Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(18):5855-5862. doi:10.7150/jca.89453 This issue Cite
Research Paper
Circulating cancer-associated macrophage-like cells and macrophage-related cytokines in obese patients with advanced breast cancer who undergo neoadjuvant chemotherapy
1. Section of Translational Breast Cancer Research, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA.
2. Diagnostic Radiology, Baylor College of Medicine, 1 Moursund St, Houston, TX 77030, USA.
3. Department of Biostatistics, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA.
4. Creatv Microtech, 9900 Belward Campus Drive, #330, Rockville, MD 20850, USA.
5. Department of Hematopathology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA.
6. Department of Internal Medicine, Greenwich Hospital, 5 Perryridge Rd Greenwich, CT 06830, USA.
7. Department of Medical Oncology, MedPark Hospital, 3333 Rama IV Rd, Khlong Toei, Bangkok 10110, Thailand.
8. Breast Center, St. Luke's International Hospital, 9-1 Akashi-cho, Chuo-ku, Tokyo 104-8560, Japan.
9. Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA.
10. Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA.
11. University of Hawai'i Cancer Center, 701 Ilalo Street Honolulu, HI 96813, USA.
Received 2023-8-24; Accepted 2024-9-5; Published 2024-9-16
Abstract
Purpose: Cancer-associated macrophage-like cells (CAMLs) are rare, gigantic, and atypical circulating cells found exclusively in the peripheral blood of patients with solid cancers. Obesity-induced hypoxia attracts macrophages to the tumor microenvironment, where they contribute to establishing chronic inflammation, leading to cancer progression. We hypothesized that obese patients with advanced breast cancer may have CAML profiles different from those of nonobese patients, and these profiles may correlate with proinflammatory markers or other macrophage-related markers.
Methods: We prospectively collected 20 mL of peripheral blood from patients diagnosed with stage 2-4 breast cancer. We identified CAMLs using the CellSieve microfiltration system and in parallel quantified the proinflammatory and macrophage-related markers using a multiplex cytokine panel. We further evaluated C-X-C chemokine receptor type 4 (CXCR4) expression in CAMLs to investigate its relationship to the macrophage differentiation. We estimated the association between CAML characteristics and body mass index (BMI), body composition, and cytokines/chemokines.
Results: Thirty patients were included in the study, and 28 samples were analyzed. Higher BMI was significantly correlated with the increased maximum CAML size (P = 0.035). Patients with higher BMIs had significantly increased macrophage-colony stimulating factor (M-CSF) levels in plasma (P = 0.007), and obese patients trended towards higher tumor necrosis factor-alpha, MIP-1α and M-CSF expression (P <0.10). Body composition analysis showed that the M-CSF and SAT amounts were significantly correlated (P = 0.010). MIP-1α expression was significantly correlated with average CXCR4 CAML expression (P = 0.003).
Conclusion: We discovered larger CAML size was associated with SAT-dominant obesity with increased macrophage-related and proinflammatory markers in obese than in nonobese breast cancer patients.
Keywords: breast neoplasm, macrophages, obesity, neoadjuvant therapy, inflammation
Introduction
Approximately 38% of adults in the United States is obesity, which defines as a body mass index (BMI) of 30 kg/m2 or more based on the World Health Organization BMI classification [1]. Notably, it is significantly more common in women than in men (40% vs. 35%) [1]. It is a common, preventable risk factor for metabolic diseases such as hyperlipidemia and diabetes mellites, and it is also significantly associated with increased morbidity and mortality in patients with breast cancer [2, 3]. A meta-analysis that evaluated the morbidity risk of obesity in postmenopausal women showed that those with an increase of 5 kg/m² in BMI had a 1.12 increased risk ratio (95% CI, 1.08-1.16) for acquiring breast cancer [4]. Obesity also negatively affects breast cancer treatment which has been observed in studies of efficacy endpoints, such as studies of patients with breast cancer who completed treatment with neoadjuvant chemotherapy showing significantly lower pathological complete response rates and shorter disease-free survival durations in patients with BMIs over 30 kg/m² compared to those with lower BMIs [5-7]. Further, adjuvant chemotherapy and endocrine therapy studies of patients with breast cancer also showed similar trends in poorer survival outcomes [8].
A potential reason for worse clinical outcomes in patients with higher BMI is obesity-induced chronic inflammation. Adipose tissue has a unique local microenvironment composed of various types of cells, including adipocytes, adipose stromal cells, and immune cells. In the cellular environment of the obese individual, oversized adipocytes induce local hypoxia because of their high oxygen demand, and hypoxia stimulates adipose stromal cells to secrete multiple types of growth factors and pro-inflammatory cytokines ‒ including insulin-like growth factor, vascular endothelial growth factor, interleukin (IL)-8, and IL-10 ‒ which can accelerate breast cancer progression [9]. Furthermore, the proinflammatory microenvironment attracts a variety of immune cells, including T cells, B cells, and macrophages, which help establish chronic inflammation.
Although the direct pathological investigation of harvested tissue would be the optimal way to evaluate the inflammatory and immune status of the tumor microenvironment (TME), tissue biopsy is an invasive procedure in most cases. Instead of an invasive tumor biopsy, recent development of liquid biopsy techniques has become a more favorable way to indirectly assess the biology of local tumor. Among the circulating components in the peripheral blood, researchers have investigated cancer-associated macrophage-like cells (CAMLs). These circulating cells are unique, giant, and pleomorphic cells that resemble macrophages for their specificity to cancer, predictive values and prognostic values as biomarkers [10]. CAMLs are characterized by specific cell-surface markers such as cluster of differentiation (CD)14 and CD45, cytokeratin, and epithelial cell adhesion molecule; their size (25-300μm); and their atypical, separated polymorphic nuclei (14-64μm in diameter) [10]. They are frequently observed, with high sensitivity and specificity, in the peripheral blood of patients with advanced solid cancers [11]. Indeed, their significant prognostic value was demonstrated in a study that showed that, in patients with solid tumors, those with more than 6 CAMLs or CAMLs over 50μm in size at initial diagnosis had significantly shorter overall survival and progression-free survival durations than did patients with fewer or smaller CAMLs [12, 13].
Although these previous studies have indicated the usefulness of CAMLs as predictive and prognostic biomarkers, the biological role of CAMLs is not fully understood. Given that CAMLs originate from tumor-associated macrophages (TAMs) [10], and TAMs play an important role in promoting obesity-induced chronic inflammation and tumor progression in the TME, we undertook this study to clarify the biological role of CAMLs and gain a better understanding of how obesity promotes breast cancer progression through systemic inflammation and macrophage-related cytokines. Moreover, we sought to elucidate the mechanism of obesity-induced breast cancer progression, which could aid the development of novel treatments targeting inflammation. Our hypothesis of the present study was that obese patients with advanced breast cancer have CAML profiles that are distinct those of non-obese patients in terms of CAML numbers and sizes. Further, we hypothesized that CAML profiles may correlate with macrophage-related peripheral blood markers and proinflammatory markers. To test this hypothesis, we conducted a prospective study in patients with advanced breast cancer who had undergone neoadjuvant chemotherapy.
Material and methods
Materials
This prospective study examined peripheral blood from patients with primary untreated breast cancer taken prior to receiving neoadjuvant or induction chemotherapy (NAC). We prospectively screened the patients in the Breast Medical Oncology clinic at The University of Texas MD Anderson Cancer Center main campus between August 2018 and January 2020. To be included in the study, patients had to be over 18 years old; be newly diagnosed with pathologically confirmed, invasive breast cancer of any subtype (including inflammatory breast cancer); have stage II to stage IV disease; be scheduled to undergo computed tomography (CT) or positron emission tomography-CT before starting NAC; and either not have started chemotherapy or have started it 4 weeks or less prior to enrollment in the study. Both men and women were eligible for inclusion. Thirty patients registered for the study, but 2 samples were of insufficient quality because of clotting or inadequate volumes. Thus, the assay was performed on 28 samples. Patient demographics, pathologic information, and response to the treatment were extracted from MD Anderson Cancer Center's electronic health records system. MD Anderson's Institutional Review Board approved this study (protocol number PA17-0542), and all patients provided written informed consent.
Detection of CAMLs and cytokines in peripheral blood
We prospectively collected 20 mL of anonymized fresh peripheral blood (two10-mL samples) from each eligible patient after enrollment and stored the samples in ethylenediaminetetraacetic acid (EDTA) or CellSave (MENARINI silicon biosystems, Pennsylvania United States) tubes for the cytokine assay or CAML detection, respectively.
The plasma from blood collected in the EDTA tubes was stored in 2 Eppendorf tubes at -80 ℃ before cytokine/chemokine levels were determined in a single batch using Bio-Plex Pro Human Cytokine 48-Plex Screening Panel (Bio-Rad Laboratories). The cytokine assay focused on proinflammatory cytokines (interleukin (IL)-1b, IL-6, and necrosis factor-alpha [TNF-α]); chemokines: macrophage-related cytokines (macrophage inflammatory protein [MIP]-1 alpha [α], MIP-1 beta [β], and macrophage-colony stimulating factor [M-CSF]).
Peripheral blood was collected in CellSave tubes and passed through the CellSieve™ microfilters that are lithographically fabricated membranes with high porosity, precise pore dimensions, and regular pore distribution to enrich for peripheral blood cells larger than 7 µm [11].
The enriched peripheral blood cells on the microfilter were subjected to staining with 4′,6-diamidino-2-phenylindole (DAPI), anti-CD14 conjugated with Cy5, anti-CD45 conjugated with Cy5, anti-C-X-C chemokine receptor type 4 (CXCR4) conjugated with Alexafluor555, and anti-Cytokeratin conjugated with AlexaFluor488. CAMLs from breast patients were defined as previously described by Adams et al. 2014 and Raghavakaimal/Adams BCR 2022), as enlarged (≥30µm in diameter), polynuclear cells with diffuse cytoplasmic cytokeratin and either CD14 positive or CD45 positive. CAMLs were differentiated from CTCs and CD45/CD14 white blood cells (WBCs) as previously described [14, 15]. Specifically, CTCs are filamented Cytokeratin positive and CD45/CD14 negative cells. WBCs are cytokeratin negative and CD45/CD14 positive cells. Expression of CXCR4, which is a receptor of C-X-C motif chemokine ligand 12 (CXCL12), was also measured to evaluate the CAMLs's biology related to macrophage-like function because the activation of CXCR4/CXCL12 signaling causes differentiation of monocytes into proangiogenic, immunosuppressive macrophages in the TME [16]. CAMLs' size, number, unique morphological characteristics (e.g., merged nuclei), and CXCR4 expression were also evaluated. CAMLs were imaged using and Olympus BX61WI fluorescent microscope with Zeiss AxioCam camera. Images were processed with expression and cell sizes measured using a pre-calibrated sizing tool function in the Zen2011 Blue software (Informer Technologies, Inc. Los Angeles, CA).
Body composition measurements
To investigate the detailed effects of obesity on the treatment response, we determined patients' body mass composition by characterizing patients' abdominal fat tissue as visceral adipose tissue (VAT) or subcutaneous adipose tissue (SAT). Estimates of the VAT and SAT (both measured in cm3) were obtained from CT imaging data provided by MD Anderson's Digital Imaging and Communications in Medicine dataset. The analyzed area for determining VAT and SAT was defined as the area from the top of the diaphragm to the navel level in the CT axial view. We also calculated the VAT to SAT (V/S) ratio to see how VAT-dominant or SAT-dominant body composition affected the cytokine/chemokine expressions and the response to the treatment. These imaging analyses were performed using MATLAB software (Mathworks, Natick, MA) and an in-house imaging analysis program, Medical Executable for the Efficient and Robust Quantification of Adipose Tissue (MEERQAT), from MD Anderson's Department of Radiation Oncology. MEERQAT can distinguish VAT from SAT by providing the volume of each component within a pre-specified area of interest. MEERQAT's detailed computing process has been described by Parikh et al. [17].
Statistical analysis
The sample size of 30 was prospectively generated based on the estimated effect size and power calculation. With 30 patients, the power to test the null hypothesis of no correlation between variables ( 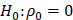 ) against moderate correlation between variables (
) against moderate correlation between variables (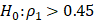 ) is 71%. Frequencies and percentages are reported for categorical variables. The Wilcoxon rank sum test and the Kruskal-Wallis test were used to compare the distributions of the continuous variables between the study groups. The Shapiro-Wilk test was performed to evaluate normality in continuous data. The Spearman correlation coefficient (SCC) was used to estimate correlations, which was based on the rank of the data and was robust to outliers and the normal distribution assumption. All tests were 2-sided. P values less than 0.05 were considered statistically significant without adjustment for multiple analyses. All analyses were conducted using SAS software, version 9.4.
) is 71%. Frequencies and percentages are reported for categorical variables. The Wilcoxon rank sum test and the Kruskal-Wallis test were used to compare the distributions of the continuous variables between the study groups. The Shapiro-Wilk test was performed to evaluate normality in continuous data. The Spearman correlation coefficient (SCC) was used to estimate correlations, which was based on the rank of the data and was robust to outliers and the normal distribution assumption. All tests were 2-sided. P values less than 0.05 were considered statistically significant without adjustment for multiple analyses. All analyses were conducted using SAS software, version 9.4.
Results
Patient characteristics
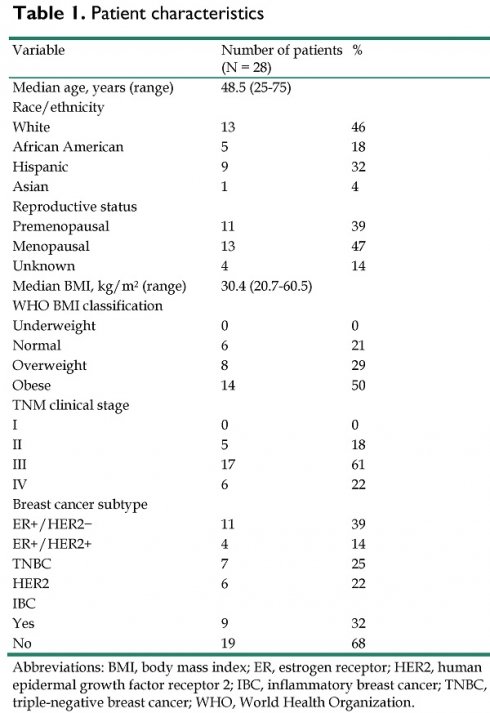
Patient characteristics are summarized in Table 1. The median BMI was 30.4, and 14 (50%) of the patients were categorized as obese according to the World Health Organization's BMI classifications. Seventeen (61%) of all patients were diagnosed with clinical stage III breast cancer, and 6 (21%) were diagnosed with clinical stage IV breast cancer. Eleven (39%) patients had the estrogen receptor (ER)+/human epidermal growth factor receptor 2 (HER2) negative subtype, 7 (25%) had the triple-receptor negative breast cancer (TNBC) subtype, 6 (21%) had the HER2 subtype, and 4 (14%) had the ER+/HER2+ subtype. Nine of these patients (32%) had inflammatory breast cancer.
CAML profiles and subtypes
The average number of CAMLs per 7.5 ml of blood (P = 0.91), the average size of CAMLs (P = 0.44), and the maximum CAML size (P = 0.47) were not significantly different among the 4 molecular subtypes (ER+/HER2-, ER+/HER2+, triple-negative breast cancer, and HER2; the P values were based on the Kruskal-Wallis test).
BMI and CAML profile correlations
The correlations between BMIs and CAML profiles are shown in Table 2. BMI was significantly positively correlated with the maximum size of CAMLs (SCC = 0.43; P = 0.035). When patients were divided into normal (BMI ≤ 25) and overweight/obese (BMI > 25) groups, the overweight/obese patients with breast cancer had a significantly higher average CAML size (P = 0.028) and maximum CAML size (P = 0.038) than did the patients with normal BMI levels (Table 3). Furthermore, to estimate the odds ratio (OR) of the association between BMI and the maximum CAML size, the continuous maximum CAML size data was dichotomized into two levels (high vs low) using the median of 58 as the cutoff. Overweight and obese patients exhibited a 9.61times higher likelihood of having a high-level maximum CAML size (≥58) compared to patients with an underweight or normal BMI (95% CI of OR =1.54 - Infinity; P = 0.019, exact logistic regression analysis).
Patient characteristics
| Variable | Number of patients(N = 28) | % |
|---|---|---|
| Median age, years (range) | 48.5 (25-75) | |
| Race/ethnicity | ||
| White | 13 | 46 |
| African American | 5 | 18 |
| Hispanic | 9 | 32 |
| Asian | 1 | 4 |
| Reproductive status | ||
| Premenopausal | 11 | 39 |
| Menopausal | 13 | 47 |
| Unknown | 4 | 14 |
| Median BMI, kg/m2 (range) | 30.4 (20.7-60.5) | |
| WHO BMI classification | ||
| Underweight | 0 | 0 |
| Normal | 6 | 21 |
| Overweight | 8 | 29 |
| Obese | 14 | 50 |
| TNM clinical stage | ||
| I | 0 | 0 |
| II | 5 | 18 |
| III | 17 | 61 |
| IV | 6 | 22 |
| Breast cancer subtype | ||
| ER+/HER2- | 11 | 39 |
| ER+/HER2+ | 4 | 14 |
| TNBC | 7 | 25 |
| HER2 | 6 | 22 |
| IBC | ||
| Yes | 9 | 32 |
| No | 19 | 68 |
Abbreviations: BMI, body mass index; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; IBC, inflammatory breast cancer; TNBC, triple-negative breast cancer; WHO, World Health Organization.
BMI and CAML profile correlations
| CAML profile | ||||
|---|---|---|---|---|
| Number | Average size | Maximum size | ||
| BMI | SCC | 0.17 | 0.39 | 0.43 |
| P | 0.38 | 0.059 | 0.035 | |
| Number of observations | 28 | 24 | 24 | |
*P was based on the Spearman correlation test.
Abbreviations: BMI, body mass index; CAML, cancer-associated macrophage-like cell; SCC, Spearman correlation coefficient.
Association between WHO BMI classifications and CAML profiles
| CAML profile | BMI | Number of patients (N = 28) | Mean ± standard deviation | P |
|---|---|---|---|---|
| Number of CAMLs | Normal | 6 | 1.3 ± 0.8 | 0.567 |
| Overweight/obese | 22 | 2.9 ± 3.3 | ||
| Average CAML size | Normal | 5 | 34.1 ± 7.6 | 00.028 |
| Overweight/obese | 19 | 59.2 ± 21.1 | ||
| Maximum CAML size | Normal | 5 | 35.4 ± 8.3 | 0.038 |
| Overweight/obese | 19 | 77.3 ± 39.0 |
Abbreviations: BMI, body mass index; CAML, cancer-associated macrophage-like cell; WHO, World Health Organization.
Body composition and CAML profile correlations
The correlations between the CAML profiles and the body composition measurements ‒ the VAT amount, the SAT amount, and the V/S ratio ‒ were not statistically significant (all P >0.05), although the SAT amount trended towards correlation with the maximum CAML size (SCC = 0.364; P = 0.088; Table 4).
Correlation between CAML profiles and body composition parameters
| CAML profiles | ||||
|---|---|---|---|---|
| Number | Average size | Maximum size | ||
| VAT amount | SCC | 0.09 | 0.12 | 0.30 |
| P* | 0.648 | 0.606 | 0.159 | |
| Number of observations | 27 | 23 | 23 | |
| SAT amount | SCC | 0.05 | 0.29 | 0.36 |
| P | 0.817 | 0.18 | 0.088 | |
| Number of observations | 27 | 23 | 23 | |
| V/S ratio | SCC | 0.09 | -0.20 | 0.02 |
| P | 0.669 | 0.349 | 0.918 | |
| Number of observations | 27 | 23 | 23 | |
*P was based on the Spearman correlation test.
Abbreviations: CAML, cancer-associated macrophage-like cell; SAT, subcutaneous adipose tissue; SCC, Spearman correlation coefficient; VAT, visceral adipose tissue; V/S, visceral adipose tissue to subcutaneous adipose tissue.
Proinflammatory cytokines/chemokines and obesity correlations
The correlation between BMI and the expression of cytokines is shown in Table 5. M-CSF expression was significantly positively correlated with BMI; specifically, patients with a higher BMI level had increased M-CSF levels (SCC = 0.50; P = 0.0074). There was a marginal positive correlation between the MIP-1α level and BMI (SCC = 0.35; P = 0.068).
Correlation between BMI and the expression of cytokines
| Variables (N = 28) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variable | BMI | CXCL12 | IL-1b | IL-6 | TNF-α | MIP-1α | MIP-1β | M-CSF |
| BMI SCC | 1.00 | 0.06 | 0.23 | 0.12 | 0.17 | 0.35 | -0.03 | 0.50 |
| P* | N/A | 0.759 | 0.230 | 0.543 | 0.388 | 0.068 | 0.886 | .007 |
| CXCL12 SCC | 1.00 | 0.11 | -0.15 | 0.51 | 0.33 | 0.16 | 0.24 | |
| P* | N/A | 0.569 | 0.438 | 0.006 | 0.087 | 0.416 | 0.219 | |
| IL-1b SCC | 1.00 | -0.18 | 0.23 | 0.13 | 0.16 | 0.04 | ||
| P* | N/A | 0.371 | 0.240 | 0.498 | 0.424 | 0.832 | ||
| IL-6 SCC | 1.00 | 0.00 | 0.20 | -0.14 | 0.40 | |||
| P* | N/A | 0.986 | 0.320 | 0.475 | 0.034 | |||
| TNF-α SCC | 1.00 | 0.44 | 0.57 | 0.44 | ||||
| P* | N/A | 0.021 | 0.001 | 0.018 | ||||
| MIP-1α SCC | 1.00 | 0.25 | 0.52 | |||||
| P* | N/A | 0.190 | 0.005 | |||||
| MIP-1β SCC | 1.00 | -0.09 | ||||||
| P* | N/A | 0.660 | ||||||
| M-CSF SCC | 1.00 | |||||||
| P* | N/A | |||||||
*P was based on the Spearman correlation test.
Abbreviations: BMI, body mass index: CXCL12, C-X-C motif chemokine ligand 12; IL, interleukin; M-CSF, macrophage-colony stimulating factor; MIP, macrophage inflammatory protein; N/A, not applicable; SCC, Spearman correlation coefficient; TNF-α, tissue necrosis factor-alpha.
The other cytokines were not significantly associated with BMI. However, there was a trend that obese patients with breast cancer were more likely than nonobese patients to have higher levels of TNF-α, MIP-1α, and M-CSF (P <0.10). The body composition analysis showed that the M-CSF and SAT levels were significantly correlated (SCC = 0.48 P = 0.0104). No other significant correlations were found between the body composition parameters and the cytokines (Supplemental Table 1).
Proinflammatory cytokines/chemokines and CAML correlations
The correlations between CAML sizes and numbers and cytokines were not statistically significant (all P >0.05; Supplemental Table 2). However, the correlation between M-CSF and the average size of CAMLs was marginally significant (SCC = 0.38; P = 0.06). In the chemokine analysis, MIP-1α was significantly correlated with the average CXCR4 expression on CAMLs (SCC = 0.593; P = 0.003) and marginally correlated with the maximum CXCR4 expression on CAMLs (SCC = 0.353; P = 0.098). No significant correlations were found between the other cytokines and CXCR4 expression (Supplemental Table 3).
Discussion
This is the first study to investigate the role of CAMLs on systemic inflammation and macrophage-related cytokines in obese patients with advanced breast cancer. We demonstrated a statistically significant correlation between BMI and the maximum size of CAMLs, BMI and M-CSF expression, and SAT-dominant body composition and M-CSF. We also found marginal correlations between BMI and MIP-1α, M-CSF and the average size of CAMLs, and maximum CXCR4 expression on CAMLs and MIP-1α. Although these marginal correlations were not statistically significant, they provided potential keys to understanding the biology of CAMLs in obese patient with advanced breast cancer.
The present study showed a significant positive correlation between BMI and the maximum CAML size. Also, a marginal correlation was observed between BMI and average CAML size. Furthermore, the detailed body composition analysis demonstrated a trend toward positive correlation between the SAT amount and the maximum CAML size. These results suggest that the size of CAMLs was associated with obesity or SAT-dominant body composition type. The SAT is the largest fat depot in the human body and is enriched by white adipose tissue that promotes chronic inflammation in obese environments. In an obese environment, the adipocytes can expand to up to 20 times larger than their normal size which increases their oxygen demand and leads to local hypoxia. Adipocytes in the hypoxic condition secrete hypoxia-inducible factor 1-alpha, which directly upregulates the production of proinflammatory cytokines such as TNF-α, IL-6, and MCP-1 [18]. These cytokines attract various immune cells, including macrophages, to the adipose tissue, and the macrophages also help establish the proinflammatory TME [19]. Intriguigly, a previous report indicated that the origin of CAMLs is the macrophages in the TME [10], which help establish obesity-induced proinflammation states. Our study result implied the link between macrophages in TME and CAMLs through the obesity-induced imflammation.
Although the present study indicated that the size of the CAMLs was associated with SAT-dominant body composition or obesity, it is unclear why there was no significant difference in the number of CAMLs in overweight and obese vs. normal-weight patients. A previous study including various types of solid tumor showed that the average CAML size increased as the clinical stage increased [20]. A similar trend of the number of CAMLs increasing as the clinical stage increased was also seen in the study, although the CAML number was not significantly different for patients with stage I vs. stage II cancer [20]. This result may indicate that the size of CAMLs increases before the number of CAMLs increases as cancer progresses. Indeed, a previous study demonstrated that CAMLs ingest necrotic tumor debris in patients with pancreatic or prostate cancer by detecting the expression of tumor-specific antigens inside the CAMLs [10]. Thus, increased CAML size could be due to their activated phagocytic activity caused by the pro-inflammatory environment.
The macrophage-related cytokines M-CSF and MIP-1α and the pro-inflammatory cytokine TNF-α had significant or marginal positive correlations to BMI, and M-CSF had a marginal correlation to the SAT amount and average CAML size. M-CSF is a primary growth factor that regulates tumor growth, proliferation, and cell differentiation in hematopoietic lineages, including macrophages [21]. It is secreted from tumor and stromal cells, such as CAMLs, and from white adipose tissue, which is a major source of M-CSF production [22]. M-CSF has a direct effect on the activation of the PI3K and MAPK signaling pathways via the colony-stimulating factor-1 receptor. Furthermore, M-CSF recruits macrophages into the TME which promotes angiogenesis and tumor growth [21]. In this study, we also observed a trend toward high expression of MIP-1α, a chemotactic chemokine produced by macrophages, in obese patients with breast cancer; this is a sign of macrophage activation. MIP-1α has various biological functions, including recruiting inflammatory cells, aiding in wound healing, inhibiting stem cells, and maintaining the effector immune response [23]. Although the present study did not investigate the interaction between M-CSF and MIP-1α in the TME, coexpression of those markers in obese patients with breast cancer indicated the activation and active trafficking of TAMs. Another essential biological finding in this study is the correlation between the CXCR4 expression on CAMLs and MIP-1α expression. CXCR4 is a receptor of CXCL12 (also known as stromal cell-derived factor 1), which is often expressed in breast cancer, and high CXCR4 expression is an indicator of poor survival prognosis [24, 25]. Importantly, the activation of the CXCR4/CXCL12 signaling pathway differentiates monocytes into proangiogenic, immunosuppressive macrophages in the TME [16]. No previous study has evaluated CXCR4 expression in circulating cells; accordingly, future studies should clarify whether the expression of CXCR4 on CAMLs represents the active differentiation of monocytes into macrophages in the TME.
In summary, the present study results generates a new hypothesis that, in the SAT-dominant body composition or obesity environment, various cells, including white adipocytes, tumor cells, and stromal cells, secrete M-CSF and MIP-1α, leading to the recruitment of macrophages to the TME. The recruited macrophages then help establish chronic inflammation and phagocytose tumor debris and dead adipocytes in the TME. These macrophages may return to the peripheral circulation as CAMLs expressing CXCR4.
The present study has several limitations. First, the small sample size of 28 prevents definitive conclusions. Also, p-values and level of significant were not adjusted for multiple analysis, increasing the risk of false positive results. The original sample size (30) was generated based on the estimated effect size and power calculation. However, the present study could not show a large effect size as originally estimated, and the obtained result is underpowered. Second, as mentioned above, the present study included patients with advanced and de novo stage IV disease, including Inflammatory Breast Cancer, which might have created inconsistencies in the study results. Future studies should involve a cohort with a standardized clinical background (i.e., clinical stage and receptor subtype) or a patient cohort large enough to be stratified by clinical background. Last, the present study only investigated the CAMLs and cytokine/chemokines in the systemic circulation, not in the TME. Therefore, the biological role of CAMLs in TME is still unknown. Further investigation of the TME including spatial analysis is needed to confirm the origin of CAMLs, their biological behavior, and their interaction with other cells such as immunocytes, adipocytes, and fibroblasts. The retrospective review of archival primary tumor tissues could be useful for this purpose.
Conclusions
The present study discovered that the size of CAMLs may be associated with SAT-dominant obesity. Furthermore, increased macrophage-related markers and proinflammatory markers were observed in obese patients with breast cancer. These findings may imply an association between CAMLs and activated macrophages in the TME and may contribute to a better understanding of the mechanism of obesity-induced breast cancer progression and, potentially, to biomarker development.
Abbreviations
CAMLs: cancer-associated macrophage-like cells; CXCR4: C-X-C chemokine receptor type 4; BMI: body mass index; M-CSF: macrophage-colony stimulating factor; SAT: subcutaneous adipose tissue; TME: tumor microenvironment; cluster of differentiation: CD; tumor-associated macrophages: TAMs; NAC: neoadjuvant chemotherapy; CT: computed tomography; EDTA: ethylenediaminetetraacetic acid; IL: interleukin; TNF-α: tissue necrosis factor-alpha; MIP: macrophage inflammatory protein; DAPI: 4′,6-diamidino-2-phenylindole; WBCs: white blood cells; CXCL12: C-X-C motif chemokine ligand 12; VAT: visceral adipose tissue; V/S: visceral adipose tissue to subcutaneous adipose tissue; MEERQAT: Medical Executable for the Efficient and Robust Quantification of Adipose Tissue; SCC: Spearman correlation coefficient; ER: estrogen receptor; HER2: human epidermal growth factor receptor 2; TNBC: triple-receptor negative breast cancer; SCC: Spearman correlation coefficient; WHO: World Health Organization; N/A: not applicable.
Supplementary Material
Supplementary tables.
Acknowledgements
We thank Ms. Laura L. Russell in the Research Medical Library, The University of Texas MD Anderson Cancer Center, and Ms. Shraddha Subramanian in the Center for Metabolic and Degenerative Diseases, The University of Texas Health Science Center at Houston, for editorial assistance.
Funding
This work was supported by the Morgan Welch Inflammatory Breast Cancer Research Program, a State of Texas Rare and Aggressive Breast Cancer Research Program grant, and by the National Institutes of Health/National Cancer Institute award 1R01CA205043-01A1 (to NTU). This study was also supported by the NIH/NCI under award number P30CA016672 and used the Biostatistics Resource Group.
Competing Interests
D.L.A. and C.-M.T. are employees of Creatv Microtech. The remaining authors declare that they have no competing interests.
References
1. Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. Jama. 2016;315:2284-91
2. Chan DSM, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A. et al. Body mass index and survival in women with breast cancer—systematic literature review and meta-analysis of 82 follow-up studies. Annals of Oncology. 2014;25:1901-14
3. Ligibel JA, Strickler HD. Obesity and Its Impact on Breast Cancer: Tumor Incidence, Recurrence, Survival, and Possible Interventions. American Society of Clinical Oncology Educational Book. 2013:52-9
4. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet (London, England). 2008;371:569-78
5. Fontanella C, Lederer B, Gade S, Vanoppen M, Blohmer JU, Costa SD. et al. Impact of body mass index on neoadjuvant treatment outcome: a pooled analysis of eight prospective neoadjuvant breast cancer trials. Breast cancer research and treatment. 2015;150:127-39
6. Iwase T, Nakamura R, Yamamoto N, Yoshi A, Itami M, Miyazaki M. The effect of molecular subtype and body mass index on neo-adjuvant chemotherapy in breast cancer patients. Breast (Edinburgh, Scotland). 2014;23:264-72
7. Litton JK, Gonzalez-Angulo AM, Warneke CL, Buzdar AU, Kau SW, Bondy M. et al. Relationship between obesity and pathologic response to neoadjuvant chemotherapy among women with operable breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26:4072-7
8. Pajares B, Pollan M, Martin M, Mackey JR, Lluch A, Gavila J. et al. Obesity and survival in operable breast cancer patients treated with adjuvant anthracyclines and taxanes according to pathological subtypes: a pooled analysis. Breast cancer research: BCR. 2013;15:R105
9. Razmkhah M, Jaberipour M, Erfani N, Habibagahi M, Talei AR, Ghaderi A. Adipose derived stem cells (ASCs) isolated from breast cancer tissue express IL-4, IL-10 and TGF-beta1 and upregulate expression of regulatory molecules on T cells: do they protect breast cancer cells from the immune response? Cellular immunology. 2011;266:116-22
10. Adams DL, Martin SS, Alpaugh RK, Charpentier M, Tsai S, Bergan RC. et al. Circulating giant macrophages as a potential biomarker of solid tumors. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:3514-9
11. Adams DL, Adams DK, Alpaugh RK, Cristofanilli M, Martin SS, Chumsri S. et al. Circulating Cancer-Associated Macrophage-Like Cells Differentiate Malignant Breast Cancer and Benign Breast Conditions. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2016;25:1037-42
12. Adams DK. Cancer-associated macrophage-like cells as prognostic indicators of overall survival in a variety of solid malignancies. Journal of Clinical Oncology. 2017;35:11503-11503
13. Tang CM, Adams DL. Clinical Applications of Cancer-Associated Cells Present in the Blood of Cancer Patients. Biomedicines. 2022;10(3):587
14. Adams DL, Stefansson S, Haudenschild C, Martin SS, Charpentier M, Chumsri S. et al. Cytometric characterization of circulating tumor cells captured by microfiltration and their correlation to the CellSearch(®) CTC test. Cytometry A. 2015;87:137-44
15. Adams DL, Alpaugh RK, Tsai S, Tang CM, Stefansson S. Multi-Phenotypic subtyping of circulating tumor cells using sequential fluorescent quenching and restaining. Sci Rep. 2016;6:33488
16. Sánchez-Martín L, Estecha A, Samaniego R, Sánchez-Ramón S, Vega M, Sánchez-Mateos P. The chemokine CXCL12 regulates monocyte-macrophage differentiation and RUNX3 expression. Blood. 2011;117:88-97
17. Parikh AM, Coletta AM, Yu ZH, Rauch GM, Cheung JP, Court LE. et al. Development and validation of a rapid and robust method to determine visceral adipose tissue volume using computed tomography images. PloS one. 2017;12:e0183515
18. Iwase T, Wang X, Shrimanker TV, Kolonin MG, Ueno NT. Body composition and breast cancer risk and treatment: mechanisms and impact. Breast cancer research and treatment. 2021;186:273-83
19. Quail DF, Dannenberg AJ. The obese adipose tissue microenvironment in cancer development and progression. Nat Rev Endocrinol. 2019;15:139-54
20. Adams D, Adams DK, Lin SH, Cristofanilli M, Bergan RC, Marks JR. et al. Cancer-associated macrophage-like cells as prognostic indicators of overall survival in a variety of solid malignancies. Journal of Clinical Oncology. 2017;35:11503 -
21. Chockalingam S, Ghosh SS. Macrophage colony-stimulating factor and cancer: a review. Tumour Biol. 2014;35:10635-44
22. Levine JA, Jensen MD, Eberhardt NL, O'Brien T. Adipocyte macrophage colony-stimulating factor is a mediator of adipose tissue growth. J Clin Invest. 1998;101:1557-64
23. Ntanasis-Stathopoulos I, Fotiou D, Terpos E. CCL3 Signaling in the Tumor Microenvironment. Adv Exp Med Biol. 2020;1231:13-21
24. Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME. et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50-6
25. Salvucci O, Bouchard A, Baccarelli A, Deschênes J, Sauter G, Simon R. et al. The role of CXCR4 receptor expression in breast cancer: a large tissue microarray study. Breast cancer research and treatment. 2006;97:275-83
Author contact
![]() Corresponding author: Naoto T. Ueno, University of Hawai'i Cancer Center, 701 Ilalo Street Honolulu, HI 96813. USA. E-mail: nuenohawaii.edu; Tel.: +1-808-586-3013.
Corresponding author: Naoto T. Ueno, University of Hawai'i Cancer Center, 701 Ilalo Street Honolulu, HI 96813. USA. E-mail: nuenohawaii.edu; Tel.: +1-808-586-3013.

 Global reach, higher impact
Global reach, higher impact