Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(14):2739-2750. doi:10.7150/jca.86718 This issue Cite
Research Paper
A Positive Feedback Loop of E2F4-Mediated Activation of MNX1 Regulates Tumour Progression in Colorectal Cancer
1. Department of General Surgery, The Third Xiangya Hospital of Central South University, Changsha, 410013, China.
2. National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China.
Received 2023-6-1; Accepted 2023-8-19; Published 2023-9-4
Abstract
Purpose: Colorectal cancer (CRC) is the 3rd most prevalent malignant tumour globally. Although significant strides have been made in diagnosis and treatment, its prognosis at the moment remains unpromising. Therefore, there is an urgent and desperate need to identify novel biomarkers of CRC and evaluate its mechanism of tumourigenesis and development.
Methods: JASPAR and RNAinter databases are used to analyze target genes associated with colorectal cancer. Western blotting, q-PCR and immunohistochemistry et, al. were used to detect the level of MNX1 in patients with colorectal cancer, and Chip-PCR was used to detect the targeted binding ability of E2F4 and MNX1. The cells and animal models overexpressed MNX1 and E2F4 were constructed by shRNA transfection.
Results: Herein, MNX1 was highly expressed and linked to favourable overall survival curves in colorectal cancer. The functional assay revealed that MNX1 overexpression could promote proliferation, migration, and invasion of CRC cells. Based on the prediction of the JASPAR and RNAinter databases, the transcription factor, E2F4, was bound to the MNX1 promoter region. The Chromatin Immunoprecipitation (ChIP) assay verified the interactions between MNX1 and E2F4 in CRC. Additionally, we found that sh-E2F4 markedly downregulated the MNX1 levels and reduced CRC progression in vivo and in vitro, which reversed MNX1 overexpression.
Conclusion: Therefore, our research discovered that E2F4-mediated abnormal MNX1 expression promotes CRC progression and could become a novel diagnostic or therapeutic target of CRC.
Keywords: MNX1, E2F4, Colorectal Cancer (CRC), diagnostic biomarker
Introduction
Colorectal cancer (CRC) is the 3rd most common malignant tumour, with more than 881000 annual fatalities across the globe [1]. It is the 5th leading cancer in China, with the highest number of cancer-related deaths in both sexes [2]. Whilst researchers have made the exciting progress in diagnosis and treatment, such as more precise personalized treatments [3] microbial-based treatments and diagnostic tools [4], CRC prognosis is currently not promising. Therefore, it is necessary to identify novel CRC biomarkers and investigate their mechanism of tumorigenesis and development to provide effective treatments.
Motor neuron and pancreas homeobox 1 (MNX1), also known as HLXB9, SCRA1, and HB9, is a member of the EHG homeobox gene family and located on chromosome 7q36.3 [5]. MNX1 is a transcription factor that regulates the development and differentiation of cells and tissues [6]. Additional studies revealed that MNX1 expression is strongly upregulated in different cancers, including breast [7], colon [8], prostate [5], and liver cancers [9]. Furthermore, recent research on CRC confirmed that MNX1-AS1 (MNX1 antisense RNA1), A LncRNA with partial exon overlap with the MNX1 gene, promotes colon adenocarcinoma progression [10]. But the mechanism by which MNX1 is improved remains unclear.
MNX1 expression in CRC was first detected using The Cancer Genome Atlas (TCGA) database and quantitative reverse transcription-polymerase chain reaction (qRT‐PCR). Based on the RNAinter database, we speculated that E2F4 could bind to the MNX1 promoter region as a potential upstream regulator. Current studies demonstrate that the E2F family comprising E2F1 and E2F4 regulates the cell cycle tumour development and progression [11]. Recent research discovered that E2F4 is a potential novel biomarker for breast cancer prognosis [12]. In colon cancer, E2F4 was found upregulated and its level was correlated with the clinical stage of colon cancer [13, 14]. Nonetheless, the regulatory role of the E2F4/MNX1 pathway in tumorigenesis and progression still needs to be explored.
This study explores the expression level and interaction of MNX1 and E2F4 in CRC using bioinformatics analyses and functional assays. Meanwhile, MNX1 and E2F4 effects on cell migration, invasion and tumour growth were examined through in vivo and in vitro assays. Our findings revealed that the E2F4/MNX1 pathway could be a promising biomarker and therapeutic target for CRC.
Methods
Bioinformatics
GSE41258 and GDS4382 datasets were downloaded from the National Centre for Biotechnology Information (https://www.ncbi.nlm.nih.gov/). Visual analysis was performed using the NetworkAnalyst software (https://www.networkanalyst.ca). GEPIA (http://gepia.cancer-pku.cn/index.html) and StarBase (http://starbase.sysu.edu.cn) were used to analyze MNX1 and E2F4 expressions in colorectal cancer and adjacent normal tissues as well as the disease-free survival (DFS) of MNX1 and E2F4 in colorectal cancer patients. The target relationship between E2F4 and MNX1 was predicted using the RNAinter website (http://www.rna-society.org/). Moreover, the ChIPBase website (http://rna.sysu.edu.cn/chipbase/) discovered a positive correlation between the two genes. The potential binding sites for MNX1 were predicated using the JASPAR website (http://jaspardev.genereg.net/).
Clinical Samples and Cell Lines
All studies were approved by the Ethics Committee of the Third Xiangya Hospital of Central South University (approval number: No. KUAI I 21007), and informed consent was obtained from all subjects. Clinical colorectal cancer and other normal tissue samples were selected from 20 patients (12 tumour patients and 8 normal patients) at the Third Xiangya Hospital of Central South University (Table S1-S4). Normal colorectal epithelial cells (NCM460) and colorectal cancer cell lines (SW480, HCT116 and SW620) were purchased from the Cell Bank of the Chinese Academy of Science (Shanghai, China). All cells were grown in RPMI-1640 medium supplemented with 10% FBS.
Inclusion criteria were as follows: (1) Patients diagnosed with colorectal cancer by histopathological examination after tumour resection; (2) Matched healthy controls were selected from asymptomatic patients with negative colonoscopy results; (3) Patients aged between 18 and 80 years, regardless of sex. The following conditions were excluded: (1) Psychiatric disorders, pregnant or lactating women; (2) Suffering from other primary digestive diseases or tumour lesions related to hereditary diseases; (3) Preoperative radiotherapy or chemotherapy; (4) Currently suffering from inflammation, autoimmune diseases, etc.
Plasmid constructs and transfections
The full-length cDNA of human MNX1 was PCR-amplified and cloned into the pcDNA 3.1 vectors to construct the overexpression cell lines. Meanwhile, human MNX1- and E2F4-targeting shRNA sequences were cloned into the pcDNA 3.1 vectors to construct knockdown cell lines. The shRNA sequences we selected were: MNX1:sh#1:GCAGGAAGCGGAGAAACAGAA; E2F4:sh#1:CGGCGGATTTACGACATT. According to the manufacturer's instructions, HCT116, and SW620 cells were seeded in 6-well plates (Eppendorf, Hamburg, Germany), then Lipofectamine™ 3000 (Invitrogen, Carlsbad CA, USA) was utilized to transfect the plasmid. The cells were collected for subsequent experiments after transfection for 48 h.
Real-time quantitative PCR
Total RNA was isolated from the above cells or frozen tissues using TRIzol reagent. Subsequently, cDNA was synthesized from 1 μg of total RNA by reverse transcription using the PrimeScript RT reagent Kit. cDNA was amplified using SYBR Green Premix following the manufacturer's instructions. PCR was performed under the following cycling conditions: 10 min at 95°C, followed by 40 cycles at 95°C for 10 s and 60°C for 30 s. Relative quantification analysis was performed using the comparative CT (2 -∆∆Ct) method. The primer sequences used in the experiment are presented in Table 1.
Immunohistochemistry assay
After paraffin sectioning, four groups of paraffin-embedded specimens were sliced, deparaffinized, rehydrated, and treated with 3% hydrogen peroxide for 15 min to block endogenous peroxidase activity. Thereafter, the sections were overnight incubated with the primary antibodies at 4°C. The sections were then washed in PBS and treated with biotinylated secondary antibodies. Eventually, the sections were counterstained with hematoxylin, dehydrated, mounted, and observed under a light microscope.
Western blotting
Proteins were isolated from CRC cells and tissues using RIPA lysis buffer (Thermo Scientific, USA) for Western blot, following the manufacturer's instructions. Protein concentrations were determined using the bicinchoninic acid assay (Beyotime Biotechnology, China). Equal amounts of protein were separated using SDS-PAGE on a 10% gel, then transferred onto polyvinylidene difluoride membranes, followed by blocking with 5% skim milk for 2 h. After incubation with primary antibodies (including MNX1 antibody: 1:1000, Abcam, ab92606, USA; E2F4 antibody: 1:500, Proteintech, 10923-1-AP, China; β-actin: 1:2000, Proteintech, 66009-1-Ig, China), the samples were washed 4 times (10 min for each wash), then, the membranes were incubated with secondary antibodies (Millipore, Bedford, USA) for 2 h at room temperature. We wrapped the PVDF membrane in the film. Under the condition of red light in the darkroom, the film is processed by developer and fixative. Once dried, the protein bands can be seen directly. Then the film image is converted into a grayscale image by Photoshop software. In the process, the background of the image is converted to gray from blue.
Proliferation assay
Cell growth was assessed using an MTT assay. The transfected cells were seeded in 96-well plates at 37°C in 5% CO2 for 24 h. Each well was incubated with 50 μl of MTT solution at 37°C for 4 h. Thus, the growth medium was removed, and 150 µL of DMSO was added to each well. Finally, the optical density of each well was recorded at 570 nm using a microplate reader.
Scratch assay
HCT116 and SW620 cells were seeded at a density of 1x105 cells/well in 24-well plates until they were 90% confluent. Scrape wounds were generated using a 20-µl pipette tip; then, cells were cultured with a serum-free medium for 48 h. Wound closure was monitored and photographed at 0, 24, and 48 h using microscope.
Migration and Invasion Experiments
Cell migration and invasion assays were conducted in 6-well Transwell chambers (8 μm filter pore, Corning) pre-coated or not precoated with Matrigel basement membrane gel. HCT116 and SW620 cells were seeded in the upper chambers, and a culture medium supplemented with 10% was added to the lower chambers. After 24 h of incubation, cells were fixed with 95% ethanol and then stained with hematoxylin. Finally, the cells were observed under a light microscope and quantified by manual counting.
Chromatin Immunoprecipitation (ChIP)-PCR
HCT116 and SW620 cells were cultured in 150 mm dishes, then 1% formaldehyde was added to the cultures and incubated at room temperature for 10 min. Thereafter, glycine was added to neutralize the remaining formaldehyde. The cells were washed using cold PBS and added to PBS containing protease inhibitor mixture. After centrifugation, the supernatant was obtained as the cell lysate. The proteins were sonicated to shear chromosomal DNA after successfully crosslinking with chromatin. The supernatants were overnight incubated with anti-E2F4 antibody and normal rabbit IgG at 4°C to couple antigen to antibody, then immunoprecipitated by protein A/G to precipitate the immune complexes. After eluting the protein-DNA complexes and reversing the crosslinks, the DNA was purified before analyzing using real-time PCR. Table 2 shows the primer sequences used in the experiment.
Primers for quantitative real-time PCR (qRT-PCR).
| Homo/Gene | Primer sequences 5′-3′ | |
|---|---|---|
| E2F4 | F | CACCACCAAGTTCGTGTCCC |
| R | GCGTACAGCTAGGGTGTCA | |
| MNX1 | F | GAGTGCGTGTGAGAAGAACC |
| R | CAGTTTGAACGCTCGTGACA | |
| β-actin | F | ACCCTGAAGTACCCCATCGAG |
| R | AGCACAGCCTGGATAGCAAC |
Primers for Chromatin Immunoprecipitation (ChIP)-PCR.
| Homo/Gene | Primer sequences 5′-3′ | |
|---|---|---|
| MNX1 | F | CTCCAGGGACCAACCAAGT |
| R | CAACGGGGAGTGGATACTC | |
| GAPDH | F | TACTAGCGGTTTTACGGGCG |
| R | TCGAACAGGAGGAGCAGAGAGCGA |
Xenograft assay
The Animal Ethics Committee approved the animal experiments of Central South University. A total of 18 BALB/c-nu mice (4-8 weeks old, half males and half females) were purchased from the SLAC Experimental Animal Limited Company (Shanghai, China). The mice were randomly divided into three groups using a random number generator. HCT116 cells (1 × 106 cells) were subcutaneously injected into the right flank of each mouse. After 20 days, tumour cells were collected, weighed, and paraffin-embedded. Eventually, fluorescence microscopy detected Ki-67-positive cells (1:800, Proteintech, 27309-1-AP, Wuhan, China).
Statistical analysis
All statistical analyses were conducted using the GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA, USA), and the experiments were independently repeated three times. The student's t-test (unpaired or paired) was used to determine the differences between the two groups. In contrast, the ANOVA test was used to determine the differences between the multiple groups. The GEPIA site examined the relationship between the expressions of two genes and the overall survival rates of colorectal cancer patients using the log-rank test. The expression correlations were analyzed through the Spearman correlation analysis from the ChipBase. Fisher's exact test was used to evaluate clinical characteristics in relation to MNX1 and E2F4. A difference with a P value of less than 0.05 (P <0.05) was considered statistically significant.
Results
MNX1 overexpression in CRC tissues and correlation with clinical stage
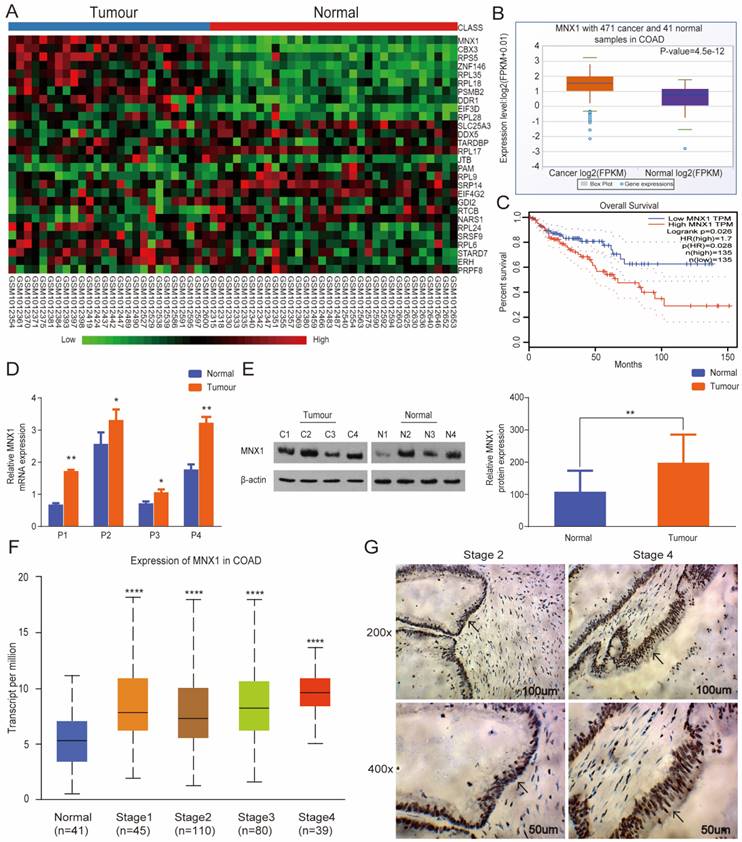
Bioinformatics assays were performed based on GSE41258 and found its overexpression in CRC tissues (Fig. 1A). At the same time, analysis from GDS4382 confirmed similar results (Fig. S1). Additionally, we analyzed the GEPIA and StarBase databases and discovered significant MNX1 upregulation in colorectal cancer. Also, low MNX1 expression was linked to a relatively longer overall survival duration (Fig. 1B, C). To further examine this issue, we first collected CRC, and normal tissues were extracted, then MNX1 expression was detected via qRT‐PCR and Western blot.
Consequently, MNX1 expression was higher in the CRC tissues than in the normal tissues (Fig. 1D, E). In contrast with that in the human colorectal epithelial mucosa cell line NCM460, the mRNA and protein expression of MNX1 was upregulated in the human CRC cell lines LOVO, HCT116, and SW620 (Fig. S2). Further, MNX1 expression correlated with the clinical stage as per the UALCAN database (Fig. 1F). Therefore, immunohistochemistry assays were used to detect MNX1 levels in tissues from different clinical stage CRC patients; consequently, MNX1 levels correlated with clinical stage in CRC patients (Fig. 1G).
MNX1 knockdown inhibits the migration and invasion of CRC cells
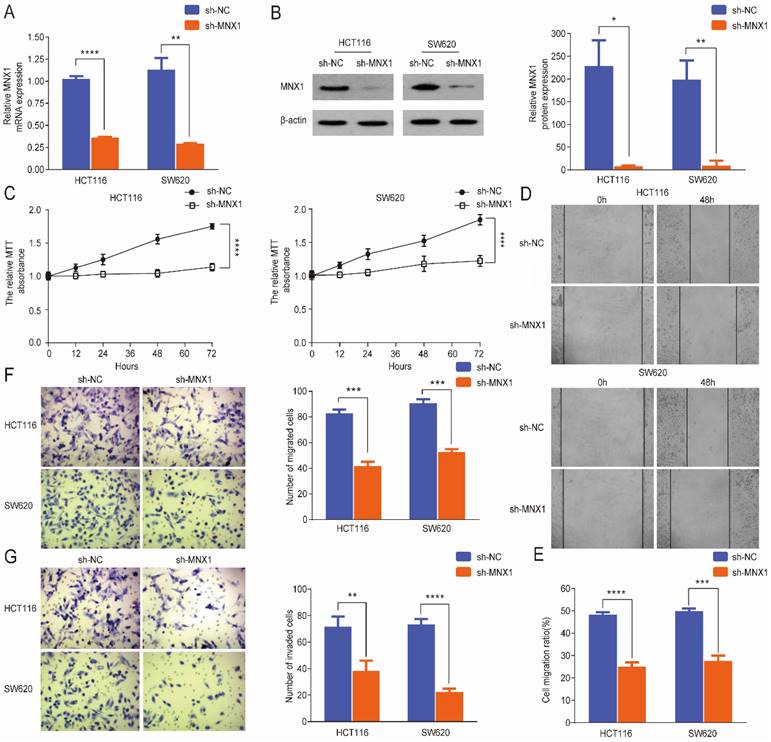
To further evaluate the effect of MNX1 in colorectal cancer progression, a small hairpin RNA (shRNA) approach was used to knockdown MNX1 in SW620 and HCT116 cell lines. qRT-PCR and Western blot results revealed that MNX1 expression was significantly downregulated in the transfected cells after treatment (Fig. 2A, B). First, MTT assays showed that MNX1 knockdown inhibited cancer cell growth (Fig. 2C). MNX1 knockdown in HCT116 and SW620 cells substantially suppressed migration of CRC cells (Fig. 2D, E). Subsequently, we further analyzed the effects of MNX1 on cell migration and invasion through Transwell analysis. As a result, HCT116 and SW620 cell migration and invasion were significantly lower than control groups (Fig. 2F, G).
MNX1 overexpression promotes the migration and invasion of CRC cells
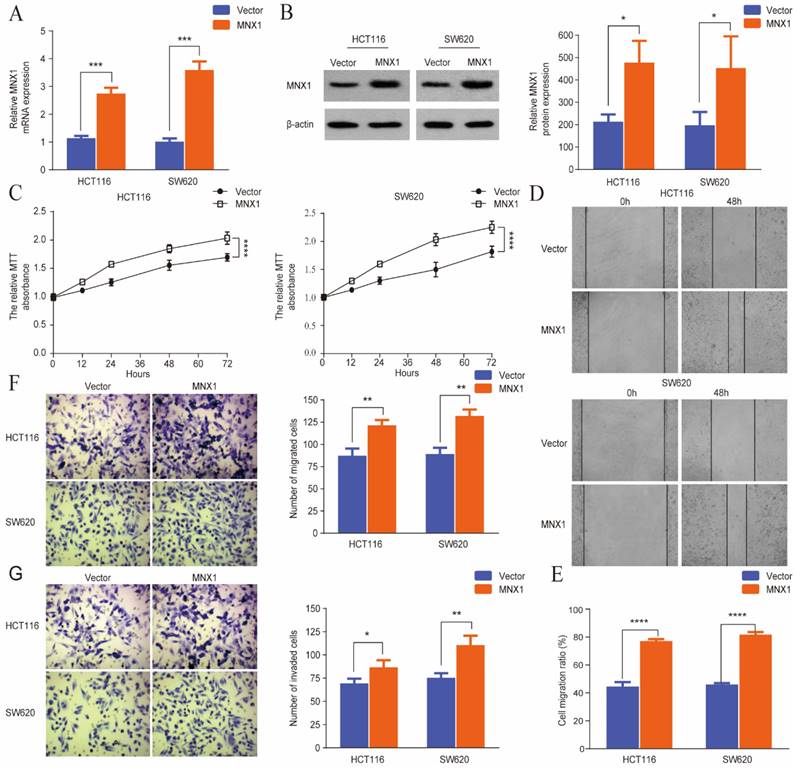
Stable MNX1 overexpression models were constructed using HCT116 and SW620 cells to confirm the biological role of MNX1 in CRC. The plasmid effectively upregulated MNX1 expression, as confirmed by the qRT-PCR and Western blot results (Fig. 3A, B). First, in the MTT assay, MNX1 overexpression increased HCT116 and SW620 cells (Fig. 3C). Through scratch wound assays, we noted that MNX1 overexpression promoted cancer cell migration (Fig. 3D, E). Moreover, Transwell assays revealed that MNX1 overexpression promoted cell migration and invasion compared to the control group (Fig. 3F, G). Summarily MNX1 could influence the migratory and invasive capacity of colorectal cancer cells.
E2F4 is an upstream gene of MNX1 in colorectal cancer
Based on the above experimental results, MNX1 was implicated in CRC cell migration and invasion. Therefore, we further evaluated the underlying regulatory mechanisms of CRC. Furthermore, we investigated the upstream regulatory gene of MNX1. Based on the prediction of the RNAinter database, MNX1 was a predicted target of E2F4, and transcription factors identify specific RNA sequences regulating gene expression and function. According to the JASPAR database, we predicted that E2F4 could bind to the promoter regions of MNX1 (Fig. 4A). Through ChIPBase database analysis, a positive correlation was found between E2F4 and MNX1 (Fig. 4B). Meanwhile, the GEPIA and StarBase databases were analyzed and found predominant upregulation of E2F4 in CRC, linked to a favorable overall survival curve (Fig. 4C, D). Therefore, we found E2F4 upregulation in colorectal cancer tissues (Fig. 4E, F) and CRC cell lines (Fig. S3A, B). Furthermore, MNX1 was dramatically reduced after the E2F4 knockdown (Fig. 4G, H). ChIP assays revealed that MNX1 promoter enrichment in the precipitates of the E2F4 antibody was lower than that in the control group. After delivery of MNX1, the MNX1 promoter enrichment was not improved (Fig. 4I, J). Based on the above results, we speculated that E2F4 could effectively bind to the MNX1 promoter.
MNX1 is highly expressed in colorectal cancer tissues. (A) Data from GSE41258: The differentially expressed mRNAs are illustrated in a heatmap. MNX1 was included in the overexpression category; (B) MNX1 was upregulated in the tumour tissues compared to the adjacent normal tissues in the StarBase database (P=4.5e-12); (C) Patients with high MNX1 expression had poor disease-free survival (P=0.026); (D, E) MNX1 expression determined by qRT-PCR and Western blot assays; (F) MNX1 expression in different stages; (G) Immunohistochemistry assay was used to detect the expression of MNX1 in colorectal tumours. Mean ± SD (n = 3 independent experiments). *P < 0.05, **P < 0.01, ****P < 0.0001.
MNX1 knockdown affects the migration and invasion of colorectal cancer cells. (A, B) MNX1 mRNA and protein levels are downregulated in cell lines; (C) MTT analysis of the HCT116 and SW620 cells for the different groups; (D, E) Wound healing assays showed that MNX1 downregulation significantly reduced cell migration; (F, G) The results of the transwell assay revealed that MNX1 downregulation in HCT116 and SW620 cells decreased cell invasion and migration. Mean ± SD (n = 3 independent experiments). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
E2F4 promotes the development of CRC by activating MNX1
Rescue experiments were performed further to verify the regulatory role of E2F4 in CRC. Western blotting and qRT-PCR results showed that E2F4 knockdown downregulated MNX1 expression in HCT116 and SW620 cell lines; however, these effects were reversed through MNX1 overexpression (Fig. 5A, B). The MTT wound healing and Transwell assays revealed that MNX1 overexpression partially reversed the decreased migration and invasion of HCT116 and SW620 cells caused by E2F4 knockdown (Fig. 5B-G).
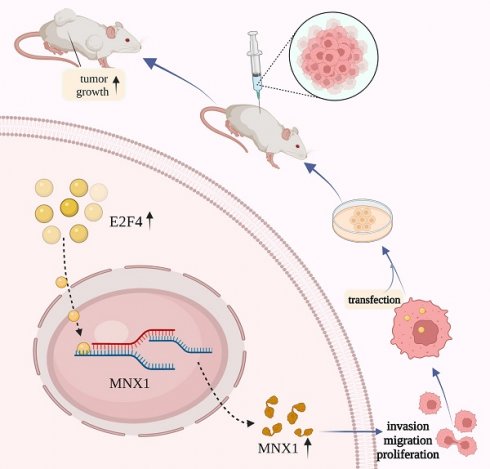
The xenograft mice models were used to explore E2F4 and MNX1 roles in vivo. shRNA-E2F4, shRNA-E2F4+MNX1, and shRNA-NC were successfully transfected using the HCT116 cells (Fig. 6A). The tumour weight and volume in the shRNA-E2F4 group were the lowest, whereas the continuous delivery of MNX1 overexpressed plasmids increased the tumour weight and volume (Fig. 6B, C). Furthermore, immunohistochemistry revealed that the number of positive cells for Ki-67 staining in the shRNA-E2F4 group was substantially lower than in the other two groups (Fig. 6D). Therefore, we believe that E2F4 activates MNX1 transcription to promote the development of CRC (Fig 7).
MNX1 overexpression affects the migration and invasion of colorectal cancer cells. (A, B) Upregulated MNX1 mRNA and protein levels in cell lines; (C) The MTT assay results showed the proliferation of the HCT116 and SW620 cells for different groups; (D, E) Wound healing assays revealed that MNX1 upregulation substantially promoted cell migration; (F, G) The results of the transwell assay showed that MNX1 upregulation in HCT116 and SW620 cells increased cell invasion and migration. Mean ± SD (n = 3 independent experiments). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Discussion
Colorectal cancer has one of the highest cancer-related mortality rates in both sexes [15, 16]. Although significant steps have been made in diagnosis and treatment, CRC prognosis is not promising. Therefore, there is a need for urgent research on the pathogenesis of this malignancy and the identification of novel indices for early diagnosis, therapy, and dynamic monitoring of colorectal cancer progression. Many genes are responsible for the occurrence and development of CRC, including NCOA5 [17], FOXC2 [18], and MNX1 [19]. Wu et al. reported that MNX1 overexpression induces the proliferation of CRC cells. Nonetheless, the mechanism of MNX1 upregulation and the comprehensive roles of MNX1 in vivo remain unclear [19].
MNX1 is a member of the homeobox gene (HOX) family, and several homeobox genes are involved in the development of various malignant tumours [20]. For instance, SIX1 is upregulated in the cervical [21] and pancreatic cancers [22]; MNX1 correlates with multiple tumours [23-25]. This work confirmed overexpressed MNX1 in CRC tissues and cell lines by qRT-PCR and Western blotting, which corroborates with the bioinformatics analysis results in CRC.
MNX1 is a direct target of E2F4. (A) Predicted E2F4: MAX DNA-binding motif in the human MNX1 promoter region; (B) The ChIPBase site indicated a positive correlation between E2F4 and MNX1; (C, D) E2F4 is upregulated in CC tissues compared to adjacent normal tissues in StarBase database (P=7.4e-14). Patients with high E2F4 expression had poor disease-free survival (P=0.025); (E, F) E2F4 expression determined by qRT-PCR and Western blot assays; (G, H) E2F4 knockdown possibly promoted MNX1 downregulation at the mRNA and protein levels; (I, J) ChIP assays revealed that MNX1 promoter enrichment in the precipitates of E2F4 antibody was lower than that in the control group. After MNX1 delivery, MNX1 promoter enrichment was not improved. Mean ± SD (n = 3 independent experiments). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
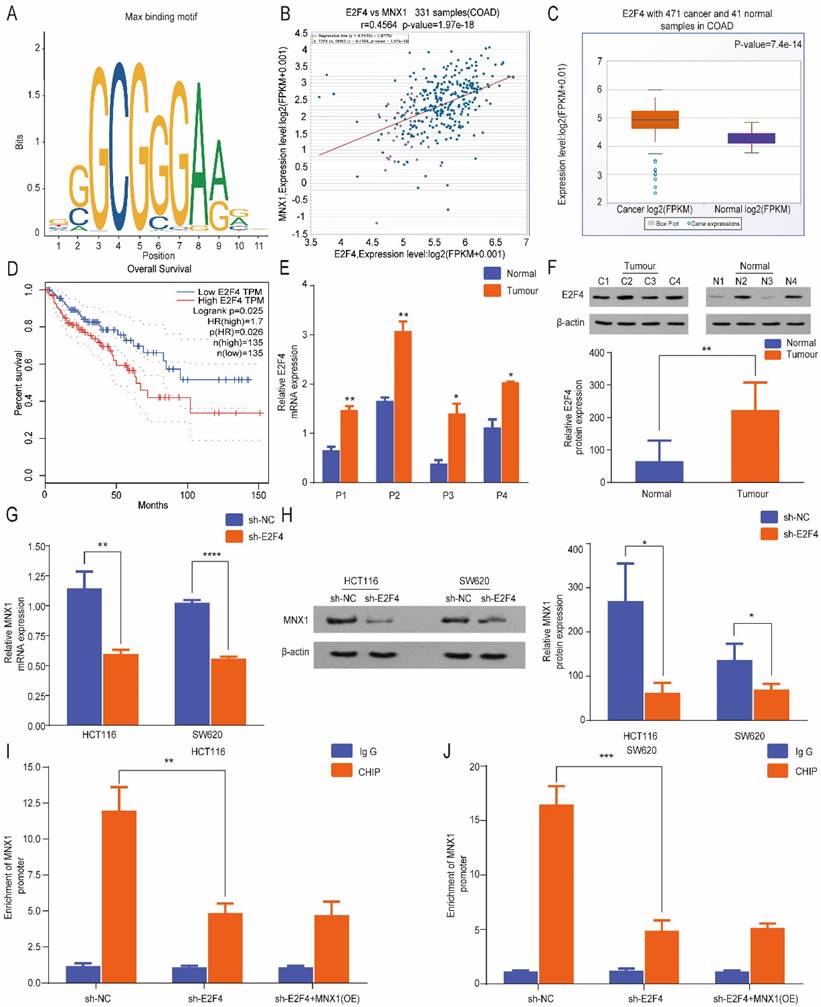
MNX1 upregulation partially recovered the malignant phenotypes of sh-E2F4 cells. (A, B) The mRNA and protein levels of MNX1 were partially reversed when MNX1 was upregulated in the sh-E2F4 cells compared to the sh-E2F4 cells alone; (C) The proliferative capacities were partially rescued after MNX1 upregulation in sh-E2F4 HCT116, and SW620 cells by MTT analysis; (D-G) The invasion and migration capacities have been improved after MNX1 upregulation in sh-E2F4 cells compared to sh-E2F4 cells alone. Error bars represent the mean ± SD values of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, NS, No significance.
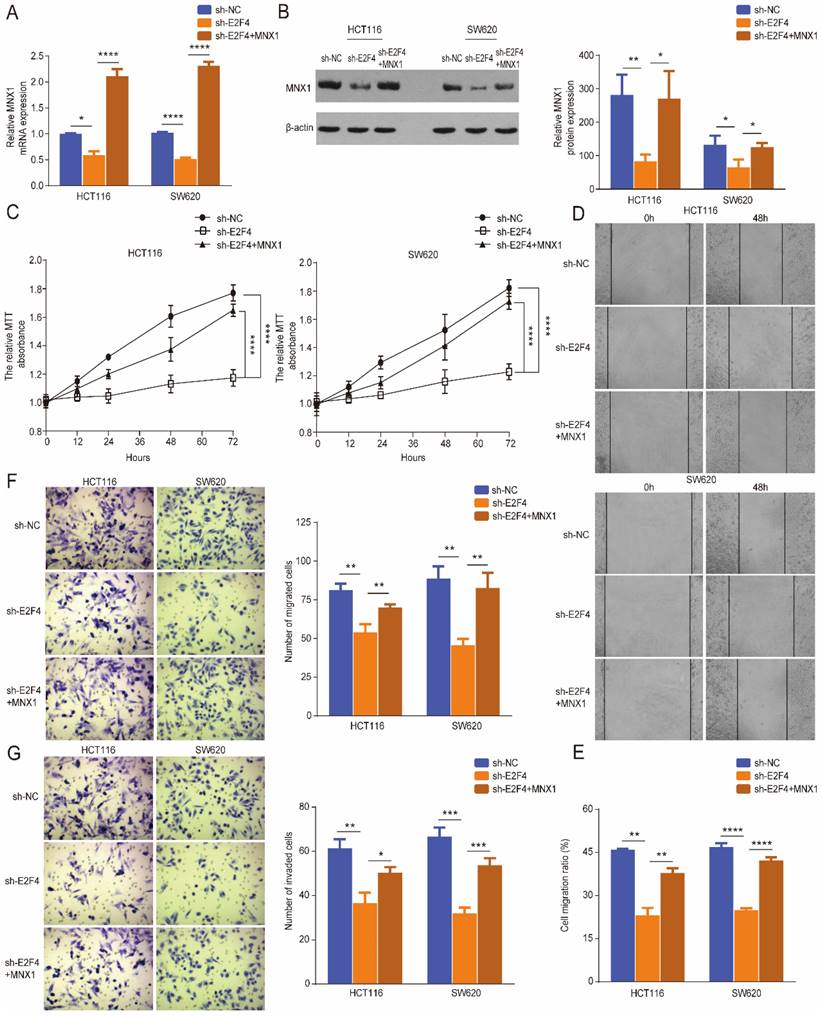
E2F4 activates MNX1 to facilitate the proliferation of CRC cells in vivo. (A) The xenograft tumours extracted from the nude mice; (B, C) The tumour weight and volumes of the mice in different groups are illustrated in the diagram; (D) Ki-67 expression in different groups is shown by Immunohistochemistry assay. Error bars represent the mean ± SD values of three independent experiments. **P < 0.01, ***P < 0.001, ****P < 0.0001.
Further, we analyzed the mechanism of MNX1 upregulation and the roles of MNX1 in CRC. In a bladder cancer study, MNX1 regulated cell proliferation by targeting the G1-S transition in vitro and in vivo [26]. In cervical cancer, MNX1 promotes cancer cell proliferation, invasion, migration, and the progression of the cell cycle by regulating the transcription of p21cip1 [27, 28]. Here, overexpressed MNX1 promoted proliferation, invasion, and migration of colorectal cancer cells, and downregulated levels yielded the opposite result. Based on the JASPAR and RNAinter databases, we speculated that as an essential upstream regulator, E2F4 stimulates MNX1 to promote CRC invasion and migration. We further confirmed this conclusion through chip assay. Current research indicates that the E2F family, including E2F1 and E2F4, regulates cell cycle, tumorigenesis and progression of multiple tumours. [29, 30]. As a transcription factor, E2F proteins regulate the expression of target genes by binding to specific sequences in the promoter of target genes. For instance, E2F often binds to the 5 '-TTTSSCGC-3' (S=C or G) sequence of the target gene promoter, expressed in several genes [31, 32]. Ye et al. reported that E2F1-mediated abnormal expression of LncRNA MNX1‐AS1 promotes colon adenocarcinoma progression [13]. Moreover, recent research indicates that the transcription factor E2F4 activates downstream genes in many cancers [33, 34]. However, for the first time, we report that E2F4 is involved in tumour progression by regulating downstream MNX1 expression, providing new insight into the mechanistic research and treatment of CRC.
Western blot and qRT‐PCR showed E2F4 upregulation in CRC tissues and cell lines. Functional assays revealed that E2F4 downregulation correspondingly downregulated MNX1 expression in CRC cells. The function of the E2F4-MNX1 axis in vivo was further explored, and we constructed the shRNA-E2F4, shRNA-E2F4+MNX1, and shRNA-NC mice models, respectively. The tumour weight and volume in the shRNA-E2F4 group were the lowest, whereas the continuous delivery of overexpressed MNX1 plasmids increased the tumour weight and volume. Therefore, we confirmed that E2F4 has a strong correlation with MNX1.
Schematic diagram of E2F4/MNX1 pathway in colorectal cancer progression.
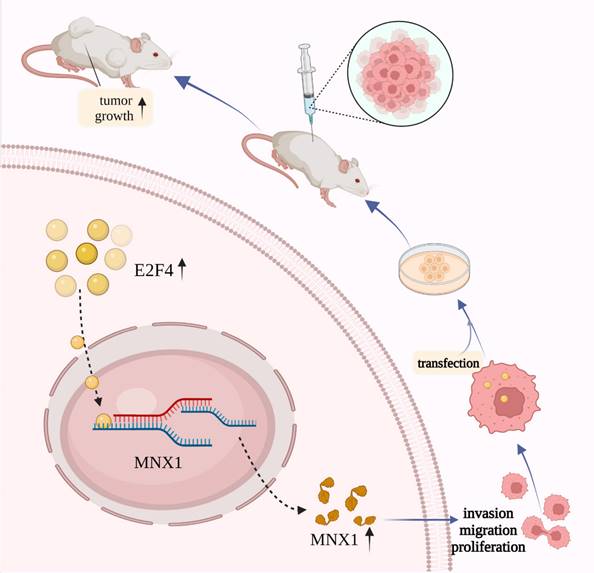
In conclusion, we proposed that E2F4 may act as a transcriptional regulator, activating MNX1 to induce colorectal cancer development. These findings consider the E2F4/MNX1 feedback loop a novel potential diagnostic biomarker of CRC. However, additional research on this perspective is essential in the future.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
We would like to thank the help of the Medical Research Centre of Xiangya Third Hospital and the National Clinical Research Center for Geriatric Disorders, Xiangya Hospital. This study was funded by the Natural Science Foundation of Hunan Province of China (No. 2020JJ4873) and the Fundamental Research Funds for the Central Universities of Central South University.
Author Contributions
(I) Conception and design: Zhuo-Tao Liang; (II) Administrative support: Hai Liu, Zhuo-Tao Liang; (III) Collection and assembly of data: Jia-Ke Li (IV) Data analysis and interpretation: Jia-Ke Li, Hui-Wen Zhang, Jun Li; (V) Manuscript writing: Jia-Ke Li; (VI) Final approval of manuscript: All authors.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: a cancer journal for clinicians. 2020;70:7-30
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F. et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-32
3. Luo C, Cen S, Ding G, Wu W. Mucinous colorectal adenocarcinoma: clinical pathology and treatment options. Cancer communications (London, England). 2019;39:13
4. Saus E, Iraola-Guzmán S, Willis JR, Brunet-Vega A, Gabaldón T. Microbiome and colorectal cancer: Roles in carcinogenesis and clinical potential. Molecular aspects of medicine. 2019;69:93-106
5. Zhang L, Wang J, Wang Y, Zhang Y, Castro P, Shao L. et al. MNX1 Is Oncogenically Upregulated in African-American Prostate Cancer. Cancer research. 2016;76:6290-8
6. Leotta CG, Federico C, Brundo MV, Tosi S, Saccone S. HLXB9 gene expression, and nuclear location during in vitro neuronal differentiation in the SK-N-BE neuroblastoma cell line. PLoS One. 2014;9:e105481
7. Neufing PJ, Kalionis B, Horsfall DJ, Ricciardelli C, Stahl J, Vivekanandan S. et al. Expression and localization of homeodomain proteins DLX4/HB9 in normal and malignant human breast tissues. Anticancer Res. 2003;23:1479-88
8. Hollington P, Neufing P, Kalionis B, Waring P, Bentel J, Wattchow D. et al. Expression and localization of homeodomain proteins DLX4, HB9 and HB24 in malignant and benign human colorectal tissues. Anticancer Res. 2004;24:955-62
9. Wilkens L, Jaggi R, Hammer C, Inderbitzin D, Giger O, von Neuhoff N. The homeobox gene HLXB9 is upregulated in a morphological subset of poorly differentiated hepatocellular carcinoma. Virchows Arch. 2011;458:697-708
10. Wu QN, Luo XJ, Liu J, Lu YX, Wang Y, Qi J. et al. MYC-Activated LncRNA MNX1-AS1 Promotes the Progression of Colorectal Cancer by Stabilizing YB1. Cancer research. 2021;81:2636-50
11. Zhou C, Liu HS, Wang FW, Hu T, Liang ZX, Lan N. et al. circCAMSAP1 Promotes Tumor Growth in Colorectal Cancer via the miR-328-5p/E2F1 Axis. Molecular therapy: the journal of the American Society of Gene Therapy. 2020;28:914-28
12. Xanthoulis A, Kotsinas A, Tiniakos D, Fiska A, Tentes AA, Kyroudi A. et al. The relationship between E2F family members and tumor growth in colorectal adenocarcinomas: A comparative immunohistochemical study of 100 cases. Applied immunohistochemistry & molecular morphology: AIMM. 2014;22:471-7
13. Ye Y, Gu B, Wang Y, Shen S, Huang W. E2F1-mediated MNX1-AS1-miR-218-5p-SEC61A1 feedback loop contributes to the progression of colon adenocarcinoma. Journal of cellular biochemistry. 2019;120:6145-53
14. Yao H, Lu F, Shao Y. The E2F family as potential biomarkers and therapeutic targets in colon cancer. PeerJ. 2020;8:e8562
15. Liu T, Yu T, Hu H, He K. Knockdown of the long non-coding RNA HOTTIP inhibits colorectal cancer cell proliferation and migration and induces apoptosis by targeting SGK1. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2018;98:286-96
16. Yamada A, Yu P, Lin W, Okugawa Y, Boland CR, Goel A. A RNA-Sequencing approach for the identification of novel long non-coding RNA biomarkers in colorectal cancer. Scientific reports. 2018;8:575
17. Sun K, Wang S, He J, Xie Y, He Y, Wang Z. et al. NCOA5 promotes proliferation, migration and invasion of colorectal cancer cells via activation of PI3K/AKT pathway. Oncotarget. 2017;8:107932-46
18. Cui YM, Jiang D, Zhang SH, Wu P, Ye YP, Chen CM. et al. FOXC2 promotes colorectal cancer proliferation through inhibition of FOXO3a and activation of MAPK and AKT signaling pathways. Cancer letters. 2014;353:87-94
19. Yang X, Pan Q, Lu Y, Jiang X, Zhang S, Wu J. MNX1 promotes cell proliferation and activates Wnt/β-catenin signaling in colorectal cancer. Cell biology international. 2019;43:402-8
20. Bhatlekar S, Fields JZ, Boman BM. HOX genes and their role in the development of human cancers. J Mol Med (Berl). 2014;92:811-23
21. Zheng XH, Liang PH, Guo JX, Zheng YR, Han J, Yu LL. et al. Expression and clinical implications of homeobox gene Six1 in cervical cancer cell lines and cervical epithelial tissues. Int J Gynecol Cancer. 2010;20:1587-92
22. Li Z, Tian T, Lv F, Chang Y, Wang X, Zhang L. et al. Six1 promotes proliferation of pancreatic cancer cells via upregulation of cyclin D1 expression. PLoS One. 2013;8:e59203
23. Shuai Y, Ma Z, Liu W, Yu T, Yan C, Jiang H. et al. TEAD4 modulated LncRNA MNX1-AS1 contributes to gastric cancer progression partly through suppressing BTG2 and activating BCL2. Molecular cancer. 2020;19:6
24. Tian T, Wang M, Zhu Y, Zhu W, Yang T, Li H. et al. Expression, Clinical Significance, and Functional Prediction of MNX1 in Breast Cancer. Molecular therapy Nucleic acids. 2018;13:399-406
25. Wang Y, Wang J, Zhang L, Karatas OF, Shao L, Zhang Y. et al. RGS12 Is a Novel Tumor-Suppressor Gene in African American Prostate Cancer That Represses AKT and MNX1 Expression. Cancer research. 2017;77:4247-57
26. Chen M, Wu R, Li G, Liu C, Tan L, Xiao K. et al. Motor neuron and pancreas homeobox 1/HLXB9 promotes sustained proliferation in bladder cancer by upregulating CCNE1/2. J Exp Clin Cancer Res. 2018;37:154
27. Xiao L, Hong L, Zheng W. Motor Neuron and Pancreas Homeobox 1 (MNX1) Is Involved in Promoting Squamous Cervical Cancer Proliferation via Regulating Cyclin E. Med Sci Monit. 2019;25:6304-12
28. Zhu B, Wu Y, Luo J, Zhang Q, Huang J, Li Q. et al. MNX1 Promotes Malignant Progression of Cervical Cancer via Repressing the Transcription of p21(cip1). Front Oncol. 2020;10:1307
29. Cristóbal I, Luque M, Sanz-Alvarez M, Rojo F, García-Foncillas J. Clinical Impact and Regulation of the circCAMSAP1/ miR-328-5p/E2F1 Axis in Colorectal Cancer. Molecular therapy: the journal of the American Society of Gene Therapy. 2020;28:1387-8
30. Bhawe K, Roy D. Interplay between NRF1, E2F4 and MYC transcription factors regulating common target genes contributes to cancer development and progression. Cellular oncology (Dordrecht). 2018;41:465-84
31. Slansky JE, Farnham PJ. Introduction to the E2F family: protein structure and gene regulation. Current topics in microbiology and immunology. 1996;208:1-30
32. Liu N, Lucibello FC, Zwicker J, Engeland K, Müller R. Cell cycle-regulated repression of B-myb transcription: cooperation of an E2F site with a contiguous corepressor element. Nucleic acids research. 1996;24:2905-10
33. Garneau H, Paquin MC, Carrier JC, Rivard N. E2F4 expression is required for cell cycle progression of normal intestinal crypt cells and colorectal cancer cells. Journal of cellular physiology. 2009;221:350-8
34. Gong J, Fan H, Deng J, Zhang Q. LncRNA HAND2-AS1 represses cervical cancer progression by interaction with transcription factor E2F4 at the promoter of C16orf74. Journal of cellular and molecular medicine. 2020;24:6015-27
Author contact
![]() Corresponding author: Zhuo-Tao Liang (liangztedu.cn), National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China.
Corresponding author: Zhuo-Tao Liang (liangztedu.cn), National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China.

 Global reach, higher impact
Global reach, higher impact