Impact Factor
ISSN: 1837-9664
J Cancer 2025; 16(1):135-145. doi:10.7150/jca.100678 This issue Cite
Research Paper
The effect of intraoperative radiotherapy in musculoskeletal malignancy: A population study from US SEER database
1. Department of Bone and Soft Tissue Tumors, Tianjin Medical University Cancer Institute &Hospital, National Clinical Research Center for Cancer, Tianjin's Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin, Tianjin 300060, China.
2. Department of Radiation Oncology, Tianjin Medical University Cancer Institute &Hospital National Clinical Research Center for Cancer, Tianjin's Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin, Tianjin 300060, China.
* First authors.
Received 2024-7-9; Accepted 2024-9-26; Published 2025-1-1
Abstract
Objective: The aim of our study was to explore the effect of IORT on survival outcome of patients with musculoskeletal malignancy. The prognostic factors of patients with IORT treatment were also identified in this study.
Methods: The retrospective analysis was conducted based on the Surveillance, Epidemiology, and End Results (SEER) database spanning from 2000 to 2020. The musculoskeletal malignancy patients who received both surgery and radiation therapy (RT) treatment were included into the study. Survival differences between groups were explored by Kaplan-Meier method and log-rank test. Potential prognostic factors of patients with IORT treatment were identified by Cox proportional hazards regression analysis.
Results: A total of 24,297 patients were selected finally, including 23,877 cases with neoadjuvant/adjuvant RT alone, 190 cases with IORT alone, and other 230 cases received both neoadjuvant/adjuvant RT and IORT. The median survival time of these patients was 141.0 (95%CI: 101.1-180.9) months. Patients who received both IORT and neoadjuvant/adjuvant RT treatment presented the best survival outcome when compared with those underwent either IORT or neoadjuvant/adjuvant RT only. Further subgroup analyses verified the survival benefit of the combination of IORT and neoadjuvant/adjuvant RT in female patients with tumor located on limb and in patients who received the performance of chemotherapy. A series of variables, including age at diagnosis, gender, primary tumor site, tumor Grade, SEER stage, T stage, N stage, IORT only or the combination of IORT and neoadjuvant/adjuvant RT, the performance of chemotherapy, were identified as independent prognostic factors of patients with IORT treatment.
Conclusions: The current study is distinguished by its large-scale analysis of the SEER database, encompassing a comprehensive cohort of musculoskeletal malignancy patients treated with IORT, as well as the rigorous subgroup analysis. We concluded that IORT during surgery procedure, accompanied with neoadjuvant/adjuvant RT, might confer a survival benefit for selected patients diagnosed with musculoskeletal malignancy.
Keywords: Bone Sarcoma, Soft Tissue Sarcoma, Intraoperative Radiotherapy, SEER Program, Survival Outcome
Introduction
Malignant musculoskeletal neoplasms, originating from bone or soft tissues, mainly included bone sarcomas and soft tissue sarcomas (STS), which accounting for nearly 1.0% of all adult tumors and about 15.0% in childhood [1]. The incidence of bone sarcomas was reported to be 0.8-0.9 per 100,000 persons while the incidence of STS was up to 1.28-1.72 per 100,000 persons [2, 3]. The prognosis of musculoskeletal malignancy was discrepant across numerous pathological subtypes due to obvious heterogeneity of histological manifestations. Nearly 9.0%-10.0% of patients would present local recurrence after tumor resection and about 7.5% of patients died of distant metastasis [3]. It was reported that the relative survival of bone sarcomas at 3-year, 5-year and 10-year were 73.3%, 67.4% and 61.9%, respectively [4]. The overall survival (OS) rate of low-grade sarcoma and high-grade sarcoma at 5 years were 87.0% and 62.0%, respectively, in STS [5].
The surgical resection was the mainstay of treatment in musculoskeletal malignancy and the 5-year disease-free survival rate after surgery was 50.0% as previously reported [6]. Nevertheless, a variety of factors could determine the therapeutic efficacy of surgery, including tumor size, anatomic tumor location, and infiltration of surrounding vessels and organs [7]. Moreover, the general condition of patients, as well as mode of surgery and surgical skills were of crucial importance to prognosis and outcomes [8]. In addition, margin status was one of the most crucial risk factors affecting local tumor recurrence. Patients with negative margin (R0 resection) can usually achieve long-term local control while in cases with R1 or R2 resection, the rate of local recurrence was significantly increased [9, 10]. The therapeutic principle of musculoskeletal malignancy has changed from amputation and similar radical surgical resection to a more comprehensive multi-modality way, and surgical wide resection plus RT has emerged as the standard approach for high-grade STS and specific types of bone sarcomas. According to the NCCN guidelines, neoadjuvant RT can be performed in patients with large high-grade STS to downstage the tumors while postoperative RT should be given in STS when the oncologically appropriate margins cannot be achieved [1].
Ionizing radiation could cause cell death by cleaving DNA [11]. However, the killing effect of radiation on cells was not selective. While killing tumor cells, normal tissues in the radiation target area could also be damaged. The adequate radiation dose is not always achievable with traditional external beam radiotherapy (EBRT) methods, primarily due to the low radiation tolerance of adjacent normal organs and tissues in target area [12, 13]. With the development of radiotherapy technology, three-dimensional radiotherapy methods, such as stereotactic body radiotherapy (SBRT), has become the main auxiliary approach to surgical treatment [14]. Nevertheless, these radiotherapy methods could only be carried out in batches outside the perioperative period when the patient's performance status is favorable. While prolonging the treatment time, it will make both normal cells and tumor cells recover to a certain extent [15]. Since the beginning of modern intraoperative radiotherapy (IORT) in the 1980s, growing evidence has demonstrated that IORT offers unique therapeutic advantages over traditional radiotherapy methods in musculoskeletal malignancy [16]. A previous study indicated that the success rate of IORT in STS was up to 90.0% [17]. In Germany, a total of 153 patients with limb STS were retrospectively analyzed and all the patients were received the treatment of intraoperative electron boost radiotherapy (IOERT) followed by EBRT [18]. After a median follow-up of 33 months, the 5-year OS rate and 5-year local control rate were 77.0% and 78.0%, respectively [18]. Nearly 23.0% of patients presented acute toxicity levels 2-4, and 17.0% had advanced toxicity level 2-4[18]. It concluded that the surgical resection combined with IORT not only improved the tumor control rate, but also resulted in acceptable radiation toxicity [18].
The Surveillance, Epidemiology, and End Results (SEER) database is one of the most representative large-scale oncology registration databases in North America, which included patients' demographic and clinicopathological characteristics as well as survival outcome information. Notably, the radiation modalities of each patient were recorded in detail. Thus, our study aimed to explore the characteristics of musculoskeletal malignancy patients who received IORT treatment based on data from the SEER database. Furthermore, we investigated the effect of IORT on survival outcome and identified the prognostic factors of musculoskeletal malignancy patients who were treated with IORT.
Materials and Methods
Data sources of SEER database
In the current study, we retrieved the clinical data of patients with musculoskeletal malignancy from the SEER database. The specific name of the database we used in the current study was illustrated as following: Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER Research Data, 17 Registries, Nov 2022 Sub (2000-2020) - Linked To County Attributes - Time Dependent (1990-2021) Income/Rurality, 1969-2021 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2023, based on the November 2022 submission.
Cohort selection and exclusion criteria of SEER analysis
We identified musculoskeletal malignancy patients diagnosed between 2000 to 2020 based on the variable 'AYA site recode 2020 Revision' in SEER*Stat software. The option was restricted to '4.Sarcomas', thus, including sarcomas originating both from soft tissue (soft tissue sarcoma category) and bone (bone sarcoma category) into the study. The soft tissue sarcoma category contained the following tumor types as SEER*Stat software recording: Ewing family of tumors originated from soft tissue, Fibromatous neoplasms, Liposarcoma, Synovial sarcoma, Leiomyosarcoma, Rhabdomyosarcoma, Gastrointestinal stromal tumor, Spindle cell sarcoma, Epithelioid sarcoma, Desmoplastic small round cell tumor, Giant cell sarcoma, Other soft tissue sarcomas. While the bone sarcoma category consisted of the following pathological types: Osteosarcoma, Chondrosarcoma, Ewing family of tumors originated from bone, Chordoma, Other bone tumors.
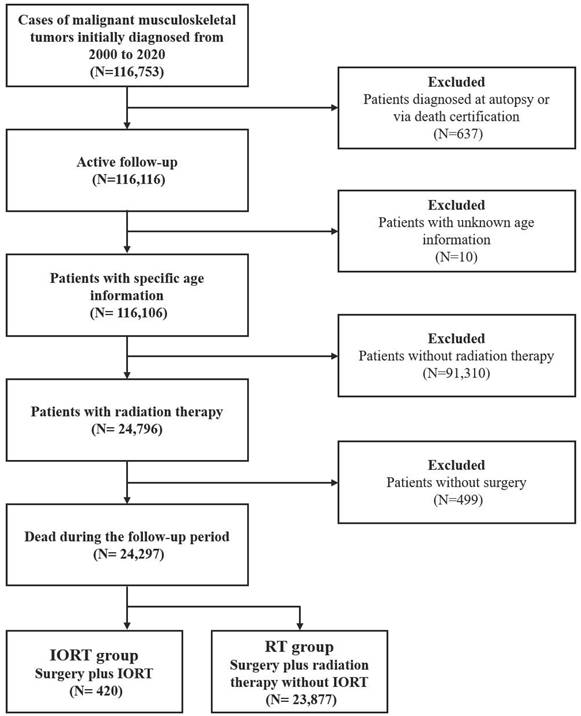
Initially, a total of 116,753 cases were selected. Patients diagnosed at autopsy, or indicated in death certification, and patients with unknown age information were routinely excluded. In order to exclude patients who did not receive either radiation therapy or surgical intervention, the variable 'RX Summ--Surg/Rad Seq' and the variable 'RX Summ--Surg Prim Site' were used. Finally, a total of 24,297 patients were included into the present study. Among them, 23,877 cases received pre/postoperative RT without IORT, thus deemed as neoadjuvant/adjuvant RT group. While the other 420 cases received IORT alone (IORT- group) or the combination of IORT and neoadjuvant/ adjuvant RT (IORT+ group). The flowchart of cohort selection was shown in supplementary Figure 1.
The flowchart of cohort selection.
Outcome Measures and statistics analysis
All the quantitative data were expressed as mean ± standard deviation (SD), while the categorical data were presented as the number and the percentage (N, %). Pearson chi-square (χ2) test or Fisher's exact test was used to evaluate the difference between categorical variables. The main outcome of the study was overall survival (OS), which was defined as the interval time between initial diagnosis and all cause of death or the last follow-up. The Kaplan-Meier method was performed and the log-rank test was employed to analyze survival difference between groups. To identify the prognostic factors of patients who received IORT, the Cox proportional hazards regression analysis was performed. Those variables with P<0.05 in univariate analysis were analyzed in a further multivariate analysis to determine the independent prognostic factors. The SPSS 26.0 (IBM Corporation, Armonk, NY) was used for statistical analyzing and all the survival curves were performed by MedCalc® Statistical Software version 20.100 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2022). All tests were two sided and values of P<0.05 were considered statistically significant.
Ethics Statement
The present study is in accordance with the 1964 Declaration of Helsinki and subsequent amendments or comparable ethical standards. The informed consent of patients was not required since the SEER dataset is an open access database available all over the world and cancer a is reportable disease in the USA.
Results
Characteristics of the patients with musculoskeletal malignancy
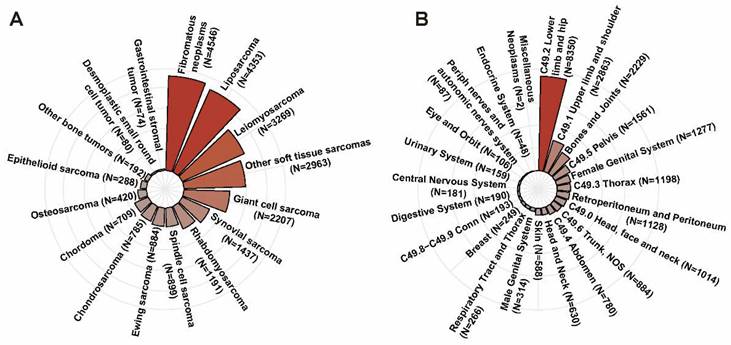
According to the inclusion and exclusion criteria, a total of 24,297 patients diagnosed with musculoskeletal malignancy in the SEER dataset were included. The mean age of all patients was 54.5±20.7 years with a slightly male predominance (N=13,030, 53.6%). As shown in Figure 2A, the top five pathologic types were fibromatous neoplasms (N=4,546, 18.7%), liposarcoma (N=4,353, 17.9%), leiomyosarcoma (N=3,269, 13.5%), giant cell sarcoma (N=2,207, 9.1%) and synovial sarcoma (N=1,437, 5.9%). As for primary tumor location, lower limb and hip was the most common tumor site accounting for 34.4% (N=8,350), followed by upper limb and shoulder (N=2,683, 11.8%), bones and joints (N=2,229, 9.2%). The distribution of cases in different primary tumor sites was described in Figure 2B.
Among all patients, a total of 23,877 patients were collected into the RT group, in which patients received both surgery without IORT and neoadjuvant/adjuvant RT. The other 420 patients received IORT during the surgical procedure. Detailed information about the baseline demographic and clinicopathological characteristics in the RT group and the IORT group were presented in Table 1. The distribution of gender, marital status, SEER stage and distant metastases were not statistically different between the RT group and the IORT group. It seemed that patients in the IORT group possessed a higher level of income than the RT group. Besides, the primary tumor site of limb was more common in IORT group and these patients were more tended to undergo chemotherapy at the same time.
The distribution of cases in different pathologic types (A) and the distribution of cases in different primary tumor sites (B).
Demographic and clinicopathological characteristics of patients with musculoskeletal malignancy in SEER database.
| Characteristics | Neoadjuvant/adjuvant RT group | IORT group | P-value |
|---|---|---|---|
| Gender | |||
| Male | 12795 (53.6) | 235 (56.0) | 0.335 |
| Female | 11082 (46.4) | 185 (44.0) | |
| Marital status | |||
| Unmarried | 9801 (41) | 181 (43.1) | 0.151 |
| Married | 13289 (55.7) | 232 (55.2) | |
| Unknown | 787 (3.3) | 7 (1.7) | |
| Race | |||
| White | 19243 (80.6) | 341 (81.2) | <0.001 |
| Black | 2337 (9.8) | 20 (4.8) | |
| Others | 2193 (9.2) | 59 (14.0) | |
| Unknown | 104 (0.4) | 0 (0) | |
| Median household income | |||
| < $65,000 | 6874 (28.8) | 100 (23.8) | <0.001 |
| $65,000 - $75,000 | 6331 (26.5) | 53 (12.6) | |
| > $75,000 | 10670 (44.7) | 267 (63.6) | |
| Unknown | 2 (0) | 0 (0) | |
| Histology | |||
| Osteosarcoma | 417 (1.7) | 3 (0.7) | <0.001 |
| Chondrosarcoma | 777 (3.3) | 8 (1.9) | |
| Ewing sarcoma | 878 (3.7) | 6 (1.4) | |
| Fibromatous neoplasms | 4473 (18.7) | 73 (17.4) | |
| Liposarcoma | 4258 (17.8) | 95 (22.6) | |
| Synovial sarcoma | 1391 (5.8) | 46 (11.0) | |
| Leiomyosarcoma | 3222 (13.5) | 47 (11.2) | |
| Rhabdomyosarcoma | 1172 (4.9) | 19 (4.5) | |
| Gastrointestinal stromal tumor | 69 (0.3) | 5 (1.2) | |
| Spindle cell sarcoma | 879 (3.7) | 20 (4.8) | |
| Epithelioid sarcoma | 283 (1.2) | 5 (1.2) | |
| Desmoplastic small round cell tumor | 80 (0.3) | 0 (0) | |
| Chordoma | 703 (2.9) | 6 (1.4) | |
| Giant cell sarcoma | 2172 (9.1) | 35 (8.3) | |
| Other soft tissue sarcomas | 2913 (12.2) | 50 (11.9) | |
| Other bone tumors | 190 (0.8) | 2 (0.5) | |
| Primary site | |||
| Limb | 11432 (47.9) | 232 (55.2) | <0.001 |
| Trunk | 5286 (22.1) | 101 (24.0) | |
| Head, face, neck | 1796 (7.5) | 2 (0.5) | |
| Other sites | 5142 (21.5) | 83 (19.8) | |
| Unknown | 221 (0.9) | 2 (0.5) | |
| Grade | |||
| Well differentiated; Grade I | 1790 (7.5) | 51 (12.1) | <0.001 |
| Moderately differentiated; Grade II | 3345 (14.0) | 84 (20.0) | |
| Poorly differentiated; Grade III | 5208 (21.8) | 109 (26.0) | |
| Undifferentiated; anaplastic; Grade IV | 6941 (29.1) | 105 (25.0) | |
| Unknown | 6593 (27.6) | 71 (16.9) | |
| SEER Stage | |||
| Localized | 12321 (51.6) | 205 (48.8) | 0.121 |
| Regional | 5434 (22.8) | 116 (27.6) | |
| Distant | 1935 (8.1) | 29 (6.9) | |
| Unknown | 4187 (17.5) | 70 (16.7) | |
| T stage | |||
| T1 | 5256 (22.0) | 86 (20.5) | <0.001 |
| T2 | 9775 (40.9) | 226 (53.8) | |
| T3 | 737 (3.1) | 9 (2.1) | |
| T4 | 526 (2.2) | 5 (1.2) | |
| Unknown | 7583 (31.8) | 94 (22.4) | |
| N stage | |||
| N0 | 16291 (68.2) | 315 (75.0) | <0.001 |
| N1 | 662 (2.8) | 19 (4.5) | |
| Unknown | 6924 (29.0) | 86 (20.5) | |
| M stage | |||
| M0 | 16145 (67.6) | 312 (74.3) | 0.003 |
| M1 | 1296 (5.4) | 26 (6.2) | |
| Unknown | 6436 (27.0) | 82 (19.5) | |
| Bone metastasis | |||
| No | 13190 (55.2) | 245 (58.3) | 0.423 |
| Yes | 293 (1.2) | 4 (1.0) | |
| Unknown | 10394 (43.5) | 171 (40.7) | |
| Brain metastasis | |||
| No | 13426 (56.2) | 250 (59.5) | 0.269 |
| Yes | 53 (0.2) | 0 (0) | |
| Unknown | 10398 (43.5) | 170 (40.5) | |
| Liver metastasis | |||
| No | 13361 (56.0) | 246 (58.6) | 0.170 |
| Yes | 110 (0.5) | 4 (1) | |
| Unknown | 10406 (43.6) | 170 (40.5) | |
| Lung metastasis | |||
| No | 12878 (53.9) | 242 (57.6) | 0.284 |
| Yes | 594 (2.5) | 8 (1.9) | |
| Unknown | 10405 (43.6) | 170 (40.5) | |
| Chemotherapy | |||
| No/Unknown | 16837 (70.5) | 271 (64.5) | 0.008 |
| Yes | 7040 (29.5) | 149 (35.5) |
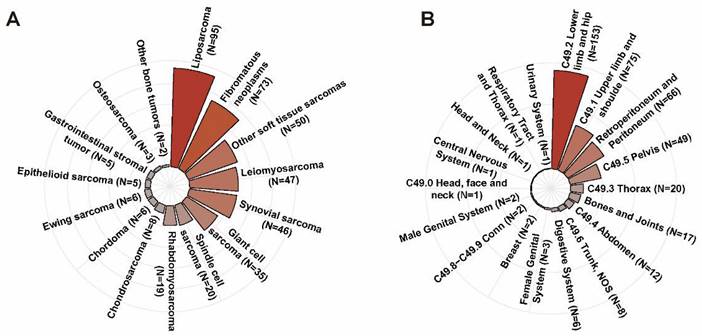
In the IORT group, the mean age of patients was 51.7±20.7 years. There were 235 male patients (56.0%) and 185 female cases (44.0%), respectively. The majority of patients were White race (N=341, 81.2%) and nearly half of them were diagnosed with localized disease (N=205, 48.8%). As shown in Figure 3A, the predominant histologic subtypes in the IORT group were liposarcoma (N=95, 22.6%), fibromatous neoplasms (N=73, 17.4%), leiomyosarcoma (N=47, 11.2%), synovial sarcoma (N=46, 11.0%) and giant cell sarcoma (N=35, 8.3%). Lower limb and hip (N=153, 36.4%), upper limb and shoulder (N=75, 17.9%), retroperitoneum and peritoneum (N=66, 15.7%) were the common tumor sites in the IORT group as described in Figure 3B. The number of patients who received neoadjuvant/adjuvant chemotherapy was 149, accounting for 35.5% in the IORT group. As for radiotherapy modes in the IORT group, 190 of 420 cases received IORT alone (IORT- group) while the other 230 patients received the combination of IORT and neoadjuvant/ adjuvant RT (IORT+ group). As presented in Supplementary Table 1, the proportion of patients receiving chemotherapy was significantly higher in the IORT+ group compared to the IORT- group (P = 0.003). No significant differences were observed between the two groups in terms of other variables.
Survival outcome and subgroup analyses
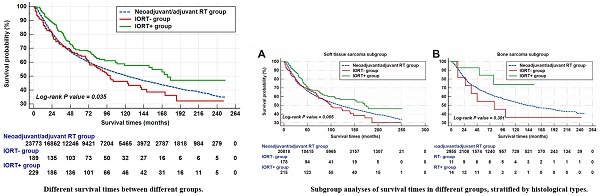
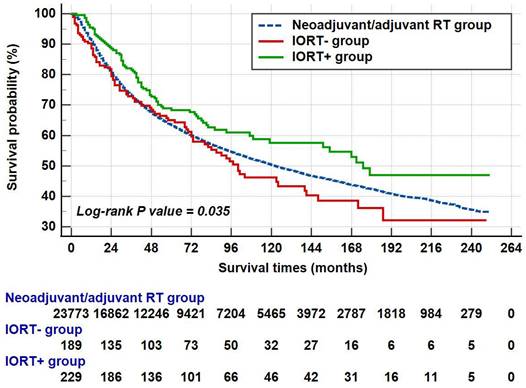
The median survival of all patients was 122.0 (95%CI: 117.1-126.9) months. In the neoadjuvant/adjuvant RT group, the mean survival and median survival time were 136.2 (95%CI: 134.6-137.7) and 122.0 (95%CI: 117.1-126.9) months, respectively. As for IORT group, the median survival time was up to 141.0 (95%CI: 101.1-180.9) months and the 1-year, 3-year, 5-year and 10-year OS rates were 92.4%, 77.7%, 66.9% and 52.3%, respectively. As shown in Figure 4, patients in the IORT+ group presented better survival outcome when compared with the neoadjuvant/adjuvant RT group and the IORT- group (P=0.035). The survival outcome of these three groups was summarized in Table 2.
The survival outcome of musculoskeletal malignancy patients who received radiation therapy in SEER database.
| Groups | Mean survival | 95%CI | Median survival | 95% CI | 1-year OS% | 3-year OS% | 5-year OS% | 10-year OS% |
|---|---|---|---|---|---|---|---|---|
| Neoadjuvant/adjuvant RT | 136.2 | 134.6 - 137.7 | 122.0 | 118.0 - 127.0 | 90.8% | 73.0% | 63.4% | 50.3% |
| IORT- | 125.8 | 108.8 - 142.8 | 100.0 | 73.0 - 141.0 | 88.6% | 72.9% | 65.0% | 46.2% |
| IORT+ | 155.2 | 139.7 - 170.8 | 175.0 | 119.0 - 179.0 | 95.5% | 81.6% | 68.3% | 57.6% |
The predominant histologic subtypes (A) and the tumor sites (B) in IORT group.
The survival times of different groups. Note: The patient counts in this figure were lower than the total numbers reported in the Abstract and other sections because patients with a survival time of less than one month were not shown. Specifically, 104 patients from the neoadjuvant/adjuvant RT group and one patient each from the IORT- and IORT+ groups were excluded.
In male patients, the median survival in the neoadjuvant/adjuvant RT group, IORT+ group, and the IORT- group were 116.0 (95%CI: 109.9-122.1), 72.0 (95%CI: 50.0-94.0) and 119.0 (95%CI: not available) months, respectively. As presented in Figure 5A, the difference between these groups was not statistically significant. In female, the median survival of the IORT+ group (179.0 months, 95%CI: not available) was higher than those in the neoadjuvant/adjuvant RT group (131.0 months, 95%CI: 123.1-138.9 months) and IORT- group (148.0 months, 95%CI: 98.4-197.6 months). The survival curve of female patients was shown in Figure 5B (P = 0.037).
Subgroup analyses of survival times in different groups, stratified by gender, primary tumor location, and chemotherapy. Note: The patient counts in this figure were lower than the total numbers reported in the Abstract and other sections because patients with a survival time of less than one month were not shown.
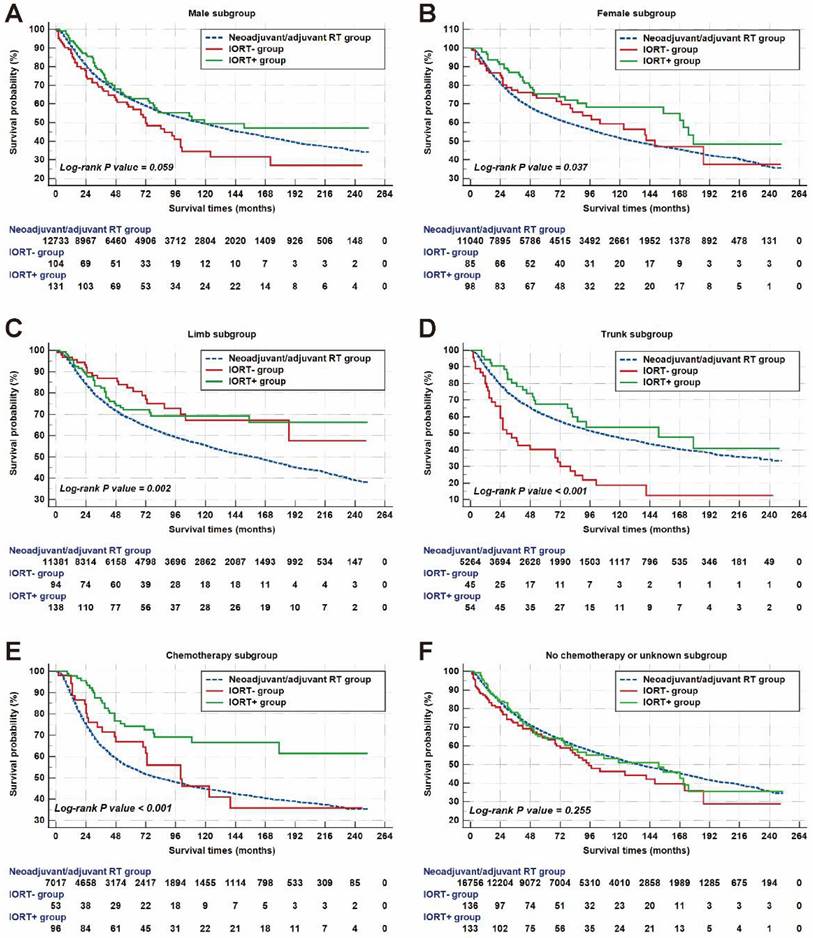
As for patients with tumors located on limb, the median survival of the neoadjuvant/ adjuvant RT group was 158.0 (95%CI: 149.6-166.4) months, which was shorter than those in the IORT- group and IORT+ group (Figure 5C, P = 0.002). In trunk subgroup, there was a statistically significant difference in survival outcome between groups with a P value < 0.001 as shown in Figure 5D. The median survival in the neoadjuvant/adjuvant RT group, the IORT- group and the IORT+ group were 103.0 (95%CI: 94.4-111.6) months, 29.0 (95%CI: 16.4-41.6) months and 151.0 (95%CI: 50.7-251.3) months, respectively.
For musculoskeletal malignancy patients who received the performance of chemotherapy, the mean survival of cases in the IORT+ group was up to 178.3 (95%CI: 155.9-200.6) months and no more than half of patients died until the last follow-up. Meanwhile, the mean survival of the neoadjuvant/adjuvant RT group and the IORT- group were 125.2 (95%CI: 122.4-128.9) and 127.4 (95%CI: 96.7-158.0) months, respectively. Patients in the IORT+ group had a longer survival time than the other two groups, as shown in Figure 5E. However, there were no significant differences between radiotherapy treatment groups in patients who did not receive chemotherapy or those without specific chemotherapy information (Figure 5F).
To further investigate the potential survival benefit, we conducted a subgroup analysis based on histological types. As illustrated in Supplementary Figure 1, the analysis compared the survival outcomes across three treatment modalities (neoadjuvant/adjuvant RT group, IORT- group, and IORT+ group) within both soft tissue sarcoma and bone sarcoma subgroups. The results showed no statistically significant differences in survival outcomes among the three groups in either the soft tissue sarcoma or bone sarcoma subgroups. However, in the soft tissue sarcoma subgroup (Supplementary Figure 1A), the IORT+ group demonstrated a longer mean survival time (153.2 months) compared to the neoadjuvant/adjuvant RT group (135.1 months) and the IORT- group (125.4 months). Meanwhile, the mean survival of the neoadjuvant/adjuvant RT group, the IORT- group and the IORT+ group in bone sarcoma subgroup were 143.4 (95%CI: 139.1-147.7), 117.1 (95%CI: 58.9-175.3) months and 127.9 (95%CI: 100.8-154.9) months, respectively (P =0.301).
Prognostic factors of patients with IORT
The Cox proportional hazards regression analysis was performed to identify the independent prognostic factors of musculoskeletal malignancy patients who underwent IORT. In the univariate analysis, age at diagnosis, gender, primary tumor site, tumor Grade, SEER stage, T stage, N stage, M stage, bone metastasis, radiotherapy method (IORT alone or IORT plus neoadjuvant/adjuvant RT), and the performance of chemotherapy were associated with patients' survival outcome. To adjust these parameters and exclude potential confounders, the multivariate analysis was performed and then several independent prognostic factors were identified. Older age (versus younger age; HR=1.02, 95% CI: 1.01-1.03, P<0.001), tumor located in the trunk (versus limb tumor location; HR=1.95, 95% CI: 1.33-2.85, P=0.001) and other sites (versus limb tumor location; HR=1.70, 95% CI: 1.10-2.61, P=0.016), higher tumor Grade (versus Grade I; Grade III: HR=3.06, 95% CI: 1.58-5.90, P=0.001; Grade IV: HR=3.63, 95% CI: 1.97-6.70, P<0.001), regional SEER stage (versus localized SEER stage; HR=2.11, 95% CI: 1.38-3.23, P=0.001) were independent prognostic factors for worse survival. Meanwhile, female gender (versus male gender; HR=0.56, 95% CI: 0.40-0.79, P=0.001) was an independent prognostic factor for better survival. Besides, higher T stage and N stage were proved to indicate worse survival when compared with their counterparts. The performance of chemotherapy was a protective factor with a 0.66-fold increased risk of death (versus No/Unknown chemotherapy; 95% CI: 0.44-1.00, P=0.048). It is worth noting that the combination of IORT and neoadjuvant/adjuvant RT presented a survival benefit for patients. There was a 0.65-fold of risk death when compared with IORT alone. Detailed information about Cox regression analysis was listed in Table 3.
Discussion
The treatment in musculoskeletal malignancy usually adopts a comprehensive model emphasizing interdisciplinary collaboration, in which surgery is the cornerstone of therapy while auxiliary radiation therapy servers as an effective mean to improve local tumor control [19]. As for IORT, two major approaches have been reported and used worldwide, thus IOERT and high-dose-rate brachytherapy. Johannes et al. summarized the previous literature about the application of brachytherapy for STS and analyzed the recurrence rate as well as local complications [20]. It reported a local control rate of 50%-90% in STS of the extremities after brachytherapy as monotherapy [20]. In another study, the perioperative high-dose-rate interstitial brachytherapy (PHDRIBT) was performed in localized STS patients after two days of tumor resection and EBRT was supplemented after one month [21]. No recurrent cases were observed during a median follow-up of 46.0 months and the 5-year disease-free survival rate was reported to be 63.0% [21]. Both excellent local control and high survival rate could be acquired in this treatment regime involving both PHDRIBT and EBRT [21]. To validate the advancements of IORT treatment, we conducted a retrospective study based on the SEER database to explore the effect of IORT in musculoskeletal malignancy. Unlike previous studies that have focused on specific clinical situations or isolated surgical challenges, our research systematically explored the application of IORT across different contexts within the musculoskeletal malignancy. We found that patients with the combination treatment of IORT and neoadjuvant/adjuvant RT presented a median survival time up to 175.0 months, which was better than that of patients who underwent IORT alone or neoadjuvant/adjuvant RT alone. Besides, the potential prognostic factors of patients with IORT treatment were identified in our study.
Identifying the independent prognostic factors of patients underwent IORT in SEER database.
| Characteristics | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P-Value | HR (95% CI) | P-Value | |
| Age at diagnosis | 1.03 (1.02-1.04) | <0.001 | 1.02 (1.01-1.03) | <0.001 |
| Gender | ||||
| Male | 1.00 (Reference) | 1.00 (Reference) | ||
| Female | 0.65 (0.48-0.89) | 0.006 | 0.56 (0.40-0.79) | 0.001 |
| Marital status | ||||
| Unmarried | 1.00 (Reference) | |||
| Married | 1.03 (0.76-1.40) | 0.856 | ||
| Unknown | 1.55 (0.56-4.24) | 0.397 | ||
| Race | ||||
| White | 1.00 (Reference) | |||
| Black | 0.58 (0.24-1.42) | 0.236 | ||
| Others | 1.36 (0.91-2.05) | 0.135 | ||
| Median household income | ||||
| < $65,000 | 1.00 (Reference) | |||
| $65,000 - $75,000 | 0.97 (0.59-1.59) | 0.900 | ||
| > $75,000 | 0.82 (0.58-1.16) | 0.264 | ||
| Histology | ||||
| Osteosarcoma | 1.00 (Reference) | |||
| Chondrosarcoma | 1.16 (0.12-11.13) | 0.900 | ||
| Ewing sarcoma | 5.85 (0.68-50.25) | 0.107 | ||
| Fibromatous neoplasms | 2.15 (0.29-15.75) | 0.450 | ||
| Liposarcoma | 1.87 (0.26-13.64) | 0.535 | ||
| Synovial sarcoma | 0.56 (0.07-4.47) | 0.583 | ||
| Leiomyosarcoma | 2.89 (0.39-21.32) | 0.297 | ||
| Rhabdomyosarcoma | 1.21 (0.15-10.08) | 0.859 | ||
| Gastrointestinal stromal tumor | 1.59 (0.17-15.33) | 0.687 | ||
| Spindle cell sarcoma | 1.52 (0.19-12.37) | 0.696 | ||
| Epithelioid sarcoma | 1.62 (0.15-17.86) | 0.695 | ||
| Chordoma | 0.62 (0.04-9.89) | 0.733 | ||
| Giant cell sarcoma | 1.31 (0.16-10.53) | 0.797 | ||
| Other soft tissue sarcomas | 2.19 (0.30-16.28) | 0.442 | ||
| Other bone tumors | - | 0.952 | ||
| Primary site | ||||
| Limb | 1.00 (Reference) | 1.00 (Reference) | ||
| Trunk | 2.60 (1.81-3.75) | <0.001 | 1.95 (1.33-2.85) | 0.001 |
| Head, face, neck | 1.46 (0.20-10.53) | 0.709 | 0.75 (0.09-6.22) | 0.792 |
| Other sites | 2.52 (1.73-3.67) | <0.001 | 1.70 (1.10-2.61) | 0.016 |
| Unknown | 4.82 (1.17-19.81) | 0.029 | 3.96 (0.90-17.47) | 0.070 |
| Grade | ||||
| Well differentiated; Grade I | 1.00 (Reference) | 1.00 (Reference) | ||
| Moderately differentiated; Grade II | 0.85 (0.43-1.69) | 0.642 | 1.44 (0.69-3.02) | 0.331 |
| Poorly differentiated; Grade III | 1.86 (1.04-3.34) | 0.036 | 3.06 (1.58-5.90) | 0.001 |
| Undifferentiated; anaplastic; Grade IV | 2.60 (1.47-4.58) | 0.001 | 3.63 (1.97-6.70) | <0.001 |
| Unknown | 1.51 (0.81-2.80) | 0.196 | 2.62 (1.32-5.17) | 0.006 |
| SEER Stage | ||||
| Localized | 1.00 (Reference) | 1.00 (Reference) | ||
| Regional | 2.74 (1.86-4.04) | <0.001 | 2.11 (1.38-3.23) | 0.001 |
| Distant | 3.45 (2.02-5.88) | <0.001 | 0.84 (0.11-6.58) | 0.865 |
| Unknown | 2.34 (1.55-3.52) | <0.001 | 0.93 (0.43-2.04) | 0.861 |
| T stage | ||||
| T1 | 1.00 (Reference) | 1.00 (Reference) | ||
| T2 | 3.22 (1.80-5.75) | <0.001 | 2.06 (1.11-3.83) | 0.022 |
| T3 | 3.54 (0.46-27.32) | 0.226 | 2.74 (0.33-22.78) | 0.352 |
| T4 | 1.63 (0.21-12.50) | 0.636 | 0.28 (0.03-2.45) | 0.252 |
| Unknown | 3.85 (2.11-7.03) | <0.001 | 2.17 (0.73-6.46) | 0.165 |
| N stage | ||||
| N0 | 1.00 (Reference) | 1.00 (Reference) | ||
| N1 | 2.34 (1.25-4.36) | 0.008 | 2.06 (1.11-3.83) | 0.022 |
| Unknown | 1.76 (1.26-2.44) | 0.001 | 2.74 (0.33-22.78) | 0.352 |
| M stage | ||||
| M0 | 1.00 (Reference) | 1.00 (Reference) | ||
| M1 | 2.73 (1.65-4.57) | <0.001 | 4.35 (0.52-36.34) | 0.175 |
| Unknown | 1.75 (1.25-2.45) | 0.001 | 1.03 (0.22-4.82) | 0.971 |
| Bone metastasis | ||||
| No | 1.00 (Reference) | 1.00 (Reference) | ||
| Yes | 5.75 (2.08-15.86) | 0.001 | 1.88 (0.56-6.38) | 0.310 |
| Unknown | 1.57 (1.14-2.18) | 0.007 | 1.25 (0.84-1.86) | 0.270 |
| Brain metastasis | ||||
| No | 1.00 (Reference) | |||
| Unknown | 1.47 (1.07-2.03) | 0.018 | ||
| Liver metastasis | ||||
| No | 1.00 (Reference) | |||
| Yes | 2.51 (0.79-8.00) | 0.119 | ||
| Unknown | 1.52 (1.10-2.10) | 0.012 | ||
| Lung metastasis | ||||
| No | 1.00 (Reference) | |||
| Yes | 1.81 (0.66-4.97) | 0.250 | ||
| Unknown | 1.51 (1.09-2.10) | 0.013 | ||
| Radiotherapy method | ||||
| IORT only | 1.00 (Reference) | 1.00 (Reference) | ||
| IORT plus neoadjuvant/adjuvant RT | 0.70 (0.52-0.94) | 0.017 | 0.65 (0.47-0.90) | 0.009 |
| Chemotherapy | ||||
| No/Unknown | 1.00 (Reference) | 1.00 (Reference) | ||
| Yes | 0.69 (0.50-0.95) | 0.023 | 0.66 (0.44-1.00) | 0.048 |
IORT is performed under the condition of fully exposing the irradiated area during surgery. It helps to set the irradiation area accurately and eliminates the time interval between surgical resection and postoperative radiation therapy [22]. During the surgical procedure, the normal tissues and radiosensitive organs surrounding to the target area could be removed or temporarily shielded, ensuring the adequate target dose to achieve local control [22]. Furthermore, it has been reported that a single high-dose irradiation during surgery could yield a 2.5-fold greater biological effect than that of conventional external irradiation [23]. Azinovic et al. conducted the one of the earliest IORT study in 2003 and 45 patients with extremity sarcomas were analyzed, including 19 patients with recurrent disease [24]. Most of them (36/45, 80.0%) were diagnosed with tumor larger than 5.0cm and nine patients relapsed until the last follow-up with an actuarial local control rate at 5-year of 88.0%. In Austria, a total of 35 patients with high-grade STS were retrospectively analyzed [25]. All of them received IORT treatment during limb-preserving surgery, and pre/postoperative radiotherapy was also performed [25]. At the last follow-up, the 2-year local control rate was up to 94.3% while the local recurrence rate for R0, R1 and R2 resections were 6.0%, 13.0% and 100.0%, respectively [25]. It concluded that the combination of IORT and pre- or postoperative radiotherapy could help for satisfactory local tumor control [25].
The American Society for Radiation Oncology (ASTRO) and the European Society of Radiotherapy & Oncology (ESTRO) have elaborated the role and advantages of IORT and proposed expert recommendation on the application of IORT [26, 27]. The ASTRO recommend that IORT should be used in conjunction with auxiliary external irradiation to limit local recurrence [26]. IORT is suitable for the situation in which the surgical margin may be positive. The dose range of IORT should be 10-17.5 Gy in the abdomen and 10-20 Gy in the limbs [26]. It is also recommended that the higher dose among appropriate doses should be used in selected cases with high risk of positive margin [26]. On this basis, ESTRO has claimed that the combination of preoperative RT and IORT could be more advantageous than postoperative RT alone in controlling local tumors and avoiding late-stage toxicities [27]. In addition, to prevent severe neurotoxic reaction, the dose of IORT should be limited to below 12.5Gy [27].
Several demographic and clinicopathological characteristics, including age, tumor Grade, clinical stage, resection margin, were found to be associated with the survival outcome of patients receiving IORT treatment in the previous studies [18, 28, 29]. In a single-center retrospective study conducted by the University of Heidelberg, a total of 183 patients with extremity STS were treated with IOERT and preceded/followed EBRT. After a median follow-up of 64.0 months, the 5-year OS and 10-year OS rate were 77.0% and 66.0%, respectively [28]. In univariate analysis, tumor Grade, metastases prior/at the time of IOERT, and clinical stage were three factors associated with OS. Further multivariate analysis concluded that tumor Grade and distant metastasis were two prognostic factors affecting survival outcome [28]. Our current findings based on SEER cohort were consistent with, and extended, the previously study. We found tumor location was associated with patients' OS while female gender and the performance of chemotherapy indicated good survival outcome. Patients with tumor located in trunk had a 1.95-fold risk of mortality compared with patients with limb-located tumors. The potential cause behind the effect of tumor location on survival might be attributed to tumor resectability in different sites.
Our study was limited by the biases arising from the SEER database and the retrospective design of study. Firstly, the end point of our study was restricted to OS since neither disease-free survival (DFS) after IORT nor local control rate was recorded in the SEER database. This limitation cannot be ignored considering the fact that IORT is an efficient method for local tumor control. Second, the information on IORT dose, resection margins as well as adjacent anatomical relationships was not available in the SEER database. These variables were significantly associated with the survival outcome of patients receiving IORT treatment [18]. However, our findings may not provide effective guidance for addressing specific clinical challenges, such as tendon involvement [30] and soft tissue reconstruction [31]. Finally, the histologic subgroup analysis was not performed adequately. Based on the classification criteria existing in the SEER database, we found no statistically significant result in our Cox proportional hazards regression analysis. Thus, further large-scale clinical studies are needed to validate the results.
Conclusions
The key strength of our study was the comprehensive analysis conducted on a large cohort derived from the SEER database, encompassing a broad range of musculoskeletal malignancy. This approach allowed us to evaluate the impact of intraoperative radiotherapy (IORT) across various tumor types and clinical scenarios, offering a more generalized understanding of its effectiveness in diverse patient populations. Despite the aforementioned limitations, we confirmed the survival benefit of the combination of IORT during surgery and neoadjuvant/adjuvant RT. Furthermore, we identified several independent prognostic factors of patients undergoing IORT treatment, which can aid in clinically making individualized treatment plans.
Supplementary Material
Supplementary figure and table.
Acknowledgements
Funding
National Natural Science Foundation of China (82303076); National Key Laboratory of Druggability Evaluation and Systematic Translational Medicine (QZ23-10); Science & Technology Development Fund of Tianjin Education Commission for Higher Education (2022KJ225).
Data availability statement
Only publicly available data were used in our study, and data sources and handling of these data are described in the Materials and Methods. Further information is available from the corresponding author upon request.
Author contributions
Conceptualization, LiMing Xu and GuoWen Wang; methodology, Yao Xu; software, Zhe Feng; validation, YongHeng Liu; formal analysis, YaoXu and Zhe Feng; investigation, JinYan Feng; resources, Yan Zhang; writing-original draft preparation, Yao Xu and Zhe Feng; writing-review and editing, Chao Zhang and XiuXin Han; visualization, Yao Xu and Zhe Feng; supervision, GuoWen Wang; funding acquisition, JinYan Feng and GuoWen Wang. All authors have read and agreed to the published version of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. NCCN Guidelines Version 3. 2024. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1464
2. Strauss SJ, Frezza AM, Abecassis N. et al. Bone sarcomas: ESMO-EURACAN-GENTURIS-ERN PaedCan Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2021;32:1520-1536
3. Vodanovich DA, PF MC. Soft-tissue Sarcomas. Indian J Orthop. 2018;52:35-44
4. Xu Y, Shi F, Zhang Y. et al. Twenty-year outcome of prevalence, incidence, mortality and survival rate in patients with malignant bone tumors. Int J Cancer. 2024;154:226-240
5. Bannasch H, Eisenhardt SU, Grosu AL, Heinz J, Momeni A, Stark GB. The diagnosis and treatment of soft tissue sarcomas of the limbs. Dtsch Arztebl Int. 2011;108:32-38
6. Chiappa A, Bertani E, Pravettoni G. et al. Aggressive Surgical Approach for Treatment of Primary and Recurrent Retroperitoneal Soft Tissue Sarcoma. Indian J Surg. 2018;80:154-162
7. Chiappa A, Zbar AP, Biffi R. et al. Primary and recurrent retroperitoneal sarcoma: factors affecting survival and long-term outcome. Hepatogastroenterology. 2004;51:1304-1309
8. Gilbert NF, Cannon CP, Lin PP, Lewis VO. Soft-tissue sarcoma. J Am Acad Orthop Surg. 2009;17:40-47
9. von Mehren M, Kane JM, Agulnik M. et al. Soft Tissue Sarcoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:815-833
10. Biermann JS, Chow W, Reed DR. et al. NCCN Guidelines Insights: Bone Cancer, Version 2.2017. J Natl Compr Canc Netw. 2017;15:155-167
11. Szatkowska M, Krupa R. Regulation of DNA Damage Response and Homologous Recombination Repair by microRNA in Human Cells Exposed to Ionizing Radiation. Cancers (Basel). 2020;12:1838
12. Davis AM, O'Sullivan B, Turcotte R. et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol. 2005;75:48-53
13. O'Sullivan B, Davis AM, Turcotte R. et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359:2235-2241
14. Bhide SA, Nutting CM. Recent advances in radiotherapy. BMC Med. 2010;8:25
15. Barney BM, Petersen IA, Dowdy SC, Bakkum-Gamez JN, Haddock MG. Long-term outcomes with intraoperative radiotherapy as a component of treatment for locally advanced or recurrent uterine sarcoma. Int J Radiat Oncol Biol Phys. 2012;83:191-197
16. Roeder F, Krempien R. Intraoperative radiation therapy (IORT) in soft-tissue sarcoma. Radiat Oncol. 2017;12:20
17. Miller ED, Xu-Welliver M, Haglund KE. The role of modern radiation therapy in the management of extremity sarcomas. J Surg Oncol. 2015;111:599-603
18. Oertel S, Treiber M, Zahlten-Hinguranage A. et al. Intraoperative electron boost radiation followed by moderate doses of external beam radiotherapy in limb-sparing treatment of patients with extremity soft-tissue sarcoma. Int J Radiat Oncol Biol Phys. 2006;64:1416-1423
19. Naghavi AO, Fernandez DC, Mesko N. et al. American Brachytherapy Society consensus statement for soft tissue sarcoma brachytherapy. Brachytherapy. 2017;16:466-489
20. Neugebauer J, Blum P, Keiler A. et al. Brachytherapy in the Treatment of Soft-Tissue Sarcomas of the Extremities-A Current Concept and Systematic Review of the Literature. Cancers (Basel). 2023;15:1133
21. Sharma DN, Deo SV, Rath GK. et al. Perioperative high-dose-rate interstitial brachytherapy combined with external beam radiation therapy for soft tissue sarcoma. Brachytherapy. 2015;14:571-577
22. Willett CG, Czito BG, Tyler DS. Intraoperative radiation therapy. J Clin Oncol. 2007;25:971-977
23. Calvo FA, Meirino RM, Orecchia R. Intraoperative radiation therapy first part: rationale and techniques. Crit Rev Oncol Hematol. 2006;59:106-115
24. Azinovic I, Martinez Monge R, Javier Aristu J. et al. Intraoperative radiotherapy electron boost followed by moderate doses of external beam radiotherapy in resected soft-tissue sarcoma of the extremities. Radiother Oncol. 2003;67:331-337
25. Dammerer D, Neugebauer J, Braito M. et al. Midterm Results of High-Dose-Rate Intraoperative Brachytherapy in the Treatment of Soft Tissue Sarcomas. Cancers (Basel). 2023;15:2854
26. Tom MC, Hepel JT, Patel R. et al. The American Brachytherapy Society consensus statement for electronic brachytherapy. Brachytherapy. 2019;18:292-298
27. Roeder F, Morillo V, Saleh-Ebrahimi L, Calvo FA, Poortmans P, Ferrer Albiach C. Intraoperative radiation therapy (IORT) for soft tissue sarcoma - ESTRO IORT Task Force/ACROP recommendations. Radiother Oncol. 2020;150:293-302
28. Roeder F, Lehner B, Saleh-Ebrahimi L. et al. Intraoperative electron radiation therapy combined with external beam radiation therapy and limb sparing surgery in extremity soft tissue sarcoma: a retrospective single center analysis of 183 cases. Radiother Oncol. 2016;119:22-29
29. Calvo FA, Sole CV, Polo A. et al. Limb-sparing management with surgical resection, external-beam and intraoperative electron-beam radiation therapy boost for patients with primary soft tissue sarcoma of the extremity: a multicentric pooled analysis of long-term outcomes. Strahlenther Onkol. 2014;190:891-898
30. Matsumine A, Tsujii M, Nakamura T. et al. Minimally invasive surgery using intraoperative electron-beam radiotherapy for the treatment of soft tissue sarcoma of the extremities with tendon involvement. World J Surg Oncol. 2016;14(1):214
31. Honig RL, Tibbo ME, Mallett KE. et al. Outcome of Soft-tissue Reconstruction in the Setting of Combined Preoperative and Intraoperative Radiotherapy for Extremity Soft-tissue Sarcomas. Anticancer Res. 2020;40(12):6941-6945
Author contact
![]() Corresponding authors: LiMing Xu (xulimingcom) and GuoWen Wang (wangguowenedu.cn).
Corresponding authors: LiMing Xu (xulimingcom) and GuoWen Wang (wangguowenedu.cn).

 Global reach, higher impact
Global reach, higher impact