3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(16):5367-5375. doi:10.7150/jca.98409 This issue Cite
Review
Research progress of epithelial-mesenchymal transformation-related transcription factors in peritoneal metastases
1. Institute of Biology and Medical Sciences, Soochow University, Suzhou 215123, China.
2. Suzhou Medical College of Soochow University, Soochow University, Suzhou 215123, China.
3. State Key Laboratory of Radiation Medicine and Protection, School of Radiation Medicine and Protection & School for Radiological and Interdisciplinary Sciences (RAD-X), Collaborative Innovation Center of Radiation Medicine of Jiangsu Higher Education Institutions, Soochow University, Suzhou 215123, Jiangsu, China.
4. Department of Pathology, The First Affiliated Hospital of Soochow University, Suzhou, 215000, China.
*These authors contributed equally to this work.
Received 2024-5-14; Accepted 2024-8-9; Published 2024-8-19
Abstract
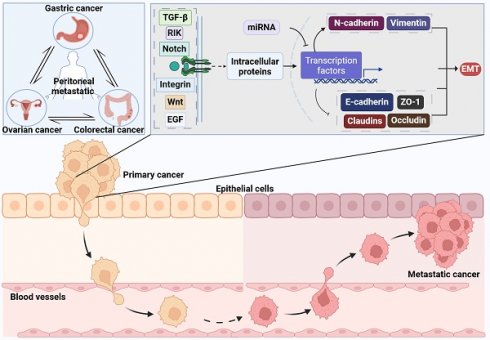
Metastasis is the leading cause of mortality in patients with malignant tumors, particularly characterized by peritoneal metastases originating from gastric, ovarian, and colorectal cancers. Regarded as the terminal phase of tumor progression, peritoneal metastasis presents limited therapeutic avenues and is associated with a dismal prognosis for patients. The epithelial-mesenchymal transition (EMT) is a crucial phenomenon in which epithelial cells undergo significant changes in both morphology and functionality, transitioning to a mesenchymal-like phenotype. This transition plays a pivotal role in facilitating tumor metastasis, with transcription factors being key mediators of EMT's effects. Consequently, we provide a retrospective summary of the efforts to identify specific targets among EMT-related transcription factors, aimed at modulating the onset and progression of peritoneal metastatic cancer. This summary offers vital theoretical underpinnings for developing treatment strategies against peritoneal metastasis.
Keywords: Peritoneal metastasis, Epithelial-mesenchymal Transition, Transcription factor
Introduction
Gastric cancer (GC) is a malignancy of epithelial origin that develops within the stomach and stands as one of the most prevalent cancers in China. Peritoneal metastasis emerges as a frequent occurrence in GC, serving as a common site for both metastatic spread and disease recurrence [1, 2], with a median survival of 3 to 6 months following peritoneal metastasis [3]. Ovarian cancer (OC) stands as the most fatal malignancy affecting the female reproductive system, characterized by a startling 70% of patients presenting with extensive intra-abdominal dissemination upon their initial diagnosis. This spread complicates surgical removal and leads to recurrent relapses, significantly impacting patient survival outcomes [4, 5]. Global cancer statistics by Siegel et al. indicates a 5-year survival rate of less than 20% for these patients [5]. Colorectal cancer (CRC) stands as a prevalent malignancy within the digestive tract. Both international and national epidemiological statistics presented by Siegel et al. and the team of Academician He Jie indicate that CRC has a high incidence and mortality rate [1, 6]. The incidence of peritoneal metastasis in CRC ranges from 4% to 19% [7-9], with a median survival of approximately 6 months [10, 11]. These findings highlight the poor prognosis associated with common peritoneal metastatic cancers.
Epithelial-mesenchymal transition (EMT) includes three distinct types: Type I EMT, originally recognized as a crucial mechanism in early embryonic morphogenesis, is involved in critical developmental phases such as gastrulation, the formation of the neural crest, and cardiac morphogenesis. Type II EMT primarily functions in inflammatory responses, including wound healing, tissue regeneration, and fibrosis. Type III EMT, critical for cancer metastasis, involves changes in tumor cell heterogeneity and the local microenvironment, leading some epithelial tumor cells at the primary site to transition toward a mesenchymal phenotype [12-14]. Through this transition, these cells gain enhanced invasive and migratory capabilities, breaking free from the primary site and entering the circulatory system to initiate the early stages of malignant metastasis [15]. Current research on EMT primarily focuses on its role in cancer metastasis. However, studies on EMT in peritoneal metastatic cancer are sparse, considering its low incidence yet high mortality. This review aims to discuss the advances in research on EMT-related transcription factors involved in the molecular regulatory mechanisms and interventions at multiple steps of peritoneal metastatic cancer.
Epithelial-mesenchymal transition (EMT)
The primary pathways of peritoneal metastatic cancer include: 1) Downregulation of different adhesion molecules on the surface of tumor cells, resulting in the detachment of tumor cells; 2) Detached cancer cells acquire more mesenchymal characteristics, enhancing their invasiveness and resistance to apoptosis; 3) Detached cancer cells invade the peritoneum via the mesothelial/lymphatic pathways; 4) Cancer cells invading the peritoneum predominantly aggregate in milky spots; 5) Peritoneal-implanted cancer cells proliferate extensively [16, 17]. In peritoneal metastatic cancer, epithelial-mesenchymal transition (EMT) primarily functions through transcriptional regulation, which is driven by EMT itself.
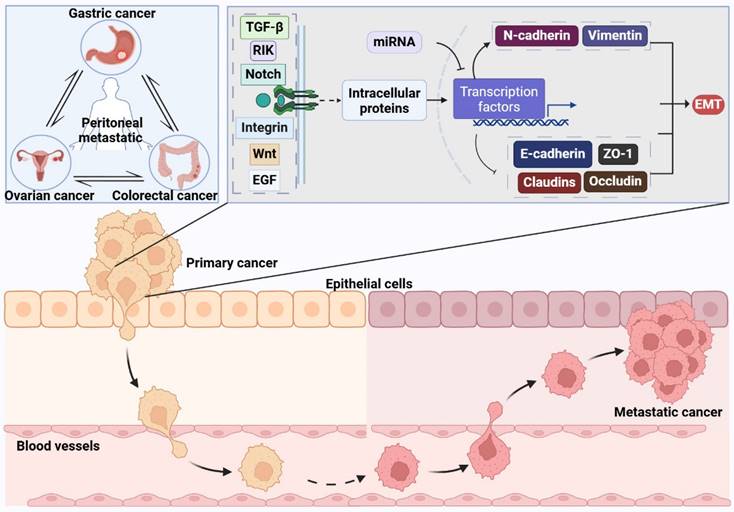
Transcription factors, a set of proteins, specifically bind to sequences located upstream of a gene's 5' region. This binding ensures that the target gene is expressed with particular intensity, timing, and spatial distribution [18]. EMT-related transcription factors primarily include the Snail family, Twist family, and zinc-finger E-box binding (ZEB) transcription factor family. Other families involved are the Kruppel-like factor family, forkhead box family, SRY-related high-mobility group, RUNX family, and GATA family. This review will sequentially discuss the role of these EMT-related transcription factors in peritoneal metastatic cancer.
Snail Family Transcription Factors
The Snail family genes encode transcription factors with zinc finger structures and are widely regarded as typical EMT-related transcription factors due to their involvement in regulating multiple physiological levels through binding to downstream genes. The Snail family primarily includes two members: Snail1 (commonly referred to as Snail) and Snail2 (also known as Slug) [19]. All members of this family encode transcriptional repressors that possess a highly conserved C-terminal domain, containing 4-6 C2H2-type zinc fingers, which bind to the E-box motifs (5'-CANNTG-3') of target gene promoters [20].
Current perspectives suggest that fibrin deposits on the peritoneal surface serve as a breeding ground for cancer dissemination in cancer patients [21]; significant upregulation of Snail is observed in both gastric cancer and ovarian cancer peritoneal metastases [22, 23]. Mechanistically, the MUC4 protein promotes peritoneal metastasis by upregulating Snail [24]; miR-22 induces extracellular matrix (ECM) remodeling and EMT by upregulating MMP14 and Snail, thereby facilitating peritoneal and lung metastasis in gastric cancer [25]. β-hCG and estrogen-related receptor alpha (ERRα) mediate ovarian cancer peritoneal metastasis through EMT and Snail [26, 27]. CST1 Promotes the EMT Process in Gastric Cancer by Upregulating Snail [28]. At the same time, strategies aimed at targeting Snail in peritoneal metastatic cancer reveal that melatonin suppresses EMT progression, diminishes Snail expression, and enhances endoplasmic reticulum (ER) stress, thereby hindering gastric cancer peritoneal metastasis [29]. The novel aryl hydrocarbon receptor inhibitor, Biseugenol, activates Calpain-10 and inhibits Aryl hydrocarbon receptor, thus inducing ER stress and obstructing gastric cancer peritoneal metastasis [30]. An ethyl acetate extract from daylily (Hemerocallis fulva), COE, inhibits gastric cancer peritoneal metastasis by suppressing the HSP27-mediated NF-κB/Snail signaling pathway [31].
Research on Slug's role in peritoneal metastatic cancer shows that ARL4C promotes gastric cancer peritoneal metastasis by activating Slug [32]; CEACAM6 enhances EMT and Slug expression via the PI3K/AKT signaling pathway to promote gastric cancer peritoneal metastasis [33]; and miR-203 targets the ERK1/2/Slug/E-cadherin signaling pathway to inhibit gastric cancer peritoneal metastasis [34].
These investigations emphasize the crucial role of the Snail family in facilitating peritoneal metastasis, especially in gastric and ovarian cancers. Research focused on targeting the Snail family for therapeutic interventions in peritoneal metastasis is making steady progress.
The Mechanisms of Epithelial-Mesenchymal Transition. The process of inducing EMT involves a variety of extracellular signaling molecules, intracellular proteins, and the miRNA group, including Transforming Growth Factor-beta (TGF-β), Wnt, Epidermal Growth Factor (EGF), integrins, Notch, and the associated kinase RIK. These components trigger a signaling cascade upon binding to specific receptors situated on the cell membrane. Crucial steps in this signaling pathway encompass the activation of diverse downstream proteins, which subsequently modulate the activity of particular transcription factors. Upon activation, transcription factors such as Snail, Slug, Twist, and members of the ZEB family prompt the reduction in cell surface adhesion molecules like E-cadherin, ZO-1, claudins, and occludin. This change leads to the disruption of tight intercellular junctions and diminished cell adhesion. Concurrently, the transcription factors also stimulate the elevation of mesenchymal markers such as N-cadherin and vimentin, thereby facilitating the acquisition of mesenchymal traits by the cells.
Twist Family Transcription Factors
Twist, a highly conserved member of the basic helix-loop-helix (bHLH) transcription factor family, is pivotal in embryonic development and tissue morphogenesis. Furthermore, Twist aids in the advancement of malignant tumors primarily by transcriptionally regulating and facilitating the epithelial-mesenchymal transition (EMT), which promotes cancer progression [35]. Twist is a highly conserved protein with 96% amino acid sequence homology between mice and humans, and its DNA binding domain is 100% conserved across different species, containing the E-box binding DNA sequence: 5'-CANNTG-3' [36, 37].
Current research on Twist in peritoneal metastatic cancer indicates that Twist promotes peritoneal metastasis in ovarian cancer [38]. Mechanistically, asparagine endopeptidase (AEP) upregulates Twist to promote peritoneal metastasis in gastric cancer [39]; EG-1 upregulates eIF4E-mediated MMP-9 and Twist to facilitate gastric cancer metastasis [40]; TrkB promotes EMT and Twist-enhanced peritoneal metastasis and apoptosis resistance in ovarian cancer [41]. Additionally, interventions targeting Twist in peritoneal metastatic cancer have been studied by Huang et al., who demonstrated that dextran sulfate (DS) inhibits hypoxia-inducible factor-1α (HIF-1α) expression, suppresses TGF-β-mediated EMT in gastric cancer cells, and thus inhibits gastric cancer peritoneal metastasis [42].
In summary, Twist significantly contributes to peritoneal metastasis in tumors, with research primarily centered on gastric and ovarian cancers. Efforts to target Twist for inhibiting tumor peritoneal metastasis are progressing, underscoring its potential as a therapeutic target in these aggressive forms of cancer.
Zinc Finger E-box-Binding (ZEB) Transcription Factors
The zinc finger E-box binding protein (ZEB) family is instrumental in the development and progression of cancers. This family comprises two primary members: ZEB1 (also known as TCF-8 or δEF1) and ZEB2 (also known as SIP1) [43]. Both genes belong to the C2H2 type zinc finger protein family, characterized by a central homology domain along with four N-terminal zinc fingers and three C-terminal zinc fingers [44]. Research indicates that both ZEB1 and ZEB2 bind to the E-box consensus sequence 5'-CANNTG-3' on the CDH1 promoter. They exert suppression of CDH1 expression by recruiting repressive protein complexes such as C-terminal binding proteins (CtBP), Polycomb proteins, CoREST, and the SWI/SNF chromatin remodeling complex BRG1[45].
Research on ZEB1 in peritoneal metastatic cancer indicates that patients with high expression of ZEB1 in gastric cancer peritoneal lavage fluids have poor prognosis [46]. Gastric cancer tissues with high ZEB1 expression also show an increased risk of peritoneal metastasis [47]. A gastric cancer stem cell peritoneal metastasis model demonstrated upregulation of ZEB1 through immunofluorescence [22]. Molecular investigations have revealed that subtypes of TGF-β (TGF-β1, TGF-β2, and TGF-β3) upregulate ZEB1 expression, promoting ovarian cancer peritoneal metastasis [48]. Tissue transglutaminase (TG2) activates NF-κB, which in turn activates ZEB1 to promote ovarian cancer peritoneal metastasis [49]. miR-34b-5p Inhibits Peritoneal Metastasis of Endometrial Cancer by Targeting ZEB1 [50]. Targeted studies on ZEB1 have shown that FOXM1 and EGFR/ERBB2 can upregulate ZEB1, promoting ovarian cancer peritoneal metastasis, while combined treatment with lapatinib (a dual kinase inhibitor of EGFR/ERBB1 and ERBB2) and thiostrepton (an inhibitor of FOXM1) can reverse this mechanism and inhibit ovarian cancer peritoneal metastasis [51].
Research on ZEB2 in peritoneal metastatic cancer shows that ZEB2 promotes peritoneal metastasis of high-grade serous ovarian cancer by regulating cancer stem-like cells [52]. Additionally, the interaction of gastric cancer cells with nearby cancer-associated fibroblasts (CAFs) leads to the production of IL-33. This cytokine, via ST2L-dependent activation of the ERK1/2-SP1-ZEB2 pathway, triggers epithelial-mesenchymal transition (EMT), subsequently increasing the migratory and invasive properties of the gastric cancer cells [53].
These findings demonstrate the ZEB family's role in promoting peritoneal metastatic cancer, with high expression of ZEB1 in ascites associated with poor prognosis. The focus of related research has predominantly been on gastric and ovarian cancers' peritoneal metastasis.
Other EMT-related transcription factors
In addition to the established EMT-related transcription factors, recent research has identified an increasing number of other transcription factors that play significant roles in the EMT process. This section will focus on these other EMT-related transcription factors that facilitate the progression of peritoneal metastatic cancer.
Transcription Factors and Signaling Pathways in the Process of EMT. Throughout the process of epithelial-mesenchymal transition (EMT), regulation encompasses a diverse array of transcription factors and signaling pathways. The principal transcription factors encompass the Snail family, Twist family, and the zinc-finger E-box binding (ZEB) transcription factor family. Furthermore, this regulatory process involves the Kruppel-like factor (KLF) family, Forkhead box (Fox) family, SRY-related HMG-box (SOX) family, RUNX family, and GATA family. During EMT, a multitude of extracellular and intracellular proteins activate various intracellular signaling pathways, thereby modulating the expression of these transcription factors and facilitating the progression of EMT.
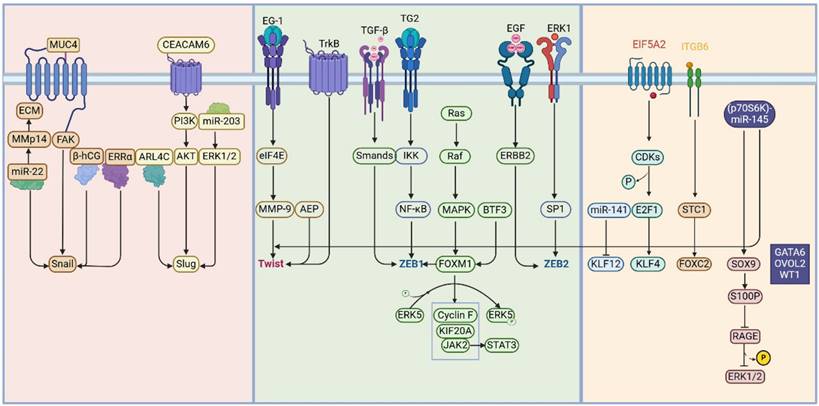
Mechanisms of action of translational factors in peritoneal metastasis
| Transcription Factor | Target gene function | Interaction signal pathways | Ref | |
|---|---|---|---|---|
| Upregulation | Downregulation | |||
| Snail | N-cadherin; Vimentin ERK1/2; Akt; MMP9; MMP15; MMP2; MMP14; TWIST; ZEB1; ZEB2 | E-cadherin; Claudins; Occludin | FAK; TGFβ/SMAD3; Wnt/β-catenin; Notch; PI3K/AKT; NF-Κb; ATK/MAPK; EGF; FGF; RTKs | [21-31] |
| Slug | N-cadherin; Vimentin; MMP9 | E-cadherin | ERK1/2/Slug/E-cadherin; PI3K/AKT | [32-34] |
| Twist | N-cadherin; MMPs; Vimentin; β-catenin | E-cadherin; E-cadherin; Claudins; Occludin; ERK1/2; Akt; MMP9 | FAK; MAPK; PI3K/AKT; Wnt/β-catenin | [35-42] |
| ZEB1 | N-cadherin; MMPs; Vimentin | E-cadherin; ZO1 Claudins; Occludin | TGFβ/SMAD3, Wnt/β-catenin; RAS/MAPK | [46-51] |
| ZEB2 | N-cadherin; Vimentin | E-cadherin | ERK1/2/SP1/ZEB2 | [52, 53] |
| KLF12 | ISG15; miR-137 | E-cadherin | Wnt/β-catenin | [57-61] |
| FOXC2 | Vimentin; N-cadherin | E-cadherin | TGFβ/SMAD3; MAPK/AKT | |
| FOXF1 | Fibronectin; N-cadherin; Snail; Vimentin | E-cadherin; Claudin1; Occludin; ZO1 | Unknown | [63] |
| FOXM1 | N-cadherin; ZEB2 KLF20A; GFR; ERBB2 | E-cadherin; ZRB1 | JAK2/pSTAT3 | [64] |
| SOX9 | S100p; SNAIL2; N-cadherin | Unknown | BMPs; PKA; PI3K/AKT; RAGE/ERK | [69, 70] |
| GATA6 | N-cadherin; MMP1 | Fibronectin; N-cadherin; SNAI1 | Wnt/β-catenin | [72, 73] |
| OVOL2 | Vimentin; α-SMA | E-cadherin | Wnt/β-catenin | [22] |
| WT1 | Unknown | Unknown | Wnt/β-catenin | [74-76] |
| SMAD3 | ANGPTL4 | Unknown | TGF-β1/SMAD3/ANGPTL4 | [77] |
The Kruppel-like factor (KLF) family consists of transcription regulators with C2H2 zinc finger structures and includes 17 members, named KLF1 through KLF17 [54]. Studies have shown that EIF5A2 promotes cancer cell stemness and peritoneal metastasis through the E2F1/KLF4 pathway [55]. Multi-omic analyses of ascites from gastric cancer patients with peritoneal metastasis revealed active super-enhancers at the ELF3, KLF5, and EHF gene loci [56]. Interestingly, existing research on KLF12 has demonstrated its role in promoting the malignant progression of both pancreatic and ovarian cancers [57-60], yet a study by Celia S. L. and colleagues revealed that high expression of miR-141 in ovarian cancer inhibits KLF12 [61], thus promoting peritoneal metastasis, indicating that the role of KLF12 requires further exploration.
The forkhead box (FOX) transcription factors are characterized by a highly conserved winged helix DNA-binding domain and consist of 50 human genes [62]. Members of the FOX family associated with EMT include FOXA1, FOXA2, FOXC1, FOXC2, FOXD2, FOXF1, FOXF2, FOXG1, FOXK1, FOXM1, FOXN2, FOXO3A, FOXQ1, FOSL1, and FOSL2. STC1 promotes ovarian cancer peritoneal metastasis via the FOXC2/ITGB6 signaling axis [63]; FOXF1 promotes peritoneal metastasis of colorectal cancer through transcriptional activation of SNAI [64]; FOXM1 regulates ZEB1 to enhance ovarian cancer peritoneal metastasis [51]; Additionally, exosomes from omental adipose tissue-derived mesenchymal stem cells have been shown to exacerbate ovarian cancer peritoneal metastasis through FOXM1 and associated Cyclin F, KIF20A, and MAPK signaling pathways, providing new insights into therapeutic interventions [65]. BTF3 induces gastric cancer peritoneal metastasis by modulating FOXM1 and the JAK2/STAT3 signaling pathway [66].
The SRY-related HMG Box (SOX) proteins are a subgroup within the high mobility group (HMG) proteins. Research indicates that the SOX family genes play significant roles in the proliferation, migration, invasion, and metastasis of various cancer cells [67]. The SOX family includes numerous transcriptional regulators that mediate DNA binding through the HMG domain. This domain comprises approximately 79 amino acid residues and features six core sequences, WWCAAW (W = A/T) [67, 68]. Current studies have identified SOX4, SOX9, and SOX11 as transcription factors related to epithelial-mesenchymal transition (EMT), with a particular focus on SOX9 in the context of peritoneal metastasis-related cancers. SOX9 transcriptionally activates S100P, which suppresses the RAGE/ERK signaling pathway and promotes EMT, thus aiding the peritoneal spread of colorectal cancer [69]. Additionally, the p70S6 kinase (p70S6K)-miR-145 pathway promotes peritoneal metastasis in ovarian cancer by targeting Twist and Sox9 [70].
The GATA family of transcription factors is a group of evolutionarily conserved zinc finger proteins that play multifaceted roles in cell differentiation and organ development during the early stages across various tissues [71] This family primarily consists of six transcription factors, GATA1 through GATA6, with initial functional studies focusing on hematopoiesis and cardiac development [72]. However, their functions and expression patterns extend well beyond these tissues. Recent research has discovered that GATA6-AS1 inhibits the epithelial-to-mesenchymal transition (EMT) of pancreatic cancer under hypoxic conditions by regulating the stability of SNAI1 mRNA [73].
Diagnostic and Therapeutic Value of Transcription Factors
| Transcription Factor | Therapeutic value | Diagnostic value |
|---|---|---|
| Snail Famliy | Ovarian cancer [23] Gastric cancer [22] Lung cancer [25] | Gastric cancer [29, 31] |
| Twist Famliy | Ovarian cancer [41] Gastric cancer [39, 40] | Gastric cancer [42] |
| ZEB Famliy | Gastric cancer [22, 47] Ovarian cancer [48, 49] Endometrial cancer [50] | Ovarian cancer [51] |
| KLF Famliy | Gastric cancer [56, 57] Pancreatic cancer [58] Ovarian cancer [55] | Ovarian cancer [61] |
| FOX Famliy | Ovarian cancer [63, 65] Colorectal cancer [64] Gastric cancer [66] | Gastric cancer [65] |
| SOX Famliy | Colorectal cancer [69] Ovarian cancer | / |
| GATA Famliy | Pancreatic cancer [73] | / |
| OVOL2 | Gastric cancer [22] | / |
| WT1 | Ovarian serous carcinoma [76] | / |
| SMAD3 | Colorectal cancer [77] | / |
Additional transcription factors associated with epithelial-mesenchymal transition (EMT) have been implicated in promoting peritoneal metastasis in cancer. Notably, OVOL2 is significantly overexpressed in a peritoneal metastasis tumor model constructed with gastric cancer stem cells [22]. Similarly, WT1 is highly expressed in the peritoneal metastasis model of ovarian low-grade serous carcinoma [74, 75], and shows a sensitivity of 93% in diagnosing metastatic ovarian cancer [76]; SMAD3, as a key factor in the TGF-β signaling pathway, plays an important role in the peritoneal metastasis of colorectal cancer [77].
In summary, other transcription factors related to epithelial-mesenchymal transition (EMT) primarily function to promote the occurrence and progression of peritoneal metastatic cancer. In the future, research on EMT-related transcription factors in peritoneal cancer will not be limited to those already identified but will also include investigations into the roles and molecular mechanisms of emerging EMT-related transcription factors in peritoneal metastasis.
Conclusion
Peritoneal metastasis is among the prognostically poorer groups in cancer patients. Taking gastric cancer peritoneal metastasis as a typical example, its early stages are predominantly characterized by metastasis, lacking effective means for early detection. As a result, a majority of patients receive diagnoses at advanced stages. After onset, peritoneal metastasis in gastric cancer progresses swiftly, drastically diminishing patient survival rates. The underlying mechanisms involve the gastric cancer cells' ability to remodel the extracellular matrix, induce the transformation of normal peritoneal cells into a tumor phenotype, induce angiogenesis around peritoneal colonization sites, regulate the immune function of the tumor microenvironment, and alter the epithelial and mesenchymal phenotypes of tumor cells. Consequently, identifying molecular targets and corresponding interventions for peritoneal metastasis remains a focus for clinical researchers.
Epithelial-mesenchymal transition (EMT) reveals that tumor-related epithelial cells undergo a series of changes and transformations to convert into mesenchymal phenotype tumor cells, depending on the tissue and signaling environment. Meanwhile, the process of mesenchymal-epithelial transition (MET), although not as evident as EMT, demonstrates the reversibility of these transformations. This review retrospectively summarizes the roles and molecular mechanisms of EMT-related transcription factors in peritoneal metastatic cancer. It is noteworthy that most studies on EMT-related transcription factors are conducted in a cell/tissue-type specific manner, and we still lack sufficient information to understand whether their functions and molecular mechanisms are consistent across different cell/tissue types or even in different environments. Therefore, further exploration into the similarities and differences in the roles of EMT-related transcription factors across various cells, tissues, and environments is essential to better elucidate their specific functions and mechanisms.
Additionally, future research should focus more on the roles of other known transcription factors in peritoneal metastasis and explore the roles of emerging EMT-related transcription factors in this context. Finally, the function of EMT is not entirely mediated by transcription factors; it also includes EMT-mediated signal transduction and EMT-related non-coding RNAs. Among these, the Transforming Growth Factor β (TGF-β) is one of the most extensively studied signaling pathways, regulating the initiation and progression of EMT through various mechanisms. Additionally, receptor tyrosine kinases (RTK), Wnt, Notch, Hedgehog, Hippo, and other signaling pathways also play critical roles in EMT regulation [78]. The interconnections among these signaling pathways collectively regulate the complex process of EMT, thereby affecting the metastatic and invasive abilities of tumor cells [79].
Research indicates that non-coding RNAs, such as the miR-200 family and the miR-34 family, exhibit significant tumor metastasis inhibition functions by regulating transcription factors like ZEB and Snail [78]. Moreover, inflammatory mediators in the tumor microenvironment, such as cytokines (e.g., IL-1, IL-6, IL-8, and TNF-α) and chemokines (including CCL5, SDF-1, CCL2, and CCL7), also promote the EMT process [80]. They regulate the metastatic and invasive abilities of cancer cells by binding to specific membrane receptors and activating internal signaling pathways [81].
Therefore, novel therapeutic strategies targeting these signaling pathways, non-coding RNAs, and inflammatory mediators have significant clinical prospects. They can effectively hinder the progression of metastatic cancer, reduce cancer recurrence, and prevent the development of treatment resistance [82]. For example, inhibitors targeting TGF-β receptor kinases combined with cytotoxic drugs such as paclitaxel can not only effectively inhibit the EMT process in breast cancer cells but also reduce their metastatic potential in the lungs [78]. Additionally, targeting inflammatory mediators with natural anti-inflammatory compounds and promoting the use of lipid-soluble drugs to block tumor metastasis is another potential strategy [83].
This is also the work our team plans to undertake next. By comprehensively summarizing the current functions and mechanisms of EMT in peritoneal metastasis can we better explore the potential of MET and provide guiding suggestions for identifying potential targets to reverse peritoneal metastasis.
Acknowledgements
The figures were created with BioRender.
Funding
This work was supported by Suzhou Basic Research Key Project (No. SKY2023009), Suzhou health youth backbone talent “national tutorial system” training project (Qngg2023005), the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and Beijing Xisike Clinical Oncology Research Foundation (Y-tongshu2021/qn-0366).
Author contributions
YHW and ML designed and conducted this review. YHW critically revised the final version of the manuscript, LW and YHW conceived and drafted this manuscript. LW and LH drew the figures. XBP and CM prepared the tables. All the authors approved the final version of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17-48
2. Bonnot PE, Piessen G, Kepenekian V, Decullier E, Pocard M, Meunier B. et al. Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer With Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analysis. J Clin Oncol. 2019;37:2028-40
3. Reutovich MY, Krasko OV, Sukonko OG. Hyperthermic intraperitoneal chemotherapy in serosa-invasive gastric cancer patients. Eur J Surg Oncol. 2019;45:2405-11
4. Mei S, Chen X, Wang K, Chen Y. Tumor microenvironment in ovarian cancer peritoneal metastasis. Cancer Cell Int. 2023;23:11
5. Pascual-Anton L, Cardenes B, Sainz de la Cuesta R, Gonzalez-Cortijo L, Lopez-Cabrera M, Cabanas C. et al. Mesothelial-to-Mesenchymal Transition and Exosomes in Peritoneal Metastasis of Ovarian Cancer. Int J Mol Sci. 2021;22:11496
6. Zheng RS, Zhang SW, Sun KX, Chen R, Wang SM, Li L. et al. [Cancer statistics in China, 2016]. Zhonghua Zhong Liu Za Zhi. 2023;45:212-20
7. Segelman J, Granath F, Holm T, Machado M, Mahteme H, Martling A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2012;99:699-705
8. van Gestel YR, Thomassen I, Lemmens VE, Pruijt JF, van Herk-Sukel MP, Rutten HJ. et al. Metachronous peritoneal carcinomatosis after curative treatment of colorectal cancer. Eur J Surg Oncol. 2014;40:963-9
9. Koppe MJ, Boerman OC, Oyen WJ, Bleichrodt RP. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg. 2006;243:212-22
10. Roth L, Russo L, Ulugoel S, Freire Dos Santos R, Breuer E, Gupta A. et al. Peritoneal Metastasis: Current Status and Treatment Options. Cancers (Basel). 2021;14:60
11. Quere P, Facy O, Manfredi S, Jooste V, Faivre J, Lepage C. et al. Epidemiology, Management, and Survival of Peritoneal Carcinomatosis from Colorectal Cancer: A Population-Based Study. Dis Colon Rectum. 2015;58:743-52
12. Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20:69-84
13. Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973-81
14. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178-96
15. Lu W, Kang Y. Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Dev Cell. 2019;49:361-74
16. Pretzsch E, Bosch F, Neumann J, Ganschow P, Bazhin A, Guba M. et al. Mechanisms of Metastasis in Colorectal Cancer and Metastatic Organotropism: Hematogenous versus Peritoneal Spread. J Oncol. 2019;2019:7407190
17. Lemoine L, Sugarbaker P, Van der Speeten K. Pathophysiology of colorectal peritoneal carcinomatosis: Role of the peritoneum. World J Gastroenterol. 2016;22:7692-707
18. Papavassiliou KA, Papavassiliou AG. Transcription Factor Drug Targets. J Cell Biochem. 2016;117:2693-6
19. Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151-61
20. Wang Y, Shi J, Chai K, Ying X, Zhou BP. The Role of Snail in EMT and Tumorigenesis. Curr Cancer Drug Targets. 2013;13:963-72
21. Shahid S, Iman A, Matti U, Rachid K, Assaf A, Eveno C. et al. Fibrin Deposit on the Peritoneal Surface Serves as a Niche for Cancer Expansion in Carcinomatosis Patients. Neoplasia. 2019;21:1091-101
22. Song XH, Chen XZ, Chen XL, Liu K, Zhang WH, Mo XM. et al. Peritoneal Metastatic Cancer Stem Cells of Gastric Cancer with Partial Mesenchymal-Epithelial Transition and Enhanced Invasiveness in an Intraperitoneal Transplantation Model. Gastroenterol Res Pract. 2020;2020:3256538
23. Takai M, Terai Y, Kawaguchi H, Ashihara K, Fujiwara S, Tanaka T. et al. The EMT (epithelial-mesenchymal-transition)-related protein expression indicates the metastatic status and prognosis in patients with ovarian cancer. J Ovarian Res. 2014;7:76
24. Ponnusamy MP, Lakshmanan I, Jain M, Das S, Chakraborty S, Dey P. et al. MUC4 mucin-induced epithelial to mesenchymal transition: a novel mechanism for metastasis of human ovarian cancer cells. Oncogene. 2010;29:5741-54
25. Zuo QF, Cao LY, Yu T, Gong L, Wang LN, Zhao YL. et al. MicroRNA-22 inhibits tumor growth and metastasis in gastric cancer by directly targeting MMP14 and Snail. Cell Death Dis. 2015;6:e2000
26. Liu N, Peng SM, Zhan GX, Yu J, Wu WM, Gao H. et al. Human chorionic gonadotropin beta regulates epithelial-mesenchymal transition and metastasis in human ovarian cancer. Oncol Rep. 2017;38:1464-72
27. Lam SS, Mak AS, Yam JW, Cheung AN, Ngan HY, Wong AS. Targeting estrogen-related receptor alpha inhibits epithelial-to-mesenchymal transition and stem cell properties of ovarian cancer cells. Mol Ther. 2014;22:743-51
28. Li D, Wang Y, Dong C, Chen T, Dong A, Ren J. et al. CST1 inhibits ferroptosis and promotes gastric cancer metastasis by regulating GPX4 protein stability via OTUB1. Oncogene. 2023;42:83-98
29. Wu SM, Lin WY, Shen CC, Pan HC, Keh-Bin W, Chen YC. et al. Melatonin set out to ER stress signaling thwarts epithelial mesenchymal transition and peritoneal dissemination via calpain-mediated C/EBPbeta and NFkappaB cleavage. J Pineal Res. 2016;60:142-54
30. Lai DW, Liu SH, Karlsson AI, Lee WJ, Wang KB, Chen YC. et al. The novel Aryl hydrocarbon receptor inhibitor biseugenol inhibits gastric tumor growth and peritoneal dissemination. Oncotarget. 2014;5:7788-804
31. Zhu Y, Liu Y, Qian Y, Dai X, Yang L, Chen J. et al. Research on the efficacy of Celastrus Orbiculatus in suppressing TGF-beta1-induced epithelial-mesenchymal transition by inhibiting HSP27 and TNF-alpha-induced NF-kappa B/Snail signaling pathway in human gastric adenocarcinoma. BMC Complement Altern Med. 2014;14:433
32. Hu Q, Masuda T, Sato K, Tobo T, Nambara S, Kidogami S. et al. Identification of ARL4C as a Peritoneal Dissemination-Associated Gene and Its Clinical Significance in Gastric Cancer. Ann Surg Oncol. 2018;25:745-53
33. Zang M, Zhang B, Zhang Y, Li J, Su L, Zhu Z. et al. CEACAM6 promotes gastric cancer invasion and metastasis by inducing epithelial-mesenchymal transition via PI3K/AKT signaling pathway. PLoS One. 2014;9:e112908
34. Gao P, Wang S, Jing F, Zhan J, Wang Y. microRNA-203 suppresses invasion of gastric cancer cells by targeting ERK1/2/Slug/ E-cadherin signaling. Cancer Biomark. 2017;19:11-20
35. Stasinopoulos IA, Mironchik Y, Raman A, Wildes F, Winnard P Jr, Raman V. HOXA5-twist interaction alters p53 homeostasis in breast cancer cells. J Biol Chem. 2005;280:2294-9
36. Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C. et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927-39
37. Castanon I, Baylies MK. A Twist in fate: evolutionary comparison of Twist structure and function. Gene. 2002;287:11-22
38. Terauchi M, Kajiyama H, Yamashita M, Kato M, Tsukamoto H, Umezu T. et al. Possible involvement of TWIST in enhanced peritoneal metastasis of epithelial ovarian carcinoma. Clin Exp Metastasis. 2007;24:329-39
39. Cui Y, Wang Y, Li H, Li Q, Yu Y, Xu X. et al. Asparaginyl endopeptidase promotes the invasion and metastasis of gastric cancer through modulating epithelial-to-mesenchymal transition and analysis of their phosphorylation signaling pathways. Oncotarget. 2016;7:34356-70
40. Wu S, Yang L, Wu D, Gao Z, Li P, Huang W. et al. AEG-1 induces gastric cancer metastasis by upregulation of eIF4E expression. J Cell Mol Med. 2017;21:3481-93
41. Bao W, Qiu H, Yang T, Luo X, Zhang H, Wan X. Upregulation of TrkB promotes epithelial-mesenchymal transition and anoikis resistance in endometrial carcinoma. PLoS One. 2013;8:e70616
42. Huang YN, Xu YY, Ma Q, Li MQ, Guo JX, Wang X. et al. Dextran Sulfate Effects EMT of Human Gastric Cancer Cells by Reducing HIF-1alpha/ TGF-beta. J Cancer. 2021;12:3367-77
43. Funahashi J, Sekido R, Murai K, Kamachi Y, Kondoh H. Delta-crystallin enhancer binding protein delta EF1 is a zinc finger-homeodomain protein implicated in postgastrulation embryogenesis. Development. 1993;119:433-46
44. Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415-28
45. Sanchez-Tillo E, Lazaro A, Torrent R, Cuatrecasas M, Vaquero EC, Castells A. et al. ZEB1 represses E-cadherin and induces an EMT by recruiting the SWI/SNF chromatin-remodeling protein BRG1. Oncogene. 2010;29:3490-500
46. Yabusaki N, Yamada S, Murai T, Kanda M, Kobayashi D, Tanaka C. et al. Clinical significance of zinc-finger E-box binding homeobox 1 mRNA levels in peritoneal washing for gastric cancer. Mol Clin Oncol. 2015;3:435-41
47. Okugawa Y, Toiyama Y, Tanaka K, Matsusita K, Fujikawa H, Saigusa S. et al. Clinical significance of Zinc finger E-box Binding homeobox 1 (ZEB1) in human gastric cancer. J Surg Oncol. 2012;106:280-5
48. Gao J, Zhu Y, Nilsson M, Sundfeldt K. TGF-beta isoforms induce EMT independent migration of ovarian cancer cells. Cancer Cell Int. 2014;14:72
49. Shao M, Cao L, Shen C, Satpathy M, Chelladurai B, Bigsby RM. et al. Epithelial-to-mesenchymal transition and ovarian tumor progression induced by tissue transglutaminase. Cancer Res. 2009;69:9192-201
50. Shi L, Yang D, Dong H, Zhang X, Yang C. miR-34b-5p suppresses the epithelial-mesenchymal transition and metastasis in endometrial cancer AN3CA cells by targeting ZEB1. Int J Clin Exp Pathol. 2024;17:137-50
51. Parashar D, Nair B, Geethadevi A, George J, Nair A, Tsaih SW. et al. Peritoneal Spread of Ovarian Cancer Harbors Therapeutic Vulnerabilities Regulated by FOXM1 and EGFR/ERBB2 Signaling. Cancer Res. 2020;80:5554-68
52. Li Y, Fei H, Lin Q, Liang F, You Y, Li M. et al. ZEB2 facilitates peritoneal metastasis by regulating the invasiveness and tumorigenesis of cancer stem-like cells in high-grade serous ovarian cancers. Oncogene. 2021;40:5131-41
53. Zhou Q, Wu X, Wang X, Yu Z, Pan T, Li Z. et al. The reciprocal interaction between tumor cells and activated fibroblasts mediated by TNF-alpha/IL-33/ST2L signaling promotes gastric cancer metastasis. Oncogene. 2020;39:1414-28
54. Hsieh PN, Fan L, Sweet DR, Jain MK. The Kruppel-Like Factors and Control of Energy Homeostasis. Endocr Rev. 2019;40:137-52
55. Wang K, Wang Y, Wang Y, Liu S, Wang C, Zhang S. et al. EIF5A2 enhances stemness of epithelial ovarian cancer cells via a E2F1/KLF4 axis. Stem Cell Res Ther. 2021;12:186
56. Tanaka Y, Chiwaki F, Kojima S, Kawazu M, Komatsu M, Ueno T. et al. Multi-omic profiling of peritoneal metastases in gastric cancer identifies molecular subtypes and therapeutic vulnerabilities. Nat Cancer. 2021;2:962-77
57. Wu G, Zhang A, Yang Y, Wu D. Circ-RNF111 aggravates the malignancy of gastric cancer through miR-876-3p-dependent regulation of KLF12. World J Surg Oncol. 2021;19:259
58. He Z, Guo X, Tian S, Zhu C, Chen S, Yu C. et al. MicroRNA-137 reduces stemness features of pancreatic cancer cells by targeting KLF12. J Exp Clin Cancer Res. 2019;38:126
59. Lu X, Han Y, Han Y, Huang M, You J, Liu Y. et al. MicroRNA-650 suppresses KLF12 expression to regulate growth and metastasis of human ovarian cancer cells. Acta Biochim Pol. 2022;69:745-51
60. Zhang Q, Wang J, Qiao H, Huyan L, Liu B, Li C. et al. ISG15 is downregulated by KLF12 and implicated in maintenance of cancer stem cell-like features in cisplatin-resistant ovarian cancer. J Cell Mol Med. 2021;25:4395-407
61. Mak CS, Yung MM, Hui LM, Leung LL, Liang R, Chen K. et al. MicroRNA-141 enhances anoikis resistance in metastatic progression of ovarian cancer through targeting KLF12/Sp1/survivin axis. Mol Cancer. 2017;16:11
62. Castaneda M, Hollander PD, Mani SA. Forkhead Box Transcription Factors: Double-Edged Swords in Cancer. Cancer Res. 2022;82:2057-65
63. Lin F, Li X, Wang X, Sun H, Wang Z, Wang X. Stanniocalcin 1 promotes metastasis, lipid metabolism and cisplatin chemoresistance via the FOXC2/ITGB6 signaling axis in ovarian cancer. J Exp Clin Cancer Res. 2022;41:129
64. Wang S, Yan S, Zhu S, Zhao Y, Yan J, Xiao Z. et al. FOXF1 Induces Epithelial-Mesenchymal Transition in Colorectal Cancer Metastasis by Transcriptionally Activating SNAI1. Neoplasia. 2018;20:996-1007
65. Qu Q, Liu L, Cui Y, Chen Y, Wang Y, Wang Y. Exosomes from Human Omental Adipose-Derived Mesenchymal Stem Cells Secreted into Ascites Promote Peritoneal Metastasis of Epithelial Ovarian Cancer. Cells. 2022;11:3392
66. Zhang DZ, Chen BH, Zhang LF, Cheng MK, Fang XJ, Wu XJ. Basic Transcription Factor 3 Is Required for Proliferation and Epithelial-Mesenchymal Transition via Regulation of FOXM1 and JAK2/STAT3 Signaling in Gastric Cancer. Oncol Res. 2017;25:1453-62
67. Xu YR, Yang WX. SOX-mediated molecular crosstalk during the progression of tumorigenesis. Semin Cell Dev Biol. 2017;63:23-34
68. Olbromski M, Podhorska-Okolow M, Dziegiel P. Role of SOX Protein Groups F and H in Lung Cancer Progression. Cancers (Basel). 2020;12:3235
69. Shen Z, Deng H, Fang Y, Zhu X, Ye GT, Yan L. et al. Identification of the interplay between SOX9 and S100P in the metastasis and invasion of colon carcinoma. Oncotarget. 2015;6:20672-84
70. Lam SS, Ip CK, Mak AS, Wong AS. A novel p70 S6 kinase-microRNA biogenesis axis mediates multicellular spheroid formation in ovarian cancer progression. Oncotarget. 2016;7:38064-77
71. Fujiwara T. GATA Transcription Factors: Basic Principles and Related Human Disorders. Tohoku J Exp Med. 2017;242:83-91
72. Tremblay M, Sanchez-Ferras O, Bouchard M. GATA transcription factors in development and disease. Development. 2018;145:dev164384
73. Zhou Y, Zhou X, Ben Q, Liu N, Wang J, Zhai Y. et al. GATA6-AS1 suppresses epithelial-mesenchymal transition of pancreatic cancer under hypoxia through regulating SNAI1 mRNA stability. J Transl Med. 2023;21:882
74. De Thaye E, Van de Vijver K, Van der Meulen J, Taminau J, Wagemans G, Denys H. et al. Establishment and characterization of a cell line and patient-derived xenograft (PDX) from peritoneal metastasis of low-grade serous ovarian carcinoma. Sci Rep. 2020;10:6688
75. Barbolina MV, Adley BP, Shea LD, Stack MS. Wilms tumor gene protein 1 is associated with ovarian cancer metastasis and modulates cell invasion. Cancer. 2008;112:1632-41
76. McKnight R, Cohen C, Siddiqui MT. Utility of paired box gene 8 (PAX8) expression in fluid and fine-needle aspiration cytology: an immunohistochemical study of metastatic ovarian serous carcinoma. Cancer Cytopathol. 2010;118:298-302
77. Zhu C, Teng L, Lai Y, Yao X, Fang Y, Wang Z. et al. Adipose-derived stem cells promote glycolysis and peritoneal metastasis via TGF-beta1/SMAD3/ANGPTL4 axis in colorectal cancer. Cell Mol Life Sci. 2024;81:189
78. Tsubakihara Y, Moustakas A. Epithelial-Mesenchymal Transition and Metastasis under the Control of Transforming Growth Factor beta. Int J Mol Sci. 2018;19:3672
79. Li D, Xia L, Huang P, Wang Z, Guo Q, Huang C. et al. Heterogeneity and plasticity of epithelial-mesenchymal transition (EMT) in cancer metastasis: Focusing on partial EMT and regulatory mechanisms. Cell Prolif. 2023;56:e13423
80. Ray I, Michael A, Meira LB, Ellis PE. The Role of Cytokines in Epithelial-Mesenchymal Transition in Gynaecological Cancers: A Systematic Review. Cells. 2023;12:416
81. Zhang J, Hu Z, Horta CA, Yang J. Regulation of epithelial-mesenchymal transition by tumor microenvironmental signals and its implication in cancer therapeutics. Semin Cancer Biol. 2023;88:46-66
82. Seo J, Ha J, Kang E, Cho S. The role of epithelial-mesenchymal transition-regulating transcription factors in anti-cancer drug resistance. Arch Pharm Res. 2021;44:281-92
83. Lee CH. Reversal of Epithelial-Mesenchymal Transition by Natural Anti-Inflammatory and Pro-Resolving Lipids. Cancers (Basel). 2019;11:1841
Author contact
![]() Corresponding authors: Yuhong Wang (wangyuhongedu.cn) and Min Li (minl_zjucom).
Corresponding authors: Yuhong Wang (wangyuhongedu.cn) and Min Li (minl_zjucom).

 Global reach, higher impact
Global reach, higher impact