Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(12):3984-3994. doi:10.7150/jca.96614 This issue Cite
Review
Exploring the evolving roles and clinical significance of circRNAs in head and neck squamous cell carcinoma
1. Shanxi Key Laboratory of Otorhinolaryngology Head and Neck Cancer, Department of Otolaryngology Head & Neck Surgery, First Hospital of Shanxi Medical University, Taiyuan 030001, Shanxi, China
2. Department of Otolaryngology Head & Neck Surgery, The Third Hospital of Shanxi Medical University (Shanxi Bethune Hospital), Taiyuan 030032, Shanxi, China
3. Department of Hepatobiliary Surgery, Shenzhen University General Hospital & Shenzhen University Clinical Medical Academy, Shenzhen University, Shenzhen 518055, Guangdong, China
4. Department of Otolaryngology Head & Neck Surgery, Shenzhen University General Hospital & Shenzhen University Clinical Medical Academy, Shenzhen University, Shenzhen 518055, Guangdong, China
5. Shenzhen Research Institute, Northwest A&F University, Shenzhen 518000, Guangdong, China
6. Department of Otolaryngology Head & Neck Surgery, Longgang Otolaryngology Hospital, Shenzhen 518172, Guangdong, China
7. Shenzhen Institute of Otolaryngology & Key Laboratory of Otolaryngology, Longgang Otolaryngology Hospital, Shenzhen 518172, Guangdong, China
8. Shenzhen University General Hospital & Shenzhen University Clinical Medical Academy, Shenzhen University, Shenzhen 518055, Guangdong, China
# These authors contributed equally to this work.
Received 2024-3-25; Accepted 2024-5-13; Published 2024-5-30
Abstract
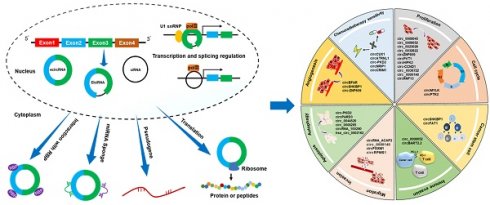
Head and neck squamous cell carcinoma (HNSCC) represents the predominant malignancies in the head and neck region, and has limited therapeutic alternatives. Circular RNAs (circRNAs), a substantial category of non-coding RNA molecules, exert influential roles in human disease development and progression, employing various mechanisms such as microRNA sponging, interaction with RNA-binding proteins, and translational capabilities. Accumulating evidence highlights the differential expression of numerous circRNAs in HNSCC, and numerous dysregulated circRNAs underscore their crucial involvement in malignant advancement and resistance to treatment. This review aims to comprehensively outline the characteristics, biogenesis, and mechanisms of circRNAs, elucidating their functional significance in HNSCC. In addition, we delve into the clinical implications of circRNAs, considering their potential as biomarkers or targets for diagnosis, prognosis, and therapeutic applications in HNSCC. The discussion extends to exploring future challenges in the clinical translation of circRNAs, emphasizing the need for further research.
Keywords: Head and neck squamous cell carcinoma, Circular RNA, Biomarker, Non-coding RNA, Immune evasion, Cancer stem cell
Introduction
Head and neck squamous cell carcinoma (HNSCC) stands as the predominant subtype of head and neck cancer, ranking seventh among global malignant tumors, with over 800,000 new cases annually[1, 2]. Influenced by environmental factors, smoking, alcohol consumption, and human papillomavirus infection, the specific pathogenic mechanism of HNSCC remains unclear[3, 4]. Due to the absence of specific symptoms, HNSCC is easily ignored in the early stage, resulting in most patients being diagnosed in the advanced clinical stage. The inherent malignant biological characteristics marked by local recurrence, lymph node metastasis, and local invasion, also contribute to poor prognosis of HNSCC. Despite available clinical treatments, including surgery, radiotherapy, chemotherapy, and immunotherapy, which have demonstrated some efficacy in improving survival time and quality of life, the overall five-year survival rate for patients with HNSCC has not significantly increased over the past decades[5-7]. Hence, there is an urgent need to elucidate the mechanisms underlying HNSCC development, identifying biomarkers and molecular targets for early diagnosis and targeted therapy.
Non-coding RNAs refers to RNA molecules in the transcriptome that are not translated into proteins[8]. Circular RNA (circRNA), a predominant class of endogenous non-coding RNA molecules widely expressed in eukaryotic cells, plays roles in various physiological and pathological processes, including neurodegenerative diseases[9, 10], cardiovascular diseases[11, 12], metabolic diseases[13, 14], and cancers[15-18]. The present study provides a comprehensive summary of biological functions and regulatory mechanisms of circRNAs in HNSCC, examining their potential applications and clinical translational value in diagnosis, prognosis, and targeted therapy. In addition, we anticipate future research directions by addressing key issues in circRNA research relevant to HNSCC.
1. Biogenesis and action mechanisms of circRNA
In 1976, Kolakofsky D[19] made the pioneering discovery of circRNA in the Sendai virus. Since then, an increasing plethora of circRNAs have been continually unveiled across diverse species, including Drosophila melanogaster, mice, and humans[20]. Initially relegated as non-functional by-products of mRNA splicing errors, the perception of circRNAs has undergone a transformative shift. The evolution of high-throughput sequencing technology and bioinformatics has progressively deepened the understanding of circRNAs. In recent years, circRNAs have emerged as a burgeoning frontier in molecular biology and oncology research[21-23].
1.1 Biological characteristics of circRNA
CircRNAs, lacking a 5' cap and 3' poly (A) tail, exhibit a structurally robust configuration that imparts high stability. This stability renders circRNAs resistant to degradation by exonucleases, resulting in an extended half-life compared to linear RNA[24-26]. Demonstrating a high degree of conservation across species, circRNAs exhibit spatiotemporal specificity in expression, with notable variations in types and abundance across different tissues, cells, and developmental stages[27, 28].
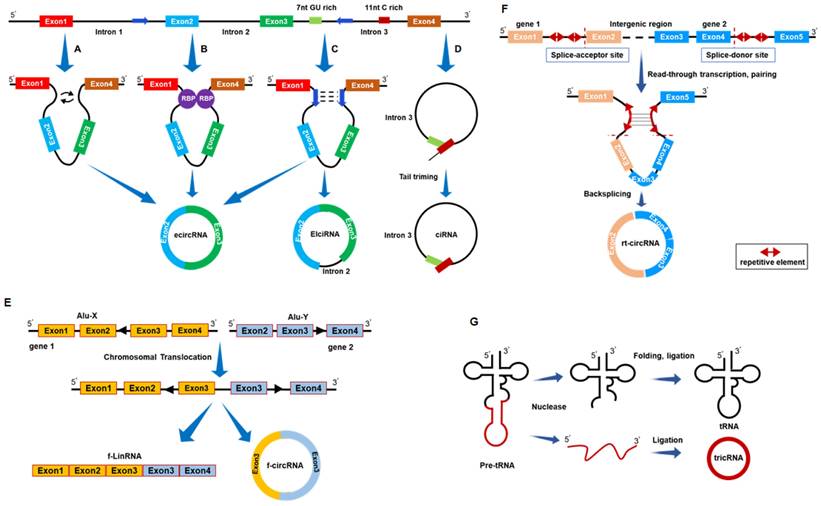
The mechanisms governing circRNA formation mainly involve three models: (1) Exon skipping or Lariat-driven model; (2) RNA-binding protein (RBP)-pairing-driven model; and (3) Intron-pairing-driven model. In the Exon skipping or Lariat-driven model, precursor mRNA (pre-mRNA) undergoes partial overlapping during transcription, leading to reverse splicing of downstream 3' splice sites with upstream 5' splice sites. This process brings non-adjacent exons into proximity, forming a circular structure[29-32] (Fig. 1A). In the RBP-pairing-driven model, RBPs bind to specific base sequences in flanking introns, regulating circularization through protein-protein interactions or dimer formation[33-35] (Fig. 1B). In the intron-pairing-driven model, the flanking introns of downstream splice donor sites and upstream splice acceptor sites contain reverse complementary sequences, such as Alu elements. Selective splicing after base pairing leads to the formation of circRNAs with or without introns[25] (Fig. 1C). In addition, intron circRNAs (ciRNAs) form through a 7 nt GU-rich sequence near the 5' splice site and an 11 nt C-rich sequence near the branch point, circularizing after the action of RNA polymerase II[30] (Fig. 1D). The connection of exons from different genes on the same or different chromosomes can produce fusion circRNAs (Fig. 1E) and read-through circRNAs[36, 37] (Fig. 1F). Based on origin and formation mechanism, circRNAs are classified into exonic circRNAs (ecircRNAs), exonic-intronic circRNAs (EIciRNAs), intronic circRNAs (ciRNAs), and tRNA intronic circRNAs (tricRNAs) (Fig. 1G). Among these, ciRNAs and EIciRNAs are predominantly localized in the cell nucleus, whereas ecircRNAs primarily distribute in the cytoplasm. ecircRNAs, accounting for over 80% of known circRNAs, have been the focus of extensive research[38-40].
1.2 Action mechanisms of circRNA
1.2.1 Transcription and splicing regulation
Introns containing circRNAs are predominantly located in the cell nucleus, where they interact with promoters and recruit transcriptional regulatory proteins, activating gene transcription[30]. Noteworthy examples include circACTN4, which recruits Y-box binding protein 1 to co-activate Frizzled-7 transcription[41]. Cia-MAF interacts with the MAFF promoter, recruiting the TIP60 chromatin remodeling complex to activate MAFF transcription[42]. circRap1b induces H3K14ac modification by recruiting acetyltransferase Kat7 to the Hoxa5 promoter region, resulting in Hoxa5 transcriptional activation and increased Fam3a expression[43]. circ_001659 recruits RBBP5 to the Vimentin promoter, enhancing H3K4 trimethylation at the Vimentin promoter, activating Vimentin transcription[44].
Introns containing circRNAs can also interact with RNA Polymerase II, exerting regulatory effects on their parent coding genes[45]. circEIF3J and circPAIP2 form an EIciRNA-U1 SnRNP complex, binding to the U1 binding site in EIciRNA and interacting with U1 snRNA, regulating parent gene transcription by interacting with the RNA Polymerase II promoter site[46]. In addition, Xu et al. reported that circSMARCA5 directly binds to its parent gene site, forming an R-loop that terminates exon 15 transcription of SMARCA5[47]. Moreover, introns containing circRNAs regulate alternative splicing by influencing splicing factors. For example, circSMARCA5 modulates the pre-mRNA splicing process of VEGFA by recruiting the splicing factor SRSF1, reducing the production of VEGFA splice isoforms[48]. These studies underscore the regulatory role of circRNAs at both transcriptional and splicing levels (Fig. 2A).
Biogenesis and classification of circRNA. (A) Lariat-driven model. (B) Intron-pairing-driven model. (C) RBP-pairing-driven model. (D) Generation of intronic circRNAs (ciRNAs). (E) Generation of fusion circRNAs (f-circRNAs). (F) Generation of read-through circRNAs (rt-circRNAs). (G) Generation of tRNA intronic circRNAs (tricRNAs). ecircRNA: exonic circRNA; EIciRNA: exonic-intronic circRNA.
1.2.2 Protein or peptide translation
Traditionally, eukaryotic mRNA translation relies on the 5' cap structure. Due to the absence of a 5' cap and 3' poly (A) tail, circRNAs have been categorized as non-coding RNAs. However, recent studies have unveiled a subset of circRNAs capable of encoding proteins or peptides, serving as templates for ribosomal translation[49, 50]. These circRNAs feature an internal ribosome entry site-driven open reading frame, facilitating direct ribosomal recruitment and translation initiation (Fig. 2A). For instance, circ-EIF6 encodes the novel peptide EIF6-224aa, EIF6-224aa directly interacted with the oncogenic protein MYH9 to decrease its degradation by inhibiting the ubiquitin-proteasome pathway, thereby promoting proliferation and metastasis in triple-negative breast cancer[51]; circDIDO1 encodes the protein DIDO1-529, and DIDO1-529 interacted with poly ADP-ribose polymerase 1 (PARP1) and inhibited its activity. Knockdown of circDIDO1 promoted gastric cancer cell proliferation, migration and invasion[52]; circMAPK14 functioned as a tumor suppressor by encoding a peptide of 175 amino acids (circMAPK14-175aa), which blocked the malignant progression and metastasis of colorectal cancer[53]; circAXIN1 encodes the protein AXIN1-295aa, which competitively interacts with APC to activate the Wnt signaling pathway, functioning as an oncogenic protein in gastric cancer[54]. Notably, recent studies found that RNA m6A modification enhances the initiation of circRNA protein translation[55]. In this context, circARHGAP35 undergoes m6A-dependent translation, producing an oncogenic protein[56]. Furthermore, m6A modification drives the translation of circMAP3K4 into the peptide circMAP3K4-455aa[57].
Schematic representation of the action mechanisms of circRNAs. (A) circRNAs exert biological functions through mechanisms such as miRNA sponges, RNA-binding protein interaction, transcription and splicing regulation, protein or peptide translation, and pseudogene generation. (B) miRNA binds to mRNA, LncRNA, pseudogene, and circRNA, forming a competitive binding relationship among RNA molecules that bind to the same miRNA.
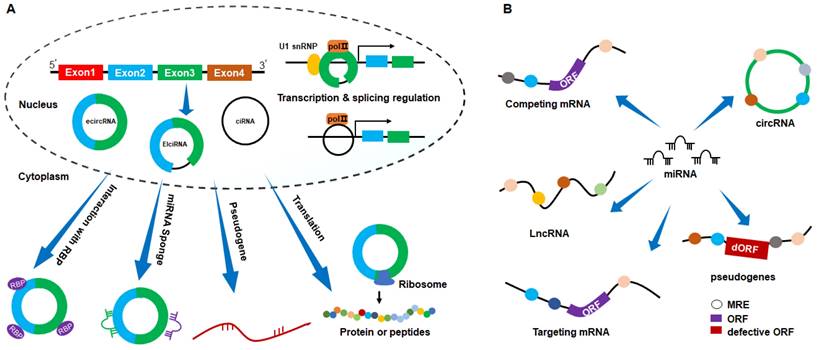
1.2.3 Interaction with RNA-binding proteins
Specific circRNAs harbor binding sites for RNA-binding proteins, enabling direct interactions[58, 59] (Fig. 2A). For instance, circDLC1 binds to the RNA-binding protein HuR. This interaction impedes the binding of HuR and MMP1 mRNA, resulting in the inhibition of MMP1 expression. Consequently, it suppresses the proliferation and metastasis of hepatocellular carcinoma[60]. circCwc27 interacts with the RNA-binding protein Pur-α. This interaction inhibits Pur-α activity, playing a role in Alzheimer's disease onset and development[61]. circSETD2 interacts with HuR, diminishing the stability of YAP1 mRNA, ultimately inhibiting the progression of breast cancer[62]. Recently, Ju et al. identified an intron containing circRNA in HNSCC, named as circGNG7. Mechanistically, circGNG7 binds to serine residues 78 and 82 of the functional heat shock protein 27 (HSP27), hindering its phosphorylation, which reduced HSP27-JNK/P38 mitogen-activated protein kinase (MAPK) oncogenic signaling[63].
1.2.4 circRNA-derived pseudogene
Pseudogenes are genomic DNA sequences closely resembling coding genes but have lost their normal function due to the absence of functional promoters or other regulatory elements, often remaining transcriptionally inert[64, 65]. Studies indicate that pseudogenes originating from linear mRNAs can undergo reverse transcription and integrate into the host genome. Similarly, circRNAs can also undergo reverse transcription transposition, resulting in pseudogenes derived from processed circRNAs being inserted into the host genome, thereby altering genomic DNA composition[66, 67] (Fig. 2A). To date, the functions and mechanisms of pseudogenes derived from circRNAs remain unclear.
1.2.5 miRNA (microRNA) sponge
miRNAs, approximately 19-24 nucleotides long, are small endogenous non-coding single-stranded RNAs that regulate translation or induce mRNA degradation by binding to the 3'UTRs of target mRNAs. This binding is mediated by miRNA response elements (MREs) on various RNAs, including lncRNAs, pseudogenes, and circRNAs. The same miRNA can bind to multiple types of RNAs, and the competitive binding of RNAs with the same MREs to miRNAs is known as the competing endogenous RNA mechanism[48, 68] (Fig. 2B). Within this mechanism, circRNA are referred to as miRNA “sponge” due to their specific adsorption of miRNAs, thus modulating the expression of downstream target genes. Numerous studies confirm the ability of circRNAs to reduce miRNA inhibitory effects on target genes, indirectly regulating target gene expression[69, 70]. Typically, a single circRNA harbors multiple binding sites for different miRNAs or multiple sites for the same miRNA. For instance, circTMEM59 inhibits colorectal cancer cell migration by adsorbing miR-668-3p and miR-410-3p. It also serves as a sponge for miR-147b, impeding the progression of pancreatic ductal adenocarcinoma[71-73]. Moreover, the same miRNA can be adsorbed by different circRNAs. For example, circKIF4A and circ_0058063 contain miR-335-5p binding sites, thereby regulating miR-335-5p target gene expression[74, 75]. To date, miRNA sponge is the most extensively studied mechanism of circRNA.
In summary, circRNAs exert their biological functions through various mechanisms, including transcription and splicing, interaction with RNA binding protein, translation of proteins or peptides, generation of pseudogenes, and acting as miRNA sponge.
2. Functional roles and mechanisms of circRNA in HNSCC
2.1 Regulation of proliferation
circRNAs exert a pivotal role in modulating the proliferation of HNSCCs. Notably, several circRNAs, including circ_0000045, circ_0000052, circ_0023028, circ_0032822, circZNF609, circPVT1, circHIPK2, and circ-CCND1, are upregulated in both HNSCC tissues and cells, actively promoting HNSCC cell proliferation[76-83]. Conversely, certain circRNAs function as tumor suppressor genes, exerting inhibitory effects on HNSCC cell proliferation. For example, circ_0036722 regulates the expression of the parental gene RHCG by sequestering miR-1248, suppressing laryngeal squamous cell carcinoma (LSCC) cell proliferation[84]. circ_0000140 inhibits the proliferation of oral squamous cell carcinoma (OSCC) cells[85]. Moreover, overexpression of circRNF13 exhibits inhibitory effects on nasopharyngeal carcinoma (NPC) cell proliferation[86].
2.2 Regulation of cell cycle transition
Cell cycle dysregulation is a hallmark of cancer cells, with cyclin D1 serving as a key regulator in the G1/S phase transition and playing a crucial role in cancer cell proliferation[87, 88]. Knockdown of circMYLK in LSCC cells results in reduced cyclin D1 expression levels, suggesting that circMYLK potentially promotes tumor cell proliferation by accelerating the cell cycle process[89]. Another study demonstrated that circPTK2 promotes cell cycle progression in LSCC cells. Knockdown of circPTK2 leads to reduced expression levels of cell cycle-related proteins, including cyclin A1, cyclin B1, and cyclin D1[90].
2.3 Regulation of invasion and migration
Invasion and migration are pivotal features of malignant tumors, contributing significantly to the mortality of patients with cancer. Epithelial-mesenchymal transition (EMT) is crucial in tumor cell dissemination, orchestrating the shift from an epithelial to a more invasive, migratory mesenchymal phenotype[91]. Ma et al. discovered that circRNA_ACAP2 regulates the EMT process through the miR-21-5p/STAT3 axis, inhibiting HNSCC cell migration[92]. circ_0000140 binds to miR-31, upregulating the target gene LATS2 expression, thereby inhibiting the EMT process in OSCC cells[85]. Furthermore, Pei et al. identified that circFOXM1 upregulates Smad2 gene expression by sequestering miR-136-5p, promoting the EMT process in NPC cells[93]. Liu et al. demonstrated that EBV-encoded circRPMS1 fosters the EMT in NPC cells by sequestering multiple miRNAs, including miR-203, miR-31, and miR-451[94].
2.4 Regulation of angiogenesis
The growth and metastasis of cancer cells rely on tumor angiogenesis, a process facilitated by the collective action of cancer cells, stromal cells, and their secretions. Given that VEGF is pivotal in promoting cancer cell growth through angiogenesis, circRNAs exert influence by directly or indirectly modulating VEGF expression levels[95, 96]. Gong et al. discovered that circBFAR promotes ki-67, MMP2, and VEGFA protein expression by binding to miR-31-5p, facilitating the generation of new blood vessels in LSCC[97]. In LSCC, silencing circSHKBP1 leads to a significant reduction in MMP2 and VEGFA expression, resulting in the inhibition of LSCC cell invasion and angiogenesis[98]. Silencing circ-ZNF609 in NPC results in decreased VEGF expression levels, along with a noticeable downregulation of VEGFR1 and VEGFR2 protein expression, suggesting that circ-ZNF609 may play a role in promoting angiogenesis in NPC[99].
2.5 Regulation of immune evasion
Immune surveillance mechanisms play a pivotal role in identifying and eliminating cancer cells. Central to this process is PD-1 (Programmed death-1), a critical immune checkpoint molecule primarily expressed in immune cells. Its interaction with the ligand PD-L1 (programmed death-ligand 1) on cancer cells prevents the activation of tumor antigen-specific T cells, contributing to the immune evasion of cancer cells[100, 101]. circ_0000052 upregulates PD-L1 expression by sequestering miR-382-3p, thereby promoting the malignant progression of HNSCC[77]. Ge et al. demonstrated that EBV-encoded circBART2.2 expression promotes PD-L1 transcription through the binding of circBART2.2 to the helicase domain of RIG-I and the activation of transcription factors IRF3 and NF-κB, resulting in immune evasion of NPC[102].
2.6 Regulation of apoptosis
circRNAs have a significant influence in regulating apoptosis in HNSCC through the modulation of pro-apoptotic and anti-apoptotic genes within apoptotic signaling pathways. Knockdown of circ_0044520 upregulates Bax (BCL2 Associated X) expression and simultaneously reduces BCL2 (B-cell lymphoma 2) expression, promoting apoptosis of LSCC cells[103]. Knockdown of circ_0000285 enhances Caspase-3 activity, upregulates Bax protein levels, and downregulates BCL2 protein levels. These alterations indicate the ability of circ_0000285 to inhibit apoptosis in NPC cells[104]. Silencing circRNA_100290 increases Caspase-9 expression in LSCC cells, suggesting that circRNA_100290 suppresses apoptosis[105]. In addition, hsa_circ_0002162 exhibits increased expression in Tongue squamous cell carcinoma. Silencing hsa_circ_0002162 leads to an increase in apoptotic protein Caspase-3 expression[106].
2.7 Regulation of autophagy
Autophagy, a cellular process crucial for maintaining homeostasis, involves the engulfment and digestion of damaged or aging proteins and organelles by lysosomal hydrolases. P62 and LC3 serve as markers reflecting autophagic activity. In conditions of low autophagy or inhibition, P62 accumulates in the cytoplasm, while the LC3-II/I ratio indicates the level of autophagy[107-110]. Studies have shown that autophagy can exert dual effects on tumor occurrence and progression[111, 112]. Overexpression of circ-PKD2 in OSCC cells results in an increased LC3-II/I ratio and decreased P62 levels, suggesting that circ-PKD2 promotes autophagy in OSCC cells[113]. Conversely, overexpression of circPARD3 leads to decreased LC3-II levels and increased P62 levels in LSCC cells, indicating that circPARD3 inhibits autophagy in LSCC[114].
2.8 Regulation of chemoradiotherapy sensitivity
Chemotherapy and radiotherapy are pivotal in cancer treatment, yet resistance poses a significant challenge, impacting treatment efficacy and contributing to poor prognosis in patients with HNSCC. circRNAs participate in the regulation of chemoradiotherapy sensitivity in cancer cells[115, 116]. circCUX1, upregulated in radiotherapy-resistant hypopharyngeal squamous cell carcinomas (HSCC) tissues, is implicated in promoting resistance. Knockdown of circCUX1 enhances the release of inflammatory cytokines IL-1β and IL-18, thereby augmenting the sensitivity of HSCC to radiotherapy[117]. circATRNL1 enhances OSCC sensitivity to radiation by promoting target gene PTEN expression, which is achieved through the sequestration of miR-23a-3p[118]. circ-PKD2 promotes the sensitivity of OSCC to cisplatin both in vitro and in vivo. Its mechanism involves inhibiting miR-646 and promoting Atg13-mediated autophagy[113]. Knockdown of circNRIP1 increases the sensitivity of NPC cells to 5-Fu and CDDP in vitro[119]. circCRIM1 competitively binds to miR-422a, counteracting its inhibitory effect on FOXQ1 and promoting resistance of NPC cells to docetaxel[120].
2.9 Regulation of stem cell properties
Cancer stem cells, with their ability for self-renewal and differentiation into diverse cancer cell types, underlie the malignancy of cancer, contributing to recurrence, metastasis, and chemoradiotherapy resistance[121]. In HNSCC, cancer stem cells are increasingly recognized as pivotal players in its pathogenesis. Chen et al. showed that the knockdown of circSHKBP1 inhibits stem-like properties in LSCC and suppresses tumor growth. This regulatory role is attributed to the ability of circSHKBP1 to sequester miR-766-5p, consequently enhancing HMGA2 expression and promoting LSCC progression[98]. circFAT1 promotes cancer stem cell characteristics by activating STAT3. Knockdown of circFAT1 reduces HNSCC cell sphere formation in vitro[122].
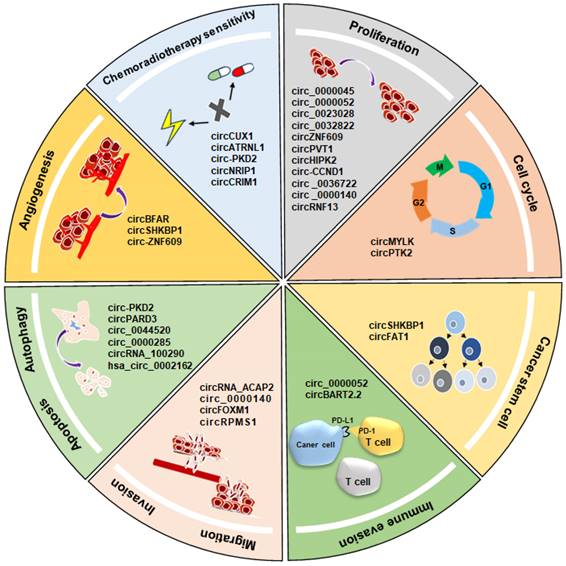
In summary, circRNAs function as either oncogenes or tumor suppressor genes, modulating HNSCC-related key signaling pathways. Their regulatory influence extends across various aspects, including cell proliferation, cell cycle transition, migration, invasion, angiogenesis, apoptosis, autophagy, and cancer stem cell maintenance. This comprehensive regulatory role underscores their significance in shaping the growth, recurrence, metastasis, and chemoradiotherapy sensitivity in HNSCC (Fig. 3).
3. Potential of circRNA as diagnostic and prognostic biomarkers in HNSCC
The identification of specific biomarkers for HNSCC is crucial for non-invasive diagnostics and accurate prognosis assessment. circRNAs, characterized by high stability, diverse types, and spatiotemporal specificity, present unique advantages as potential biomarkers in HNSCC due to their presence in various bodily fluids. Increasing evidence suggests that circRNAs have significant potential in HNSCC diagnosis and prognosis, potentially evolving into early screening and prognostic markers for patients with HNSCC[123-125]. hsa_circ_0003829 exhibits significantly lower expression in OSCC tissues compared to adjacent normal tissues, and the expression level of hsa_circ_0003829 was correlated with lymph node metastasis and clinical staging. Receiver operating characteristic curve analysis yields an area under the curve (AUC) of 0.81, sensitivity of 70%, and specificity of 80%, suggesting that hsa_circ_0003829 may serve as a potential diagnostic marker for OSCC[126]. circRNA_103862 upregulates in LSCC tissues and is closely linked to clinical staging and lymph node metastasis. It demonstrates an AUC of 0.805, with a sensitivity of 0.823 and a specificity of 0.694[127]. Furthermore, circ0019201, circ0011773, and circ0122790 upregulated in the plasma of patients with LSCC, with AUC of 0.766, 0.864, and 0.908, respectively, suggesting their potential as predictive biomarkers for LSCC[128]. Moreover, circMORC3 downregulation in HSCC tissues and plasma, with an AUC of 0.767, suggests its potential as an early diagnostic biomarker for HNSCC[129] (Table 1).
4. Potential of circRNAs as molecular target for HNSCC treatment
The pivotal regulatory role of circRNAs in governing various aspects of HNSCC, including cell proliferation, invasion, migration, apoptosis, glucose metabolism, underscores their potential as molecular targets for HNSCC treatment[130-137]. Notably, circMTCL1 was upregulated in LSCC tissues. In vivo and in vitro experiments showed that circMTCL1 promotes the proliferation, invasion, and migration of LSCC cells, suggesting it serves as a potential therapeutic target for LSCC[138] (Table 2).
Regulatory role of circRNAs in HNSCC, including cell proliferation, cell cycle, invasion, migration, angiogenesis, immune evasion, apoptosis, autophagy, and cancer stem cell maintenance.
Potential circRNA biomarker for diagnosis and prognosis of HNSCC
| circRNAs | Expression | Cancer type | Function | Clinical relevance | Reference |
|---|---|---|---|---|---|
| hsa_circ_0023305 | up | LSCC | Promotes proliferation, invasion, migration | Clinical stage, lymph node metastasis | [123] |
| hsa_circ_0066755 | up | NPC | Promotes proliferation, invasion, migration | Clinical stage | [124] |
| hsa_circ_0028007 | up | NPC | Promotes migration, and invasion | Aggressive infiltration, and metastatic lymph nodes | [125] |
| hsa_circ_0003829 | down | OSCC | - | Lymphatic metastasis, TNM stage | [126] |
| circRNA_103862 | up | LSCC | Promotes proliferation, migration, invasion | Survival time | [127] |
| circ_0019201, circ_0011773, circ_0122790 | up | LSCC | - | High diagnostic ability for single circRNA and combined | [128] |
| circMORC3 | down | HSCC | - | T stage, tumor size | [129] |
circRNA serves as potential therapeutic target in HNSCC
| circRNAs | Expression | Cancer type | Target genes | Function | Reference |
|---|---|---|---|---|---|
| circDHTKD1 | up | OSCC | miR-326/GAB1 | Promotes cell growth and migration, inhibits apoptosis | [130] |
| circ_0008068 | up | OSCC | miR-153-3p/AGK | Promotes proliferation, migration, invasion, tube formation, glycolysis, inhibits apoptosis | [131] |
| hsa_circ_0042666 | down | LSCC | miR-223/TGFBR3 | Inhibits proliferation and invasion | [132] |
| circFLNA | up | LSCC | miR-486-3p/FLNA | Promotes migration | [133] |
| circ_0000215 | up | NPC | miR-512-5p/PIK3R1 | Promotes proliferation, migration | [134] |
| circRNA CDR1as | up | NPC | miR-7-5p/E2F3 | Promotes proliferation, glucose metabolism | [135] |
| hsa_circ_0046263 | up | NPC | miR-133a-5p/IGFBP3 | Promotes proliferation, invasion, migration | [136] |
| circSOX9 | up | NPC | miR-485-3p/SOX9 | Promotes invasion and proliferation | [137] |
| circMTCL1 | up | LSCC | C1QBP/β-catenin | Promotes proliferation, invasion, migration | [138] |
5. Conclusions and perspectives
A growing body of evidence highlights significant dysregulation of circRNAs in HNSCC, with both in vitro and in vivo studies illustrating their regulatory effects on downstream target genes and signaling pathways. These circRNAs play crucial roles in governing processes such as cell proliferation, invasion, metastasis, apoptosis, and autophagy, influencing the occurrence, development, and sensitivity to chemoradiotherapy in HNSCC. Moreover, several circRNAs exhibit a significant association with clinical features and prognosis, showcasing their potential as promising biomarkers and therapeutic targets for HNSCC diagnosis, prognosis, and targeted therapy.
However, as research on circRNAs in HNSCC expands, several challenges and future research directions become apparent: (1) While circRNAs exhibit multiple mechanisms of action, current studies primarily focus on their role as miRNA sponges. The broader impact of circRNAs on transcription, splicing, protein interactions, and encoding proteins or peptides in HNSCC remains understudied. (2) The upstream regulation of circRNA expression dysregulation in HNSCC, including variable splicing and post-transcriptional modification, requires further investigation. (3) Understanding the regulatory role of circRNAs in HNSCC stem cells, considered the root of malignant behaviors and treatment resistance, offers potential insights for clinical diagnosis and treatment. (4) Compared with 2D cell models and animal models, the application of organoids and organ-on-a-chip technology presents an exciting avenue for studying the spatial structure and tissue analog of circRNAs in HNSCC, offering potential clinical transformation insights. (5) Current studies on circRNA biomarkers often feature small sample sizes. Large-scale, multi-center clinical samples are needed to validate the utility of circRNAs as biomarkers for early diagnosis and prognosis assessment. (6) Addressing the urgent challenge of altering circRNA expression levels in target cells is essential for circRNA transformation research. In conclusion, as circRNA research deepens, it holds substantial promise for clinical diagnosis and treatment of HNSCC in the future.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 82073101), Basic and Applied Basic Research Foundation of Guangdong Province (No. 2023A1515010342, 2021A1515012161), Shenzhen Science and Technology Innovation Program (RCJC20210706091950028, JCYJ20220531102815036, JCYJ20220530153604010), Shenzhen Key Medical Discipline Construction Fund (SZXK039), Longgang District science and technology innovation special fund (LGKCYLWS2023026, LGKCYLWS2023010), Shenzhen Natural Science Fund (the Stable Support Plan Program) (20220810151804002), Sanming Project of Medicine in Shenzhen (SZSM202003009).
Author contributions
Y.Y.W., W.G., and S.X.W. conceived the study. Y.Y.W., W.G., P.X.L., Q.B.G., J.W.H., H.L. designed literature search strategy and project implementation plan. P.X.L., Q.B.G., J.W.H., H.L., Y.F.C., F.W., Z.H. conducted the literature search, wrote the manuscript. P.X.L., Q.B.G., X.L.Z., N.N.Z. prepared figures 1-3, Tables 1 and 2. Y.F.C., F.W., Z.H., X.L.Z., N.N.Z. provided suggestions in literature search. Y.Y.W., W.G., and S.X.W. directed the writing of the manuscript. All authors reviewed and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Nisar S, Yousuf P, Masoodi T. et al. Chemokine-Cytokine Networks in the Head and Neck Tumor Microenvironment. Int J Mol Sci. 2021;22:4584
2. Pei R, Shi Y, Lv S. et al. Nivolumab vs Pembrolizumab for Treatment of US Patients With Platinum-Refractory Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: A Network Meta-analysis and Cost-effectiveness Analysis. JAMA Netw Open. 2021;4:e218065
3. Haring CT, Kana LA, Dermody SM. et al. Patterns of recurrence in head and neck squamous cell carcinoma to inform personalized surveillance protocols. Cancer. 2023;129:2817-27
4. Milan TM, Eskenazi A, Oliveira LD. et al. Interplay between EZH2/β-catenin in stemness of cisplatin-resistant HNSCC and their role as therapeutic targets. Cell Signal. 2023;109:110773
5. Xu Y, Zhu G, Maroun CA. et al. Programmed Death-1/Programmed Death-Ligand 1-Axis Blockade in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma Stratified by Human Papillomavirus Status: A Systematic Review and Meta-Analysis. Front Immunol. 2021;12:645170
6. Wei Z, Wang Y, Peng J. et al. CircRFWD3 promotes HNSCC metastasis by modulating miR-27a/b/PPARγ signaling. Cell Death Discov. 2022;8:285
7. Reverdy T, Varnier R, de Talhouët S. et al. Analysis of the benefit of salvage chemotherapy after progression on nivolumab in patients with squamous cell carcinoma of the head and neck. Oral Oncol. 2023;145:106533
8. Nemeth K, Bayraktar R, Ferracin M. et al. Non-coding RNAs in disease: from mechanisms to therapeutics. Nat Rev Genet. 2024;25:211-32
9. Wang ZY, Wen ZJ, Xu HM. et al. Exosomal noncoding RNAs in central nervous system diseases: biological functions and potential clinical applications. Front Mol Neurosci. 2022;15:1004221
10. Wu DP, Zhao YD, Yan QQ. et al. Circular RNAs: emerging players in brain aging and neurodegenerative diseases. J Pathol. 2023;259:1-9
11. Rai AK, Lee B, Hebbard C. et al. Decoding the complexity of circular RNAs in cardiovascular disease. Pharmacol Res. 2021;171:105766
12. Ding C, Zhou Y. Insights into circular RNAs: Biogenesis, function and their regulatory roles in cardiovascular disease. J Cell Mol Med. 2023;27:1299-314
13. Shu H, Zhang Z, Liu J. et al. Circular RNAs: An emerging precise weapon for diabetic nephropathy diagnosis and therapy. Biomed Pharmacother. 2023;168:115818
14. Zhong Y, Xia J, Liao L. et al. Non-coding RNAs and exosomal non-coding RNAs in diabetic retinopathy: A narrative review. Int J Biol Macromol. 2024;259:128182
15. Ning J, Luo Y, Chen L. et al. CircRNAs and lung cancer: Insight into their roles in metastasis. Biomed Pharmacother. 2023;166:115260
16. Zhang Y, Luo J, Yang W. et al. CircRNAs in colorectal cancer: potential biomarkers and therapeutic targets. Cell Death Dis. 2023;14:353
17. Zhang L, Zhang Y, Li X. et al. CircRNA-miRNA-VEGFA: an important pathway to regulate cancer pathogenesis. Front Pharmacol. 2023;14:1049742
18. Cheng J, Li G, Wang W. et al. Circular RNAs with protein-coding ability in oncogenesis. Biochim Biophys Acta Rev Cancer. 2023;1878:188909
19. Kolakofsky D. Isolation and characterization of Sendai virus DI-RNAs. Cell. 1976;8:547-55
20. Lyu L, Zhang S, Deng Y. et al. Regulatory mechanisms, functions, and clinical significance of CircRNAs in triple-negative breast cancer. J Hematol Oncol. 2021;14:41
21. Kristensen LS, Jakobsen T, Hager H. et al. The emerging roles of circRNAs in cancer and oncology. Nat Rev Clin Oncol. 2022;19:188-206
22. Zhai X, Zhang Y, Xin S. et al. Insights Into the Involvement of Circular RNAs in Autoimmune Diseases. Front Immunol. 2021;12:622316
23. Nielsen AF, Bindereif A, Bozzoni I. et al. Best practice standards for circular RNA research. Nat Methods. 2022;19:1208-20
24. Zheng S, Zhang X, Odame E. et al. CircRNA-Protein Interactions in Muscle Development and Diseases. Int J Mol Sci. 2021;22:3262
25. Li T, Wang WC, McAlister V. et al. Circular RNA in colorectal cancer. J Cell Mol Med. 2021;25:3667-79
26. Wang S, Dong Y, Gong A. et al. Exosomal circRNAs as novel cancer biomarkers: Challenges and opportunities. Int J Biol Sci. 2021;17:562-73
27. Liu R, Zhang L, Zhao X. et al. circRNA: Regulatory factors and potential therapeutic targets in inflammatory dermatoses. J Cell Mol Med. 2022;26:4389-400
28. Tang X, Ren H, Guo M. et al. Review on circular RNAs and new insights into their roles in cancer. Comput Struct Biotechnol J. 2021;19:910-28
29. Qu Z, Meng F, Shi J. et al. A Novel Intronic Circular RNA Antagonizes Influenza Virus by Absorbing a microRNA That Degrades CREBBP and Accelerating IFN-β Production. mBio. 2021;12:e0101721
30. He AT, Liu J, Li F. et al. Targeting circular RNAs as a therapeutic approach: current strategies and challenges. Signal Transduct Target Ther. 2021;6:185
31. Misir S, Wu N, Yang BB. Specific expression and functions of circular RNAs. Cell Death Differ. 2022;29:481-91
32. Zhang P, Dai M. CircRNA: a rising star in plant biology. J Genet Genomics. 2022;49:1081-92
33. Chen R, Wang SK, Belk JA. et al. Engineering circular RNA for enhanced protein production. Nat Biotechnol. 2023;41:262-72
34. Knupp D, Cooper DA, Saito Y. et al. NOVA2 regulates neural circRNA biogenesis. Nucleic Acids Res. 2021;49:6849-62
35. Wu C, Wang S, Cao T. et al. Newly discovered mechanisms that mediate tumorigenesis and tumour progression: circRNA-encoded proteins. J Cell Mol Med. 2023;27:1609-20
36. Vo JN, Cieslik M, Zhang Y. et al. The Landscape of Circular RNA in Cancer. Cell. 2019;176:869-81
37. Vidal AF. Read-through circular RNAs reveal the plasticity of RNA processing mechanisms in human cells. RNA Biol. 2020;17:1823-6
38. Caba L, Florea L, Gug C. et al. Circular RNA-Is the Circle Perfect. Biomolecules. 2021;11:1755
39. Jiao K, Walsh LJ, Ivanovski S. et al. The Emerging Regulatory Role of Circular RNAs in Periodontal Tissues and Cells. Int J Mol Sci. 2021;22:4636
40. Tian M, Cao Z, Pang H. Circular RNAs in Sudden Cardiac Death Related Diseases: Novel Biomarker for Clinical and Forensic Diagnosis. Molecules. 2021;26:1155
41. Chen Q, Wang H, Li Z. et al. Circular RNA ACTN4 promotes intrahepatic cholangiocarcinoma progression by recruiting YBX1 to initiate FZD7 transcription. J Hepatol. 2022;76:135-47
42. Chen Z, Lu T, Huang L. et al. Circular RNA cia-MAF drives self-renewal and metastasis of liver tumor-initiating cells via transcription factor MAFF. J Clin Invest. 2021;131:e148020
43. Zhang FF, Zhang L, Zhao L. et al. The circular RNA Rap1b promotes Hoxa5 transcription by recruiting Kat7 and leading to increased Fam3a expression, which inhibits neuronal apoptosis in acute ischemic stroke. Neural Regen Res. 2023;18:2237-45
44. He B, Chao W, Huang Z. et al. Hsa_circ_001659 serves as a novel diagnostic and prognostic biomarker for colorectal cancer. Biochem Biophys Res Commun. 2021;551:100-6
45. Zeng Y, Zou Y, Gao G. et al. The biogenesis, function and clinical significance of circular RNAs in breast cancer. Cancer Biol Med. 2021;19:14-29
46. Wang Z, Tan W, Li B. et al. Exosomal non-coding RNAs in angiogenesis: Functions, mechanisms and potential clinical applications. Heliyon. 2023;9:e18626
47. Xu X, Zhang J, Tian Y. et al. CircRNA inhibits DNA damage repair by interacting with host gene. Mol Cancer. 2020;19:128
48. Liu Y, Liu X, Lin C. et al. Noncoding RNAs regulate alternative splicing in Cancer. J Exp Clin Cancer Res. 2021;40:11
49. Li H, Peng K, Yang K. et al. Circular RNA cancer vaccines drive immunity in hard-to-treat malignancies. Theranostics. 2022;12:6422-36
50. Wang Y, Wu C, Du Y. et al. Expanding uncapped translation and emerging function of circular RNA in carcinomas and noncarcinomas. Mol Cancer. 2022;21:13
51. Li Y, Wang Z, Su P. et al. circ-EIF6 encodes EIF6-224aa to promote TNBC progression via stabilizing MYH9 and activating the Wnt/beta-catenin pathway. Mol Ther. 2022;30:415-30
52. Zhang Y, Jiang J, Zhang J. et al. CircDIDO1 inhibits gastric cancer progression by encoding a novel DIDO1-529aa protein and regulating PRDX2 protein stability. Mol Cancer. 2021;20:101
53. Wang L, Zhou J, Zhang C. et al. A novel tumour suppressor protein encoded by circMAPK14 inhibits progression and metastasis of colorectal cancer by competitively binding to MKK6. Clin Transl Med. 2021;11:e613
54. Peng Y, Xu Y, Zhang X. et al. A novel protein AXIN1-295aa encoded by circAXIN1 activates the Wnt/β-catenin signaling pathway to promote gastric cancer progression. Mol Cancer. 2021;20:158
55. Ghafouri-Fard S, Taheri M, Hussen BM. et al. Function of circular RNAs in the pathogenesis of colorectal cancer. Biomed Pharmacother. 2021;140:111721
56. Li Y, Chen B, Zhao J. et al. HNRNPL Circularizes ARHGAP35 to Produce an Oncogenic Protein. Adv Sci (Weinh). 2021;8:2001701
57. Duan JL, Chen W, Xie JJ. et al. A novel peptide encoded by N6-methyladenosine modified circMAP3K4 prevents apoptosis in hepatocellular carcinoma. Mol Cancer. 2022;21:93
58. Wang S, Sun Z, Lei Z. et al. RNA-binding proteins and cancer metastasis. Semin Cancer Biol. 2022;86:748-68
59. Qin H, Ni H, Liu Y. et al. RNA-binding proteins in tumor progression. J Hematol Oncol. 2020;13:90
60. Liu H, Lan T, Li H. et al. Circular RNA circDLC1 inhibits MMP1-mediated liver cancer progression via interaction with HuR. Theranostics. 2021;11:1396-411
61. Song C, Zhang Y, Huang W. et al. Circular RNA Cwc27 contributes to Alzheimer's disease pathogenesis by repressing Pur-α activity. Cell Death Differ. 2022;29:393-406
62. Jing L, Yang L, Jianbo C. et al. CircSETD2 inhibits YAP1 by interaction with HuR during breast cancer progression. Cancer Biol Ther. 2023;24:2246205
63. Ju H, Hu Z, Wei D. et al. A novel intronic circular RNA, circGNG7, inhibits head and neck squamous cell carcinoma progression by blocking the phosphorylation of heat shock protein 27 at Ser78 and Ser82. Cancer Commun (Lond). 2021;41:1152-72
64. Bruford EA, Braschi B, Denny P. et al. Guidelines for human gene nomenclature. Nat Genet. 2020;52:754-8
65. Zhang Z, Harrison PM, Liu Y. et al. Millions of years of evolution preserved: a comprehensive catalog of the processed pseudogenes in the human genome. Genome Res. 2003;13:2541-58
66. Liu J, Liu T, Wang X. et al. Circles reshaping the RNA world: from waste to treasure. Mol Cancer. 2017;16:58
67. Liu Y, Wang X, Bi L. et al. Identification of Differentially Expressed Circular RNAs as miRNA Sponges in Lung Adenocarcinoma. J Oncol. 2021;2021:5193913
68. Ye J, Li J, Zhao P. Roles of ncRNAs as ceRNAs in Gastric Cancer. Genes (Basel). 2021;12:1036
69. Mao M, Zhang J, Xiang Y. et al. Role of exosomal competitive endogenous RNA (ceRNA) in diagnosis and treatment of malignant tumors. Bioengineered. 2022;13:12156-68
70. Li J, Han Y, Wang S. et al. Circular RNAs: Biogenesis, Biological Functions, and Roles in Myocardial Infarction. Int J Mol Sci. 2023;24:4233
71. Wang L, Wu X, Ruan Y. et al. Exosome-transmitted hsa_circ_0012634 suppresses pancreatic ductal adenocarcinoma progression through regulating miR-147b/HIPK2 axis. Cancer Biol Ther. 2023;24:2218514
72. Feng Y, Wang X, Huang C. et al. Upregulated circTMEM59 Inhibits Cell Growth and Metastasis by miR-668-3p/ID4 Axis in Colorectal Cancer. Oxid Med Cell Longev. 2022;2022:7242124
73. Liu J, Li J, Su Y. et al. CircTMEM59 Serves as miR-410-3p Sponge to Inhibit the Proliferation and Metastasis of Colorectal Cancer by Regulating HOXD8. Biochem Genet. 2022;60:2399-415
74. Sun M, Liu X, Zhao W. et al. Circ_0058063 contributes to cisplatin-resistance of bladder cancer cells by upregulating B2M through acting as RNA sponges for miR-335-5p. BMC Cancer. 2022;22:313
75. Luo K, Liu A, Wu H. et al. CircKIF4A promotes glioma growth and temozolomide resistance by accelerating glycolysis. Cell Death Dis. 2022;13:740
76. Sun R, Zhou Y, Cai Y. et al. circ_0000045 promotes proliferation, migration, and invasion of head and neck squamous cell carcinomas via regulating HSP70 and MAPK pathway. BMC Cancer. 2022;22:799
77. Zhang DJ, Fu ZM, Guo YY. et al. Circ_0000052/miR-382-3p axis induces PD-L1 expression and regulates cell proliferation and immune evasion in head and neck squamous cell carcinoma. J Cell Mol Med. 2023;27:113-26
78. Zheng Y, Duan L, Yang Y. et al. Circ_0023028 contributes to the progression of laryngeal squamous cell carcinoma by upregulating LASP1 through miR-486-3p. Mol Cell Biochem. 2021;476:2951-61
79. Zhang S, Han J, Fu J. The circ_0032822 Promotes the Proliferation of Head and Neck Squamous Cell Carcinoma Cells Through miR-141/EF3 Signaling Axis. Front Oncol. 2021;11:662496
80. Yin X, Wang J, Shan C. et al. Circular RNA ZNF609 promotes laryngeal squamous cell carcinoma progression by upregulating epidermal growth factor receptor via sponging microRNA-134-5p. Bioengineered. 2022;13:6929-41
81. Verduci L, Ferraiuolo M, Sacconi A. et al. The oncogenic role of circPVT1 in head and neck squamous cell carcinoma is mediated through the mutant p53/YAP/TEAD transcription-competent complex. Genome Biol. 2017;18:237
82. Zhang D, Huang H, Sun Y. et al. CircHIPK2 promotes proliferation of nasopharyngeal carcinoma by down-regulating HIPK2. Transl Cancer Res. 2022;11:2348-58
83. Zang Y, Li J, Wan B. et al. circRNA circ-CCND1 promotes the proliferation of laryngeal squamous cell carcinoma through elevating CCND1 expression via interacting with HuR and miR-646. J Cell Mol Med. 2020;24:2423-33
84. Guo Y, Huang Q, Zheng J. et al. Diagnostic Role of Dysregulated Circular RNA hsa_circ_0036722 in Laryngeal Squamous Cell Carcinoma. Onco Targets Ther. 2020;13:5709-19
85. Peng QS, Cheng YN, Zhang WB. et al. circRNA_0000140 suppresses oral squamous cell carcinoma growth and metastasis by targeting miR-31 to inhibit Hippo signaling pathway. Cell Death Dis. 2020;11:112
86. Mo Y, Wang Y, Zhang S. et al. Circular RNA circRNF13 inhibits proliferation and metastasis of nasopharyngeal carcinoma via SUMO2. Mol Cancer. 2021;20:112
87. Liu J, Peng Y, Wei W. Cell cycle on the crossroad of tumorigenesis and cancer therapy. Trends Cell Biol. 2022;32:30-44
88. Montalto FI, De Amicis F. Cyclin D1 in Cancer: A Molecular Connection for Cell Cycle Control, Adhesion and Invasion in Tumor and Stroma. Cells. 2020;9:2648
89. Duan X, Shen N, Chen J. et al. Circular RNA MYLK serves as an oncogene to promote cancer progression via microRNA-195/cyclin D1 axis in laryngeal squamous cell carcinoma. Biosci Rep. 2019;39:BSR20190227
90. Yang Z, Jin J, Chang T. CircPTK2 (hsa_circ_0003221) Contributes to Laryngeal Squamous Cell Carcinoma by the miR-1278/YAP1 Axis. J Oncol. 2021;2021:2408384
91. Iser IC, Pereira MB, Lenz G. et al. The Epithelial-to-Mesenchymal Transition-Like Process in Glioblastoma: An Updated Systematic Review and In Silico Investigation. Med Res Rev. 2017;37:271-313
92. Ma C, Shi T, Qu Z. et al. CircRNA_ACAP2 Suppresses EMT in Head and Neck Squamous Cell Carcinoma by Targeting the miR-21-5p/STAT3 Signaling Axis. Front Oncol. 2020;10:583682
93. Pei S, Ma C, Chen J. et al. CircFOXM1 acts as a ceRNA to upregulate SMAD2 and promote the progression of nasopharyngeal carcinoma. Mol Genet Genomic Med. 2022;10:e1914
94. Liu Q, Shuai M, Xia Y. Knockdown of EBV-encoded circRNA circRPMS1 suppresses nasopharyngeal carcinoma cell proliferation and metastasis through sponging multiple miRNAs. Cancer Manag Res. 2019;11:8023-31
95. Zhang XP, Pei JP, Zhang CD. et al. Exosomal circRNAs: A key factor of tumor angiogenesis and therapeutic intervention. Biomed Pharmacother. 2022;156:113921
96. Meng D, Jia R, Yuan S. et al. Research progress on the circRNA-mediated regulation of tumor angiogenesis through ceRNA mechanisms (Review). Oncol Rep. 2023;49:12
97. Gong H, Wu W, Fang C. et al. CircBFAR correlates with poor prognosis and promotes laryngeal squamous cell cancer progression through miR-31-5p/COL5A1 axis. Laryngoscope Investig Otolaryngol. 2022;7:1951-62
98. Chen F, Zhang H, Wang J. Circular RNA CircSHKBP1 accelerates the proliferation, invasion, angiogenesis, and stem cell-like properties via modulation of microR-766-5p/high mobility group AT-hook 2 axis in laryngeal squamous cell carcinoma. Bioengineered. 2022;13:11551-63
99. Wang J, Lin Y, Jiang DH. et al. CircRNA ZNF609 promotes angiogenesis in nasopharyngeal carcinoma by regulating miR-145/STMN1 axis. Kaohsiung J Med Sci. 2021;37:686-98
100. Pauken KE, Torchia JA, Chaudhri A. et al. Emerging concepts in PD-1 checkpoint biology. Semin Immunol. 2021;52:101480
101. Xu Y, Song G, Xie S. et al. The roles of PD-1/PD-L1 in the prognosis and immunotherapy of prostate cancer. Mol Ther. 2021;29:1958-69
102. Ge J, Wang J, Xiong F. et al. Epstein-Barr Virus-Encoded Circular RNA CircBART2.2 Promotes Immune Escape of Nasopharyngeal Carcinoma by Regulating PD-L1. Cancer Res. 2021;81:5074-88
103. Yang H, Yu G, Wang Y. et al. Circ_0044520 regulates the progression of laryngeal squamous cell carcinoma via the miR-338-3p/ROR2 axis. Histol Histopathol. 2022;37:513-26
104. Zeng Q, Ji X, Li X. et al. Circ_0000285 regulates nasopharyngeal carcinoma progression through miR-1278/FNDC3B axis. Hum Exp Toxicol. 2023;42:9603271221141689
105. Wang Z, Huang C, Zhang A. et al. Overexpression of circRNA_100290 promotes the progression of laryngeal squamous cell carcinoma through the miR-136-5p/RAP2C axis. Biomed Pharmacother. 2020;125:109874
106. Zhang C, Yao Y, Bi L. Hsa_circ_0002162 has a critical role in malignant progression of tongue squamous cell carcinoma through targeting miR-33a-5p. Braz J Med Biol Res. 2021;54:e10093
107. Xu Y, Wan W. Acetylation in the regulation of autophagy. Autophagy. 2023;19:379-87
108. Berkamp S, Mostafavi S, Sachse C. Structure and function of p62/SQSTM1 in the emerging framework of phase separation. FEBS J. 2021;288:6927-41
109. Bai J, Geng B, Wang X. et al. Exercise Facilitates the M1-to-M2 Polarization of Microglia by Enhancing Autophagy via the BDNF/AKT/mTOR Pathway in Neuropathic Pain. Pain Physician. 2022;25:E1137-E1151
110. Wu S, Zhao K, Wang J. et al. Recent advances of tanshinone in regulating autophagy for medicinal research. Front Pharmacol. 2022;13:1059360
111. Wang Y, Mo Y, Peng M. et al. The influence of circular RNAs on autophagy and disease progression. Autophagy. 2022;18:240-53
112. Miller DR, Thorburn A. Autophagy and organelle homeostasis in cancer. Dev Cell. 2021;56:906-18
113. Gao L, Zhang Q, Li S. et al. Circ-PKD2 promotes Atg13-mediated autophagy by inhibiting miR-646 to increase the sensitivity of cisplatin in oral squamous cell carcinomas. Cell Death Dis. 2022;13:192
114. Gao W, Guo H, Niu M. et al. circPARD3 drives malignant progression and chemoresistance of laryngeal squamous cell carcinoma by inhibiting autophagy through the PRKCI-Akt-mTOR pathway. Mol Cancer. 2020;19:166
115. Cui C, Yang J, Li X. et al. Functions and mechanisms of circular RNAs in cancer radiotherapy and chemotherapy resistance. Mol Cancer. 2020;19:58
116. Lin H, Wang Y, Wang P. et al. Mutual regulation between N6-methyladenosine (m6A) modification and circular RNAs in cancer: impacts on therapeutic resistance. Mol Cancer. 2022;21:148
117. Wu P, Fang X, Liu Y. et al. N6-methyladenosine modification of circCUX1 confers radioresistance of hypopharyngeal squamous cell carcinoma through caspase1 pathway. Cell Death Dis. 2021;12:298
118. Chen G, Li Y, He Y. et al. Upregulation of Circular RNA circATRNL1 to Sensitize Oral Squamous Cell Carcinoma to Irradiation. Mol Ther Nucleic Acids. 2020;19:961-73
119. Lin J, Qin H, Han Y. et al. CircNRIP1 Modulates the miR-515-5p/IL-25 Axis to Control 5-Fu and Cisplatin Resistance in Nasopharyngeal Carcinoma. Drug Des Devel Ther. 2021;15:323-30
120. Hong X, Liu N, Liang Y. et al. Circular RNA CRIM1 functions as a ceRNA to promote nasopharyngeal carcinoma metastasis and docetaxel chemoresistance through upregulating FOXQ1. Mol Cancer. 2020;19:33
121. He B, Liang J, Qin Q. et al. IL-13/IL-13RA2 signaling promotes colorectal cancer stem cell tumorigenesis by inducing ubiquitinated degradation of p53. Genes Dis. 2024;11:495-508
122. Jia L, Wang Y, Wang CY. circFAT1 Promotes Cancer Stemness and Immune Evasion by Promoting STAT3 Activation. Adv Sci (Weinh). 2021;8:2003376
123. Zhang Y, Tian K, Zhou E. et al. hsa_circ_0023305 Enhances Laryngeal Squamous Cell Carcinoma Progression and Modulates TRPM7 via miR-218-5p Sponging. Biomed Res Int. 2021;2021:9968499
124. Wang J, Kong J, Nie Z. et al. Circular RNA Hsa_circ_0066755 as an Oncogene via sponging miR-651 and as a Promising Diagnostic Biomarker for Nasopharyngeal Carcinoma. Int J Med Sci. 2020;17:1499-507
125. Qiongna D, Jiafeng Z, Yalin H. et al. Implication of hsa_circ_0028007 in reinforcing migration, invasion, and chemo-tolerance of nasopharyngeal carcinoma cells. J Clin Lab Anal. 2020;34:e23409
126. Zhang H, Shen Y, Zhang B. et al. Hsa_circ_0003829 serves as a potential diagnostic predictor for oral squamous cell carcinoma. J Int Med Res. 2020;48:300060520936880
127. Wang X, Wu T, Wang P. et al. Circular RNA 103862 Promotes Proliferation and Invasion of Laryngeal Squamous Cell Carcinoma Cells Through the miR-493-5p/GOLM1 Axis. Front Oncol. 2020;10:1064
128. Han J, Lin Q, Dong C. Plasma cell-free circRNAs panel act as fingerprint predicts the occurrence of laryngeal squamous cell carcinoma. Aging (Albany NY). 2021;13:17328-36
129. Guo Y, Huang Q, Zheng J. et al. Diagnostic Significance of Downregulated circMORC3 as a Molecular Biomarker of Hypopharyngeal Squamous Cell Carcinoma: A Pilot Study. Cancer Manag Res. 2020;12:43-9
130. Wu Z, He X, Chen S. Oncogenic circDHTKD1 promotes tumor growth and metastasis of oral squamous cell carcinoma in vitro and in vivo via upregulating miR-326-mediated GAB1. Braz J Med Biol Res. 2021;54:e10837
131. Long Y, Li C, Zhu B. Circ_0008068 facilitates the oral squamous cell carcinoma development by microRNA-153-3p/acylgycerol kinase (AGK) axis. Bioengineered. 2022;13:13055-69
132. Wei Z, Chang K, Fan C. Hsa_circ_0042666 inhibits proliferation and invasion via regulating miR-223/TGFBR3 axis in laryngeal squamous cell carcinoma. Biomed Pharmacother. 2019;119:109365
133. Wang JX, Liu Y, Jia XJ. et al. Upregulation of circFLNA contributes to laryngeal squamous cell carcinoma migration by circFLNA-miR-486-3p-FLNA axis. Cancer Cell Int. 2019;19:196
134. Chen X, Xu W, Ma Z. et al. Circ_0000215 Exerts Oncogenic Function in Nasopharyngeal Carcinoma by Targeting miR-512-5p. Front Cell Dev Biol. 2021;9:688873
135. Zhong Q, Huang J, Wei J. et al. Circular RNA CDR1as sponges miR-7-5p to enhance E2F3 stability and promote the growth of nasopharyngeal carcinoma. Cancer Cell Int. 2019;19:252
136. Yin L, Chen J, Ma C. et al. Hsa_circ_0046263 functions as a ceRNA to promote nasopharyngeal carcinoma progression by upregulating IGFBP3. Cell Death Dis. 2020;11:562
137. Sun Y, Liu Y, Du Z. et al. CircSOX9 acts as a molecular sponge of miR-485-3p to promote the progression of nasopharyngeal carcinoma. Aging (Albany NY). 2022;14:4914-26
138. Wang Z, Sun A, Yan A. et al. Circular RNA MTCL1 promotes advanced laryngeal squamous cell carcinoma progression by inhibiting C1QBP ubiquitin degradation and mediating beta-catenin activation. Mol Cancer. 2022;21:92
Author contact
![]() Corresponding authors: Yongyan Wu, Ph.D., Professor. Email: wuyongyanorg, ORCID: 0000-0003-1669-3860. Wei Gao, M.D., Professor. Email: gaoweisxentorg, ORCID: 0000-0001-7836-2851. Shuxin Wen, M.D., Professor. Email: wsxsxorg, ORCID: 0000-0002-8377-2481.
Corresponding authors: Yongyan Wu, Ph.D., Professor. Email: wuyongyanorg, ORCID: 0000-0003-1669-3860. Wei Gao, M.D., Professor. Email: gaoweisxentorg, ORCID: 0000-0001-7836-2851. Shuxin Wen, M.D., Professor. Email: wsxsxorg, ORCID: 0000-0002-8377-2481.

 Global reach, higher impact
Global reach, higher impact