxia. Cell Death Dis. 2023;14:147
49. Hu N, Cai Z, Jiang X, Wang C, Tang T, Xu T. et al. Hypoxia-pretreated ADSC-derived exosome-embedded hydrogels promote angiogenesis and accelerate diabetic wound healing. Acta biomaterialia. 2023;157:175-86
50. Nguyen TH, Pham PV, Vu NB. Exosomes from adipose-derived stem cells promote angiogenesis and reduce necrotic grade in hindlimb ischemia mouse models. Iranian journal of basic medical sciences. 2023;26:429-37
51. Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe'er J. et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. The American journal of pathology. 1999;155:739-52
52. Zhang X, Song Q, Wei C, Qu J. LRIG1 inhibits hypoxia-induced vasculogenic mimicry formation via suppression of the EGFR/PI3K/AKT pathway and epithelial-to-mesenchymal transition in human glioma SHG-44 cells. Cell stress & chaperones. 2015;20:631-41
53. Jiang J, Wang S, Meng QH, Yu R, Wei SC, Wang J. et al. [Study on the expression of non-coding microRNA-376b-3p in serum exosomes of patients with malignant glioma and the mechanism of anti-angiogenesis]. Zhonghua yi xue za zhi. 2020;100:1634-9
54. Zhang Z, Guo X, Guo X, Yu R, Qian M, Wang S. et al. MicroRNA-29a-3p delivery via exosomes derived from engineered human mesenchymal stem cells exerts tumour suppressive effects by inhibiting migration and vasculogenic mimicry in glioma. Aging. 2021;13:5055-68
55. Kore RA, Edmondson JL, Jenkins SV, Jamshidi-Parsian A, Dings RPM, Reyna NS. et al. Hypoxia-derived exosomes induce putative altered pathways in biosynthesis and ion regulatory channels in glioblastoma cells. Biochemistry and biophysics reports. 2018;14:104-13
56. Tomaszewski W, Sanchez-Perez L, Gajewski TF, Sampson JH. Brain Tumor Microenvironment and Host State: Implications for Immunotherapy. Clinical cancer research: an official journal of the American Association for Cancer Research. 2019;25:4202-10
57. D'Souza-Schorey C, Clancy JW. Tumor-derived microvesicles: shedding light on novel microenvironment modulators and prospective cancer biomarkers. Genes & development. 2012;26:1287-99
58. Qian M, Wang S, Guo X, Wang J, Zhang Z, Qiu W. et al. Hypoxic glioma-derived exosomes deliver microRNA-1246 to induce M2 macrophage polarization by targeting TERF2IP via the STAT3 and NF-κB pathways. Oncogene. 2020;39:428-42
59. Qiu W, Guo X, Li B, Wang J, Qi Y, Chen Z. et al. Exosomal miR-1246 from glioma patient body fluids drives the differentiation and activation of myeloid-derived suppressor cells. Molecular therapy: the journal of the American Society of Gene Therapy. 2021;29:3449-64
60. Hegde S, Leader AM, Merad M. MDSC: Markers, development, states, and unaddressed complexity. Immunity. 2021;54:875-84
61. Guo X, Qiu W, Wang J, Liu Q, Qian M, Wang S. et al. Glioma exosomes mediate the expansion and function of myeloid-derived suppressor cells through microRNA-29a/Hbp1 and microRNA-92a/Prkar1a pathways. International journal of cancer. 2019;144:3111-26
62. Mirzaei R, Sarkar S, Dzikowski L, Rawji KS, Khan L, Faissner A. et al. Brain tumor-initiating cells export tenascin-C associated with exosomes to suppress T cell activity. Oncoimmunology. 2018;7:e1478647
63. Spelat R, Jihua N, Sánchez Triviño CA, Pifferi S, Pozzi D, Manzati M. et al. The dual action of glioma-derived exosomes on neuronal activity: synchronization and disruption of synchrony. Cell Death Dis. 2022;13:705
64. Kaina B, Christmann M, Naumann S, Roos WP. MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA repair. 2007;6:1079-99
65. Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M. et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. The New England journal of medicine. 2005;352:997-1003
66. Barile L, Vassalli G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacology & therapeutics. 2017;174:63-78
67. Beermann J, Piccoli MT, Viereck J, Thum T. Non-coding RNAs in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiological reviews. 2016;96:1297-325
68. Viereck J, Bang C, Foinquinos A, Thum T. Regulatory RNAs and paracrine networks in the heart. Cardiovascular research. 2014;102:290-301
69. Wu P, Guo J, Yang H, Yuan D, Wang C, Wang Z. Exosomes Derived from Hypoxic Glioma Cells Reduce the Sensitivity of Glioma Cells to Temozolomide Through Carrying miR-106a-5p. Drug design, development and therapy. 2022;16:3589-98
70. Wang J, Li T, Wang B. Exosomal transfer of miR-25-3p promotes the proliferation and temozolomide resistance of glioblastoma cells by targeting FBXW7. International journal of oncology. 2021;59:e64
71. Yin J, Zeng A, Zhang Z, Shi Z, Yan W, You Y. Exosomal transfer of miR-1238 contributes to temozolomide-resistance in glioblastoma. EBioMedicine. 2019;42:238-51
72. Yue X, Lan F, Xia T. Hypoxic Glioma Cell-Secreted Exosomal miR-301a Activates Wnt/β-catenin Signaling and Promotes Radiation Resistance by Targeting TCEAL7. Molecular therapy: the journal of the American Society of Gene Therapy. 2019;27:1939-49
73. Hajjari M, Khoshnevisan A, Shin YK. Molecular function and regulation of long non-coding RNAs: paradigms with potential roles in cancer. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35:10645-63
74. Li Q, Shao Y, Zhang X, Zheng T, Miao M, Qin L. et al. Plasma long noncoding RNA protected by exosomes as a potential stable biomarker for gastric cancer. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2015;36:2007-12
75. Zhang Z, Yin J, Lu C, Wei Y, Zeng A, You Y. Exosomal transfer of long non-coding RNA SBF2-AS1 enhances chemoresistance to temozolomide in glioblastoma. Journal of experimental & clinical cancer research: CR. 2019;38:166
76. Hsiao KY, Sun HS, Tsai SJ. Circular RNA - New member of noncoding RNA with novel functions. Experimental biology and medicine (Maywood, NJ). 2017;242:1136-41
77. Ding C, Yi X, Chen X, Wu Z, You H, Chen X. et al. Warburg effect-promoted exosomal circ_0072083 releasing up-regulates NANGO expression through multiple pathways and enhances temozolomide resistance in glioma. Journal of experimental & clinical cancer research: CR. 2021;40:164
78. Geng X, Zhang Y, Lin X, Zeng Z, Hu J, Hao L. et al. Exosomal circWDR62 promotes temozolomide resistance and malignant progression through regulation of the miR-370-3p/MGMT axis in glioma. Cell Death Dis. 2022;13:596
79. Han C, Wang S, Wang H, Zhang J. Exosomal circ-HIPK3 Facilitates Tumor Progression and Temozolomide Resistance by Regulating miR-421/ZIC5 Axis in Glioma. Cancer biotherapy & radiopharmaceuticals. 2021;36:537-48
80. Si J, Li W, Li X, Cao L, Chen Z, Jiang Z. Heparanase confers temozolomide resistance by regulation of exosome secretion and circular RNA composition in glioma. Cancer science. 2021;112:3491-506
81. Oldrini B, Vaquero-Siguero N, Mu Q, Kroon P, Zhang Y, Galán-Ganga M. et al. MGMT genomic rearrangements contribute to chemotherapy resistance in gliomas. Nature communications. 2020;11:3883
82. Li Z, Ye L, Wang L, Quan R, Zhou Y, Li X. Identification of miRNA signatures in serum exosomes as a potential biomarker after radiotherapy treatment in glioma patients. Annals of diagnostic pathology. 2020;44:151436
83. Catelan S, Olioso D, Santangelo A, Scapoli C, Tamanini A, Pinna G. et al. miRNAs in Serum Exosomes for Differential Diagnosis of Brain Metastases. Cancers. 2022;14:3493
84. Lan F, Yue X, Xia T. Exosomal microRNA-210 is a potentially non-invasive biomarker for the diagnosis and prognosis of glioma. Oncology letters. 2020;19:1967-74
85. Lan F, Qing Q, Pan Q, Hu M, Yu H, Yue X. Serum exosomal miR-301a as a potential diagnostic and prognostic biomarker for human glioma. Cell Oncol (Dordr). 2018;41:25-33
86. Wang Q, Hu B, Hu X, Kim H, Squatrito M, Scarpace L. et al. Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer cell. 2017;32:42-56.e6
87. Gutmann DH, Kettenmann H. Microglia/Brain Macrophages as Central Drivers of Brain Tumor Pathobiology. Neuron. 2019;104:442-9
88. Zhang M, Hu S, Liu L, Dang P, Liu Y, Sun Z. et al. Engineered exosomes from different sources for cancer-targeted therapy. Signal transduction and targeted therapy. 2023;8:124
89. Liu T, Hu J, Han B, Tan S, Jia W, Xin Y. A positive feedback loop of lncRNA-RMRP/ZNRF3 axis and Wnt/β-catenin signaling regulates the progression and temozolomide resistance in glioma. Cell Death Dis. 2021;12:952
90. Arranja AG, Pathak V, Lammers T, Shi Y. Tumor-targeted nanomedicines for cancer theranostics. Pharmacological research. 2017;115:87-95
91. Edgar JR. Q&A: What are exosomes, exactly? BMC biology. 2016;14:46
92. Wu JY, Li YJ, Hu XB, Huang S, Luo S, Tang T. et al. Exosomes and biomimetic nanovesicles-mediated anti-glioblastoma therapy: A head-to-head comparison. Journal of controlled release: official journal of the Controlled Release Society. 2021;336:510-21
93. Wu W, Klockow JL, Zhang M, Lafortune F, Chang E, Jin L. et al. Glioblastoma multiforme (GBM): An overview of current therapies and mechanisms of resistance. Pharmacological research. 2021;171:105780
94. Liang S, Xu H, Ye BC. Membrane-Decorated Exosomes for Combination Drug Delivery and Improved Glioma Therapy. Langmuir: the ACS journal of surfaces and colloids. 2022;38:299-308
95. Yu L, Gui S, Liu Y, Qiu X, Zhang G, Zhang X. et al. Exosomes derived from microRNA-199a-overexpressing mesenchymal stem cells inhibit glioma progression by down-regulating AGAP2. Aging. 2019;11:5300-18
96. Zeng A, Wei Z, Yan W, Yin J, Huang X, Zhou X. et al. Exosomal transfer of miR-151a enhances chemosensitivity to temozolomide in drug-resistant glioblastoma. Cancer Lett. 2018;436:10-21
97. Rehman FU, Liu Y, Yang Q, Yang H, Liu R, Zhang D. et al. Heme Oxygenase-1 targeting exosomes for temozolomide resistant glioblastoma synergistic therapy. Journal of controlled release: official journal of the Controlled Release Society. 2022;345:696-708
98. M HR, Bayraktar E, G KH, Abd-Ellah MF, Amero P, Chavez-Reyes A. et al. Exosomes: From Garbage Bins to Promising Therapeutic Targets. International journal of molecular sciences. 2017;18:538
99. Zhan Q, Yi K, Cui X, Li X, Yang S, Wang Q. et al. Blood exosomes-based targeted delivery of cPLA2 siRNA and metformin to modulate glioblastoma energy metabolism for tailoring personalized therapy. Neuro-oncology. 2022;24:1871-83
100. Wolfers J, Lozier A, Raposo G, Regnault A, Théry C, Masurier C. et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nature medicine. 2001;7:297-303
101. Bu N, Wu H, Sun B, Zhang G, Zhan S, Zhang R. et al. Exosome-loaded dendritic cells elicit tumor-specific CD8+ cytotoxic T cells in patients with glioma. J Neurooncol. 2011;104:659-67
102. Isaac R, Reis FCG, Ying W, Olefsky JM. Exosomes as mediators of intercellular crosstalk in metabolism. Cell metabolism. 2021;33:1744-62
103. Shao X, Hua S, Feng T, Ocansey DKW, Yin L. Hypoxia-Regulated Tumor-Derived Exosomes and Tumor Progression: A Focus on Immune Evasion. International journal of molecular sciences. 2022;23:11789
104. Lin Z, Wu Y, Xu Y, Li G, Li Z, Liu T. Mesenchymal stem cell-derived exosomes in cancer therapy resistance: recent advances and therapeutic potential. Molecular cancer. 2022;21:179
105. Zhang F, Guo J, Zhang Z, Qian Y, Wang G, Duan M. et al. Mesenchymal stem cell-derived exosome: A tumor regulator and carrier for targeted tumor therapy. Cancer Lett. 2022;526:29-40
106. Gunassekaran GR, Poongkavithai Vadevoo SM, Baek MC, Lee B. M1 macrophage exosomes engineered to foster M1 polarization and target the IL-4 receptor inhibit tumor growth by reprogramming tumor-associated macrophages into M1-like macrophages. Biomaterials. 2021;278:121137
107. Wang Y, Xiang Y, Xin VW, Wang XW, Peng XC, Liu XQ. et al. Dendritic cell biology and its role in tumor immunotherapy. J Hematol Oncol. 2020;13:107
108. Li J, Li J, Peng Y, Du Y, Yang Z, Qi X. Dendritic cell derived exosomes loaded neoantigens for personalized cancer immunotherapies. Journal of controlled release: official journal of the Controlled Release Society. 2023;353:423-33
109. Reyes-Ruiz JM, Osuna-Ramos JF, De Jesús-González LA, Hurtado-Monzón AM, Farfan-Morales CN, Cervantes-Salazar M. et al. Isolation and characterization of exosomes released from mosquito cells infected with dengue virus. Virus research. 2019;266:1-14
110. Xu X, Qian J, Ding J, Li J, Nan F, Wang W. et al. Detection of viral components in exosomes derived from NDV-infected DF-1 cells and their promoting ability in virus replication. Microbial pathogenesis. 2019;128:414-22
111. Li X, Wang N, Leng H, Yuan H, Xu L. Hsa_circ_0043949 reinforces temozolomide resistance via upregulating oncogene ITGA1 axis in glioblastoma. Metabolic brain disease. 2022;37:2979-93
112. Jia G, Han Y, An Y, Ding Y, He C, Wang X. et al. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials. 2018;178:302-16
113. Wang J, Tang W, Yang M, Yin Y, Li H, Hu F. et al. Inflammatory tumor microenvironment responsive neutrophil exosomes-based drug delivery system for targeted glioma therapy. Biomaterials. 2021;273:120784
114. Shan S, Chen J, Sun Y, Wang Y, Xia B, Tan H. et al. Functionalized Macrophage Exosomes with Panobinostat and PPM1D-siRNA for Diffuse Intrinsic Pontine Gliomas Therapy. Advanced science (Weinheim, Baden-Wurttemberg, Germany). 2022;9:e2200353
115. Lee H, Bae K, Baek AR, Kwon EB, Kim YH, Nam SW. et al. Glioblastoma-Derived Exosomes as Nanopharmaceutics for Improved Glioma Treatment. Pharmaceutics. 2022;14:1002
116. Thakur A, Sidu RK, Zou H, Alam MK, Yang M, Lee Y. Inhibition of Glioma Cells' Proliferation by Doxorubicin-Loaded Exosomes via Microfluidics. International journal of nanomedicine. 2020;15:8331-43
117. Zhang C, Song J, Lou L, Qi X, Zhao L, Fan B. et al. Doxorubicin-loaded nanoparticle coated with endothelial cells-derived exosomes for immunogenic chemotherapy of glioblastoma. Bioengineering & translational medicine. 2021;6:e10203
118. Wang R, Liang Q, Zhang X, Di Z, Wang X, Di L. Tumor-derived exosomes reversing TMZ resistance by synergistic drug delivery for glioma-targeting treatment. Colloids and surfaces B, Biointerfaces. 2022;215:112505
Author contact
![]() Corresponding author: Jie Zhou, Department of Neurosurgery, The Affiliated Hospital of Southwest Medical University, Southwest Medical University, Taiping Street 25#, Luzhou 646000, Sichuan Province, China. Email: zhoujieedu.cn.
Corresponding author: Jie Zhou, Department of Neurosurgery, The Affiliated Hospital of Southwest Medical University, Southwest Medical University, Taiping Street 25#, Luzhou 646000, Sichuan Province, China. Email: zhoujieedu.cn.

 Global reach, higher impact
Global reach, higher impact