Impact Factor
ISSN: 1837-9664
J Cancer 2025; 16(3):735-747. doi:10.7150/jca.99319 This issue Cite
Research Paper
Third generation vs first generation EGFR-TKIs in the first line treatment for EGFR-mutated locally advanced or metastatic non-small cell lung cancer: a meta-analysis based on randomized controlled trials
1. Department of Oncology, The Second People's Hospital of Jingdezhen, Jingdezhen, China.
2. Department of Respiratory and Critical Care Medicine, The Second People's Hospital of Jingdezhen, Jingdezhen, China.
3. Department of thoracic surgery, The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, China.
Received 2024-6-6; Accepted 2024-7-28; Published 2025-1-1
Abstract
Background: The prevailing belief is that third-generation tyrosine kinase inhibitors (TKIs) targeting the epidermal growth factor receptor (EGFR) (TGET) outperform first-generation EGFR-TKIs (FGET) in managing advanced-stage EGFR-mutated non-small cell lung cancer (NSCLC). However, this standpoint lacks substantiation in evidence-based medicine. Therefore, this meta-analysis was conducted to compare the efficacy and adverse effects (AEs) of these two categories.
Methods: We searched seven databases for relevant randomized controlled trials (RCTs), focusing on primary endpoints such as progression-free survival (PFS), overall survival (OS), and central nervous system PFS (CNS-PFS). Additional factors considered included treatment responses and AEs.
Results: We analyzed 15 studies from 6 RCTs on six third-generation TKIs: Osimertinib, Lazertinib, Furmonertinib, Aumolertinib, Naquotinib, and Befotertinib. TGET showed better efficacy in PFS (hazard ratio [HR]: 0.55 [0.41, 0.75]), CNS-PFS (HR: 0.48 [0.35, 0.66]), CNS-objective response rate (CNS-ORR, risk ratio [RR]: 1.40 [1.19, 1.65]), and duration of response (DOR, HR: 0.52 [0.38, 0.72]). Most subgroups confirmed the PFS advantage. With longer survival time, the superiority in PFS, OS, and CNS-PFS of TGETs became more evident. Both groups had similar OS (HR: 0.86), ORR, CNS-DOR, total AEs, and grade 3-5 AEs. However, TGETs had more severe AEs (RR: 1.17 [1.02, 1.35]). Additionally, there were more grade 3-4 cases of diarrhea, decreased platelet count, pulmonary embolism, fatigue, decreased neutrophil count, and rash, and fewer grade 3-4 increases in alanine transaminase (ALT) and aspartate transaminase (AST) in the TGET group. The top 5 AEs in the TGET group were diarrhea (36.32%), rash (30.24%), decreased platelet count (29.15%), elevated serum creatinine (23.63%), and decreased white blood cell count (22.02%).
Conclusions: Except for Naquotinib, TGETs demonstrate superiority over FGETs in treating EGFR-mutated locally advanced or metastatic NSCLC, showing improved survival and responses. However, the increased incidence of AEs necessitates careful consideration.
Keywords: Tyrosine kinase inhibitors, Third-generation, First-generation, Non-small cell lung cancer, Meta-analysis
Introduction
During the past decade, 80% of diagnosed cases of non-small cell lung cancer (NSCLC) were either locally advanced or metastatic, significantly contributing to cancer-related mortality [1,2]. Approximately 51.4% of all NSCLC cases are attributed to mutations in the epidermal growth factor receptor (EGFR) [3]. The standard treatment approach for EGFR-mutated NSCLC is EGFR-tyrosine kinase inhibitors (TKIs) [4]. Over a decade ago, first-generation TKIs such as Erlotinib and Gefitinib were employed in the treatment of EGFR-mutated NSCLC, confirming their superior efficacy and safety compared to chemotherapy [5]. However, their ability to prolong patient survival remains unsatisfactory. With subsequent drug iterations, third-generation TKIs have increasingly been utilized as first-line treatments for EGFR-mutated advanced NSCLC in recent years [6-11]. Many perspectives suggest that the first-line use of TGETs may lead to better clinical outcomes [12].
In the latest versions of the NCCN Clinical Practice Guidelines and ESMO Clinical Practice Guidelines, both TGET and FGET are recommended for the first-line treatment of EGFR-mutated advanced NSCLC [4,13]. Studies such as FLAURA (Osimertinib) and LASER301 (Lazertinib) reported better survival outcomes, including progression-free survival (PFS), in the TGET group [6,7]. Similar results were confirmed by trials such as FURLONG (Furmonertinib) and AENEAS (Aumolertinib) [8,9]. Lu et al. reported that although the third-generation TKI (Befotertinib) may exhibit superior survival efficacy, it is associated with a higher incidence of total/grade 3-5 adverse effects (AEs) [11]. However, the SOLAR trial (Naquotinib) reported worse survival outcomes and a higher incidence of grade 3-5 AEs in the TGET group [10].
To address this clinical controversy, this meta-analysis was conducted to compare the two groups in terms of survival, responses, and safety.
Materials and Methods
In compliance with PRISMA guidelines, this study was registered in PROSPERO (ID: CRD42024533158) and conducted accordingly (Table S1).
Search strategy
The search strategy utilized the keywords 'lung cancer,' 'randomized,' and TGETs (including Osimertinib, Nazartinib, Rociletinib, Mavelertinib, Lazertinib, Olmutinib, Naquotinib, Almonertinib, Furmonertinib, Abivertinib, Rezivertinib, Limertinib, Befotertinib, Olafertinib, Keynatinib, Oritinib, and TAS-121). Thorough searches were conducted across seven databases (PubMed, ScienceDirect, Ovid MEDLINE, the Cochrane Library, Scopus, EMBASE, and Web of Science) for eligible RCTs from the inception of each database until January 20, 2024 (Table S2). Additionally, the reference lists of the included RCTs were reviewed to identify additional eligible studies.
Selection criteria
English-published studies were chosen based on PICOS criteria:
(1) Participants (P): patients with EGFR-mutated advanced NSCLC.
(2) Intervention (I): first line treatment with TGET.
(3) Control (C): first line treatment with FGET.
(4) Outcomes (O): survival (PFS, overall survival [OS], central nervous system PFS [CNS-PFS]), responses, and AEs.
(5) Study design (S): RCTs.
Articles lacking primary data, as well as meta-analyses and case reports, were excluded. Multiple studies reporting on the same trial with diverse outcomes were included, but only the most recent data were used for identical outcomes in the analysis.
Data extraction
Two investigators independently collected data, including study characteristics (publication date, first author, etc.), participant details (sex, age, etc.), cancer specifics (histopathology, stage, etc.), antitumor effectiveness (PFS, OS, CNS-PFS, responses, etc.), and adverse event counts (total AEs, serious AEs, etc.). Any discrepancies were resolved through re-evaluation and discussion.
Outcome assessments
The primary endpoints analyzed were PFS, OS, and CNS-PFS. Additionally, we examined PFS within specific subgroups: Age (<65 or >65 years), Sex (Female or Male), Smoking status (Active/Former smoker or Non-smoker), Eastern Cooperative Oncology Group (ECOG) PS (0 or 1), CNS metastases at baseline (Yes or No), EGFR mutation (Ex19del or L858R), Race category (Asian or Non-Asian), and TGETs (Osimertinib, Lazertinib, Furmonertinib, Aumolertinib, Befotertinib, or Naquotinib). Meanwhile, comparisons were conducted between the two groups for PFS rate (PFSR), OS rate (OSR), and CNS-PFS rate (CNS-PFSR) at 6-36 months. The PFSR was also analyzed in subgroups according to CNS metastases at baseline (Yes or No) and EGFR mutation (Ex19del or L858R).
Quality assessment
We evaluated RCT quality using both the Jadad scale, a 5-point system reflecting randomization, blinding, and patient inclusion, with ≥3 points considered indicative of high quality [14], and the Cochrane Risk Assessment Tool, categorizing risk as low, unclear, or high for bias related to selection, performance, detection, attrition, and reporting [15]. The bias graph illustrates the findings.
The quality of the results was assessed using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) method, primarily considering bias, indirectness, inaccuracy, and publication bias. Outcomes are categorized into four levels: very low, low, medium, and high [16].
Statistical analysis
Pooled data were evaluated using Review Manager 5.3. For the analysis of survival data (PFS, OS, CNS-PFS, etc.), hazard ratio (HR) was employed. Favorable outcomes were indicated by an HR < 1 in the TGET group. Dichotomous variables (PFSR, OSR, ORR, AEs, etc.) were analyzed using risk ratio (RR). Mean difference (MD) was used for analyzing continuous variables (depth of response, etc.). Heterogeneity was assessed using the I2 statistic and χ2 test. When I2 was less than 50% or P was greater than 0.1, indicating no significant heterogeneity, a fixed-effects model was applied; otherwise, a random-effects model was used. Publication bias was assessed by visually examining funnel plots. A p-value < 0.05 denoted statistical significance.
Results
Search results
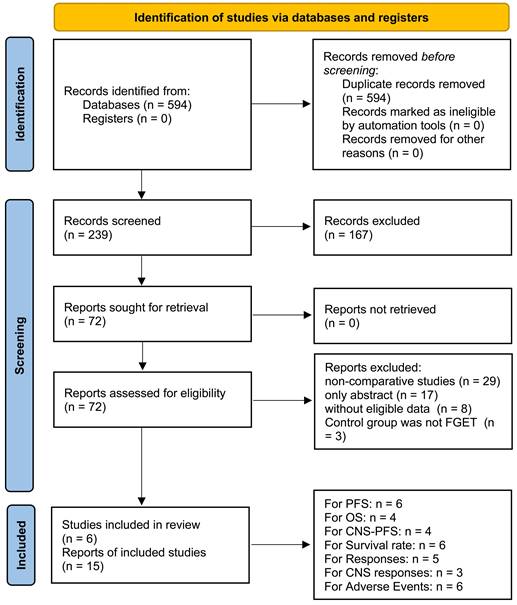
In the final analysis, fifteen studies based on six RCTs investigating 6 TGETs (Osimertinib, Lazertinib, Furmonertinib, Aumolertinib, Naquotinib, and Befotertinib) were included (Figure 1) [6-11,17-25]. The TGET group comprised 1316 patients, and the FGET group included 1311 patients. All six RCTs were of high quality according to the Jadad scale and Cochrane Risk Assessment Tool (Figure S1, Table S3). The quality of all results fell within the medium-high range as per the GRADE method (Table S4). Table 1 summarizes the baseline information for the included studies. At the time of data cutoff, 397 patients (30.17%) continued treatment in the TGET group, and 145 patients (11.06%) continued treatment in the FGET group (Figure S2).
Study selection flow.
Characteristics of the included studies.
| Study | Country | Groups | Patients | Sex (M/F) | Age (Mean, year) | Smoking (Yes/No) | ECOG PS (0/1/2) | Histologic type (Adeno/Others) | Stage (IIIB/IV) | CNS metastases (Yes/No) | EGFR mutation (Ex19del/L858R/T790M) | Follow up (months) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT02296125(FLAURA, 2014.12-2016.03) | |||||||||||||
| Cheng 2021 [17] | Global multicenter-China Subset | Osimertinib | 71 | 28/43 | 60 | 18/53 | 7/64/0 | 70/1 | 2/69 | 17/54 | 36/35/0 | 35.8 | |
| Gef | 65 | 19/46 | 61 | 15/50 | 13/52/0 | 64/1 | 0/65 | 21/44 | 33/32/0 | 27 | |||
| Ramalingam 2020 [18] | Global multicenter | Osimertinib | 279 | 101/178 | 64 | 97/182 | 112/167/0 | 275/4 | 14/265 | 53/226 | 175/104/0 | 35.8 | |
| Gef/Erl | 277 | 195/172 | 64 | 102/175 | 116/161/0 | 272/0 | 15/262 | 63/214 | 174/103/0 | 27 | |||
| Ohe 2019 [19] | Global multicenter-Japen Subset | Osimertinib | 65 | 22/43 | 67 | 30/65 | 38/27/0 | 65/0 | 5/60 | 14/51 | 33/32/0 | 15 | |
| Gef | 55 | 27/28 | 67 | 26/29 | 34/21/0 | 55/0 | 2/53 | 13/42 | 30/25/0 | 9.7 | |||
| Cho 2019 [20] | Global multicenter-Asian Subset | Osimertinib | 162 | 61/101 | 64 | 58/104 | 65/97/0 | 162/0 | 8/154 | 39/123 | - | 15 | |
| Gef/Erl | 160 | 69/91 | 64 | 65/95 | 62/98/0 | 160/0 | 4/156 | 33/127 | - | 9.7 | |||
| Ohe 2018 [6] | Global multicenter | Osimertinib | 279 | 101/178 | 64 | 97/182 | 112/167/0 | 275/4 | 14/265 | 53/226 | 175/104/0 | 15 | |
| Gef/Erl | 277 | 195/172 | 64 | 102/175 | 116/161/0 | 272/0 | 15/262 | 63/214 | 174/103/0 | 9.7 | |||
| Reungwetwattana 2018 [21] | Global multicenter-CNS Subset | Osimertinib | 61 | 23/38 | 63 | - | 16/45/0 | 61/0 | 0/61 | 61/0 | 40/21/0 | 15 | |
| Gef/Erl | 67 | 26/41 | 63 | - | 27/39/0 | 67/0 | 0/67 | 67/0 | 45/22/0 | 9.7 | |||
| NCT04248829(LASER301, 2020.02-2021.09) | |||||||||||||
| Lee 2024 [22] | Global multicenter-Korean Subset | Lazertinib | 87 | 36/51 | 67 | 32/55 | 18/69/0 | 87/0 | 2/85 | 31/56 | 50/37/0 | 23.3 | |
| Gef | 85 | 42/43 | 66 | 26/59 | 20/65/0 | 85/0 | 1/84 | 25/60 | 48/37/0 | 26.1 | |||
| Soo 2023 [23] | Global multicenter-CNS Subset | Lazertinib | 45 | 14/31 | 66 | - | 8/37/0 | 45/0 | 0/45 | 45/0 | 25/20/0 | 17.8 | |
| Gef | 41 | 17/24 | 59 | - | 12/29/0 | 41/0 | 0/41 | 41/0 | 23/18/0 | 12.2 | |||
| Reungwetwattana 2023 [24] | Global multicenter-Asian Subset | Lazertinib | 129 | 49/80 | 66 | 43/86 | 30/99/0 | 129/0 | 3/126 | 39/90 | 77/52/0 | 21 | |
| Gef | 129 | 57/72 | 64 | 34/95 | 31/98/0 | 129/0 | 3/126 | 31/98 | 77/52/0 | 22.1 | |||
| Cho 2023 [7] | Global multicenter | Lazertinib | 196 | 64/132 | 67 | 61/135 | 49/147/0 | 196/0 | 5/191 | 51/145 | 121/75/0 | 20.5 | |
| Gef | 197 | 78/119 | 64 | 48/149 | 53/144/0 | 197/0 | 5/192 | 48/149 | 122/75/0 | 20.6 | |||
| NCT03787992(FURLONG, 2019.05-2019.12) | |||||||||||||
| Shi 2022 [8] | China multicenter | Furmonertinib | 178 | 62/116 | 59 | 41/137 | 39/138/1 | 178/0 | 10/168 | 62/115 | 91/87/0 | 21 | |
| Gefitinib | 179 | 68/111 | 60 | 44/135 | 28/151/0 | 179/0 | 7/172 | 58/121 | 92/87/0 | 21 | |||
| Shi 2022 CNS [25] | China multicenter-CNS Subset | Furmonertinib | 65 | 25/40 | 58 | 14/51 | 14/51/0 | 65/0 | 0/65 | 65/0 | 35/30/0 | 21 | |
| Gefitinib | 62 | 23/39 | 60 | 18/44 | 9/53/0 | 62/0 | 0/62 | 62/0 | 32/30/0 | 21 | |||
| NCT03849768(AENEAS, 2018.11-2019.09) | |||||||||||||
| Lu 2022 [9] | China multicenter | Aumolertinib | 214 | 80/134 | 59 | 58/156 | 51/160/0 | 210/4 | 12/202 | 56/158 | 140/74/0 | 20.5 | |
| Gefitinib | 215 | 80/135 | 62 | 71/144 | 54/159/0 | 211/4 | 17/198 | 59/156 | 141/74/0 | 20.7 | |||
| NCT02588261(SOLAR, 2016.02-2017.12) | |||||||||||||
| Kelly 2019 [10] | Global multicenter | Naquotinib | 267 | 96/171 | 68 | 96/171 | 103/155/9 | 267/0 | 14/253 | - | 134/111/4 | 3.6 | |
| Gef/Erl | 263 | 110/153 | 67 | 92/171 | 103/152/8 | 263/0 | 16/247 | - | 129/108/6 | 3.6 | |||
| NCT04206072(2019.12-2020.12) | |||||||||||||
| Lu 2023 [11] | China multicenter | Befotertinib | 182 | 72/110 | 60 | 61/121 | 35/146/0 | 182/0 | 17/165 | 47/135 | 117/65/0 | 20.7 | |
| Icotinib | 180 | 72/108 | 58 | 55/125 | 39/141/0 | 180/0 | 6/174 | 45/135 | 117/63/0 | 19.4 | |||
Abbreviations: CNS: Central nervous system; ECOG: Eastern Cooperative Oncology Group; EGFR: Epidermal growth factor receptor; Erl: Erlotinib; Gef: Gefitinib; M/F: Male/Female.
Survival
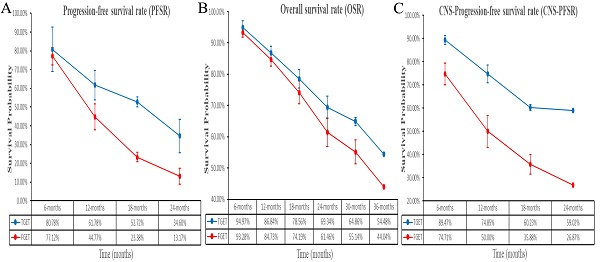
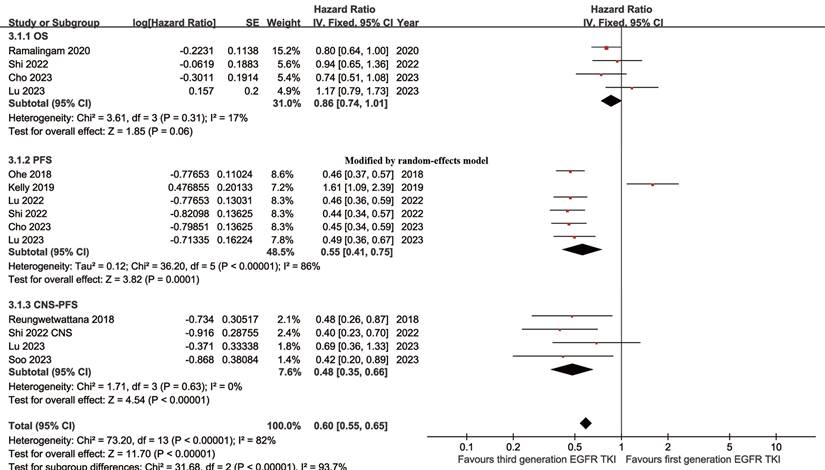
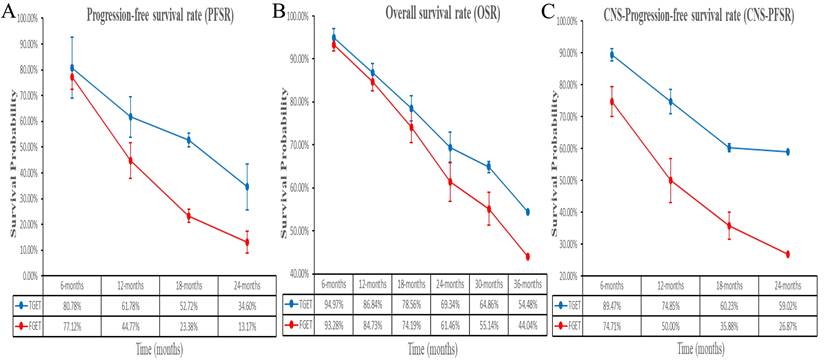
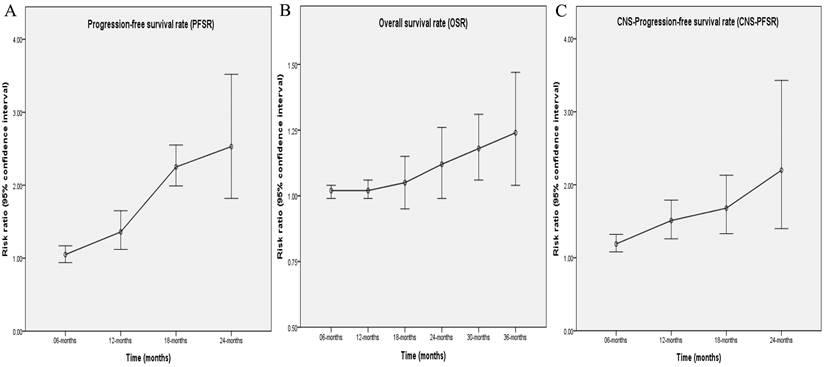
Better PFS was found in the TGET group (HR: 0.55 [0.41, 0.75], p = 0.0001; Figure 2). At 12-24 months, PFSR favored the TGET group (Figure S3). In terms of extended survival, TGET displayed a growing advantage in PFSR compared to FGET (Figure 3A, 4A).
OS tended to favor the TGET group without statistical significance (HR: 0.86 [0.74, 1.01], p = 0.06; Figure 2). At 30-36 months, OSR favored the TGET group (Figure S4). In terms of extended survival, TGET displayed a growing advantage in OSR compared to FGET (Figure 3B, 4B).
Better CNS-PFS was found in the TGET group (HR: 0.48 [0.35, 0.66], p < 0.00001; Figure 2). At 6-24 months, CNS-PFSR favored the TGET group (Figure S5). In terms of extended survival, TGET displayed a growing advantage in CNS-PFSR compared to FGET (Figure 3C, 4C).
Forest plots of OS, PFS, and CNS-PFS associated with TGET versus FGET.
Comparisons of PFSR (6-24 months), OSR (3-36 months), and CNS-PFSR (6-24 months) associated with TGET versus FGET.
Subgroup analysis
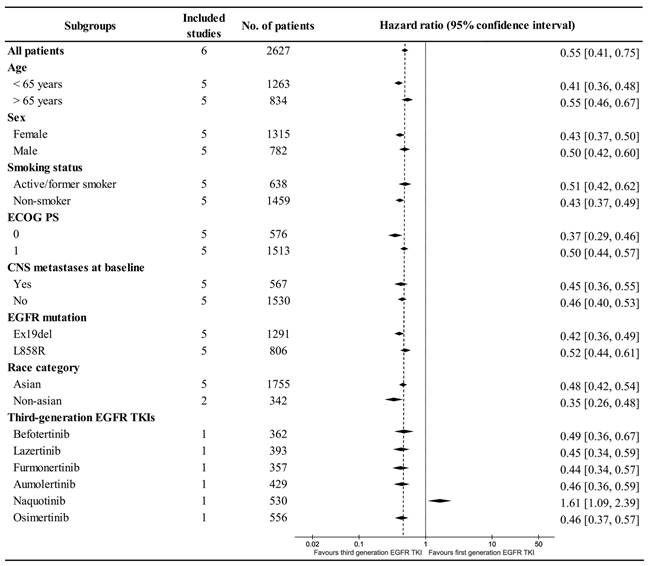
Subgroup analysis showed that PFS was better with TGETs in most groups. Age (< 65 years), sex (Female), smoking status (Non-smoker), ECOG PS (0), EGFR mutation (Ex19del), and race category (Non-Asian) might benefit more from TGET treatment. However, in the subgroup of TGET-Naquotinib, PFS tended to favor the FGET group (Figure 5).
PFSR tends to favor the TGET group in all subgroups (with or without CNS metastases, Ex19del or L858R mutations) at 6-24 months (Figure S6-S10). Meanwhile, in terms of extended survival, TGET shows an increasing advantage in PFSR compared to FGET in all subgroups (Figure S11).
Responses
In the response analysis, the ORR (RR: 0.98 [0.90, 1.07]), DCR (RR: 0.99 [0.96, 1.03]), CR (RR: 1.66 [0.61, 4.54]), PR (RR: 0.98 [0.90, 1.06]), SD (RR: 1.01 [0.79, 1.29]), and PD (RR: 0.86 [0.42, 1.77]) showed similarity between the two groups (Figure S12). The TGET group had favorable outcomes in terms of duration of response (DOR, HR: 0.52 [0.38, 0.72]) and depth of response (MD: -0.05 [-0.06, -0.04] %) (Figure S13, S14). The TGET group also exhibited a higher estimated percentage remaining in response (EPR) at 6-24 months (Figure S15).
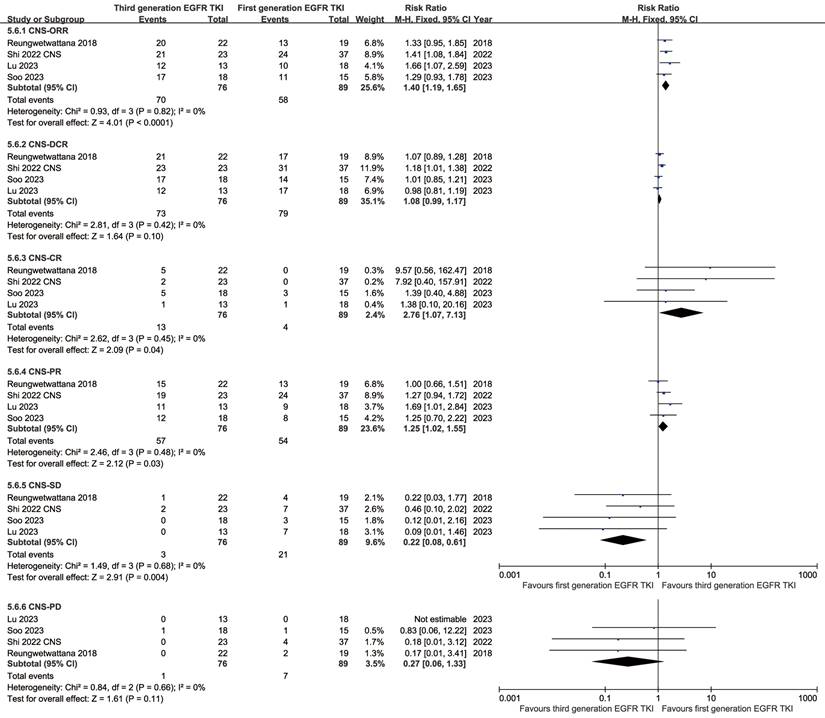
In the analysis of CNS responses, the TGET group tended to surpass the FGET group in CNS-ORR (RR: 1.40 [1.19, 1.65]), CNS-DCR (RR: 1.08 [0.99, 1.17]), CNS-CR (RR: 2.76 [1.07, 7.13]), and CNS-PR (RR: 1.25 [1.02, 1.55]). Conversely, the TGET group showed lower CNS-SD (RR: 0.22 [0.08, 0.61]) and CNS-PD (RR: 0.27 [0.06, 1.33]) (Figure 6). While the CNS-DOR (HR: 0.73 [0.26, 2.08]) and depth of response (MD: -0.12 [-0.28, 0.04] %) tended to favor the TGET group, statistical significance was not observed (Figure S13, S14). Similarly, the estimated percentage remaining in CNS response (CNS-EPR) favored the TGET group at 6-24 months without statistical significance (Figure S16).
Toxicity
In summary, total AEs (RR: 0.99 [0.98, 1.01]), grade 3-5 AEs (RR: 1.10 [0.93, 1.29]), fatal AEs (RR: 1.22 [0.81, 1.83]), treatment-related AEs (RR: 1.01 [0.96, 1.06]), grade 3-5 treatment-related AEs (RR: 1.24 [0.73, 2.12]), serious treatment-related AEs (RR: 1.15 [0.62, 2.12]), fatal treatment-related AEs (RR: 2.10 [0.47, 9.32]), discontinuation due to AEs (RR: 1.20 [0.79, 1.84]), dose reduction due to AEs (RR: 1.58 [0.81, 3.09]), dose interruption due to AEs (RR: 1.04 [0.84, 1.29]) were similar between the two groups. More serious AEs (RR: 1.17 [1.02, 1.35]) were found in the TGET group (Table 2).
Trend of risk ratios in the comparisons of PFSR (6-24 months), OSR (3-36 months), and CNS-PFSR (6-24 months) associated with TGET versus FGET.
Summary of adverse events.
| Adverse events | Studies involved | TGET | FGET | Risk ratio [95% CI] | P | ||
|---|---|---|---|---|---|---|---|
| Event/total | % | Event/total | % | ||||
| Total adverse events | 6 | 1282/1316 | 97.42% | 1289/1311 | 98.32% | 0.99 [0.98, 1.01] | 0.48 |
| Grade 3-5 adverse events | 6 | 568/1316 | 43.16% | 519/1311 | 39.59% | 1.10 [0.93, 1.29] | 0.28 |
| Serious adverse events | 5 | 310/1134 | 27.34% | 264/1131 | 23.34% | 1.17 [1.02, 1.35] | 0.03 |
| Fatal adverse events | 5 | 49/1134 | 4.32% | 40/1131 | 3.54% | 1.22 [0.81, 1.83] | 0.34 |
| Discontinuation due to adverse events | 6 | 134/1316 | 10.18% | 116/1311 | 8.85% | 1.20 [0.79, 1.84] | 0.39 |
| Dose reduction due to adverse events | 6 | 174/1316 | 13.22% | 100/1311 | 7.63% | 1.58 [0.81, 3.09] | 0.18 |
| Dose interruption due to adverse events | 6 | 368/1316 | 27.96% | 348/1311 | 26.54% | 1.04 [0.84, 1.29] | 0.7 |
| Treatment-related adverse events | 7 | 1073/1173 | 91.47% | 1057/1154 | 91.59% | 1.01 [0.96, 1.06] | 0.78 |
| Grade 3-5 treatment-related adverse events | 6 | 272/959 | 28.36% | 192/939 | 20.45% | 1.24 [0.73, 2.12] | 0.43 |
| Serious treatment-related adverse events | 6 | 96/991 | 9.69% | 75/974 | 7.70% | 1.15 [0.62, 2.12] | 0.67 |
| Fatal treatment-related adverse events | 5 | 5/777 | 0.64% | 2/759 | 0.26% | 2.10 [0.47, 9.32] | 0.33 |
Abbreviations: CI: confidence interval; FGET: First generation EGFR tyrosine kinase inhibitor; P: Probability; TGET: Third generation EGFR tyrosine kinase inhibitor.
Subgroup analysis of PFS.
In analyzing AEs of any grade, more occurrences of platelet count decrease, elevated serum creatinine, white blood cell count decrease, peripheral sensory neuropathy, deep vein thrombosis, hyponatremia, lipid metabolism diseases, renal symptoms, hyperuricemia, anemia, upper respiratory tract infection, headache, fatigue, constipation, elevated fibrin D-dimer, neutrophil count decrease, muscle spasms, blood lactate dehydrogenase increase, vomiting, nasopharyngitis, dyspnea, ECG QTc prolongation, lymphocyte count decrease, edema, gastrointestinal diseases, and pulmonary embolism were found in the TGET group. More rash, ALT increase, AST increase, hypokalemia, gamma-glutamyl transferase increase, and blood bilirubin increase were found in the FGET group (Table S5). AEs of any grade, exceeding a 10% occurrence rate, were listed in Table 3.
In analyzing grade 3-5 AEs, more diarrhea, platelet count decrease, pulmonary embolism, fatigue, and neutrophil count decrease were found in the TGET group. More ALT increase, AST increase, and rash were found in the FGET group (Table S6). Grade 3-5 AEs, exceeding a 1% occurrence rate, were listed in Table 4.
Sensitivity analysis
The analysis of PFS, ORR, DCR, DOR, total AEs, and grade 3-5 AEs revealed notable heterogeneity. Omitting any study did not alter the stability or reliability of the results according to the sensitivity analysis (Figure S17).
Publication bias
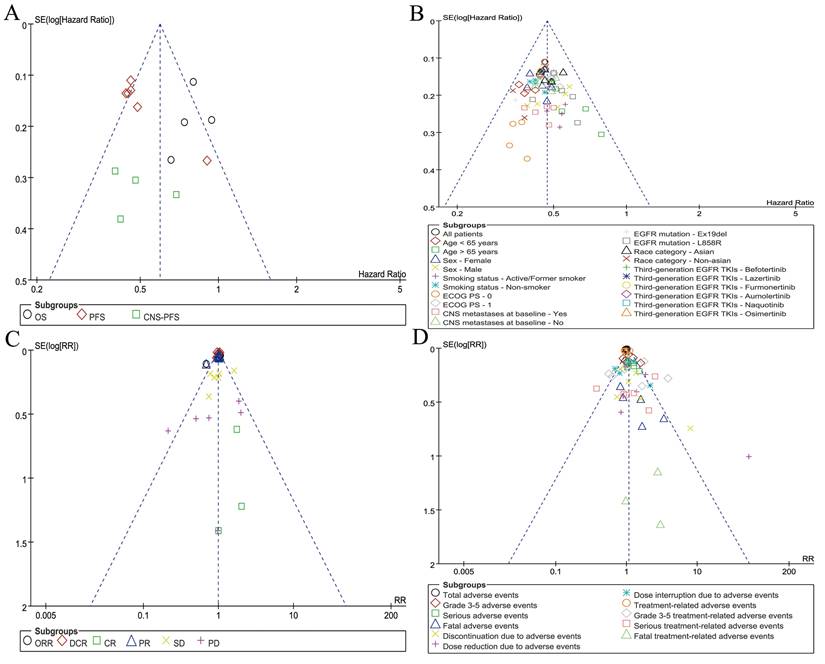
Symmetrical funnel plots were observed for survival summary (Figure 7A), subgroup analysis of PFS (Figure 7B), responses (Figure 7C), and summary of AEs (Figure 7D), indicating acceptable publication bias.
Discussion
With the introduction of various TGETs, an increasing number of patients with EGFR-mutated locally advanced or metastatic NSCLC are receiving first-line treatment with these agents. The superiority of TGETs over FGETs for patients in this stage is widely recognized in clinical practice [26,27]. However, whether this perspective is accurate, whether it applies to all TGETs, where the superiority of TGETs over FGETs lies, and what limitations exist remain under-explored in evidence-based medicine [28,29]. This study represents the first meta-analysis comparing TGET with FGET for advanced NSCLC. The results suggest that TGET achieves superior efficacy in PFS, CNS-PFS, CNS-ORR, and DOR. The survival advantage of PFS was confirmed in almost all subgroups. Similar OS, ORR, CNS-DOR, total AEs, grade 3-5 AEs, and fatal AEs were found between the two groups. However, more serious AEs were found in the TGET group.
Prolongation of survival time is widely recognized as the most important reason for the acceptance of third-generation drugs. In recent years, some drugs have been shown to significantly improve PFS but fail to improve OS, a phenomenon that has been perplexing [11,30]. In this study, we found that the TGET group exhibited higher PFS and CNS-PFS compared to the FGET group and tended to have higher OS without statistical significance. Osimertinib was the only TGET to demonstrate a positive OS outcome [18]. The advantage in PFS was observed in almost all TGETs, except for Naquotinib. The SOLAR study was prematurely terminated due to the inferior PFS of Naquotinib compared to FGET [10].
Total adverse events with an incidence of greater than 10% according to the TGET groups.
| Adverse events | Studies involved | TGET | FGET | Risk ratio [95% CI] | P | ||
|---|---|---|---|---|---|---|---|
| Event/total | % | Event/total | % | ||||
| Diarrhea | 6 | 478/1316 | 36.32% | 537/1311 | 40.96% | 0.79 [0.60, 1.04] | 0.09 |
| Rash | 6 | 398/1316 | 30.24% | 699/1311 | 53.32% | 0.54 [0.34, 0.86] | 0.01 |
| Platelet count decreased | 4 | 188/645 | 29.15% | 35/639 | 5.48% | 5.08 [1.72, 14.96] | 0.003 |
| Elevated serum creatinine | 1 | 43/182 | 23.63% | 8/180 | 4.44% | 5.32 [2.57, 10.99] | <0.00001 |
| White blood cell count decreased | 4 | 142/645 | 22.02% | 81/639 | 12.68% | 1.77 [1.19, 2.64] | 0.005 |
| Peripheral sensory neuropathy | 3 | 141/645 | 21.86% | 22/640 | 3.44% | 5.61 [1.38, 22.81] | 0.02 |
| Dry skin | 3 | 161/742 | 21.70% | 190/737 | 25.78% | 0.80 [0.42, 1.54] | 0.51 |
| Deep vein thrombosis | 1 | 39/182 | 21.43% | 1/180 | 0.56% | 38.57 [5.36, 277.75] | 0.0003 |
| ALT increased | 6 | 272/1316 | 20.67% | 479/1311 | 36.54% | 0.56 [0.41, 0.78] | 0.0005 |
| Hyponatremia | 2 | 69/338 | 20.41% | 4/328 | 1.22% | 15.08 [5.88, 38.64] | <0.00001 |
| Lipid metabolism diseases | 1 | 36/182 | 19.78% | 17/180 | 9.44% | 2.09 [1.22, 3.59] | 0.007 |
| Paronychia | 3 | 146/742 | 19.68% | 195/737 | 26.46% | 0.45 [0.14, 1.47] | 0.18 |
| Urinary tract infection | 4 | 124/645 | 19.22% | 106/639 | 16.59% | 1.16 [0.92, 1.47] | 0.21 |
| Cough | 3 | 119/639 | 18.62% | 100/636 | 15.72% | 1.18 [0.93, 1.51] | 0.17 |
| AST increased | 6 | 244/1316 | 18.54% | 437/1311 | 33.33% | 0.56 [0.45, 0.70] | <0.00001 |
| Renal symptoms | 1 | 50/279 | 17.92% | 32/277 | 11.55% | 1.55 [1.03, 2.34] | 0.04 |
| Hyperuricaemia | 1 | 32/182 | 17.58% | 14/180 | 7.78% | 2.26 [1.25, 4.09] | 0.007 |
| Anemia | 5 | 184/1049 | 17.54% | 95/1048 | 9.06% | 1.90 [1.26, 2.87] | 0.002 |
| Upper respiratory tract infection | 4 | 149/853 | 17.47% | 106/851 | 12.46% | 1.40 [1.12, 1.77] | 0.004 |
| Weight increased | 2 | 62/360 | 17.22% | 66/359 | 18.38% | 0.94 [0.68, 1.28] | 0.68 |
| Increased blood creatine phosphokinase | 3 | 94/574 | 16.38% | 33/574 | 5.75% | 2.12 [0.79, 5.69] | 0.14 |
| Headache | 4 | 137/853 | 16.06% | 51/851 | 5.99% | 2.58 [1.14, 5.80] | 0.02 |
| Fatigue | 3 | 113/728 | 15.52% | 65/720 | 9.03% | 1.72 [1.29, 2.29] | 0.0002 |
| Stomatitis | 4 | 139/920 | 15.11% | 143/916 | 15.61% | 0.85 [0.39, 1.88] | 0.69 |
| Decreased appetite | 5 | 170/1138 | 14.94% | 147/1132 | 12.99% | 1.16 [0.80, 1.68] | 0.45 |
| Proteinuria | 2 | 37/253 | 14.62% | 22/245 | 8.98% | 1.63 [0.99, 2.68] | 0.05 |
| Constipation | 4 | 120/835 | 14.37% | 84/833 | 10.08% | 1.42 [1.10, 1.85] | 0.008 |
| Elevated fibrin D-dimer | 1 | 26/182 | 14.29% | 8/180 | 4.44% | 3.21 [1.50, 6.91] | 0.003 |
| Neutrophil count decreased | 3 | 65/463 | 14.04% | 35/459 | 7.63% | 1.84 [1.24, 2.72] | 0.002 |
| Nausea | 6 | 183/1316 | 13.91% | 134/1311 | 10.22% | 1.41 [0.91, 2.18] | 0.12 |
| Pruritus | 5 | 140/1049 | 13.35% | 125/1048 | 11.93% | 0.98 [0.62, 1.55] | 0.94 |
| Musculoskeletal pain | 3 | 85/639 | 13.30% | 22/636 | 3.46% | 2.62 [0.49, 14.06] | 0.26 |
| Muscle spasms | 1 | 26/196 | 13.27% | 7/197 | 3.55% | 3.73 [1.66, 8.40] | 0.001 |
| Blood lactate dehydrogenase increase | 1 | 26/214 | 12.15% | 14/215 | 6.51% | 1.87 [1.00, 3.47] | 0.05 |
| Vomiting | 4 | 97/853 | 11.37% | 62/851 | 7.29% | 1.56 [1.15, 2.11] | 0.004 |
| Insomnia | 1 | 31/279 | 11.11% | 21/277 | 7.58% | 1.47 [0.86, 2.49] | 0.16 |
| Nasopharyngitis | 1 | 31/279 | 11.11% | 16/277 | 5.78% | 1.92 [1.08, 3.44] | 0.03 |
| Dermatitis acneiform | 1 | 21/196 | 10.71% | 27/197 | 13.71% | 0.78 [0.46, 1.33] | 0.37 |
| Back pain | 2 | 48/457 | 10.50% | 48/456 | 10.53% | 1.00 [0.68, 1.46] | 0.99 |
| Pyrexia | 2 | 48/461 | 10.41% | 31/457 | 6.78% | 1.48 [0.48, 4.62] | 0.5 |
| Hematuria | 2 | 26/253 | 10.28% | 37/245 | 15.10% | 0.81 [0.32, 2.03] | 0.65 |
| Dyspnea | 3 | 65/639 | 10.17% | 41/636 | 6.45% | 1.58 [1.08, 2.29] | 0.02 |
Abbreviations: ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; CI: confidence interval; ECG: Electrocardiogram; FGET: First generation EGFR tyrosine kinase inhibitor; P: Probability; TGET: Third generation EGFR tyrosine kinase inhibitor.
Forest plots of CNS responses (ORR, DCR, CR, PR, SD, and PD) associated with TGET versus FGET.
Additionally, we found that the DOR and depth of response were significantly superior in the TGET group compared with the FGET group. Three reasons may explain this survival advantage: 1. TGETs have better blood-brain barrier permeability, leading to significantly improved control of intracranial metastases due to higher drug solubility in the brain [31,32]; 2. TGETs also have a certain therapeutic effect on the T790M mutation after conventional Ex19del and L858R mutations, which may prolong the duration of drug response [33,34]; 3. TGETs not only inhibit the tyrosine kinase activity of EGFR but also have inhibitory effects on multiple other targets, allowing for a more comprehensive blockade of tumor cell signaling pathways, effectively inhibiting tumor cell growth and spread [35]. In subgroup analysis of PFS, PFS tended to favor the TGET group across most subgroups. Age (< 65 years), sex (Female), smoking status (Non-smoker), ECOG PS (0), EGFR mutation (Ex19del), and race category (Non-Asian) might benefit more from TGET treatment.
Brain metastases occur at a significantly high rate in patients with advanced NSCLC (up to 40%) [36]. Additionally, the prognosis for patients with brain metastases is often poor. Therefore, controlling brain metastases is of utmost importance and greatly influences the OS of patients at this stage of cancer [37]. Our study found that the notable survival advantage of TGET over FGET lies in CNS-PFS. This finding was corroborated in the brain metastasis subgroups of FLAURA, LASER301, and FURLONG [21,23,25]. Furthermore, we observed that TGET demonstrated superior DOR and depth of response for measurable intracranial lesions. The enhanced control of CNS lesions with third-generation drugs is primarily attributed to their improved blood-brain barrier permeability, allowing TGETs to achieve higher CNS concentration and brain-to-plasma concentration ratios [31,32].
Safety is another crucial consideration in drug selection, as drugs with good efficacy but significant side effects are common in clinical practice. Our study revealed that the top 5 AEs in the TGET group were diarrhea (36.32%), rash (30.24%), decreased platelet count (29.15%), elevated serum creatinine (23.63%), and decreased white blood cell count (22.02%). The incidence of 26 AEs, including decreased platelet count, was higher in the TGET group. Among them, the decline in blood cell counts (red blood cells, white blood cells, and platelets) was the most pronounced difference compared to FGET. Additionally, more serious AEs and more discontinuations due to AEs were also observed in the TGET group. Among these six TGETs, the incidence rates of grade 3-5 AEs ranked from highest to lowest were Naquotinib (54.31%), Befotertinib (47.25%), Osimertinib (41.94%), Lazertinib (40.82%), Aumolertinib (36.45%), and Furmonertinib (34.83%) [6-11]. The ability of third-generation EGFR-TKIs to control intracranial lesions in EGFR-mutated advanced NSCLC patients is accompanied by significant CNS toxicities such as headaches, dizziness, and cognitive impairments due to enhanced drug penetration across the blood-brain barrier [38]. Additionally, patients may experience radiation necrosis or leukoencephalopathy, particularly if they have undergone prior radiotherapy, necessitating careful monitoring and management [6,39]. Resistance mechanisms, such as C797S mutations, can lead to CNS relapse, requiring a combination of systemic therapy and localized treatments [40]. Therefore, although TGETs can substantially improve survival, close monitoring and management of AEs still require high attention.
Funnel plots of survival summary (A), subgroup analysis of PFS (B), responses (C), and summary of AEs (D) associated with TGET versus FGET.
Grade 3-5 adverse events with an incidence of greater than 1% according to the TGET groups.
| Adverse events | Studies involved | TGET | FGET | Risk ratio [95% CI] | P | ||
|---|---|---|---|---|---|---|---|
| Event/total | % | Event/total | % | ||||
| Hyponatremia | 2 | 63/546 | 11.54% | 6/540 | 1.11% | 7.50 [0.54, 105.21] | 0.13 |
| Diarrhea | 6 | 34/1249 | 2.72% | 17/1243 | 1.37% | 1.79 [0.89, 3.60] | 0.02 |
| Platelet count decreased | 4 | 23/853 | 2.70% | 3/851 | 0.35% | 3.77 [0.85, 16.76] | 0.0009 |
| Increased blood creatine phosphokinase | 3 | 15/574 | 2.61% | 2/574 | 0.35% | 2.76 [0.07, 115.54] | 0.59 |
| Pulmonary embolism | 3 | 16/639 | 2.50% | 2/636 | 0.31% | 5.89 [1.53, 22.71] | 0.005 |
| ALT increased | 6 | 31/1249 | 2.48% | 99/1243 | 7.96% | 0.25 [0.09, 0.68] | 0.007 |
| Lipid metabolism diseases | 1 | 4/182 | 2.20% | 1/180 | 0.56% | 3.96 [0.45, 35.05] | 0.22 |
| Hypertension | 4 | 18/853 | 2.11% | 12/851 | 1.41% | 1.59 [0.43, 5.87] | 0.49 |
| Anemia | 5 | 20/982 | 2.04% | 11/980 | 1.12% | 1.63 [0.72, 3.66] | 0.12 |
| Pneumonia | 4 | 17/853 | 1.99% | 18/851 | 2.12% | 0.98 [0.45, 2.14] | 0.86 |
| Renal symptoms | 1 | 5/279 | 1.79% | 1/277 | 0.36% | 4.96 [0.58, 42.22] | 0.14 |
| Ejection fraction decrease | 1 | 5/279 | 1.79% | 1/277 | 0.36% | 4.96 [0.58, 42.22] | 0.14 |
| Fatigue | 3 | 12/728 | 1.65% | 3/720 | 0.42% | 3.38 [0.56, 20.26] | 0.03 |
| Decreased appetite | 5 | 16/1071 | 1.49% | 9/1064 | 0.85% | 1.66 [0.72, 3.83] | 0.17 |
| Neutrophil count decreased | 3 | 10/671 | 1.49% | 1/671 | 0.15% | 5.22 [1.14, 23.82] | 0.03 |
| Hypokalemia | 3 | 10/671 | 1.49% | 12/671 | 1.79% | 0.84 [0.36, 1.95] | 0.67 |
| Pathological fracture | 1 | 4/279 | 1.43% | 2/277 | 0.72% | 0.20 [0.01, 4.12] | 0.3 |
| White blood cell count decreased | 4 | 12/853 | 1.41% | 2/851 | 0.24% | 3.77 [1.04, 13.60] | 0.02 |
| ECG QTc prolongation | 4 | 12/853 | 1.41% | 10/851 | 1.18% | 1.22 [0.51, 2.88] | 0.66 |
| Gamma-glutamyl transferase increase | 3 | 8/671 | 1.19% | 6/671 | 0.89% | 1.42 [0.24, 8.56] | 0.6 |
| Lymphocyte count decreased | 2 | 5/457 | 1.09% | 0/456 | 0.00% | 5.45 [0.63, 47.00] | 0.1 |
| Deep vein thrombosis | 2 | 5/461 | 1.08% | 1/457 | 0.22% | 2.84 [0.31, 25.75] | 0.16 |
| Cataract disorder | 1 | 3/279 | 1.08% | 1/277 | 0.36% | 2.98 [0.31, 28.46] | 0.34 |
| Sepsis | 1 | 3/279 | 1.08% | 3/277 | 1.08% | 1.99 [0.37, 10.75] | 0.43 |
| Peripheral sensory neuropathy | 3 | 6/578 | 1.04% | 1/572 | 0.17% | 2.86 [0.52, 15.60] | 0.12 |
Abbreviations: ALT: Alanine aminotransferase; CI: confidence interval; ECG: Electrocardiogram; FGET: First generation EGFR tyrosine kinase inhibitor; P: Probability; TGET: Third generation EGFR tyrosine kinase inhibitor.
This meta-analysis has several limitations. Firstly, it initially included only English articles, which may introduce language bias. Secondly, the TGET group comprised only six types of TGETs, potentially excluding other varieties. Thirdly, the data for analysis were solely derived from previous publications, leading to data heterogeneity. Fourthly, the absence of individual patient data hindered an individual patient data meta-analysis, possibly reducing the clinical value. Fifthly, the differences in median follow-up times among studies could contribute to data heterogeneity. Lastly, the majority of study populations were Asian (84% in each group), raising uncertainties about the generalizability to other populations.
Conclusion
TGETs appear to outperform FGETs in EGFR-mutated locally advanced or metastatic NSCLC, demonstrating superior survival and responses. This superiority, particularly evident in CNS-PFS, is consistent across most subgroups, except for the TGET-Naquotinib subgroup. However, the TGET group exhibits a high incidence of AEs, particularly hematologic AEs, which necessitates careful consideration. Validation of these results in large-scale RCTs is necessary due to the aforementioned limitations.
Abbreviations
AEs: Adverse effects; ALT: Alanine aminotransferase; ALTD: AEs leading to treatment discontinuation; AST: Aspartate aminotransferase; CI: confidence interval; CNS: Central nervous system; CNS-DOR: Duration of central nervous system response; CNS-PFS: Central nervous system-Progression-free survival; CNS-PFSR: CNS-PFS: Central nervous system-Progression-free survival rate; CR: Complete response; DCR: Disease control rate; DOR: Duration of response; ECG: Electrocardiogram; ECOG: Eastern Cooperative Oncology Group; EGFR: Epidermal growth factor receptor; EPR: Estimated percentage remaining in response; Erl: Erlotinib; FGET: First generation EGFR tyrosine kinase inhibitor; Gef: Gefitinib; GRADE: Grading of Recommendations, Assessment, Development, and Evaluation; HR: Hazard ratio; MD: Mean difference; M/F: Male/Female; NSCLC: Non-small cell lung cancer; ORR: Objective response rate; OS: Overall survival; OSR: Overall survival rate; P: Probability; PD: Progressive disease; PFS: Progression-free survival; PFSR: Progression-free survival rate; PICOS: Participants, Intervention, Control, Outcome and Study design; PR: Partial response; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analysis; RCT: Randomized controlled trial; RR: Risk ratio; SD: Stable disease; TGET: Third generation EGFR tyrosine kinase inhibitor; TKI: Tyrosinkinase inhibitor.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
The authors thank professor Yiping Wei, MD (Department of Thoracic Surgery, The second affiliated hospital of Nanchang University) for his data collection and statistical advice.
Funding
This study was supported by Natural Science Foundation of Jiangxi Province (Grant number: 20212BAB206050). The funding had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Availability of data and materials
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author contributions
Tanggui Feng had full access to all of the data in the manuscript and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Wenjie Hu, Yi Qin, Taoming Dong, Xueying Lin, Yuan Chen, and Tanggui Feng.
Acquisition, analysis, or interpretation of data: Wenjie Hu, Yi Qin, Taoming Dong, Xueying Lin, Yuan Chen, Wenxiong Zhang and Tanggui Feng.
Drafting of the manuscript: Wenjie Hu, Yi Qin, and Tanggui Feng.
Critical revision of the manuscript for important intellectual content: Wenjie Hu, Yi Qin, and Tanggui Feng.
Statistical analysis: Wenjie Hu, Yi Qin, and Taoming Dong.
Supervision: Wenjie Hu, and Tanggui Feng.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12-49
2. Korn AR, Walsh-Bailey C, Correa-Mendez M, DelNero P, Pilar M, Sandler B. et al. Social determinants of health and US cancer screening interventions: A systematic review. CA Cancer J Clin. 2023;73(5):461-479
3. Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT. et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9(2):154-62
4. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A. et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20(5):497-530
5. Lee CK, Davies L, Wu YL, Mitsudomi T, Inoue A, Rosell R. et al. Gefitinib or Erlotinib vs Chemotherapy for EGFR Mutation-Positive Lung Cancer: Individual Patient Data Meta-Analysis of Overall Survival. J Natl Cancer Inst. 2017;109(6):djw279
6. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH. et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378(2):113-125
7. Cho BC, Ahn MJ, Kang JH, Soo RA, Reungwetwattana T, Yang JC. et al. Lazertinib Versus Gefitinib as First-Line Treatment in Patients With EGFR-Mutated Advanced Non-Small-Cell Lung Cancer: Results From LASER301. J Clin Oncol. 2023;41(26):4208-4217
8. Shi Y, Chen G, Wang X, Liu Y, Wu L, Hao Y. et al. Furmonertinib (AST2818) versus gefitinib as first-line therapy for Chinese patients with locally advanced or metastatic EGFR mutation-positive non-small-cell lung cancer (FURLONG): a multicentre, double-blind, randomised phase 3 study. Lancet Respir Med. 2022;10(11):1019-1028
9. Lu S, Dong X, Jian H, Chen J, Chen G, Sun Y. et al. AENEAS: A Randomized Phase III Trial of Aumolertinib Versus Gefitinib as First-Line Therapy for Locally Advanced or MetastaticNon-Small-Cell Lung Cancer With EGFR Exon 19 Deletion or L858R Mutations. J Clin Oncol. 2022;40(27):3162-3171
10. Kelly RJ, Shepherd FA, Krivoshik A, Jie F, Horn L. A phase III, randomized, open-label study of ASP8273 versus erlotinib or gefitinib in patients with advanced stage IIIB/IV non-small-cell lung cancer. Ann Oncol. 2019;30(7):1127-1133
11. Lu S, Zhou J, Jian H, Wu L, Cheng Y, Fan Y. et al. Befotertinib (D-0316) versus icotinib as first-line therapy for patients with EGFR-mutated locally advanced or metastatic non-small-cell lung cancer: a multicentre, open-label, randomised phase 3 study. Lancet Respir Med. 2023;11(10):905-915
12. Cheng Z, Cui H, Wang Y, Yang J, Lin C, Shi X. et al. The advance of the third-generation EGFR-TKI in the treatment of non-small cell lung cancer (Review). Oncol Rep. 2024;51(1):16
13. Hendriks LE, Kerr KM, Menis J, Mok TS, Nestle U, Passaro A. et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(4):339-357
14. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ. et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1-12
15. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD. et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928
16. Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380-382
17. Cheng Y, He Y, Li W, Zhang HL, Zhou Q, Wang B. et al. Osimertinib Versus Comparator EGFR TKI as First-Line Treatment for EGFR-Mutated Advanced NSCLC: FLAURA China, A Randomized Study. Target Oncol. 2021;16(2):165-176
18. Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y. et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med. 2020;382(1):41-50
19. Ohe Y, Imamura F, Nogami N, Okamoto I, Kurata T, Kato T. et al. Osimertinib versus standard-of-care EGFR-TKI as first-line treatment for EGFRm advanced NSCLC: FLAURA Japanese subset. Jpn J Clin Oncol. 2019;49(1):29-36
20. Cho BC, Chewaskulyong B, Lee KH, Dechaphunkul A, Sriuranpong V, Imamura F. et al. Osimertinib versus Standard of Care EGFR TKI as First-Line Treatment in Patients with EGFRm Advanced NSCLC: FLAURA Asian Subset. J Thorac Oncol. 2019;14(1):99-106
21. Reungwetwattana T, Nakagawa K, Cho BC, Cobo M, Cho EK, Bertolini A. et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2018:JCO2018783118.
22. Lee KH, Cho BC, Ahn MJ, Lee YG, Lee Y, Lee JS. et al. Lazertinib versus Gefitinib as First-Line Treatment for EGFR-mutated Locally Advanced or Metastatic NSCLC: LASER301 Korean Subset. Cancer Res Treat. 2024;56(1):48-60
23. Soo RA, Cho BC, Kim JH, Ahn MJ, Lee KH, Zimina A. et al. Central Nervous System Outcomes of Lazertinib Versus Gefitinib in EGFR-Mutated Advanced NSCLC: A LASER301 Subset Analysis. J Thorac Oncol. 2023;18(12):1756-1766
24. Reungwetwattana T, Cho BC, Lee KH, Pang YK, Fong CH, Kang JH. et al. Lazertinib Versus Gefitinib Tyrosine Kinase Inhibitors in Treatment-Naíve Patients With EGFR-Mutated Advanced NSCLC: Analysis of the Asian Subpopulation in LASER301. J Thorac Oncol. 2023;18(10):1351-1361
25. Shi Y, Chen G, Wang X, Liu Y, Wu L, Hao Y. et al. Central Nervous System Efficacy of Furmonertinib (AST2818) Versus Gefitinib as First-Line Treatment for EGFR-Mutated NSCLC: Results From the FURLONG Study. J Thorac Oncol. 2022;17(11):1297-1305
26. Uryu K, Imamura Y, Shimoyama R, Mase T, Fujimura Y, Hayashi M. et al. Stepwise prolongation of overall survival from first to third generation EGFR-TKIs for EGFR mutation-positive non-small-cell lung cancer: the Tokushukai REAl-world Data project (TREAD 01). Jpn J Clin Oncol. 2024;54:319-328
27. Zhang D, Liu X, Shen F, Zhao D, Shi Y, Zhang H. et al. Osimertinib versus comparator first-generation epidermal growth factor receptor tyrosine kinase inhibitors as first-line treatment in patients with advanced EGFR-mutated non-small cell lung cancer: a Chinese, multicenter, real-world cohort study. Transl Lung Cancer Res. 2023;12(11):2229-2244
28. Tatineni V, O'Shea PJ, Ozair A, Khosla AA, Saxena S, Rauf Y. et al. First- versus Third-Generation EGFR Tyrosine Kinase Inhibitors in EGFR-Mutated Non-Small Cell Lung Cancer Patients with Brain Metastases. Cancers (Basel). 2023;15(8):2382
29. Vaid AK, Gupta A, Momi G. Overall survival in stage IV EGFR mutation-positive NSCLC: Comparing first-, second- and third-generation EGFR-TKIs (Review). Int J Oncol. 2021;58(2):171-184
30. Lu S, Wang J, Yu Y, Yu X, Hu Y, Ai X. et al. Tislelizumab Plus Chemotherapy as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC (RATIONALE 304): A Randomized Phase 3 Trial. J Thorac Oncol. 2021;16(9):1512-1522
31. Zhang Y, Zhang Y, Niu W, Ge X, Huang F, Pang J. et al. Experimental Study of Almonertinib Crossing the Blood-Brain Barrier in EGFR-Mutant NSCLC Brain Metastasis and Spinal Cord Metastasis Models. Front Pharmacol. 2021;12:750031
32. Popat S, Ahn MJ, Ekman S, Leighl NB, Ramalingam SS, Reungwetwattana T. et al. Osimertinib for EGFR-Mutant Non-Small-Cell Lung Cancer Central Nervous System Metastases: Current Evidence and Future Perspectives on Therapeutic Strategies. Target Oncol. 2023;18(1):9-24
33. Park S, Jung HA, Lee SH, Ahn JS, Ahn MJ, Sun JM. Real-world clinical evidence of lazertinib use in acquired EGFR T790M mutated non-small cell lung cancer. Transl Lung Cancer Res. 2023;12(9):1912-1922
34. Zhou Q, Zhang HL, Jiang LY, Shi YK, Chen Y, Yu JM. et al. Real-world evidence of osimertinib in Chinese patients with EGFR T790M-positive non-small cell lung cancer: a subgroup analysis from ASTRIS study. J Cancer Res Clin Oncol. 2023;149(12):10771-10780
35. Liu CY, Lin HF, Lai WY, Lin YY, Lin TW, Yang YP. et al. Molecular target therapeutics of EGF-TKI and downstream signaling pathways in non-small cell lung cancers. J Chin Med Assoc. 2022;85(4):409-413
36. Jablonska PA, Das A. Management of brain metastases in non-small cell lung cancer without actionable driver mutations-the need to dive deeper in the right 'pool'. Transl Lung Cancer Res. 2023;12(10):1966-1971
37. Li Z, Lu S. Third-Generation EGFR Tyrosine Kinase Inhibitor for Central Nervous System Metastases EGFR-Mutant NSCLC: Current Evidence and Future Perspectives. J Thorac Oncol. 2023;18(12):1625-1628
38. John T, Grohé C, Goldman JW, Shepherd FA, de Marinis F, Kato T. et al. Three-Year Safety, Tolerability, and Health-Related Quality of Life Outcomes of Adjuvant Osimertinib in Patients With Resected Stage IB to IIIA EGFR-Mutated NSCLC: Updated Analysis From the Phase 3 ADAURA Trial. J Thorac Oncol. 2023;18(9):1209-1221
39. Mok TS, Wu Y-L, Ahn M-J, Garassino MC, Kim HR, Ramalingam SS. et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med. 2017;376(7):629-640
40. Thress KS, Paweletz CP, Felip E, Cho BC, Stetson D, Dougherty B. et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med. 2015;21(6):560-2
Author contact
![]() Corresponding author: Tanggui Feng, MD, Department of Respiratory and Critical Care Medicine, The Second People's Hospital of Jingdezhen, 3 North Square Road, Jingdezhen, China, 333000, E-mail: vekin008com, 4203119067ncu.edu.cn.
Corresponding author: Tanggui Feng, MD, Department of Respiratory and Critical Care Medicine, The Second People's Hospital of Jingdezhen, 3 North Square Road, Jingdezhen, China, 333000, E-mail: vekin008com, 4203119067ncu.edu.cn.

 Global reach, higher impact
Global reach, higher impact