Impact Factor
ISSN: 1837-9664
J Cancer 2025; 16(4):1379-1396. doi:10.7150/jca.104412 This issue Cite
Research Paper
Risk Prediction of Myelosuppression Following First-line Chemotherapy in Colorectal Cancer
1. Department of Oncology, Guang'anmen Hospital, China Academy of Chinese Medical Sciences, Beijing 100053, China.
2. Beijing University of Chinese Medicine, Beijing 100029, China.
†These authors contributed equally to this work and share first authorship.
Received 2024-9-29; Accepted 2024-12-23; Published 2025-1-20
Abstract
Background: Colorectal cancer (CRC) is a leading cause of cancer-related deaths, with over 1.9 million new cases and 904,000 deaths in 2022. Chemotherapy is a primary treatment for CRC but often leads to myelosuppression, significantly affecting treatment efficacy and patient outcomes. Predictive tools for chemotherapy-induced myelosuppression are currently lacking.
Methods: This retrospective study analyzed 855 CRC patients from Guang'anmen Hospital who received first-line chemotherapy (CapeOx, FOLFOX, FOLFIRI) between April 2020 and July 2024. Patients were divided into training (684) and validation (171) groups. Univariate analysis, LASSO regression, and multivariable logistic regression identified risk factors for myelosuppression, and a predictive nomogram was developed and validated using ROC curves, calibration curves, and decision curve analysis. Propensity score matching (PSM) was employed to minimize baseline differences between groups, followed by multivariate logistic regression analysis on the post-PSM data.
Results: The incidence of myelosuppression was similar in both groups (33.04% vs. 32.16%). Significant predictors included age, smoking, diabetes, BMI, tumor location, lung metastasis, albumin (ALB) levels, and carcinoembryonic antigen (CEA) levels. The nomogram demonstrated good predictive performance with AUC values of 0.78 and 0.80 for the training and validation groups, respectively, showing consistent and clinically useful predictions. PSM further validated the robustness of the model, confirming BMI as a consistently significant predictor of myelosuppression.
Conclusions: The study identified key risk factors for chemotherapy-induced myelosuppression in CRC patients and developed a nomogram for prediction. This tool can help clinicians assess risk and guide treatment decisions. Limitations include potential selection bias and the need for external validation in diverse populations. Future studies should further refine and validate this predictive model.
Keywords: Colorectal cancer (CRC), Chemotherapy-induced myelosuppression, Predictive nomogram, Risk factors, Retrospective study
Introduction
Colorectal cancer (CRC) refers to malignant tumors occurring in the colon or rectum. It ranks third in incidence, following lung cancer and breast cancer, and is the second leading cause of cancer-related deaths. Statistics indicate that in 2022, there were over 1.9 million new cases of colorectal cancer (including anal cancer) and 904,000 deaths, accounting for nearly one-tenth of all cancer cases and deaths[1]. With the aging population and changes in lifestyle, the incidence and mortality rates of CRC are expected to increase significantly in the coming decades. By 2040, the global incidence of CRC is projected to rise to 3.2 million new cases, with 1.6 million deaths[2-3]. Evidence suggests that in many high-income countries, the incidence of CRC among individuals under 50 years old has been increasing annually[4-5]. CRC has thus become an increasingly serious public health issue.
Chemotherapy is one of the main treatment options for CRC. For patients with early-stage CRC (Stage I and Stage II), surgical resection is the primary treatment modality. However, for high-risk Stage II patients, adjuvant chemotherapy post-surgery may be recommended to reduce the risk of recurrence. For patients with locally advanced CRC (Stage III), the standard treatment regimen includes surgical resection combined with adjuvant chemotherapy. In cases of metastatic CRC (Stage IV), systemic chemotherapy is the preferred approach, often in combination with targeted therapies such as bevacizumab or cetuximab[6-7]. The first-line treatment regimens for CRC include FOLFOX (fluorouracil [5-FU], leucovorin, and oxaliplatin), FOLFIRI (fluorouracil [5-FU], leucovorin, and irinotecan), and CapeOx (capecitabine and oxaliplatin)[6]. Although the aforementioned regimens demonstrate significant clinical efficacy[8-9], they are still associated with side effects such as myelosuppression, gastrointestinal reactions, dermatological lesions, and peripheral neurotoxicity[10-12]. In some cases, these regimens can lead to life-threatening cardiotoxicity[13] and central nervous system toxicity[14]. Among the side effects, myelosuppression is one of the most common adverse reactions following first-line chemotherapy regimens for CRC. Myelosuppression is a side effect induced by chemotherapy or radiotherapy that results in a reduced ability of the bone marrow to produce blood cells, leading to pancytopenia. The primary cause of myelosuppression is that chemotherapy, while attacking tumor cells, also inhibits the highly proliferative and poorly differentiated bone marrow cells, suppressing all immature cells with proliferative capabilities[15]. Specific manifestations of myelosuppression include neutropenia, anemia, and thrombocytopenia[16], which can also cause adverse reactions such as fever, rash, and bone pain[17-18]. More severely, the reduction of neutrophils and platelets can significantly increase the risk of infection and bleeding, directly threatening the patient's life. Furthermore, when severe myelosuppression occurs, it necessitates a reduction in chemotherapy dosage or a delay in treatment, impacting the clinical benefits of the therapy. Therefore, early diagnosis of chemotherapy-induced myelosuppression is of paramount importance.
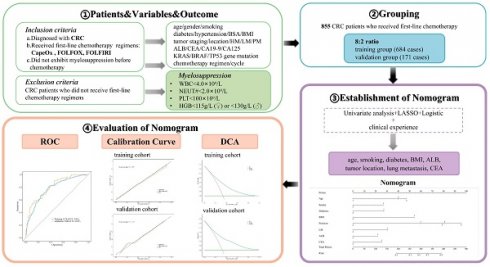
Considering the crucial role of chemotherapy in treating CRC patients and the adverse effects of chemotherapy-induced myelosuppression, it is imperative to identify predictive factors for myelosuppression in this population. Currently, there are no reliable methods to accurately assess the risk of myelosuppression in CRC patients. Creating a reliable predictive tool for assessing myelosuppression risk after chemotherapy would be of significant clinical importance. This study aims to develop and validate a model for predicting the myelosuppression after first-line chemotherapy for colorectal cancer. The study protocol of this article is shown in Figure 1.
Methods
Patients and study design
This study retrospectively selected 855 inpatients from the Oncology Department of Guang'anmen Hospital, China Academy of Chinese Medical Sciences, from April 2020 to July 2024. To maximize the accuracy, sensitivity, and specificity of the predictive model and enhance its overall performance, including its utility in clinical decision-making, this study utilized the randomizr package in R to perform simple randomization of the included cases (training group: validation group = 8:2). This approach reduces selection bias, improves comparability between groups, and enhances the predictive power of the model's features with respect to the outcome indicators, thereby increasing the external validity of the nomogram predictive model. The patients were randomly divided into a training group and a validation group at a ratio of 8:2. Inclusion criteria: ① Diagnosed with CRC according to the diagnostic criteria of the "Chinese Protocol of Diagnosis and Treatment of Colorectal Cancer (2023 Edition)"; ② Received first-line chemotherapy regimens: CapeOx (capecitabine and oxaliplatin), FOLFOX (fluorouracil, leucovorin, and oxaliplatin), FOLFIRI (fluorouracil, leucovorin, and irinotecan); ③ Did not exhibit myelosuppression before chemotherapy, defined as meeting all of the following criteria: adult peripheral blood leukocyte count ≥4.0×10^9/L, absolute neutrophil count ≥2.0×10^9/L, platelet count ≥100×10^9/L, hemoglobin ≥115g/L (female) or hemoglobin ≥130g/L (male). Exclusion criteria: CRC patients who did not receive first-line chemotherapy regimens.
The training group consisted of 684 patients and was used to develop and train the model. The validation group consisted of 171 patients and was used to validate the model.
The study was approved by the ethical committee of our hospital (Approval Number: 2022-215-KY).
Flowchart of the study protocol.
Outcome definition
The outcome variable of this study is antineoplastic drug-related myelosuppression, defined as adult peripheral blood leukocyte count <4.0×10^9/L, absolute neutrophil count <2.0×10^9/L, platelet count <100×10^9/L, and hemoglobin <115g/L (female) or hemoglobin <130g/L (male). Meeting any one of these four criteria qualifies as a diagnosis of antineoplastic drug-related myelosuppression[16]. Laboratory tests were evaluated between the start of each chemotherapy cycle and the next cycle, typically conducted 3-7 days after chemotherapy, to assess the severity of myelosuppression following first-line chemotherapy.
Demographic and clinical measures for prediction and defnition
The predictive variables include patient-specific and chemotherapy regimen-related risk factors. The candidate predictors include age, gender, smoking, diabetes, hypertension, body surface area (BSA), body mass index (BMI), tumor staging, tumor location, hepatic metastasis, lung metastasis, peritoneal metastasis, albumin (ALB), carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), carbohydrate antigen 125 (CA125), KRAS gene mutation, BRAF gene mutation, TP53 gene mutation, chemotherapy regimen, and chemotherapy cycle. These predictive variables were assessed and recorded before each chemotherapy session.
Age was categorized into three groups: <60 years, 60-74 years, and ≥75 years. Smoking status included both past and current smoking habits. BSA was divided into three categories: <1.6 m², 1.6-1.8 m², and >1.8 m². According to the Chinese standard[19], BMI was categorized into non-overweight and overweight, with a cutoff point of 24.0 kg/m². CRC staging was based on the AJCC Cancer Staging Manual, 8th Edition[20], and divided into four stages: Stage I, II, III, and IV. Tumor location was categorized into five anatomical sites: ascending colon, transverse colon, descending colon, sigmoid colon, and rectum. The first-line chemotherapy regimens for CRC were categorized into three groups: CapeOx (capecitabine and oxaliplatin), FOLFOX (fluorouracil, leucovorin, and oxaliplatin), and FOLFIRI (fluorouracil, leucovorin, and irinotecan). The number of chemotherapy cycles was divided into three categories: 1-2 cycles, 3-4 cycles, and 5 or more cycles.
Statistical analysis
Univariate analysis was conducted to preliminarily identify potential risk factors for myelosuppression following first-line chemotherapy in CRC patients. Subsequently, to identify the most predictive factors associated with myelosuppression in CRC patients undergoing first-line chemotherapy, we applied the Least Absolute Shrinkage and Selection Operator (LASSO) regression model. This statistical approach is particularly effective in selecting relevant non-zero features, thereby enhancing the model's predictive accuracy by focusing on the most significant variables contributing to myelosuppression risk in this patient population[21-22]. Based on the univariate analysis and the non-zero features identified, combined with clinical experience, a multivariable logistic regression model was established for further analysis. In the multivariable logistic regression analysis, the relationship between each factor and the outcome variable was determined using forward selection, backward elimination, and stepwise selection methods. The likelihood ratio test with the minimum Akaike Information Criterion (AIC) value was used as the stopping rule[23]. A nomogram model was then constructed, incorporating the results from both the LASSO regression analysis and the multivariable logistic regression analysis.
To assess the discriminatory capability of the developed nomogram, we analyzed the area under the receiver operating characteristic (ROC) curve. This metric provides a quantitative measure of the nomogram's ability to distinguish between different clinical outcomes, offering a robust evaluation of its predictive accuracy and effectiveness in clinical practice. Calibration was assessed by comparing observed outcomes with predicted probabilities, with an optimal fit indicated by an intercept of α=0 and a slope of β=1. To thoroughly evaluate the clinical utility and applicability of the nomogram, we employed decision curve analysis (DCA). This analytical method offers a nuanced approach to assessing the net benefit by quantifying it across a comprehensive spectrum of threshold probabilities present within the dataset. By doing so, DCA provides a more detailed and practical understanding of the nomogram's performance in various clinical scenarios, ensuring that its predictive accuracy and potential benefits are effectively validated in real-world settings. This approach underscores the robustness and reliability of the nomogram in guiding clinical decision-making[24-25].Due to certain differences in specific factors between patients with myelosuppression and those without, we employed a propensity score matching (PSM) analysis model using a caliper of 0.2 to balance the intergroup variability of these factors. This approach was aimed at eliminating potential selection bias and enhancing the evidence level of our retrospective study. Matching was performed using the "nearest-neighbor" method, with a PSM ratio of 1:1. Subsequently, a multivariable logistic regression analysis was conducted on the post-PSM baseline data to further validate the original logistic regression model and the nomogram.
The organization of data and execution of statistical analyses were conducted using Zstats software alongside R version 4.4.0. For the univariate analysis, continuous variables were assessed using the Wilcoxon rank-sum test, while categorical variables were analyzed with the χ² test. In the multivariable logistic regression analysis, we employed forward selection, backward elimination, and stepwise selection techniques, determining the optimal model based on the minimum AIC value. The calibration curve was assessed using the unreliable U test, ensuring the model's calibration accuracy. The "rms" package in R facilitated the creation of the nomogram and calibration curve. A p-value of less than 0.05 was considered statistically significant throughout the analysis.
Results
Characteristics of patients
In this study, a retrospective selection of 684 patients was made for inclusion in the training group, while 171 patients were assigned to the validation group. There were no statistically significant differences in demographic and clinical characteristics between the two groups (P>0.05). Additionally, the incidence of myelosuppression did not differ significantly between the training and validation groups (33.04% vs. 32.16%, P=0.827) (Table 1).
Feature selection
In the univariate analysis of the training group of CRC patients after first-line chemotherapy, significant differences were observed between the myelosuppression and non-myelosuppression groups in terms of age, gender, smoking, diabetes, body surface area(BSA), body mass index(BMI), tumor staging, tumor location, hepatic metastasis, lung metastasis, albumin(ALB), carcinoembryonic antigen(CEA), and the number of chemotherapy cycles. No statistically significant differences were found between the two groups in terms of hypertension, peritoneal metastasis, carbohydrate antigen 19-9(CA19-9), carbohydrate antigen 125(CA125), KRAS gene mutation, BRAF gene mutation, TP53 gene mutation, or chemotherapy regimen (Table 2).
Balance test between the training and validation groups.
| Variables | Total (n = 855) | Validation group (n = 171) | Training group (n = 684) | Statistic | P | |
|---|---|---|---|---|---|---|
| Age (y) | χ²=5.27 | 0.072 | ||||
| <60 | 302 (35.32) | 72 (42.11) | 230 (33.63) | |||
| 60~74 | 481 (56.26) | 83 (48.54) | 398 (58.19) | |||
| ≥75 | 72 (8.42) | 16 (9.36) | 56 (8.19) | |||
| Gender | χ²=0.00 | 0.945 | ||||
| Male | 507 (59.30) | 101 (59.06) | 406 (59.36) | |||
| Female | 348 (40.70) | 70 (40.94) | 278 (40.64) | |||
| Smoking | χ²=0.00 | 0.972 | ||||
| Yes | 306 (35.79) | 61 (35.67) | 245 (35.82) | |||
| Not | 549 (64.21) | 110 (64.33) | 439 (64.18) | |||
| Diabetes | χ²=0.94 | 0.333 | ||||
| Yes | 141 (16.49) | 24 (14.04) | 117 (17.11) | |||
| Not | 714 (83.51) | 147 (85.96) | 567 (82.89) | |||
| Hypertension | χ²=0.03 | 0.862 | ||||
| Yes | 345 (40.35) | 68 (39.77) | 277 (40.50) | |||
| Not | 510 (59.65) | 103 (60.23) | 407 (59.50) | |||
| BSA (m2) | χ²=3.39 | 0.183 | ||||
| <1.6 | 291 (34.04) | 48 (28.07) | 243 (35.53) | |||
| 1.6~1.8 | 309 (36.14) | 67 (39.18) | 242 (35.38) | |||
| >1.8 | 255(29.82) | 56 (32.75) | 199 (29.09) | |||
| BMI | χ²=2.81 | 0.094 | ||||
| Overweight | 416 (48.65) | 93 (54.39) | 323 (47.22) | |||
| Not overweight | 439 (51.35) | 78 (45.61) | 361 (52.78) | |||
| Tumor(T) | χ²=1.66 | 0.646 | ||||
| 1 | 18 (2.11) | 3 (1.75) | 15 (2.19) | |||
| 2 | 97 (11.35) | 21 (12.28) | 76 (11.11) | |||
| 3 | 447 (52.28) | 95 (55.56) | 352 (51.46) | |||
| 4 | 293 (34.27) | 52 (30.41) | 241 (35.23) | |||
| Node(N) | - | 0.700 | ||||
| 0 | 180 (21.05) | 38 (22.22) | 142 (20.76) | |||
| 1 | 367 (42.92) | 67 (39.18) | 300 (43.86) | |||
| 2 | 303 (35.44) | 65 (38.01) | 238 (34.80) | |||
| 3 | 5 (0.58) | 1 (0.58) | 4 (0.58) | |||
| Metastasis(M) | χ²=0.01 | 0.918 | ||||
| 0 | 393 (45.96) | 78 (45.61) | 315 (46.05) | |||
| 1 | 462 (54.04) | 93 (54.39) | 369 (53.95) | |||
| Tumor staging | χ²=2.34 | 0.504 | ||||
| I | 15 (1.77) | 5 (2.94) | 10 (1.48) | |||
| II | 73 (8.62) | 12 (7.06) | 61 (9.01) | |||
| III | 296 (34.95) | 58 (34.12) | 238 (35.16) | |||
| IV | 463 (54.66) | 95 (55.88) | 368 (54.36) | |||
| Tumor location | χ²=2.77 | 0.596 | ||||
| Ascending colon | 167 (19.53) | 32 (18.71) | 135 (19.74) | |||
| Transverse colon | 22 (2.57) | 2 (1.17) | 20 (2.92) | |||
| Descending colon | 28 (3.27) | 4 (2.34) | 24 (3.51) | |||
| Sigmoid colon | 234 (27.37) | 51 (29.82) | 183 (26.75) | |||
| Rectum | 404 (47.25) | 82 (47.95) | 322 (47.08) | |||
| Hepatic metastasis | χ²=0.22 | 0.640 | ||||
| Yes | 293 (34.27) | 56 (32.75) | 237 (34.65) | |||
| Not | 562 (65.73) | 115 (67.25) | 447 (65.35) | |||
| Lung metastasis | χ²=0.07 | 0.789 | ||||
| Yes | 237 (27.72) | 46 (26.90) | 191 (27.92) | |||
| Not | 618 (72.28) | 125 (73.10) | 493 (72.08) | |||
| Peritoneum metastasis | χ²=0.42 | 0.516 | ||||
| Yes | 51 (5.96) | 12 (7.02) | 39 (5.70) | |||
| Not | 804 (94.04) | 159 (92.98) | 645 (94.30) | |||
| ALB | χ²=0.00 | 0.968 | ||||
| Normal | 654 (76.49) | 131 (76.61) | 523 (76.46) | |||
| Abnormal | 201 (23.51) | 40 (23.39) | 161 (23.54) | |||
| CEA | χ²=1.06 | 0.304 | ||||
| Normal | 455 (53.22) | 85 (49.71) | 370 (54.09) | |||
| Abnormal | 400 (46.78) | 86 (50.29) | 314 (45.91) | |||
| CA19-9 | χ²=0.31 | 0.575 | ||||
| Normal | 255 (29.82) | 48 (28.07) | 207 (30.26) | |||
| Abnormal | 600 (70.18) | 123 (71.93) | 477 (69.74) | |||
| CA125 | χ²=1.13 | 0.287 | ||||
| Normal | 89 (10.41) | 14 (8.19) | 75 (10.96) | |||
| Abnormal | 766 (89.59) | 157 (91.81) | 609 (89.04) | |||
| KRAS | χ²=1.92 | 0.166 | ||||
| Yes | 140 (16.37) | 34 (19.88) | 106 (15.50) | |||
| Not | 715 (83.63) | 137 (80.12) | 578 (84.50) | |||
| BRAF | χ²=0.20 | 0.659 | ||||
| Yes | 16 (1.87) | 2 (1.17) | 14 (2.05) | |||
| Not | 839 (98.13) | 169 (98.83) | 670 (97.95) | |||
| TP53 | χ²=0.04 | 0.850 | ||||
| Yes | 68 (7.95) | 13 (7.60) | 55 (8.04) | |||
| Not | 787 (92.05) | 158 (92.40) | 629 (91.96) | |||
| Myelosuppression | χ²=0.05 | 0.827 | ||||
| Yes | 574 (67.13) | 116 (67.84) | 458 (66.96) | |||
| Not | 281 (32.87) | 55 (32.16) | 226 (33.04) | |||
| Chemotherapy cycle | χ²=1.53 | 0.465 | ||||
| First or Second | 344 (40.23) | 66 (38.60) | 278 (40.64) | |||
| Third or Fourth | 267 (31.23) | 60 (35.09) | 207 (30.26) | |||
| Fifth and Above | 244 (28.54) | 45 (26.32) | 199 (29.09) | |||
| Chemotherapy regimen | χ²=0.35 | 0.840 | ||||
| CapeOx | 639 (74.74) | 126 (73.68) | 513 (75.00) | |||
| FOLFOX | 93 (10.88) | 18 (10.53) | 75 (10.96) | |||
| FOLFIRI | 123 (14.39) | 27 (15.79) | 96 (14.04) |
χ²: Chi-square test, -: Fisher exact
BSA: Body Surface Area; BMI: Body Mass Index; ALB: Albumin; CEA: Carcinoembryonic Antigen; CA19-9: Carbohydrate Antigen 19-9; CA125: Carbohydrate Antigen 125.
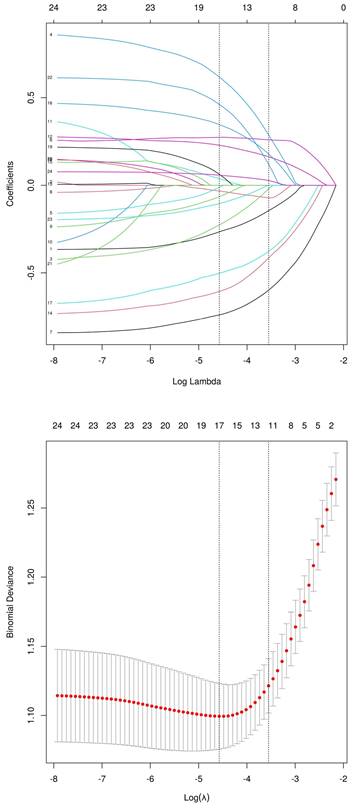
Using the LASSO regression model, the 24 sociodemographic and clinical characteristics were reduced to 12 potential risk factors with non-zero coefficients (approximately 2:1 ratio) (Figure 2). These features include age, smoking, diabetes, BSA, BMI, tumor staging, tumor location, lung metastasis, ALB, CEA, TP53 gene mutation, and the number of chemotherapy cycles.
Clinical characteristics of patients in both the training and validation groups.
| Characteristics | Training group | Validation group | |||||
|---|---|---|---|---|---|---|---|
| Myelosuppression (n=458) | Non-myelosuppression (n=226) | P -value | Myelosuppression (n=116) | Non-myelosuppression (n=55) | P -value | ||
| Age (y) | 0.001 | 0.067 | |||||
| <60 | 136 (29.69%) | 94 (41.59%) | 43 (37.07%) | 29 (52.73%) | |||
| 60~74 | 276 (60.26%) | 122 (53.98%) | 59 (50.86%) | 24 (43.64%) | |||
| ≥75 | 46 (10.04%) | 10 (4.42%) | 14 (12.07%) | 2 (3.64%) | |||
| Gender | <.001 | <.001 | |||||
| Male | 244 (53.28%) | 162 (71.68%) | 58 (50.00%) | 43 (78.18%) | |||
| Female | 214 (46.72%) | 64 (28.32%) | 58 (50.00%) | 12 (21.82%) | |||
| Smoking | 0.001 | 0.134 | |||||
| Not | 313 (68.34%) | 126 (55.75%) | 79 (68.10%) | 31 (56.36%) | |||
| Yes | 145 (31.66%) | 100 (44.25%) | 37 (31.90%) | 24 (43.64%) | |||
| Diabetes | 0.006 | 0.200 | |||||
| Not | 367 (80.13%) | 200 (88.50%) | 97 (83.62%) | 50 (90.91%) | |||
| Yes | 91 (19.87%) | 26 (11.50%) | 19 (16.38%) | 5 (9.09%) | |||
| Hypertension | 0.083 | 0.167 | |||||
| Not | 283 (61.79%) | 124 (54.87%) | 74 (63.79%) | 29 (52.73%) | |||
| Yes | 175 (38.21%) | 102 (45.13%) | 42 (36.21%) | 26 (47.27%) | |||
| BSA (m2) | <.001 | <.001 | |||||
| <1.6 | 184 (40.17%) | 59 (26.11%) | 39 (33.62%) | 9 (16.36%) | |||
| 1.6-1.8 | 181 (39.52%) | 61 (26.99%) | 51 (43.97%) | 16 (29.09%) | |||
| >1.8 | 93 (20.31%) | 106 (46.90%) | 26 (22.41%) | 30 (54.55%) | |||
| BMI | <.001 | <.001 | |||||
| Not overweight | 281 (61.35%) | 80 (35.40%) | 52 (44.83%) | 41 (74.55%) | |||
| Overweight | 177 (38.65%) | 146 (64.60%) | 64 (55.17%) | 14 (25.45%) | |||
| Tumor staging | <.001 | 0.032 | |||||
| I | 4 (0.89%) | 6 (2.65%) | 4 (3.48%) | 1 (1.82%) | |||
| II | 53 (11.75%) | 8 (3.54%) | 11 (9.57%) | 1 (1.82%) | |||
| III | 171 (37.92%) | 67 (29.65%) | 44 (38.26%) | 14 (25.45%) | |||
| IV | 223 (49.45%) | 145 (64.16%) | 56 (48.70%) | 39 (70.91%) | |||
| Tumor location | <.001 | <.001 | |||||
| Ascending colon | 110 (24.02%) | 25 (11.06%) | 31 (26.72%) | 1 (1.82%) | |||
| Transverse colon | 18 (3.93%) | 2 (0.88%) | 1 (0.86%) | 1 (1.82%) | |||
| Descending colon | 18 (3.93%) | 6 (2.65%) | 0 (0.00%) | 4 (7.27%) | |||
| Sigmoid colon | 117 (25.55%) | 66 (29.20%) | 36 (31.03%) | 15 (27.27%) | |||
| Rectum | 195 (42.58%) | 127 (56.19%) | 48 (41.38%) | 34 (61.82%) | |||
| Hepatic metastasis | 0.046 | 0.730 | |||||
| Not | 311 (67.90%) | 136 (60.18%) | 79 (68.10%) | 36 (65.45%) | |||
| Yes | 147 (32.10%) | 90 (39.82%) | 37 (31.90%) | 19 (34.55%) | |||
| Lung metastasis | <.001 | 0.022 | |||||
| Not | 355 (77.51%) | 138 (61.06%) | 91 (78.45%) | 34 (61.82%) | |||
| Yes | 103 (22.49%) | 88 (38.94%) | 25 (21.55%) | 21 (38.18%) | |||
| Peritoneum metastasis | 0.968 | 0.682 | |||||
| Not | 432 (94.32%) | 213 (94.25%) | 109 (93.97%) | 50 (90.91%) | |||
| Yes | 26 (5.68%) | 13 (5.75%) | 7 (6.03%) | 5 (9.09%) | |||
| ALB | 0.001 | 0.047 | |||||
| Normal | 367 (80.13%) | 156 (69.03%) | 94 (81.03%) | 37 (67.27%) | |||
| Abnormal | 91 (19.87%) | 70 (30.97%) | 22 (18.97%) | 18 (32.73%) | |||
| CEA | <.001 | 0.012 | |||||
| Normal | 222 (48.47%) | 148 (65.49%) | 50 (43.10%) | 35 (63.64%) | |||
| Abnormal | 236 (51.53%) | 78 (34.51%) | 66 (56.90%) | 20 (36.36%) | |||
| CA19-9 | 0.415 | 0.194 | |||||
| Normal | 134 (29.26%) | 73 (32.30%) | 29 (25.00%) | 19 (34.55%) | |||
| Abnormal | 324 (70.74%) | 153 (67.70%) | 87 (75.00%) | 36 (65.45%) | |||
| CA125 | 0.643 | 1.000 | |||||
| Normal | 52 (11.35%) | 23 (10.18%) | 9 (7.76%) | 5 (9.09%) | |||
| Abnormal | 406 (88.65%) | 203 (89.82%) | 107 (92.24%) | 50 (90.91%) | |||
| KRAS | 0.497 | 0.701 | |||||
| Not | 384 (83.84%) | 194 (85.84%) | 92 (79.31%) | 45 (81.82%) | |||
| Yes | 74 (16.16%) | 32 (14.16%) | 24 (20.69%) | 10 (18.18%) | |||
| BRAF | 0.222 | 1.000 | |||||
| Not | 446 (97.38%) | 224 (99.12%) | 114 (98.28%) | 55 (100.00%) | |||
| Yes | 12 (2.62%) | 2 (0.88%) | 2 (1.72%) | 0 (0.00%) | |||
| TP53 | 0.065 | 0.098 | |||||
| Not | 415 (90.61%) | 214 (94.69%) | 104 (89.66%) | 54 (98.18%) | |||
| Yes | 43 (9.39%) | 12 (5.31%) | 12 (10.34%) | 1 (1.82%) | |||
| Chemotherapy regimen | 0.111 | 0.002 | |||||
| CapeOx | 174 (37.99%) | 104 (46.02%) | 93 (80.17%) | 33 (60.00%) | |||
| FOLFOX | 142 (31.00%) | 65 (28.76%) | 6 (5.17%) | 12 (21.82%) | |||
| FOLFIRI | 142 (31.00%) | 57 (25.22%) | 17 (14.66%) | 10 (18.18%) | |||
| Chemotherapy cycle | <.001 | 0.268 | |||||
| First or Second | 371 (81.00%) | 142 (62.83%) | 40 (34.48%) | 26 (47.27%) | |||
| Third or Fourth | 29 (6.33%) | 46 (20.35%) | 44 (37.93%) | 16 (29.09%) | |||
| Fifth and Above | 58 (12.66%) | 38 (16.81%) | 32 (27.59%) | 13 (23.64%) | |||
BSA: Body Surface Area; BMI: Body Mass Index; ALB: Albumin; CEA: Carcinoembryonic Antigen; CA19-9: Carbohydrate Antigen 19-9; CA125: Carbohydrate Antigen 125.
Demographic and Clinical Characteristics Screening Using the LASSO Regression Model. (A) LASSO regression coefficient path diagram of risk factors. (B) Cross-validation curve for the LASSO regression.
Risk factors for myelosuppression following first-line chemotherapy in CRC patients
In the multivariate analysis, this study employed a multivariate logistic regression analysis method, using myelosuppression as the dependent variable. The independent variables included age, smoking, diabetes, BSA, BMI, tumor staging, tumor location, hepatic metastasis, lung metastasis, ALB, CEA, and the number of chemotherapy cycles. The results indicated that age, smoking, diabetes, BMI, tumor location, lung metastasis, ALB, and CEA were independent risk factors for myelosuppression in CRC patients undergoing first-line chemotherapy (Table 3).
Multivariate logistic regression analysis.
| Intercept and variable | β | P | Odds ratio (95% CI) |
|---|---|---|---|
| Intercept | 1.32 | 0.214 | 3.73 (0.47 ~ 29.84) |
| Age (y) | |||
| <60 | 1.00 (Reference) | ||
| 60~74 | -0.16 | 0.442 | 0.85 (0.56 ~ 1.29) |
| ≥75 | -1.05 | 0.012 | 0.35 (0.15 ~ 0.79) |
| Smoking | |||
| Yes | 1.00 (Reference) | ||
| Not | -0.60 | 0.005 | 0.55 (0.36 ~ 0.84) |
| Diabetes | |||
| Yes | 1.00 (Reference) | ||
| Not | 0.61 | 0.025 | 1.83 (1.08 ~ 3.11) |
| BMI | |||
| Overweight | 1.00 (Reference) | ||
| Not overweight | -1.21 | <.001 | 0.30 (0.20 ~ 0.44) |
| Tumor location | |||
| Ascending colon | 1.00 (Reference) | ||
| Transverse colon | -1.33 | 0.121 | 0.26 (0.05 ~ 1.42) |
| Descending colon | 0.42 | 0.477 | 1.52 (0.48 ~ 4.85) |
| Sigmoid colon | 0.47 | 0.136 | 1.60 (0.86 ~ 2.96) |
| Rectum | 0.80 | 0.005 | 2.22 (1.28 ~ 3.84) |
| Tumor staging | |||
| I | 1.00 (Reference) | ||
| II | -2.22 | 0.026 | 0.11 (0.02 ~ 0.76) |
| III | -1.33 | 0.152 | 0.26 (0.04 ~ 1.64) |
| IV | -1.42 | 0.133 | 0.24 (0.04 ~ 1.54) |
| Lung metastasis | |||
| Yes | 1.00 (Reference) | ||
| Not | -0.68 | 0.006 | 0.50 (0.31 ~ 0.82) |
| ALB | |||
| Normal | 1.00 (Reference) | ||
| Abnormal | 0.49 | 0.032 | 1.63 (1.04 ~ 2.54) |
| CEA | |||
| Normal | 1.00 (Reference) | ||
| Abnormal | -0.56 | 0.019 | 0.57 (0.35 ~ 0.91) |
| Chemotherapy regimen | |||
| CapeOx | 1.00 (Reference) | ||
| FOLFOX | 1.11 | <.001 | 3.03 (1.58 ~ 5.82) |
| FOLFIRI | -0.13 | 0.650 | 0.88 (0.50 ~ 1.54) |
β is the regression coefficient. CI: Confidence Interval
BMI: Body Mass Index; ALB: Albumin; CEA: Carcinoembryonic antigen.
Establishment of the nomogram for predicting myelosuppression after first-line chemotherapy in CRC patients
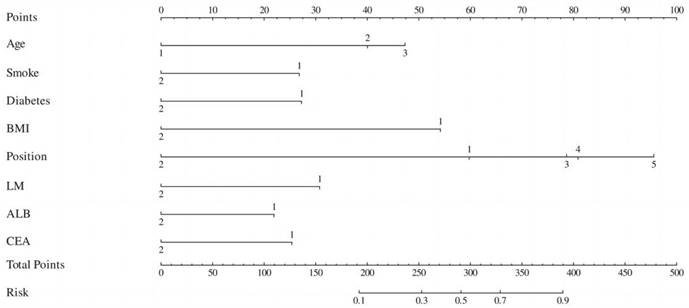
By synthesizing the outcomes of univariate analysis, LASSO regression, and multivariate logistic regression, and incorporating clinical expertise, the study identified age, smoking, diabetes, BMI, tumor location, lung metastasis, ALB, and CEA as independent risk factors for myelosuppression following first-line chemotherapy in CRC patients. These eight risk factors were incorporated into a predictive model, culminating in the creation of a nomogram for forecasting myelosuppression in CRC patients after first-line chemotherapy (Figure 2).
The model uses the 8 risk factors as variable axes. Each variable's score is vertically aligned with the score axis, which ranges from 0 to 100 points. The total score of the eight variables corresponds to the total score axis, ranging from 0 to 500 points. The total score is then vertically aligned with the probability axis, which ranges from 0.1 to 0.9, indicating the probability of myelosuppression. The total score of each variable corresponds to the risk probability line to obtain the probability of risk of myelosuppression. For example, for a 90-year-old CRC patient with lung metastasis, a BMI of 25 kg/m², a history of type 2 diabetes, chronic smoking, and an albumin level (ALB) of 32 g/L, the nomogram scores are as follows: age 90 years (47.5 points), lung metastasis (32 points), BMI 25 kg/m² (54 points), type 2 diabetes (27.5 points), smoking (27 points), and ALB 32 g/L (22 points), totaling 210 points, resulting in a 0.13 (13%) probability of myelosuppression. Thus, both doctors and patients can use this intuitive and user-friendly scoring system to predict the risk of myelosuppression and rationalize clinical decisions regarding any adjunctive treatment before or during chemotherapy. Therefore, this nomogram has clinical value for predicting and preventing myelosuppression in CRC patients after first-line chemotherapy.
Evaluation of the nomogram for predicting myelosuppression after first-line chemotherapy in CRC patients in the training and validation groups
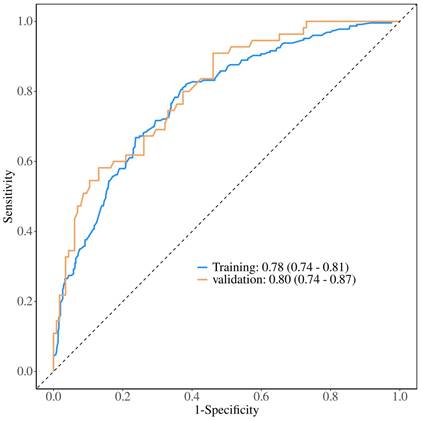
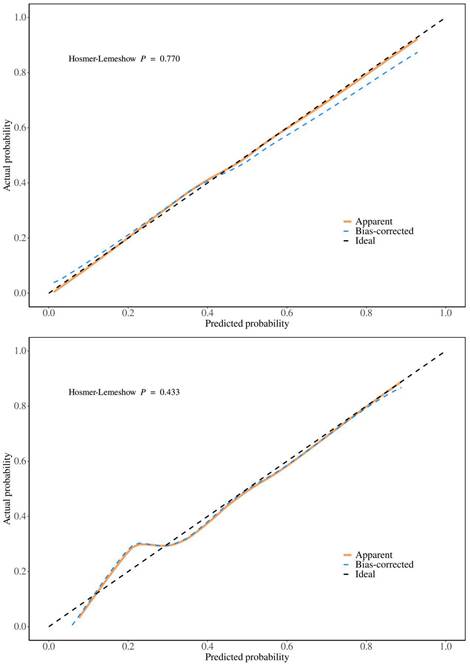
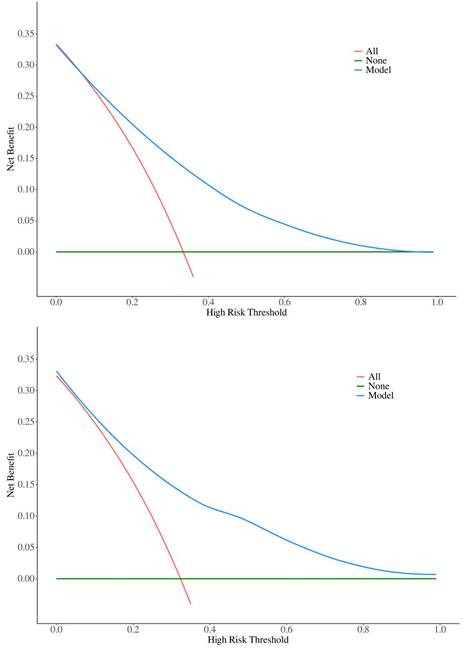
To validate the sensitivity and specificity of the nomogram model, this study evaluated the predictive nomogram using the area under the ROC curve (AUC). The AUC values of the nomogram for the training and validation groups were found to be 0.78 and 0.80, respectively (Figure 5). The calibration curves demonstrated acceptable consistency between the predicted and actual values in two groups of CRC patients receiving first-line chemotherapy (Figure 4). DCA showed that if the threshold probability (Pt) in the training and validation groups ranged between 10-90%, timely clinical intervention based on the predicted probability from the nomogram before first-line chemotherapy could provide a higher net benefit compared to either the full intervention or no intervention strategies (Figure 6). Therefore, the nomogram model exhibits good discriminative ability, acceptable calibration, and clinical utility in predicting myelosuppression in CRC patients following chemotherapy.
Risk factors for myelosuppression after first-line chemotherapy in CRC patients following PSM
After PSM, all variables achieved balance between the groups, with P-values greater than 0.05 and standardized mean differences (SMDs) generally below 0.10 (Table 4).
Nomogram for predicting myelosuppression induced by first-line chemotherapy in CRC. BMI: body mass index; LM: Lung metastasis; ALB: albumin; CEA: carcinoembryonic antigen. (Age: 1=<60y 2=60~74y 3=≥75y; Smoking: 1=Yes 2=Not; Diabetes: 1=Yes 2=Not: BMI: 1=<1.6 m² 2=1.6-1.8 m² 3=>1.8 m²; Location: 1=Ascending colon 2=Transverse colon 3=Descending colon 4=Sigmoid colon 5=Rectum; LM: 1=Yes 2=Not; ALB: 1=Normal 2=Abnormal; CEA: 1=Normal 2=Abnormal).
ROC curves for the training and validation groups. ROC curve for the training group, AUC = 0.78; ROC curve for the validation group, AUC = 0.80.
Calibration curves for training and validation groups. (A) Calibration curve for the training group (B) Calibration curve for the validation group.
We conducted a multivariate logistic regression analysis on data after PSM and reconfirmed that BMI is a significant risk factor for bone marrow suppression in CRC patients following first-line chemotherapy. It is important to emphasize that BMI consistently emerged as a significant risk factor in both the initial model and the model optimized for baseline variables, indicating its critical role in the occurrence of chemotherapy-induced bone marrow suppression.
Discussion
Despite recent advancements in treatment options, CRC continues to significantly impact public health due to its high incidence and mortality rates. Currently, standard treatment regimens rely on chemotherapy protocols containing highly myelotoxic drugs, such as FOLFOX and FOLFIRI. These treatments often lead to myelosuppression, particularly in elderly patients with multiple comorbidities. Therefore, identifying risk factors for myelosuppression in CRC patients following first-line chemotherapy is crucial for optimizing treatment outcomes and improving survival rates.
This study aimed to identify risk factors for chemotherapy-induced myelosuppression in CRC patients. Through univariate and multivariate logistic regression analyses, we identified eight significant risk factors: age, smoking status, diabetes, BMI, tumor location, lung metastasis, ALB, and CEA. Nomograms, which graphically represent the relationships between multiple variables using a statistical model, are widely used in clinical practice due to their user-friendly interface, high accuracy, ease of prognostic interpretation, and utility in guiding clinical decision-making. This study seeks to develop and validate such a nomogram, aiming to predict the probability of myelosuppression and aid in individualized treatment decisions. The predictive model incorporates eight easily obtainable demographic and clinical variables, enabling accurate and personalized risk assessment for myelosuppression post-chemotherapy in CRC patients. Meanwhile, the large sample size of our group enhances the nomogram's applicability and accuracy. This study also utilized AUC, calibration curves, and DCA to validate the model. The AUC is a widely used metric for evaluating the performance of predictive models, providing a single value that quantifies the model's ability to distinguish between positive and negative cases[26]. The AUC of the training group in this model is 0.78, indicating good generalization without overfitting, while the AUC of the validation group is 0.80, confirming strong performance on new data. The small difference between the training and validation AUCs suggests that the model is robust. Calibration curves assess how well predicted probabilities align with actual outcomes. A well-calibrated model produces predictions that match observed results, ideally falling on a diagonal line representing perfect calibration[27]. In this model, both the training and validation groups show calibration curves that closely follow the ideal line, indicating good calibration. The p-values for the training and validation groups (0.770 and 0.433, respectively) further support that there is no significant deviation from good calibration. DCA evaluates the clinical utility of the model by comparing its net benefit across different risk thresholds to two extreme strategies: treating all patients or treating none[28]. The model provides a higher net benefit within the threshold probability range of 10-90%, indicating that it supports more effective decision-making, helps to personalize interventions, reduces unnecessary treatments, and balances the benefits and risks of clinical interventions.
Decision curves for the training and validation groups. (A) Decision curve for the training group (B) Decision curve for the validation group.
Clinical characteristics of patients in both the myelosuppression and non-myelosuppression patients.
| Variable | Total (n = 440) | 0 (n = 220) | 1 (n = 220) | Statistic | P | SMD |
|---|---|---|---|---|---|---|
| Age, n (%) | χ²=0.395 | 0.821 | ||||
| 1 | 164 (37.27) | 84 (38.18) | 80 (36.36) | -0.038 | ||
| 2 | 250 (56.82) | 122 (55.45) | 128 (58.18) | 0.055 | ||
| 3 | 26 (5.91) | 14 (6.36) | 12 (5.45) | -0.040 | ||
| Sex, n (%) | χ²=2.103 | 0.147 | ||||
| 1 | 306 (69.55) | 160 (72.73) | 146 (66.36) | -0.135 | ||
| 2 | 134 (30.45) | 60 (27.27) | 74 (33.64) | 0.135 | ||
| Smoke, n (%) | χ²=0.000 | 1.000 | ||||
| 1 | 180 (40.91) | 90 (40.91) | 90 (40.91) | 0.000 | ||
| 2 | 260 (59.09) | 130 (59.09) | 130 (59.09) | 0.000 | ||
| Diabetes, n (%) | χ²=0.090 | 0.764 | ||||
| 1 | 50 (11.36) | 26 (11.82) | 24 (10.91) | -0.029 | ||
| 2 | 390 (88.64) | 194 (88.18) | 196 (89.09) | 0.029 | ||
| Hypertension, n (%) | χ²=0.758 | 0.384 | ||||
| 1 | 183 (41.59) | 96 (43.64) | 87 (39.55) | -0.084 | ||
| 2 | 257 (58.41) | 124 (56.36) | 133 (60.45) | 0.084 | ||
| BSA, n (%) | χ²=1.692 | 0.429 | ||||
| 1 | 117 (26.59) | 54 (24.55) | 63 (28.64) | 0.090 | ||
| 2 | 152 (34.55) | 82 (37.27) | 70 (31.82) | -0.117 | ||
| 3 | 171 (38.86) | 84 (38.18) | 87 (39.55) | 0.028 | ||
| BMI, n (%) | χ²=0.231 | 0.631 | ||||
| 1 | 249 (56.59) | 122 (55.45) | 127 (57.73) | 0.046 | ||
| 2 | 191 (43.41) | 98 (44.55) | 93 (42.27) | -0.046 | ||
| Tumor(T), n (%) | - | 0.314 | ||||
| 1 | 1 (0.23) | 0 (0.00) | 1 (0.45) | 0.068 | ||
| 2 | 51 (11.59) | 30 (13.64) | 21 (9.55) | -0.139 | ||
| 3 | 234 (53.18) | 111 (50.45) | 123 (55.91) | 0.110 | ||
| 4 | 154 (35) | 79 (35.91) | 75 (34.09) | -0.038 | ||
| Node(N), n (%) | - | 0.374 | ||||
| 0 | 79 (17.95) | 35 (15.91) | 44 (20.00) | 0.102 | ||
| 1 | 212 (48.18) | 110 (50.00) | 102 (46.36) | -0.073 | ||
| 2 | 147 (33.41) | 75 (34.09) | 72 (32.73) | -0.029 | ||
| 3 | 2 (0.45) | 0 (0.00) | 2 (0.91) | 0.096 | ||
| Metastasis(M), n (%) | χ²=0.091 | 0.763 | ||||
| 0 | 151 (34.32) | 74 (33.64) | 77 (35.00) | 0.029 | ||
| 1 | 289 (65.68) | 146 (66.36) | 143 (65.00) | -0.029 | ||
| Staging, n (%) | - | 0.382 | ||||
| 1 | 4 (0.91) | 2 (0.91) | 2 (0.91) | 0.000 | ||
| 2 | 12 (2.73) | 3 (1.36) | 9 (4.09) | 0.138 | ||
| 3 | 131 (29.77) | 67 (30.45) | 64 (29.09) | -0.030 | ||
| 4 | 293 (66.59) | 148 (67.27) | 145 (65.91) | -0.029 | ||
| Position, n (%) | χ²=0.756 | 0.944 | ||||
| 1 | 47 (10.68) | 22 (10.00) | 25 (11.36) | 0.043 | ||
| 2 | 8 (1.82) | 5 (2.27) | 3 (1.36) | -0.078 | ||
| 3 | 19 (4.32) | 9 (4.09) | 10 (4.55) | 0.022 | ||
| 4 | 137 (31.14) | 69 (31.36) | 68 (30.91) | -0.010 | ||
| 5 | 229 (52.05) | 115 (52.27) | 114 (51.82) | -0.009 | ||
| HM, n (%) | χ²=0.755 | 0.385 | ||||
| 1 | 185 (42.05) | 97 (44.09) | 88 (40.00) | -0.084 | ||
| 2 | 255 (57.95) | 123 (55.91) | 132 (60.00) | 0.084 | ||
| LM, n (%) | χ²=0.254 | 0.614 | ||||
| 1 | 149 (33.86) | 72 (32.73) | 77 (35.00) | 0.048 | ||
| 2 | 291 (66.14) | 148 (67.27) | 143 (65.00) | -0.048 | ||
| PM, n (%) | χ²=0.164 | 0.686 | ||||
| 1 | 26 (5.91) | 14 (6.36) | 12 (5.45) | -0.040 | ||
| 2 | 414 (94.09) | 206 (93.64) | 208 (94.55) | 0.040 | ||
| ALB, n (%) | χ²=0.106 | 0.745 | ||||
| 1 | 325 (73.86) | 164 (74.55) | 161 (73.18) | -0.031 | ||
| 2 | 115 (26.14) | 56 (25.45) | 59 (26.82) | 0.031 | ||
| CEA, n (%) | χ²=0.356 | 0.551 | ||||
| 1 | 282 (64.09) | 138 (62.73) | 144 (65.45) | 0.057 | ||
| 2 | 158 (35.91) | 82 (37.27) | 76 (34.55) | -0.057 | ||
| CA199, n (%) | χ²=0.010 | 0.920 | ||||
| 1 | 151 (34.32) | 75 (34.09) | 76 (34.55) | 0.010 | ||
| 2 | 289 (65.68) | 145 (65.91) | 144 (65.45) | -0.010 | ||
| CA125, n (%) | χ²=0.084 | 0.771 | ||||
| 1 | 54 (12.27) | 28 (12.73) | 26 (11.82) | -0.028 | ||
| 2 | 386 (87.73) | 192 (87.27) | 194 (88.18) | 0.028 | ||
| KRAS, n (%) | χ²=1.087 | 0.297 | ||||
| 1 | 51 (11.59) | 22 (10.00) | 29 (13.18) | 0.094 | ||
| 2 | 389 (88.41) | 198 (90.00) | 191 (86.82) | -0.094 | ||
| BRAF, n (%) | χ²=0.000 | 1.000 | ||||
| 1 | 3 (0.68) | 2 (0.91) | 1 (0.45) | -0.068 | ||
| 2 | 437 (99.32) | 218 (99.09) | 219 (99.55) | 0.068 | ||
| TP53, n (%) | χ²=0.927 | 0.336 | ||||
| 1 | 18 (4.09) | 7 (3.18) | 11 (5.00) | 0.083 | ||
| 2 | 422 (95.91) | 213 (96.82) | 209 (95.00) | -0.083 | ||
| CF, n (%) | χ²=0.842 | 0.656 | ||||
| 1 | 182 (41.36) | 88 (40.00) | 94 (42.73) | 0.055 | ||
| 2 | 143 (32.5) | 76 (34.55) | 67 (30.45) | -0.089 | ||
| 3 | 115 (26.14) | 56 (25.45) | 59 (26.82) | 0.031 | ||
| CR, n (%) | χ²=1.822 | 0.402 | ||||
| 1 | 307 (69.77) | 160 (72.73) | 147 (66.82) | -0.125 | ||
| 2 | 73 (16.59) | 33 (15.00) | 40 (18.18) | 0.082 | ||
| 3 | 60 (13.64) | 27 (12.27) | 33 (15.00) | 0.076 |
BSA: Body Surface Area; BMI: Body Mass Index; ALB: Albumin; CEA: Carcinoembryonic Antigen; CA19-9: Carbohydrate Antigen 19-9; CA125: Carbohydrate Antigen 125.
Multivariate logistic regression analysis after PSM
| Variables | β | P | OR (95%CI) |
|---|---|---|---|
| Intercept | -1.25 | 0.549 | 0.29 (0.00 ~ 17.24) |
| Age | |||
| 1 | 1.00 (Reference) | ||
| 2 | -0.00 | 0.990 | 1.00 (0.62 ~ 1.60) |
| 3 | -0.61 | 0.200 | 0.55 (0.22 ~ 1.38) |
| Sex | |||
| 1 | 1.00 (Reference) | ||
| 2 | -0.26 | 0.485 | 0.77 (0.37 ~ 1.61) |
| Smoke | |||
| 1 | 1.00 (Reference) | ||
| 2 | -0.18 | 0.548 | 0.84 (0.47 ~ 1.50) |
| Diabetes | |||
| 1 | 1.00 (Reference) | ||
| 2 | 0.39 | 0.219 | 1.48 (0.79 ~ 2.75) |
| Hypertension | |||
| 1 | 1.00 (Reference) | ||
| 2 | -0.00 | 0.993 | 1.00 (0.63 ~ 1.59) |
| BSA | |||
| 1 | 1.00 (Reference) | ||
| 2 | -0.21 | 0.532 | 0.81 (0.41 ~ 1.58) |
| 3 | -0.03 | 0.942 | 0.97 (0.41 ~ 2.29) |
| BMI | |||
| 1 | 1.00 (Reference) | ||
| 2 | -0.71 | 0.008 | 0.49 (0.29 ~ 0.83) |
| Tumor(T) | |||
| 1 | 1.00 (Reference) | ||
| 2 | 1.39 | 0.260 | 4.02 (0.36 ~ 45.48) |
| 3 | 1.50 | 0.215 | 4.50 (0.42 ~ 48.34) |
| 4 | 1.37 | 0.259 | 3.93 (0.36 ~ 42.32) |
| Node(N) | |||
| 0 | 1.00 (Reference) | ||
| 1 | -0.02 | 0.952 | 0.98 (0.48 ~ 1.99) |
| 2 | -0.28 | 0.473 | 0.75 (0.35 ~ 1.64) |
| Metastasis(M) | |||
| 0 | 1.00 (Reference) | ||
| 1 | -0.72 | 0.562 | 0.49 (0.04 ~ 5.52) |
| Staging | |||
| 1 | 1.00 (Reference) | ||
| 2 | -1.51 | 0.164 | 0.22 (0.03 ~ 1.85) |
| 3 | -0.95 | 0.366 | 0.39 (0.05 ~ 3.03) |
| 4 | -0.17 | 0.917 | 0.84 (0.03 ~ 20.45) |
| Position | |||
| 1 | 1.00 (Reference) | ||
| 2 | -0.73 | 0.450 | 0.48 (0.07 ~ 3.20) |
| 3 | 0.12 | 0.846 | 1.13 (0.34 ~ 3.78) |
| 4 | 0.19 | 0.629 | 1.21 (0.56 ~ 2.63) |
| 5 | 0.44 | 0.197 | 1.55 (0.80 ~ 3.01) |
| HM | |||
| 1 | 1.00 (Reference) | ||
| 2 | 0.07 | 0.823 | 1.07 (0.59 ~ 1.93) |
| LM | |||
| 1 | 1.00 (Reference) | ||
| 2 | -0.37 | 0.218 | 0.69 (0.39 ~ 1.24) |
| PM | |||
| 1 | 1.00 (Reference) | ||
| 2 | -0.10 | 0.837 | 0.91 (0.36 ~ 2.31) |
| ALB | |||
| 1 | 1.00 (Reference) | ||
| 2 | 0.28 | 0.253 | 1.33 (0.82 ~ 2.17) |
| CEA | |||
| 1 | 1.00 (Reference) | ||
| 2 | -0.47 | 0.104 | 0.63 (0.36 ~ 1.10) |
| CA199 | |||
| 1 | 1.00 (Reference) | ||
| 2 | 0.05 | 0.858 | 1.05 (0.62 ~ 1.78) |
| CA125 | |||
| 1 | 1.00 (Reference) | ||
| 2 | 0.33 | 0.345 | 1.39 (0.70 ~ 2.75) |
| KRAS | |||
| 1 | 1.00 (Reference) | ||
| 2 | 0.03 | 0.933 | 1.03 (0.50 ~ 2.12) |
| BRAF | |||
| 1 | 1.00 (Reference) | ||
| 2 | 0.15 | 0.903 | 1.16 (0.11 ~ 11.99) |
| TP53 | |||
| 1 | 1.00 (Reference) | ||
| 2 | 0.28 | 0.608 | 1.33 (0.45 ~ 3.89) |
| CF | |||
| 1 | 1.00 (Reference) | ||
| 2 | -0.13 | 0.581 | 0.87 (0.54 ~ 1.41) |
| 3 | -0.28 | 0.270 | 0.75 (0.45 ~ 1.25) |
| CR | |||
| 1 | 1.00 (Reference) | ||
| 2 | 0.56 | 0.140 | 1.76 (0.83 ~ 3.71) |
| 3 | -0.26 | 0.454 | 0.77 (0.39 ~ 1.53) |
OR: Odds Ratio, CI: Confidence Interval
BSA: Body Surface Area; BMI: Body Mass Index; ALB: Albumin; CEA: Carcinoembryonic Antigen; CA19-9: Carbohydrate Antigen 19-9; CA125: Carbohydrate Antigen 125.
Finally, to minimize the impact of baseline imbalance on the results of multivariate analysis, we employed PSM to balance baseline characteristics. In the matched dataset with balanced baselines, we conducted a multivariate logistic regression analysis again, and the results indicated that BMI remained a significant risk factor. Although several other significant factors were identified in the initial model, this does not imply that they were false associations due to confounding bias. On the contrary, these factors may indeed have an influence on the occurrence of myelosuppression in specific subgroups of patients, but their significance was weakened in the dataset with balanced baseline characteristics. The persistent significance of BMI in the optimized model reflects its consistency and independence under varying baseline conditions.
Age is one of the risk factors for chemotherapy-induced myelosuppression identified in this study. Chemotherapy-related myelosuppression in elderly patients may be associated with decreased metabolism of myelosuppressive drugs, leading to drug accumulation and increased toxicity[29]. Decline in bone marrow function and depletion of hematopoietic stem cell reserves are also reasons for increased susceptibility to myelosuppression in the elderly. This decline results from changes in the bone marrow microenvironment and alterations in the intrinsic properties of hematopoietic stem cells. Chronic low-grade inflammation and elevated levels of oxidative stress commonly present in the elderly[30], as well as diminished DNA damage repair capacity[31], can impair the bone marrow microenvironment and hematopoietic stem cell function, making myelosuppression more likely. Furthermore, elderly patients often have multiple chronic diseases, and medications used to treat these conditions, such as methotrexate and allopurinol, may suppress bone marrow function or exacerbate myelosuppression through drug interactions[32].
The impact of smoking on chemotherapy-induced myelosuppression is also noteworthy. While smoking is generally associated with altered peripheral blood cell counts, such as increased neutrophils and erythrocytes[33], this does not imply a protective effect of smoking on bone marrow function. On the contrary, smoking exacerbates chemotherapy-induced myelosuppression through various mechanisms. Chronic inflammation and oxidative stress caused by smoking can damage bone marrow stem cells. Free radicals and toxic compounds produced by tobacco combustion induce systemic inflammatory responses, altering the bone marrow microenvironment and making it more susceptible to chemotherapeutic agents. Additionally, chemicals in tobacco, such as benzene, carbon monoxide, and nicotine, exert direct toxic effects on bone marrow stem cells by damaging DNA and interfering with cell signaling pathways, thereby inhibiting their function. Moreover, smoking suppresses immune system function, making the body more vulnerable to chemotherapy-induced bone marrow damage. Immunosuppression also increases the risk of infection, which further exacerbates myelosuppression[32-34]. In summary, smokers are more likely to experience severe neutropenia and thrombocytopenia during chemotherapy.
Consistent with previous studies, diabetic patients are more prone to experiencing chemotherapy-related myelosuppression[35]. The exacerbation of chemotherapy-induced myelosuppression in diabetic patients may be related to systemic chronic inflammation and metabolic disorders caused by chronic hyperglycemia. Chronic hyperglycemia in diabetic patients induces a state of low-grade systemic inflammation, primarily mediated by endotoxins, free fatty acids, and cholesterol through the activation of Toll-like receptor (TLR) and nuclear factor κB (NF-κB) pathways. This activation leads to the release of numerous pro-inflammatory cytokines that disrupt the bone marrow microenvironment, resulting in reduced production of red blood cells, white blood cells, and platelets. These inflammatory cytokines also directly induce apoptosis and dysfunction of bone marrow cells, thereby exacerbating chemotherapy-induced myelosuppression[36-37].
Our predictive model indicates that overweight patients are more likely to experience chemotherapy-induced myelosuppression. Additionally, we have demonstrated the robustness and significance of this result under different statistical methods. Previous studies have shown that obese patients typically have a slower clearance rate of chemotherapeutic drugs, resulting in an extended drug elimination half-life[38]. High BMI is also usually associated with increased bone marrow fat content, which may affect the hematopoietic function of the bone marrow[36]. Contradictorily, however, many studies have shown no significant correlation between high BMI and myelosuppression[40-41]. Some evidence even suggests that BMI is inversely related to myelosuppression[42]. The primary reason for these conflicting findings may be related to variations in chemotherapy dosing, among other factors, and the exact mechanisms need further investigation.
Previous studies have not discussed the correlation between specific tumor locations in CRC and chemotherapy-induced myelosuppression. Our research is the first to propose this association. Our model indicates that malignancies in the rectum and sigmoid colon are more prone to chemotherapy-induced myelosuppression, which may be related to differences in treatment modalities. Rectal cancer often requires concurrent chemoradiotherapy, and the dose-volume parameters of radiotherapy (such as V20 and V25 for pelvic bone marrow) significantly affect the incidence of myelosuppression. Treatment for rectal cancer typically involves high-dose radiotherapy to the pelvic area, increasing the risk of myelosuppression. In contrast, cancers of the ascending colon, transverse colon, and descending colon generally do not require radiotherapy, with chemotherapy being the primary treatment modality. Therefore, myelosuppression in these cases is solely related to chemotherapeutic drugs.
It is evident that lung metastasis, elevated CEA, and decreased albumin levels in CRC are risk factors for chemotherapy-induced myelosuppression. We believe this is likely related to a higher tumor burden. CRC with lung metastasis has already progressed to an advanced stage, and CEA levels are proportional to the severity of CRC. A high tumor burden leads to significant albumin consumption, and such patients typically have poorer overall health, lower immune function, and reduced tolerance to chemotherapeutic drugs, making them more susceptible to myelosuppression[43-44].
In this study, we chose not to include the chemotherapy regimen in the final predictive model, despite its statistical significance, as it contradicts common sense and clinical experience. Although the p-value for this factor was less than 0.05, our team's clinical experience suggests that this variable does not align with existing theories and common sense, possibly indicating a false-positive result due to data issues or chance. Additionally, previous studies have presented conflicting findings regarding the differences in myelosuppression caused by the FOLFOX, CapeOx, and FOLFIRI regimens[45-49]. For these reasons, we decided to exclude the chemotherapy regimen variable from the model.
Limitations
We acknowledge the limitations of our study. First, as a retrospective investigation, our study inevitably carries selection bias, even though we applied the same inclusion and selection criteria in both the validation and training groups. Second, our findings may not be representative of all CRC patients, as the patients in this study were primarily from northern China, potentially limiting the generalizability to other populations. Furthermore, although we attempted to include all possible influencing factors, it is unavoidable that some potential factors affecting chemotherapy-induced myelosuppression were not included. Additionally, in this study, a small number of patients began interventions for myelosuppression to prevent severe cases. While this introduces some bias, it better reflects the real-world data rules. Lastly, although we performed internal validation, the robustness of our nomogram has not been verified in other groups, and its applicability to other populations of CRC patients undergoing first-line chemotherapy in different regions and countries remains uncertain. This necessitates external evaluation in a broader population. We believe it is essential to conduct further prospective studies to identify more new practical factors and validate the nomogram across multiple centers.
Conclusions
The nomogram predictive model developed in this study, based on general information and relevant laboratory test results from colorectal cancer patients, demonstrates strong clinical decision-making capabilities. It accurately predicts the likelihood of myelosuppression after first-line chemotherapy, thereby assisting clinicians in formulating subsequent treatment plans. This approach is aimed at ensuring the smooth progression of the treatment process, reducing risks, and improving patients' quality of life.
Abbreviations
CRC: Colorectal cancer; BSA: body surface area; BMI: body mass index; T: Tumor; N: Node; M: Metastasis; ALB: albumin; CA125: carbohydrate antigen 125; CA19-9: carbohydrate antigen 19-9; CEA: carcinoembryonic antigen; AJCC: the American Joint Committee on Cancer; LASSO: the Least Absolute Shrinkage and Selection Operator; AIC: Akaike Information Criterion; ROC: the receiver operating characteristic; DCA: decision curve analysis; AUC: the area under the ROC curve; DNA: deoxyribonucleic acid; TLR: the activation of Toll-like receptor; NF-κB: nuclear factor κB.
Acknowledgements
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the High Level Chinese Medical Hospital Promotion Project (No. HLCMHPP2023085); the National Natural Scientifc Foundation of China (No. 82174463); the (National Administration of Traditional Chinese Medicine) under Grant (ZYYCXTD-C-C202205); the (China Academy of Chinese Medicine Sciences) under Grant (CI2021A01804 and 2022S469).
Author contributions
YD: Data curation, Writing-original draft, Writing-review & editing. YL: Data curation, Writing-original draft, Writing-review & editing. RF: Writing-original draft, Writing-review & editing. LC: Writing-original draft, Writing-review & editing. YS: Writing-review & editing. SM: Data curation, Writing-review & editing. JG: Writing-review & editing. HZ: Writing-review & editing. BL: Data curation. HY: Writing-review & editing. HX: Writing-review & editing. HZ: Conceptualization, Investigation, Writing-original draft, Writing-review & editing.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Bray F, Laversanne M, Sung H. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229-263
2. Arnold M, Abnet CC, Neale RE. et al. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology. 2020;159(1):335-349.e15
3. Morgan E, Arnold M, Gini A. et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72(2):338-344
4. Siegel RL, Torre LA, Soerjomataram I. et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut. 2019;68(12):2179-2185
5. Howren A, Sayre EC, Loree JM. et al. Trends in the Incidence of Young-Onset Colorectal Cancer With a Focus on Years Approaching Screening Age: A Population-Based Longitudinal Study. J Natl Cancer Inst. 2021;113(7):863-868
6. Benson AB, Venook AP, Adam M. et al. Colon Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2024;22(2 D):e240029
7. You YN, Hardiman KM, Bafford A. et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Rectal Cancer. Dis Colon Rectum. 2020;63(9):1191-1222
8. Tournigand C, André T, Achille E. et al. FOLFIRI Followed by FOLFOX6 or the Reverse Sequence in Advanced Colorectal Cancer: A Randomized GERCOR Study. J Clin Oncol. 2023;41(19):3469-3477
9. Colucci G, Gebbia V, Paoletti G. et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell'Italia Meridionale. J Clin Oncol. 2005;23(22):4866-4875
10. Negarandeh R, Salehifar E, Saghafi F. et al. Evaluation of adverse effects of chemotherapy regimens of 5-fluoropyrimidines derivatives and their association with DPYD polymorphisms in colorectal cancer patients. BMC Cancer. 2020;20(1):560
11. Akdeniz N, Kaplan MA, Uncu D. et al. The comparison of FOLFOX regimens with different doses of 5-FU for the adjuvant treatment of colorectal cancer: a multicenter study. Int J Colorectal Dis. 2021;36(6):1311-1319
12. Hwang JJ. Irinotecan and 5-FU/ leucovorin in metastatic colorectal cancer: balancing efficacy, toxicity, and logistics. Oncology (Williston Park). 2004;18(14 Suppl 14):26-34
13. Polk A, Shahmarvand N, Vistisen K. et al. Incidence and risk factors for capecitabine-induced symptomatic cardiotoxicity: a retrospective study of 452 consecutive patients with metastatic breast cancer. BMJ Open. 2016;6(10):e012798
14. Cordier PY, Nau A, Ciccolini J. et al. 5-FU-induced neurotoxicity in cancer patients with profound DPD deficiency syndrome: a report of two cases. Cancer Chemother Pharmacol. 2011;68(3):823-826
15. Yamashina T, Baghdadi M, Yoneda A. et al. Cancer stem-like cells derived from chemoresistant tumors have a unique capacity to prime tumorigenic myeloid cells. Cancer Res. 2014;74(10):2698-2709
16. Shah S. Common terminology criteria for adverse events[J]. National Cancer Institute: USA. 2022;784:785
17. Kadakia MP. Human herpesvirus 6 infection and associated pathogenesis following bone marrow transplantation. Leuk Lymphoma. 1998;31(3-4):251-266
18. Zeng SY, Li L, Zhong ML, Jiang W, Wu YY, Liu Y. Zhonghua Zhong Liu Za Zhi. 2011;33(7):517-519.
19. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies [published correction appears in Lancet. 2004 Mar 13;363(9412):902]. Lancet. 2004;363(9403):157-163
20. Amin MB, Greene FL, Edge SB. et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67(2):93-99
21. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173-e180
22. Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26(8):1364-1370
23. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350:g7594
24. Vickers AJ, van Calster B, Steyerberg EW. A simple, step-by-step guide to interpreting decision curve analysis. Diagn Progn Res. 2019;3:18
25. Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26(6):565-574
26. Linden A. Measuring diagnostic and predictive accuracy in disease management: an introduction to receiver operating characteristic (ROC) analysis. J Eval Clin Pract. 2006;12(2):132-139
27. Alba AC, Agoritsas T, Walsh M. et al. Discrimination and Calibration of Clinical Prediction Models: Users' Guides to the Medical Literature. JAMA. 2017;318(14):1377-1384
28. Vickers AJ, Van Calster B, Steyerberg EW. Net benefit approaches to the evaluation of prediction models, molecular markers, and diagnostic tests. BMJ. 2016;352:i6
29. Chan A, Lee CP, Chiang J, Ng R. Breakthrough febrile neutropenia and associated complications among elderly cancer patients receiving myelosuppressive chemotherapy for solid tumors and lymphomas. Support Care Cancer. 2013;21(8):2137-2143
30. Wang L, Jiang C, Wang N. et al. "Moderate" adjuvant chemotherapy-induced leukopenia is beneficial for survival of patients with early breast cancer: a retrospective study. BMC Cancer. 2023;23(1):1227
31. Wang X, Gu S, Wen J, Zhang L, Qi X. Association between chemotherapy-induced myelosuppression and curative efficacy of 2-cycle chemotherapy in small cell lung cancer. Cancer Chemother Pharmacol. 2024;93(2):151-159
32. Epstein RS, Aapro MS, Basu Roy UK. et al. Patient Burden and Real-World Management of Chemotherapy-Induced Myelosuppression: Results from an Online Survey of Patients with Solid Tumors. Adv Ther. 2020;37(8):3606-3618
33. Xie J, Broxmeyer HE, Feng D. et al. Human adipose-derived stem cells ameliorate cigarette smoke-induced murine myelosuppression via secretion of TSG-6. Stem Cells. 2015;33(2):468-478
34. Cairo MS. Dose reductions and delays: limitations of myelosuppressive chemotherapy. Oncology (Williston Park). 2000;14(9 Suppl 8):21-31
35. Srokowski TP, Fang S, Hortobagyi GN, Giordano SH. Impact of diabetes mellitus on complications and outcomes of adjuvant chemotherapy in older patients with breast cancer. J Clin Oncol. 2009;27(13):2170-2176
36. Lega IC, Austin PC, Fischer HD. et al. The Impact of Diabetes on Breast Cancer Treatments and Outcomes: A Population-Based Study. Diabetes Care. 2018;41(4):755-761
37. Guan J, Abudouaini H, Lin K, Yang K. Emerging insights into the role of IL-1 inhibitors and colchicine for inflammation control in type 2 diabetes. Diabetol Metab Syndr. 2024;16(1):140
38. Powis G, Reece P, Ahmann DL, Ingle JN. Effect of body weight on the pharmacokinetics of cyclophosphamide in breast cancer patients. Cancer Chemother Pharmacol. 1987;20(3):219-222
39. Lemoine L, Buckinx F, Aidoud A, Leroy V, Fougère B, Aubertin-Leheudre M. Relationships between obesity markers and bone parameters in community-dwelling older adults. Aging Clin Exp Res. 2024;36(1):49
40. Kamimura K, Matsumoto Y, Zhou Q, Moriyama M, Saijo Y. Myelosuppression by chemotherapy in obese patients with gynecological cancers. Cancer Chemother Pharmacol. 2016;78(3):633-641
41. Li N, Liu X, Zhai F. et al. Association between dose-volume parameters and acute bone marrow suppression in rectal cancer patients treated with concurrent chemoradiotherapy. Oncotarget. 2017;8(54):92904-92913
42. Li M, Chen J, Deng Y. et al. Risk prediction models based on hematological/body parameters for chemotherapy-induced adverse effects in Chinese colorectal cancer patients. Support Care Cancer. 2021;29(12):7931-7947
43. Pettengell R, Bosly A, Szucs TD. et al. Multivariate analysis of febrile neutropenia occurrence in patients with non-Hodgkin lymphoma: data from the INC-EU Prospective Observational European Neutropenia Study. Br J Haematol. 2009;144(5):677-685
44. Jiang N, Chen XC, Zhao Y. Analysis of the risk factors for myelosuppression after concurrent chemoradiotherapy for patients with advanced non-small cell lung cancer. Support Care Cancer. 2013;21(3):785-791
45. Ten Berg MJ, van den Bemt PM, Shantakumar S. et al. Thrombocytopenia in adult cancer patients receiving cytotoxic chemotherapy: results from a retrospective hospital-based group study. Drug Saf. 2011;34(12):1151-1160
46. Kilpatrick K, Shaw JL, Jaramillo R. et al. Occurrence and Management of Thrombocytopenia in Metastatic Colorectal Cancer Patients Receiving Chemotherapy: Secondary Analysis of Data From Prospective Clinical Trials. Clin Colorectal Cancer. 2021;20(2):170-176
47. Eckstrom J, Bartels T, Abraham I. et al. A single-arm, retrospective analysis of the incidence of febrile neutropenia using same-day versus next-day pegfilgrastim in patients with gastrointestinal cancers treated with FOLFOX or FOLFIRI. Support Care Cancer. 2019;27(3):873-878
48. Kim HS, Kim JH, Kim HJ. et al. Oxaliplatin, 5-fluorouracil and leucovorin (modified FOLFOX-6) as first-line chemotherapy for advanced gastric cancer patients with poor performance status. Oncol Lett. 2012;3(2):425-428
49. Smith RE. Trends in recommendations for myelosuppressive chemotherapy for the treatment of solid tumors. J Natl Compr Canc Netw. 2006;4(7):649-658
Author biography
Honggang Zheng is a chief physician with more than 20 years of work experience in the field of traditional Chinese medicine in preventing and treating tumors. He is mainly engaged in clinical practice, scientific research and teaching in the prevention and treatment of colorectal cancer, lung cancer, liver cancer, malignant melanoma, etc. He has presided over more than 10 major national and provincial-level projects. He once conducted a one-year visiting research work at the University of Kentucky in the United States, during which he was engaged in tumor molecular biology-related research.
Yanyuan Du is a researcher in Dr. Honggang Zheng's team. He is mainly engaged in clinical and scientific research on colorectal cancer, liver cancer, etc., mechanism research on cancer pain, and the establishment of related prediction models for malignant tumors.
Yuming Liu is a researcher in Dr. Honggang Zheng's team. He is mainly engaged in clinical and scientific research on colorectal cancer, liver cancer, etc., and the establishment of related prediction models for malignant tumors.
![]() Corresponding author: Honggang Zheng (Corresponding author): Department of Oncology, Guang'anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China. Post Address: No.5, Beixiange, Xicheng District, Beijing, 100053; Tel.: 86-10-88001138; Email address: honggangzhengcom.
Corresponding author: Honggang Zheng (Corresponding author): Department of Oncology, Guang'anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China. Post Address: No.5, Beixiange, Xicheng District, Beijing, 100053; Tel.: 86-10-88001138; Email address: honggangzhengcom.

 Global reach, higher impact
Global reach, higher impact