3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(1):90-102. doi:10.7150/jca.90090 This issue Cite
Research Paper
Incidence of HER2-targeted antibody-drug conjugates-related cardiac events: a meta-analysis
1. Department of Pharmacy, Hunan Cancer Hospital, the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha 410011, Hunan, China.
2. Department of Pharmacy, the Second Xiangya Hospital, Central South University, Changsha 410011, Hunan, China.
3. Department of Pharmacy, Yantai Hospital of Traditional Chinese Medicine, Yantai 264000, Shandong, China.
Abstract
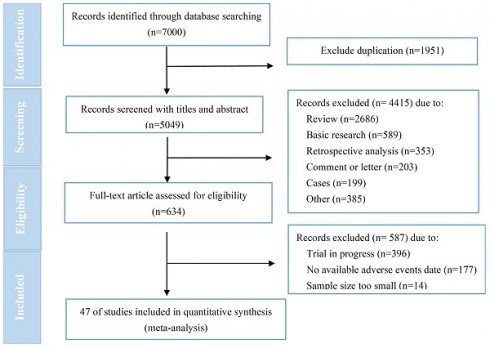
Background: Human epidermal growth factor receptor 2 (HER2)-targeted antibody-drug conjugate (ADC) has emerged as a hotspot for research and brought breakthroughs in the treatment of breast cancer and other solid tumors. While the occurrence of cardiac events (CEs) has yet not been systematically reported.
Methods: The prospective clinical trials of marketed HER2-targeted ADCs were systematically searched in PubMed, Embase, Cochrane Library, and ClinicalTrials.gov from inception to May 2023. Two investigators independently extracted data with priority given to ClinicalTrials.gov, followed by peer-reviewed articles. Stata 15.0 software was used to perform the meta-analysis. The effect statistics were estimated as pooled incidence with 95% confidence intervals (CI). The primary objectives were to assess the incidence of all-grade and ≥3 /serious grades CEs related to HER2-targeted ADC. Our study strictly adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and has been registered on PROSPERO (NO. CRD42023440448).
Results: After conducting a comprehensive literature search, initially 7000 relevant studies were identified, and eventually a total of 47 trials involving 10594 patients were included for analysis. The pooled incidence of all-grade and ≥3/serious grades CEs respectively were 4.7% [95% CI, 3.7-5.8%] and 0.6% (95% CI, 0.5-0.8%). The pooled incidence of CEs leading to dosage discontinuation was 0.8% (95% CI, 0.4-1.3%). Subgroup analysis revealed a significantly higher incidence of all-grade CEs in T-DXd treatment compared to T-DM1 treatment (7.7% versus 3.6%; p=0.017), as well as in phase I/II trials compared to phase III trials (6.9% versus 3.2%; p=0.002) and combination therapy compared to monotherapy (7.6% versus 3.9%; p=0.013). The electrocardiogram QT corrected interval prolonged was identified as the CE with the highest pooled incidence, occurring at a rate of 5.9% (95% CI, 3.3-8.5%).
Conclusions: The incidence of CEs associated with HER2-targeted ADC is relatively low. However, it is crucial to enhance surveillance measures, particularly for T-DXd treatment and combination therapy.
Keywords: human epidermal growth factor receptor 2, antibody-drug conjugate, cardiac events, trastuzumab emtansine, trastuzumab deruxtecan

 Global reach, higher impact
Global reach, higher impact